Published online Mar 27, 2022. doi: 10.4254/wjh.v14.i3.570
Peer-review started: September 27, 2021
First decision: December 2, 2021
Revised: December 10, 2021
Accepted: February 16, 2022
Article in press: February 16, 2022
Published online: March 27, 2022
Processing time: 178 Days and 12.6 Hours
Acute kidney injury (AKI) has serious consequences on the prognosis of patients undergoing liver transplantation. Recently, artificial neural network (ANN) was reported to have better predictive ability than the classical logistic regression (LR) for this postoperative outcome.
To identify the risk factors of AKI after deceased-donor liver transplantation (DDLT) and compare the prediction performance of ANN with that of LR for this complication.
Adult patients with no evidence of end-stage kidney dysfunction (KD) who underwent the first DDLT according to model for end-stage liver disease (MELD) score allocation system was evaluated. AKI was defined according to the International Club of Ascites criteria, and potential predictors of postoperative AKI were identified by LR. The prediction performance of both ANN and LR was tested.
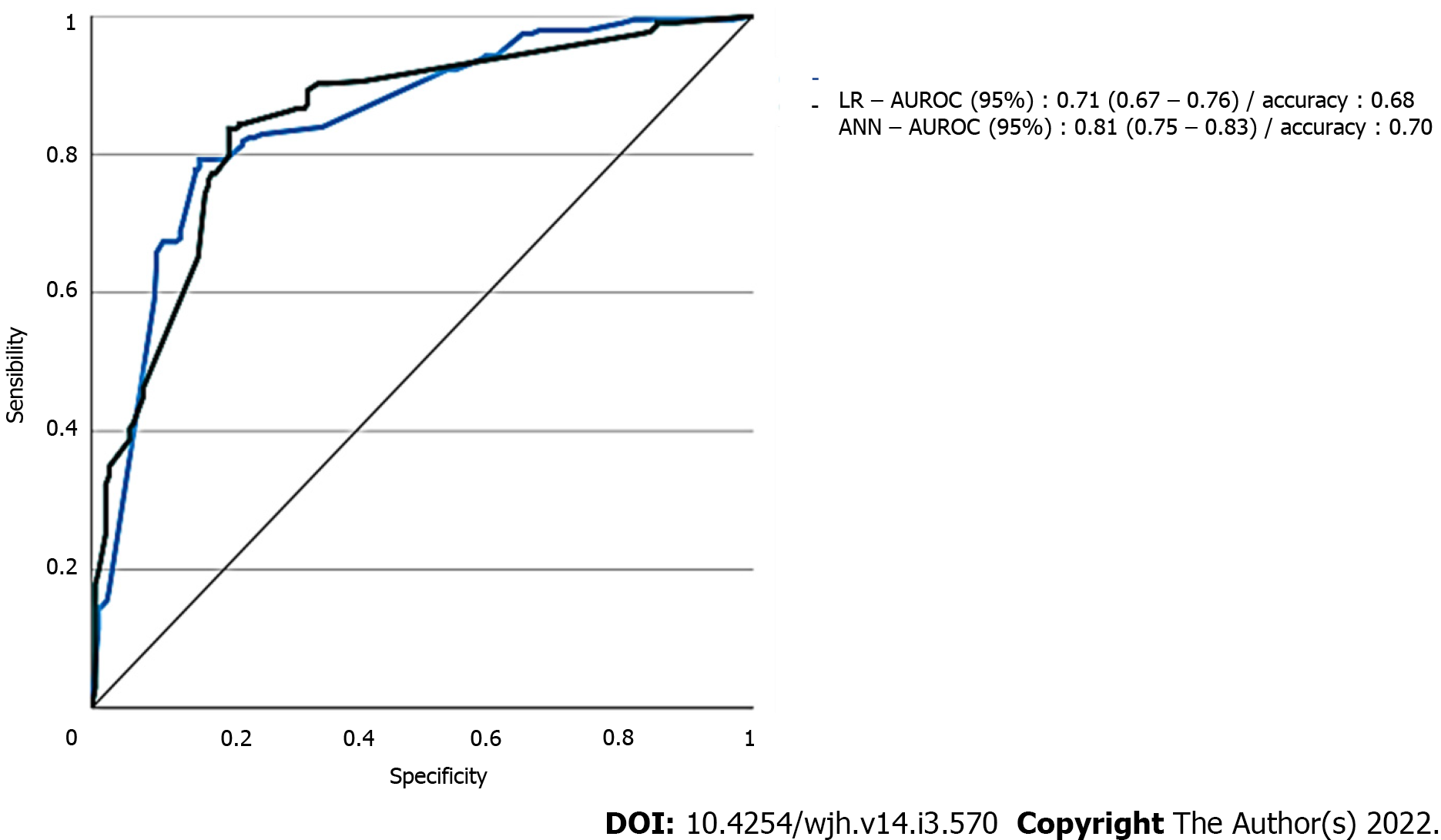
The incidence of AKI was 60.6% (n = 88/145) and the following predictors were identified by LR: MELD score > 25 (odds ratio [OR] = 1.999), preoperative kidney dysfunction (OR = 1.279), extended criteria donors (OR = 1.191), intraoperative arterial hypotension (OR = 1.935), intraoperative massive blood transfusion (MBT) (OR = 1.830), and postoperative serum lactate (SL) (OR = 2.001). The area under the receiver-operating characteristic curve was best for ANN (0.81, 95% confidence interval [CI]: 0.75-0.83) than for LR (0.71, 95%CI: 0.67-0.76). The root-mean-square error and mean absolute error in the ANN model were 0.47 and 0.38, respectively.
The severity of liver disease, pre-existing kidney dysfunction, marginal grafts, hemodynamic instability, MBT, and SL are predictors of postoperative AKI, and ANN has better prediction performance than LR in this scenario.
Core Tip: This study aimed to identify the risk factors of acute kidney injury (AKI) after deceased-donor liver transplantation and compare the performance of artificial neural network (ANN) with that of logistic regression (LR) analysis to predict this complication. LR analysis revealed the following predictors of AKI: Previous kidney dysfunction, marginal grafts, intra-operative arterial hypotension, massive blood transfusion, and serum lactate. ANN prediction had better performance than LR in this scenario.
- Citation: Bredt LC, Peres LAB, Risso M, Barros LCAL. Risk factors and prediction of acute kidney injury after liver transplantation: Logistic regression and artificial neural network approaches . World J Hepatol 2022; 14(3): 570-582
- URL: https://www.wjgnet.com/1948-5182/full/v14/i3/570.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i3.570
Among the possible complications of complex abdominal and liver procedures, acute kidney injury (AKI) should be considered a major cause of postoperative morbidity and mortality[1-6]. Updated data report a 0.9%-17.9% incidence of AKI after liver resection[7-9], and 4%-94% after LT[10,11], either living-donor (LDLT) or deceased-donor LT (DDLT). Although there is a lack of a reported standard definition of postoperative AKI[12] after DDLT, it is of fundamental importance to identify patients at risk for AKI after LT, ideally by the set of preoperative clinical evaluation, as well as by the complementary information of the intraoperative period, thus enabling the adoption of preventive measures or early therapies for AKI in the postoperative period.
There are many studies available based on deep learning models for different clinical purposes in distinct fields of medicine, such as for complex imaging acquisition and processing[13-17], and artificial neural network (ANN) as a deep learning modality is commonly used to solve complex problems, where the behavior of variables is not rigorously known. In the specific field of AKI after LT, along with other machine learning techniques (gradient boosting machine, random forest, decision tree, support vector machine, naïve Bayes, and deep belief network), ANN has already been compared to multivariable logistic regression (LR) regarding their prediction performance[18]. We hypothesized that ANN would be a feasible alternative with higher performance than the classic LR model, reinforcing the wide applicability of ANN and its ability to learn from input data with or without supervision.
The multifactorial origin of AKI after LT makes it complex to predict which candidate for the procedure has an increased risk of this complication, and in the face of this complexity, along with the classical LR, ANN would be a very reliable prognostic tool for AKI risk assessment, where the relative risk term is parameterized by an ANN instead of regression, enabling the application of deep learning, whereas comparative studies evaluating such a promising tool for predicting AKI following LT are scarce[19-20].
In face of this serious postoperative complication, this retrospective study of patients who underwent only-first DDLT aimed to identify the risk factors for postoperative AKI and compare the prediction performance of ANN with that of LR for this complication.
A retrospective study was conducted on patients of both sexes, aged > 18 yr, diagnosed with liver cirrhosis and portal hypertension (platelets < 100000/mm3, splenomegaly and/or esophageal varices), eventually associated with hepatocellular carcinoma (HCC), and undergoing the first DDLT at a tertiary referral hospital between September 2017 and June 2021. The patients were allocated according to Model for End-Stage Liver Disease (MELD) score, with no evidence of end-stage kidney disease. The MELD score was dichotomized at 25 points for statistical purposes according to Romano et al[21], and the minimum hospital stay was 7 d according to Wong et al[22] and the International Club of Ascites (ICA) definitions for the onset of AKI[23].
Kidney dysfunction (KD) subtypes were defined according to Wong et al[22] (Table 1) and the ICA definitions (Table 2)[23], and both the acute deterioration of renal function and the background CKD could be structural or functional in nature, including hepatorenal syndrome (HRS) types 1 and 2 (Table 3)[23]. Estimated glomerular filtration rate (eGFR) was calculated by the Modified Diet in Renal Disease 6 (MDRD6) formula: eGFR = 198 × [serum creatinine (mg/dL)−0.858 × age−0.167 × 0.822 if patient is female × 1.178 if patient is black] × [serum urea nitrogen concentration (mg/dL)]−0.293 × [urine urea nitrogen excretion (g/d)]0.249[3].
| Diagnosis | Definition |
| AKI | Rise in serum creatinine of > 50% from baseline or rise of sCr by > 26.4 mmol/L (> 0.3 mg/dL) in < 48 h; HRS type 1 is a specific form of AKI |
| CKD | eGFR of < 60 mL/min for > 3 mo calculated using MDRD6 formula; HRS type 2 is a specific form of CKD |
| ACKD | Rise in serum creatinine of > 50% from baseline or rise of sCr by > 26.4 mmol/L (> 0.3 mg/dL) in < 48 h in a patient with cirrhosis whose eGFR is < 60 ml/min for > 3 mo calculated using MDRD6 formula |
| Baseline sCr | A sCr value obtained in 3 mo prior to hospital admission, with preference to the value dated the closest to hospital admission. In patients without a previous sCr value, the value on admission should be used |
| AKI definition | Increase in sCr ≥ 0.3 mg/dL (≥ 26.5 µmol/L) within 48 h; or the percentage increase in sCr ≥ 50%, which occurred in the last 7 d |
| Stage 1 AKI | Increase in sCr ≥ 0.3 mg/dL (26.5 µmol/L) or an increase of 1.5 to 2 times the baseline value |
| Stage 2 AKI | Increase of sCr 2 to 3 times the baseline value |
| Stage 3 AKI | Increase in sCr > 3 times the baseline or sCr ≥ 4.0 mg/dL (353.6 µmol/L), with acute increase in sCr ≥ 0.3 mg/dL (26.5 µmol/L) or onset of RRT |
| Diagnostic criteria for HRS | HRS subtype |
| 1) Presence of cirrhosis or ascites; 2) sCr > 1.5 mg/dL or 133 µmoles/L; 3) No improvement in sCr (below 1.5 mg/dL) after at least 48 h of diuretic withdrawal and volume expansion with albumin; 4) Absence of shock; 5) Has not undergone recent treatment with nephrotoxic drugs; 6) Absence of parenchymal kidney disease as indicated by proteinuria less than 500 mg/d, microhematuria (less than 50 erythrocytes/high-magnification field), and/or abnormal renal ultrasound findings | HRS type 1-Rapidly progressive renal failure defined as the doubling of initial serum creatinine to a level greater than 2.5 mg/dL or 220 µmoles/L in less than 3 wk, and associated with a very poor prognosis; HRS type 2-Moderate renal failure (sCr > 1.5 mg/dL or 133 µmoles/L), following a stable or slowly progressive course, often associated with refractory ascites |
Marginal liver grafts of extended criteria donor (ECD) were defined as grafts with three or more of the following donor features: > 60 yr, body mass index (BMI) > 27-30 kg/m2, macrovesicular steatosis > 30%, intensive care unit (ICU) stay > 4 d, sustained arterial hypotension > 1 h, cold ischemia times (CIT) > 8 h, warm ischemia times (WIT) > 40-45 min, controlled sepsis, history of alcoholism, serum creatinine > 1.2 mg/dL, arterial hypotensive episodes < 60 mmHg for > 1 h, bilirubin > 2.0 mg/dL, alanine transaminase (ALT) > 170 U/L and aspartate transaminase (AST) > 140 U/L, the use of dopamine doses > 10 microg/kg per min, and peak serum sodium > 155 mEq/L[24-26].
Routine biopsy was performed on the donor allograft for all patients included in the study. Liver specimens were evaluated by hematoxylin and eosin staining using either frozen or permanent section. Macrovesicular steatosis was defined as a single vacuole larger than the nucleus, replacing most of the hepatocyte cytoplasm and displacing the nucleus to the cell membrane[27]. Macrosteatosis was categorized as no steatosis (< 5%), mild steatosis (10%-29%), moderate steatosis (30%-60%), and severe steatosis (> 60%)[28].
Fluid administration consisted of a baseline infusion of a balanced crystalloid (Plasmalyte, Baxter, Belgium) with or without 4% albumin (depending on patient conditions). Rapid infusers, perfusion heaters, and a Cell Saver (Haemonetics, Massachusetts, EUA) for blood recovery were ready for use prior to induction. In accordance to American Society of Anaesthesiologists (ASA) guidelines, Cell Saver has effectiveness in reducing the volume of allogeneic blood transfused[29].
A Flow Trac/EV1000 System (Edwards Lifesciences, Irvine, USA) was inserted and hemodynamic interventions were guided using continuous cardiac index (CCI), stroke volume index (SVI), mixed venous oxygen saturation (SvO2), central venous pressure (CVP), and mean arterial pressure (MAP). Fluids were administered if SVI was < 30 mL/m2 and/or CCI < 2 L/min/m2 for compensation for blood loss via 250-500 mL fluid boluses of Plasmalyte, to strictly maintain MAP > 65 mmHg, avoiding hemodynamic instability as described elsewhere[30,31].
Blood loss monitoring consisted of visual assessment of the surgical field, including the extent of blood present, presence of microvascular bleeding, surgical sponges, clot size and shape, and volume in suction canister. In case of active hemorrhage, blood product administration was guided by using rotational thromboelastometry monitoring via ROTEM (Tem Innovations GmbH, Munich, Germany), hemoglobin/hematocrit monitoring, coagulation tests (international normalized ratio [INR]), activated partial thromboplastin time [aPTT], fibrinogen concentration [normal range: 200 to 400 mg/dL], and platelet count[29]. Whereas there is no clear evidence that ROTEM improved survival in LT patients, it was effective in reducing bleeding and fewer patients required both platelets and fresh frozen plasma (FFP) transfusion[32]. Monitoring for perfusion of vital organs included standard ASA monitoring, renal monitoring (urine output), and analysis of arterial blood gases and serum (SL) level (cutoff of 2.0 mmol/L)[29].
Massive blood transfusion (MBT) protocol for avoidance of dilutional coagulopathy was activated when hemorrhage was expected to be massive (anticipated need to replace 50% or more of blood volume within 2 h), or bleeding continued after the transfusion of 4 units of packed red blood cells (PRBC) within a short period of time (1-2 h), or systolic blood pressure (SBP) was below 90 mmHg and heart rate was above 120 beats per minute in the presence of uncontrolled bleeding[33]. According to the Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) study group recommendations, blood transfusion of RBC, fresh frozen plasma (FFP), and platelets were at a 1:1:1 ratio[34].
Postreperfusion (PRS) was defined as a decrease in MAP > 30% below the baseline value, for at least 1 min, occurring during the first 5 min after reperfusion of the liver graft, asystole, or hemodynamically significant arrhythmias, or the need to start the infusion of vasopressors during the postreperfusion period[35]. Intraoperative arterial hypotension (IOAH) was defined as MAP less than 65-60 mmHg for at least 5 min, or any exposure to MAP less than 55-50 mmHg[31], irrespective of the cause: Prolonged surgery time, massive bleeding, PRS, and/or hemodynamic instability because of end-stage liver disease.
The baseline characteristics of the patients are expressed in absolute values, the mean ± SD, and percentages, when appropriate. The comparison between groups was performed for continuous variables using the Kruskal-Wallis test and the Mann-Whitney test. The assumptions were made to perform or not the parametric tests, and the categorical variables were compared using the chi-square test. Independent variables with significance in the univariate model was selected for the bootstrap classical LR model to assess the effect of bivariate independent variables (graft quality, patients characteristics, and intraoperative events) on the incidence of postoperative AKI. The results of the model are expressed by odds ratio (OR), together with the corresponding 95% confidence intervals [CIs], Nagelkerke R2 statistic, and Hosmer and Lemeshow goodness of fit test. P values < 0.05 were considered significant. A relationship map between the significant variables in the LR model was also constructed.
The explanatory variables selected in the LR model were used for the ANN machine learning. Before developing prediction models, our collected data were divided into 70% of training dataset cases and 30% of test dataset cases. The cases in the training dataset were used for developing machine learning models. The ANN method had its own hyperparameters (number of layers in multilayer perceptron ANN), with a 10-fold cross-validation. This cross-validation process was used for developing the model, and performance was evaluated. The activation function of the hidden layer was made by hyperbolic tangent activation function, and Softmax for the output layer. All possible combinations of hyperparameters were investigated, and the hyperparameters with the highest average validation AUROC (area under the receiver-operating characteristic curve) were considered as optimal hyperparameters, and after that, the final model was tested for performance by root-mean-square error (RMSE) and mean absolute error (MAE) calculation. The importance of variables for the model was calculated. ANN structural model was constructed according to Haykin[36].
Our primary analysis attempted to analyze the prediction ability of machine learning and LR model in terms of AUROC. Accuracy was defined as the sum of the number of cases with true positive and true negative results divided by the total number of test sets. Statistical calculations were performed using the SPSS 28.0 software for Windows.
During the period from September 2017 to June 2021, 145 DDLT cases were included in the present study. Of the total patients included, 88 (60.6%) presented any further stage of postoperative AKI during the 7-d follow-up, 22 (15.1%) developed stage 1 AKI, 36 (24.8%) developed stage 2, and 30 (20.6%) developed stage 3 AKI (Table 4); renal replacement therapy (RRT) was required in 12 patients (8.7%). All patients’ preoperative baseline information, donors, and grafts characteristics according to the occurrence of AKI are shown in Tables 5 and 6. The intraoperative data related to IOAH, blood derivatives transfusion, and piggy-back clamping, and laboratorial tests until the seventh postoperative (PO) day are shown in Table 7.
| Overall incidence (n = 88) | Stage 1 (n = 22) | Stage 2 (n = 36) | Stage 3/RRT (n = 30/12) |
| 60.6% | 15.1% | 24.8% | 20.6/8.7% |
| No AKI (n = 57) | AKI (n = 88) | P value | |
| Male gender, n (%) | 29 (50.8) | 49 (55.6) | 0.441 |
| Age (yr), mean (± SD) | 53.2 (± 13.56) | 56.2 (± 13.26) | 0.352 |
| BMI, mean (± SD) | 18.2(± 4.54) | 22.7 (± 4.92) | 0.065 |
| Biological MELD score, mean (± SD) | 21.67 (± 2.15) | 26.05 (± 3.05) | < 0.001 |
| Previous ascites, n (%) | 24 (42.1) | 52 (59.0) | 0.013 |
| Previous encephalopathy, n (%) | 18 (31.5) | 39 (44.3) | 0.025 |
| Previous upper digestive bleeding, n (%) | 21 (36.8) | 45 (51.1) | 0.018 |
| Preexisting KD, n (%) | 15 (26.3) | 60 (68.1) | < 0.001 |
| HCC, n (%) | 20 (35.0) | 37 (42.0) | 0.069 |
| Systemic arterial hypertension, n (%) | 28 (49.1) | 46 (52.2) | 0.083 |
| Diabetes mellitus, n (%) | 23 (40.3) | 43 (48.8) | 0.254 |
| No AKI (n = 57) | AKI (n = 88) | P value | |
| Donor > 60 yr, n (%) | 16 (28.0) | 31 (35.2) | 0.346 |
| Donor BMI > 27-30 kg/m2, n (%) | 14(24.5) | 28 (31.8) | 0.039 |
| Graft macrosteatosis > 30%, n (%) | 11 (19.2) | 32 (36.3) | 0.024 |
| GCIT > 8 h, n (%) | 0 | 0 | - |
| GWIT > 40-45 min | 38 (66.6) | 54 (61.3) | 0.349 |
| Donor ICU stay > 4 d, n (%) | 11 (19.2) | 22 (25.0) | 0.088 |
| Donor controlled sepsis, n (%) | 05 (8.7) | 11 (12.5) | 0.061 |
| History of alcoholism of donor, n (%) | 08 (14.0) | 15 (17.0) | 0.255 |
| Donor sCr > 1.2 mg/dL, n (%) | 16 (28.0) | 31 (35.2) | 0.024 |
| Donor hypotensive episodes (< 60 mmHg) > 1 h, n (%) | 10 (17.5) | 18 (20.4) | 0.127 |
| Donor serum bilirubin > 2.0 mg/dL, n (%) | 25 (43.8) | 48 (54.5) | 0.087 |
| Donor serum ALT > 170 U/L, n (%) | 11 (19.2) | 22 (25.0) | 0.073 |
| Donor serum AST > 140 U/L, n (%) | 05 (8.7) | 13 (14.7) | 0.023 |
| Use of dopamine doses > 10 microg/kg per min, n (%) | 10 (17.5) | 13 (14.7) | 0.176 |
| Donor peak serum sodium > 155 mEq/L, n (%) | 02 (3.5) | 5 (5.6) | 0.219 |
| ECD (3 or more factors above), n (%) | 07 (12.2) | 31 (35.2) | < 0.001 |
| Without AKI (n = 57) | With AKI (n = 88) | P value | |
| IOAH (bleeding/PRS), n (%) | 14 (24.5) | 54 (61.3) | < 0.001 |
| MBT, n (%) | 5 (8.7) | 15 (17.0) | < 0.001 |
| Vasoactive drugs, n (%) | 38(66.6) | 48 (54.5) | 0.197 |
| Cryoprecipitate transfusion, n (%) | 10 (17.5) | 18 (20.4) | 0.169 |
| Piggy-back clamping, n (%) | 30 (52.6) | 48 (54.5) | 0.072 |
| SL (mmol/L) at the end of LT, mean (± SD) | 1.4 (± 0.3) | 2.8 (± 0.7) | < 0.001 |
| Lower serum fibrinogen (mg/dL), mean (± SD) | 242 (± 34) | 214 (± 24) | 0.090 |
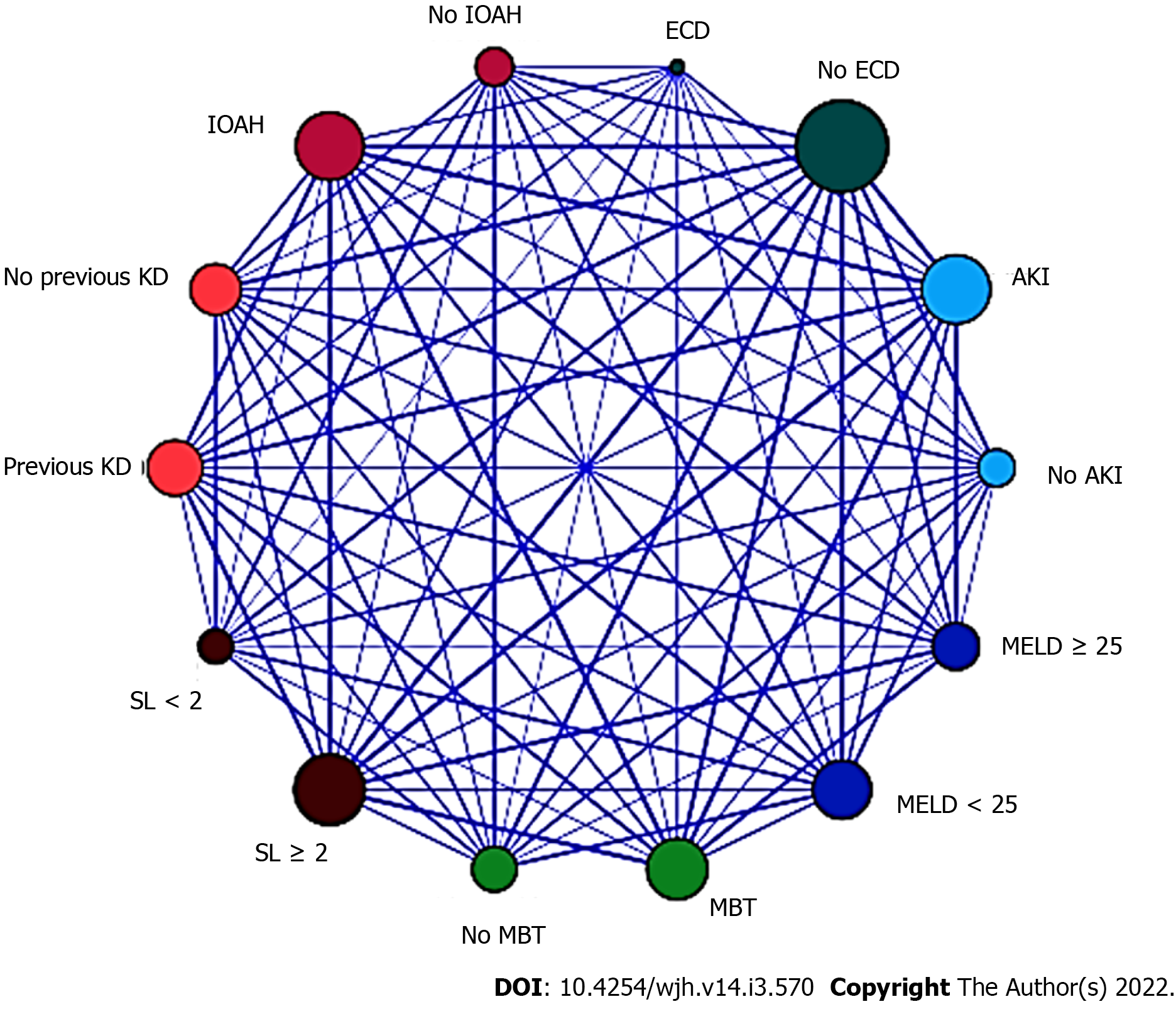
In the LR analysis, Nagelkerke R2 statistic was 0.147. Hosmer and Lemeshow goodness of fit test was not significant at 5% (P = 0.247). The six following factors were confirmed as predictors (Table 8): Biological (not adjusted) MELD score ≥ 25 (OR = 1.999, 95%CI = 1.586-2.503, P < 0.001), pre-existing KD (OR = 1.279, 95%CI = 0.916-1.686, P < 0.001), ECD (OR = 1.191, 95%CI = 0.711-1.787, P = 0.002), IOAH (OR = 1.935, 95%CI = 1.505-2.344, P < 0.001), MBT (OR = 1.830, 95%CI = 1.428-2.241, P < 0.001), serum lactate at the end of LT (OR = 2.001, 95%CI = 1.616-2.421, P < 0.001). The relationships between the significant variables were explored by a relationship map detailed in Figure 1.
| Logistic regression | Beta coeficient | OR | 95%CI | P value | |
| Biological MELD score ≥ 25 | 0.194 | 1.999 | 1.586 | 2.503 | < 0.001 |
| Pre-existing KD, n (%) | 0.115 | 1.279 | 0.916 | 1.686 | < 0.001 |
| ECD (3 or more factors above) | 0.911 | 1.191 | 0.711 | 1.787 | 0.002 |
| IOAH (bleeding/PRS), n (%) | 0.169 | 1.935 | 1.505 | 2.344 | < 0.001 |
| MBT, n (%) | 0.125 | 1.830 | 1.428 | 2.241 | < 0.001 |
| SL (mmol/L) ≥ 2.0 at the end of LT | 0.110 | 2.001 | 1.616 | 2.421 | < 0.001 |
Data of the two models with regard to AUROC for predicting AKI of all stages are detailed in Figure 2. ANN had the largest test AUROC (0.81, 95%CI: 0.75-0.83) and highest accuracy (0.68) than LR analysis [AUROC (0.71, 95%CI: 0.67 to 0.76), accuracy = 0.68].
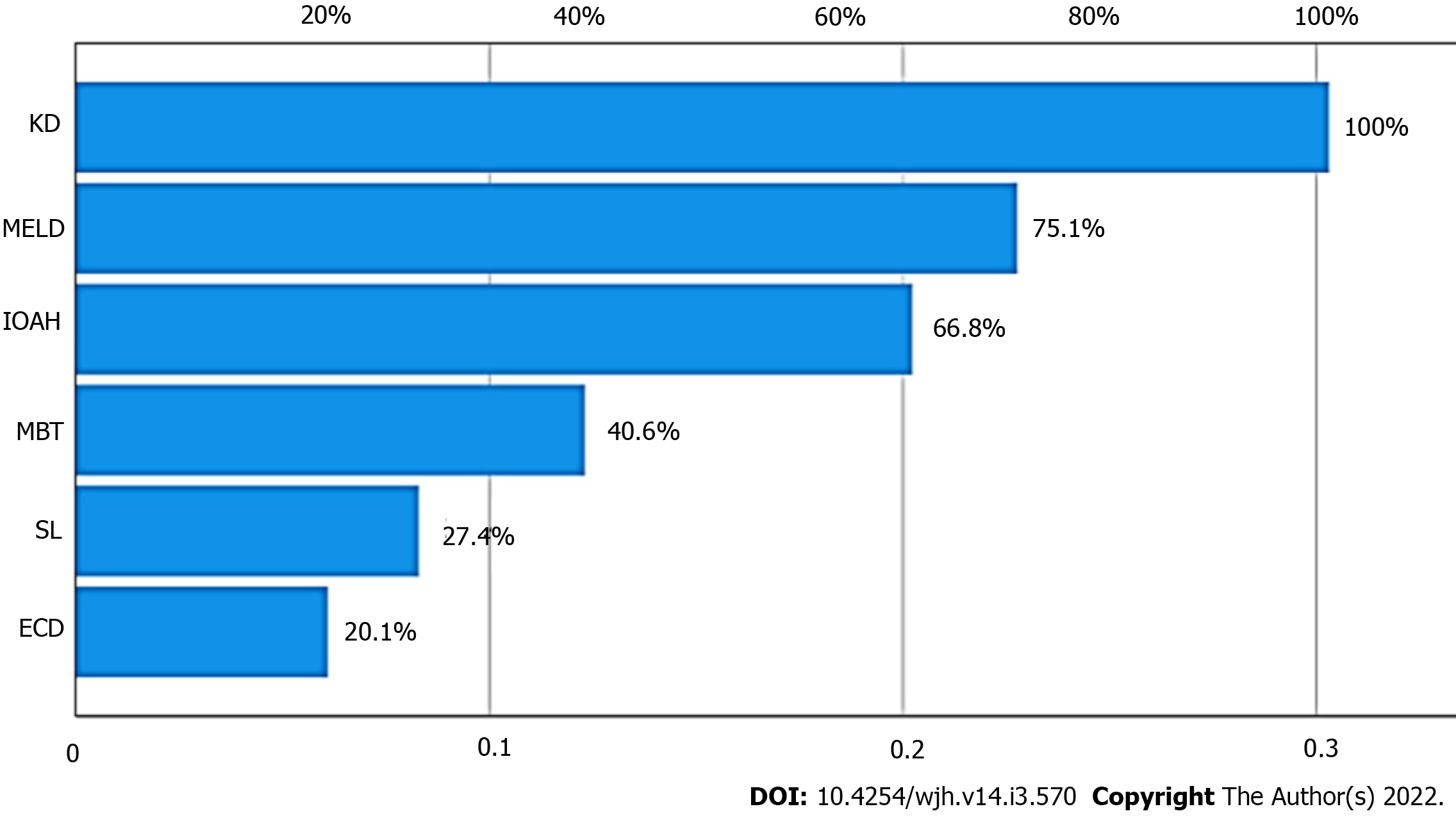
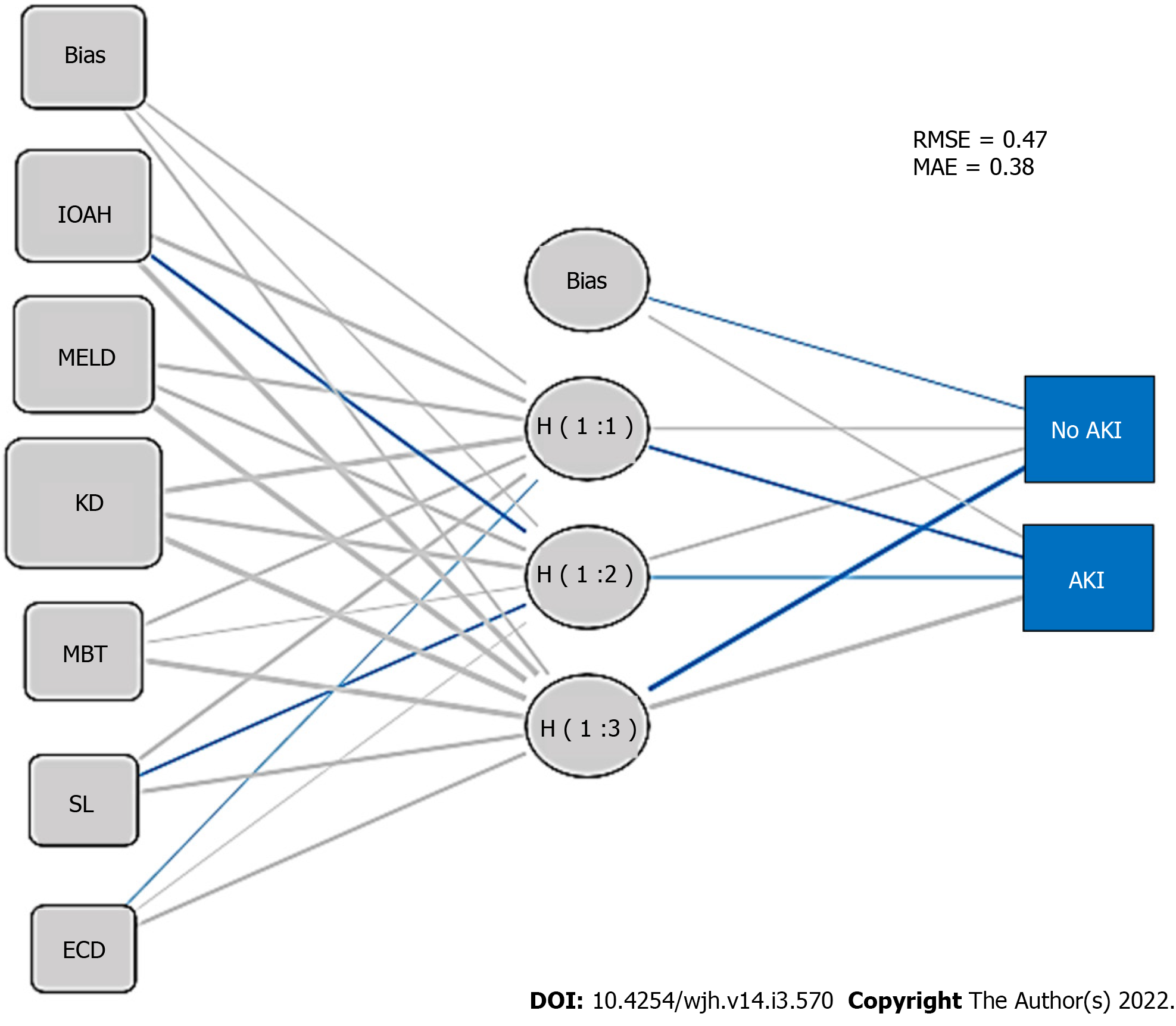
Importance plot for ANN is shown in Figure 3 (KD and MELD score ranked first and second, respectively). Multilayer perceptron ANN presented one hidden layer by hyperbolic tangent activation function with four nodes in the layer, as presented in the ANN structural model diagram (Figure 4), and the prediction RMSE was 0.47 and the prediction MAE was 0.38.
As described elsewhere[36], the findings in the present study demonstrated a high incidence of postoperative AKI, and the predictive ability of ANN and LR models for this complication. An important point in this research is that AKI prediction was focused on the identification of significant risk factors at the end of the procedure, thus enabling the adoption of preventive measures or early therapies for AKI in the postoperative period.
In the present study, the severity of chronic liver disease, pre-existing KD, marginal grafts, hemodynamic instability, MBT, and consequent inadequate tissue perfusion during LT were predictors of AKI after DDLT, and the relationship map illustrated through a visual pattern, the relationship between the variables, although it is important to understand that a visual relationship does not always mean statistical causation. As demonstrated in our study, in the case of machine learning-based techniques, the importance of each variable in the dataset can be indicated by the characteristic importance measure, which can improve the transparency of the algorithm according to He et al[20].
According to our results, ANN had larger AUROC and higher accuracy to predict AKI after DDLT than LR, which is consistent with the previous study with different machine learning tools, whereas the performance of the ANN was inferior to that of all other machine learning techniques in prediction of AKI after LT[19]. Multilayer perceptron has already been associated to a good performance in predicting in-hospital mortality, reinforcing the good performance of ANN to predict clinical outcomes, although there have been some reports that the performance of the machine learning techniques is not superior to that of LR model in predicting mortality[18].
Regarding the risk factors identified in the present research, several other authors have already described that higher MELD scores[37] were associated with AKI after LT[20,38]. Xu et al[21] showed that MELD score > 25 was a predictor of AKI, and in patients with MELD scores > 30, the most required RRT[11,39]. Moreover, in the cirrhosis scenario, the functional renal disorders can be added as risk factors for AKI, such as recipient HRS[11,23,40]. Donor marginal liver grafts of ECD were identified elsewhere as a strong predictor of PGD[24-26] and post-LT AKI[20]. Patients undergoing LT can experience IOAH and consequent AKI because of multiple factors, including the duration of surgery, massive bleeding[16,40-42], the severity of the PRS[36,43,44], and the severity of the end-stage liver disease[21,45-49]. In addition, MBT may be an additional risk factor for postoperative AKI[34,49,50].
The present retrospective study has important limitations, regarding sample size and moreover, the lack of evaluation of clinical outcomes of patients according to the occurrence of post-LT AKI, either for short or long-term evolution of patients. Despite these limitations, the high incidence of AKI reported highlights the importance of this issue, and the predictors identified may provide a focus for further research. ANN methods may provide feasible tools for forecasting AKI after LT, and perhaps provide a high-performance predictive model that may ultimately improve perioperative management of these patients at risk for this serious complication.
According to our results, the severity of chronic liver disease, pre-existing KD, marginal grafts, hemodynamic instability, MBT, and inadequate tissue perfusion during LT are predictors of AKI after DDLT, and ANN has better prediction performance than LR in this scenario.
Acute kidney injury (AKI) post-liver transplantation (LT) is a serious complication, and its prediction with validated tools is crucial.
To improve the perioperative management of patient candidates for LT.
To identify the risk factors for AKI after deceased-donor liver transplantation (DDLT) and validate a prediction tool for this complication.
Logistic regression (LR) analysis for predictor identification, and comparative analysis of artificial neural network (ANN) and LR prediction performance were performed.
The severity of liver disease, preexisting kidney dysfunction, marginal grafts, hemodynamic instability, massive blood transfusion, and SL were predictors of postoperative AKI, and ANN had better prediction performance than LR.
ANN has better performance than the classical LR for AKI prediction after DDLT.
A risk score of AKI after DDLT can be developed according to these identified predictors.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Balakrishnan DS, Byeon H, Kumar I S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 733] [Article Influence: 40.7] [Reference Citation Analysis (1)] |
| 2. | Thongprayoon C, Kaewput W, Thamcharoen N, Bathini T, Watthanasuntorn K, Lertjitbanjong P, Sharma K, Salim SA, Ungprasert P, Wijarnpreecha K, Kröner PT, Aeddula NR, Mao MA, Cheungpasitporn W. Incidence and Impact of Acute Kidney Injury after Liver Transplantation: A Meta-Analysis. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 3. | Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11183] [Cited by in RCA: 11819] [Article Influence: 454.6] [Reference Citation Analysis (0)] |
| 4. | Bihorac A, Yavas S, Subbiah S, Hobson CE, Schold JD, Gabrielli A, Layon AJ, Segal MS. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 382] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 5. | Abelha FJ, Botelho M, Fernandes V, Barros H. Outcome and quality of life of patients with acute kidney injury after major surgery. Nefrologia. 2009;29:404-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 6. | Barri YM, Sanchez EQ, Jennings LW, Melton LB, Hays S, Levy MF, Klintmalm GB. Acute kidney injury following liver transplantation: definition and outcome. Liver Transpl. 2009;15:475-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 188] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 7. | Tomozawa A, Ishikawa S, Shiota N, Cholvisudhi P, Makita K. Perioperative risk factors for acute kidney injury after liver resection surgery: an historical cohort study. Can J Anaesth. 2015;62:753-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Lim C, Audureau E, Salloum C, Levesque E, Lahat E, Merle JC, Compagnon P, Dhonneur G, Feray C, Azoulay D. Acute kidney injury following hepatectomy for hepatocellular carcinoma: incidence, risk factors and prognostic value. HPB (Oxford). 2016;18:540-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Bredt LC, Peres LAB. Risk factors for acute kidney injury after partial hepatectomy. World J Hepatol. 2017;9:815-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Kalisvaart M, Schlegel A, Umbro I, de Haan JE, Polak WG, IJzermans JN, Mirza DF, Perera MTP, Isaac JR, Ferguson J, Mitterhofer AP, de Jonge J, Muiesan P. The AKI Prediction Score: a new prediction model for acute kidney injury after liver transplantation. HPB (Oxford). 2019;21:1707-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Zhou J, Zhang X, Lyu L, Ma X, Miao G, Chu H. Modifiable risk factors of acute kidney injury after liver transplantation: a systematic review and meta-analysis. BMC Nephrol. 2021;22:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Caragata R, Wyssusek KH, Kruger P. Acute kidney injury following liver transplantation: a systematic review of published predictive models. Anaesth Intensive Care. 2016;44:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Bellotti R, De Carlo F, Tangaro S, Gargano G, Maggipinto G, Castellano M, Massafra R, Cascio D, Fauci F, Magro R, Raso G, Lauria A, Forni G, Bagnasco S, Cerello P, Zanon E, Cheran SC, Lopez Torres E, Bottigli U, Masala GL, Oliva P, Retico A, Fantacci ME, Cataldo R, De Mitri I, De Nunzio G. A completely automated CAD system for mass detection in a large mammographic database. Med Phys. 2006;33:3066-3075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Wang J, Yang X, Cai H, Tan W, Jin C, Li L. Discrimination of Breast Cancer with Microcalcifications on Mammography by Deep Learning. Sci Rep. 2016;6:27327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 146] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 15. | Kooi T, Litjens G, van Ginneken B, Gubern-Mérida A, Sánchez CI, Mann R, den Heeten A, Karssemeijer N. Large scale deep learning for computer aided detection of mammographic lesions. Med Image Anal. 2017;35:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 462] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 16. | Chang PD, Kuoy E, Grinband J, Weinberg BD, Thompson M, Homo R, Chen J, Abcede H, Shafie M, Sugrue L, Filippi CG, Su MY, Yu W, Hess C, Chow D. Hybrid 3D/2D Convolutional Neural Network for Hemorrhage Evaluation on Head CT. AJNR Am J Neuroradiol. 2018;39:1609-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 17. | Rachmadi MF, Valdés-Hernández MDC, Li H, Guerrero R, Meijboom R, Wiseman S, Waldman A, Zhang J, Rueckert D, Wardlaw J, Komura T. Limited One-time Sampling Irregularity Map (LOTS-IM) for Automatic Unsupervised Assessment of White Matter Hyperintensities and Multiple Sclerosis Lesions in Structural Brain Magnetic Resonance Images. Comput Med Imaging Graph. 2020;79:101685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Lee CK, Hofer I, Gabel E, Baldi P, Cannesson M. Development and Validation of a Deep Neural Network Model for Prediction of Postoperative In-hospital Mortality. Anesthesiology. 2018;129:649-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 19. | Lee HC, Yoon SB, Yang SM, Kim WH, Ryu HG, Jung CW, Suh KS, Lee KH. Prediction of Acute Kidney Injury after Liver Transplantation: Machine Learning Approaches vs. Logistic Regression Model. J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 20. | He ZL, Zhou JB, Liu ZK, Dong SY, Zhang YT, Shen T, Zheng SS, Xu X. Application of machine learning models for predicting acute kidney injury following donation after cardiac death liver transplantation. Hepatobiliary Pancreat Dis Int. 2021;20:222-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Romano TG, Schmidtbauer I, Silva FM, Pompilio CE, D'Albuquerque LA, Macedo E. Role of MELD score and serum creatinine as prognostic tools for the development of acute kidney injury after liver transplantation. PLoS One. 2013;8:e64089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Wong F, Nadim MK, Kellum JA, Salerno F, Bellomo R, Gerbes A, Angeli P, Moreau R, Davenport A, Jalan R, Ronco C, Genyk Y, Arroyo V. Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut. 2011;60:702-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 287] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 23. | Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, Moreau R, Jalan R, Sarin SK, Piano S, Moore K, Lee SS, Durand F, Salerno F, Caraceni P, Kim WR, Arroyo V, Garcia-Tsao G; International Club of Ascites. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut. 2015;64:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 387] [Article Influence: 38.7] [Reference Citation Analysis (1)] |
| 24. | Briceño J, Ciria R, de la Mata M, Rufián S, López-Cillero P. Prediction of graft dysfunction based on extended criteria donors in the model for end-stage liver disease score era. Transplantation. 2010;90:530-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (35)] |
| 25. | Chen XB, Xu MQ. Primary graft dysfunction after liver transplantation. Hepatobiliary Pancreat Dis Int. 2014;13:125-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 26. | Chu MJ, Dare AJ, Phillips AR, Bartlett AS. Donor Hepatic Steatosis and Outcome After Liver Transplantation: a Systematic Review. J Gastrointest Surg. 2015;19:1713-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 27. | Rajamani AS, Rammohan A, Sai VR, Rela M. Non-invasive real-time assessment of hepatic macrovesicular steatosis in liver donors: Hypothesis, design and proof-of-concept study. World J Hepatol. 2021;13:1208-1214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Ploeg RJ, D'Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, Sasaki T, Sollinger HW, Belzer FO, Kalayoglu M. Risk factors for primary dysfunction after liver transplantation--a multivariate analysis. Transplantation. 1993;55:807-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 804] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 29. | American Society of Anesthesiologists Task Force on Perioperative Blood Management. Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management. Anesthesiology. 2015;122:241-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 485] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 30. | Salmasi V, Maheshwari K, Yang D, Mascha EJ, Singh A, Sessler DI, Kurz A. Relationship between Intraoperative Hypotension, Defined by Either Reduction from Baseline or Absolute Thresholds, and Acute Kidney and Myocardial Injury after Noncardiac Surgery: A Retrospective Cohort Analysis. Anesthesiology. 2017;126:47-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 722] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 31. | Joosten A, Lucidi V, Ickx B, Van Obbergh L, Germanova D, Berna A, Alexander B, Desebbe O, Carrier FM, Cherqui D, Adam R, Duranteau J, Saugel B, Vincent JL, Rinehart J, Van der Linden P. Intraoperative hypotension during liver transplant surgery is associated with postoperative acute kidney injury: a historical cohort study. BMC Anesthesiol. 2021;21:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Afshari A, Wikkelsø A, Brok J, Møller AM, Wetterslev J. Thrombelastography (TEG) or thromboelastometry (ROTEM) to monitor haemotherapy versus usual care in patients with massive transfusion. Cochrane Database Syst Rev. 2011;CD007871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 33. | Pacheco LD, Saade GR, Costantine MM, Clark SL, Hankins GD. An update on the use of massive transfusion protocols in obstetrics. Am J Obstet Gynecol. 2016;214:340-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, Cohen MJ, Cotton BA, Fabian TC, Inaba K, Kerby JD, Muskat P, O'Keeffe T, Rizoli S, Robinson BR, Scalea TM, Schreiber MA, Stein DM, Weinberg JA, Callum JL, Hess JR, Matijevic N, Miller CN, Pittet JF, Hoyt DB, Pearson GD, Leroux B, van Belle G; PROPPR Study Group. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313:471-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1527] [Cited by in RCA: 1685] [Article Influence: 168.5] [Reference Citation Analysis (0)] |
| 35. | Uemura T, Randall HB, Sanchez EQ, Ikegami T, Narasimhan G, McKenna GJ, Chinnakotla S, Levy MF, Goldstein RM, Klintmalm GB. Liver retransplantation for primary nonfunction: analysis of a 20-year single-center experience. Liver Transpl. 2007;13:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2067] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 38. | Wiesner RH, McDiarmid SV, Kamath PS, Edwards EB, Malinchoc M, Kremers WK, Krom RA, Kim WR. MELD and PELD: application of survival models to liver allocation. Liver Transpl. 2001;7:567-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 673] [Cited by in RCA: 605] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 39. | Nadim MK, Genyk YS, Tokin C, Fieber J, Ananthapanyasut W, Ye W, Selby R. Impact of the etiology of acute kidney injury on outcomes following liver transplantation: acute tubular necrosis versus hepatorenal syndrome. Liver Transpl. 2012;18:539-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 40. | Durand F, Graupera I, Ginès P, Olson JC, Nadim MK. Pathogenesis of Hepatorenal Syndrome: Implications for Therapy. Am J Kidney Dis. 2016;67:318-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 41. | Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361:1279-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 542] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 42. | Xu ZD, Xu HT, Yuan HB, Zhang H, Ji RH, Zou Z, Fu ZR, Shi XY. Postreperfusion syndrome during orthotopic liver transplantation: a single-center experience. Hepatobiliary Pancreat Dis Int. 2012;11:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Valentine E, Gregorits M, Gutsche JT, Al-Ghofaily L, Augoustides JG. Clinical update in liver transplantation. J Cardiothorac Vasc Anesth. 2013;27:809-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Park MH, Shim HS, Kim WH, Kim HJ, Kim DJ, Lee SH, Kim CS, Gwak MS, Kim GS. Clinical Risk Scoring Models for Prediction of Acute Kidney Injury after Living Donor Liver Transplantation: A Retrospective Observational Study. PLoS One. 2015;10:e0136230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 45. | Lebrón Gallardo M, Herrera Gutierrez ME, Seller Pérez G, Curiel Balsera E, Fernández Ortega JF, Quesada García G. Risk factors for renal dysfunction in the postoperative course of liver transplant. Liver Transpl. 2004;10:1379-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 46. | Utsumi M, Umeda Y, Sadamori H, Nagasaka T, Takaki A, Matsuda H, Shinoura S, Yoshida R, Nobuoka D, Satoh D, Fuji T, Yagi T, Fujiwara T. Risk factors for acute renal injury in living donor liver transplantation: evaluation of the RIFLE criteria. Transpl Int. 2013;26:842-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 47. | Lee SK, Park JB, Kim SJ, Choi GS, Kim DJ, Kwon CH, Lee SK, Joh JW. Early postoperative renal dysfunction in the adult living donor liver transplantation. Transplant Proc. 2007;39:1517-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Licata A, Mazzola A, Ingrassia D, Calvaruso V, Cammà C, Craxì A. Clinical implications of the hyperdynamic syndrome in cirrhosis. Eur J Intern Med. 2014;25:795-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Karkouti K, Wijeysundera DN, Yau TM, McCluskey SA, Chan CT, Wong PY, Crowther MA, Hozhabri S, Beattie WS. Advance targeted transfusion in anemic cardiac surgical patients for kidney protection: an unblinded randomized pilot clinical trial. Anesthesiology. 2012;116:613-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 50. | van de Watering L. Red cell storage and prognosis. Vox Sang. 2011;100:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |