Published online Feb 27, 2022. doi: 10.4254/wjh.v14.i2.429
Peer-review started: November 20, 2021
First decision: December 26, 2021
Revised: January 8, 2022
Accepted: February 15, 2022
Article in press: February 15, 2022
Published online: February 27, 2022
Processing time: 93 Days and 13.8 Hours
Portal hypertension, a common complication associated with liver cirrhosis, can result in variceal bleeding, which greatly impacts patient survival. Recently, β-arrestin-2 has been shown to predict the acute hemodynamic response to nonselective β-blocker therapy for cirrhotic portal hypertension. However, more data is needed on the long-term effects of and changes in β-arrestin-2 following nonselective β-blocker therapy.
To investigate the expression and role of β-Arrestin-2 in predicting the long-term response to nonselective β-blockers in cirrhotic portal hypertensive patients.
We prospectively enrolled 91 treatment-naïve patients with cirrhotic portal hypertension. Baseline clinical and laboratory data were obtained. Gastroscopy was performed for grading and treating varices and obtaining gastric antral biopsies. We measured the serum and antral expression of β-arrestin-2 and obtained Doppler measurement of the portal vein congestion index. Treatment with nonselective β-blockers was then started. The patients were followed up for 18 mo, after which they have undergone a repeat antral biopsy and re-evaluation of the portal vein congestion index.
A higher serum level and antral expression of β-arrestin-2 was associated with longer bleeding-free intervals, greater reduction in the portal vein congestion index, and improved grade of varices. Among patients with a low β-arrestin-2 expression, 17.6% were nonselective β-blocker responders, whereas, among those with high expression, 95.1% were responders (P < 0.001). A serum β-arrestin-2 value ≥ 2.23 ng/mL was associated with a lower likelihood of variceal bleeding (90% sensitivity and 71% specificity). β-arrestin-2 expression significantly decreased after nonselective β-blocker therapy.
β-arrestin-2 expression in cirrhotic portal hypertension predicts the clinical response to long-term nonselective β-blocker treatment. Serum β-arrestin-2 is a potential noninvasive biomarker for selecting the candidate patients for nonselective β-blockers.
Core Tip: Gastric antral β-Arrestin-2 (β-Arr-2) expression correlates to portal hypertension in terms of esophageal varices and portal gastropathy. A stronger β-Arr-2 expression is associated with a sustained clinical response to nonselective β-blockers (NSBB) with a longer variceal bleeding-free interval. Patients who experienced variceal bleeding while on NSBB had lower baseline serum and tissue expression of β-Arr-2. In patients with responded to NSBB, the expression of β-Arr-2 was reduced after long-term treatment. The serum level of β-Arr-2 correlates to its antral expression and showed high sensitivity and specificity for defining the subgroup of patients who will respond to NSBB.
- Citation: Lashen SA, Shamseya MM, Madkour MA, Abdel Salam RM, Mostafa SS. β-arrestin-2 predicts the clinical response to β-blockers in cirrhotic portal hypertension patients: A prospective study. World J Hepatol 2022; 14(2): 429-441
- URL: https://www.wjgnet.com/1948-5182/full/v14/i2/429.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i2.429
Portal hypertension (PHT) is a risk factor for esophageal varices (EV). Variceal bleeding can significantly affect patient survival. The main pathophysiology underlying PHT is the increased resistance and/or blood flow in the portal circulation[1,2]. Once the hepatic venous pressure gradient (HVPG) exceeds 12 mmHg, variceal bleeding occurs[3], with a 30%-50% mortality risk after the first episode and a 70% rate of early rebleeding. Therefore, the prevention of formation, growth, and rupture of varices is important in PHT management[4].
Nonselective β-blockers (NSBB) are the standard of care for primary or secondary prophylaxis against variceal bleeding[5,6]. The risk of bleeding or rebleeding is greatly diminished when HVPG is reduced by ≥ 20% or to < 12 mmHg[7]. However, there are special concerns regarding NSBB use in patients with refractory ascites or spontaneous bacterial peritonitis (SBP), in terms of patient survival and quality of life[8].
Only approximately 40% of patients with PHT show a clinical response to NSBB[9]. Therefore, several patients are exposed to unfavorable side effects without clinical benefit. Identifying who will respond to NSBB is an important unresolved question having a clinical impact. Currently, the only way to identify responders is by HVPG measurement, which is an invasive technique with limited access[10]. Therefore, the search for noninvasive predictors for NSBB response is clinically desirable.
Recently, β-arrestins-2 (β-Arr-2) has been studied as a predictor using the acute propranolol challenge in a few patients[11]. However, the long-term impact of β-Arr-2 expression on portal hemodynamics is not yet clear. Moreover, the changes in β-Arr-2 expression after long-term NSBB treatment have not been studied. We designed our study to investigate the long-term changes in β-Arr-2 and its predictive accuracy for identifying potential responders to NSBB treatment.
We prospectively enrolled 120 patients with cirrhotic PHT having no previous history of endoscopic or NSBB treatment for varices. The enrollment was done between December 2017 and November 2019, with the last follow-up being performed on April 2021. We also included 40 healthy volunteers to assess normal serum β-Arr-2 levels. The study was conducted at the Internal Medicine Department, Main University Hospital, Faculty of Medicine, as well as the Endoscopy Unit at the Medical Research Institute, Alexandria University. The study was approved by the local ethics committee [Institutional Review Board: 00007555; Review Number: 0303608]. The study was conducted following the 1975 Helsinki Declaration (as revised in 2008), and the Good Clinical Practice guidelines. Informed consent was obtained from all study participants.
We excluded patients with non-cirrhotic PHT, portal vein thrombosis, endoscopic stigmata of active or recent bleeding, previous endoscopic or NSBB variceal treatment, contraindications for NSBB treatment, bradycardia with < 50 beats/minute, SBP of < 90 mmHg, coagulopathy, malignancy, or cardiorenal disease.
A history of melena or hematemesis was recorded. We performed a complete clinical examination and laboratory investigations, including complete blood count and assessment of aminotransferases, serum albumin and bilirubin levels, and international normalization ratio. The severity of liver disease was assessed using Child-Pugh classification. The Aspartate aminotransferase (AST)/platelet ratio index (APRI) was calculated. Serum β-Arr-2 levels were measured using an ELISA kit (Human ARRB2, Catalog #MBS765831, My BioSource, Inc., CA, United States) following the manufacturer’s instructions[12].
Doppler ultrasound of the portal circulation was obtained using the Acuson X-300TM color Doppler machine (Siemens, CA, United States) to measure the portal vein congestion index (PVCI) at both baseline and last follow-up visits[13].
Esophagogastroduodenoscopy (EGD) was performed to evaluate the presence and grade of EV, the risk signs for variceal bleeding, presence of gastric varices (GV), and presence and grade of portal hypertensive gastropathy (PHG)[5,14]. Mucosal biopsies from the gastric antrum, body (corpus), and duodenum were taken during baseline endoscopic evaluation.
Patients with nonbleeding small varices with red wale marks, cherry-red spots, or decompensated cirrhosis and those with medium or large EV were started on propranolol primary prophylaxis (40 mg/d over 2 doses)[5,15]. The dose was then increased on alternate days to reach a target pulse of 55 beats/minutes or a maximally tolerated dose (but not exceeding 360 mg/d). The dose was maintained until the study ended if it was tolerated and an SBP > 90 mmHg was sustained.
The follow-up duration of the study was 18 mo (540 d), calculated from the first dose of NSBB. During this period, EGD was performed every 12 wk or whenever variceal bleeding occurred.
The primary endpoint was the occurrence of variceal bleeding necessitating intervention, such as endoscopic variceal ligation or sclerotherapy (EVL/EST). Patients who bled were designated as “NSBB non-responders”. Conversely, patients who did not experience variceal bleeding in the 540 days of follow-up were designated as “NSBB responders”.
At the end of the study (either when variceal bleeding occurred or the end of follow-up), EGD was performed for EV grading/treatment and obtaining a second antral mucosal biopsy to re-assess β-Arr-2 expression. Variceal bleeding was defined as hematemesis, melena, or hematochezia with endoscopic evidence of the variceal source after excluding nonvariceal sources, including the biopsy site.
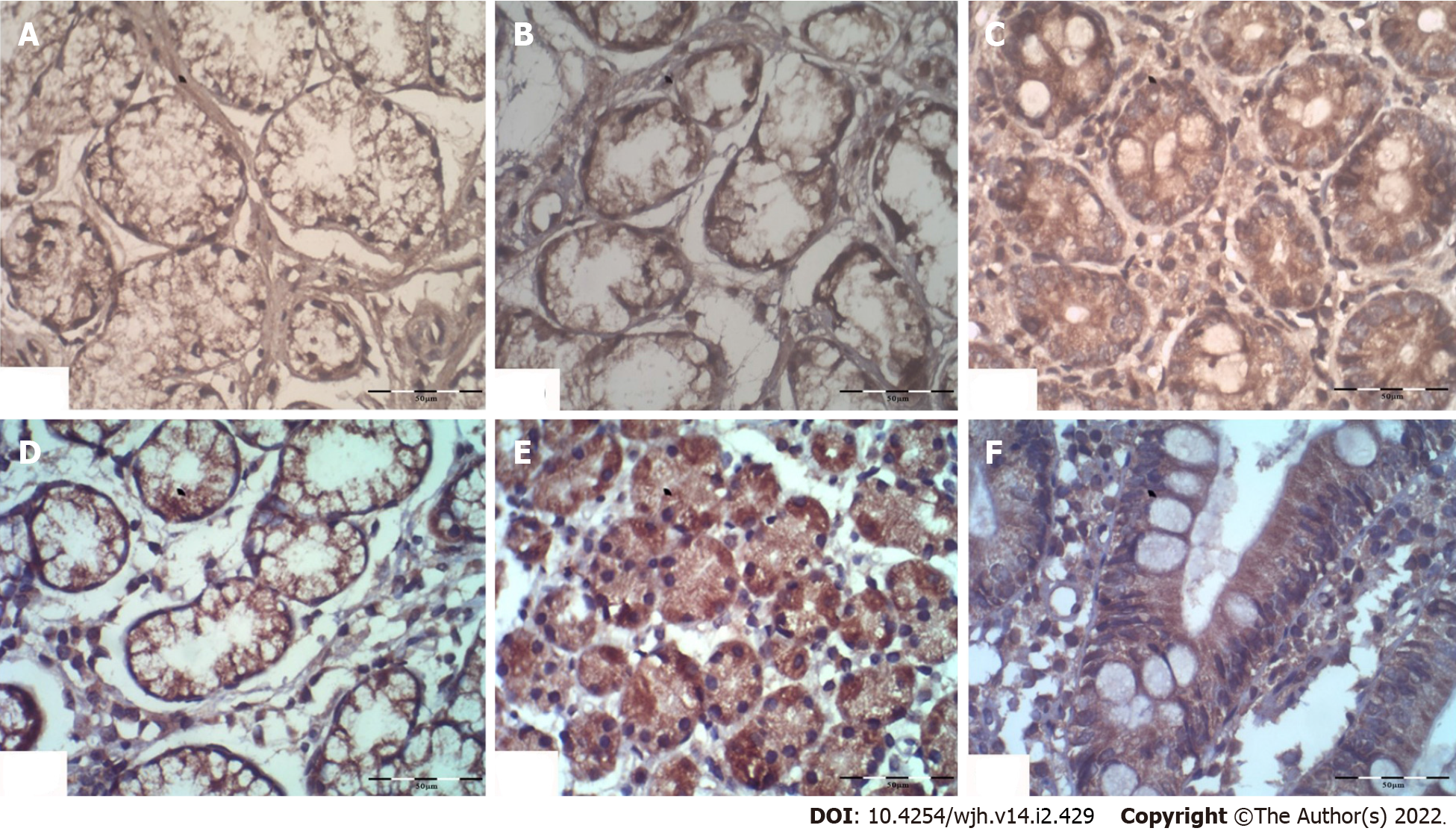
The histopathological expression of β-Arr-2 was evaluated by immunohistochemical staining using human ARRB2 antibody (Β-arrestin-2, Cat. #PA002135LA01HU, CUSABIO, United States) following the manufacturer's instructions. The degree of β-Arr-2 expression was semiquantitatively expressed as (+) low, (++) moderate, and (+++) strong staining[16]. The endoscopic and pathologic evaluations were blinded.
The response to NSBB was evaluated clinically (signs of bleeding during the follow-up), endoscopically (changes in variceal grade), and by Doppler ultrasound assessment of changes in the PVCI i.e. ΔPVCI.
The sample size was calculated using Stata/MP v.15.1 software (StataCorp LLC, United States) with a statistical power of 90%, a two-tailed α level of 0.05, and assuming a value of 0.5 as a hazard ratio. Normality of distribution was assessed, and data were expressed as a mean ± SD or proportions. The student’s t-test or the ANOVA test was used as appropriate. The Chi-square (χ2), Fisher’s Exact (FET), or McNemar (Bowker’s) test was used to compare proportions. The sensitivity and specificity of serum β-Arr-2 were assessed by receiver-operating characteristic (ROC) curve. Correlations between variables were analyzed by Pearson’s or Spearman’s test as appropriate. Multivariate regression, Cox regression, Kaplan-Meier analysis were done.
During the study, 29 patients were lost to follow-up (during the COVID-19 pandemic). The analysis was done for 91 patients (per-protocol analysis). In total, 31 (34.1%) patients experienced variceal bleeding (NSBB non-responders), and 60 (65.9%) patients did not bleed (NSBB responders). At baseline, small, medium, and large EVs were present in 17 (18.7%), 48 (52.7%), and 26 (28.6%) patients, respectively. Mild and severe PHG was detected in 32 (35.2%) and 33 (36.2%) patients, respectively. GV was seen in 14 (15.4%) patients. Table 1 shows the baseline clinical and laboratory data.
| PPA (n = 91) | NSBB responders (n = 60) | NSBB non-responders (n = 31) | P value | |
| Male sex n (%) | 65 (71.4) | 44 (73.3) | 21 (67.7) | 0.371 |
| Age (years) | 55.16 ± 4.0 | 55.43 ± 3.74 | 54.64 ± 4.57 | 0.382 |
| Ascites n (%) | 29 (31.9) | 19 (31.7) | 10 (32.3) | 0.571 |
| HB (g/dL) | 11.07 ± 1.44 | 11.10 ± 1.66 | 11.02 ± 0.91 | 0.812 |
| Platelets (× 103/mm3) | 83.13 ± 12.33 | 85.32 ± 15.46 | 78.90 ± 12.19 | 0.022 |
| WBCs (× 103/mm3) | 4.85 ± 1.05 | 4.88 ± 1.11 | 4.78 ± 0.96 | 0.662 |
| ALT (IU/L) | 40.01 ± 9.74 | 40.75 ± 9.98 | 38.58 ± 9.23 | 0.322 |
| AST (IU/L) | 54.40 ± 14.00 | 55.48 ± 14.24 | 52.29 ± 13.47 | 0.312 |
| APRI-score | 1.94 ± 0.46 | 1.92 ± 0.30 | 1.97 ± 0.56 | 0.632 |
| Albumin (g/dL) | 2.94 ± 0.18 | 2.93 ± 0.17 | 2.97 ± 0.18 | 0.252 |
| Bilirubin (mg/dL) | 1.73 ± 0.62 | 1.65 ± 0.55 | 1.89 ± 0.72 | 0.112 |
| INR | 1.35 ± 0.18 | 1.34 ± 0.17 | 1.39 ± 0.16 | 0.192 |
| CTP A/B/C | 48/36/7 | 34/22/4 | 14/14/3 | 0.563 |
| Baseline PVCI | 0.533 ± 0.10 | 0.545 ± 0.11 | 0.509 ± 0.09 | 0.112 |
| Baseline esophageal varices grade: n (%) | ||||
| Small | 17(18.7) | 10 (16.7) | 7 (22.6) | 0.061 |
| Medium | 48 (52.7) | 28 (46.6) | 20 (64.5) | |
| large | 26 (28.6) | 22 (36.7) | 4 (12.9) | |
| Baseline portal hypertensive gastropathy grade: n (%) | ||||
| No | 26 (28.6) | 13 (21.7) | 13 (41.9) | 0.0121 |
| Mild | 32 (35.2) | 19(31.7) | 13 (41.9) | |
| Severe | 33 (36.2) | 28 (46.6) | 5 (16.2) | |
| GV presence n (%) | 14 (15.4) | 12 (20.0) | 2 (6.5) | 0.083 |
| NSBB dose (min-max): (mg/d) | 64.84 ± 14.63 (30-100) | 65.33 ± 15.45 (30-100) | 63.87 ± 13.08 (40-80) | 0.652 |
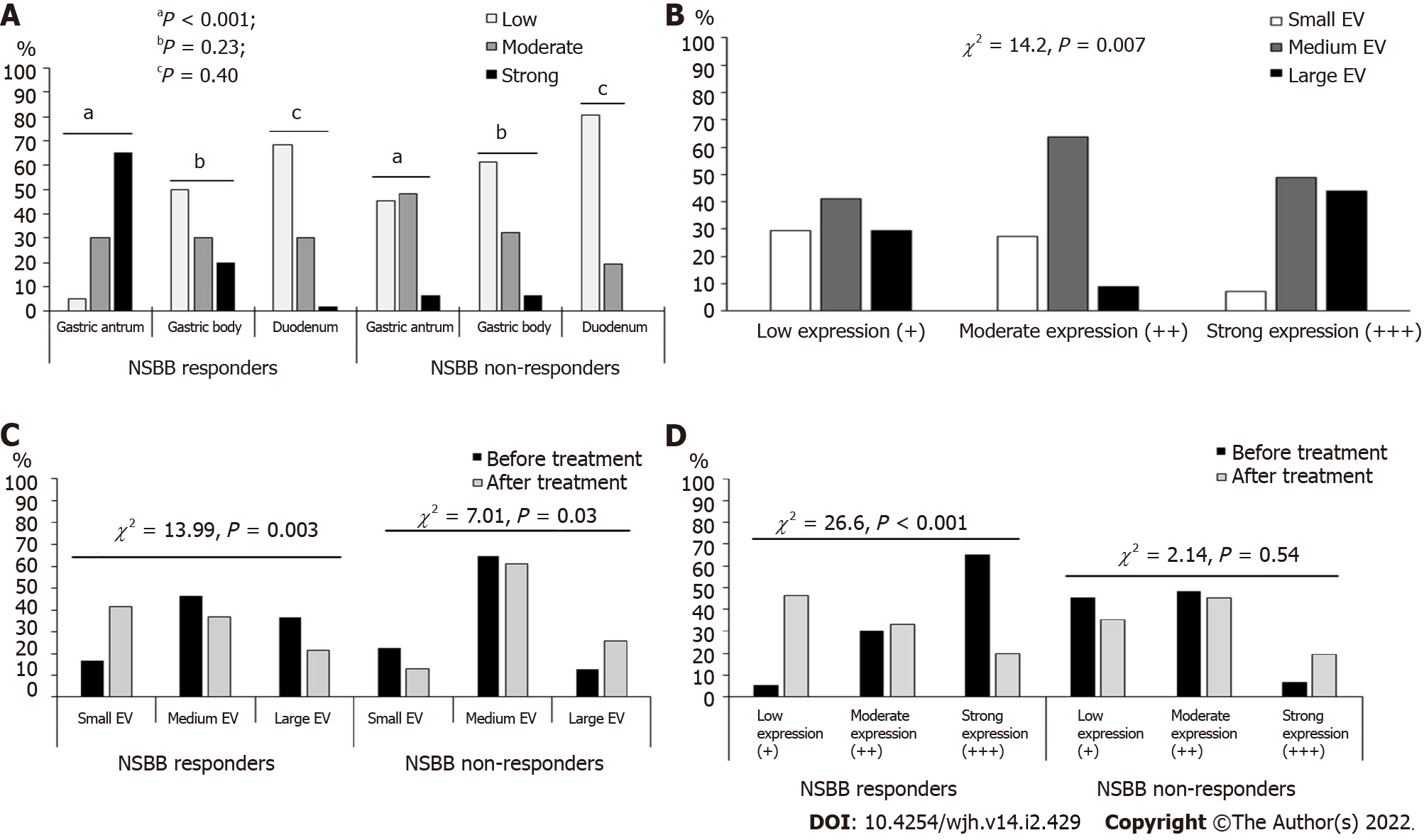
The serum β-Arr-2 levels in patients were higher than those in healthy controls (mean ± SD), 2.57 ± 0.48 vs 1.59 ± 1.29 ng/mL, respectively, P < 0.001). At baseline, serum β-Arr-2 levels in the responders were higher than those in the non-responders (mean ± SD, 2.79 ± 0.40 vs 2.13 ± 0.28 ng/mL, respectively, P < 0.001, 95%CI: -0.80 to -0.51) (Supplementary Figure 1). The tissue expression of β-Arr-2 in the gastric antrum was significantly different between subgroups [(5%, 30% and 65% among responders vs 45.2%, 48.4% and 6.4% among non-responders) for low (+), moderate (++), and strong (+++) expressions, respectively, χ2 = 30.1, P < 0.001]. No significant difference was found in terms of β-Arr-2 expression in the gastric body (P = 0.23) or duodenum (P = 0.40). Therefore, the statistical analysis subsequently focused on β-Arr-2 expression in the gastric antrum (Figure 1, Figure 2A).
Patients were stratified according to the baseline grade of antral β-Arr-2 expression (n = 91). The comparison between these strata showed that stronger antral β-Arr-2 expression was associated with a higher EV grade at baseline (43.9% of patients with strong β-Arr-2 expression (n = 41) had large EV vs 29.4% and 9.1% for low (n = 17) and moderate (n = 33) β-Arr-2 expression, respectively, χ2 = 14.2, P = 0.007) (Figure 2B). However, there was no significant difference between these strata as regards the presence of GV (P = 0.11).
The serum β-Arr-2 levels were higher in patients with large EVs than in those with medium and small EVs and patients with medium EVs than in those with small EVs (mean ± SD, 2.90 ± 0.42 vs 2.50 ± 0.44 and 2.24 ± 0.42 ng/mL respectively, P < 0.001) (Supplementary Figure 2). Patients with GV showed a higher mean serum β-Arr-2 levels than patients without GV (2.85 ± 0.37 vs 2.51 ± 0.48 ng/mL, P = 0.007). However, the number of patients with GV in the current study was too small (n = 14) for detailed analysis.
Patients with strong antral β-Arr-2 expression showed higher mean values of PVCI than those with moderate and low expression (0.566 ± 0.09 vs 0.517 ± 0.11 and vs 0.483 ± 0.08 cm2/s, P = 0.04 and P < 0.005 respectively). There was no difference between patients with low and moderate expression of β-Arr-2 in terms of mean values of PVCI (P = 0.24).
The mean value of PVCI after NSBB treatment in NSBB-responders significantly decreased compared with the baseline (0.492 ± 0.11 vs 0.545 ± 0.10 cm2/s, P < 0.001, 95%CI: 0.04 - 0.06), whereas in non-responders, there was no difference (0.511 ± 0.09 vs 0.509 ± 0.08 cm2/s, P = 0.76). Also, the mean value of ΔPVCI among responders was higher than that among non-responders (0.0538 ± 0.06 vs 0.002 ± 0.04 cm2/s, P < 0.001).
At baseline, the frequency of small, medium, and large EV between patients (n = 91) was 18.7%, 52.7%, and 28.6% of cases, respectively. At the end of the study, the frequency of small, medium, and large EV was changed to 31.9%, 45.1%, and 23.1% of cases, respectively (P = 0.049, χ2 = 7.6, Supplementary Figure 3).
Before NSBB treatment, the EV grades were not significantly different between responders and non-responders (Table 1). However, after treatment, a significant difference in favor of the responders appeared (the frequency decreased from 36.7% to 21.7% and from 46.7% to 36.7% for large and medium varices, respectively, P = 0.003). Among non-responders, there was a progression in the EV grades compared with baseline (P = 0.03) (Figure 2C).
There was a significant change in the antral expression of β-Arr-2 after treatment with NSBB compared with baseline. At baseline, 18.6% of patients showed low expression, 36.3% showed moderate expression, and 45.1% showed strong expression. After NSBB treatment, 42.9% of patients showed low expression, 37.4% showed moderate expression, and 19.7% showed strong expression (McNemar Bowker’s χ2 = 16.18, P = 0.001). Among the NSBB-responders, the frequency of strong β-Arr-2 expression significantly decreased (20% strong expression post-treatment vs 65% at baseline, χ2 = 26.6, P < 0.001); whereas in the non-responders, there was no significant difference between baseline and post-treatment expression (P = 0.54) (Figure 2D). Multivariate regression showed that NSBB dose (P = 0.02, OR = 1.01, 95%CI: 0.91-1.05) and the ΔPVCI (P = 0.005, OR = 1.58, 95%CI: 0.001- 0.002) were the only independent predictors of reduced β-Arr-2 expression (Table 2).
| Parameters | Univariate analysis | Multivariate analysis | |||
| P value | OR | P value | OR | 95%CI | |
| ALT | 0.51 | - | - | - | - |
| AST | 0.20 | - | - | - | - |
| Platelets | 0.26 | - | - | - | - |
| APRI | 0.06 | - | - | - | - |
| Albumen | 0.25 | - | - | - | - |
| Bilirubin | 0.69 | - | - | - | - |
| Child-Turcotte-Pugh class | 0.73 | - | - | - | - |
| Baseline EV grade | 0.003 | 1.35 | - | - | - |
| Baseline β-Arr-2 expression | < 0.001 | 2.41 | - | - | - |
| NSBB dose | 0.049 | 6.5 | 0.02 | 1.01 | 0.91-1.05 |
| ΔPVCI | < 0.001 | 10.34 | 0.005 | 1.58 | 0.001- 0.002 |
Serum and antral expression of β-Arr-2 were directly correlated to each other (rs = 0.72, P < 0.001). Both serum and antral expression of β-Arr-2 showed a direct correlation with baseline EV grade, baseline PHG, PVCI, and APRI score. They also showed a negative correlation with platelet count and serum AST (P < 0.05) (Table 3). In addition, β-Arr-2 expression intensity after NSBB therapy was directly correlated with the severity of PHG (rs = 0.35, P < 0.001).
| Serum β-Arr-2 level | β-Arr-2 expression in the gastric antrum | |||
| rs | P value | rs | P value | |
| Age | 0.101 | 0.43 | 0.16 | 0.14 |
| Child-Pugh class | 0.11 | 0.34 | 0.08 | 0.45 |
| INR | 0.121 | 0.19 | 0.11 | 0.34 |
| Serum albumin | -0.191 | 0.07 | -0.12 | 0.26 |
| Serum bilirubin | 0.121 | 0.19 | 0.03 | 0.77 |
| Presence of ascites | 0.11 | 0.30 | 0.14 | 0.18 |
| Platelets count | -0.381 | < 0.001 | -0.28 | 0.008 |
| Baseline EV grade | 0.48 | < 0.001 | 0.30 | 0.004 |
| Baseline PHG grade | 0.33 | 0.002 | 0.38 | < 0.001 |
| Baseline congestion index | 0.361 | < 0.001 | 0.32 | 0.002 |
| Antral β-Arr-2expression | 0.72 | < 0.001 | - | - |
| Corpus β-Arr-2 expression | 0.17 | 0.10 | 0.11 | 0.28 |
| Duodenal β-Arr-2 expression | 0.19 | 0.07 | 0.13 | 0.23 |
| AST | -0.231 | 0.03 | -0.25 | 0.019 |
| APRI score | 0.341 | < 0.001 | 0.25 | 0.039 |
| Non-selective B-blocker dose | 0.151 | 0.15 | 0.03 | 0.73 |
At baseline, 17 patients had low β-Arr-2 expression; among them, 14 patients (82.4%) experienced variceal bleeding. Further, 33 patients had moderate β-Arr-2 expression; among them, 15 (45.5%) patients experienced variceal bleeding. Similarly, 41 patients had strong β-Arr-2 expression, and among them, 2 (4.9%) patients experienced variceal bleeding (χ2 = 35.10, P < 0.001).
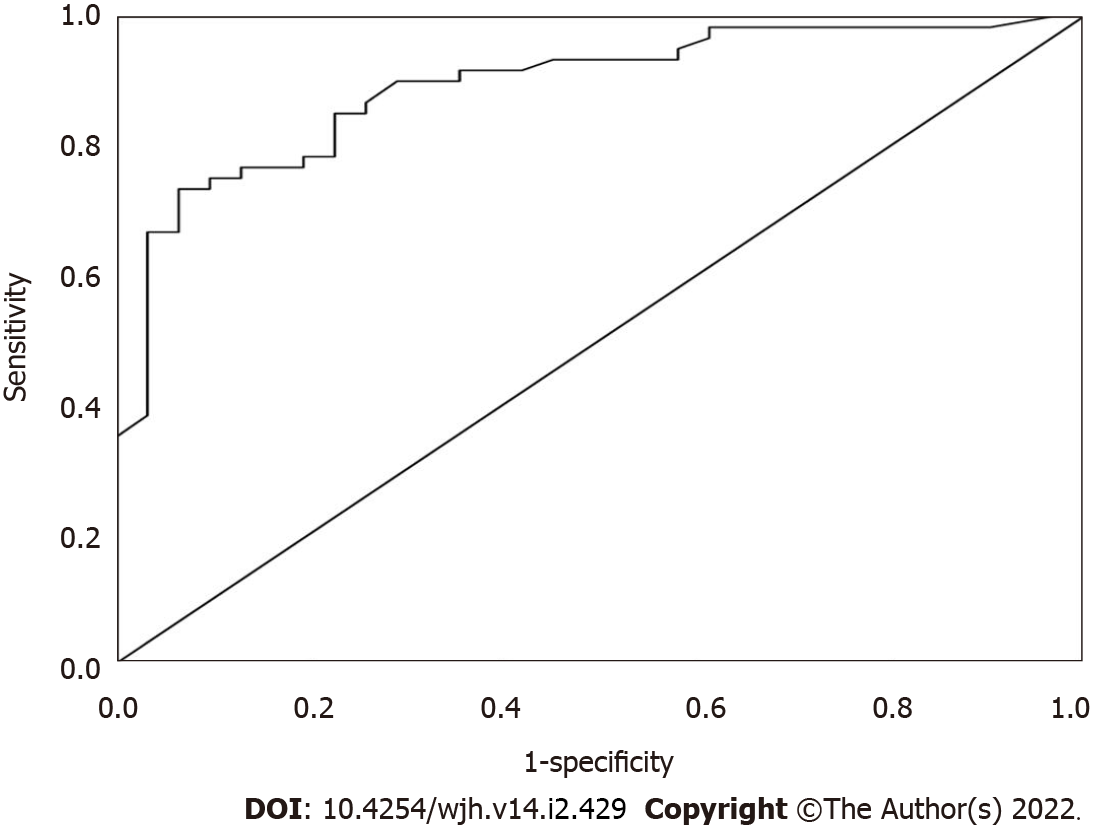
At a cut-off value of ≥ 2.23 ng/mL, serum β-Arr-2 could be used to identify patients at low risk of variceal bleeding with a sensitivity and specificity of 90% and 71%, respectively. The positive predictive value was 79%, and the negative predictive value was 85.7% (AUC = 89.2%, P < 0.001, 95%CI: 0.83-0.96) (Figure 3).
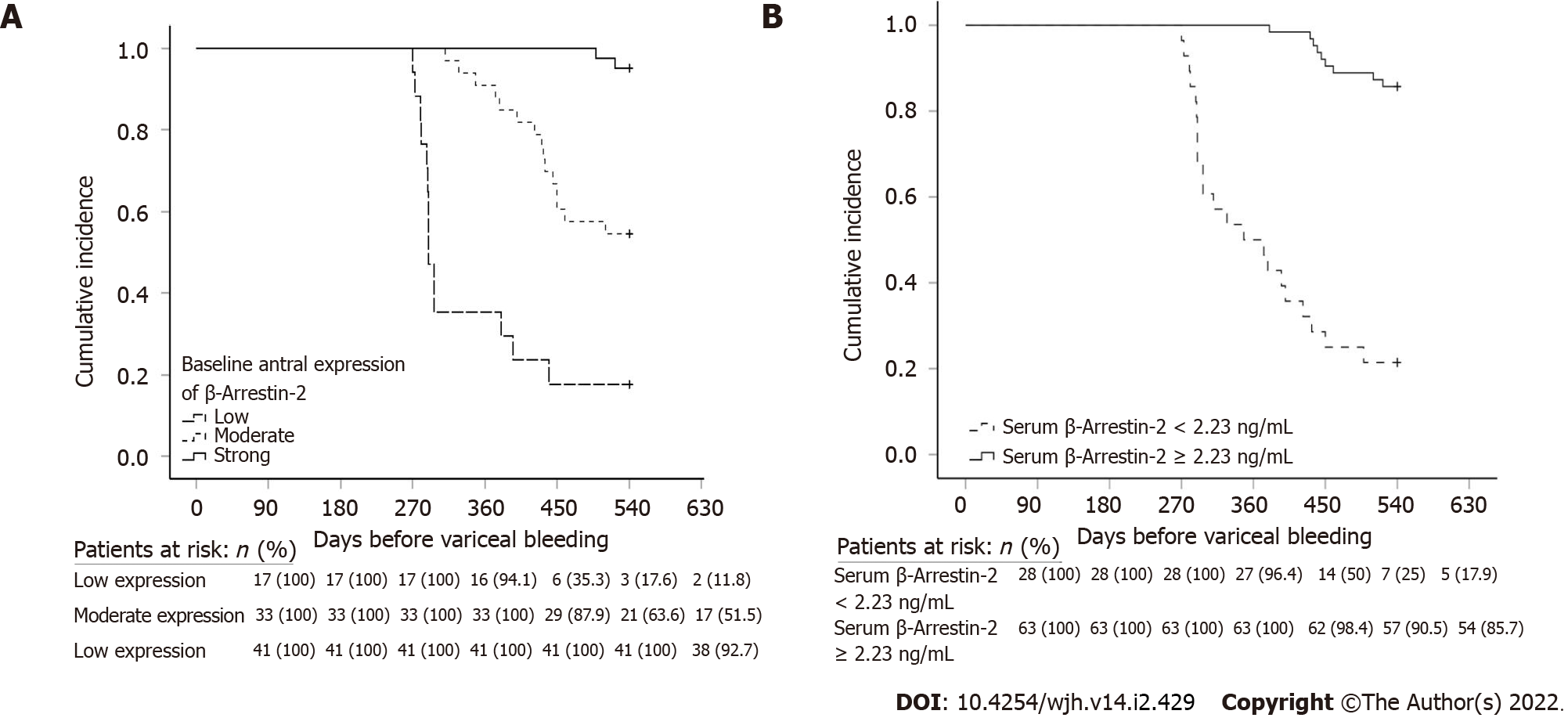
Kaplan-Meier analysis with log-rank test was performed (n = 91). Patients with strong baseline antral β-Arr-2 expression and receiving NSBB treatment had a longer variceal bleeding-free interval. The mean (median) time interval before variceal bleeding for low, moderate, and strong antral β-Arr-2 expression was 351.7 (290), 481.6 (540), and 538.5 (540) days, respectively (χ2 = 62.02, P < 0.001) (Figure 4A).
In addition, patients with a serum β-Arr-2 level of ≥ 2.23 ng/mL (as obtained from ROC analysis, n = 63) had a longer bleeding-free interval compared with patients who had a serum β-Arr-2 level of < 2.23 ng/mL (n = 28). The mean (median) variceal bleeding-free interval was 527.5 (540) and 382.8 (360) days respectively, (χ2 = 57.6, P < 0.001) (Figure 4B).
Via Cox-regression analysis, serum β-Arr-2 level (P < 0.001, OR = 0.13, 95%CI: 0.09-0.13), the intensity of β-Arr-2 expression in the gastric antrum (P < 0.001, OR = 0.15, 95%CI: 0.1-0.3), and platelet count (P = 0.006, OR = 0.93, 95%CI: 0.85-0.99), were the only independent predictors for variceal bleeding (Table 4).
| Baseline parameters | P value | OR | 95%CI |
| Alanine aminotransferase | 0.32 | - | - |
| Aspartate aminotransferase | 0.19 | - | - |
| Platelets count | 0.006 | 0.93 | 0.85- 0.99 |
| APRI score | 0.11 | - | - |
| Child-Turcotte-Pugh class | 0.54 | - | - |
| Baseline esophageal varices grade | 0.29 | - | - |
| Baseline portal vein congestion index | 0.49 | - | - |
| Β-arrestin-2 antral expression | < 0.001 | 0.15 | 0.1- 0.3 |
| Serum β-arrestin-2 | < 0.001 | 0.13 | 0.09-0.13 |
Among patients who completed the follow-up (n = 91), we had 7 (7.7%) patients who developed SBP. This subgroup of patients had Child-Pugh class B (2 patients) and C (5 patients). One patient (14.3%) of them had a strong β-Arr-2 expression, four (57.1%) patients had a moderate expression, and two (28.6%) patients had low expression. They also have a mean serum β-Arr-2 of 2.15 ± 0.3 ng/mL (vs 2.60 ± 0.47 ng/mL for patients without SBP, P = 0.006). This subgroup of patients received a mean dose of NSBB = 60 ± 19.1 mg (vs 65.23 ± 14.26 mg for patients without SBP, P = 0.36).
In addition, we have 5 (5.5%) patients who developed refractory ascites. This subgroup of patients had Child-Pugh class B (1 patient) and C (4 patients). Two (40%) patients had low β-Arr-2 expression and 3 (60%) patients had moderate expression (no patients showed strong expression). They had a mean serum β-Arr-2 of 2.10±0.19 ng/mL (vs 2.59 ± 0.48 ng/mL for patients without SBP, P = 0.002). This subgroup of patients received a mean dose of NSBB = 70 ± 10 mg (vs 64.5 ± 14.85 mg for patients without refractory ascites, P = 0.42).
Nonselective β-blockers reduce portal pressure by minimizing the cardiac output via the blockade of β1 cardiac receptors and enhancing splanchnic vasoconstriction via the blockade of β2 receptors, leaving an unopposed α-adrenergic activity[17].
The core findings in our study were as follows: (1) Gastric antral β-Arr-2 expression is more related to the portal hemodynamics than corpus or duodenal expression; (2) β-Arr-2 expression correlates with the degree of PHT in terms of EV and PHG grades; (3) Stronger β-Arr-2 expression is associated with sustained clinical response to NSBB, decrease in the PVCI, better EV control, and longer variceal bleeding-free interval; (4) Patients who experienced variceal bleeding while on NSBB therapy had a lower baseline serum and tissue expression of β-Arr-2; (5) Patients who did not bleed during NSBB therapy (NSBB responders) showed a reduction in the expression of β-Arr-2 after long-term treatment, highlighting the link between PHT dynamics and β-Arr-2 expression; and (6) the serum level of β-Arr-2 directly correlates with the antral expression of β-Arr-2 and show high sensitivity and specificity for defining the subgroup of patients who will respond to NSBB with a low likelihood for variceal bleeding. These results suggest that serum and gastric antral β-Arr-2 are potentially simple and minimally invasive markers for PHT patients who may show a favorable response to NSBB.
Although NSBB minimizes the risk of variceal bleeding, this is observed only in approximately 40% of cases, leaving 60% of patients vulnerable to the drug’s adverse effects without any benefits. Therefore, identifying such patients is essential, especially among patients with refractory ascites and spontaneous bacterial peritonitis[8,18].
The pathophysiology of PHT involves the down-regulation of vasoactive proteins [RhoA/Rho kinase (ROCK)] and up-regulation of vasodilators [e.g. Nitric oxide (NO)]. This contributes to splanchnic vasodilation and induction of the renin-angiotensin-aldosterone signaling (RAAS) pathway[19]. However, the vasoconstrictor angiotensin II (AT-2) and other vasoactive substances [e.g., endothelin 1 (ET-1)] fail to induce splanchnic vasoconstriction due to the down-regulation of downstream pathways involving RhoA and ROCK. This probably extends to the mucosal vasculature and is not limited to the large splanchnic vessels[19].
β-Arr-2 expression is increased in splanchnic vessels of animals and humans with cirrhosis and could suppress the vasoactive signaling via the desensitization of the AT-2 and ET-1 receptors[19,20]. This explains the direct correlation between the serum levels and tissue expression of β-Arr-2 on one side and the grade of varices and PHG on the other side, which was demonstrated in our study, corroborating previous research[11,21,22].
As it determines the probability of achieving more benefit than damage, the timing of initiation of NSBB therapy is clinically important. In the early stages of PHT, RAAS system activation is minimal. This results in a milder form splanchnic and systemic hyperdynamic state; therefore, the splanchnic circulation sensitivity to NSBB remains turned off[23,24]. This clinically important aspect can be identified indirectly via a minimally invasive technique through the correlation between β-Arr-2 expression and PHT severity. Also, serum and tissue expression of β-Arr-2 can aid in selecting patients who will benefit from NSBB in cases of advanced cirrhosis, in which life-threatening complications of NSBB can occur[8,24].
The mechanisms by which NSBB lowers the portal pressure[17] are driven by their increased affinity to β adrenergic receptors-1 and 2 (β1 and 2-AR)[25]. β-Arr-2 signaling has been linked to β1-AR up-regulation. In mice, β-Arr-2 overexpression has been found to restore the inotropic properties of β1-AR. In patients with heart failure, β-Arr-2 could upregulate β1-ARs (so that they are more ready for β-blocker binding). Also, β-Arr-2 can bind and inhibit β1-AR through the kinase pathway (synergistic effect with β-blockers)[26-28].
With regards to β2-AR, the overexpression of β-arr-1 or βarr-2 in human airway smooth muscle (ASM) cultures causes β2-AR desensitization and β agonist-stimulated signaling attenuation[29]. In in vivo and ex vivo murine models of ASM contractile regulation, β-Arr-2 appeared to antagonize β agonist-mediated ASM relaxation[30]. β-Arr-2 can preferentially bind to PIP5K-Iα and μ2-adaptin proteins, which regulate G protein-coupled receptor trafficking and enhance β2-AR endocytosis. These effects synergize in the attenuation of the physiological functions of β1 and β2-AR[31].
These interactions might also apply for splanchnic β2-AR and may explain the enhanced response of patients with PHT, who have a stronger expression of β-Arr-2, to NSBB treatment. With the lack of a correlation between NSBB dose and β-Arr-2 expression in our study, we suggest that β-Arr-2 might manipulate portal hemodynamics through a direct synergistic effect and by enhancing the affinity of β2-AR for binding NSBB rather than a dose-related effect. This is supported by the absence of a significant difference in the mean dose of NSBB between responders and non-responders in our results.
In the current study, few patients developed SBP and refractory ascites. The lack of significant difference in NSBB dose in these events among subgroups may support the possible hypothesis of β2-AR receptors readiness for NSBB therapy rather than a dose-dependent effect. This again, emphasizes the role of β-Arr-2 as a marker to select patients with PHT who will tolerate NSBB therapy without complications. However, the number of cases with SBP and refractory ascites in our cohort is too low to provide a conclusion as regards this point and more validation on a wide scale is reco
In the current study, the antral expression of β-Arr-2 decreased significantly in the NSBB responders. Similarly, Trebicka et al[11,21] found in their study that β-Arr-2 expression in the gastric antrum decreased after performing a trans-jugular intrahepatic portosystemic shunt. Following a decrease in the portal pressure, they found a reversal of the vasoactive protein expression toward normal. In another study, however, β-Arr-2 expression remained unchanged despite HVPG reduction. Further investigation of the changes in β-Arr-2 expression is recommended to resolve these inconsistencies.
This study has some limitations. We did not perform HVPG measurement due to its invasiveness and unavailability in our institute. Further, we did not assess other vasoactive substances, such as NO and RhoA, due to financial constraints. Nevertheless, the current study still has notable strengths. In addition to the prospective design, we studied a relatively large number of patients and had a relatively long follow-up period. This is also the first study to provide the measurement of β-Arr-2 in the serum of patients with PHT with good sensitivity and specificity.
Antral β-Arr-2 expression in PHT patients correlates to the severity of PHT. Stronger expression is associated with a better response to NSBB and longer variceal bleeding-free interval. We suggest assessing serum β-Arr-2 level as a potential, noninvasive biomarker for identifying PHT patients who are good candidates for NSBB therapy. In addition, we recommend future studies to validation of the current results on a larger scale of patients.
Variceal bleeding is a life-threatening complication of portal hypertension (PHT). Nonselective β-blockers (NSBB) are used as primary or secondary prophylaxis in patients with PHT. The use of NSBB has been associated with the development of refractory ascites and spontaneous bacterial peritonitis in a subgroup of patients. β-arrestin-2 (β-Arr-2) has been shown to predict the short-term response to NSBB in a few studies.
There is a gap of knowledge still present. The previous research about β-Arr-2 was about the acute hemodynamic response to NSBB infusion, but no data about the long-term effects. About two-thirds of patients with PHT fail to respond to NSBB, with the exposure to undesirable side effects. Identifying this subset of patients noninvasively is of clinical importance. Again, the long-term changes in β-Arr-2 expression after NSBB therapy have not yet been investigated.
We aimed to investigate the role of both serum and tissue expression of β-Arr-2 as a minimally invasive to predict the long-term clinical response of PHT to NSBB therapy, as well as to investigate the long-term changes in β-Arr-2 expression after NSBB therapy.
We prospectively enrolled 120 patients with cirrhotic PHT. Full history and clinical evaluation were done. Laboratory investigations including serum β-Arr-2 were done. Doppler ultrasound of the portal circulation to measure the portal vein congestion index (PVCI) was obtained. Esophagogastroduodenoscopy (EGD) was performed to evaluate the presence and grade of varices and to obtain mucosal biopsies to define the expression of β-Arr-2. NSBB therapy was initiated. A follow-up for 18 mo (540 d) was done. Another endoscopic biopsy was obtained at the end of the study to re-assess the tissue expression of β-Arr-2. Patients were designated as “NSBB responders” if they didn’t experience variceal bleeding until the end of follow-up; or “NSBB non-responders” if they had bled. PVCI was re-evaluated at the end of the study.
A higher serum level and antral expression of β-Arr-2 were associated with better clinical response to NSBB (longer bleeding-free intervals, and improved grade of varices). Only 17.6% of patients with low baseline β-arr-2 expression responded to NSBB, whereas, 95.1% of patients with strong β-arr-2 expression were responders (P < 0.001). A serum β-Arr-2 value ≥ 2.23 ng/mL was associated with a lower likelihood of variceal bleeding with 90% sensitivity and 71% specificity. β-arrestin-2 expression significantly decreased after nonselective β-blocker therapy. Serum β-Arr-2 level (P < 0.001), the intensity of β-Arr-2 expression in the gastric antrum (P < 0.001), and platelet count (P = 0.006), were the only independent predictors for variceal bleeding
The serum level and tissue expression of β-Arr-2 in the gastric antrum are correlated to the severity of PHT. The lower β-Arr-2 expression can predict non-response to NSBB therapy. Stronger expression is linked to a better long-term clinical response to NSBB in terms of variceal bleeding-free interval. We introduce serum β-Arr-2 level as a potential, noninvasive biomarker for selecting patients with PHT who are potentially good candidates for NSBB therapy.
Future studies are needed to validate the results of our study on a wider scale of patients. Prospective research is needed to explore the relation between the expression of β-Arr-2 and the development of spontaneous bacterial peritonitis and hepatorenal syndrome in cirrhotic PHT.
Provenance and peer review: Invited article; externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cao HC, Ferrarese A S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Triantos CK, Burroughs AK. Prevention of the development of varices and first portal hypertensive bleeding episode. Best Pract Res Clin Gastroenterol. 2007;21:31-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Rajekar H. Complication of cirrhosis portal hypertension: a review. J Liver. 2015;4:188. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Koh C, Heller T. Approach to the diagnosis of portal hypertension. Clin Liver Dis (Hoboken). 2012;1:133-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Toubia N, Sanyal AJ. Portal hypertension and variceal hemorrhage. Med Clin North Am. 2008;92:551-574, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2293] [Article Influence: 229.3] [Reference Citation Analysis (3)] |
| 6. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1441] [Article Influence: 180.1] [Reference Citation Analysis (3)] |
| 7. | Suk KT. Hepatic venous pressure gradient: clinical use in chronic liver disease. Clin Mol Hepatol. 2014;20:6-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 8. | Sersté T, Melot C, Francoz C, Durand F, Rautou PE, Valla D, Moreau R, Lebrec D. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010;52:1017-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 376] [Article Influence: 25.1] [Reference Citation Analysis (1)] |
| 9. | Sharma P, Kumar A, Sharma BC, Sarin SK. Early identification of haemodynamic response to pharmacotherapy is essential for primary prophylaxis of variceal bleeding in patients with 'high-risk' varices. Aliment Pharmacol Ther. 2009;30:48-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Bosch J, Garcia-Pagan JC. Complications of cirrhosis. Portal hypertension. J Hepatol. 2000;32:141-156. [RCA] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 338] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 11. | Trebicka J, von Heydebrand M, Lehmann J, Tofteng F, Busk T, Jensen HL, Rohde J, Reiberger T, Mortensen C, Schierwagen R, Klein S, Møller S, Bendtsen F, Krag A. Assessment of response to beta-blockers by expression of βArr2 and RhoA/ROCK2 in antrum mucosa in cirrhotic patients. J Hepatol. 2016;64:1265-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Wu Z, Tong W, Tan Z, Wang S, Lin P. [The clinical significance of β-arrestin 2 expression in the serum of non-small cell lung cancer patients]. Zhongguo Fei Ai Za Zhi. 2011;14:497-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Moriyasu F, Nishida O, Ban N, Nakamura T, Sakai M, Miyake T, Uchino H. "Congestion index" of the portal vein. AJR Am J Roentgenol. 1986;146:735-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 153] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Primignani M, Tosetti G. Portal hypertensive gastropathy after variceal eradication: more bleeding risk or just more reddening? Hepatol Int. 2016;10:847-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1818] [Article Influence: 259.7] [Reference Citation Analysis (2)] |
| 16. | Jing X, Zhang H, Hu J, Su P, Zhang W, Jia M, Cheng H, Li W, Zhou G. β-arrestin 2 is associated with multidrug resistance in breast cancer cells through regulating MDR1 gene expression. Int J Clin Exp Pathol. 2015;8:1354-1363. [PubMed] |
| 17. | Miñano C, Garcia-Tsao G. Clinical pharmacology of portal hypertension. Gastroenterol Clin North Am. 2010;39:681-695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Mandorfer M, Bota S, Schwabl P, Bucsics T, Pfisterer N, Kruzik M, Hagmann M, Blacky A, Ferlitsch A, Sieghart W, Trauner M, Peck-Radosavljevic M, Reiberger T. Nonselective β blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology. 2014;146:1680-90.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 280] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 19. | Hennenberg M, Trebicka J, Sauerbruch T, Heller J. Mechanisms of extrahepatic vasodilation in portal hypertension. Gut. 2008;57:1300-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Hennenberg M, Trebicka J, Biecker E, Schepke M, Sauerbruch T, Heller J. Vascular dysfunction in human and rat cirrhosis: role of receptor-desensitizing and calcium-sensitizing proteins. Hepatology. 2007;45:495-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Trebicka J, Wix C, von Heydebrand M, Hittatiya K, Reiberger T, Klein S, Schierwagen R, Kristiansen G, Peck-Radosavljevic M, Fischer HP, Møller S, Bendtsen F, Krag A, Sauerbruch T. Expression of vasoactive proteins in gastric antral mucosa reflects vascular dysfunction in patients with cirrhosis and portal hypertension. Liver Int. 2015;35:1393-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Ho HL, Huang HC. Molecular mechanisms of circulatory dysfunction in cirrhotic portal hypertension. J Chin Med Assoc. 2015;78:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, Escorsell A, Garcia-Pagan JC, Patch D, Matloff DS, Gao H, Makuch R; Portal Hypertension Collaborative Group. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254-2261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 653] [Article Influence: 32.7] [Reference Citation Analysis (1)] |
| 24. | Rodrigues SG, Mendoza YP, Bosch J. Beta-blockers in cirrhosis: Evidence-based indications and limitations. JHEP Rep. 2019;20:100063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 25. | Rajoriya N, Tripathi D. Current concepts and controversies. World J Pharmacol. 2016;5:15-31. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 26. | McCrink KA, Maning J, Vu A, Jafferjee M, Marrero C, Brill A, Bathgate-Siryk A, Dabul S, Koch WJ, Lymperopoulos A. β-Arrestin2 Improves Post-Myocardial Infarction Heart Failure via Sarco(endo)plasmic Reticulum Ca2+-ATPase-Dependent Positive Inotropy in Cardiomyocytes. Hypertension. 2017;70:972-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 27. | Zhabyeyev P, Zhang H, Oudit GY. Is β-Arrestin 2 a Magic Bullet for Heart Failure Treatment? Hypertension. 2017;70:887-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990;248:1547-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 910] [Cited by in RCA: 910] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 29. | Penn RB, Pascual RM, Kim YM, Mundell SJ, Krymskaya VP, Panettieri RA Jr, Benovic JL. Arrestin specificity for G protein-coupled receptors in human airway smooth muscle. J Biol Chem. 2001;276:32648-32656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Deshpande DA, Theriot BS, Penn RB, Walker JK. Beta-arrestins specifically constrain beta2-adrenergic receptor signaling and function in airway smooth muscle. FASEB J. 2008;22:2134-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Kang DS, Tian X, Benovic JL. Role of β-arrestins and arrestin domain-containing proteins in G protein-coupled receptor trafficking. Curr Opin Cell Biol. 2014;27:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |