Published online Feb 27, 2022. doi: 10.4254/wjh.v14.i2.372
Peer-review started: May 27, 2021
First decision: July 6, 2021
Revised: July 21, 2021
Accepted: January 25, 2022
Article in press: January 25, 2022
Published online: February 27, 2022
Processing time: 270 Days and 23 Hours
Hepatocellular carcinoma (HCC) is the most prevalent primary malignancy in patients suffering from chronic liver diseases and cirrhosis. Recent attention has been paid to the involvement of the gut-liver axis (GLA) in HCC pathogenesis. This axis results from a bidirectional, anatomical and functional relationship between the gastrointestinal system and the liver. Moreover, the complex network of interactions between the intestinal microbiome and the liver plays a crucial role in modulation of the HCC-tumor microenvironment, contributing to the pathogenesis of HCC by exposing the liver to pathogen-associated molecular patterns, such as bacterial lipopolysaccharides, DNA, peptidoglycans and flagellin. Indeed, the alteration of gut microflora may disturb the intestinal barrier, bringing several toll-like receptor ligands to the liver thus activating the inflammatory response. This review explores the new therapeutic opportunities that may arise from novel insights into the mechanisms by which microbiota immunomodulation, represented by probiotics, and prebiotics, affects HCC through the GLA.
Core Tip: In patients with chronic liver disease and cirrhosis, hepatocellular carcinoma (HCC) is the most common primary malignancy. Recent attention has been paid to the involvement of the gut-liver axis (GLA) in HCC pathogenesis. This review explores the potential for new treatment options as a result of novel insights into the processes by which microbiota immunomodulation, represented by probiotics and prebiotics, affects HCC through the GLA.
- Citation: Russo E, Fiorindi C, Giudici F, Amedei A. Immunomodulation by probiotics and prebiotics in hepatocellular carcinoma. World J Hepatol 2022; 14(2): 372-385
- URL: https://www.wjgnet.com/1948-5182/full/v14/i2/372.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i2.372
Hepatocellular carcinoma (HCC) is the leading cause of cancer-related death worldwide and it is the most common primary tumor in people with cirrhosis and chronic liver disease[1]. Males are diagnosed with HCC at a greater rate than females (2.4:1) in Eastern and Southern Asia, Middle and Western Africa, Micronesia/Poly-nesia and Melanesia[2]. Today, infection with hepatitis B virus (HBV) and hepatitis C virus (HCV), as well as dietary aflatoxin and alcohol abuse, are all significant risk factors for HCC occurrence. Despite the fact that HBV and HCV account for 80%-90% of total HCC cases, the obesity epidemic, the development of effective direct acting antivirals for HCV, and the availability of a universal HBV vaccination may alter HCC epidemiology in the future[3].
Recently, an increase in the incidence of non-alcoholic steatohepatitis (NASH) and non-alcoholic fatty liver disease (NAFLD) has been accompanied by an increase in the incidence of NASH-related HCC[4]. Aristolochic acid and tobacco have also been identified as probable pathogenetic cofactors in HCC, according to several findings on mutational signatures[5].
Specific advances in our understanding of the processes related to NASH-associated HCC have offered new insights into the tumor microenvironment contributions, generated by a mutual interplay between the immune system and gut microbiota (GM), defined as the assemblage of microorganisms such as bacteria, eukaryotes, archaea, and viruses inhabiting the intestine[6-9]. Indeed, important factors linked to the immune-microbiome interplay such as leaky gut, endotoxemia, toll-like receptors (TLR), dysbiosis, and immunomodulation have been associated with HCC develop-ment[10]. Although the liver does not come into direct contact with bacteria, it is anatomically connected to the gut[11]. Of note, the gastrointestinal tract influences homeostasis, preserving an intact barrier against bacterial lipopolysaccharides (LPS) and intestinal bacteria. The most often utilized marker for the translocation of inflammatory bacterial microbiota-associated molecular patterns (MAMPs) is LPS.
LPS is an element found in gram-negative bacteria cell walls that triggers inflammation via TLR4. Low-grade exposure to GM-derived metabolites and MAMPs occurs during the physiologic transit of nutrient-rich blood from the colon to the liver, despite the extremely effective multi-level intestinal barrier[11]. Bacterial translocation and LPS build up cause intestinal bacterial overgrowth and changes in GM composition when intestinal permeability is enhanced. Furthermore, a leaky gut allows dysbiotic microbiota-associated bacterial metabolites and MAMPs to more easily translocate and reach the liver. Degradation, detoxification, and clearance of LPS and other microbial products are all hampered in patients with chronic liver diseases or cirrhosis, since the damaged liver is exposed to a wider spectrum of TLR ligands, as well as other bacterial products and metabolites[12]. Indeed, it has been noted that altered microbiota is generally present in HCC patients[13]. In addition, unlike bacterial species, it was discovered that the iron transport, microbial metabolism, and energy-producing system of HCC patients and healthy controls varied considerably[13].
TLR4 produced by activated stellate cells reacts to low LPS concentrations, causing fibrosis and cirrhosis development. An animal model of hepatocarcinogenesis showed that the GM and TLR4 activation have been shown to enhance HCC development by increasing cell proliferation and suppressing apoptosis[14].
As the gut-liver axis (GLA) is involved in HCC pathogenesis, the study of the mutual interplay between the microbiota and immune response and their cross-talk with the tumor microenvironment are an important focus of current clinical research. Our review investigates and analyzes the potential therapeutic benefits of emerging insights into the mechanisms by which microbiota immunomodulation, as represented by probiotics and prebiotics, impacts HCC via the GLA.
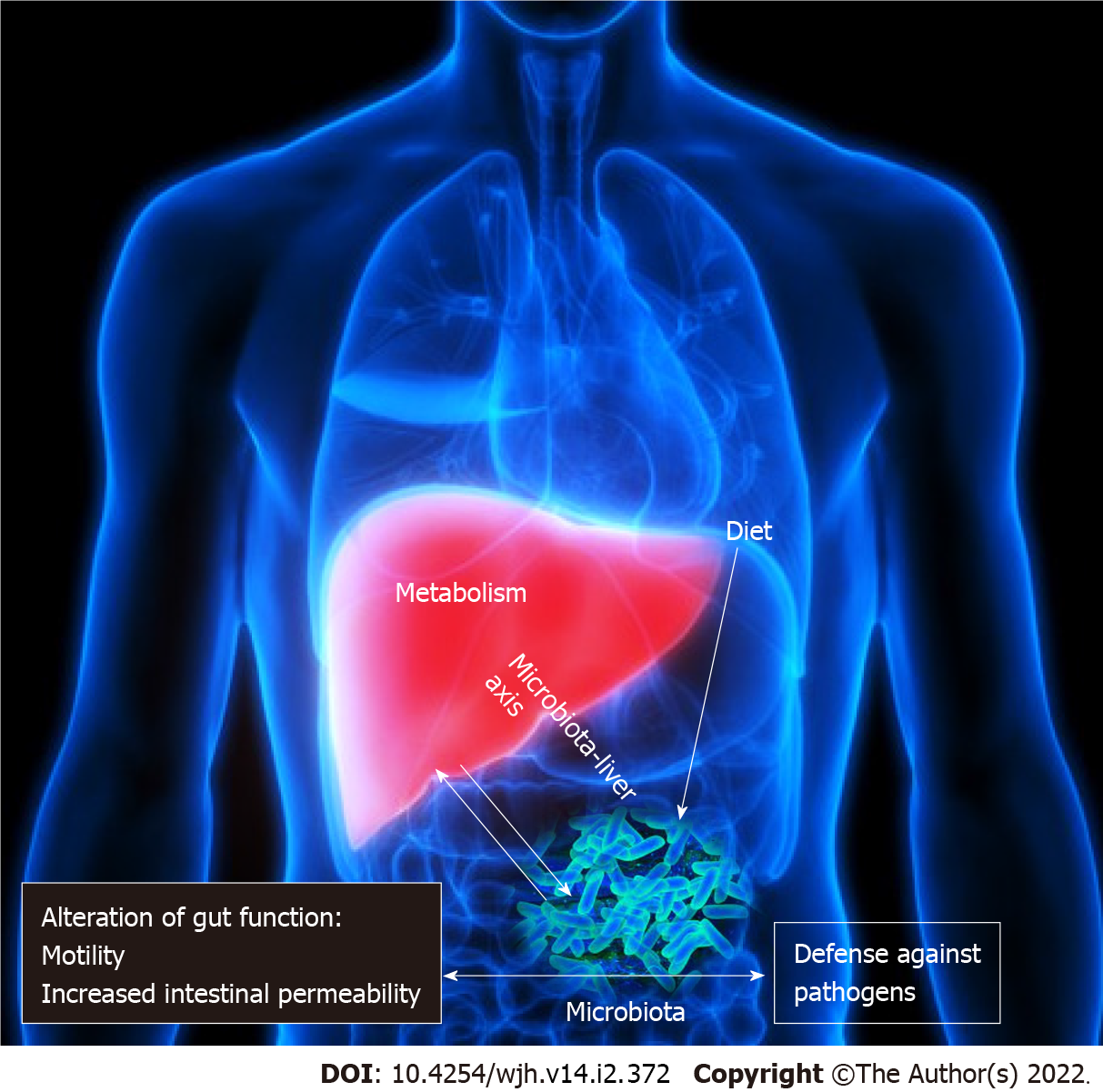
The GM as a “virtual metabolic organ” establishes an axis with several extraintestinal organs, such as the brain, kidneys, bone and cardiovascular system. However, in recent years, the GLA has received considerable interest[15]. The GLA is the result of a bidirectional, anatomical and functional relationship between the gastrointestinal system and the liver, largely via the portal circulation (Figure 1). A complex network of interactions between the enteric microbiome and the liver regulates and stabilizes their symbiotic connection, which includes metabolic and immunological crosstalk[16,17]. Antigens (from harmful microorganisms or food) enter through these linkages and are identified by dendritic cells, which then activate the adaptive immune system by regulating T cell responses. Pathogen-associated molecular patterns (PAMPs) (e.g., LPS, DNA and flagellin activate nuclear factor kappa B and peptidoglycans) via nod-like receptors and TLRs, as a result, inflammatory cytokines and chemokines are secreted and reach the portal circulation. PAMPs can activate stellate cells implicated in fibrosis development and progression, in addition to hepatocyte injury, and Kupffer cells are much more susceptible to LPS than hepatocytes[18].
As previously reported, the GM contribution to HCC etiopathogenesis is complex and elaborate. A disturbed intestinal barrier brings a series of TLR ligands to the liver and activates the inflammatory response in different ways: (1) Via upregulation of hepatic stellate cell proliferation and downregulation of hepatocyte apoptosis, the TLR signaling pathways induce liver tumorigenesis[14]; and (2) Lastly, in HCC, inadequate immunosurveillance is linked to an aberrant intestinal microbiota. Furthermore, via increasing oxidative stress, inflammatory response and steatosis, the microbiota dysbiosis might be linked to HCC development[19].
In general, changes in the makeup of microbial profiles are thought to have a role in tumorigenesis[9]. In fact, recent research has revealed a link between certain bacterial profiles and HCC patients[20], showing high amounts of Escherichia coli and other gram-negative bacteria in the intestinal bacterial flora, which are linked to elevated LPS levels in serum[21]. Moreover, Fusobacterium and Oribacterium are the bacteria most often identified from a tongue swab of HCC affected subjects. On the other hand, the intestinal HCC microbiome showed reduced amounts of Lactobacillus spp., Bifidobacterium spp., and Enterococcus spp.[22]. A more recent report examined bacterial diversity in cirrhosis and HCC patients[20]. A decrease in the fecal microbial diversity from healthy controls to cirrhosis and an increase from cirrhosis to early HCC with cirrhosis (both induced by chronic HBV infection) was observed. In addition, different microbiota markers were detected[20]. Moreover, the authors observed a decrease in Verrucomicrobia with a simultaneous increase in Actinobacteria.
Furthermore, augmented levels of Bacteroides and Ruminococcaceae and increased levels of Akkermansia and Bifidobacterium were detected in patients with NASH-induced cirrhosis and HCC, in comparison to NASH-induced cirrhosis without HCC[23]. In the same study, the authors discovered a link between enteric microbiota patterns and calprotectin levels as well as systemic inflammation. The GM of patients with HBV-associated HCC and non-HBV non-HCV (NBNC) associated HCC was compared in further research. In comparison to healthy controls or to patients with NBNC-related HCC, those with HBV-related HCC had a substantially richer fecal microbiota. Patients with NBNC-related HCC had higher amounts of pro-inflammatory bacteria (Escherichia coli, Enterococcus) and lower amounts of anti-inflammatory bacteria (Faecalibacterium, Ruminococcus, Ruminoclostridium), leading to lower quantities of anti-inflammatory short-chain fatty acids (SCFA) in their feces[24]. The GM of HCC patients receiving liver transplantation was also compared to the intestinal microbiota of individuals who did not have HCC but had a similar etiology of cirrhosis. An augmented abundance of fecal Escherichia coli was linked with HCC[25]. Additionally, Helicobacter spp., was found in liver HCC suggesting that intestine translocation might be a possible cause of carcinogenesis[26]. To this end, the enteric microflora profile might potentially indicate reaction rates in HCC patients undergoing treatment with immune checkpoint inhibitors[27], suggesting that the microbiome could be used for liver cancer immunotherapy[28].
Overall, alterations in microbial profiles in HCC did not appear to be consistent among investigations, presumably due to differences in etiologies, geographical locations, and dietary intakes. Differences between patients with cirrhosis and HCC, as well as cirrhosis alone, appear to be lower than those between healthy individuals and those with cirrhosis. As a result, microbiome-based diagnostic tools are anticipated to be more potent for cirrhosis identification than for HCC diagnosis. However, rather than particular HCC-associated abnormalities, the microbiome's functional effect on HCC development is more likely to be linked to cirrhosis-related changes, which may increase HCC advancement.
A recent study has shown that antibiotics can help with small-intestinal bacterial overgrowth-related liver damage, demonstrating the connection between intestinal microbiota and liver disorders[29]. Probiotics are microorganisms that have a beneficial effect on humans and are presently being studied as a potential therapy for chronic liver disease[16,29]. Indeed, they can support the growth of enteric microbes producing anti-inflammatory metabolites, which exert an immune suppressive effect.
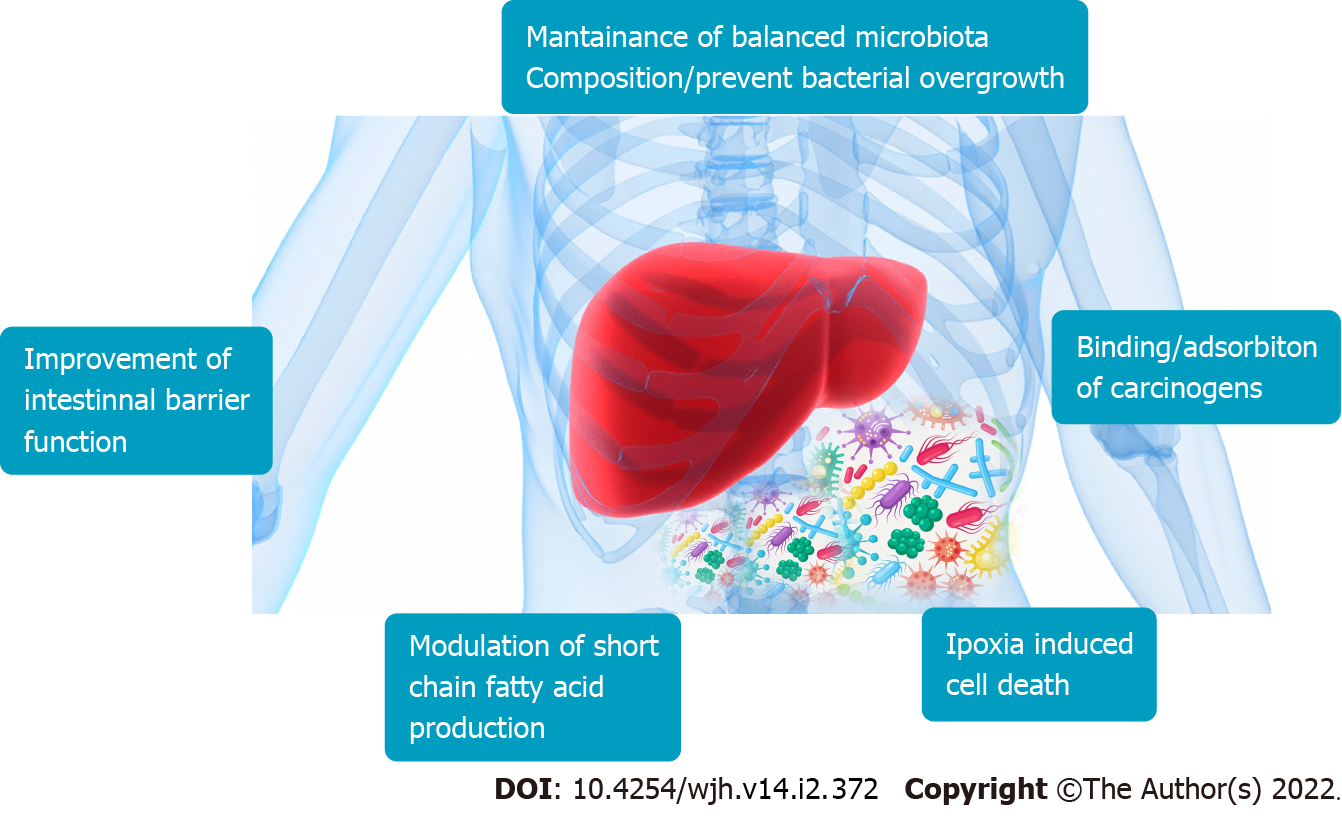
Probiotics can help microbiota generate anti-inflammatory compounds with tumor-suppressing properties. Probiotics have a significant influence on the GLA, with anti-inflammatory and immunomodulatory effects on GM and gut barrier function, as well as a metabolic influence on organs beyond the gastrointestinal tract. They can affect the generation of immunomodulating GM compounds that show anti-tumor properties (Figure 2). Furthermore, supplementation with probiotics increased the expression of some anti-inflammatory cytokines, such as interleukin (IL)-13, IL-10, and IL-27.
Downregulation of angiogenic factors and receptors, VEGFA, Fms related receptor tyrosine kinase 1, ANGPT2, and kinase insert domain receptor, were seen in mice fed a particular probiotic combination[30]. However, not all probiotic species have the same immunomodulatory effect on the gut microflora. Different strains of Lactobacillus spp., for example, are linked to both pro-obesity and anti-obesity effects[31]. Research on mice with diet-induced obesity found that distinct targeted enteric bacteria modifi-cation using vancomycin vs probiotic strain Lactobacillus salivarius resulted in divergent metabolic effects, although microbiota modifications were identical. By contrast, a recent human investigation in obese adolescents focussing on the impact of Lactobacillus salivarius Ls-33 on a number of inflammatory biomarkers, found no evidence of an effect on the metabolic syndrome[32,33]. These findings indicate that the role of the same probiotics could be different due to various conditions; therefore, attention should be paid to evaluating the different impacting factors.
The use of antimicrobials and probiotics in chronic liver illnesses should be based on the GLA pathophysiology. Despite a consistent number of findings from animal and human research, further case-control prospective studies with a large number of patients are needed to fully understand this issue. Obesity-induced intestinal microbial dysbiosis can lead to HCC, according to a mouse model[34]. Aflatoxin-induced HCC is the subject of research in relation to therapeutic HCC prevention with probiotics. In fact, aflatoxin contamination of foods is an etiological risk factor for HCC in under
The binding of aflatoxin metabolites to probiotic bacteria may have resulted in lower intestinal absorption, inducing a reduction in aflatoxin metabolites in urine[36].
In addition, the ability of probiotic bacteria to facilitate epigenetic modification of host gene expression is advantageous in reducing HCC development[37]. The crosstalk between host and enteric microflora, where gene expression is controlled by several methods such as DNA methylation and histone modification, demonstrates bacterial control of host gene expression[38]. In mice treated with a colon carcinogen, it has been observed that Lactobacillus acidophilus (L. acidophilus) and Bifidobacterium bifidum can decrease the expression of Kristen rat sarcoma viral oncogene homolog, oncomirs (microRNA-221and microRNA 155) and the oncogenes BCL2-like 2 (Bcl-w) in the liver. Moreover, mice treated with these probiotic bacteria had higher levels of the tumor suppressor microRNA-122 and the tumor suppressor gene transcription factor PU.1.[37]. Probiotic supplementation may minimize the incidence of HCC by safeguarding the hepatocyte genome, which is important in the pathophysiology of HCC. Probiotic fermented milk and chlorophyllin were shown to reduce the expression of rasp-21, c-myc, cyclin D1, and Bcl-2 in an HCC rat model, slowing tumor development and volume by 40%. Mice treated with probiotics have significant amounts of fecal Oscillibacter and Prevotella[39].
Microbial PAMPs can induce liver cancer progression via TLR-mediated inflammatory responses, as previously described. The treatment with bacteria having probiotic properties has been demonstrated to decrease the development of HCC in the liver by reducing the expression of TLR-induced inflammation. When rats with induced liver cirrhosis were treated with L plantarum probiotic bacteria, they showed lower TLR4 expression and less liver damage[40]. In addition, gut sterilization and TLR4 inactivation reduced HCC by 80% to 90%, indicating that they might be used as HCC preventive methods[14].
Furthermore, the antiviral action of probiotic bacteria may slow HCC progression by avoiding persistent HBV infection. The release of HBV surface antigen (HBsAg) was diminished by a cell extract of Bifidobacterium adolescentis by reducing the transcription of HBsAg gene, which contrasted the infection. Despite the fact that the amount of HBV DNA in the cells did not change considerably, probiotic therapy drastically reduced the amount of extracellular HBV DNA available[41]. Furthermore, treatment with Lactobacillus bulgaricus induced lowered viral load and cellular deterioration[42]. On the other hand, probiotic bacteria supplements can also help liver function during HCV infection. In addition, HCV-positive patients, treated with Enterococcus faecalis lowered blood levels of the aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (liver damage indicators)[43].
Furthermore, NAFLD is also a key etiological risk factor in HCC development. Supplementation with the probiotic bacteria L. acidophilus and Bifidobacterium lactis can help NAFLD patients with liver damage, as seen by lower ALT, AST, and total cholesterol blood levels[44]. Probiotic therapy lowered body weight and total body fat content in obese NAFLD patients. By suppressing the pro-inflammatory cytokine tumor necrosis factor-α, probiotics also reduced hepatic inflammation in obese NAFLD patients[45]. Additionally, GM changes seem to be the cause of the hepatoprotective and anti-inflammatory actions of probiotic bacteria in NAFLD patients. In rats fed with a high-sucrose and a high-fat diet, probiotic meals improved diet-induced loss of intestinal microbiota diversity, intestinal epithelial barrier function and colonization resistance. Restoration of enteric microbiota and gut epithelial barrier function reduced NAFLD progression by lowering serum LPS levels and reducing TLR4-mediated hepatic inflammation[36].
Finally, probiotic cell components and metabolites increase the production of tight junction proteins, which helps to maintain gut epithelial integrity. Tight junction integrity is critical for preventing translocation of pathogens or exogenous substances across intestinal epithelia and consequently intestinal inflammation. Constituents of tight junctions comprise integral membrane proteins, such as occludin, claudin (CLDN) family members, JAMs 1-3, cingulin, and cytoplasmic plaque proteins, such as zonular occludins-1 (ZO-1) and ZO-2[46,47]. Different components of the CLDN family have been reported to be affected during hepatocarcinogenesis[48-51]. However, in HCC patients undergoing hepatectomy, downregulation of ZO-1 is linked to a poor prognosis[52]. Probiotic supplementation can elicit considerable upregulation and relocalization of interepithelial tight junction proteins by activating numerous TLR produced by the intestinal epithelium. In terms of the effect of probiotic therapy on tight junctions, it was discovered that a VSL#3 combination maintained the epithelial barrier in acute colitis by avoiding reduced tight junction protein expression and an increased apoptotic ratio[53]. Moreover, in a mouse model of high-fat diet or obesity-induced liver steatosis, supplementation with a multispecies probiotic (including Bifidobacteria, Lactobacilli and Streptococcus) formulation helped to maintain tight-junction proteins ZO-1 and ZO-2, and reduced hepatic triglyceride concentration compared with a high-fat diet alone[54]. In older rats, a probiotic cocktail including Lactobacillus and Enterococcus strains reduced microbiota dysbiosis, leaky gut, inflammation, metabolic dysfunctions, and physical function loss caused by a high-fat diet. The GM regulated by probiotics decreased leaky gut by strengthening tight junctions, which lowered inflammation[55].
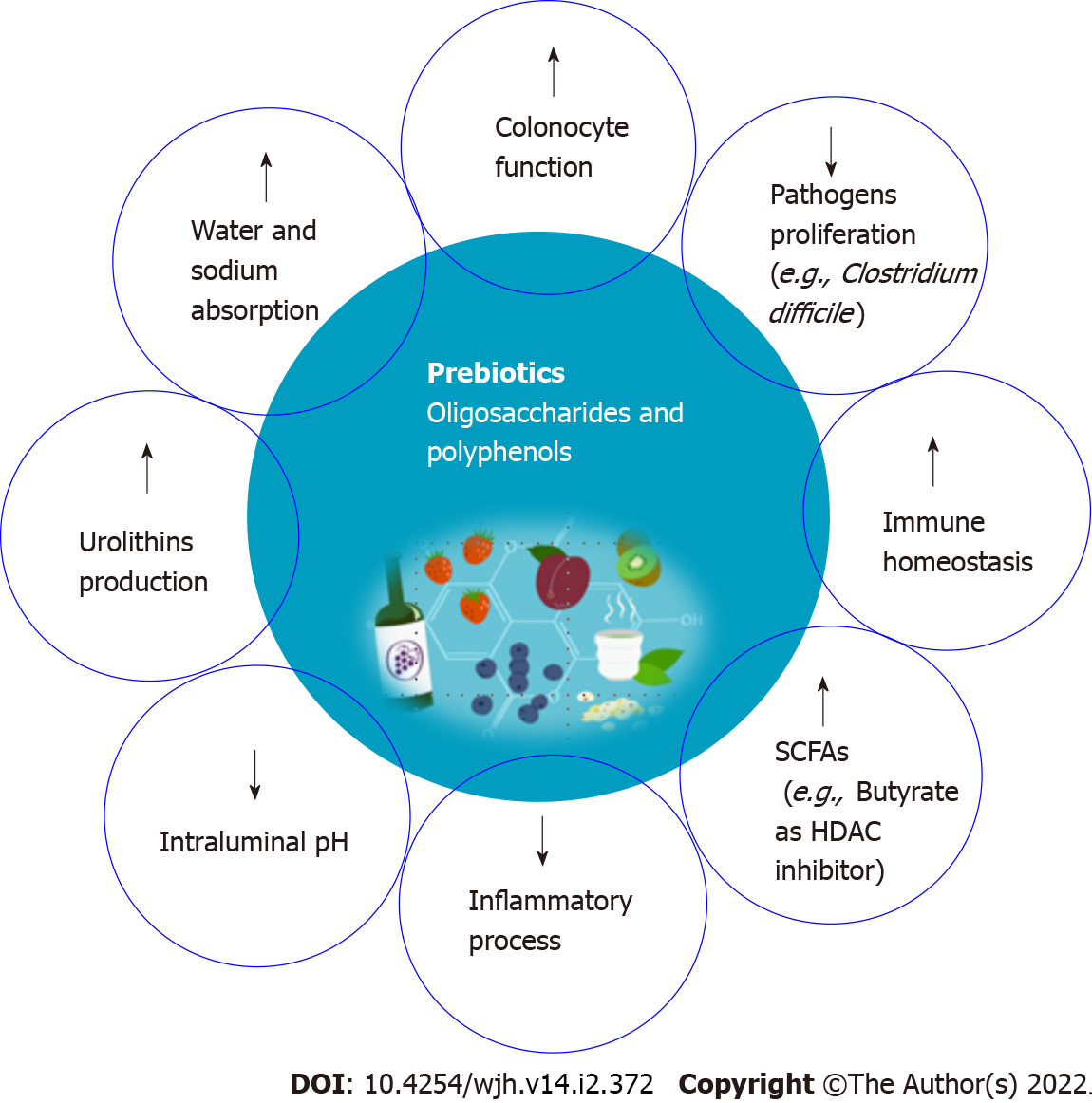
Prebiotics are substrates that are used selectively by host bacteria to provide a health benefit[56]. Health effects are mostly related to the production of SCFA through prebiotics fermentation by a limited number of genera/species of the intestinal microbiota such as Lactobacilli and Bifidobacteria[57]. Bifidobacteria are the most common bacterial species in the human GM, and they are thought to benefit human health by preserving the resident microbiota's balance. SCFAs such as acetate, propionate, and butyrate exert several biologic activities that positively influence the structure and function of the microbial community. Some of these beneficial effects include controlling colonocyte function, promoting water and electrolyte absorption, decreasing the intraluminal pH, inhibiting pathogen proliferation, modifying the immune homeostasis of the gut and modulating the inflammatory processes[56].
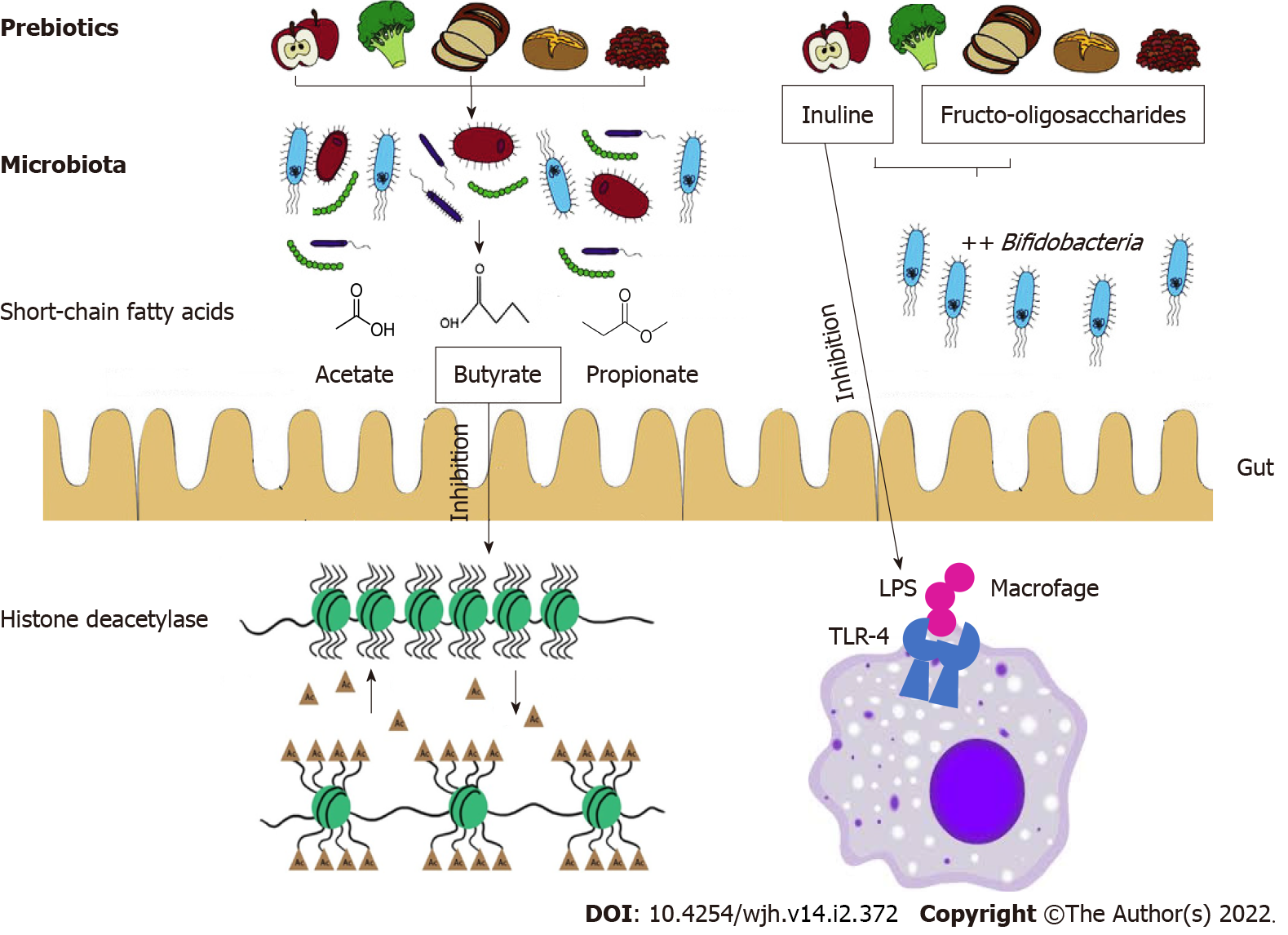
The improvement of mucosal barrier functions exerted by the modulation of GM composition improves conditions such as cirrhosis and consequently may prevent HCC. Prebiotics such as butyrate are not metabolized in the mitochondria of tumor cells but enter the cell nucleus to regulate gene expression. In fact, the functions of butyrate including inhibition of histone deacetylase, decrease tumorigenesis (Figure 3)[58].
The most studied prebiotic classes are galactans (galacto-oligosaccharides) and inulin-type fructans [e.g., fructo-oligosaccharides (FOS), inulin, oligofructose]. There are other oligosaccharides such as isomalto-oligosaccharides and soybean oligosaccharides that have recently gained interest. Dietary sources of prebiotic oligosaccharides are various types of vegetables, fruits, grains and legumes. Depending on their water solubility, dietary fibers are classed as insoluble (e.g., hemicellulose, cellulose, and lignin) and soluble (e.g., fructans, gums, and pectins). Only soluble fibers can be metabolized by the GM and have “prebiotic” functions which can affect host health[59].
In particular, the two prebiotics most researched are FOS and inulin, which have been shown to stimulate the growth of beneficial Bifidobacteria that are implicated in cancer prevention (Figure 3)[60].
Inulin is a non-digestible functional polysaccharide, present in plants such as onions, chicory, Jerusalem artichoke, leeks, garlic, bananas, rye, barley and wheat[61]. A recent study showed that inulin modulates GM and hepatic fatty acid composition limiting dysbiosis and its negative effect on host health[62].
FOS are mostly present in plants such as garlic, onions, leeks, chicory root, Jerusalem artichokes, asparagus, and bananas. New findings suggest that FOS can help to achieve colonization resistance against Clostridium difficile. Several studies have reported the association between liver cancer and intestinal microflora and indicate that Clostridium cluster XIVa, Clostridium cluster XI, or other strains catalyzing the transformation from primary to secondary bile acids could be triggers of hepatic cancerogenesis[34,63,64]. Moreover, research has shown that Clostridia species are enriched in the guts of mice with liver cancer, documenting the association between cancer and dysbiosis[65].
A study evaluating the relationship between food groups and liver cancer risk reported that specific subgroups of vegetables, rich in inulin and FOS, such as celery, mushrooms, Chinese chives, onions, garlic, garlic shoots, asparagus, lettuce, garland chrysanthemum, legumes, squash and carrots, had a strong inverse relationship with liver cancer, indicating their protective effects against HCC[66] (Figure 4). A cohort study involving 125455 individuals from the Nurses' Health Study and the Health Professionals Follow-up Study found that wholegrain, bran, and cereal fiber consump-tion may help reduce HCC[67].
Moreover, eating whole grains and dietary fiber, particularly cereal fiber, has been linked to a decreased risk of obesity and NAFLD, both of which are known predisposing factors for HCC, as previously documented[68]. In fact, long-term consumption of whole grains has been suggested to reduce the risk of HCC by improving gut integrity and changing GM composition[69]. Moreover, a meta-analysis of 19 studies involving 1290045 participants (3912 with HCC) showed that every 100 g/d increase in vegetable intake decreases the risk of HCC by 8%[70].
A potential role of diet in HCC development has recently been clarified by a systematic review (30 studies involving 5222534 participants) that investigated the association between diet and HCC. In particular, certain dietary patterns, such as the Mediterranean diet, the Urban Prudent Dietary Pattern, the Alternative Healthy Eating Index-2010, and the Traditional Cantonese Dietary Pattern, were found to lower the incidence of HCC[71].
Recently, other substances besides non-digestible oligosaccharides, such as plant polyphenols are considered to have prebiotic effects. These compounds are not absorbed in the small intestine and, therefore, reach the colon where they are processed by the colonic microbiota conferring benefit to the host health[71]. They comprise flavonoids, phenolic acids and lignins found in nuts, tea, wine, vegetables and fruits. Polyphenols, due their immunomodulation activity, can have chemopre-ventive effects in HCC progression[72]. In particular, polyphenols contained in tea have been reported to have positive effects on GM homeostasis by decreasing the growth of pathogenic bacteria[73]. One of them is the antioxidant ellagic acid, present especially in nuts and berries, which is metabolized by microbiota in the colon into urolithins that have cancer preventive properties[74].
A prospective cohort study found that a higher consumption of tree nuts (hazelnuts, almonds, macadamias, pecans, cashews, and pistachios) (mean 1.25 serving per week) was associated with a reduced risk of HCC[75,76].
Coffee contains phenolic agents which have anticarcinogenic properties and its consumption has been demonstrated by various studies to reduce HCC risk[77].
Moreover, the cancer preventive effect of the association of oligosaccharides with polyphenols was also recently investigated. A study examined the effect of the combination of FOS and pectins with raspberry polyphenols on cecal microbial fermentation and regulation of liver lipid metabolism and inflammation, and concluded that FOS and pectins enhanced the effects of the raspberry polyphenolic extract against disorders related to NAFLD[78].
Furthermore, prebiotics use has been explored as an approach to modulate intestinal barrier function through maintaining tight junction (TJ) integrity. Prebiotics have demonstrated effects on GM composition that could lead to beneficial changes in TJ protein expression and distribution. However, the effect of prebiotics on TJ by microbiota composition modulation is less recognized than those produced by probiotic supplementation.
The prebiotics which showed beneficial effects on intestinal TJs in various studies are FOS, inulin, and galacto-oligosaccharide. A recent study investigated the effect of FOS on AMP-activated protein kinase (AMPK) activity and TJ assembly under non-inflammatory and inflammatory conditions using T84 cells as an intestinal epithelial cell model, and showed that FOS promoted intestinal epithelial TJ assembly through AMPK activation[79]. In addition, other studies demonstrated that prebiotic supplementation results in robust activation of AMPK[80,81].
A study showed that inulin fermentation increased occludin, CLDN-3 and ZO-1 RNA expression that could reinforce the barrier function[82]. Even galacto-oligosaccharide supplementation increased ZO-1, occludin and CLDN-1 protein expression[83].
HCC development is a complicated process influenced by a number of etiological risk factors. However, several studies have shown that probiotics and prebiotics have a beneficial effect on GM regulation and decrease procarcinogenic factors in the liver. It is noteworthy that the most remarkable aspect is the importance of a balanced diet with proper nutrition (which includes higher intake of vegetables, fish, white meat, and coffee, and reduced intake of fat, red meat, and alcohol), especially for those patients suffering liver, and especially chronic, diseases[84]. A Mediterranean diet has recently been shown to reduce the incidence of HCC, providing a new paradigm for future research[85]. Moreover, scientific investigations have revealed the possibility of developing cancer-preventive symbiotic (combination of probiotic and prebiotic) functional foods[86]. Of course, further research is needed to fully identify the bioactive probiotic metabolites (post-biotics) of certain dietary phytochemicals and to understand the possible mechanism(s) by which these post-biotics interact with the host[87]. In the future, the development of dietary methods and adjunct treatments to prevent the development of HCC may be supported by the creation of combinations of symbiotics or post-biotics with improved anticancer potential.
The authors thank Dr. Elisangela Miceli, PhD for English revisions.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vairappan B, Zhang L S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Wang JJ
| 1. | Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477-491.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 1216] [Article Influence: 202.7] [Reference Citation Analysis (1)] |
| 2. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11835] [Article Influence: 845.4] [Reference Citation Analysis (4)] |
| 3. | Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010;42 Suppl 3:S206-S214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 404] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 4. | Cholankeril G, Patel R, Khurana S, Satapathy SK. Hepatocellular carcinoma in non-alcoholic steatohepatitis: Current knowledge and implications for management. World J Hepatol. 2017;9:533-543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (3)] |
| 5. | Schulze K, Imbeaud S, Letouzé E, Alexandrov LB, Calderaro J, Rebouissou S, Couchy G, Meiller C, Shinde J, Soysouvanh F, Calatayud AL, Pinyol R, Pelletier L, Balabaud C, Laurent A, Blanc JF, Mazzaferro V, Calvo F, Villanueva A, Nault JC, Bioulac-Sage P, Stratton MR, Llovet JM, Zucman-Rossi J. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1006] [Cited by in RCA: 1341] [Article Influence: 134.1] [Reference Citation Analysis (0)] |
| 6. | Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019;16:411-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 965] [Article Influence: 160.8] [Reference Citation Analysis (0)] |
| 7. | Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 2916] [Article Influence: 416.6] [Reference Citation Analysis (1)] |
| 8. | Russo E, Taddei A, Ringressi MN, Ricci F, Amedei A. The interplay between the microbiome and the adaptive immune response in cancer development. Therap Adv Gastroenterol. 2016;9:594-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Russo E, Amedei A. The Role of the Microbiota in the Genesis of Gastrointestinal Cancers. Front Anti-Infective Drug Discov. 2018;7:1-44. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Wan MLY, El-Nezami H. Targeting gut microbiota in hepatocellular carcinoma: probiotics as a novel therapy. Hepatobiliary Surg Nutr. 2018;7:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 11. | Pradere JP, Troeger JS, Dapito DH, Mencin AA, Schwabe RF. Toll-like receptor 4 and hepatic fibrogenesis. Semin Liver Dis. 2010;30:232-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Tao X, Wang N, Qin W. Gut Microbiota and Hepatocellular Carcinoma. Gastrointest Tumors. 2015;2:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Lu H, Ren Z, Li A, Zhang H, Jiang J, Xu S, Luo Q, Zhou K, Sun X, Zheng S, Li L. Deep sequencing reveals microbiota dysbiosis of tongue coat in patients with liver carcinoma. Sci Rep. 2016;6:33142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A, Bataller R, Lefkowitch JH, Bower M, Friedman R, Sartor RB, Rabadan R, Schwabe RF. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 854] [Cited by in RCA: 1028] [Article Influence: 79.1] [Reference Citation Analysis (0)] |
| 15. | Konturek PC, Harsch IA, Konturek K, Schink M, Konturek T, Neurath MF, Zopf Y. Gut⁻Liver Axis: How Do Gut Bacteria Influence the Liver? Med Sci (Basel). 2018;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 16. | Vajro P, Paolella G, Fasano A. Microbiota and gut-liver axis: their influences on obesity and obesity-related liver disease. J Pediatr Gastroenterol Nutr. 2013;56:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 17. | Golonka RM, Vijay-Kumar M. Atypical immunometabolism and metabolic reprogramming in liver cancer: Deciphering the role of gut microbiome. Adv Cancer Res. 2021;149:171-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Yiu JH, Dorweiler B, Woo CW. Interaction between gut microbiota and toll-like receptor: from immunity to metabolism. J Mol Med (Berl). 2017;95:13-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 19. | Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, Knight R. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15:397-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 970] [Article Influence: 138.6] [Reference Citation Analysis (0)] |
| 20. | Ren Z, Li A, Jiang J, Zhou L, Yu Z, Lu H, Xie H, Chen X, Shao L, Zhang R, Xu S, Zhang H, Cui G, Sun R, Wen H, Lerut JP, Kan Q, Li L, Zheng S. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut. 2019;68:1014-1023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 542] [Cited by in RCA: 508] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 21. | Grąt M, Wronka KM, Krasnodębski M, Masior Ł, Lewandowski Z, Kosińska I, Grąt K, Stypułkowski J, Rejowski S, Wasilewicz M, Gałęcka M, Szachta P, Krawczyk M. Profile of Gut Microbiota Associated With the Presence of Hepatocellular Cancer in Patients With Liver Cirrhosis. Transplant Proc. 2016;48:1687-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 145] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 22. | Zhang HL, Yu LX, Yang W, Tang L, Lin Y, Wu H, Zhai B, Tan YX, Shan L, Liu Q, Chen HY, Dai RY, Qiu BJ, He YQ, Wang C, Zheng LY, Li YQ, Wu FQ, Li Z, Yan HX, Wang HY. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats. J Hepatol. 2012;57:803-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 214] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 23. | Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F, Sanguinetti M, Morelli D, Paroni Sterbini F, Petito V, Reddel S, Calvani R, Camisaschi C, Picca A, Tuccitto A, Gasbarrini A, Pompili M, Mazzaferro V. Hepatocellular Carcinoma Is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology. 2019;69:107-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 473] [Article Influence: 78.8] [Reference Citation Analysis (1)] |
| 24. | Liu Q, Li F, Zhuang Y, Xu J, Wang J, Mao X, Zhang Y, Liu X. Alteration in gut microbiota associated with hepatitis B and non-hepatitis virus related hepatocellular carcinoma. Gut Pathog. 2019;11:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 25. | Sheng L, Jena PK, Hu Y, Liu HX, Nagar N, Kalanetra KM, French SW, Mills DA, Wan YY. Hepatic inflammation caused by dysregulated bile acid synthesis is reversible by butyrate supplementation. J Pathol. 2017;243:431-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 26. | Krüttgen A, Horz HP, Weber-Heynemann J, Vucur M, Trautwein C, Haase G, Luedde T, Roderburg C. Study on the association of Helicobacter species with viral hepatitis-induced hepatocellular carcinoma. Gut Microbes. 2012;3:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragón L, Jacquelot N, Qu B, Ferrere G, Clémenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryffel B, Minard-Colin V, Gonin P, Soria JC, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G, Zitvogel L. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2493] [Cited by in RCA: 3794] [Article Influence: 474.3] [Reference Citation Analysis (0)] |
| 28. | Russo E, Nannini G, Dinu M, Pagliai G, Sofi F, Amedei A. Exploring the food-gut axis in immunotherapy response of cancer patients. World J Gastroenterol. 2020;26:4919-4932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 29. | Sajjad A, Mottershead M, Syn WK, Jones R, Smith S, Nwokolo CU. Ciprofloxacin suppresses bacterial overgrowth, increases fasting insulin but does not correct low acylated ghrelin concentration in non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2005;22:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Li J, Sung CY, Lee N, Ni Y, Pihlajamäki J, Panagiotou G, El-Nezami H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci U S A. 2016;113:E1306-E1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 425] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 31. | Million M, Angelakis E, Paul M, Armougom F, Leibovici L, Raoult D. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb Pathog. 2012;53:100-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 320] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 32. | Gøbel RJ, Larsen N, Jakobsen M, Mølgaard C, Michaelsen KF. Probiotics to adolescents with obesity: effects on inflammation and metabolic syndrome. J Pediatr Gastroenterol Nutr. 2012;55:673-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 33. | Larsen N, Vogensen FK, Gøbel RJ, Michaelsen KF, Forssten SD, Lahtinen SJ, Jakobsen M. Effect of Lactobacillus salivarius Ls-33 on fecal microbiota in obese adolescents. Clin Nutr. 2013;32:935-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 34. | Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, Honda K, Ishikawa Y, Hara E, Ohtani N. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1318] [Cited by in RCA: 1649] [Article Influence: 137.4] [Reference Citation Analysis (0)] |
| 35. | Huang L, Duan C, Zhao Y, Gao L, Niu C, Xu J, Li S. Reduction of Aflatoxin B1 Toxicity by Lactobacillus plantarum C88: A Potential Probiotic Strain Isolated from Chinese Traditional Fermented Food "Tofu". PLoS One. 2017;12:e0170109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 36. | Nduti NN, McMillan AM, Seney S, Sumarah MW, Njeru PN, Mwaniki MW, Reid G. Investigating probiotic yoghurt to reduce an aflatoxin B1 biomarker among school children in Eastern Kenya: Preliminary study. Int Dairy J. 2016;63:124-129. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Heydari Z, Rahaie M, Alizadeh AM. Different anti-inflammatory effects of Lactobacillus acidophilus and Bifidobactrum bifidioum in hepatocellular carcinoma cancer mouse through impact on microRNAs and their target genes. J Nutr Intermed Metab. 2019;16:100096. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Miro-Blanch J, Yanes O. Epigenetic Regulation at the Interplay Between Gut Microbiota and Host Metabolism. Front Genet. 2019;10:638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 39. | Kumar M, Verma V, Nagpal R, Kumar A, Gautam SK, Behare PV, Grover CR, Aggarwal PK. Effect of probiotic fermented milk and chlorophyllin on gene expressions and genotoxicity during AFB₁-induced hepatocellular carcinoma. Gene. 2011;490:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Elshaer AM, El-Kharashi OA, Hamam GG, Nabih ES, Magdy YM, Abd El Samad AA. Involvement of TLR4/ CXCL9/ PREX-2 pathway in the development of hepatocellular carcinoma (HCC) and the promising role of early administration of lactobacillus plantarum in Wistar rats. Tissue Cell. 2019;60:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Lee DK, Kang JY, Shin HS, Park IH, Ha NJ. Antiviral activity of Bifidobacterium adolescentis SPM0212 against Hepatitis B virus. Arch Pharm Res. 2013;36:1525-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | El-Adawi HN, I. ; Fattouh, F.; El-Deeb, N. . Investigation of the antiviral bioactivity of Lactobacillus bulgaricus 761N extracellular extract against hepatitis C virus (HCV). Int J Pharmacol. 2015;11:19-26. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Oo KM, Lwin AA, Kyaw YY, Tun WM, Fukada K, Goshima A, Shimada T, Okada S. Safety and long-term effect of the probiotic FK-23 in patients with hepatitis C virus infection. Biosci Microbiota Food Health. 2016;35:123-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Nabavi S, Rafraf M, Somi MH, Homayouni-Rad A, Asghari-Jafarabadi M. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J Dairy Sci. 2014;97:7386-7393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 45. | Ahn SB, Jun DW, Kang BK, Lim JH, Lim S, Chung MJ. Randomized, Double-blind, Placebo-controlled Study of a Multispecies Probiotic Mixture in Nonalcoholic Fatty Liver Disease. Sci Rep. 2019;9:5688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 140] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 46. | König J, Wells J, Cani PD, García-Ródenas CL, MacDonald T, Mercenier A, Whyte J, Troost F, Brummer RJ. Human Intestinal Barrier Function in Health and Disease. Clin Transl Gastroenterol. 2016;7:e196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 593] [Article Influence: 65.9] [Reference Citation Analysis (0)] |
| 47. | Song CH, Kim N, Sohn SH, Lee SM, Nam RH, Na HY, Lee DH, Surh YJ. Effects of 17β-Estradiol on Colonic Permeability and Inflammation in an Azoxymethane/Dextran Sulfate Sodium-Induced Colitis Mouse Model. Gut Liver. 2018;12:682-693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 48. | Bouchagier KA, Assimakopoulos SF, Karavias DD, Maroulis I, Tzelepi V, Kalofonos H, Kardamakis D, Scopa CD, Tsamandas AC. Expression of claudins-1, -4, -5, -7 and occludin in hepatocellular carcinoma and their relation with classic clinicopathological features and patients' survival. In Vivo. 2014;28:315-326. [PubMed] |
| 49. | Holczbauer Á, Gyöngyösi B, Lotz G, Törzsök P, Kaposi-Novák P, Szijártó A, Tátrai P, Kupcsulik P, Schaff Z, Kiss A. Increased expression of claudin-1 and claudin-7 in liver cirrhosis and hepatocellular carcinoma. Pathol Oncol Res. 2014;20:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Huang GW, Ding X, Chen SL, Zeng L. Expression of claudin 10 protein in hepatocellular carcinoma: impact on survival. J Cancer Res Clin Oncol. 2011;137:1213-1218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Cheung ST, Leung KL, Ip YC, Chen X, Fong DY, Ng IO, Fan ST, So S. Claudin-10 expression level is associated with recurrence of primary hepatocellular carcinoma. Clin Cancer Res. 2005;11:551-556. [PubMed] |
| 52. | Nagai T, Arao T, Nishio K, Matsumoto K, Hagiwara S, Sakurai T, Minami Y, Ida H, Ueshima K, Nishida N, Sakai K, Saijo N, Kudo K, Kaneda H, Tamura D, Aomatsu K, Kimura H, Fujita Y, Haji S, Kudo M. Impact of Tight Junction Protein ZO-1 and TWIST Expression on Postoperative Survival of Patients with Hepatocellular Carcinoma. Dig Dis. 2016;34:702-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, Bruewer M. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1140-G1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 370] [Article Influence: 23.1] [Reference Citation Analysis (1)] |
| 54. | Briskey D, Heritage M, Jaskowski LA, Peake J, Gobe G, Subramaniam VN, Crawford D, Campbell C, Vitetta L. Probiotics modify tight-junction proteins in an animal model of nonalcoholic fatty liver disease. Therap Adv Gastroenterol. 2016;9:463-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 55. | Ahmadi S, Wang S, Nagpal R, Wang B, Jain S, Razazan A, Mishra SP, Zhu X, Wang Z, Kavanagh K, Yadav H. A human-origin probiotic cocktail ameliorates aging-related leaky gut and inflammation via modulating the microbiota/taurine/tight junction axis. JCI Insight. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 154] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 56. | Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K, Reid G. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2256] [Cited by in RCA: 3189] [Article Influence: 398.6] [Reference Citation Analysis (0)] |
| 57. | Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B, Guarner F, Respondek F, Whelan K, Coxam V, Davicco MJ, Léotoing L, Wittrant Y, Delzenne NM, Cani PD, Neyrinck AM, Meheust A. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104 Suppl 2:S1-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1349] [Cited by in RCA: 1313] [Article Influence: 87.5] [Reference Citation Analysis (0)] |
| 58. | Bultman SJ. The microbiome and its potential as a cancer preventive intervention. Semin Oncol. 2016;43:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 59. | Makki K, Deehan EC, Walter J, Bäckhed F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe. 2018;23:705-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 898] [Cited by in RCA: 1556] [Article Influence: 222.3] [Reference Citation Analysis (0)] |
| 60. | Davis CD, Milner JA. Gastrointestinal microflora, food components and colon cancer prevention. J Nutr Biochem. 2009;20:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 61. | Sousa V, Santos E, Sgarbieri V. The Importance of Prebiotics in Functional Foods and Clinical Practice. Food Sci Nutr. 2011;2. [RCA] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 62. | Albouery M, Bretin A, Buteau B, Grégoire S, Martine L, Gambert S, Bron AM, Acar N, Chassaing B, Bringer MA. Soluble Fiber Inulin Consumption Limits Alterations of the Gut Microbiota and Hepatic Fatty Acid Metabolism Caused by High-Fat Diet. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 63. | Loo TM, Kamachi F, Watanabe Y, Yoshimoto S, Kanda H, Arai Y, Nakajima-Takagi Y, Iwama A, Koga T, Sugimoto Y, Ozawa T, Nakamura M, Kumagai M, Watashi K, Taketo MM, Aoki T, Narumiya S, Oshima M, Arita M, Hara E, Ohtani N. Gut Microbiota Promotes Obesity-Associated Liver Cancer through PGE2-Mediated Suppression of Antitumor Immunity. Cancer Discov. 2017;7:522-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 344] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 64. | Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, Ritz T, Longerich T, Theriot CM, McCulloch JA, Roy S, Yuan W, Thovarai V, Sen SK, Ruchirawat M, Korangy F, Wang XW, Trinchieri G, Greten TF. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 1037] [Article Influence: 148.1] [Reference Citation Analysis (0)] |
| 65. | Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol. 2014;30:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 769] [Cited by in RCA: 934] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 66. | Zhang W, Xiang YB, Li HL, Yang G, Cai H, Ji BT, Gao YT, Zheng W, Shu XO. Vegetable-based dietary pattern and liver cancer risk: results from the Shanghai women's and men's health studies. Cancer Sci. 2013;104:1353-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 67. | Yang W, Ma Y, Liu Y, Smith-Warner SA, Simon TG, Chong DQ, Qi Q, Meyerhardt JA, Giovannucci EL, Chan AT, Zhang X. Association of Intake of Whole Grains and Dietary Fiber With Risk of Hepatocellular Carcinoma in US Adults. JAMA Oncol. 2019;5:879-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 68. | Makarova-Rusher OV, Altekruse SF, McNeel TS, Ulahannan S, Duffy AG, Graubard BI, Greten TF, McGlynn KA. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer. 2016;122:1757-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 251] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 69. | Costabile A, Klinder A, Fava F, Napolitano A, Fogliano V, Leonard C, Gibson GR, Tuohy KM. Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: a double-blind, placebo-controlled, crossover study. Br J Nutr. 2008;99:110-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 304] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 70. | Yang Y, Zhang D, Feng N, Chen G, Liu J, Zhu Y. Increased intake of vegetables, but not fruit, reduces risk for hepatocellular carcinoma: a meta-analysis. Gastroenterology. 2014;147:1031-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 71. | George ES, Sood S, Broughton A, Cogan G, Hickey M, Chan WS, Sudan S, Nicoll AJ. The Association between Diet and Hepatocellular Carcinoma: A Systematic Review. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 72. | Ferguson LR, Philpott M. Cancer prevention by dietary bioactive components that target the immune response. Curr Cancer Drug Targets. 2007;7:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 73. | Darvesh AS, Bishayee A. Chemopreventive and therapeutic potential of tea polyphenols in hepatocellular cancer. Nutr Cancer. 2013;65:329-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 74. | Larrosa M, González-Sarrías A, García-Conesa MT, Tomás-Barberán FA, Espín JC. Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities. J Agric Food Chem. 2006;54:1611-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 190] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 75. | Acharya S, Adamová D, Adhya SP, Adler A, Adolfsson J, Aggarwal MM, Aglieri Rinella G, Agnello M, Agrawal N, Ahammed Z, Ahmad S, Ahn SU, Aiola S, Akindinov A, Al-Turany M, Alam SN, Albuquerque DSD, Aleksandrov D, Alessandro B, Alfanda HM, Alfaro Molina R, Ali B, Ali Y, Alici A, Alkin A, Alme J, Alt T, Altenkamper L, Altsybeev I, Anaam MN, Andrei C, Andreou D, Andrews HA, Andronic A, Angeletti M, Anguelov V, Anson C, Antičić T, Antinori F, Antonioli P, Anwar R, Apadula N, Aphecetche L, Appelshäuser H, Arcelli S, Arnaldi R, Arratia M, Arsene IC, Arslandok M, Augustinus A, Averbeck R, Aziz S, Azmi MD, Badalà A, Baek YW, Bagnasco S, Bailhache R, Bala R, Baldisseri A, Ball M, Baral RC, Barbera R, Barioglio L, Barnaföldi GG, Barnby LS, Barret V, Bartalini P, Barth K, Bartsch E, Bastid N, Basu S, Batigne G, Batyunya B, Batzing PC, Bauri D, Bazo Alba JL, Bearden IG, Bedda C, Behera NK, Belikov I, Bellini F, Bellwied R, Beltran LGE, Belyaev V, Bencedi G, Beole S, Bercuci A, Berdnikov Y, Berenyi D, Bertens RA, Berzano D, Betev L, Bhasin A, Bhat IR, Bhatt H, Bhattacharjee B, Bianchi A, Bianchi L, Bianchi N, Bielčík J, Bielčíková J, Bilandzic A, Biro G, Biswas R, Biswas S, Blair JT, Blau D, Blume C, Boca G, Bock F, Bogdanov A, Boldizsár L, Bolozdynya A, Bombara M, Bonomi G, Bonora M, Borel H, Borissov A, Borri M, Botta E, Bourjau C, Bratrud L, Braun-Munzinger P, Bregant M, Broker TA, Broz M, Brucken EJ, Bruna E, Bruno GE, Buckland MD, Budnikov D, Buesching H, Bufalino S, Buhler P, Buncic P, Busch O, Buthelezi Z, Butt JB, Buxton JT, Caffarri D, Caines H, Caliva A, Calvo Villar E, Camacho RS, Camerini P, Capon AA, Carnesecchi F, Castillo Castellanos J, Castro AJ, Casula EAR, Catalano F, Ceballos Sanchez C, Chakraborty P, Chandra S, Chang B, Chang W, Chapeland S, Chartier M, Chattopadhyay S, Chauvin A, Cheshkov C, Cheynis B, Chibante Barroso V, Chinellato DD, Cho S, Chochula P, Chowdhury T, Christakoglou P, Christensen CH, Christiansen P, Chujo T, Cicalo C, Cifarelli L, Cindolo F, Cleymans J, Colamaria F, Colella D, Collu A, Colocci M, Concas M, Conesa Balbastre G, Conesa Del Valle Z, Contin G, Contreras JG, Cormier TM, Corrales Morales Y, Cortese P, Cosentino MR, Costa F, Costanza S, Crkovská J, Crochet P, Cuautle E, Cunqueiro L, Dabrowski D, Dahms T, Dainese A, Damas FPA, Dani S, Danisch MC, Danu A, Das D, Das I, Das S, Dash A, Dash S, Dashi A, De S, De Caro A, de Cataldo G, de Conti C, de Cuveland J, De Falco A, De Gruttola D, De Marco N, De Pasquale S, De Souza RD, Deb S, Degenhardt HF, Deisting A, Deja KR, Deloff A, Delsanto S, Dhankher P, Di Bari D, Di Mauro A, Diaz RA, Dietel T, Dillenseger P, Ding Y, Divià R, Djuvsland Ø, Dmitrieva U, Dobrin A, Domenicis Gimenez D, Dönigus B, Dordic O, Dubey AK, Dubla A, Dudi S, Duggal AK, Dukhishyam M, Dupieux P, Ehlers RJ, Elia D, Engel H, Epple E, Erazmus B, Erhardt F, Erokhin A, Ersdal MR, Espagnon B, Eulisse G, Eum J, Evans D, Evdokimov S, Fabbietti L, Faggin M, Faivre J, Fantoni A, Fasel M, Fecchio P, Feldkamp L, Feliciello A, Feofilov G, Fernández Téllez A, Ferrero A, Ferretti A, Festanti A, Feuillard VJG, Figiel J, Filchagin S, Finogeev D, Fionda FM, Fiorenza G, Flor F, Foertsch S, Foka P, Fokin S, Fragiacomo E, Francisco A, Frankenfeld U, Fronze GG, Fuchs U, Furget C, Furs A, Fusco Girard M, Gaardhøje JJ, Gagliardi M, Gago AM, Gal A, Galvan CD, Ganoti P, Garabatos C, Garcia-Solis E, Garg K, Gargiulo C, Garner K, Gasik P, Gauger EF, Gay Ducati MB, Germain M, Ghosh J, Ghosh P, Ghosh SK, Gianotti P, Giubellino P, Giubilato P, Glässel P, Goméz Coral DM, Gomez Ramirez A, Gonzalez V, González-Zamora P, Gorbunov S, Görlich L, Gotovac S, Grabski V, Graczykowski LK, Graham KL, Greiner L, Grelli A, Grigoras C, Grigoriev V, Grigoryan A, Grigoryan S, Groettvik OS, Gronefeld JM, Grosa F, Grosse-Oetringhaus JF, Grosso R, Guernane R, Guerzoni B, Guittiere M, Gulbrandsen K, Gunji T, Gupta A, Gupta R, Guzman IB, Haake R, Habib MK, Hadjidakis C, Hamagaki H, Hamar G, Hamid M, Hamon JC, Hannigan R, Haque MR, Harlenderova A, Harris JW, Harton A, Hassan H, Hatzifotiadou D, Hauer P, Hayashi S, Heckel ST, Hellbär E, Helstrup H, Herghelegiu A, Hernandez EG, Herrera Corral G, Herrmann F, Hetland KF, Hilden TE, Hillemanns H, Hills C, Hippolyte B, Hohlweger B, Horak D, Hornung S, Hosokawa R, Hristov P, Huang C, Hughes C, Huhn P, Humanic TJ, Hushnud H, Husova LA, Hussain N, Hussain SA, Hussain T, Hutter D, Hwang DS, Iddon JP, Ilkaev R, Inaba M, Ippolitov M, Islam MS, Ivanov M, Ivanov V, Izucheev V, Jacak B, Jacazio N, Jacobs PM, Jadhav MB, Jadlovska S, Jadlovsky J, Jaelani S, Jahnke C, Jakubowska MJ, Janik MA, Jercic M, Jevons O, Jimenez Bustamante RT, Jin M, Jonas F, Jones PG, Jusko A, Kalinak P, Kalweit A, Kang JH, Kaplin V, Kar S, Karasu Uysal A, Karavichev O, Karavicheva T, Karczmarczyk P, Karpechev E, Kebschull U, Keidel R, Keil M, Ketzer B, Khabanova Z, Khan AM, Khan S, Khan SA, Khanzadeev A, Kharlov Y, Khatun A, Khuntia A, Kileng B, Kim B, Kim D, Kim DJ, Kim EJ, Kim H, Kim JS, Kim J, Kim M, Kim S, Kim T, Kindra K, Kirsch S, Kisel I, Kiselev S, Kisiel A, Klay JL, Klein C, Klein J, Klein S, Klein-Bösing C, Klewin S, Kluge A, Knichel ML, Knospe AG, Kobdaj C, Kofarago M, Köhler MK, Kollegger T, Kondratyev A, Kondratyeva N, Kondratyuk E, Konopka PJ, Konyushikhin M, Koska L, Kovalenko O, Kovalenko V, Kowalski M, Králik I, Kravčáková A, Kreis L, Krivda M, Krizek F, Krizkova Gajdosova K, Krüger M, Kryshen E, Krzewicki M, Kubera AM, Kučera V, Kuhn C, Kuijer PG, Kumar L, Kumar S, Kundu S, Kurashvili P, Kurepin A, Kurepin AB, Kushpil S, Kvapil J, Kweon MJ, Kwon Y, La Pointe SL, La Rocca P, Lai YS, Langoy R, Lapidus K, Lardeux A, Larionov P, Laudi E, Lavicka R, Lazareva T, Lea R, Leardini L, Lee S, Lehas F, Lehner S, Lehrbach J, Lemmon RC, León Monzón I, Lettrich M, Lévai P, Li X, Li XL, Lien J, Lietava R, Lim B, Lindal S, Lindenstruth V, Lindsay SW, Lippmann C, Lisa MA, Litichevskyi V, Liu A, Liu S, Ljunggren HM, Llope WJ, Lodato DF, Loginov V, Loizides C, Loncar P, Lopez X, López Torres E, Luettig P, Luhder JR, Lunardon M, Luparello G, Lupi M, Maevskaya A, Mager M, Mahmood SM, Mahmoud T, Maire A, Majka RD, Malaev M, Malik QW, Malinina L, Mal'Kevich D, Malzacher P, Mamonov A, Manko V, Manso F, Manzari V, Mao Y, Marchisone M, Mareš J, Margagliotti GV, Margotti A, Margutti J, Marín A, Markert C, Marquard M, Martin NA, Martinengo P, Martinez JL, Martínez MI, Martínez García G, Martinez Pedreira M, Masciocchi S, Masera M, Masoni A, Massacrier L, Masson E, Mastroserio A, Mathis AM, Matuoka PFT, Matyja A, Mayer C, Mazzilli M, Mazzoni MA, Mechler AF, Meddi F, Melikyan Y, Menchaca-Rocha A, Meninno E, Meres M, Mhlanga S, Miake Y, Micheletti L, Mieskolainen MM, Mihaylov DL, Mikhaylov K, Mischke A, Mishra AN, Miśkowiec D, Mitu CM, Mohammadi N, Mohanty AP, Mohanty B, Khan MM, Mondal MM, Mordasini C, Moreira De Godoy DA, Moreno LAP, Moretto S, Morreale A, Morsch A, Mrnjavac T, Muccifora V, Mudnic E, Mühlheim D, Muhuri S, Mulligan JD, Munhoz MG, Münning K, Munzer RH, Murakami H, Murray S, Musa L, Musinsky J, Myers CJ, Myrcha JW, Naik B, Nair R, Nandi BK, Nania R, Nappi E, Naru MU, Nassirpour AF, Natal da Luz H, Nattrass C, Nayak K, Nayak R, Nayak TK, Nazarenko S, Negrao De Oliveira RA, Nellen L, Nesbo SV, Neskovic G, Ng F, Nielsen BS, Nikolaev S, Nikulin S, Nikulin V, Noferini F, Nomokonov P, Nooren G, Noris JCC, Norman J, Nowakowski P, Nyanin A, Nystrand J, Ogino M, Ohlson A, Oleniacz J, Oliveira Da Silva AC, Oliver MH, Onderwaater J, Oppedisano C, Orava R, Ortiz Velasquez A, Oskarsson A, Otwinowski J, Oyama K, Pachmayer Y, Pacik V, Pagano D, Paić G, Palni P, Pan J, Pandey AK, Panebianco S, Papikyan V, Pareek P, Park J, Parkkila JE, Parmar S, Passfeld A, Pathak SP, Patra RN, Paul B, Pei H, Peitzmann T, Peng X, Pereira LG, Pereira Da Costa H, Peresunko D, Perez GM, Perez Lezama E, Peskov V, Pestov Y, Petráček V, Petrovici M, Pezzi RP, Piano S, Pikna M, Pillot P, Pimentel LODL, Pinazza O, Pinsky L, Pisano S, Piyarathna DB, Płoskoń M, Planinic M, Pliquett F, Pluta J, Pochybova S, Poghosyan MG, Polichtchouk B, Poljak N, Poonsawat W, Pop A, Poppenborg H, Porteboeuf-Houssais S, Pozdniakov V, Prasad SK, Preghenella R, Prino F, Pruneau CA, Pshenichnov I, Puccio M, Punin V, Puranapanda K, Putschke J, Quishpe RE, Ragoni S, Raha S, Rajput S, Rak J, Rakotozafindrabe A, Ramello L, Rami F, Raniwala R, Raniwala S, Räsänen SS, Rascanu BT, Rath R, Ratza V, Ravasenga I, Read KF, Redlich K, Rehman A, Reichelt P, Reidt F, Ren X, Renfordt R, Reshetin A, Revol JP, Reygers K, Riabov V, Richert T, Richter M, Riedler P, Riegler W, Riggi F, Ristea C, Rode SP, Rodríguez Cahuantzi M, Røed K, Rogalev R, Rogochaya E, Rohr D, Röhrich D, Rokita PS, Ronchetti F, Rosas ED, Roslon K, Rosnet P, Rossi A, Rotondi A, Roukoutakis F, Roy A, Roy P, Rueda OV, Rui R, Rumyantsev B, Rustamov A, Ryabinkin E, Ryabov Y, Rybicki A, Rytkonen H, Saarinen S, Sadhu S, Sadovsky S, Šafařík K, Saha SK, Sahoo B, Sahoo P, Sahoo R, Sahoo S, Sahu PK, Saini J, Sakai S, Sambyal S, Samsonov V, Sandoval A, Sarkar A, Sarkar D, Sarkar N, Sarma P, Sarti VM, Sas MHP, Scapparone E, Schaefer B, Schambach J, Scheid HS, Schiaua C, Schicker R, Schmah A, Schmidt C, Schmidt HR, Schmidt MO, Schmidt M, Schmidt NV, Schmier AR, Schukraft J, Schutz Y, Schwarz K, Schweda K, Scioli G, Scomparin E, Šefčík M, Seger JE, Sekiguchi Y, Sekihata D, Selyuzhenkov I, Senyukov S, Serradilla E, Sett P, Sevcenco A, Shabanov A, Shabetai A, Shahoyan R, Shaikh W, Shangaraev A, Sharma A, Sharma M, Sharma N, Sheikh AI, Shigaki K, Shimomura M, Shirinkin S, Shou Q, Sibiriak Y, Siddhanta S, Siemiarczuk T, Silvermyr D, Simatovic G, Simonetti G, Singh R, Singh VK, Singhal V, Sinha T, Sitar B, Sitta M, Skaali TB, Slupecki M, Smirnov N, Snellings RJM, Snellman TW, Sochan J, Soncco C, Song J, Songmoolnak A, Soramel F, Sorensen S, Sputowska I, Stachel J, Stan I, Stankus P, Steffanic PJ, Stenlund E, Stocco D, Storetvedt MM, Strmen P, Suaide AAP, Sugitate T, Suire C, Suleymanov M, Suljic M, Sultanov R, Šumbera M, Sumowidagdo S, Suzuki K, Swain S, Szabo A, Szarka I, Tabassam U, Taillepied G, Takahashi J, Tambave GJ, Tang S, Tarhini M, Tarzila MG, Tauro A, Tejeda Muñoz G, Telesca A, Terrevoli C, Thakur D, Thakur S, Thomas D, Thoresen F, Tieulent R, Tikhonov A, Timmins AR, Toia A, Topilskaya N, Toppi M, Torales-Acosta F, Torres SR, Tripathy S, Tripathy T, Trogolo S, Trombetta G, Tropp L, Trubnikov V, Trzaska WH, Trzcinski TP, Trzeciak BA, Tsuji T, Tumkin A, Turrisi R, Tveter TS, Ullaland K, Umaka EN, Uras A, Usai GL, Utrobicic A, Vala M, Valle N, van der Kolk N, van Doremalen LVR, van Leeuwen M, Vande Vyvre P, Varga D, Vargas A, Vargyas M, Varma R, Vasileiou M, Vasiliev A, Vázquez Doce O, Vechernin V, Veen AM, Vercellin E, Vergara Limón S, Vermunt L, Vernet R, Vértesi R, Vickovic L, Viinikainen J, Vilakazi Z, Villalobos Baillie O, Villatoro Tello A, Vino G, Vinogradov A, Virgili T, Vislavicius V, Vodopyanov A, Volkel B, Völkl MA, Voloshin K, Voloshin SA, Volpe G, von Haller B, Vorobyev I, Voscek D, Vrláková J, Wagner B, Wang M, Watanabe Y, Weber M, Weber SG, Wegrzynek A, Weiser DF, Wenzel SC, Wessels JP, Westerhoff U, Whitehead AM, Widmann E, Wiechula J, Wikne J, Wilk G, Wilkinson J, Willems GA, Willsher E, Windelband B, Witt WE, Wu Y, Xu R, Yalcin S, Yamakawa K, Yang S, Yano S, Yin Z, Yokoyama H, Yoo IK, Yoon JH, Yuan S, Yuncu A, Yurchenko V, Zaccolo V, Zaman A, Zampolli C, Zanoli HJC, Zardoshti N, Zarochentsev A, Závada P, Zaviyalov N, Zbroszczyk H, Zhalov M, Zhang X, Zhang Y, Zhang Z, Zhao C, Zherebchevskii V, Zhigareva N, Zhou D, Zhou Y, Zhou Z, Zhu H, Zhu J, Zhu Y, Zichichi A, Zimmermann MB, Zinovjev G, Zurlo N; A Large Ion Collider Experiment Collaboration. Investigations of Anisotropic Flow Using Multiparticle Azimuthal Correlations in pp, p-Pb, Xe-Xe, and Pb-Pb Collisions at the LHC. Phys Rev Lett. 2019;123:142301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 76. | Lamuel-Raventos RM, Onge MS. Prebiotic nut compounds and human microbiota. Crit Rev Food Sci Nutr. 2017;57:3154-3163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 77. | Bøhn SK, Blomhoff R, Paur I. Coffee and cancer risk, epidemiological evidence, and molecular mechanisms. Mol Nutr Food Res. 2014;58:915-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 78. | Fotschki B, Juśkiewicz J, Jurgoński A, Sójka M. Fructo-Oligosaccharides and Pectins Enhance Beneficial Effects of Raspberry Polyphenols in Rats with Nonalcoholic Fatty Liver. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 79. | Wongkrasant P, Pongkorpsakol P, Ariyadamrongkwan J, Meesomboon R, Satitsri S, Pichyangkura R, Barrett KE, Muanprasat C. A prebiotic fructo-oligosaccharide promotes tight junction assembly in intestinal epithelial cells via an AMPK-dependent pathway. Biomed Pharmacother. 2020;129:110415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 80. | Wang J, Tian S, Yu H, Wang J, Zhu W. Response of Colonic Mucosa-Associated Microbiota Composition, Mucosal Immune Homeostasis, and Barrier Function to Early Life Galactooligosaccharides Intervention in Suckling Piglets. J Agric Food Chem. 2019;67:578-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 81. | Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139:1619-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1443] [Cited by in RCA: 1344] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 82. | Julie Uerlingsac MS, Els Willemsb, Sofie Tangheb, Geert Bruggemanb, Jérôme Bindellea, Nadia Everaert. Differential effects of inulin or its fermentation metabolites on gut barrier and immune function of porcine intestinal epithelial cells. J Funct Foods. 2020;67:103855. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 83. | Wang G, Sun W, Pei X, Jin Y, Wang H, Tao W, Xiao Z, Liu L, Wang M. Galactooligosaccharide pretreatment alleviates damage of the intestinal barrier and inflammatory responses in LPS-challenged mice. Food Funct. 2021;12:1569-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 84. | Mandair DS, Rossi RE, Pericleous M, Whyand T, Caplin M. The impact of diet and nutrition in the prevention and progression of hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2014;8:369-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 85. | Turati F, Trichopoulos D, Polesel J, Bravi F, Rossi M, Talamini R, Franceschi S, Montella M, Trichopoulou A, La Vecchia C, Lagiou P. Mediterranean diet and hepatocellular carcinoma. J Hepatol. 2014;60:606-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 86. | Thilakarathna WPDW, Langille MG, Rupasinghe HPV. Polyphenol-based prebiotics and synbiotics: Potential for cancer chemoprevention. Curr Opin Food Sci. 2018;20:51-57. [RCA] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 87. | Russo E, Giudici F, Fiorindi C, Ficari F, Scaringi S, Amedei A. Immunomodulating Activity and Therapeutic Effects of Short Chain Fatty Acids and Tryptophan Post-biotics in Inflammatory Bowel Disease. Front Immunol. 2019;10:2754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 135] [Article Influence: 22.5] [Reference Citation Analysis (0)] |