Published online Feb 27, 2022. doi: 10.4254/wjh.v14.i2.464
Peer-review started: July 5, 2021
First decision: August 18, 2021
Revised: September 4, 2021
Accepted: January 13, 2022
Article in press: January 13, 2022
Published online: February 27, 2022
Processing time: 232 Days and 0.8 Hours
Emphysematous hepatitis (EH) is a rare, rapidly progressive fulminant gas-forming infection of the liver parenchyma. It is often fatal and mostly affects diabetes patients.
We report a case of EH successfully managed by a step-up approach consisting of aggressive hemodynamic support, intravenous antibiotics, and percutaneous drainage, ultimately followed by laparoscopic deroofing. Of 11 documented cases worldwide, only 1 of the patients survived, treated by urgent laparotomy and surgical debridement.
EH is a life-threatening infection. Its high mortality rate makes timely diagnosis essential, in order to navigate treatment accordingly.
Core Tip: Emphysematous hepatitis (EH) is a very rare, rapidly progressive fulminant gas-forming infection of the liver parenchyma. There is a paucity of literature with regard to pathogenesis, involved organisms, imaging appearance, and management of this condition. We report the successful treatment of a patient diagnosed with EH by adopting a multimodal step-up approach including rigorous fluid resuscitation, early hemodynamic support, broad-spectrum antimicrobial therapy, and percutaneous radiologically guided drainage followed by minimal invasive surgical treatment.
- Citation: Francois S, Aerts M, Reynaert H, Van Lancker R, Van Laethem J, Kunda R, Messaoudi N. Step-up approach in emphysematous hepatitis: A case report. World J Hepatol 2022; 14(2): 464-470
- URL: https://www.wjgnet.com/1948-5182/full/v14/i2/464.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i2.464
Emphysematous hepatitis (EH) is a rare life-threatening condition that results from a necrotizing gas-forming infection of the liver parenchyma. The pathogenesis is poorly understood, although rare published case series have shown that diabetes mellitus was present in most patients[1-6]. Diagnosis of EH is based on radiological findings on computed tomography demonstrating hepatic intraparenchymal gas without the typical fluid-air level seen in pyogenic abscesses. Early recognition is crucial in attempts to decrease mortality, although there is still discussion regarding the appropriate management, as almost all documented cases evolved unfavorably[1-4,6-11]. This report presents the successful management of a critically ill patient with EH using a step-up approach.
A 70-year-old woman presented to the emergency department with acute epigastric pain of 1 h duration.
The patient had a history of well-controlled diabetes mellitus, cholecystectomy, and heterozygote alpha-1 antitrypsin deficiency.
Clinical examination revealed the patient to be in no distress, fully alert and oriented, and presenting with epigastric tenderness without signs of peritonitis. She had no fever, a pulse rate of 82 beats/min, blood pressure of 128/68 mmHg, and normal respiratory rate and oxygen saturation.
Laboratory investigation performed within a few hours after onset of pain showed a total bilirubin of 0.28 mg/dL, glutamic oxaloacetic transaminase of 45 U/L, glutamic pyruvic transaminase of 30 U/L, γ-glutamyltransferase of 91 U/L, alkaline phosphatase of 99 U/L and lipase of 3518 U/L. Complete blood count, C-reactive protein, coagulation, and renal function were within normal limits. Cardiac evaluation by electrocardiogram and cardiac enzymes confirmed normal findings. As acute pancreatitis was suspected, the patient was initially managed conservatively, via intravenous fluid resuscitation and pain relief. However, within hours after admission, the patient deteriorated rapidly, developing signs of severe septic shock. She was transferred to the intensive care unit, requiring mechanical ventilation and aggressive hemodynamic support.
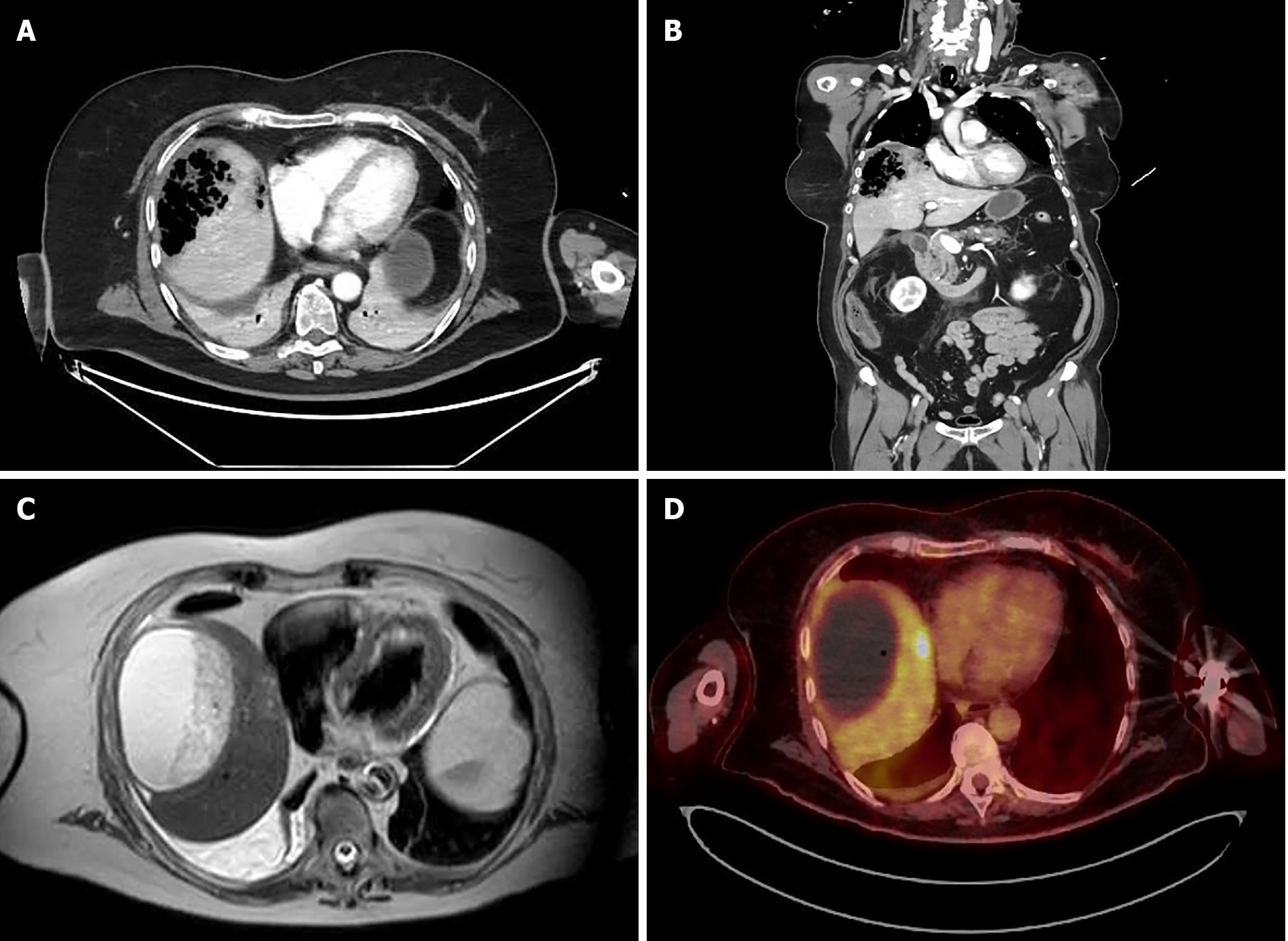
After initial resuscitation, a computed tomography (CT) scan was performed, showing a large (9 cm) air-filled cavity in the right liver lobe (Figure 1A and B). The bile duct was only mildly dilated in this cholecystectomized patient but nonradiopaque choledocholithiasis could not be ruled out. No apparent inflammation surrounding the pancreas was visible on scans.
As soon as the patient clinically deteriorated, multidisciplinary consultations between gastroenterology, hepatobiliary surgery, intensive care, interventional radiology and microbiology were performed repeatedly.
Based on the CT-graphic findings, a diagnosis of EH was made.
The patient was treated with broad-spectrum intravenous antibiotics (meropenem, vancomycin, and amikacin). Subsequent CT-guided percutaneous pigtail catheter drainage yielded no significant amount of fluid or pus. The pigtail drain was then flushed by continuous irrigation of 1 L saline solution per 24 h. Because of elevated serum lipase suggesting pancreatitis and a mildly dilated bile duct, albeit without biochemical cholestasis, an endoscopic retrograde cholangiopancreatography was performed in this rapidly deteriorating patient. A cholangiogram showed normal biliary anatomy, and clear bile was visible after endoscopic sphincterotomy of the papilla Vateri.
Antibiotics were rationalized to ceftriaxone and metronidazole after blood and fluid cultures revealed Escherichia coli, Streptococcus anginosus, and Klebsiella oxytoca as microbial pathogens. Continuous pigtail irrigation was stopped after 3 d, and the drain was removed after 5 d because of no output. The patient was weaned from the ventilator after a week and transferred to the ward after 2 wk in intensive care.
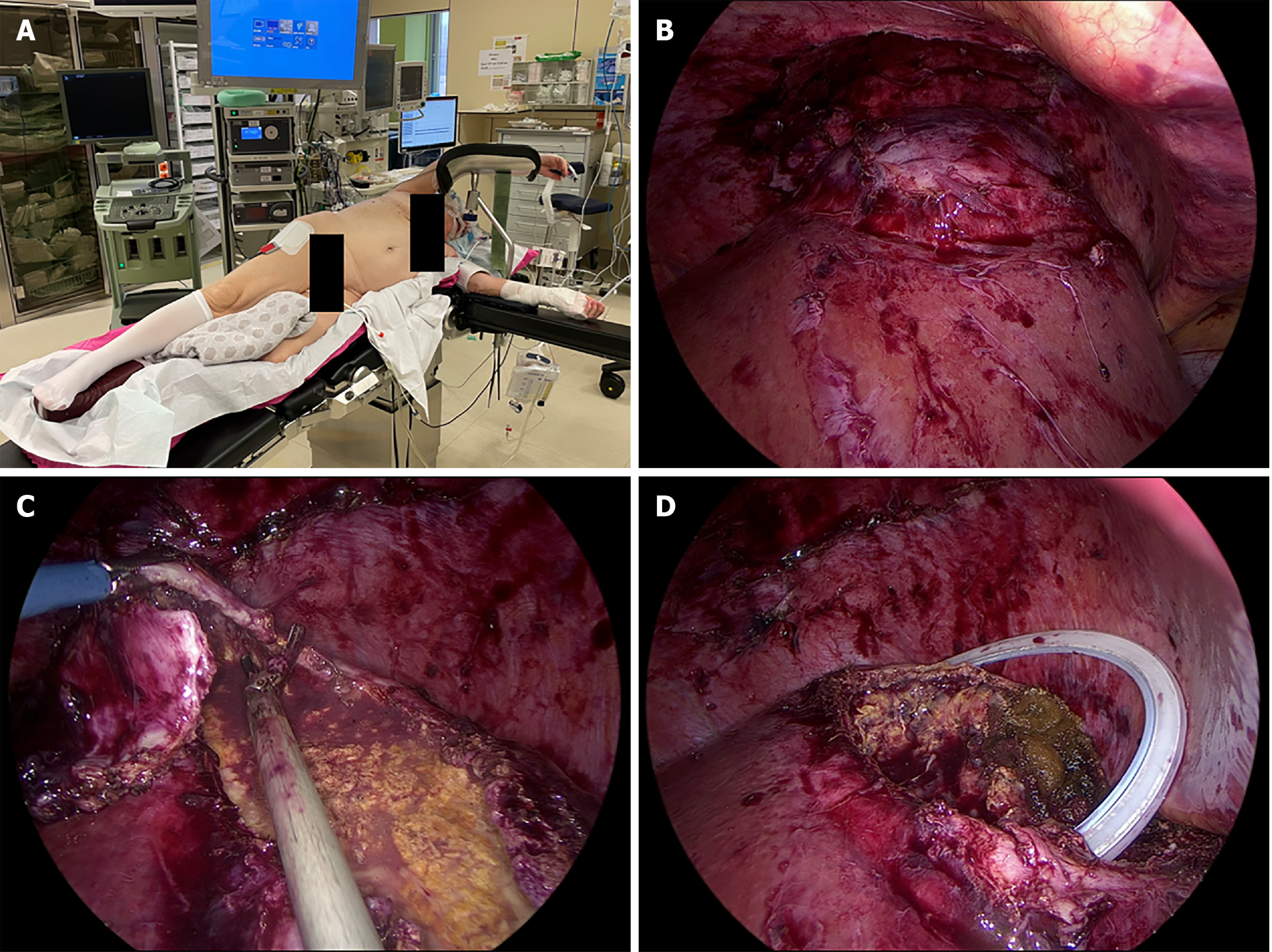
The patient continued to recover favorably and was discharged 1 mo after admission, with intravenous antibiotics at home. During her stay, a colonoscopy and transthoracic echocardiogram were performed to rule out other potential etiologies. At the time of discharge, a CT scan showed a 90 mm × 47 mm liquefied collection in segment VII. Follow-up included magnetic resonance imaging (MRI) and positron emission tomography (PET). MRI, performed 6 wk after initial admission, showed a 10-cm cystic formation in liver segments VII and VIII that contained both fluid and necrotic debris (Figure 1C) that were not metabolically active on the PET scan. However, in segment VIII, a 2-cm PET-positive nodule was detected (Figure 1D). Surgical drainage was performed because of the heterogenous content of the large intrahepatic collection in segments VII-VIII and the undetermined nature of the 2-cm lesion in segment VIII. Three mo after onset, the patient underwent laparoscopic deroofing and debridement of the hepatic collection in segment VII and partial hepatectomy of the 2-cm lesion in segment VIII (Figure 2). No malignancy was found in the resected specimens, and microbiological cultures were sterile. Hence, antibiotics were discontinued after a total treatment of 14 wk. The patient had an uneventful recovery but was hospitalized again after a few weeks for coronavirus disease 2019 infection. To date, the patient is asymptomatic and without recurrence on follow-up imaging.
EH is a severe, life-threatening infection of the liver parenchyma by gas-forming bacteria. To the best of our knowledge, 11 cases of EH have been previously reported in the literature (Table 1)[1-11]. Remarkably, only 1 of those patients, treated by urgent laparotomy and surgical debridement, was reported to have survived this dismal clinical entity[5]. However, a mixed collection of necrotic debris and air was diagnosed by CT and intraoperatively, making the probability of a pyogenic liver abscess or at least coexistence of both entities more likely. The other patients all died within 3 d of severe multiple organ failure in the setting of fulminant septic shock. This case report describes the favorable outcome of a patient diagnosed with EH and managed by a step-up approach consisting of initial aggressive resuscitation, systemic antimicrobial therapy, and percutaneous radiologically guided drainage followed by laparoscopic surgical debridement.
| Ref. | Year | Age/sex | History | Imaging | Treatment | Pathogen(s) | Outcome |
| Blachar et al[1] | 2002 | 43/F | Diabetes mellitus, hyperlipidemia, short-gut duet of multiple ischemic episodes, peripheral vascular disease | CT: Extensive hepatic gas right lobe without fluid collection | IV antibiotics; Radiological drainage | Blood and liver aspirate: Klebsiella pneumoniae | Died 3 d after admission |
| Lopez Zarraga et al[2] | 2006 | 72/F | Diabetes mellitus | CT: Total gas content in multiple abscesses | NA | Culture of liver lesion post mortem: Klebsiella oxytoca | Died 24 h after admission |
| Létourneau-Guillon et al[7] | 2010 | 53/M | Three mo before admission: Left hepatectomy with hepaticojejunostomy for hilar cholangiocarcinoma; No adjuvant chemotherapy1 wk before admission: Cellulitis at surgical incision treated with oral cephalexin | CT: 8 cm air-filled cavity in the right lobe, no fluid collection | IV antibiotics | Blood culture: Enterobacter cloacae, Clostridium perfringens | Died 36 h after admission |
| Chauhan et al[3] | 2012 | 77/F | Diabetes mellitus | CT: Air collection in segment VI and VII without fluid collection | IV antibiotics; Radiological drainage | NA | Died 3 d after admission |
| Jung Ho et al[8] | 2012 | 80/F | Hilar cholangiocarcinoma; ERCP + stenting was performed 3 mo before admission followed by radiotherapy for 17 d after admission | CT: Hepatic parenchymal gas 6.3 cm × 4.4 cm in the right liver (sVII/sVIII) | IV antibiotics; Radiological drainage | Blood culture: Clostridium perfringens, Escherichia coli | Died 3 d after admission |
| Dimitriou et al[4] | 2014 | 72/M | Diabetes mellitus | CT: Replacement of liver parenchyma by gas without fluid collection | IV antibiotics | NA | Died within hours after admission |
| Nada et al[9] | 2017 | 73/F | Pancreatic adenocarcinoma; Whipple performed 8 mo before admission. Lung- and liver metastasis diagnosed 6 wk prior to admission. COPD, hypertension, chronic hepatitis C, pulmonary embolism | CT: Hepatic gas in the right liver lobe, sparing the hepatic metastasis | IV antibiotics | Blood culture: Streptococcus mutans, Enterococcus faecalis | Died within 24 h after admission |
| Ghosn et al[5] | 2019 | 38/F | Diabetes mellitus, cholecystectomy | CT: Mixed collection 8 cm × 7 cm × 5.5 cm, containing necrotic debris and air | IV antibiotics; Laparotomy urgent | Perioperative fluid: Escherichia coli, Enterococcus faecium | Survived. Discharged 13 d after admission |
| Calderon et al[6] | 2020 | 80/F | Hypertension, diabetes mellitus, chronic kidney disease | CT at presentation: Indeterminate, scattered, hypo-enhancing lesions in the liver. CT 5 h after admission (clinical deterioration): Gas in the right liver lobe | IV antibiotics | Blood culture: Clostridium perfringens | Died within 16 h after admission |
| Azri et al[7] | 2020 | 75/F | Hilar cholangiocarcinoma; ERCP + stenting 14 mo prior to admission. Followed by stereotactic radiotherapy until 4 mo prior to admission | CT: Left hepatic parenchymal emphysema and pneumoperitoneum | NA | Blood culture: Klebsiella pneumoniae, Escherichia coli, Enterococcus faecalis, Clostridium perfringens, Aeromonas ichtiosmia | Died |
| Gonçalos et al[11] | 2020 | 74/M | Hypertension, gastroesophageal reflux | CT: Two areas of gas within the right lobe of the liver | IV antibiotics | Blood culture: Escherichia coli | Died 3 d after admission |
EH occurs predominantly in women, and diabetes mellitus seems to be a predisposing condition. Abdominal pain and fever are the most common clinical manifestations of the disease. Diagnosis of EH is confirmed by the presence of parenchymal gas in the liver on CT in the absence of intrahepatic fluid collection. CT is the imaging modality of choice for diagnosing EH, as it permits early detection, evaluation of the extent and location of liver involvement, and excludes other etiologies of acute abdominal pain causing septic shock. Importantly, parenchymal gas in the liver has to be differentiated from air in other liver structures. Air can be observed within bile ducts (e.g., following endoscopic sphincterotomy), portal veins (e.g., as a result of bowel infarction), in infarcted liver (e.g., after liver transplantation), and in pyogenic liver abscesses. In contrast to EH, characteristics of pyogenic liver abscesses on CT scans include peripheral enhancing and centrally hypoattenuating (dense or) liquefied collections containing gas bubbles or air-fluid boundaries[12].
Emphysematous infections in the abdomen are known to occur in the gallbladder, stomach, pancreas, and urinary tract[13]. Clinically, pathologically, and radiologically, EH shares features with emphysematous pyelonephritis. The latter is defined as an acute necrotizing, gas-forming infection in the kidney associated with a poor prognosis. Bacterial pathogens cultured in emphysematous pyelonephritis include Escherichia coli and members of the genera Klebsiella, Enterobacter, Pseudomonas, Proteus and Streptococcus[1,14]. As shown in Table 1, the same causative pathogens can be found in EH.
The pathophysiology of emphysematous infections is believed to be caused by mixed acid bacterial fermentation from tissue necrosis resulting in the production of hydrogen (15%), nitrogen (60%), oxygen (5%), and carbon dioxide (5%). Diabetes is known to predispose to emphysematous infections by providing high levels of glucose used as a substrate by the microorganisms[3,15]. Last, diabetes as well as other risk factors for microangiopathy, may contribute to slow transport of catabolic products, leading to accumulation of gas[3,15]. Similar to most previously published cases of EH, our patient was diabetic. Noteworthy was the well-controlled disease state, with glycated hemoglobin of 5.8%, suggesting that factors other than circulating glucose levels may have been of importance.
With advances in cross-sectional imaging and localization, percutaneous drainage has now become the treatment of choice of pyogenic abscesses of the liver. By analogy, less invasive means seem to be a first-line approach in EH as well. Although often ineffective in previously reported cases, our patient responded to early aggressive medical management and radiologically guided drainage. Surgical intervention in this case was not intended as a salvage therapy but rather as a step-up to initial conservative management. Laparoscopic deroofing and debridement of necrosis was undertaken 3 mo after the initial presentation. Given the dorsal localization of the hepatic area involved in our patient, a semi-prone position was chosen to allow laparoscopic visualization of posterior segments and partial hepatectomy of segment VII and VIII, avoiding laparotomy via a right subcostal incision.
In conclusion, EH is a serious potentially life-threatening infection of the liver. We report the successful treatment of a patient diagnosed with EH by adopting a multimodal step-up approach including rigorous fluid resuscitation, early hemodynamic support, broad-spectrum antimicrobial therapy and percutaneous radiologically guided drainage followed by minimally invasive surgical treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Belgium
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li FX S-Editor: Li X L-Editor: A P-Editor: Li X
| 1. | Blachar A, Federle MP, Brancatelli G. Acute fulminant hepatic infection causing fatal "emphysematous hepatitis": case report. Abdom Imaging. 2002;27:188-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | López Zárraga F, Aisa P, Saenz de Ormijana J, Diez Orive M, Añorbe E, Aguirre X, Paraiso M, Morales Bravo M. Fulminant infection with emphysematous changes in the biliary tract and air-filled liver abscesses. Abdom Imaging. 2006;31:90-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Chauhan U, Prabhu SM, Shetty GS, Solanki RS, Udiya AK, Singh A. Emphysematous hepatitis--a fatal infection in diabetic patients: case report. Clin Res Hepatol Gastroenterol. 2012;36:e114-e116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Dimitriou C, Papadimitriou A, Nalmpantidou C, Katsiba D, Kaitartzis C, Arvaniti M. Emphysematous hepatitis in a diabetic patient. Eurorad. 2014;. |
| 5. | Ghosn Y, Abdallah A, Hussein Kamareddine M, Geahchan A, Baghdadi A, El-Rassi Z, Chamseddine A, Ashou R. Gas-Forming Liver Abscess versus Emphysematous Hepatitis: A Radiologic Diagnostic Dilemma-A Case Report and Review of the Literature. Case Reports Hepatol. 2019;2019:5274525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Calderon H, Serfin J. 13-Hour progression of emphysematous hepatitis as depicted on repeat computerized tomography. J Surg Case Rep. 2020;2020:rjaa089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Létourneau-Guillon L, Audet P, Plasse M, Lepanto L. Answer to case of the month #162. Emphysematous infection of the liver parenchyma. Can Assoc Radiol J. 2010;61:117-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Kim JH, Jung ES, Jeong SH, Kim JS, Ku YS, Hahm KB, Kim JH, Kim YS. A case of emphysematous hepatitis with spontaneous pneumoperitoneum in a patient with hilar cholangiocarcinoma. Korean J Hepatol. 2012;18:94-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Nada KM, El Husseini I, Abu Hishmeh ME, Shah NS, Ibragimova N, Basir R. A Rare Case of Septic Shock Secondary to Emphysematous Hepatitis. Case Rep Crit Care. 2017;2017:3020845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Azri A, Pichon J, Fartoukh M, Djibré M. Fatal emphysematous hepatitis with spontaneous pneumoperitoneum. Liver Int. 2020;40:1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Miranda G, Dionísio AC, Azevedo C, Carvalho E, Semião M, Branco V, Castelo-Branco M. Fulminant Emphysematous Hepatitis - A Rare Cause of Septic Shock. Eur J Case Rep Intern Med. 2020;7:001539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Bächler P, Baladron MJ, Menias C, Beddings I, Loch R, Zalaquett E, Vargas M, Connolly S, Bhalla S, Huete Á. Multimodality Imaging of Liver Infections: Differential Diagnosis and Potential Pitfalls. Radiographics. 2016;36:1001-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 13. | Grayson DE, Abbott RM, Levy AD, Sherman PM. Emphysematous infections of the abdomen and pelvis: a pictorial review. Radiographics. 2002;22:543-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 197] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Mynbaev OA, Micali S, Zordani A, Bianchi G. Re: Critical Analysis of Early Recurrence after Laparoscopic Radical Cystectomy in a Large Cohort by the ESUT: S. Albisinni, L. Fossion, M. Oderda, O. M. Aboumarzouk, F. Aoun, T. Tokas, V. Varca, R. Sanchez-Salas, X. Cathelineau, P. Chlosta, F. Gaboardi, U. Nagele, T. Piechaud, J. Rassweiler, P. Rimington, L. Salomon and R. van Velthoven J Urol 2016;195:1710-1717. J Urol. 2016;196:1319-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Huang JJ, Tseng CC. Emphysematous pyelonephritis: clinicoradiological classification, management, prognosis, and pathogenesis. Arch Intern Med. 2000;160:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 446] [Article Influence: 17.8] [Reference Citation Analysis (1)] |