Published online Dec 27, 2022. doi: 10.4254/wjh.v14.i12.2025
Peer-review started: August 26, 2022
First decision: October 11, 2022
Revised: October 18, 2022
Accepted: November 7, 2022
Article in press: November 7, 2022
Published online: December 27, 2022
Processing time: 119 Days and 9.7 Hours
Acute-on-chronic liver failure (ACLF) is a syndrome characterized by de
To investigate the role of the EASL-CLIF definition for ACLF and the ability of CLIF-SOFA, CLIF-C ACLF, and CLIF-C AD scores for prognosticating ACLF or AD.
This study is a literature review using a standardized search method, conducted using the steps following the guidelines for reporting systematic reviews set out by the PRISMA statement. For specific keywords, relevant articles were found by searching PubMed, ScienceDirect, and BioMed Central-BMC. The databases were searched using the search terms by one reviewer, and a list of potentially eligible studies was generated based on the titles and abstracts screened. The data were then extracted and assessed on the basis of the Reference Citation Analysis (https://www.referencecitationanalysis.com/).
Most of the included studies used the EASL-CLIF definition for ACLF to identify cirrhotic patients with a significant risk of short-term mortality. The primary outcome in all reviewed studies was mortality. Most of the study findings were based on an area under the receiver operating characteristic curve (AUROC) analysis, which revealed that CLIF-SOFA, CLIF-C ACLF, and CLIF-C AD scores were preferable to other models predicting 28-d mortality. Their AUROC scores were higher and able to predict all-cause mortality at 90, 180, and 365 d. A total of 50 articles were included in this study, which found that the CLIF-SOFA, CLIF-C ACLF and CLIF-C AD scores in more than half of the articles were able to predict short-term and long-term mortality in patients with either ACLF or AD.
CLIF-SOFA score surpasses other models in predicting mortality in ACLF patients, especially in the short-term. CLIF-SOFA, CLIF-C ACLF, and CLIF-C AD are accurate short-term and long-term mortality prognosticating scores.
Core Tip: Acute-on-chronic liver failure (ACLF) is a serious medical challenge worldwide, and its occurrence is a difficult clinical incident due to its severe presentation, quick disease course, and elevated short-term mortality. The European Association for the Study of Liver-Chronic-Liver Failure (EASL-CLIF) Consortium proposal has gained considerable acceptance as a diagnostic criteria for ACLF. CLIF-SOFA has increased the ability to detect patients with ACLF. Unless presenting with renal impairment and/or mild to moderate hepatic encephalopathy, cirrhotic patients with acute decompensation and single liver failure (or any other single "non-renal" organ failure) had a minimum mortality risk. These results suggest that CLIF-SOFA score surpasses other models in predicting mortality in ACLF patients, especially in the short-term.
- Citation: Rashed E, Soldera J. CLIF-SOFA and CLIF-C scores for the prognostication of acute-on-chronic liver failure and acute decompensation of cirrhosis: A systematic review. World J Hepatol 2022; 14(12): 2025-2043
- URL: https://www.wjgnet.com/1948-5182/full/v14/i12/2025.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i12.2025
Acute-on-chronic liver failure (ACLF) is a syndrome characterized by liver decompensation in individuals with chronic liver disease. It is associated with one or more extra-hepatic organ failures and an elevated mortality rate[1-4].
Acute decompensation (AD) is the term used for the occurrence of one or more significant complications of liver disease in a short period of time (i.e., bacterial infection, gastrointestinal haemorrhage, ascites, encephalopathy)[5-9]. It is the most common reason for hospital admission in cirrhotic patients. Most of these patients will develop AD without any other significant features, while others will develop AD associated with multiple organ failures (i.e., kidney failure, declining liver function, and/or other organ failures). Nevertheless, AD patients with extra-hepatic organ failures are at greater risk for short-term mortality[10-12].
In Europe and America, the primary cause of ACLF is alcohol, while viral hepatitis infection is the main cause of ACLF in Asia, particularly in China[13]. Despite procedures such as haemodialysis and liver transplantation significantly increasing short-term survival, they are not widely available in medical care due to their high cost, the requirement for hospital admission, and the limited availability of liver resources[14]. ACLF places a significant financial burden on patients and on the healthcare system.
A European prospective multi-centric study named CANONIC developed and published in 2013 definitions and a classification and grading of ACLF. The most common reasons for cirrhosis were alcoholic liver disease, chronic hepatitis C, and/or both[15]. Hepatic (alcoholic liver injury) and extra-hepatic disorders (gastrointestinal bleeding or bacterial infection) were the most common precipitating disorders for decompensation of cirrhosis, with or without ACLF. The most common organ failures (OFs) were kidney (55.8% of ACLF patients) and liver failure (43.6%), then coagulation (27.7%) and cerebral failure (24.1%). Heart and respiratory failures were the least common, around 16.8% and 9.2%, respectively[15]. Twenty-eight-day transplant-free mortality rate in ACLF patients was 32.8%, while in patients without ACLF, it was 1.9%[15].
Ascites, a higher model for end-stage liver disease (MELD) score, low haemoglobin (Hb) levels, and low mean arterial pressure were defined as predictive factors for ACLF development in a large single-centre Italian prospective cohort of cirrhotic outpatients[16]. The European Association for the Study of Liver-Chronic-Liver Failure (EASL-CLIF) consortium has stated that today's global mortality rate of ACLF ranges from 30% to 50%.
The aim of the current study is to provide an overview of research into the role of the EASL-CLIF definition for ACLF, as well as the ability of CLIF-Sequential Organ Failure Assessment (SOFA), CLIF-C ACLF and CLIF-C AD scores to predict adverse outcomes associated with chronic liver disease.
Various predictive scores have previously been developed. Nearly fifty years ago, the Child-Turcotte-Pugh (CTP) (Table 1) score was established as the most relevant liver-specific score[17]. Wiesner's study evaluated data to develop the MELD score that outperformed the CTP score in predicting 90-d death in individuals with chronic end-stage liver disease[18]. The MELD-Na score (Table 2), which combines the MELD score with serum sodium content, has enhanced predictive accuracy in patients with cirrhosis awaiting liver transplantation[19]. The CLIF-SOFA score, a new scoring system that is an adaptation of the original SOFA score, was used to describe ACLF in the EASL-CLIF CANONIC study of ACLF in cirrhotic patients (Table 3). It has been used to distinguish AD from ACLF, classifying it into three grades[15]. The EASL-CLIF consortium also established the CLIF consortium organ failure (CLIF-C OF) score.
| Points | 1 | 2 | 3 |
| Ascites | Absent | Slight | Moderate |
| Serum Bilirubin (mg/dL) | < 2 | 2-3 | > 3 |
| Serum Albumin (g/dL) | > 3.5 | 2.8-3.5 | < 2.8 |
| PT ratio or | < 4 | 4-6 | > 6 |
| INR | < 1.7 | 1.7–2.3 | > 2.3 |
| HE | None | Grade I-II | Grade III-IV |
| MELD | Mortality rate (%) | MELD-Na | Mortality rate (%) (90-d) |
| ≤ 9 | 1.9 | < 17 | < 2 |
| 10-19 | 6 | 17-20 | 3-4 |
| 20-29 | 19.6 | 21-22 | 7-10 |
| 30-39 | 52.6 | 23-26 | 14-15 |
| ≥ 40 | 71.3 | 27-31 | 27-32 |
| ≥ 32 | 65-66 | ||
| Points | 0 | 1 | 2 |
| Liver Bilirubin (mg/dL) | < 1.2 | ≥ 1.2 - < 2.0 | ≥ 2.0 - < 6.0 |
| Renal Creatinine (mg/dL) | < 1.2 | ≥ 1.2 - < 2.0 | ≥ 2.0 - < 3.5 |
| Neurological HE grade | - | 1 | 2 |
| Haematological INR | < 1.1 | ≥ 1.1 - < 1.25 | ≥ 1.25 - < 1.5 |
| Circulation MAP (mmHg) | ≥ 70 | < 70 | Dopamine ≤ 5 or Dobutamine or Terlipressin |
| Respiratory PaO2 /FiO2 or SpO2 /FiO2 | > 400; > 512 | > 300-≤ 400; > 357 - ≤ 512 | > 200 - ≤ 300; > 214 - ≤ 357 |
Jalan et al[20], described that age and white blood cell (WBC) counts are independent risk factors for death in subsequent investigations and developed the CLIF-C ACLF score. The EASL-CLIF Group created an online calculator for calculating CLIF-SOFA and either CLIF-C ACLF or CLIF-C AD (https://www.clifresearch.com/ToolsCalculators.aspx).
CLIF-C ACLF Score Formula: The CLIF-C ACLF Score Formula[21] combines (CLIF-C OF score, age, and WBC) with the following formula: CLIF-C ACLF = 10 × [0.33 × CLIF-OFs + 0.04 × Age + 0.63 × Ln (WBC)] – 2.
CLIF-C AD Score Formula: The CLIF-C AD Score Formula (non-ACLF patients with AD) combines (Age, Creatinine, international normalized ratio (INR), WBC, and Sodium) with the following formula[22,23]: CLIF-C AD = 10 × [0.03 × Age + 0.66 × Ln (Creatinine mg/dL) + 1.71 × Ln (INR) + 0.88 × Ln (WBC 109 cells/L) – 0.05 × (Sodium mmol/L) + 8].
ACLF Grades[15]: Grade I ACLF: Only kidney failure. [According to Shah et al[24], grade 1 could be with one of the following: Liver failure, kidney failure, coagulation, circulatory, or lung failure, with creatinine (1.5 - 1.9 mg/dL), or hepatic encephalopathy (grade 1 or 2), or brain failure with creatinine (1.5 - 1.9 mg/dL)]. Grade II ACLF: Two organ failures. Grade III ACLF: Three organ failures.
This study is a literature review using a standardized search method, conducted using the steps following the guidelines for reporting systematic reviews set out by the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-analyses)[25].
For relevant original studies, a literature search was conducted using PubMed, ScienceDirect, and BioMed Central-BMC databases. The search command used was a combination of words and Boolean characters: ("CLIF-SOFA" OR "CLIF-C ACLF" OR "CLIF-C AD") AND ("acute-on-chronic liver failure"). Reference Citation Analysis (https://www.referencecitationanalysis.com/) was used to supplement the search.
Studies were included if they analyzed data of patients more than 18 years old from the emergency department or inpatient settings. They needed to report data using ACLF definitions and scores published by the EASL-CLIF group and had a full text available. Studies were excluded if they used only scores other than CLIF-SOFA and CLIF-C AD or CLIF-C ACLF, if they were not written in English or if they were reviews, letters, editorials, opinion articles, conference abstracts, and in-vitro studies.
The databases were searched using the above search terms by one reviewer, and a list of potentially eligible studies was generated based on the titles and abstracts screened. Then, a full-text review was conducted, using the inclusion and exclusion criteria.
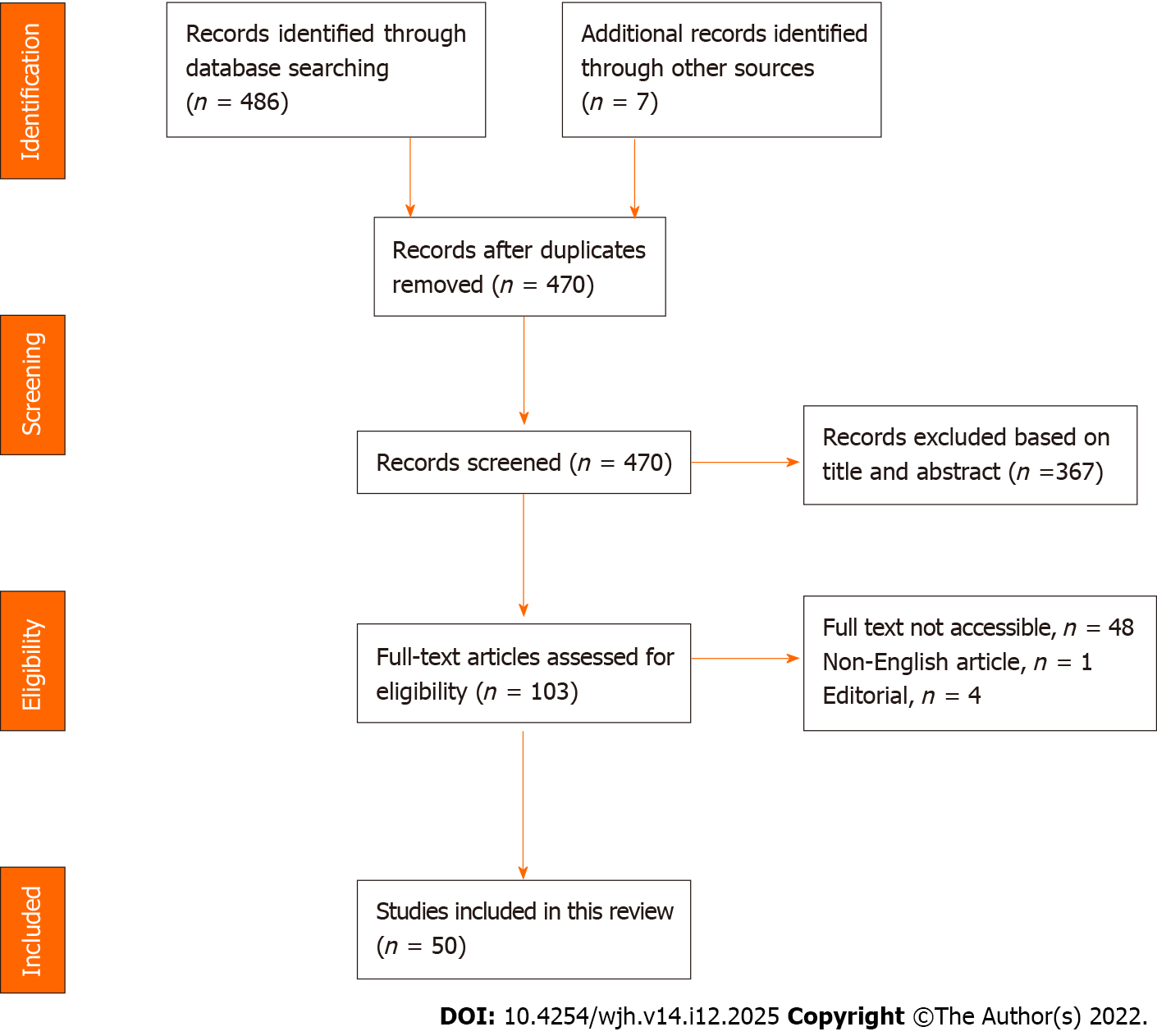
Figure 1 shows the study search and the selection process, including the reasons for exclusion after a full-text review. A total of 50 related articles were included in the final review.
Most of the included studies used the EASL-CLIF definition for ACLF to identify patients with cirrhosis who had a significant risk of short-term mortality. Some articles used the Asian Pacific Association for the Study of the Liver and Chinese Group on the Study of Severe Hepatitis B-ACLF (COSSH-ACLF) prognostic criteria. The included studies were not assessed using a quality assessment tool, although they were considered to be good quality.
The primary outcome in all reviewed studies was mortality. Most of the studies' findings were based on an area under the receiver operating characteristic curve (AUROC) analysis, which revealed that CLIF-SOFA, CLIF-C ACLF, and CLIF-C AD scores were preferable to other models predicting 28-d mortality (Table 4). They had the greatest AUROC scores predicting overall mortality at 90, 180, and 365 d.
| Ref. | Year | Country | Aim | Setting | Results | Conclusions |
| Kuo et al[65] | 2021 | Taiwan | Assess the predictive value and clinical reliability of three different scores | ACLF patients admitted to the ICU | Non-survivor: CLIF-C ACLF, CLIF-C ACLF lactate, and CLIF-C ACLF-D were 58.85 ± 11.40, 60.88 ± 13.71, and 34.03 ± 1.57, respectively. Survivor: 44.55 ± 9.14, 46.91 ± 11.66, and 32.29 ± 1.17, respectively, (all P values < 0.01) | The CLIF-C ACLF-D score may be a better predictor of short- and long-term mortality |
| Li et al[66] | 2017 | China | Assess various prognostic scores, such as the CLIF-C OFs, CLIF-SOFAs, CLIF-C ACLFs, ACLF grade, and MELD, predicted short-term (28-d) mortality | CHB patients with ACLF | Scores in no ACLF group and for ACLF group grades 1, 2, and 3, respectively: CLIF-C OFs: 7, 9, 10, and 13; CLIF-C ACLFs: 29, 37, 44, and 60; CLIF-SOFAs: 5, 7, 9, and 13; MELDs: 16, 22, 30, and 37 | CLIF-C OF score outperforms other scores |
| Dong et al[67] | 2020 | China | Determine the characteristics and outcomes of ACLF | ACLF patients who have or do not have cirrhosis | COSSH ACLF score (AUROC = 0.778 or 0.792, 95%CI 0.706-0.839 or 0.721–0.851) displayed the better prognostic ability for EASL ACLF patients with non-cirrhosis. CLIF-C ACLF score (AUROC = 0.757 or 0.796, 95%CI 0.701–0.807 or 0.743-0.843) still was the best prognostic scoring system in EASL ACLF patients with cirrhosis | CLIF-C ACLF score was better at predicting short-term mortality in ACLF patients with cirrhosis, while the COSSH ACLF score was better for ACLF patients without cirrhosis |
| Grochot et al[68] | 2020 | Brazil | Determine the accuracy of the presence of ACLF in predicting mortality. | Patients with cirrhosis | CLIF-SOFA score at 28-, 90-, and 365-d was 1.32, 1.3, and 1.2, respectively. CLIF-C AD/ACLF score was 1.0, 1.0, and 1.0, respectively | CLIF-SOFA score increased mortality by 1.3 times for each point |
| Jacques et al[41] | 2020 | Brazil | Assess and compare the liver-specific scores ability to predict mortality | Cirrhotic patients with SBP | CLIF-SOFA was able to predict mortality at 30-, 90-, and 365-d, with an AUROC of 0.75, 0.64, and 0.64, respectively. CLIF-C AD or CLIF ACLF scores 0.59, 0.51, and 0.52, respectively | CLIF-SOFA outperformed other liver-specific measures |
| Terres et al[39] | 2022 | Brazil | Assess and compare the significance of liver-specific scores in predicting mortality | HRS patients who received terlipressin | CTP at 30-, 90- and 365-d mortality 0.76, 0.75 and 0.72, respectively. CLIF-SOFA 0.66, 0.63, and 0.57. CLIF-C ACLF 0.60, 0.55, and 0.53. MELD 0.67, 0.64, and 0.5. MELD-Na 0.65, 0.63, and 0.52 | CTP was able to predict increased mortality at |
| Terres et al[40] | 2021 | Brazil | Evaluate the liver-specific scores to predict mortality | AOVH patients who received terlipressin | AUROC at 30- and 90-d: MELD-Na 0.77 and 0.78. CLIF-SOFA 0.76 and 0.75. CLIF-C AD or ACLF 0.64 and 0.60. MELD 0.75 and 0.77. CTP 0.75 and 0.76 | CLIF-SOFA was better in ACLF patients. CTP performed better in AD patients |
| Grochot et al[56] | 2019 | Brazil | Assess the validity of CLIF SOFA in predicting mortality and compare it to other liver-specific scores | AD and ACLF patients | AUROC at 28-, 90- and 365-d, respectively: CLIF-SOFA 0.71, 0.75 and 0.66. CLIF-C AD/ACLF 0.52, 0.51, and 0.56. MELD 0.54, 0.50, and 0.52. MELD-Na 0.57, 0.54, and 0.55 | CLIF-SOFA predicted 90-d mortality better than other scores |
| Jacques et al[69] | 2021 | Brazil | Evaluate the relation between ACLF and mortality | Cirrhotic patients with SBP | Scores for 28- and 90-d mortality, respectively: MELD 0.83 and 0.87. CLIF-SOFA 1.1 and 1.1. CTP 31 and 8.3 | Elevated CLIF-SOFA scores and the presence of ACLF were related to higher 28- and 90-d mortality |
| Engelmann et al[21] | 2018 | United Kingdom | Assess if the currently available scores can identify patients with ACLF | Patients with ACLF | AUROC of 28-d mortality prediction: CLIF-C ACLF 0.8. CLIF-C OF 0.75. MELD, 0.68. CP 0.66 | CLIF-C ACLF accurately predicted 28-d mortality |
| Barosa et al[70] | 2017 | Portugal | Evaluate CLIF-C ACLF, MELD, MELD-Na, and CTP scores for short/medium-term mortality, to identify ACLF frequency and to compare mortality between non-ACLF and ACLF patients | Patients admitted for AD of cirrhosis | Cut-off point in 28- and 90-d mortality, respectively: CLIF-C ACLF 50 and 50. CTP 10 and 10. MELD 17 and 14. MELD-Na 22 and 22 | CLIF-C ACLF score outperformed other scores |
| Ferreira Cardoso et al[71] | 2019 | Portugal | Validate the EASL-CLIF C scores | Patients with and without ACLF | AUROC for CLIF-C ACLF score for 28-d mortality was (0.856 ± 0.071) | CLIF-C AD score of 60 was related to an increased risk of developing ACLF |
| Maipang et al[57] | 2019 | Thailand | Assess ACLF prognostic models and investigation of their discriminative capacities in ACLF patients | Cirrhotic patients with AD and ACLF | Scores for 28-d, 90-d, 6-mo, and 1-yr mortality, respectively: CLIF-SOFA: 0.84, 0.85, 0.80, 0.80. CLIF-C OF: 0.83, 0.82, 0.78, and 0.78. CLIF-C ACLF: 0.79, 0.80, 0.77, and 0.77. CTP: 0.7, 0.67, 0.64, and 0.63. MELD: 0.63, 0.60, 0.56, and 0.56. MELD-Na: 0.63, 0.59, 0.56, and 0.56. iMELD: 0.73, 0.71, 0.67, and 0.68. APACHE II: 0.69, 0.65, 0.63, and 0.63 | The CLIF-SOFA had similar predictive accuracy for 28-d mortality as the CLIF-C OF |
| Li et al[36] | 2016 | China | Assess if CLIF-C OFs criteria can be used to identify patients and if the CLIF-C ACLF score can be used to predict prognosis | HBV cirrhotic patients with ACLF | Assess patients with ACLF for 28-, 90-, 180-, and 360-d mortality, respectively: HBV-ACLF: 0.654, 0.645, 0.644, and 0.640. CLIF-C ACLF: 0.704, 0.685, 0.687, and 0.682. MELD: 0.554, 0.543, 0.543, and 0.540. MELD-Na: 0.549, 0.541, 0.541, and 0.537. Patients without ACLF: for 28-, 90-, 180-, and 360-d mortality, respectively: HBV-AD: 0.737, 0.716, 0.720, and 0.721. CLIF-C AD: 0.733, 0.724, 0.728, and 0.728. MELD: 0.667, 0.653, 0.657, and 0.639. MELD-Na: 0.719, 0.710, 0.701, and 0.682 | CLIF-C ACLFs were found to be more accurate in predicting short-term mortality |
| Chirapongsathorn et al[49] | 2022 | Thailand | Collect epidemiological data and assess a scoring system for predicting mortality | ACLF patients. | AUROC of prognostic scores for 30- and 90-d mortality, respectively: CLIF-SOFA: 0.64 and 0.61 (95%CI: 0.585-0.704). CLIF-OF: 0.62 and 0.59. CLIF-C: 0.62 and 0.61. MELD: 0.60 and 0.56. MELD-Na: 0.60 and 0.57 | CLIF-SOFA score had a higher AUROC than the other scores |
| Zhang et al[31] | 2018 | China | Assess bacterial infection and predictors of mortality | ACLF patients with autoimmune liver disease | CLIF-SOFA score for 28-d mortality was 1.362 and 1.093, respectively.Scores for 90-d mortality were, respectively: CLIF-SOFA 2.936 and 1.578. MELD 1.232 and 0.664. CP 2.003 and 0.595 | All scores of ACLF patients with bacterial infection were high |
| Shin et al[72] | 2020 | South Korea | To look into the risk factors for mortality in cirrhotic patients and to see how ACLF affected their prognosis | Cirrhotic patients with variceal bleeding | Prediction of mortality at 28- and 90-d with AUROC were, respectively: CTP 0.842 and 0.846. MELD 0.857 and 0.867. MELD-Na 0.828 and 0.834. CLIF-SOFA 0.895 (95%CI, 0.829-0.962) and 0.897 (95%CI, 0.842-0.951) | CLIF-SOFA model well predicted 28-d or 90-d mortality |
| Gao et al[73] | 2018 | China | Investigate the CLIF-SOFA lung score's predictive value and determine the best voriconazole regimen | ACLF patients with IPA | CLIF-SOFA 10 (P = 0.083). CLIF-C ACLF 46.8 (P = 0.028). MELD 27.2 (P = 0.145). MELD-Na 28.6 (P = 0.064) | Patients with a CLIF-SOFA lung score of less than 2 had a superior 28-d survival rate than those with a lung score of more than 1 (P = 0.001) |
| Chen et al[74] | 2021 | China | Create a predictive nomogram | HBV-ACLF patients undergoing LT | CP score (0.626), MELD (0.627), MELD-Na (0.583), CLIF-C OF (0.674), and CLIF-C ACLF (0.684) | The nomogram's concordance index for predicting 1-yr survival was 0.707, which was significantly greater than that of other prognostic models. The nomogram could be helpful in determining which HBV-ACLF patients may improve after LT |
| Yu et al[75] | 2021 | China | Multicenter study to develop and evaluate a novel scoring system that uses baseline and dynamic data to predict short-term prognosis | ACLF patients | For 90-d prognosis: DP-ACLF with an AUC value of 0.907, CTP (0.601/74.6%), MELD (0.721/76.2%), MELD-Na (0.740/73.8%), CLIF-SOFA (0.701/76.9%), CLIF-C ACLF (0.694/74.6%), and COSSH-ACLF (0.724/77.7%) (P < 0.001) | The validation group had a higher predictive accuracy of DP-ACLF on ACLF prognosis and an accuracy rate of 85.4%, according to ROC analysis |
| Liu et al[35] | 2020 | China | Assess different prognostic models to predict short-term mortality | ACLF patients | The AUROCS of the CLIF-SOFA score, PWR, ALBI score, and MELD score was 0.804, 0.759, 0.710, and 0.670, respectively | CLIF-SOFA was the best model for predicting 28-d mortality |
| Zhang et al[76] | 2015 | China | Examine and contrast the various ACLF diagnostic criteria currently in use. Also, to identify predictors of the progress from ACLF at enrolment defined by APASL alone or by both APASL and CMA | Selected patients were cirrhotic, fulfilling at least APASL criteria for ACLF | CTP 12 and 11 (P = 0.53). MELD 17.8 and 16.0 (P = 0.02). MELD-Na 20.1 and 18.7 (P = 0.02). CLIF-SOFA 7 and 7 (P = 0.01) | The maximum rise in the CLIF-SOFA score, MELD-Na score, and total bilirubin were all independent predictors of progression into post-enrollment EASL-CLIF ACLF from ACLF at enrollment |
| Li et al[77] | 2020 | China | Randomized study to assess the scoring systems for predicting short-term results | HBV-ACLF patients | ALBI score (30-d mortality: HR = 3.452; 90-d mortality: HR = 3.822), MELD (30-d mortality: HR = 1.073; 90-d mortality: HR = 1.082), CLIF-C ACLF score (30-d mortality: HR = 1.061; 90-d mortality: HR = 1.065) | All scores accurately predicted 30-d and 90-d mortality. A higher CLIF-C ACLF score was linked to a lower overall survival rate |
| Zhang et al[14] | 2020 | China | Find prognostic scores that can be used to predict short- and long-term outcomes | ACLF patients with cirrhosis | Scores for survivors and [non-survivors] at 28-d, 3- and 6-mo, respectively: CTP 10 [12] (P = 0.001), 10 [11] (P = 0.028) and 10 [11] (P = 0.033). MELD 16 [24] (P = 0.004), 15 [23] (P = 0.001) and 15 [23] (p=0.002). MELD-Na 18 [24] (P = 0.081), 16.54 [23.27] (P = 0.011) and 17.27 [23] (P = 0.020). CLIF-C OF 9 [11] (P = < 0.001), 9 [10.00] (P = 0.001) and 9 [10] (P = 0.001). CLIF-SOFA 8 [12] (P ≤ 0.001), 8.55 [11.46] (P ≤ 0.001) and 8.53 [11.33] (P ≤ 0.001). CLIF-C ACLF 45.01 [53.98] (P ≤ 0.001), 44.39 [52.85] (P ≤ 0.001) and 44.11 [52.56] (P = 0.001) | The CLIF-SOFA score was particularly useful for assessing 28-d mortality |
| Kim et al[42] | 2016 | South Korea | A comparative study to evaluate the performance of suggested ACLF-specific scores in predicting short-term mortality | Alcoholic hepatitis patients | The AUROC of CLIF-SOFA, CLIF-C OFs, DF, ABIC, GAHS, MELD, and MELD-Na was 0.86 (0.81-0.90), 0.89 (0.84-0.92), 0.79 (0.74-0.84), 0.78 (0.72-0.83), 0.81 (0.76-0.86), 0.83 (0.78-0.88), and 0.83 (0.78-0.88), respectively, for 28-d mortality. CLIF-SOFA score of 8 had (78.1% Sn and 79.7% Sp), and CLIF-C OFs of 10 had (68.8% Sn and 91.4% Sp) for predicting 28-d mortality | CLIF-SOFA and CLIF-C OF scores performed well for short-term mortality |
| Costa E Silva et al[78] | 2021 | Brazil | Assess how well prognostic scores predict mortality | Cirrhotic patients admitted to the ICU | AUC revealed in all patients: CTP 0.701, APACHE II 0.695, MELD 0.727, MELD-Na 0.729, MESO index 0.723, iMELD 0.640, SOFA 0.753, CLIF-SOFA 0.776, CLIF-C OF 0.807 and CCI 0.627. CLIF-C OF in ACLF patients (0.749). CLIF-SOFA in AD patients (0.716) and CLIF-C AD (0.695) | CLIF-C OF and CLIF-SOFA had the best ability to predict mortality in all patients |
| Chen et al[38] | 2020 | Taiwan | Compare the eight prognostic scores | Cirrhotic patients with ACLF | Score on admission to ICU median (IQR) (P ≤ 0.001): CTP 9.0, MELD 23.0, CLIF-C OF 10.0, CLIF-C ACLF 49.2, SAP III 51.0, MPM0-III 0.0 (P = 0.001), APACHE II 16.0, and APACHE III 81.0. Predict overall mortality by AUROC: CTP 0.719, MELD 0.702, CLIF-C OF 0.721, CLIF-C ACLF 0.772, MPM0-III 0.607, SAP III 0.739, APACHE II 0.756 and APACHE III 0.817 | APACHE III and CLIF-C ACLF scores were superior to other models for predicting overall mortality |
| Sheng et al[79] | 2021 | China | Create a new and effective prognosis model and identify new prognostic factors | HRS with AD patients | AUROC in derivation and validation, respectively: GIMNS (0.830 and 0.732), MELD (0.759 and 0.623), CLIF-SOFA (0.767 and 0.661), COSSH-ACLF (0.759 and 0.674). Mortality at 28-d according to the developed GIMNS score: (GIMNS ≥ 2) 100.0%, (GIMNS 1-2) 73.8%, (GIMNS 0-1) 57.1% and (GIMNS < 0) 30.3% | GIMNS had a higher accuracy AUROC and outperformed MELD and CLIF-SOFA |
| Hong et al[80] | 2016 | South Korea | Evaluate the features and outcomes of ACLF patients | ACLF patients with underlying liver disease | Scores in Type A (non-cirrhosis), B (cirrhosis), and C (cirrhosis with the previous decompensation), respectively: MELD 29, 27 and 26. Hepatic CLIF-SOFA 19, 34 and 21. Extra-hepatic CLIF-SOFA 7, 11 and 31 | The 30-d overall survival rate for types A, B, and C, respectively, was 85.3%, 81.1%, and 83.7% |
| Sy et al[54] | 2016 | Canada | Assess if the CLIF-SOFA score could predict survival | Severely ill patients with ACLF | APACHE II 23; MELD 26; CTP 12; SOFA 15 and CLIF-SOFA 17. The CLIF-SOFA (AUROC 0.865). SOFA (AUROC 0.935) | CLIF-SOFA outperformed the other scores |
| Cai et al[2] | 2019 | China | Evaluate prognostic scoring models and create prediction models | Various causes of AD in cirrhotic patients | Hepatitis B group, AUROC for 28-d mortality for MELD, CLIF-C-AD, MELD-Na, AARC-ACLF, and the newly developed AD scores was 0.663, 0.673, 0.657, 0.662, and 0.773, respectively. Alcoholic liver disease group, 0.731, 0.737, 0.735, 0.689, and 0.778, respectively. Others group 0.765, 0.767, 0.814, 0.720, and 0.814, respectively | In predicting the prognosis of AD cirrhosis, the newly developed scoring models for short-term mortality outperformed the other models |
| Marciano et al[81] | 2017 | Argentina | Compare the predictive accuracy for 28- and 90-d transplant-free mortality of a modified CLIF-SOFA score with that of the classic CLIF-SOFA and KDIGO scores | AKI in cirrhotic patients with AD | Classic CLIF-SOFA and modified CLIF-SOFA by AUCROC: In 28-d transplant-free, 0.93 and 0.92 (P = 0.34), respectively. In 90-d transplant-free, 0.79 and 0.78 (P = 0.78), respectively. In AKI 28-d and 90-d transplant-free mortality by AUCROC, 0.67 (P = 0.002) and 0.63 (P = 0.02) | Both CLIF-SOFA scores were extremely accurate in predicting 28-d and 90-d transplant-free mortality |
| Xu et al[82] | 2018 | China | Recognizing mortality risk variables and optimizing stratification are crucial for increasing survival rates | Cirrhotic patients with pneumonia | Scores by AUROC for predicting mortality in 30-d and 90-d respectively: CLIF-SOFA 0.890 and 0.900. MELD 0.853 and 0.889. MELD-Na 0.801 and 0.849, qSOFA 0.854 and 0.777, PSI 0.867 and 0.831. CTP 0.726 and 0.768 | CLIF-SOFA outperformed the other models in predicting mortality |
| Silva et al[83] | 2021 | Brazil | Assess the prognostic scores predicting mortality | Cirrhotic patients who were admitted to the ICU without being pre-screened | ROC curves SOFA 0.88, MELD-Na 0.76, MELD 0.75, CPS 0.71 and SAPS 3 (0.51). In patients with ACLF, CLIF-ACLF 0.74, CLIF-OF 0.70, MELD-Na 0.73 and MELD 0.69, SAPS 3 (0.55), SOFA 0.63 and CLIF-SOFA 0.66 | In patients with and without ACLF, CLIF-ACLF and SOFA had higher accuracy in predicting mortality |
| McPhail et al[46] | 2015 | United Kingdom | Compare the capabilities of SOFA and CLIF-SOFA scores to predict patient survival and evaluate CLIF-SOFA | Cirrhotic patients | At the time of admission, with AUROC values, CLIF-SOFA and SOFA scores were 0.813 and 0.799, respectively. At 48 h after admission were 0.853 and 0.840, respectively. After 1 wk were 0.842 and 0.844, respectively | SOFA and CLIF-SOFA scores appear to have equal ability to predict patient survival |
| Yang et al[52] | 2022 | China | Estimate the short-term prognosis of ACLF patients | ACLF patients who had undergone LT | AUROC of MELDs 0.704, ABIC: 0.607, CLIF-C OFs 0.606, CLIF-C ACLFs 0.653 and CLIF-SOFAs 0.633 of the 90-d outcome | MELDs had a higher AUROC than others for predicting the 90-d outcome in ACLF patients after LT |
| Moreau et al[15] | 2013 | 12 European countries | Multicenter study to establish ACLF diagnostic criteria and characterize the progression of the disease | Cirrhotic patients with AD | The increased 28-d mortality rate was linked to three risk variables identified from the CLIF-SOFA score at enrollment: ≥ 2 organ failures, kidney failure alone, a combination of renal dysfunction, and a single organ failure other than kidney and/or hepatic encephalopathy (mild-moderate) | In patients with ACLF, higher CLIF-SOFA scores and leukocyte counts were predictors of mortality. The mortality rates at 28-d and 90-d, respectively: No ACLF 4.7% and 14%. ACLF g1: 22.1% and 40.7%. ACLF g2: 32% and 52.3%. ACLF g3: 76.7% and 79.1% |
| Li et al[37] | 2021 | China | Create a new simple prognostic score that can accurately predict outcomes | HBV-ACLF patients | The C-indices of the new score for 28- and 90-d mortality (0.826 and 0.809), COSSH-ACLF 0.793 and 0.784; CLIF-C ACLF 0.792 and 0.770; MELD 0.731 and 0.727; MELD-Na 0.730 and 0.726 (all P < 0.05) | The C-indices of the new score were significantly higher than other existing scores for 28-d and 90-d mortality |
| Perdigoto et al[58] | 2019 | Identify and characterize ACLF, and compare the CLIF-C OF score to the MELD-Na and the CP score. Also, to assess the CLIF-C ACLF and CLIF-C AD scores | Patients with ACLF | In the whole study group, the AUC: For 28-d mortality, the scores MELD, CLIF-C OF, and CP were 0.908, 0.844, and 0.753, respectively. For 90-d mortality 0.902, 0.814, and 0.724, respectively (P < 0.0001 for AUC in all scores) | CLIF-C OF shows good accuracy and diagnoses ACLF. MELD performed better in terms of 90-d mortality prediction | |
| Ramzan et al[84] | 2020 | Evaluate the CLIF-C CLF score and compare it to the MELD score | ACLF patients in ICU | MELD scores 30, 40 and 50 at 48 h were 0.532, 0.594 and 0.529, respectively. CLIF-C ACLF ≥ 70 at 0 h, 24 h, and 48 h were 0.498, 0.605, and 0.643, respectively | CLIF-C ACLF score of 70 or higher accurately predicts mortality | |
| Verma et al[85] | 2021 | Assess the prognostic models | ACLF patients | Day-7 AARC model had the numerically highest c-index, 0.872, best accuracy of 84.0%, Day-7 NACSELD-ACLF sensitivity (100%) but with a lower PPV (70%) for mortality | Patients having an AARC score of > 12 on day 7 had the lowest 30-d survival rate. All model performance parameters were better on day 7 | |
| Picon et al[59] | 2017 | Brazil | Assess prognostic scores | Patients with AD of cirrhosis and ACLF | Patients with ACLF, at 28-d from the diagnosis: CLIF-C ACLF with an AUC of 0.71. Patients with AD, regarding 28-d mortality: CLIF-C AD 0.75; CP 0.72; MELD 0.75; MELD-Na 0.76; CLIF-C OF 0.74. Patients with AD regarding 90-d mortality: CLIF-C AD 0.70; CP 0.73; MELD 0.7; MELD-Na 0.73; CLIF-C OF 0.65 | The CLIF-C ACLF score is the most accurate for predicting 28-d death in patients with ACLF. The CLIF-C AD score was also good in predicting death in cirrhosis with AD |
| Gupta et al[44] | 2017 | India | Assess the variations in mortality outcomes and predictors | Patients admitted with AD and ACLF caused by hepatic or extra-hepatic insults | AUROC for 28-d mortality in the extrahepatic ACLF group for CLIF-SOFA, MELD, iMELD, APACHE-11, and CTP was 0.788, 0.724, 0.718, 0.634, and 0.726, respectively. AUROC for 28-d mortality in the hepatic ACLF group for CLIF-SOFA, MELD, iMELD, APACHE-11, and CTP was 0.786, 0.625, 0.802, 0.761, and 0.648, respectively | iMELD and CLIF-SOFA were the best for predicting 28-d mortality |
| Niewiński et al[45] | 2020 | Poland | Use the available prognostic scores to find the best mortality risk factor(s) | Critically unwell ACLF patients | Predictive 90-d mortality: MELD 1.10, SOFA 1.33, CLIF-SOFA 1.40, and CLIF-C OF 1.64 | SOFA score surpassed the CLIF-C values |
| Kulkarni et al[55] | 2018 | India | Determine the in-hospital predictors of 28-d mortality | ACLF patients admitted to the Medical ICU | MELD 0.783 (Sn 75% and Sp 82.1%). CLIF-SOFA 0.947 (Sn 83.3% and Sp 96.4%). CTP 0.795 (Sn 94.4% and Sp 57.1%). APACHE-II 0.876 (Sn 91.6% and Sp 78.5%) | CLIF-SOFA and APACHE-II scores had a superior ability to predict mortality |
| Dhiman et al[86] | 2014 | India | Assess the efficacy of the CLIF-SOFA and APASL definitions of ACLF in predicting the short-term prognosis of ACLF patients | Patients selected were cirrhotic with AD | AUROCs for 28-d mortality were 0.795, 0.787, 0.739, and 0.710 for CLIF-SOFA, APACHE-II, CTP, and MELD, respectively | The strongest predictor of short-term mortality was the CLIF-SOFA score |
| Safi et al[87] | 2018 | Germany | Evaluate how infection detected at the time of admission, as well as other clinical baseline factors, affected the mortality | Cirrhotic patients with emergency admissions | Predictors of mortality up to 90 d (all patients): HR, 95%Cl, and P, respectively: SOFA 0.15, 0.03-0.69 and 0.015. CLIF C ACLF 1.09, 1.06-1.13 and < 0.001. Infection and CLIF-SOFA and infection and CLIF-C-ACLF: HR, 95%CI and P, respectively: CLIF-SOFA 1.33, 1.17- 1.51 and < 0.001 CLIF-SOFA: Infection 0.85, 0.71-1.02 and 0.074. CLIF-C-ACLF 1.09, 1.06-1.12 and < 0.001 CLIF-C-ACLF: Infection 0.96, 0.92-1.01 and 0.082 | Infection reduced the significant relation between mortality and CLIF-C-ACLF or CLIF-SOFA-score |
| Leão et al[88] | 2019 | Brazil | Assess how different ACLF diagnostic criteria performed in terms of predicting mortality | Cirrhotic patients with AD | AUROC at 28-d for CLIF-C, AARC and NACSELD criteria were 0.710, 0.560 and 0.561 (P = 0.002), respectively. AUROC at 90-d mortality were 0.760, 0.554 and 0.555 respectively (P < 0.001) | CLIF-C performed better in predicting mortality at 28-d and 90-d |
| Bartoletti et al[89] | 2018 | Different European countries | Summarize the current epidemiology of BSI, and assess predictors of 30-d mortality and antibiotic resistance risk factors | Cirrhotic patients | In a Cox regression model, CLIF-SOFA scores were (HR 1.35; 95%CI 1.28-1.43; P < 0.001) | The SOFA and CLIF-SOFA scores were the best predictors of 30-d mortality |
| Mendizabal et al[47] | 2021 | 11 Latin American countries | Evaluate whether SARS-CoV-2 infection affects the outcome and assess the effectiveness of the different prognostic models in predicting mortality | Hospitalized cirrhotic patients | AUROC for performance evaluation in predicting 28-d mortality for CLIF-C, NACSELD, CTP score and MELD-Na were 0.85, 0.75, 0.69, 0.67; respectively (P < 0.0001) | In patients with cirrhosis and SARS-CoV-2 infection, CLIF-C performed better than other models |
ACLF has become a serious medical challenge, and it remains a complex clinical scenario for hepatologists and specialists in different related departments due to its severe presentation, and quick disease course with high short-term mortality. Regional differences when defining ACLF and understanding its diagnostic methods has led to many clinical phenotypes. The current therapeutic management of ACLF patients primarily focuses on treating and supporting multiple organ failures[26].
The CANONIC study introduced accurate criteria for the diagnosis of this condition. The CLIF-SOFA score was developed and evaluated for the prognosis of ACLF in the CANONIC research[15]. This development has increased the ability to distinguish patients with ACLF from those with AD using the CLIF-SOFA parameters[15].
Every scoring system has advantages and disadvantages. Even though the CLIF-SOFA score has a significant prognosticative accuracy, its calculation is challenging due to the combination of many indicators[14]. The CTP score is calculated by the ascites, serum bilirubin, albumin, prothrombin time, and hepatic encephalopathy (HE) levels[17]. The presence of HE and ascites is a component of the CTP score; nevertheless, these are subjective, without a defined cut-off value. The MELD score includes three laboratory markers: INR, bilirubin, and creatinine; nevertheless, it is susceptible to confounding factors such as haemorrhage, ascites, and diuretic treatment, and there are no obviously defined cut-off levels for identifying patients with cirrhosis[27]. The MELD score does not include subjective indicators, which may diminish evaluating reliability[28].
Hyponatraemia is strongly associated with the prognosis of cirrhotic patients, especially those with ascites; thus, the MELD-Na score was developed to improve on the MELD score[29].
Jalan et al[20] in 2014, showed that the CLIF-C OF accuracy is similar to the CLIF-SOFA score in predicting mortality. The CLIF-C ACLF score does not consider only the role of extra-hepatic organ injuries, circulatory system failure, and coagulation impairment on prognosis, but also includes the WBC count, in order to assess the level of inflammation. In this study, the CLIF-C ACLF score outperformed the CTP, MELD and MELD-Na scores[30].
This was also true of the CANONIC study data, which demonstrated that CLIF-SOFA, CLIF-C OF and CLIF-C ACLF scores were able to outperform CTP, MELD, and MELD-Na scores when predicting short- and long-term mortality in ACLF patients[15,20].
Zhang et al[31] in 2018, assessed the relationship between bacterial infection and predictors of mortality in ACLF patients with autoimmune liver disease. No significant association was found between 28-d and 90-d transplant-free mortality and any predictor. The CTP, MELD, and CLIF-SOFA scores of ACLF patients with bacterial infection were all high[31].
Ascites at admission were a potential risk for post-enrollment development of ACLF in the study by Moreau et al, as it is an independent prognostic factor of renal failure following bacterial infection[15,32,33]. CLIF-SOFA scores at enrollment and ACLF diagnosis were significant independent predictors for post-enrollment ACLF development and ACLF-associated death, respectively[15].
The albumin-bilirubin (ALBI) score, which uses albumin and bilirubin values to indicate liver injury, effectively predicts the outcome of hepatocellular carcinoma[34]. The ALBI score and the CLIF-SOFA score had a comparable effect in predicting the outcome of ACLF patients, according to the findings of Liu et al[35].
Hepatitis B virus (HBV) is the most common etiology of ACLF in the East, which differed from patients in Western societies. HBV-ACLF is a pan-Asian and African condition associated with excessively elevated short-term mortality[36]. In 2021, Li et al[37] created a new simple prognostic score that can accurately predict outcomes in HBV-ACLF patients. The C-indices of the new score were significantly higher than the C-indices of four existing scores (COSSH-ACLF, CLIF-C ACLF, MELD, and MELD-Na) for 28- and 90-d mortality. Without assessing organ failure, the novel prognostic score can correctly predict short-term mortality in patients with HBV-ACLF and could be used to guide clinical care[37]. In Taiwan, a viral hepatitis endemic country[38], a study demonstrated that APACHE III, CLIF-OF and CLIF-C ACLF scores have outperformed other models for predicting 28-d overall mortality[38].
Terres et al[39] assessed and compared the significance of liver-specific scores in predicting mortality in hepatorenal syndrome (HRS) patients who received terlipressin. CTP was superior to CLIF-SOFA, CLIF-ACLF, MELD, and MELD-Na in estimating 30-d, 90-d, and 365-d mortality[39].
CTP was superior to CLIF-SOFA, CLIF-ACLF, MELD, and MELD-Na in estimating 30-d and 90-d mortality in AD patients, while CLIF-SOFA was better in ACLF patients with acute oesophageal variceal haemorrhage (AOVH) who received terlipressin[40].
CLIF-SOFA has demonstrated superior performance in spontaneous bacterial peritonitis (SBP)[41] and alcoholic hepatitis[42].
Both the standard and the modified CLIF-SOFA scores demonstrated remarkable accuracy for the prognostication of 28-d transplant free-mortality evaluation (AUC-ROC greater than 0.9) in acute kidney injury (AKI) patients with cirrhosis and AD. Nevertheless, it presents a reduced effectiveness in 90-d mortality assessment (AUC-ROC 0.78). These results are comparable to the results reported by Angeli et al[43] in 2015.
A study by Gupta et al[44] in 2017, that included hepatic and extra-hepatic ACLF patients showed that, in the hepatic group, iMELD was the best indicator of 28-d mortality. On the other hand, CLIF-SOFA was the strongest predictor of death in the extra-hepatic ACLF cohort. The majority of patients in this cohort were decompensated, and infection was the most frequent extra-hepatic event, leading to systemic inflammation and extra-hepatic organ involvement with fewer liver failures[44].
In predicting 90-d mortality, the SOFA score surpassed the more commonly used prognostic liver-specific scores (MELD, SOFA, CLIF-SOFA, CLIF-C OF, and CLIF-C ACLF/CLIF-C AD) in a study conducted to describe the best mortality risk factor(s) in critically unwell ACLF patients[45]. The CLIF-C ACLF, CLIF-C OF and ACLF grades varied widely between ACLF patients who underwent liver transplantation and those who died waiting for an organ. At the time of admission, those with two or three organ failures had survival rates ranging from 30% to 55%, whereas patients with more than three organ failures had mortality rates approaching 80%[46].
Mendizabal et al[47] performed a study to evaluate whether severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection affects the outcome of hospitalized cirrhotic patients and to assess the effectiveness of the different prognostic models in predicting mortality. CLIF-C scores performed better than North American Consortium for the Study of End-Stage Liver Disease (NACSELD)–ACLF score, CTP, and MELD-Na.
Aggressive alcohol intake, alcoholic hepatitis, and bacterial infection were the most common causes of ACLF in alcohol liver disease[48]. The AUROCs of the CLIF-SOFA, CLIF-OF, and CLIF-C scores showed a slight superior effect in estimating short-term mortality; however, they were equivalent to MELD and MELD-Na[49]. To clarify this finding, Chirapongsathorn et al[49] had elevated short- and long-term mortality rates. In patients with ACLF, as per the CLIF-C definition, the prediction accuracy of the CLIF-SOFA, CLIF-OF and CLIF-C scoring tools were no better than the accuracy of MELD and MELD-Na scores. In a retrospective investigation by Lee et al[50] the CLIF-SOFA score surpassed other scoring systems in estimating short-term mortality in alcoholic cirrhotic patients with AD.
The MELD score is commonly used in liver transplantation (LT) as a scoring method for organ allocation and is the standard model prognostic tool for predicting 3-mo to 6-mo survival in patients with liver failure[51]. Nevertheless, ACLF has a distinct clinical characteristic (Table 5); therefore, the MELD score for patients with ACLF is not expected to be optimal[52].
| Liver transplantation ACLF | Liver transplantation AD | P value | |
| Total | 22 (73.3%) | 7 (26.7%) | - |
| Age (yr) | 57.0 (IQR 11.0) | 54.0 (IQR 5.0) | n.s. |
| MELD | 30.7 (IQR 5.0) | 12.9 (IQR 7.3) | < 0.001 |
| iMELD | 53.1 (IQR 8.7) | 36.5 (IQR 15.6) | < 0.001 |
| MELD-Na | 34.4 (IQR 18.7) | 14.3 (IQR 17.6) | 0.002 |
| CPC | 13.0 (IQR 1.0) | 9.0 (IQR 3.0) | < 0.001 |
| SOFA | 8.0 (IQR 3.0) | 4.0 (IQR 3.0) | < 0.001 |
| CLIF-SOFA | 12.0 (IQR 3.0) | 5.0 (IQR 3.0) | < 0.001 |
| CLIF-C OF | 11.5 (IQR 2.0) | 7.0 (IQR 1.0) | < 0.001 |
The MELD score was associated with post-transplant survival but is considered to have poor prediction accuracy[53]. No more trials demonstrated that CLIF-SOFA, CLIF-C ACLF, or CLIF-C OF had good prognostic value for short-term survival after LT[52].
Despite the excellent predictive accuracy of CLIF-C ACLF and CLIF-C OF scores, they were developed analyzing data from patients generally with alcohol-related liver disease from Europe and the United States, and more research is necessary to confirm whether this is appropriate for Asian populations. However, according to the study by Zhang et al[14], the scores were also applicable in Asian populations.
A higher CLIF-SOFA was separately associated with higher mortality; this is consistent with previous research, which found that the CLIF-SOFA was better than other liver-specific scores in predicting mortality[42,54,55]. It has been shown by other researchers that CLIF-C ACLF or CLIF-C AD, MELD, and MELD-Na are preferred, even for extra-hepatic injuries[56,57].
In the study by Zhang et al[14], the prognostication accuracy and power of the six scores (CTP score, MELD score, MELD-Na, CLIF-ACLF score, CLIF-C OF score and CLIF-SOFA score) were analyzed and compared for 28-, 90- and 180-d overall mortality. The AUROC of CLIF-SOFA was superior to other predictive scores at 28-, 90-, and 180-d mortality, particularly at 28 d. The CLIF-SOFA score provides an overall and efficient evaluation of the severity of multi-organ failure in patients with ACLF by considering various systems, including the hepatic, respiratory, coagulation, circulatory, nervous, and renal systems. Zhang et al[14] and other researchers found that at all times, the CLIF-SOFA scores AUROCs were higher than those of other scores. A study performed by Perdigoto et al[58] showed that when ACLF is present, the CLIF-C OF score has good accuracy and is able to diagnose ACLF. MELD, on the other hand, performed better in terms of 90-d mortality prediction.
The CLIF-C ACLF score is the most accurate way to predict 28-d mortality in patients with ACLF. The CLIF-C AD score was also beneficial in predicting death in cirrhotic individuals with AD who did not meet diagnostic criteria for ACLF, although it did not outperform other well-established prognostication measures[59].
The CANONIC study found that 28-d mortality was 33.9%, while two Brazilian studies found that mortality rates in ACLF patients were 39%[56,60].
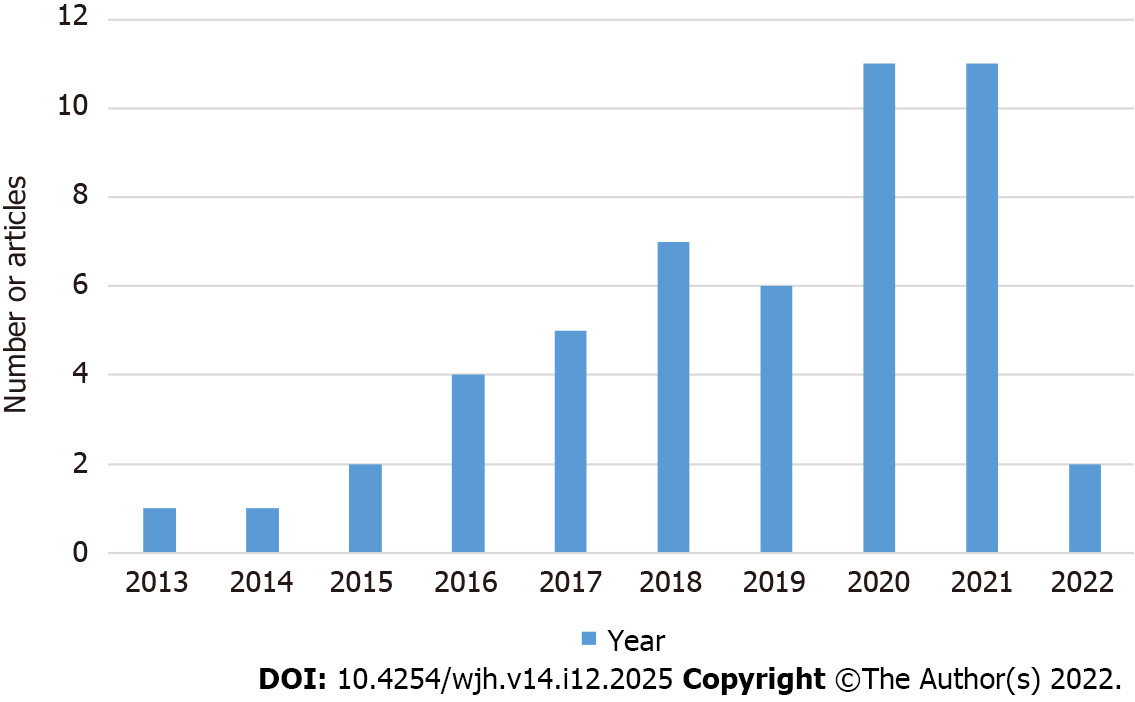
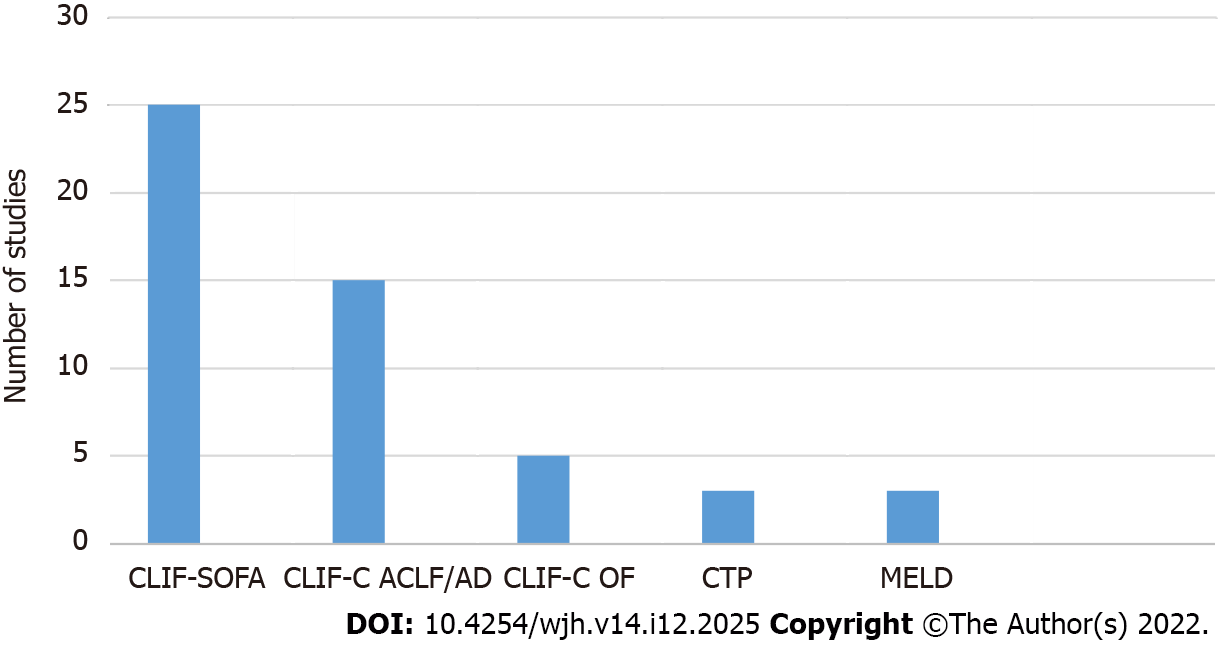
Within the included articles in this study from 2013 to 2022 (Figure 2), CLIF-SOFA was superior to other scores for predicting mortality (mostly in the short-term) in ACLF patients in more than 50% of the included articles, followed by CLIF-C ACLF and CLIF-C AD (30% of the articles)[61-89]. CLIF-C OF was more accurate at 10%. CTP accurately prognosticated ACLF patients with HRS and AOVH patients with AD. The MELD score accurately predicted short-term mortality in ACLF patients who underwent LT (Figure 3).
The CLIF-SOFA score surpasses other predictive models in prognosticating short-term mortality in ACLF patients. CLIF-SOFA, CLIF-C ACLF, and CLIF-C AD are accurate in predicting scores for short-term and long-term mortality in patients with ACLF and in predicting adverse outcomes associated with chronic liver disease.
Acute-on-chronic liver failure is a syndrome characterized by decompensation in individuals with chronic liver disease, and is generally secondary to one or more extra-hepatic organ failures, implying an elevated mortality rate. Acute decompensation is the term used for one or more significant consequences of liver disease in a short time and is the most common reason for hospital admission in cirrhotic patients.
The European Association for the Study of Liver-Chronic-Liver Failure (EASL-CLIF) Group modified the intensive care Sequential Organ Failure Assessment score into CLIF-SOFA, which detects the presence of acute-on-chronic liver failure (ACLF) in patients with or without acute decompensation (AD), classifying it into three grades.
To investigate the role of the EASL-CLIF definition for ACLF and the ability of CLIF-SOFA, CLIF-C ACLF, and CLIF-C AD scores for prognosticating ACLF or AD.
This study is a literature review using a standardized search method, conducted using the steps following the guidelines for reporting systematic reviews set out by the PRISMA statement. Using specific keywords, relevant articles were found by searching PubMed, ScienceDirect, and BioMed Central-BMC. The databases were searched using the search terms by one reviewer (MSc student), and a list of potentially eligible studies was generated based on the titles and abstracts screened.
Most of the included studies used the EASL-CLIF definition for ACLF to identify cirrhotic patients with a significant risk of short-term mortality. The primary outcome in all reviewed studies was mortality. Most of the studies' findings were based on an AUROC analysis, which revealed that the CLIF-SOFA, CLIF-C ACLF, and CLIF-C AD scores were preferable to other models in predicting 28-d mortality. They had the greatest AUROC scores predicting overall mortality at 90, 180, and 365 d. A total of 50 articles were included in this study, which found that the CLIF-SOFA, CLIF-C ACLF, and CLIF-C AD scores could predict short-term and long-term mortality in patients with ACLF or AD in more than 50% of the articles found.
The CLIF-SOFA score surpassed other predictive models in predicting short-term prognosis in ACLF patients. CLIF-SOFA, CLIF-C ACLF, and CLIF-C AD are accurate in predicting scores for short-term and long-term mortality in patients with ACLF and in predicting adverse outcomes associated with chronic liver disease.
Within the included articles in this study from 2013 to 2022, CLIF-SOFA was superior to other scores for predicting mortality (mainly in the short-term) in ACLF patients in more than 50% of the included articles, followed by CLIF-C ACLF and CLIF-C AD (30% of the articles). CLIF-C OF was accurate at 10%. CTP accurately predicted the score for ACLF patients with HRS and AOVH patients with AD. The MELD score accurately predicted short-term mortality in ACLF patients who underwent LT.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta T, India; Narciso-Schiavon JL, Brazil; Wang Y, China S-Editor: Liu JH L-Editor: Webster JR P-Editor: Liu JH
| 1. | Praktiknjo M, Clees C, Pigliacelli A, Fischer S, Jansen C, Lehmann J, Pohlmann A, Lattanzi B, Krabbe VK, Strassburg CP, Arroyo V, Merli M, Meyer C, Trebicka J. Sarcopenia Is Associated With Development of Acute-on-Chronic Liver Failure in Decompensated Liver Cirrhosis Receiving Transjugular Intrahepatic Portosystemic Shunt. Clin Transl Gastroenterol. 2019;10:e00025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 2. | Cai JJ, Wang K, Jiang HQ, Han T. Characteristics, Risk Factors, and Adverse Outcomes of Hyperkalemia in Acute-on-Chronic Liver Failure Patients. Biomed Res Int. 2019;2019:6025726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Wilde B, Katsounas A. Immune Dysfunction and Albumin-Related Immunity in Liver Cirrhosis. Mediators Inflamm. 2019;2019:7537649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Patil V, Jain M, Venkataraman J. Paracentesis-induced acute kidney injury in decompensated cirrhosis - prevalence and predictors. Clin Exp Hepatol. 2019;5:55-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, Angeli P, Porayko M, Moreau R, Garcia-Tsao G, Jimenez W, Planas R, Arroyo V. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003;38:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 611] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 6. | Blei AT, Córdoba J; Practice Parameters Committee of the American College of Gastroenterology. Hepatic Encephalopathy. Am J Gastroenterol. 2001;96:1968-1976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 425] [Article Influence: 17.7] [Reference Citation Analysis (1)] |
| 7. | Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362:823-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 636] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 8. | Arvaniti V, D'Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246-1256, 1256.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 835] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 9. | Gustot T, Durand F, Lebrec D, Vincent JL, Moreau R. Severe sepsis in cirrhosis. Hepatology. 2009;50:2022-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 324] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 10. | Jalan R, Williams R. Acute-on-chronic liver failure: pathophysiological basis of therapeutic options. Blood Purif. 2002;20:252-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 222] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 11. | Olson JC, Kamath PS. Acute-on-chronic liver failure: concept, natural history, and prognosis. Curr Opin Crit Care. 2011;17:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Jalan R, Gines P, Olson JC, Mookerjee RP, Moreau R, Garcia-Tsao G, Arroyo V, Kamath PS. Acute-on chronic liver failure. J Hepatol. 2012;57:1336-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 456] [Article Influence: 35.1] [Reference Citation Analysis (1)] |
| 13. | Zhao RH, Shi Y, Zhao H, Wu W, Sheng JF. Acute-on-chronic liver failure in chronic hepatitis B: an update. Expert Rev Gastroenterol Hepatol. 2018;12:341-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 14. | Zhang Y, Nie Y, Liu L, Zhu X. Assessing the prognostic scores for the prediction of the mortality of patients with acute-on-chronic liver failure: a retrospective study. PeerJ. 2020;8:e9857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2162] [Article Influence: 180.2] [Reference Citation Analysis (5)] |
| 16. | Piano S, Tonon M, Vettore E, Stanco M, Pilutti C, Romano A, Mareso S, Gambino C, Brocca A, Sticca A, Fasolato S, Angeli P. Incidence, predictors and outcomes of acute-on-chronic liver failure in outpatients with cirrhosis. J Hepatol. 2017;67:1177-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 17. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5726] [Article Influence: 110.1] [Reference Citation Analysis (2)] |
| 18. | Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM, Wolfe RA, Krom R; United Network for Organ Sharing Liver Disease Severity Score Committee. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1825] [Cited by in RCA: 1863] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 19. | Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, Edwards E, Therneau TM. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1047] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 20. | Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P, Levesque E, Durand F, Angeli P, Caraceni P, Hopf C, Alessandria C, Rodriguez E, Solis-Muñoz P, Laleman W, Trebicka J, Zeuzem S, Gustot T, Mookerjee R, Elkrief L, Soriano G, Cordoba J, Morando F, Gerbes A, Agarwal B, Samuel D, Bernardi M, Arroyo V; CANONIC study investigators of the EASL-CLIF Consortium. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 730] [Article Influence: 66.4] [Reference Citation Analysis (1)] |
| 21. | Engelmann C, Thomsen KL, Zakeri N, Sheikh M, Agarwal B, Jalan R, Mookerjee RP. Validation of CLIF-C ACLF score to define a threshold for futility of intensive care support for patients with acute-on-chronic liver failure. Crit Care. 2018;22:254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (1)] |
| 22. | Jalan R, Pavesi M, Saliba F, Amorós A, Fernandez J, Holland-Fischer P, Sawhney R, Mookerjee R, Caraceni P, Moreau R, Ginès P, Durand F, Angeli P, Alessandria C, Laleman W, Trebicka J, Samuel D, Zeuzem S, Gustot T, Gerbes AL, Wendon J, Bernardi M, Arroyo V; CANONIC Study Investigators; EASL-CLIF Consortium. The CLIF Consortium Acute Decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J Hepatol. 2015;62:831-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 256] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 23. | European Foundation for the Study of Chronic Liver Failure. Available from: https://www.efclif.com/scientific-activity/score-calculators/clif-c-ad. |
| 24. | Shah NJ, Mousa OY, Syed K, John S. Acute On Chronic Liver Failure. 2022 May 2. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. [PubMed] |
| 25. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 39563] [Article Influence: 9890.8] [Reference Citation Analysis (2)] |
| 26. | Zaccherini G, Weiss E, Moreau R. Acute-on-chronic liver failure: Definitions, pathophysiology and principles of treatment. JHEP Rep. 2021;3:100176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 27. | Cholongitas E, Papatheodoridis GV, Vangeli M, Terreni N, Patch D, Burroughs AK. Systematic review: The model for end-stage liver disease--should it replace Child-Pugh's classification for assessing prognosis in cirrhosis? Aliment Pharmacol Ther. 2005;22:1079-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 292] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 28. | Durand F, Valla D. Assessment of prognosis of cirrhosis. Semin Liver Dis. 2008;28:110-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 218] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 29. | Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T, Benson J, Therneau T, Kremers W, Wiesner R, Kamath P, Klintmalm G. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130:1652-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 546] [Article Influence: 28.7] [Reference Citation Analysis (1)] |
| 30. | Hernaez R, Solà E, Moreau R, Ginès P. Acute-on-chronic liver failure: an update. Gut. 2017;66:541-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 438] [Article Influence: 54.8] [Reference Citation Analysis (1)] |
| 31. | Zhang X, Chen P, Gao H, Hao S, Yang M, Zhao H, Hu J, Ma W, Li L. Bacterial Infection and Predictors of Mortality in Patients with Autoimmune Liver Disease-Associated Acute-On-Chronic Liver Failure. Can J Gastroenterol Hepatol. 2018;2018:5108781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Moreau R, Lebrec D. Acute renal failure in patients with cirrhosis: perspectives in the age of MELD. Hepatology. 2003;37:233-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 166] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 33. | Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361:1279-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 540] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 34. | Antkowiak M, Gabr A, Das A, Ali R, Kulik L, Ganger D, Moore C, Abecassis M, Katariya N, Mouli S, Mahalingam D, Lewandowski RJ, Salem R, Riaz A. Prognostic Role of Albumin, Bilirubin, and ALBI Scores: Analysis of 1000 Patients with Hepatocellular Carcinoma Undergoing Radioembolization. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 35. | Liu LX, Zhang Y, Nie Y, Zhu X. Assessing the Prediction Effect of Various Prognosis Model for 28-Day Mortality in Acute-on-Chronic Liver Failure Patients. Risk Manag Healthc Policy. 2020;13:3155-3163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 36. | Li H, Chen LY, Zhang NN, Li ST, Zeng B, Pavesi M, Amorós À, Mookerjee RP, Xia Q, Xue F, Ma X, Hua J, Sheng L, Qiu DK, Xie Q, Foster GR, Dusheiko G, Moreau R, Gines P, Arroyo V, Jalan R. Characteristics, Diagnosis and Prognosis of Acute-on-Chronic Liver Failure in Cirrhosis Associated to Hepatitis B. Sci Rep. 2016;6:25487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 37. | Li J, Liang X, You S, Feng T, Zhou X, Zhu B, Luo J, Xin J, Jiang J, Shi D, Lu Y, Ren K, Wu T, Yang L, Li J, Li T, Cai Q, Sun S, Guo B, Chen J, He L, Li P, Yang H, Hu W, An Z, Jin X, Tian J, Wang B, Chen X, Xin S; Chinese Group on the Study of Severe Hepatitis B (COSSH). Development and validation of a new prognostic score for hepatitis B virus-related acute-on-chronic liver failure. J Hepatol. 2021;75:1104-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 38. | Chen BH, Tseng HJ, Chen WT, Chen PC, Ho YP, Huang CH, Lin CY. Comparing Eight Prognostic Scores in Predicting Mortality of Patients with Acute-On-Chronic Liver Failure Who Were Admitted to an ICU: A Single-Center Experience. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Terres AZ, Balbinot RS, Muscope ALF, Longen ML, Schena B, Cini BT, Luis Rost G Jr, Balensiefer JIL, Eberhardt LZ, Balbinot RA, Balbinot SS, Soldera J. Evidence-based protocol for diagnosis and treatment of hepatorenal syndrome is independently associated with lower mortality. Gastroenterol Hepatol. 2022;45:25-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Terres AZ, Balbinot RS, Muscope ALF, Eberhardt LZ, Balensiefer JIL, Cini BT, Rost Jr. GL, Longen ML, Schena B, Balbinot RA, Balbinot SS, Soldera J. Predicting mortality for cirrhotic patients with acute oesophageal variceal haemorrhage using liver-specific scores. GastroHep. 2021;3:236-246. [DOI] [Full Text] |
| 41. | Jacques ROC, Massignan LS, Winkler MS, Balbinot RS, Balbinot RA, Balbinot SS, Solder J. Liver-specific scores as predictors of mortality in spontaneous bacterial peritonitis. GastroHep. 2020;. [DOI] [Full Text] |
| 42. | Kim TY, Song DS, Kim HY, Sinn DH, Yoon EL, Kim CW, Jung YK, Suk KT, Lee SS, Lee CH, Kim TH, Kim JH, Choe WH, Yim HJ, Kim SE, Baik SK, Lee BS, Jang JY, Suh J 3rd, Kim HS, Nam SW, Kwon HC, Kim YS, Kim SG, Chae HB, Yang JM, Sohn JH, Lee HJ, Park SH, Han BH, Choi EH, Kim CH, Kim DJ; Korean Acute-on-Chronic Liver Failure Study Group. Characteristics and Discrepancies in Acute-on-Chronic Liver Failure: Need for a Unified Definition. PLoS One. 2016;11:e0146745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 43. | Angeli P, Rodríguez E, Piano S, Ariza X, Morando F, Solà E, Romano A, García E, Pavesi M, Risso A, Gerbes A, Willars C, Bernardi M, Arroyo V, Ginès P; CANONIC Study Investigators of EASL-CLIF Consortium. Acute kidney injury and acute-on-chronic liver failure classifications in prognosis assessment of patients with acute decompensation of cirrhosis. Gut. 2015;64:1616-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 44. | Gupta T, Dhiman RK, Rathi S, Agrawal S, Duseja A, Taneja S, Chawla Y. Impact of Hepatic and Extrahepatic Insults on the Outcome of Acute-on-Chronic Liver Failure. J Clin Exp Hepatol. 2017;7:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 45. | Niewiński G, Morawiec S, Janik MK, Grąt M, Graczyńska A, Zieniewicz K, Raszeja-Wyszomirska J. Acute-On-Chronic Liver Failure: The Role of Prognostic Scores in a Single-Center Experience. Med Sci Monit. 2020;26:e922121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 46. | McPhail MJ, Shawcross DL, Abeles RD, Chang A, Patel V, Lee GH, Abdulla M, Sizer E, Willars C, Auzinger G, Bernal W, Wendon JA. Increased Survival for Patients With Cirrhosis and Organ Failure in Liver Intensive Care and Validation of the Chronic Liver Failure-Sequential Organ Failure Scoring System. Clin Gastroenterol Hepatol. 2015;13:1353-1360.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 47. | Mendizabal M, Ridruejo E, Piñero F, Anders M, Padilla M, Toro LG, Torre A, Montes P, Urzúa A, Gonzalez Ballerga E, Silveyra MD, Michelato D, Díaz J, Peralta M, Pages J, García SR, Gutierrez Lozano I, Macias Y, Cocozzella D, Chavez-Tapia N, Tagle M, Dominguez A, Varón A, Vera Pozo E, Higuera-de la Tijera F, Bustios C, Conte D, Escajadillo N, Gómez AJ, Tenorio L, Castillo Barradas M, Schinoni MI, Bessone F, Contreras F, Nazal L, Sanchez A, García M, Brutti J, Cabrera MC, Miranda-Zazueta G, Rojas G, Cattaneo M, Castro-Narro G, Rubinstein F, Silva MO. Comparison of different prognostic scores for patients with cirrhosis hospitalized with SARS-CoV-2 infection. Ann Hepatol. 2021;25:100350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 48. | Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6591] [Cited by in RCA: 7739] [Article Influence: 266.9] [Reference Citation Analysis (1)] |
| 49. | Chirapongsathorn S, Teerasarntipan T, Tipchaichatta K, Suttichaimongkol T, Chamroonkul N, Bunchorntavakul C, Siramolpiwat S, Chainuvati S, Sobhonslidsuk A, Leerapun A, Piratvisuth T, Sukeepaisarnjaroen W, Tanwandee T, Treeprasertsuk S. Acute-on-chronic liver failure: Epidemiology, prognosis, and outcome of a multicenter study in Thai population. JGH Open. 2022;6:205-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Lee M, Lee JH, Oh S, Jang Y, Lee W, Lee HJ, Yoo JJ, Choi WM, Cho YY, Cho Y, Lee DH, Lee YB, Yu SJ, Yi NJ, Lee KW, Kim YJ, Yoon JH, Suh KS, Lee HS. CLIF-SOFA scoring system accurately predicts short-term mortality in acutely decompensated patients with alcoholic cirrhosis: a retrospective analysis. Liver Int. 2015;35:46-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 51. | Deo SV, Al-Kindi SG, Altarabsheh SE, Hang D, Kumar S, Ginwalla MB, ElAmm CA, Sareyyupoglu B, Medalion B, Oliveira GH, Park SJ. Model for end-stage liver disease excluding international normalized ratio (MELD-XI) score predicts heart transplant outcomes: Evidence from the registry of the United Network for Organ Sharing. J Heart Lung Transplant. 2016;35:222-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 52. | Yang M, Peng B, Zhuang Q, Li J, Liu H, Cheng K, Ming Y. Models to predict the short-term survival of acute-on-chronic liver failure patients following liver transplantation. BMC Gastroenterol. 2022;22:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 53. | Klein KB, Stafinski TD, Menon D. Predicting survival after liver transplantation based on pre-transplant MELD score: a systematic review of the literature. PLoS One. 2013;8:e80661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 54. | Sy E, Ronco JJ, Searle R, Karvellas CJ. Prognostication of critically ill patients with acute-on-chronic liver failure using the Chronic Liver Failure-Sequential Organ Failure Assessment: A Canadian retrospective study. J Crit Care. 2016;36:234-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Kulkarni S, Sharma M, Rao PN, Gupta R, Reddy DN. Acute on Chronic Liver Failure-In-Hospital Predictors of Mortality in ICU. J Clin Exp Hepatol. 2018;8:144-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 56. | Grochot RM, Luz LB, Garcia R, Balbinot RA, Balbinot SS, Soldera J. CLIF-SOFA is superior to other liver-specific scores for predicting mortality in acute-on-chronic liver failure and decompensated cirrhosis. Austin. J Gastroenterol. 2019;6:1105. |
| 57. | Maipang K, Potranun P, Chainuvati S, Nimanong S, Chotiyaputta W, Tanwandee T, Charatcharoenwitthaya P. Validation of the prognostic models in acute-on-chronic liver failure precipitated by hepatic and extrahepatic insults. PLoS One. 2019;14:e0219516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 58. | Perdigoto DN, Figueiredo P, Tomé L. The Role of the CLIF-C OF and the 2016 MELD in Prognosis of Cirrhosis with and without Acute-on-Chronic Liver Failure. Ann Hepatol. 2019;18:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | Picon RV, Bertol FS, Tovo CV, de Mattos ÂZ. Chronic liver failure-consortium acute-on-chronic liver failure and acute decompensation scores predict mortality in Brazilian cirrhotic patients. World J Gastroenterol. 2017;23:5237-5245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 60. | Silva PE, Fayad L, Lazzarotto C, Ronsoni MF, Bazzo ML, Colombo BS, Dantas-Correa EB, Narciso-Schiavon JL, Schiavon LL. Single-centre validation of the EASL-CLIF consortium definition of acute-on-chronic liver failure and CLIF-SOFA for prediction of mortality in cirrhosis. Liver Int. 2015;35:1516-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 61. | Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1-85. [PubMed] |
| 62. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3671] [Article Influence: 153.0] [Reference Citation Analysis (0)] |
| 63. | MdCalc. MELD Score (Model For End-Stage Liver Disease) (12 and older). Available from: https://www.mdcalc.com/meld-score-model-end-stage-liver-disease-12-older#evidence. |
| 64. | Jeong JH, Park IS, Kim DH, Kim SC, Kang C, Lee SH, Kim TY, Lee SB. CLIF-SOFA score and SIRS are independent prognostic factors in patients with hepatic encephalopathy due to alcoholic liver cirrhosis. Medicine (Baltimore). 2016;95:e3935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 65. | Kuo CC, Huang CH, Chang C, Chen PC, Chen BH, Chen WT, Ho YP. Comparing CLIF-C ACLF, CLIF-C ACLFlactate, and CLIF-C ACLF-D Prognostic Scores in Acute-on-Chronic Liver Failure Patients by a Single-Center ICU Experience. J Pers Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Li N, Huang C, Yu KK, Lu Q, Shi GF, Zheng JM. Validation of prognostic scores to predict short-term mortality in patients with HBV-related acute-on-chronic liver failure: The CLIF-C OF is superior to MELD, CLIF SOFA, and CLIF-C ACLF. Medicine (Baltimore). 2017;96:e6802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 67. | Dong X, He J, Chen W, Su R, Xu Y, Sheng X, Li L, Cao H. Characteristics and outcomes of acute-on-chronic liver failure patients with or without cirrhosis using two criteria. Sci Rep. 2020;10:8577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 68. | Grochot RM, Luz LB, Garcia R, Balbinot RA, Balbinot SS, Soldera J. Acute-on-chronic liver failure data from a teaching hospital in Brazil. A Historical Cohort. 2020; Available from: https://www.worldwidejournals.com/international. |
| 69. | Jacques ROC, Massignan LDS, Winkler MS, Balbinot RS, Balbinot SS, Soldera J. Acute-on-chronic liver failure is independently associated with lower survival in patients with spontaneous bacterial peritonitis. Arq Gastroenterol. 2021;58:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Reference Citation Analysis (0)] |
| 70. | Barosa R, Roque Ramos L, Patita M, Nunes G, Fonseca J. CLIF-C ACLF score is a better mortality predictor than MELD, MELD-Na and CTP in patients with Acute on chronic liver failure admitted to the ward. Rev Esp Enferm Dig. 2017;109:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 71. | Ferreira Cardoso M, Alexandrino G, Carvalho E Branco J, Anapaz V, Carvalho R, Horta D, Martins A. The impact and evolution of acute-on-chronic liver failure in decompensated cirrhosis: A Portuguese single-center study. Gastroenterol Hepatol. 2019;42:296-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 72. | Shin J, Yu JH, Jin YJ, Yim HJ, Jung YK, Yang JM, Song DS, Kim YS, Kim SG, Kim DJ, Suk KT, Yoon EL, Lee SS, Kim CW, Kim HY, Jang JY, Jeong SW. Acute-on-chronic liver failure as a major predictive factor for mortality in patients with variceal bleeding. Clin Mol Hepatol. 2020;26:540-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 73. | Gao J, Zhang Q, Wu Y, Li Y, Qi T, Zhu C, Liu S, Yu R, He Q, Wen W, Zhou F, Chen Y, Chen J, Hou J. Improving survival of acute-on-chronic liver failure patients complicated with invasive pulmonary aspergillosis. Sci Rep. 2018;8:876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 74. | Chen L, Zhang J, Lu T, Cai J, Zheng J, Yao J, Yi S, Li H, Chen G, Zhao H, Zhang Y, Yang Y. A nomogram to predict survival in patients with acute-on-chronic hepatitis B liver failure after liver transplantation. Ann Transl Med. 2021;9:555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 75. | Yu Z, Zhang Y, Cao Y, Xu M, You S, Chen Y, Zhu B, Kong M, Song F, Xin S, Duan Z, Han T. A dynamic prediction model for prognosis of acute-on-chronic liver failure based on the trend of clinical indicators. Sci Rep. 2021;11:1810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 76. | Zhang Q, Li Y, Han T, Nie C, Cai J, Liu H, Liu Y. Comparison of current diagnostic criteria for acute-on-chronic liver failure. PLoS One. 2015;10:e0122158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 77. | Li H, Zheng J, Chen L, Cai J, Zhang M, Wang G. The scoring systems in predicting short-term outcomes in patients with hepatitis B virus-related acute-on-chronic liver failure. Ann Palliat Med. 2020;9:3048-3058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 78. | Costa E Silva PP, Codes L, Rios FF, Esteve CP, Valverde Filho MT, Lima DOC, de Almeida Filho GF, Morais MCA, Lima BC, Chagas PBO, Boa-Sorte N, Bittencourt PL. Comparison of General and Liver-Specific Prognostic Scores in Their Ability to Predict Mortality in Cirrhotic Patients Admitted to the Intensive Care Unit. Can J Gastroenterol Hepatol. 2021;2021:9953106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 79. | Sheng XY, Lin FY, Wu J, Cao HC. Development and validation of a prognostic model for patients with hepatorenal syndrome: A retrospective cohort study. World J Gastroenterol. 2021;27:2615-2629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 80. | Hong YS, Sinn DH, Gwak GY, Cho J, Kang D, Paik YH, Choi MS, Lee JH, Koh KC, Paik SW. Characteristics and outcomes of chronic liver disease patients with acute deteriorated liver function by severity of underlying liver disease. World J Gastroenterol. 2016;22:3785-3792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 81. | Marciano S, Mauro E, Dirchwolf M, Debernardi ME, Giunta D, Pagotto V, Rojas L, Gadano A. A Dynamic Definition of Acute Kidney Injury Does not Improve Prognosis Assessment in Acutely Decompensated Patients with Cirrhosis. J Clin Exp Hepatol. 2017;7:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 82. | Xu L, Ying S, Hu J, Wang Y, Yang M, Ge T, Huang C, Xu Q, Zhu H, Chen Z, Ma W. Pneumonia in patients with cirrhosis: risk factors associated with mortality and predictive value of prognostic models. Respir Res. 2018;19:242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 83. | Silva P, Rios F, Codes L, Esteve C, Filho M, Lima D, Filho G, Lima B, Chagas P, Morais M, Bittencourt P. Accuracy of prognostic scores in prediction of mortality in cirrhotic patients admitted to the intensive care unit. 2021. [DOI] [Full Text] |
| 84. | Ramzan M, Iqbal A, Murtaza HG, Javed N, Rasheed G, Bano K. Comparison of CLIF-C ACLF Score and MELD Score in Predicting ICU Mortality in Patients with Acute-On-Chronic Liver Failure. Cureus. 2020;12:e7087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 85. | Verma N, Dhiman RK, Singh V, Duseja A, Taneja S, Choudhury A, Sharma MK, Eapen CE, Devarbhavi H, Al Mahtab M, Shukla A, Hamid SS, Jafri W, Butt AS, Ning Q, Chen T, Tan SS, Lesmana LA, Lesmana CRA, Sahu MK, Hu J, Lee GH, Sood A, Midha V, Goyal O, Ghazinian H, Kim DJ, Treeprasertsuk S, Mohan Prasad VG, Dokmeci AK, Sollano JD, Shah S, Payawal DA, Rao PN, Kulkarni A, Lau GK, Duan Z, Chen Y, Yokosuka O, Abbas Z, Karim F, Chowdhury D, Prasad AS, Sarin SK; APASL ACLF Working Party. Comparative accuracy of prognostic models for short-term mortality in acute-on-chronic liver failure patients: CAP-ACLF. Hepatol Int. 2021;15:753-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 86. | Dhiman RK, Agrawal S, Gupta T, Duseja A, Chawla Y. Chronic Liver Failure-Sequential Organ Failure Assessment is better than the Asia-Pacific Association for the Study of Liver criteria for defining acute-on-chronic liver failure and predicting outcome. World J Gastroenterol. 2014;20:14934-14941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 87. | Safi W, Elnegouly M, Schellnegger R, Umgelter K, Geisler F, Reindl W, Saugel B, Hapfelmeier A, Umgelter A. Infection and Predictors of Outcome of Cirrhotic Patients after Emergency Care Hospital Admission. Ann Hepatol. 2018;17:948-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 88. | Leão GS, Lunardi FL, Picon RV, Tovo CV, de Mattos AA, de Mattos ÂZ. Acute-on-chronic liver failure: A comparison of three different diagnostic criteria. Ann Hepatol. 2019;18:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 89. | Bartoletti M, Giannella M, Lewis R, Caraceni P, Tedeschi S, Paul M, Schramm C, Bruns T, Merli M, Cobos-Trigueros N, Seminari E, Retamar P, Muñoz P, Tumbarello M, Burra P, Torrani Cerenzia M, Barsic B, Calbo E, Maraolo AE, Petrosillo N, Galan-Ladero MA, D'Offizi G, Bar Sinai N, Rodríguez-Baño J, Verucchi G, Bernardi M, Viale P; ESGBIS/BICHROME Study Group. A prospective multicentre study of the epidemiology and outcomes of bloodstream infection in cirrhotic patients. Clin Microbiol Infect. 2018;24:546.e1-546.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |