Published online Dec 27, 2022. doi: 10.4254/wjh.v14.i12.2012
Peer-review started: July 3, 2022
First decision: September 30, 2022
Revised: October 21, 2022
Accepted: December 21, 2022
Article in press: December 21, 2022
Published online: December 27, 2022
Processing time: 172 Days and 20 Hours
Coronavirus disease (COVID-19) patients exhibit different patterns of liver impairment, according to growing evidence.
In this study, we sought to provide a comprehensive analysis of liver test para
We performed a meta-analysis of published liver manifestations and described the liver damage in COVID-19. We searched PubMed, Google Scholar, Embase, Cochrane Library, medRxiv, bioRxiv, and three Chinese electronic databases through April 18, 2020, in accordance with the Preferred Reporting Items for Meta-Analyses. We analyzed pooled data on liver chemistries stratified by COVID-19 severity using a fixed or random-effects model.
A meta-analysis of 56 studies, including 11052 patients, found that the pooled mean alanine aminotransferase (ALT) in severe COVID-19 cases was 35.9 IU/L whereas in non-severe COVID-19 cases was 27.3 IU/L. Average aspa
Severe COVID-19 was more likely to be associated with abnormal liver test results. Monitoring liver chemistry closely can help detect disease progression early.
Core Tip: Data on abnormal liver chemistries related to coronavirus disease (COVID-19) are cumulating but are potentially confusing. We performed a meta-analysis of 56 studies that included a total of 11052 patients with COVID-19. We noted that patients with abnormal liver test results are at higher risk of progression to severe disease and close monitoring of liver chemistries provides early warning against disease progression.
- Citation: Dong X, Zeng DY, Xing QQ, Hong MZ, Pan JS. Liver chemistries in severe or non-severe cases of COVID-19: A systematic review and meta-analysis. World J Hepatol 2022; 14(12): 2012-2024
- URL: https://www.wjgnet.com/1948-5182/full/v14/i12/2012.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i12.2012
According to World Health Organization, as of April 18, 2020, 2160207 coronavirus disease (COVID-19) cases were confirmed globally, of which 146088 led to deaths[1]. Although effectively controlled in mainland China, COVID-19 has spread and risen dramatically in most other countries. Similarly, the other two previously identified coronaviruses, namely severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East Respiratory Syndrome-CoV, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes severe viral pneumonia in humans. As no specific acquired immunity exists in the general population, SARS-CoV-2 has high infectivity, which has resulted in an ongoing global health crisis.
Apart from the respiratory system, gastrointestinal tract, the urinary system, and even the central nervous system are the probable target organs of SARS-CoV-2, which utilizes the angiotensin-converting enzyme 2 (ACE2) receptors located in the respiratory and gastrointestinal tracts as the entry point for epithelial cells[2]. Among patients’ common complaints related to COVID-19 are gastr
Furthermore, there are differences in liver chemistry between patients with severe and non-severe COVID-19 based on cumulative observations. Although liver manifestations of COVID-19 pose an immense diagnostic challenge to clinicians when treating patients with symptoms related to COVID-19, these are potentially useful for recognizing severe cases of COVID-19 in the early stage.
Considering the diverse clinical manifestations and increasing number of reported COVID-19 cases, a systematic summary of the liver manifestations of COVID-19 is urgently needed. Liver chemistries generally consist of hepatocellular injury-related indexes, including ALT and AST; cholestatic injury-related indexes, comprised of alkaline phosphatase (ALP) and GGT; and hepatocellular function-related indexes such as albumin (ALB) level and prothrombin time (PT)[13]. In general, international standardized ratio (INR), TBIL, direct bilirubin (DBIL), and globulin (GLB) levels, and are also assessed in clinical practice. However, there are few observations to comprehensively analyze liver chemistries in patients with COVID-19 patients. We therefore aimed to provide a comprehensive overview of liver test parameters in patients with severe and non-severe COVID-19. It is possible to develop more effective therapies and holistic approaches to care with a better understanding of the disease.
The following databases were searched from December 1, 2019, through April 18, 2020: PubMed, Google Scholar, Embase, Cochrane Library, medRxiv, bioRxiv, and three Chinese electronic databases (CQVIP, Wanfang Data, and Chinese National Knowledge Infrastructure). “Coronavirus,” “COVID-19,” “2019-nCoV-2,” “SARS-CoV-2,” or novel coronavirus were used as search keywords. Potential studies were retrieved in accordance with the Systematic reviews and Meta-Analyses guideline[14]. Details of the database search are listed in the Supplementary file. The retrieved articles were imported to Endnote X9.3 (Thompson and Reuters, Philadelphia, Pennsylvania), and duplicates were removed. The Reference Citation Analysis had be used to further improve the manuscript content when revised the manuscript (https://www.referencecitationanalysis.com/).
The eligibility of the potential studies was determined independently by two authors (XD and DYZ), and dissonance was arbitrated by the third author (JSP). The inclusion criteria were as follows: (1) Study population: adult COVID-19 patients; (2) study design: case series, case report, prospective cohort study, retrospective cohort study, case-control study, and randomized controlled trial; and (3) language: Studies published in English or Chinese. The exclusion criteria were as follows: (1) Pediatric patients or pregnant women; (2) patients without nucleic acid data or serology evidence of SARS-CoV2 infection; (3) asymptomatic patients with SARS-CoV2 infection; and (3) study design: Review article, meta-analysis, editorial, or commentary. Studies that only reported the percentages of the indexes related to liver chemistries rather than the mean or median values of the corresponding indexes were also excluded.
For the eligible articles, we recorded the following items: first author, study location, sample size, patient age and sex, and liver chemistry-related indexes such as ALT, AST, TBIL, DBIL, GGT, ALP, and ALB levels. The severity of COVID-19 was also recorded. Severe disease was defined according to the American Thoracic Society and Infectious Disease Society of America guidelines for community-acquired pneumonia, and the guidelines for diagnosis and management of COVID-19 released by National Health Commission of China, need of intensive care unit admission, mechanical ventilation[15,16].
The statistical analyses were performed using the R version 3.2.3 statistical software (R Foundation for Statistical Computing). The continuous variables that showed a normal distribution were expressed as mean ± SD, while those that conformed to a skewed distribution were expressed as median [interquartile range (IQR)]. For the studies that provided summary data of median, minimum, and maximum values, we used the method developed by Luo et al[17] to estimate the sample mean and SD for the continuous outcomes. The online tool used is provided at http://www.math.hkbu.edu.hk/~tongt/papers/median2mean.html. The 95% confidence interval (CI) was presented as a Forest plot. The Cochran Q test was used to detect the heterogeneity among studies, with a p value of < 0.10 indicating significant heterogeneity. The I2 statistics was calculated to measure the proportion of total variation among the studies to which the heterogeneity was attributed. I2 values of < 25%, 25%-75%, and > 75% represent low, moderate, and high heterogeneity, respectively[18]. Publication bias was evaluated using a funnel plot. A subgroup analysis was performed according to disease severity.
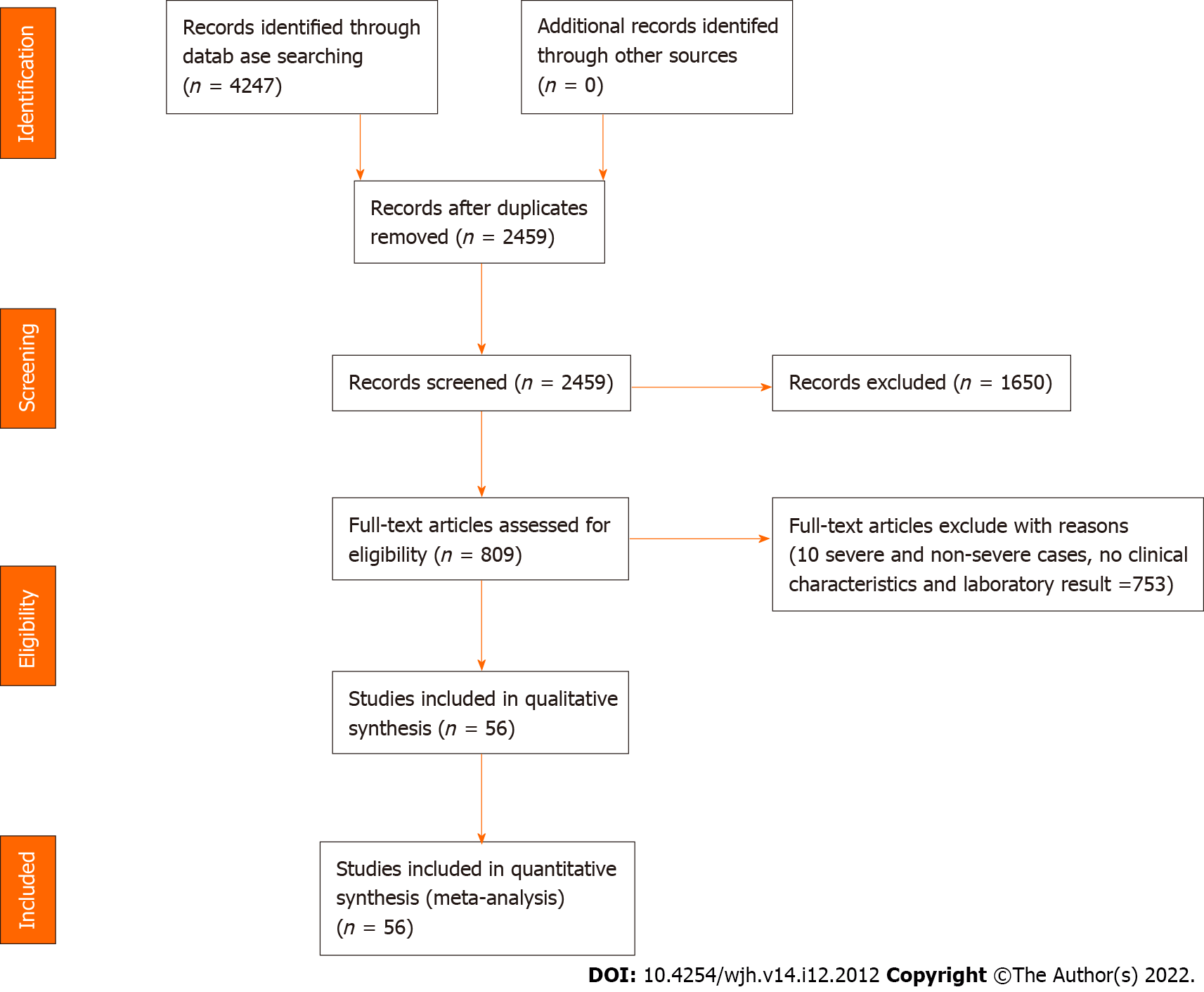
Screening process of the potential studies was shown in Figure 1. The meta-analysis consisted 56 studies, whose characteristics were listed in Supplementary Table 1. Information, including the study location, sample size, patient age and sex, disease severity, TBIL, DBIL, ALB, GLB, ALT, AST, GGT, ALP, INR, and PT levels, was recorded. The mean ages of patients with non-severe and severe COVID-19 were 50.1 and 63.2 years, respectively (Supplementary Figure 1). Male patients accounted for 50.7% in the enrolled studies. Among the studies that reported disease severity, severe disease accounted for 25.3% of the cases.
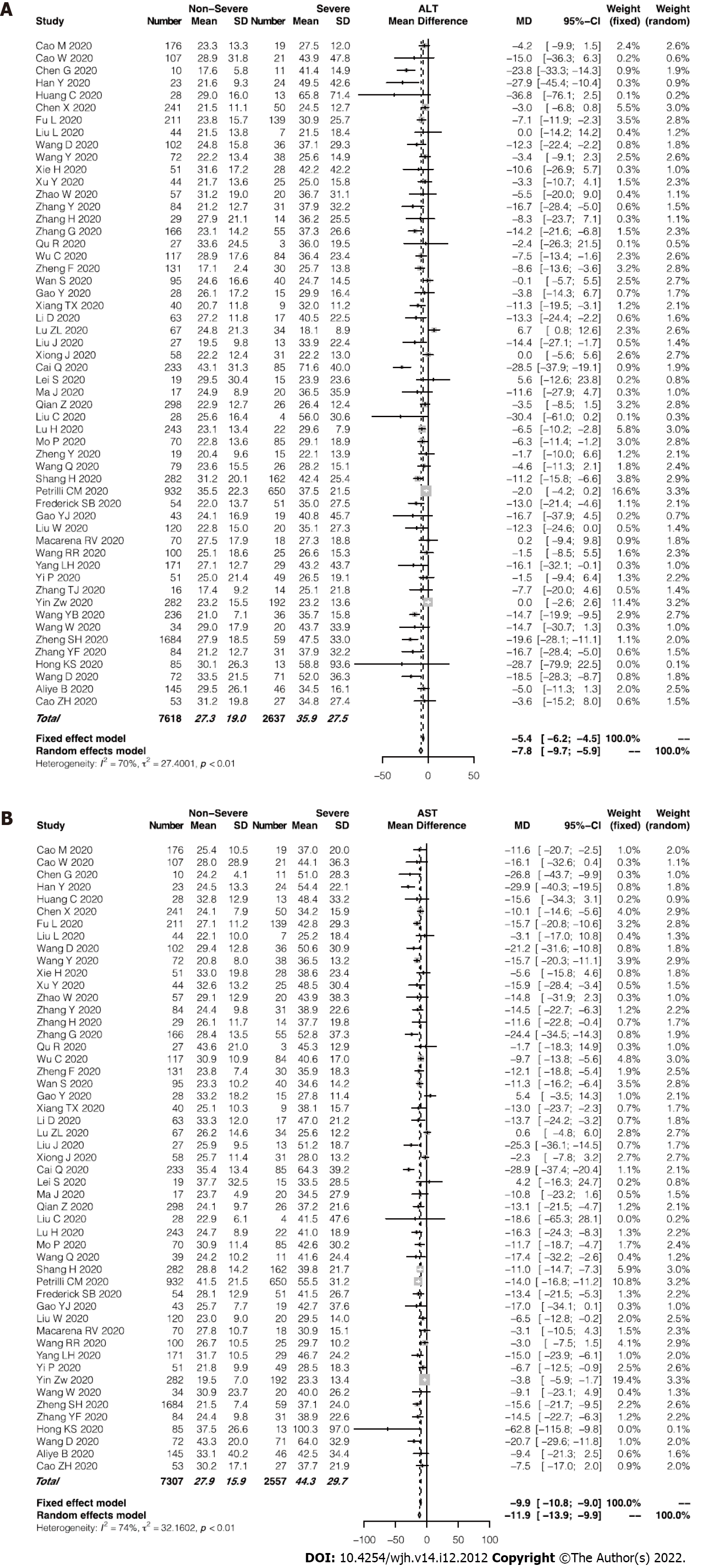
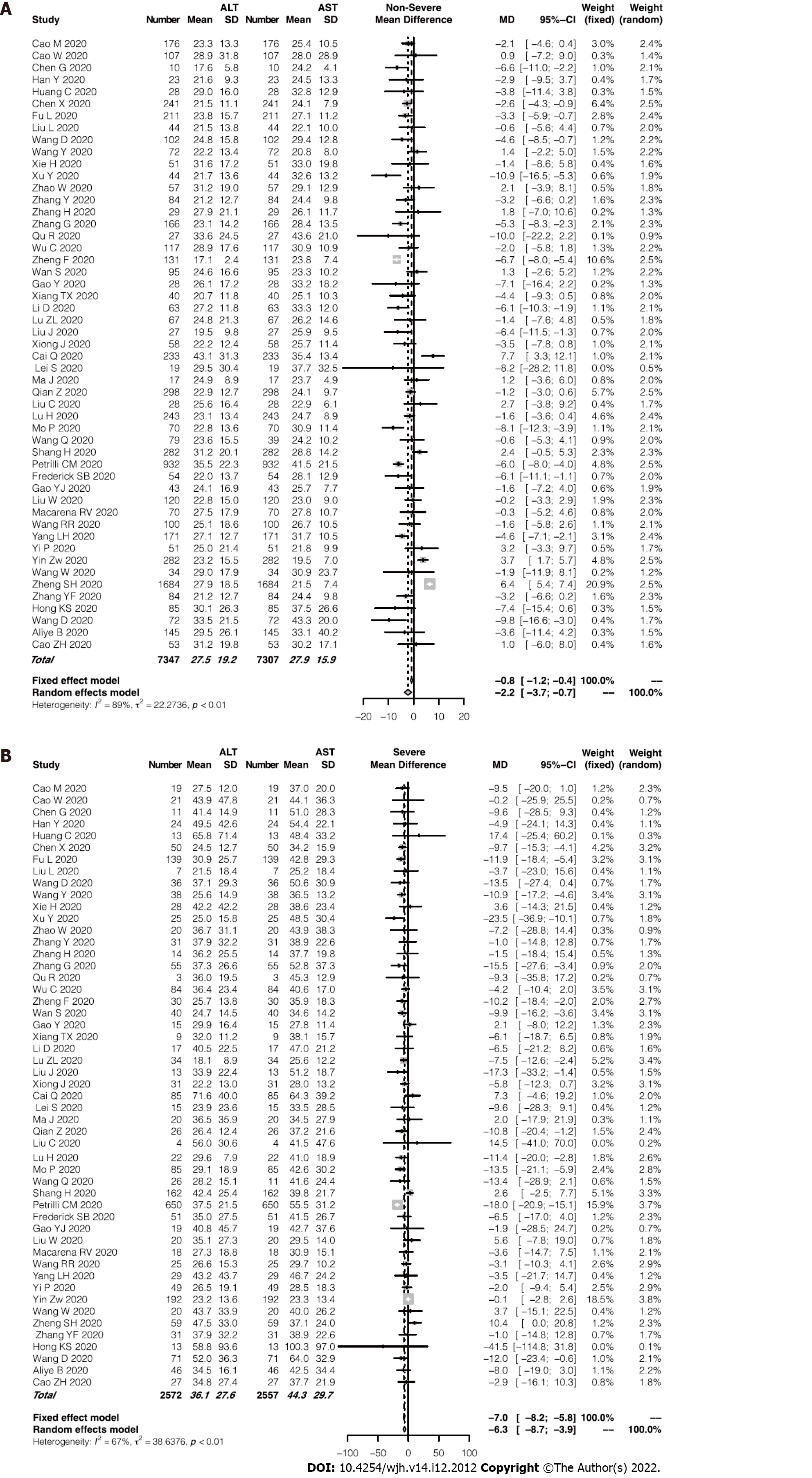
Of the enrolled studies, 56 reported assays of ALT or AST in a total of 6235 patients with COVID-19. The pooled mean ALT level was 35.9 IU/L in the patients with severe COVID-19 and 27.3 IU/L in the patients with non-severe COVID-19 (95%CI: -9.7 to -5.9, P < 0.0001; Figure 2A), with significant heterogeneity among the studies (I2 = 70%, P < 0.01). Similarly, the pooled mean AST level was 44.3 IU/L in the severe cases and 27.9 IU/L in the non-severe cases (95%CI: -13.9 to -9.9, P < 0.0001; Figure 2B). Among the studies, significant heterogeneity for the AST levels was observed (I2 = 74%, P < 0.01). Using a funnel plot, potential publication bias was evaluated (Supplementary Figure 2). Average AST level tended to be higher than average ALT level in both the severe and non-severe groups. Furthermore, the severe group showed an even greater difference between levels of AST and ALT (44.3 and 36.1 IU/L, respectively; Figure 3). Supplementary Figure 3 presented the evaluation of publication bias.
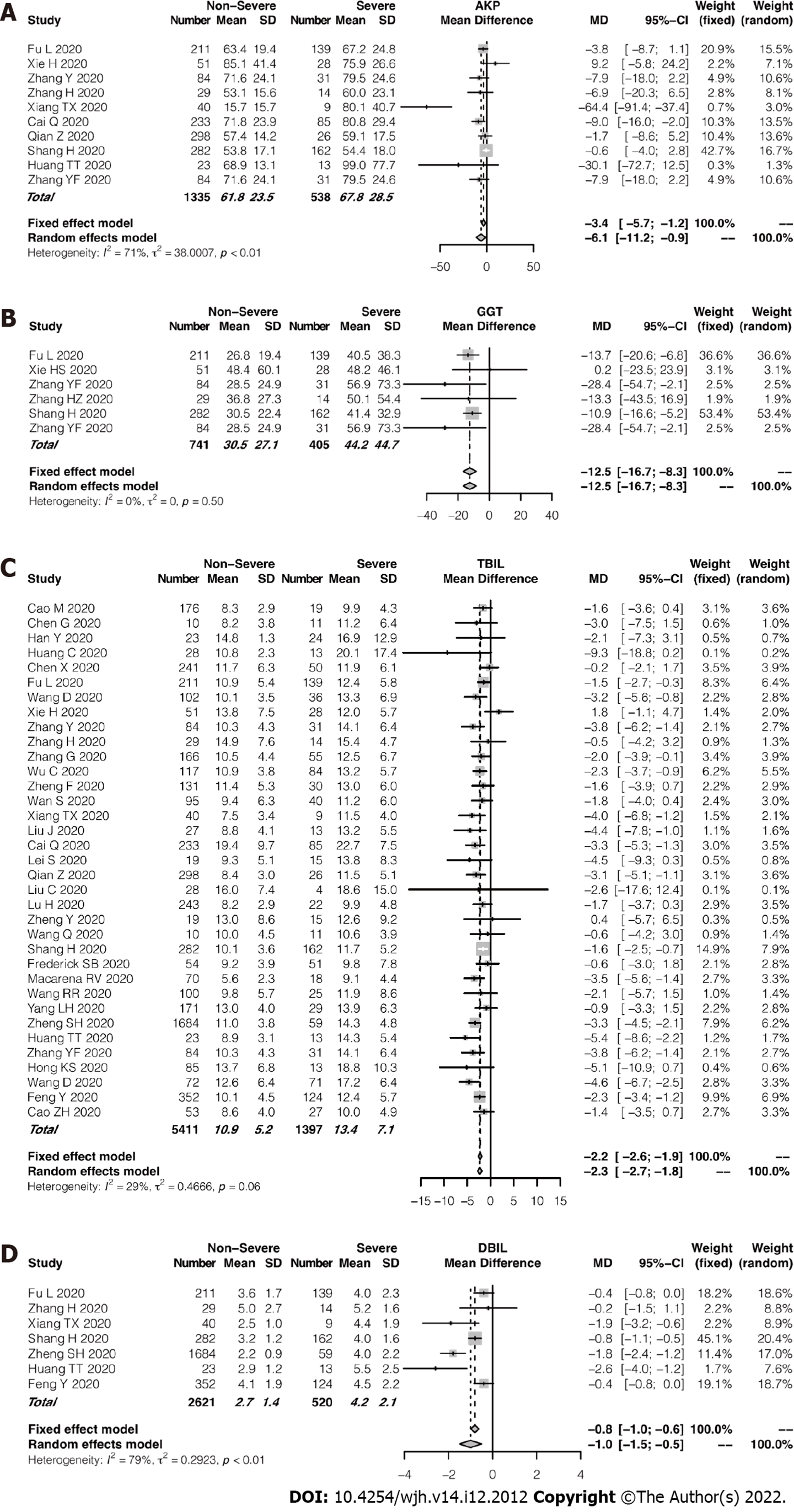
Compared with the studies that frequently reported ALT and AST levels, cholestasis-related indexes such as ALP, GGT, and DBIL levels were presented in rather fewer studies. Among the enrolled studies, 10 reported ALP assays and 6 studies reported GGT measurements. The pooled mean ALP level was 67.8 IU/L in the patients with severe COVID-19 and 61.8 IU/L in those with non-severe COVID-19 (95%CI: -11.2 to 0.9, P = 0.02; Figure 4A). Figure 4B showed that the pooled mean GGT level was 44.2 IU/L in the severe group while 30.5 IU/L in the non-severe group. As compared with the non-severe group, the severe group had a slightly higher pooled mean TBIL level. However, TBIL levels remained within normal ranges in both groups (Figure 4C). Even fewer studies reported DBIL values in patients with COVID-19. In fact, no significant difference in mean DBIL level was found between the 2 groups (Figure 4D). In terms of TBIL levels, the studies showed low heterogeneity (I2 = 29%, P = 0.06). Supplementary Figure 4 showed a funnel plot of TBIL levels.
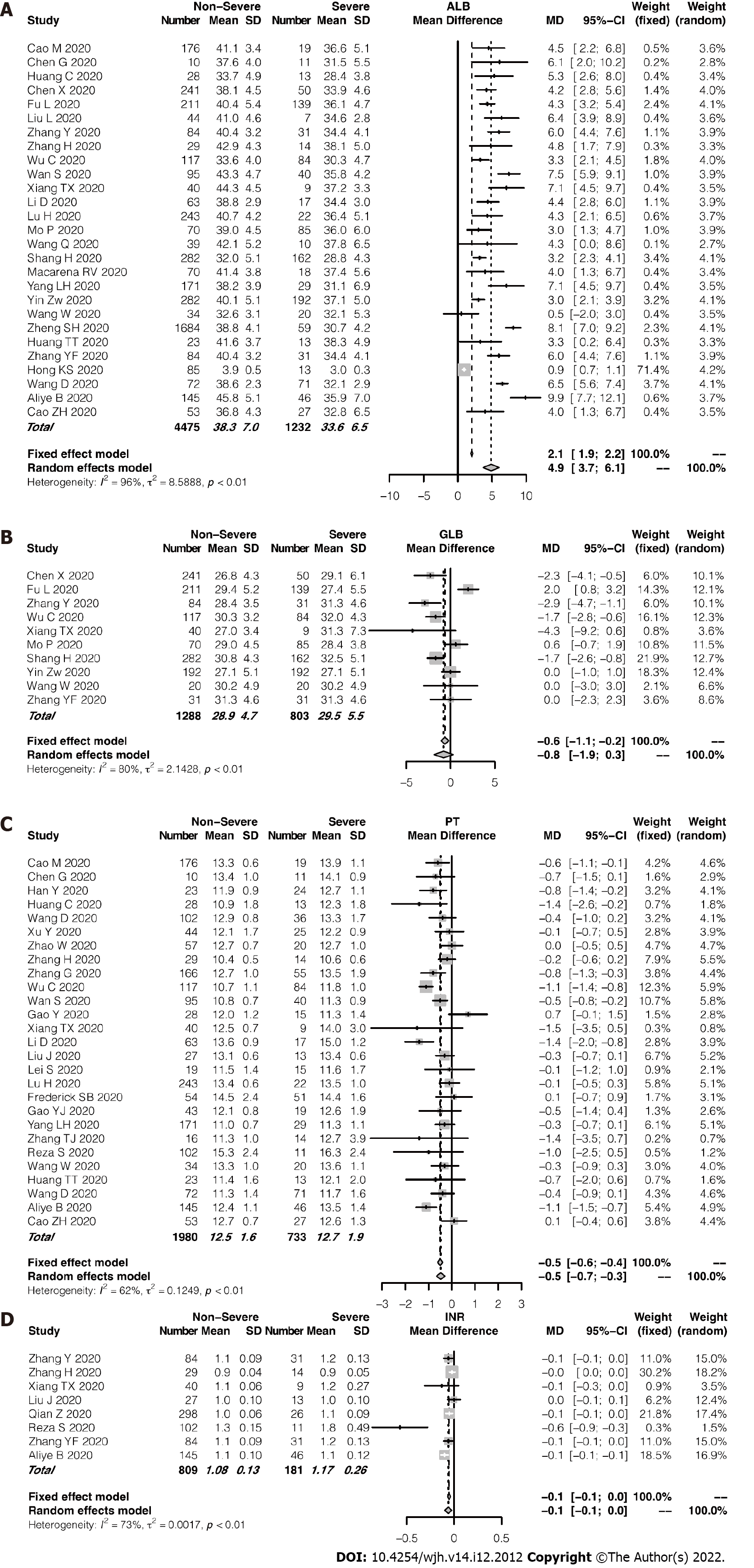
27 studies compared the mean ALB levels according to COVID-19 severity, between 1232 and 4475 severe and non-severe cases, respectively (Figure 5A). Across the studies, a significant heterogeneity was observed (I2 = 96%, P < 0.01). Average ALB level in the patients with severe disease was significantly lower than that in the patients with non-severe disease. No significant difference in GLB level was found between the groups (P = 0.14; Figure 5B). However, PT and INR, the coagulation-related indexes, showed no significant differences were found between the severity groups. The patients in the severe group tended to have longer PT or higher INR (Figure 5C and D). An evaluation of publication bias in relation to ALB level and PT is shown in Supplementary Figure 5. Supplem
In this meta-analysis, 56 studies that consisted a total of 11052 patients with COVID-19 from China, United States, Chile, Iran and South Korea were enrolled. According to the pooled analysis, hepatocellular injury, hepatocellular dysfunction, and cholestasis, three patterns of liver impairment, can develop in quite a part of patients with COVID-19 at variable severity. In brief, the patients with severe COVID-19 tended to have higher ALT/AST, ALP/GGT, and TBIL levels; higher INR; and prolonged PT. However, the severe cases had lower ALB levels than the non-severe cases. Particularly in severe cases of COVID-19, the AST levels were often higher than the ALT levels. We also observed a tendency of the severe cases to arise in the elderly.
Although the liver may act as the latent target of SARS-CoV-2, the actual prevalence of abnormal liver chemistries could be underestimated since many studies did not report cholestasis-related indexes such as ALP and GGT levels, and synthetic function-related indexes such as ALB level and INR. Moreover, most studies reported ALT/AST levels on the day of admission while not the entire disease course. This issue further compromises the role of liver chemistries in disease monitoring and provides an early warning against severe cases. As SARS-CoV-2 can lead to bile duct damage by conquering the ACE2 expressed on cholangiocytes and induce a subsequent cholestatic liver injury[8], cholestasis-related abnormalities could be overlooked.
Cumulating studies have linked abnormal liver chemistries to the severity of COVID-19[9]. It is more likely that patients with abnormal liver test results will progress to severe cases[9]. In fact, coronavirus infection can cause direct damage to liver cells[11]. Moreover, several underlying diseases, comorbidities, and complications that develop in the course of the disease, such as sepsis and multiple-organ failure, and drugs that can cause potential liver damage also increase the risk of liver injury. Lopinavir/ritonavir use during hospitalization has been reported to possible lead to liver damage[9,19]. The liver chemistry tests in the enrolled studies were all performed on admission, which suggests that the influences of the drugs on the liver tests, if any, should be minor.
ALB level and PT are known to reflect hepatocellular function. Albumin, which has a circulating half-life of 3 weeks, is a plasma protein exclusively synthesized by the liver[20]. Hypoalbuminemia results from and reflects the inflammatory state, which leads to inflammatory exudate. Effective nutrition support helps to correct hypoalbuminemia[21]. Our meta-analysis revealed that ALB level was lower in the severe cases than in the non-severe cases, which indicated that the severe cases tended to have more intense inflammation and require more solid nutrition support. PT is a far more sensitive measure of hepatocellular function than ALB level because PT may be prolonged in patients with severe liver disease duration of < 24 h[13]. In accordance with the alteration of the ALB level, PT was prolonged in the severe cases, which further indicated impairment of hepatocellular function in the severe cases[20].
According to our meta-analysis, another interesting feature of liver impairment related to COVID-19 is that the AST level often overrides the ALT level, especially in severe cases. By contrast, in patients with chronic hepatitis B or nonalcoholic fatty liver disease, the ALT level is generally higher than the AST level. While ALT is primarily present in the liver and is a more specific indicator for hepatocellular injury, the distribution of AST is far wider than that of ALT, including the cardiac muscle, skeletal muscle, kidney, and brain[13]. An elevated AST level accompanied by a normal ALT level often suggests cardiac or muscle disease. In fact, cardiac injury is frequent in severe cases of COVID-19, especially in deceased patients[22,23].
This study has some substantial merits. First, acomprehensive review of COVID-19 literature, which is rapidly developing and sometimes confusing, was presented in this meta-analysis regarding the manifestation of liver chemistries. The extensive coverage of 37 studies allowed a more precise evaluation of the abnormalities of liver chemistries. Our subgroup analysis revealed that the abnormal liver chemistries were associated with a more severe disease course. It is imperative that liver chemistries should be monitored more closely for diagnostic and prognostic purposes.
Second, this analysis extensively covered hepatocellular injury, hepatocellular dysfunction, and cholestasis, three patterns of liver impairment. Most observations focused on ALT, AST, and ALB levels. However, cholestasis-related impairment (e.g., abnormal ALP and GGT levels) tended to be inadvertently ignored. Moreover, we also compared hepatocellular dysfunction between the severe and non-severe cases. The alarmingly high prevalence of hypoalbuminemia in the severe cases prompts further nutrition support in severe cases. In addition, coagulation dysfunction in severe cases requires vigilance. Third, the enrolled studies included multiple observations not only from mainland China but also from other ethnic groups. This facilitates the assessment of abnormal liver chemistries related to COVID-19 in a broader ethnic context. Fourth, eligible studies preprinted in medRxiv and bioRxiv were also covered. As a result, our analysis has a clear leading position. However, our study has a few limitations. As mentioned earlier, cholestasis-related indexes such as ALP/GGT level may be under-reported in quite a number of studies, which may lead to less precise pooled data. Second, most studies were conducted in mainland China. It was difficult to determine if liver chemistry was abnormal in other ethnic groups. Most of the studies that came from mainland China seem to have an adverse impact. On the contrary, this helps to abate the heterogeneity caused by the disease grouping, as some potential discrepancies may exist in the definition of severe and non-severe cases of COVID-19 between different countries.
In this meta-analysis, we comprehensively described hepatocellular injury, hepatocellular dysfunction, cholestasis, three patterns of liver impairment, related to COVID-19. Severe COVID-19 was more likely to be associated with abnormal liver test results. A close monitoring of liver chemistries can provide an early warning of disease progression.
According to the World Health Organization released situation report, many of people were confirmed coronavirus disease (COVID-19) globally.
Severe COVID-19 was more likely to be associated with abnormal liver test results.
A close monitoring of liver chemistries can provide an early warning of disease progression.
We used 56 studies, which included a total of 11052 patients for Meta-Analyses to explored the difference of liver chemistries from severe cases of COVID-19 to non-severe cases.
This article showed that severe cases of COVID-19 tended to have higher alanine aminotransferase or aspartate aminotransferase, alkaline phosphatase/γ-glutamyltransferase, and total bilirubin levels; prolonged prothrombin time; and higher international standardized ratio. However, the severe cases had lower albumin levels than the non-severe cases.
Severe COVID-19 was more likely to be associated with abnormal liver test results.
In the future, more targeted therapies and holistic care approaches may be developed as a result of better knowledge.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Kitamura K, Japan; Okasha H, Egypt; Papalexis PG, Greece; Reddy NNR, India S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report–89. World Health Organization, 19 April 2020. |
| 2. | Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol. 2020;94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3085] [Cited by in RCA: 2915] [Article Influence: 583.0] [Reference Citation Analysis (0)] |
| 3. | Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, Ng YY, Chu MY, Chung TWH, Tam AR, Yip CCY, Leung KH, Fung AY, Zhang RR, Lin Y, Cheng HM, Zhang AJX, To KKW, Chan KH, Yuen KY, Leung WK. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology. 2020;159:81-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1113] [Cited by in RCA: 1132] [Article Influence: 226.4] [Reference Citation Analysis (1)] |
| 4. | Cholankeril G, Podboy A, Aivaliotis VI, Tarlow B, Pham EA, Spencer SP, Kim D, Hsing A, Ahmed A. High Prevalence of Concurrent Gastrointestinal Manifestations in Patients With Severe Acute Respiratory Syndrome Coronavirus 2: Early Experience From California. Gastroenterology. 2020;159:775-777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 165] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 5. | Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, Hao SR, Jia HY, Cai H, Zhang XL, Yu GD, Xu KJ, Wang XY, Gu JQ, Zhang SY, Ye CY, Jin CL, Lu YF, Yu X, Yu XP, Huang JR, Xu KL, Ni Q, Yu CB, Zhu B, Li YT, Liu J, Zhao H, Zhang X, Yu L, Guo YZ, Su JW, Tao JJ, Lang GJ, Wu XX, Wu WR, Qv TT, Xiang DR, Yi P, Shi D, Chen Y, Ren Y, Qiu YQ, Li LJ, Sheng J, Yang Y. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002-1009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 944] [Cited by in RCA: 870] [Article Influence: 174.0] [Reference Citation Analysis (0)] |
| 6. | Lin L, Jiang X, Zhang Z, Huang S, Fang Z, Gu Z, Gao L, Shi H, Mai L, Liu Y, Lin X, Lai R, Yan Z, Li X, Shan H. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997-1001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 630] [Cited by in RCA: 658] [Article Influence: 131.6] [Reference Citation Analysis (0)] |
| 7. | Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158:1831-1833.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1995] [Article Influence: 399.0] [Reference Citation Analysis (1)] |
| 8. | Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J, Cai J, Lan F. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. bioRxiv 2020: 2020.2002.2003.931766. [DOI] [Full Text] |
| 9. | Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 661] [Article Influence: 132.2] [Reference Citation Analysis (0)] |
| 10. | Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 357] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 11. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1295] [Article Influence: 259.0] [Reference Citation Analysis (4)] |
| 12. | Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 310] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 13. | Kwo PY, Cohen SM, Lim JK. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am J Gastroenterol. 2017;112:18-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 716] [Article Influence: 89.5] [Reference Citation Analysis (0)] |
| 14. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13353] [Article Influence: 834.6] [Reference Citation Analysis (0)] |
| 15. | Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, Cooley LA, Dean NC, Fine MJ, Flanders SA, Griffin MR, Metersky ML, Musher DM, Restrepo MI, Whitney CG. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1279] [Cited by in RCA: 2186] [Article Influence: 437.2] [Reference Citation Analysis (36)] |
| 16. | National Health Commission of the People's Republic of China Handbook of Prevention and Treatment of the Pneumonia Caused by the Novel Coronavirus (2019-nCoV) (in Chinese) 2020. February 6, 2020. Available from: http://en.nhc.gov.cn/2020-02/06/c_76295.htm. |
| 17. | Luo D, Wan X, Liu J, Tong T. How to estimate the sample mean and standard deviation from the sample size, median, extremes or quartiles? Zhongguo Xunzheng Yixue Zazhi. 2017;17:1350-1356. [DOI] [Full Text] |
| 18. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 25800] [Article Influence: 1121.7] [Reference Citation Analysis (0)] |
| 19. | Meraviglia P, Schiavini M, Castagna A, Viganò P, Bini T, Landonio S, Danise A, Moioli MC, Angeli E, Bongiovanni M, Hasson H, Duca P, Cargnel A. Lopinavir/ritonavir treatment in HIV antiretroviral-experienced patients: evaluation of risk factors for liver enzyme elevation. HIV Med. 2004;5:334-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. II. Recommendations for use of laboratory tests in screening, diagnosis, and monitoring. Clin Chem. 2000;46:2050-2068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 234] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 21. | Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: Pathogenesis and Clinical Significance. JPEN J Parenter Enteral Nutr. 2019;43:181-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 708] [Article Influence: 101.1] [Reference Citation Analysis (0)] |
| 22. | Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2289] [Cited by in RCA: 2550] [Article Influence: 510.0] [Reference Citation Analysis (2)] |
| 23. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30117] [Article Influence: 6023.4] [Reference Citation Analysis (3)] |