Published online Mar 27, 2022. doi: 10.4254/wjh.v14.i3.516
Peer-review started: June 25, 2021
First decision: July 27, 2021
Revised: August 4, 2021
Accepted: February 23, 2022
Article in press: February 23, 2022
Published online: March 27, 2022
Processing time: 272 Days and 13 Hours
Hepatitis E virus (HEV) infections are generally self-limited. Rare cases of hepatitis E induced fulminant liver failure requiring liver transplantation are reported in the literature. Even though HEV infection is generally encountered among developing countries, a recent uptrend is reported in developed countries. Consumption of unprocessed meat and zoonosis are considered to be the likely transmission modalities in developed countries. Renal involvement of HEV generally holds a benign and self-limited course. Although rare cases of cryoglobulinemia are reported in immunocompetent patients, glomerular manifestations of HEV infection are frequently encountered in immunocompromised and solid organ transplant recipients. The spectrum of renal manifestations of HEV infection include pre-renal failure, glomerular disorders, tubular and interstitial injury. Kidney biopsy is the gold standard diagnostic test that confirms the pattern of injury. Management predominantly includes conservative approach. Reduction of immunosuppressive medications and ribavirin (for 3-6 mo) is considered among patients with solid organ transplants. Here we review the clinical course, pathogenesis, renal manifestations, and management of HEV among immunocompetent and solid organ transplant recipients.
Core Tip: Hepatitis E virus (HEV) infection is infrequently associated with significant mortality and morbidity. HEV infection is not only restricted to developing countries, but is also identified among developed nations and predominantly holds zoonotic transmission. Renal manifestations of HEV infection range from acute tubular necrosis to immune-mediated glomerular injury. Conservative approach is routinely employed in management of acute kidney injury from HEV. Ribavirin and reduction of immunosuppression are considered among patients with solid organ transplants as they are prone to develop chronic hepatitis E infection. Plasma exchange and pulse steroids are sometimes used in management of crescentic glomerular nephritis associated with HEV infection.
- Citation: Kovvuru K, Carbajal N, Pakanati AR, Thongprayoon C, Hansrivijit P, Boonpheng B, Pattharanitima P, Nissaisorakarn V, Cheungpasitporn W, Kanduri SR. Renal manifestations of hepatitis E among immunocompetent and solid organ transplant recipients. World J Hepatol 2022; 14(3): 516-524
- URL: https://www.wjgnet.com/1948-5182/full/v14/i3/516.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i3.516
Hepatitis E virus (HEV) has a pronounced worldwide distribution. It is a spherical, single-strand RNA virus consisting of three partially overlapping open reading frames (ORF) ORF1, ORF2, and ORF3[1]. HEV belongs to hepeviridae family, and eight genotypes of HEV (HEV1 to HEV 8) have been identified[2,3]. Genotypes HEV1 and HEV2 are routinely encountered in developing countries and are transmitted through fecal-oral route. HEV3 and HEV4 are associated with sporadic autochthonous infection among western countries and are predominantly transmitted through animal reservoirs and ingestion of uncooked meat[4-6]. Additionally, HEV genome 3 related infection is associated with solid organ transplant recipients and immunocompromised patients. Other uncommon modalities of transmission could occur through blood products and solid organ transplants[7,8]. Transfusion-related transmission is not common in the United States, but is reported in countries like China and Japan[9,10]. Lastly, vertical transmission of HEV infection from mother to fetus could be up to 100%, as reported by Kumar et al[11] and is associated with fatal outcomes.
HEV infection commonly holds a benign, self-limiting course, and the case-fatality rate in developing countries is estimated to be 0.5%-4%[12,13]. Clinical presentation of HEV infection is similar to that of hepatitis A. Majority of the infected patients sustain mild and asymptomatic course. Acute HEV infection is accompanied by jaundice, icteric eyes, malaise, anorexia, and abdominal discomfort. Severe infection is usually reported among patients with underlying chronic liver disease and is associated with increased mortality[14]. Additionally, solid organ transplant recipients encounter a more sustained course[15]. Among such patients, HEV antibody production could be delayed, often leading to sustained viremia with progression to chronic hepatitis and cirrhosis[16,17].
Pregnant women can suffer a complicated course with fulminant HEV infection and sustain higher mortality rates compared to non-pregnant cohorts. It is estimated that fatality rates reach 10%-40% among pregnant women[11,18]. Both obstetric and non-obstetric complications are encountered. Non-obstetric complications include fulminant hepatic failure, acute liver failure, acute cerebral edema and obstetric complications include pre-term delivery, antepartum hemorrhage, intrauterine fetal demise[19-21].
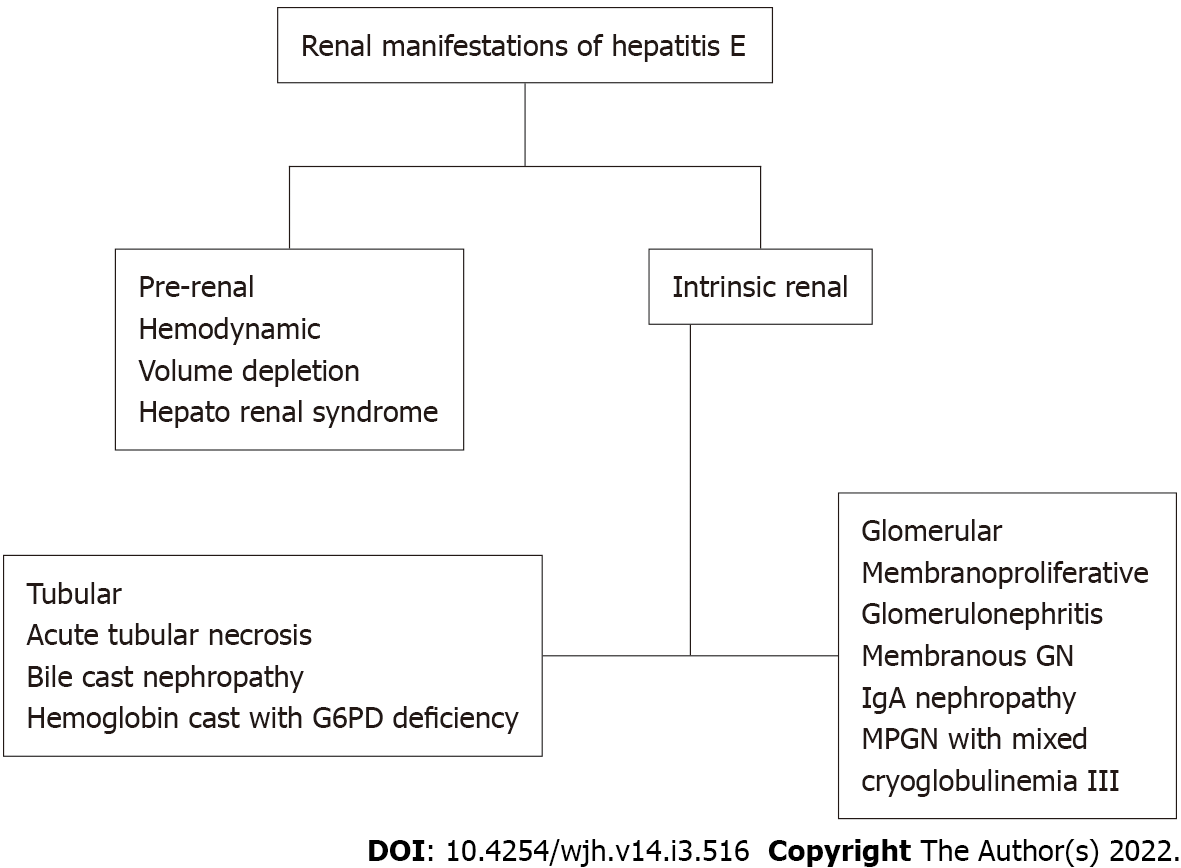
Renal manifestations of hepatitis B and hepatitis C (HBV, HCV) infection are well described. The association between HEV infection and kidney is established as the HEV particles are isolated from the urine of infected patients[22,23]. Additionally, when urine of infected monkeys was induced into healthy animals, the development of HEV infection was well appreciated and confirmed the infectious nature of the viral particles shed in the urine[23]. HEV-associated renal manifestations include prerenal or intrinsic renal disorders. Among intrinsic renal conditions, glomeruli and tubules are the affected sites[24,25].
HEV infection is less commonly associated with the progression of kidney disease in immunocompetent patients. Chronic HEV infection and subsequent development of decompensated liver cirrhosis are frequently encountered among solid organ transplant recipients. Hepatorenal physiology secondary to increased circulating vasoactive agents like nitric oxide is often noted. Similar to other cirrhotic patients, HEV-associated liver dysfunction patients could have increased vasodilatory mediators released secondary to shear stress on the portal vasculature, leading to splanchnic vasodilatation, portosystemic shunting, and bacterial translocation. Additionally, reduction in effective arterial blood volume perpetuates decrease in renal perfusions that ultimately leads to renal vasoconstriction[26]. Urine sodium levels remain low, indicating prerenal failure. However, prolongation of renal hypoperfusion contributes to ischemic injury of the proximal tubule with manifestations of acute tubular necrosis[13].
Bile cast nephropathy, also called cholemic nephrosis, is typically encountered among patients with cholestasis secondary to advanced cirrhosis or acute liver failure. Nayak et al[27] reported a case of cholemic nephrosis secondary to acute HEV infection. Historically, the diagnosis is made by kidney biopsy with the presence of bile cast obstructing distal tubules. The pathogenesis of cholemic nephrosis is not completely understood, however, it is hypothesized secondary to intraluminal obstruction of the bile cast along with direct tubular toxicity[28,29].
Cases of hemolysis and subsequent renal failure are reported with HEV infection. Karki et al[30] reported a case of massive hemolysis in a patient with glucose-6-phosphate dehydrogenase (G6PD) deficiency, heme pigment causing direct proximal tubular toxicity. Development of hemoglobin cast further leads to intratubular obstruction and subsequent development of acute kidney injury. It is hypothesized that the liver dysfunction secondary to acute HEV leads to accumulation of toxins along with the depletion of antioxidants like glutathione. Additionally, if patients have underlying G6PD deficiency, massive hemolysis, and acute kidney injury are encountered[31] (Figure 1).
Glomerular manifestations of HEV infection are reported among solid organ transplant recipients associated with HEV genotype 3. However, it is unclear if renal manifestations and presentation differ among various organ transplant recipients. While glomerular manifestations are commonly noted among immunocompromised patients[32,33], autochthonous HEV-induced membranoproliferative glomerular pattern was reported in an immunocompetent individual[33].
Study by Kamar et al[34] evaluated the renal function of patients with HEV infection in solid organ transplants recipients. Out of total 51 cases of genotype 3 HEV infections, 43.2% were cleared of the virus spontaneously within 6 mo of infection, whereas 56.8% progressed to chronic hepatitis. Among 36 kidney and kidney-pancreas-transplant patients, glomerular filtration rate (GFR) significantly decreased from baseline of 52.9 ± 17.7 mL/min at four-month median before HEV infection to 48.8 ± 18.7 mL/min during acute HEV infection (P = 0.04). Acute rejection episode, infection, modification in immunosuppressant type or dose, and functional renal insufficiency were ruled out, and the GFR decline is attributed to acute HEV infection. Proteinuria levels significantly increased in four kidney-transplant patients at HEV diagnosis, which subsequently improved with improvement in renal functions and HEV clearances.
Kidney biopsy performed during acute phase revealed patterns of membranoproliferative glomerulonephritis, cryoglobulinemia II and III types, and IgA nephropathy[34]. Additionally, among patients who developed chronic hepatitis, 12 patients who received anti-viral therapy with ribavirin for three months had clearances of HEV with subsequent improvement in GFR at 6 mo follow up. Interestingly, In the subgroup who received anti- viral therapy, cryoglobulinemia was detected in 70% of patients before therap, eventually became undetectable in all patients after viral clearance. Renal manifestations of the reported cases of HEV infection among immunocompetent and solid organ recipients are summarized in Table 1.
| Case study | Status | Age | Sex | Country | Serum creatinine/eGFR | Renal manifestations | Treatment | Follow up | Outcomes |
| Karki et al[30] | I.C | 48 yr | M | India | 8.1 mg/dL | ATN(Hemoglobin Cast) | Hemodialysis; Supportive care | 3 mo | Improved kidney function |
| Verschuuren et al[13] | I.C | 34 yr | F | Netherlands | 10 mg/dL | ATN | Hemodialysis; Supportive care | 3 wk | Complete kidney function recovery |
| Biliotti et al[51] | I.C | 57 yr | M | Italy | 44 mL/min | NR | Sofosbuvir; Ribavarin | 3 wk | Patient died from MRSA infection |
| Guinault et al[33] | I.C | 48 yr | M | France | 3.6 mg/dL | MPGN | Steroids | 4 mo | |
| Kamar et al[34] | K.T | 33 yr | M | France | 2.1 mg/dL | MPGN | Steroids | 16 mo | Improved kidney function |
| Kamar et al[34] | K.T | 26 yr | M | France | 2.4 mg/dL | IgAN | Ribavarin 3 mo | 9 mo | Stable kidney function |
| Kamar et al[34] | K.T | 40 yr | M | France | 2.1 mg/dL | IgAN | Change in IS + Rituximab | 3 mo | |
| Kamar et al[34] | K.T | 24 yr | M | France | 2.3 mg/dL | MPGN | Rituximab | 3 yr | Renal replacement therapy |
| Kamar et al[52] | K.T | 28 yr | M | France | 2.4 mg/dL | ATN | None | 3 mo | Serum creatinine returned to baseline |
| Del Bello et al[32] | K.T | 46 yr | M | France | 2 mg/dL | MPGN | Ribavarin 30 mo | 12 mo | Improved serum creatinine |
Pathophysiology of HEV-induced kidney injury is not completely known. HEV-mediated renal manifestations were thought to be a result of direct cytopathic injury due to the viral infection per se or related to immune-mediated mechanisms. Similar to HBV and HCV, it is hypothesized that HEV plays a role in precipitating glomerular injury through immune complex-mediated mechanisms[35]. The study by El- Mokhtar et al[36] assessed the role of immune-mediated mechanisms in HEV-induced renal dysfunction. CD10 and CD13 positive proximal tubular epithelial cells were isolated and challenged in vitro with HEV inoculum. HEV infection minimally upregulated inflammatory markers in the absence of peripheral blood mononuclear cells, and no measurable changes were noted in lactate dehydrogenase (LDH) levels, kidney injury molecules, or transcription of chemokines. However, when the HEV infected proximal tubular cells were inoculated with peripheral blood mononuclear cells, there was upregulation of inflammatory molecules, kidney injury markers, and LDH levels, indicating that HEV infection per se might not be completely responsible for glomerular injury. Thus, it is the intersection between immune cells, HEV infection, and proximal tubular epithelial cells that contribute to renal injury[36].
Over the recent years, HEV laboratory testing has been refined drastically. Two main methods for testing HEV currently are indirect and direct serological tests. With regards to indirect studies, there are commercially available kits for serological testing for the presence of anti-HEV IgM and anti-HEV IgG that relies on the presence of antibodies in the serum to detect infection[37]. In addition, indirect studies rely heavily on patient’s immune response to HEV infection, decreasing sensitivity in immunocompromised patients to some degree[38]. Direct testing predominately uses more advanced nucleic acid testing, that works via detecting the presence of viral genetic material in the form of nucleic acid sequences (HEV RNA) to determine the presence or absence of infection along with detection of viral capsid antigens[39,40].
In Immunocompetent patients, it is advised to check anti-HEV IgM initially for suspected HEV infection[41]. A negative test rules out the disease, however, if the test is positive, HEV RNA analysis is needed. On the other hand, among immunocompromised patients, it is recommended to test HEV-RNA even with negative anti-HEV IgM in blood and in stool before ruling out HEV infection[37]. Urine studies and electrolytes give subtle clues in identifying various causes of AKI. Urine microscopy adds an additional advantage to diagnose patients with acute tubular necrosis in the presence of muddy brown granular cast. Kidney biopsy remains the gold standard diagnostic testing for glomerular disorders and tubular obstructions, including bile cast nephropathy, while evaluating renal manifestations of HEV. Patients with acute or chronic hepatitis with new-onset proteinuria should be considered for kidney biopsy[42].
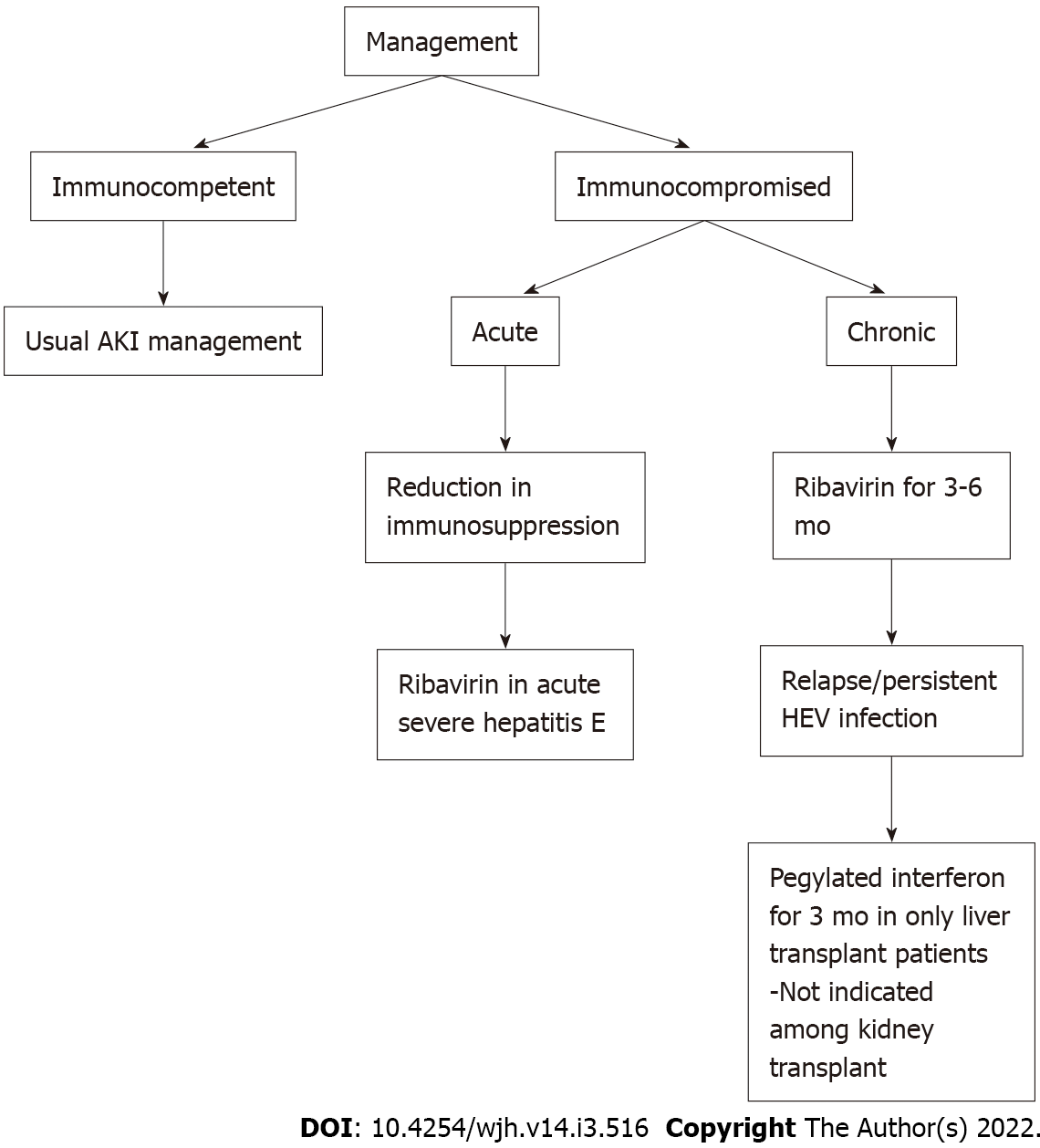
Management of HEV-associated renal manifestations depends on the clinical presentation. Treatment is predominantly based on a conservative approach given benign course of the disease. Acute infection with HEV usually does not require anti-viral therapy. In patients with severe acute infection or acute on chronic liver disease, ribavirin therapy is considered[42]. For patients with acute kidney injury secondary to acute tubular necrosis or bile cast nephropathy, routine care to maintain mean arterial pressures, avoid nephrotoxic agents, and further insults are recommended. Indications for initiation of renal replacement therapy are similar to routine indications of dialysis initiation. Management of HEV-associated glomerular disorders should be based on underlying pathology. Guinault et al[33] reported a case of HEV–induced cryoglobulinemic glomerulonephritis in an immunocompetent patient with serum monoclonal IgG k light chain type II cryoglobulin. Renal biopsy results were consistent with lobular membranoproliferative exudative glomerulonephritis with fibrinoid necrosis and cellular crescents with a ruptured Bowman capsule. The patient was subsequently treated with seven sessions of plasma exchange along with pulse steroids with improvement in HEV RNA titers and cryoglobulinemic levels. Occasionally acute HEV infection follows a fulminant course as reported in pregnant individuals and could manifest as acute cerebral edema, seizures, acute fatty liver and are associated with increased mortality[43].
While managing patients with solid organ transplants, benefits of treatment need to be weighed against risks of rejection. Reduction of immunosuppression is considered the first-line approach[44], allowing HEV clearance in about one-third of patients. Ribavirin, an anti-viral agent, is considered in patients with severe acute or acute on chronic liver failure[45,46]. It has also been postulated that ribavirin acts by inhibiting HEV viral replication and increases the expression of interferon stimulating genes leading to immune modulation[47]. In a study done by Kamar et al[34], patients who received anti-viral therapy with ribavirin, cryoglobulinemia was detected in 70% of patients before therapy and became undetectable in all patients after viral clearance. Ribavirin is also used successfully to treat HEV-associated membranoproliferative glomerulonephritis in a solid organ transplant recipient[32] (Figure 2).
In a multicenter retrospective study by Karmer et al, solid-organ transplant recipients were treated with ribavirin at a median dose of 600 (range, 29-1200) mg/d for three months. Similar virological remission was observed in patients who received ribavirin for three months as compared to those who were treated for more than three months. In patients with detectable HEV RNA in the serum and/or in the stool, at the end of three months, ribavirin monotherapy can be continued for an additional three months[48] Hence it is indicated to treat with ribavirin initially for three months and evaluate the response. With non-sustained virological remission, ribavirin is recommended to be continued for a total of 6 mo. Among liver transplant recipients, interferon (IFN) α has shown to achieve sustained virological remission among patients with HEV after liver transplant. However, the use of IFNα is not recommended among other solid organ transplant recipients due to the risk of graft rejection (Table 1).
Sofosbuvir, a nucleotide analog, is evaluated along with ribavirin in patients who failed ribavirin monotherapy. Wezel et al[49] evaluated two solid organ transplant recipients who failed ribavirin monotherapy and observed that sofosbuvir showed variable antiviral activity in chronic HEV patients. Sofosbuvir was ineffective in achieving sustained virological response. Pegylated IFNα has shown efficacy in achieving a sustained virological response in patients with hemodialysis and liver transplants[50]. However, given the concern of interference with graft and risk of acute rejection, interferon α is contraindicated in patients with other solid organ transplants[47].
HEV infection is a global health concern and is uncommonly associated with mortality and morbidity. HEV infection is restricted not only to developing countries, but is increasingly identified among developed countries. Renal manifestations of HEV range from prerenal failure, acute tubular necrosis, glomerular disorders, and intratubular obstruction form bile cast nephropathy. Similar to HBV and HCV infections, immune-mediated mechanisms are hypothesized in development of HEV-associated glomerular diseases. Conservative approach is routinely employed in cases of renal involvement from acute hepatitis in immunocompetent patients. Among solid organ transplant recipients, ribavirin is considered in patients with chronic HEV infection for a duration of 3-6 mo along with reduction of immunosuppression. IFNα has shown to achieve sustained virological remission among patients with HEV after liver transplant. However, the use of IFNα is not recommended among other solid organ transplant recipients secondary to the risk of graft rejection. In patients who failed monotherapy with ribavirin, sofosbuvir has been evaluated in conjunction with ribavirin with variable anti-viral effects. Plasma exchange, in addition to pulse steroids is occasionally used in management of crescentic glomerular nephritis associated with HEV infection.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kayesh MEH, Manrai M, Tanaka Y S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Lu L, Li C, Hagedorn CH. Phylogenetic analysis of global hepatitis E virus sequences: genetic diversity, subtypes and zoonosis. Rev Med Virol. 2006;16:5-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 592] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 2. | Smith DB, Simmonds P, Izopet J, Oliveira-Filho EF, Ulrich RG, Johne R, Koenig M, Jameel S, Harrison TJ, Meng XJ, Okamoto H, Van der Poel WHM, Purdy MA. Proposed reference sequences for hepatitis E virus subtypes. J Gen Virol. 2016;97:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 324] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 3. | Sayed IM, Vercouter AS, Abdelwahab SF, Vercauteren K, Meuleman P. Is hepatitis E virus an emerging problem in industrialized countries? Hepatology. 2015;62:1883-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Meng XJ, Purcell RH, Halbur PG, Lehman JR, Webb DM, Tsareva TS, Haynes JS, Thacker BJ, Emerson SU. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci U S A. 1997;94:9860-9865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 853] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 5. | Pavio N, Meng XJ, Doceul V. Zoonotic origin of hepatitis E. Curr Opin Virol. 2015;10:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (3)] |
| 6. | Domanović D, Tedder R, Blümel J, Zaaijer H, Gallian P, Niederhauser C, Sauleda Oliveras S, O'Riordan J, Boland F, Harritshøj L, Nascimento MSJ, Ciccaglione AR, Politis C, Adlhoch C, Flan B, Oualikene-Gonin W, Rautmann G, Strengers P, Hewitt P. Hepatitis E and blood donation safety in selected European countries: a shift to screening? Euro Surveill. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 7. | Pourbaix A, Ouali N, Soussan P, Roque Afonso AM, Péraldi MN, Rondeau E, Peltier J. Evidence of hepatitis E virus transmission by renal graft. Transpl Infect Dis. 2017;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, Dalton HR. Hepatitis E. Lancet. 2012;379:2477-2488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 744] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 9. | Satake M, Matsubayashi K, Hoshi Y, Taira R, Furui Y, Kokudo N, Akamatsu N, Yoshizumi T, Ohkohchi N, Okamoto H, Miyoshi M, Tamura A, Fuse K, Tadokoro K. Unique clinical courses of transfusion-transmitted hepatitis E in patients with immunosuppression. Transfusion. 2017;57:280-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Zhang L, Jiao S, Yang Z, Xu L, Liu L, Feng Q, Zhang X, Hou Y, He S, Saldanha J, Wang S, Wang B. Prevalence of hepatitis E virus infection among blood donors in mainland China: a meta-analysis. Transfusion. 2017;57:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Kumar RM, Uduman S, Rana S, Kochiyil JK, Usmani A, Thomas L. Sero-prevalence and mother-to-infant transmission of hepatitis E virus among pregnant women in the United Arab Emirates. Eur J Obstet Gynecol Reprod Biol. 2001;100:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Krawczynski K, Aggarwal R, Kamili S. Hepatitis E. Infect Dis Clin North Am. 2000;14:669-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Verschuuren EA, Haagsma EB, Zijlstra JG, Stegeman CA. Non-oliguric acute renal failure associated with hepatitis E. Nephrol Dial Transplant. 1997;12:799-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Péron JM, Bureau C, Poirson H, Mansuy JM, Alric L, Selves J, Dupuis E, Izopet J, Vinel JP. Fulminant liver failure from acute autochthonous hepatitis E in France: description of seven patients with acute hepatitis E and encephalopathy. J Viral Hepat. 2007;14:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 15. | Hering T, Passos AM, Perez RM, Bilar J, Fragano D, Granato C, Medina-Pestana JO, Ferraz ML. Past and current hepatitis E virus infection in renal transplant patients. J Med Virol. 2014;86:948-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Pas SD, de Man RA, Mulders C, Balk AH, van Hal PT, Weimar W, Koopmans MP, Osterhaus AD, van der Eijk AA. Hepatitis E virus infection among solid organ transplant recipients, the Netherlands. Emerg Infect Dis. 2012;18:869-872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 17. | Versluis J, Pas SD, Agteresch HJ, de Man RA, Maaskant J, Schipper ME, Osterhaus AD, Cornelissen JJ, van der Eijk AA. Hepatitis E virus: an underestimated opportunistic pathogen in recipients of allogeneic hematopoietic stem cell transplantation. Blood. 2013;122:1079-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | Khuroo MS, Kamili S, Jameel S. Vertical transmission of hepatitis E virus. Lancet. 1995;345:1025-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 182] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Navaneethan U, Al Mohajer M, Shata MT. Hepatitis E and pregnancy: understanding the pathogenesis. Liver Int. 2008;28:1190-1199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 317] [Cited by in RCA: 280] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 20. | Khuroo MS, Teli MR, Skidmore S, Sofi MA, Khuroo MI. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70:252-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 363] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 21. | Khuroo MS, Kamili S, Khuroo MS. Clinical course and duration of viremia in vertically transmitted hepatitis E virus (HEV) infection in babies born to HEV-infected mothers. J Viral Hepat. 2009;16:519-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Ma H, Zheng L, Liu Y, Zhao C, Harrison TJ, Ma Y, Sun S, Zhang J, Wang Y. Experimental infection of rabbits with rabbit and genotypes 1 and 4 hepatitis E viruses. PLoS One. 2010;5:e9160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Geng Y, Zhao C, Huang W, Harrison TJ, Zhang H, Geng K, Wang Y. Detection and assessment of infectivity of hepatitis E virus in urine. J Hepatol. 2016;64:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 24. | Wilkinson SP, Davies MH, Portmann B, Williams R. Renal failure in otherwise uncomplicated acute viral hepatitis. Br Med J. 1978;2:338-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Geltner D, Naot Y, Zimhoni O, Gorbach S, Bar-Khayim Y. Acute oliguric renal failure complicating type A nonfulminant viral hepatitis. A case presentation and review of the literature. J Clin Gastroenterol. 1992;14:160-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Velez JCQ, Therapondos G, Juncos LA. Reappraising the spectrum of AKI and hepatorenal syndrome in patients with cirrhosis. Nat Rev Nephrol. 2020;16:137-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 27. | Nayak S, Sharma M, Kataria A, Tiwari SC, Rastogi A, Mukund A. Cholemic Nephrosis from Acute Hepatitis E Virus Infection: A Forgotten Entity? Indian J Nephrol. 2018;28:250-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | van der Wijngaart H, van Dam B, van den Berg JG, Krul-Poel YH, Klemt-Kropp M, Bax WA. A 73-year-old male with jaundice and acute kidney injury. Bile cast nephropathy. Neth J Med. 2014;72:95, 99. [PubMed] |
| 29. | Betjes MG, Bajema I. The pathology of jaundice-related renal insufficiency: cholemic nephrosis revisited. J Nephrol. 2006;19:229-233. [PubMed] |
| 30. | Karki P, Malik S, Mallick B, Sharma V, Rana SS. Massive Hemolysis Causing Renal Failure in Acute Hepatitis E Infection. J Clin Transl Hepatol. 2016;4:345-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Arese P, De Flora A. Pathophysiology of hemolysis in glucose-6-phosphate dehydrogenase deficiency. Semin Hematol. 1990;27:1-40. [PubMed] |
| 32. | Del Bello A, Guilbeau-Frugier C, Josse AG, Rostaing L, Izopet J, Kamar N. Successful treatment of hepatitis E virus-associated cryoglobulinemic membranoproliferative glomerulonephritis with ribavirin. Transpl Infect Dis. 2015;17:279-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 33. | Guinault D, Ribes D, Delas A, Milongo D, Abravanel F, Puissant-Lubrano B, Izopet J, Kamar N. Hepatitis E Virus-Induced Cryoglobulinemic Glomerulonephritis in a Nonimmunocompromised Person. Am J Kidney Dis. 2016;67:660-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (2)] |
| 34. | Kamar N, Weclawiak H, Guilbeau-Frugier C, Legrand-Abravanel F, Cointault O, Ribes D, Esposito L, Cardeau-Desangles I, Guitard J, Sallusto F, Muscari F, Peron JM, Alric L, Izopet J, Rostaing L. Hepatitis E virus and the kidney in solid-organ transplant patients. Transplantation. 2012;93:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 35. | Ozkok A, Yildiz A. Hepatitis C virus associated glomerulopathies. World J Gastroenterol. 2014;20:7544-7554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 36. | El-Mokhtar MA, Seddik MI, Osman A, Adel S, Abdel Aziz EM, Mandour SA, Mohammed N, Zarzour MA, Abdel-Wahid L, Radwan E, Sayed IM. Hepatitis E Virus Mediates Renal Injury via the Interaction between the Immune Cells and Renal Epithelium. Vaccines (Basel). 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Kar P, Karna R. A Review of the Diagnosis and Management of Hepatitis E. Curr Treat Options Infect Dis. 2020;1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 38. | Abravanel F, Chapuy-Regaud S, Lhomme S, Miedougé M, Peron JM, Alric L, Rostaing L, Kamar N, Izopet J. Performance of anti-HEV assays for diagnosing acute hepatitis E in immunocompromised patients. J Clin Virol. 2013;58:624-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 39. | Trémeaux P, Lhomme S, Chapuy-Regaud S, Peron JM, Alric L, Kamar N, Izopet J, Abravanel F. Performance of an antigen assay for diagnosing acute hepatitis E virus genotype 3 infection. J Clin Virol. 2016;79:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 40. | Sauleda S, Ong E, Bes M, Janssen A, Cory R, Babizki M, Shin T, Lindquist A, Hoang A, Vang L, Piron M, Casamitjana N, Koppelman M, Danzig L, Linnen JM. Seroprevalence of hepatitis E virus (HEV) and detection of HEV RNA with a transcription-mediated amplification assay in blood donors from Catalonia (Spain). Transfusion. 2015;55:972-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 41. | Goel A, Aggarwal R. Advances in hepatitis E - II: Epidemiology, clinical manifestations, treatment and prevention. Expert Rev Gastroenterol Hepatol. 2016;10:1065-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 42. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on hepatitis E virus infection. J Hepatol. 2018;68:1256-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 442] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 43. | El Sayed Zaki M, El Razek MM, El Razek HM. Maternal-Fetal Hepatitis E Transmission: Is It Underestimated? J Clin Transl Hepatol. 2014;2:117-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Kamar N, Abravanel F, Selves J, Garrouste C, Esposito L, Lavayssière L, Cointault O, Ribes D, Cardeau I, Nogier MB, Mansuy JM, Muscari F, Peron JM, Izopet J, Rostaing L. Influence of immunosuppressive therapy on the natural history of genotype 3 hepatitis-E virus infection after organ transplantation. Transplantation. 2010;89:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 45. | Gerolami R, Borentain P, Raissouni F, Motte A, Solas C, Colson P. Treatment of severe acute hepatitis E by ribavirin. J Clin Virol. 2011;52:60-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 46. | Goyal R, Kumar A, Panda SK, Paul SB, Acharya SK. Ribavirin therapy for hepatitis E virus-induced acute on chronic liver failure: a preliminary report. Antivir Ther. 2012;17:1091-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 47. | Rostaing L, Izopet J, Baron E, Duffaut M, Puel J, Durand D, Suc JM. Preliminary results of treatment of chronic hepatitis C with recombinant interferon alpha in renal transplant patients. Nephrol Dial Transplant. 1995;10 Suppl 6:93-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Kamar N, Izopet J, Tripon S, Bismuth M, Hillaire S, Dumortier J, Radenne S, Coilly A, Garrigue V, D'Alteroche L, Buchler M, Couzi L, Lebray P, Dharancy S, Minello A, Hourmant M, Roque-Afonso AM, Abravanel F, Pol S, Rostaing L, Mallet V. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N Engl J Med. 2014;370:1111-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 374] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 49. | van Wezel EM, de Bruijne J, Damman K, Bijmolen M, van den Berg AP, Verschuuren EAM, Ruigrok GA, Riezebos-Brilman A, Knoester M. Sofosbuvir Add-on to Ribavirin Treatment for Chronic Hepatitis E Virus Infection in Solid Organ Transplant Recipients Does Not Result in Sustained Virological Response. Open Forum Infect Dis. 2019;6:ofz346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 50. | Kamar N, Abravanel F, Garrouste C, Cardeau-Desangles I, Mansuy JM, Weclawiak H, Izopet J, Rostaing L. Three-month pegylated interferon-alpha-2a therapy for chronic hepatitis E virus infection in a haemodialysis patient. Nephrol Dial Transplant. 2010;25:2792-2795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 51. | Biliotti E, Franchi C, Spaziante M, Garbuglia AR, Volpicelli L, Palazzo D, De Angelis M, Esvan R, Taliani G. Autochthonous acute hepatitis E: treatment with sofosbuvir and ribavirin. Infection. 2018;46:725-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 52. | Kamar N, Mansuy JM, Esposito L, Legrand-Abravanel F, Peron JM, Durand D, Rostaing L, Izopet J. Acute hepatitis and renal function impairment related to infection by hepatitis E virus in a renal allograft recipient. Am J Kidney Dis. 2005;45:193-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |