Published online Nov 27, 2022. doi: 10.4254/wjh.v14.i11.1977
Peer-review started: May 21, 2022
First decision: July 25, 2022
Revised: August 8, 2022
Accepted: October 27, 2022
Article in press: October 27, 2022
Published online: November 27, 2022
Processing time: 186 Days and 17.2 Hours
Hepatic infarctions (HI) are ischemic events of the liver in which a disruption in the blood flow to the hepatocytes leads to focal ischemia and necrosis. Most HI are due to occlusive events in the liver’s blood vessels, but non-occlusive HI may occur. They are associated with disruption of microvasculature, such as in diabetic ketoacidosis. While HI usually presents as peripheral lesions with clear borders, irregular nodular lesions may occur, indistinguishable from liver neoplasms and presenting a diagnostic challenge.
We report a case of multiple extensive HI in a patient with poorly controlled diabetes mellitus, who first presented to the emergency room with diabetic ketoacidosis. He then developed jaundice, thrombocytopenia, and a marked elevation of serum aminotransferases. An ultrasound of the liver showed the presence of multiple irregular lesions. Further investigation with a computerized tomography scan confirmed the presence of multiple hypoattenuating nodules with irregular borders and heterogeneous appearance. These lesions were considered highly suggestive of a primary neoplasm of the liver. While the patient was clinically stable, his bilirubin levels remained persistently elevated, and he underwent an ultrasound-guided percutaneous biopsy of the largest lesion. Biopsy results revealed extensive ischemic necrosis of hepatocytes, with no signs of associated malignancy. Three months after the symptoms, the patient showed great improvement in all clinical and laboratory parameters and extensive regression of the lesions on imaging exams.
This case highlights that diabetic ketoacidosis can cause non-occlusive HI, possibly presenting as nodular lesions indistinguishable from neoplasms.
Core Tip: Hepatic infarction (HI) is usually caused by occlusion of the blood vessels supplying the liver. Non-occlusive HI secondary to diabetic ketoacidosis is an exceedingly rare occurrence, with few cases described in the literature. We report a case of HI secondary to diabetic ketoacidosis, whose diagnosis was complicated by the atypical aspect of the infarction areas on the imaging exams. The appearance of multiple irregular and heterogenous nodules was suggestive of metastatic liver neoplasm, and correct diagnosis could only be obtained by biopsy. This case demonstrates a rare cause of HI, and highlights the diagnostic challenges posed by its atypical presentations.
- Citation: Gomes VMDS, Ferreira GSA, Barros LCTR, Santos BMRTD, Vieira LPB. Multiple hepatic infarctions secondary to diabetic ketoacidosis: A case report. World J Hepatol 2022; 14(11): 1977-1984
- URL: https://www.wjgnet.com/1948-5182/full/v14/i11/1977.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i11.1977
Hepatic infarctions (HI) are ischemic events of the liver in which a disruption in the blood flow to the hepatocytes leads to focal ischemia, necrosis, and, in severe cases, hepatocellular dysfunction[1]. Due to the dual blood supply that the liver receives from the hepatic artery and the portal vein, HI occurs less commonly than infarctions in other abdominal organs[2]. Most HI is a consequence of occlusive events in either blood vessels supplying the liver. Common causes are portal vein thrombosis, hepatic artery thrombosis, trauma, pancreatitis, surgery (liver transplantation in particular), or hilarious neoplasms[1,3-5]. However, non-occlusive HI may rarely occur[3,4,6]. These uncommon events are associated with disruption of the liver microvasculature and can be secondary to rheumatologic diseases (polyarteritis nodosa, scleroderma, systemic lupus erythematosus, Churg-Strauss syndrome), infection, polycythemia vera, hemodynamic shock, and severe preeclampsia, among other causes[6].
Diabetic ketoacidosis (DK) has been described as a potential cause of non-occlusive HI in a limited number of cases reported in the medical literature[3,6-8]. The pathophysiology of HI in patients with DK is not completely understood but is thought to be multifactorial. Elevated levels of catecholamines released in DK might induce vasoconstriction and liver ischemia[3]. Dehydration and hypotension often present in DK decrease blood flow to the liver, further contributing to ischemia[3]. The low levels of 2, 3-diphosphoglycerate in patients with DK may affect hepatocyte oxygenation, and widespread atherosclerosis, endothelial dysfunction, and hypercoagulability–that are commonly found in patients with diabetes–can also play a role in the occurrence of HI[3,6,7]. Abdominal pain, nausea, jaundice, and fever are the most common symptoms of HI[3,6]. Transaminase levels are elevated, and hyperbilirubinemia, leukocytosis, and disorders of hemostasis are also frequent findings in HI[3,6,8].
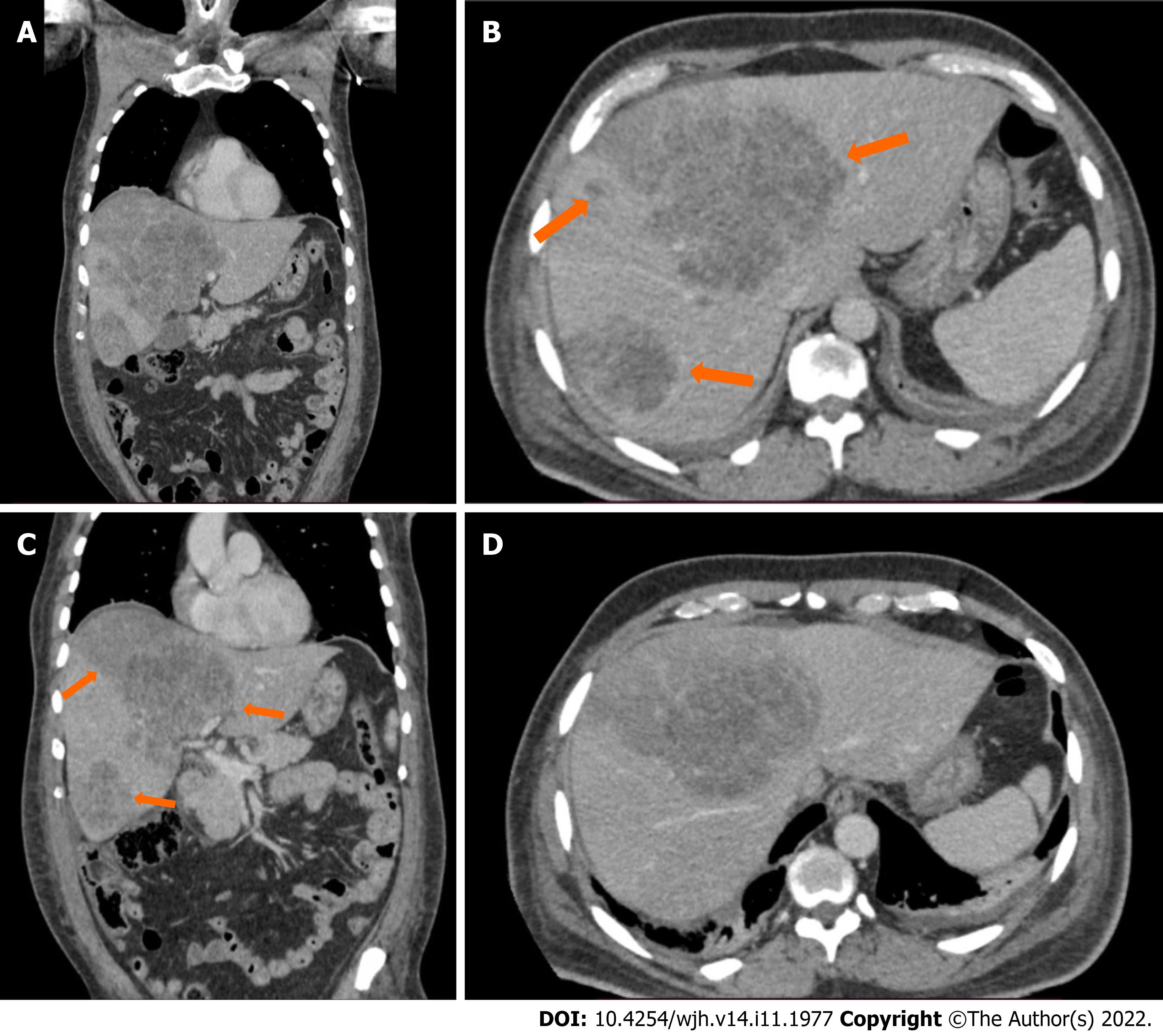
An ultrasound of the liver with doppler evaluation of the hepatic vessels showed multiple heterogeneous nodular lesions in both lobes, with no signs of the hepatic artery or portal vein thrombosis. He then underwent a computerized abdominal tomography (CT) scan on the same date, which revealed the presence of multiple heterogenous lesions in both lobes of the liver, which were hypoattenuating with slight peripheral enhancement in the late phase of the study (Figure 1A and B). Of note, there was a clear wedge-shaped delineation between affected parenchyma and normal areas in the periphery of the liver (Figure 1C and D). The largest lesions were located on liver segments IV and VI, measuring 127 mm and 95 mm, respectively. Based on the imaging exams, primary metastatic neoplasm of the liver (most likely cholangiocarcinoma) or multiple liver abscesses were considered the most likely diagnoses. However, given the lack of clinical and laboratory markers of infection and the sudden onset of symptoms associated with elevation of transaminases, HI was also considered a differential diagnosis. The patient was discharged from the intensive care unit (ICU) 6 d after admission. A control CT scan was obtained 10 days after admission, with no difference in the aspect of the liver lesions but an additional finding of subsegmental pulmonary thromboembolism in the right lung. Anticoagulation with therapeutical doses of enoxaparin was initiated while the patient remained asymptomatic. A magnetic resonance imaging (MRI) scan 16 d after admission showed the same irregular nodular lesions, with a slight peripheral enhancement of the lesions by the contrast medium (gadolinium). As the patient remained clinically well but with significant cholestasis, the decision was made to perform an ultrasound-guided liver biopsy to determine the lesions’ definitive diagnosis, which was made 20 days after patient admission.
A 57-year-old male patient was transferred to the ICU of a tertiary hospital due to a diagnosis of DK with hemodynamical instability. He had first presented to an emergency medical service complaining of diffuse abdominal pain.
Blood and urine exams obtained at arrival at the emergency department (Table 1) showed marked ketonuria, hyperglycemia (470 mg/dL), acidosis (pH of 7.27 and bicarbonate of 15 mEq/L), the elevation of aminotransferases [aspartate aminotransferase (AST) of 2356 U/L and alanine aminotransferase (ALT) of 2438 U/L], and thrombocytopenia (9380 platelets/mcL). At admission to the ICU, there was a decrease in aminotransferase levels (AST of 1121 U/L and ALT of 1546 U/L) but an increase in bilirubin levels (total bilirubin of 1.59 mg/dL). A serological panel for viral hepatitis, dengue fever, and yellow fever yielded negative results. While the patient remained hemodynamically stable, he developed jaundice as his bilirubin levels steadily increased, and he underwent an abdominal ultrasound 2 d after admission.
| Variable | Reference range | Admission on the emergency room | Admission on the ICU | One week after admission | Two weeks after admission | Three weeks after admission | Three months after admission |
| Hemoglobin (g/dL) | 13-16.9 | 13.8 | 13.6 | 11.8 | 9.8 | 9.5 | 11.2 |
| Leukocytes (leukocytes/mm3) | 4000-10200 | 4580 | 4353 | 14350 | 9615 | 8172 | 5414 |
| Platelets (platelets/mm3) | 140000-400000 | 9380 | 15000 | 110000 | 262000 | 306000 | 290000 |
| Glucose (mg/dL) | 70-99 | 470 | 425 | ||||
| AST (U/L) | 5-40 | 2356 | 805 | 135 | 149 | 108 | |
| ALT (U/L) | 7-56 | 2438 | 1489 | 56 | 56 | 58 | |
| Bilirubin: total /direct (mg/dL) | 0-1.2/0-0.3 | 1.24/0.91 | 2.5/2.2 | 10.2/8.3 | 12.5/10.6 | 10.1/8.9 | 3.6/1.4 |
| Alkaline phosphatase (U/L) | 40-150 | 168 | 193 | 376 | 407 | ||
| Gamma-glutamyl transferase (U/L) | 7-45 | 178 | 770 | 1665 | |||
| Creatinine (mg/dL) | 0.7-1.3 | 1.6 | 1.3 | 1.1 | 0.9 | 1.2 | |
| Arterial blood pH | 7.36-7.44 | 7.27 | 7.43 | 7.42 | |||
| Arterial blood bicarbonate (mEq/L) | 22-28 | 15 | 16.7 | 18 | |||
| Lactate (mmol/L) | 0.5-2.2 | 4.4 | 2 | ||||
| Albumin (g/dL) | 3.4-5.4 | 2.3 | 1.7 | 3.4 | |||
| International normalized ratio | 0.8-1.1 | 1.41 | 1.37 | 1.09 | |||
| Carcinoembryonic antigen (ng/mL) | 0-2.5 | 1 | |||||
| Cancer antigen 19-9 (U/mL) | 0-37 | 4 | |||||
| Alpha-fetoprotein (ng/mL) | 1.3-7.8 | 8 |
On arrival at the ICU, physical examination was unremarkable, except for light tenderness on deep palpation of the right upper quadrant during the abdominal exam. Vital signs were within the normal range of values, and the patient was afebrile.
The patient suffered from hypertension and poorly controlled diabetes mellitus, with irregular use of metformin. He had a previous history of smoking tobacco but was abstinent for more than 20 years and ingested small amounts of alcohol once per week.
At the moment of his arrival in the emergency room, the patient was noticed to be tachycardic and hypotensive. He was placed in close monitoring and was diagnosed with monomorphic ventricular tachycardia, being subject to successful synchronized electrical cardioversion, improving his hemodynamical condition. Treatment with ceftriaxone was started and intravenous insulin, as he had significant hyperglycemia (470 mg/dL). After this procedure, he was transferred to the ICU of a tertiary hospital for stabilization and further investigation.
The patient complained of diffuse abdominal pain that had started 2 d prior and progressively worsened, associated with malaise, asthenia, nausea, and vomiting.
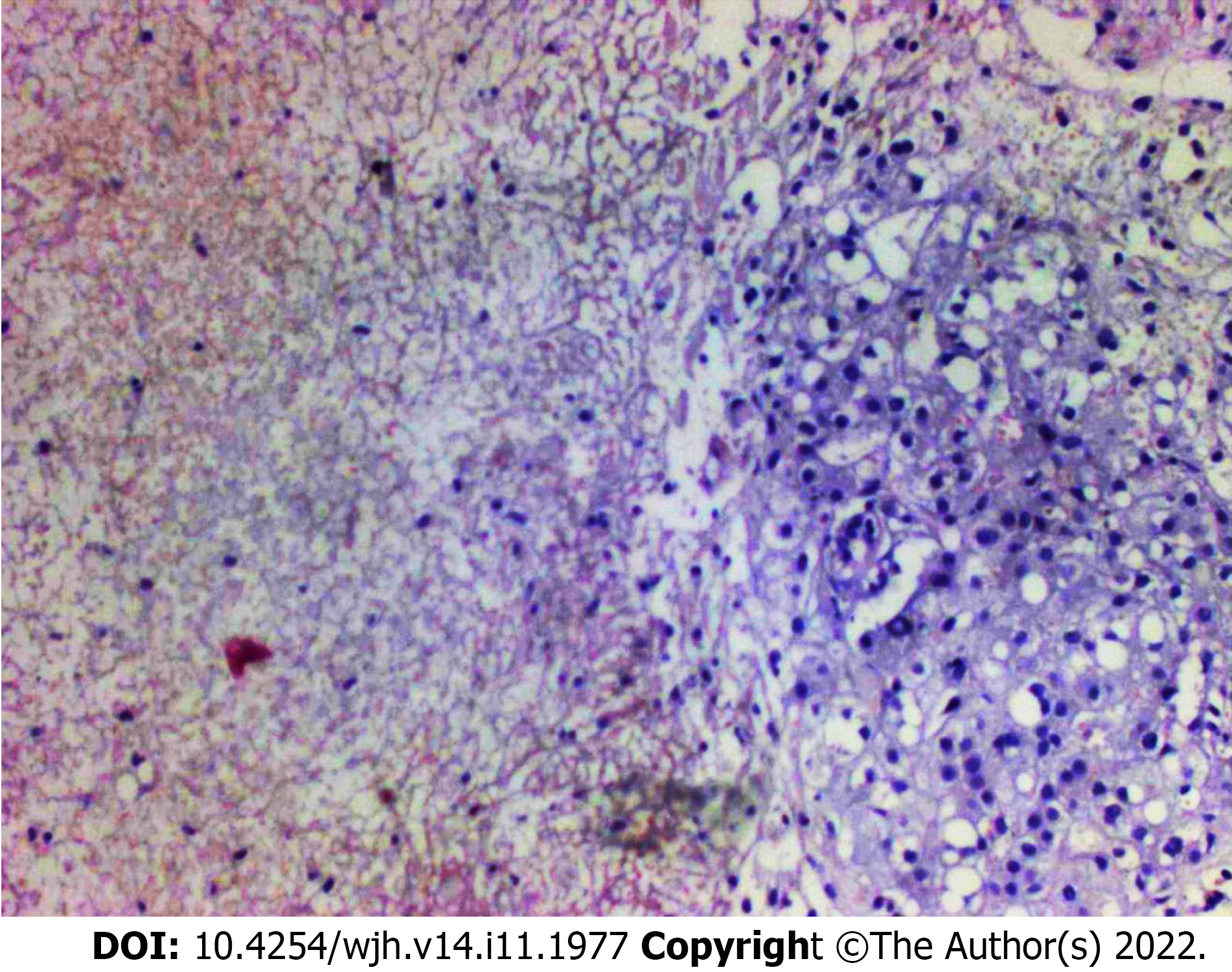
Histology of the liver biopsy showed extensive mononuclear infiltration of the liver, associated with intracellular cholestasis, and areas of ischemic necrosis, with no signs of associated malignancy (Figure 2). Tissue cultures obtained at the same moment showed no signs of bacterial growth. These results confirmed the diagnosis of non-occlusive HI, secondary to DK.
The patient was discharged from the hospital 21 days after admission, with optimized control of diabetes and anticoagulation with oral rivaroxaban.
While the patient still had significant cholestasis at the moment of discharge, his jaundice began to improve 1 mo after the onset of the symptoms, and bilirubin levels returned to normal after another month. The patient remains asymptomatic and well during two months of outpatient follow-up, and an ultrasound scan obtained 3 mo after the onset of the symptoms revealed small, focal areas of heterogeneity on the right lobe of the liver, measuring no more than 4 cm, therefore showing significant regression of the lesions.
Non-occlusive HI secondary to DK is a rare occurrence, with a small number of cases reported in the literature (Table 2). Its correct diagnosis depends on a high index of clinical suspicion during the evaluation of diabetic patients presenting with abdominal pain and elevation of aminotransferases. While imaging exams can usually correctly determine the presence of HI, atypical presentations may pose a diagnostic challenge. Prolonged hypotension, as described in the case reported, can be a significant factor in the occurrence of HI[8]. CT scan is the most commonly used imaging exam in the diagnosis of HI. While findings of peripheral lesions with clearly limited borders are characteristic of HI, with triangular or wedge-shaped areas of low attenuation, irregular nodular lesions of central location may be present in extensive infarction, indistinguishable from liver neoplasms[3,9,10]. These central parenchyma pseudo nodular lesions are found in about 25% of HI[10]. Enhancement of HI by the contrast medium is generally patchy and heterogenous, with areas of more extensive necrosis remaining hypoattenuating in all phases, while areas that remain isoattenuating in the portal venous phase are suggestive of viable liver tissue[1,10]. A high attenuation, thin subcapsular rim may be present in some cases, which must be distinguished from liver abscesses[9]. Gas formation has been described in both sterile and infected infarcts, and the presence of gas is not an unequivocal marker of infected necrosis of the liver[1,10]. Bile lakes may be present as a late complication of large infarcts due to ischemic necrosis of bile duct epithelium, with jaundice persisting for several weeks[1].
| Journal | Ref. | Year | Patient | Diagnosis | Outcome |
| Gastroenterology | Sundaram et al[6] | 1978 | 36-year-old male | Laparotomy and biopsy | Recovery |
| World Journal of Gastroenterology | Deng et al[3] | 2006 | 53-year-old male | Hepatectomy | Recovery |
| Brazilian Journal of Intensive Care | Paes et al[7] | 2007 | 67-year-old female | Necropsy | Death |
| Practical Diabetes International | Chen et al[8] | 2007 | 53-year-old female | CT | Death |
| International Journal of Clinical and Experimental Medicine | Xu et al[12] | 2017 | 45-year-old female | Laparoscopic biopsy | Recovery |
| Open Journal of Case Reports | Tiwari et al[11] | 2021 | 37-year-old male | MRI | Recovery |
In some cases reported in the literature, diagnosis of hepatic infarction was only established postoperatively, with resection being performed due to the aspect of the lesion being highly suggestive of a liver neoplasm in the imaging exams[3,6]. MRI can be helpful in the diagnosis of HI, showing lesions of heterogeneous intensity, with the center of the lesion being more apparent than the rim, restricted diffusion, no significant enhancement, and little or no mass effect, which helps in differentiating HI from liver neoplasms[3,11,12]. Using a gadoxetate disodium contrast medium may further increase specificity in the differential diagnosis of HI[5].
In the case we reported, both CT and MRI were unable to differentiate the lesions from the liver's primary neoplasms or liver abscesses. Besides the clinical history of acute onset of symptoms with no signs of infection, there was also one finding in the imaging exams that were suggestive of HI: The wedge shape marked delimitation between the areas affected by the infarction and normal liver parenchyma, which was visible in the peripheral areas of the liver and coexisted with the nodular areas which were more centrally located. The use of percutaneous biopsy to confirm the diagnosis of HI is a novel aspect in this case report, as in previously reported cases, HI was diagnosed either by imaging exams or surgical exploration (Table 2). Since correct diagnosis could not be confirmed by imaging exams and considering a high clinical suspicion of HI, liver biopsy was seen as the next step in the investigation to avoid unnecessary surgical exploration with significant morbidity to the patient and also to avoid missing a diagnosis of liver neoplasm, which could coexist or even be the cause of a liver infarction. Histological analysis of HI is characterized by the presence of a centrilobular zone of parenchymal necrosis, in contrast to a peripheral zone with relative preservation of portal tracts, hepatic veins, and intralobular stroma[2,9].
Non-occlusive liver infarcts usually regress after a while as regeneration of the liver occurs. While the necrotic tissue present at the site of a HI is usually sterile, an infection may occur due to biliary tract or hematogenous dissemination of bacteria, with progression to a liver abscess that may require treatment with antibiotics and/or percutaneous drainage[6]. In the case we reported, the patient showed no signs of infection, and tissue cultures obtained at the moment of the liver biopsy showed no signs of bacterial growth. His persistently elevated bilirubin levels may be attributed to the formation of bile lakes in the central areas of necrosis and the significant disruption of the biliary drainage of the areas of liver parenchyma adjacent to the areas most affected by the HI. The benefits of anticoagulant therapy in the management of HI are uncertain, and unless the infarction is associated with vascular occlusion or a thrombotic etiology, the use of anticoagulants is not generally recommended[13]. In this case, the patient received anticoagulation due to concomitant pulmonary thromboembolism. This thromboembolic event raises the question of whether a hypercoagulable state may also play a role in the genesis of HI associated with DK, as microvascular thrombosis of the liver may aggravate the ischemic insult already present due to the other mechanisms of aggression in DK that were previously discussed.
DK is a rare cause of non-occlusive HI that must be remembered in diabetic patients with abdominal pain and elevated markers of hepatic injury. While in the imaging exams, HI usually presents itself as wedge-shaped areas of hypoattenuation on the periphery of the liver, atypical presentations with irregular nodular areas of central location may occur, which are indistinguishable from liver neoplasms. Using ultrasound-guided percutaneous biopsy may provide the correct diagnosis in these cases, avoiding unnecessary surgical exploration.
The authors would like to acknowledge the important contribution of Dr. Neto RT and the CONLAB laboratory in the diagnostic investigation of the case reported and in the elaboration of this manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Feng X, China; Surani S, United States S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Torabi M, Hosseinzadeh K, Federle MP. CT of nonneoplastic hepatic vascular and perfusion disorders. Radiographics. 2008;28:1967-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | CARROLL R. Infarction of the human liver. J Clin Pathol. 1963;16:133-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Deng YG, Zhao ZS, Wang M, Su SO, Yao XX. Diabetes mellitus with hepatic infarction: a case report with literature review. World J Gastroenterol. 2006;12:5091-5093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Francque S, Condat B, Asselah T, Vilgrain V, Durand F, Moreau R, Valla D. Multifactorial aetiology of hepatic infarction: a case report with literature review. Eur J Gastroenterol Hepatol. 2004;16:411-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Maruyama M, Yamada A, Kuraishi Y, Shibata S, Fukuzawa S, Yamada S, Arakura N, Tanaka E, Kadoya M, Kawa S. Hepatic infarction complicated with acute pancreatitis precisely diagnosed with gadoxetate disodium-enhanced magnetic resonance imaging. Intern Med. 2014;53:2215-2221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Sundaram M, Srivisal S, Lagos JA, Ho JE. Angiographic demonstration of non-occlusive hepatic infarction with scintigraphic and microscopic correlation. Gastrointest Radiol. 1978;3:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Paes T, Gazoni FM, Pinheiro Junior Nde F, Guimarães HP, Lopes RD, Lanzoni VP, Vendrame LS, Lopes AC. [Liver ischemic necrosis and diabetes mellitus: case report]. Rev Bras Ter Intensiva. 2007;19:490-493. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 8. | Chen M, Croxson S. Triad: diabetic ketoacidosis, elevated liver enzymes and abdominal pain–think liver infarct! Pract Diab Int. 2007;24:302-303. [DOI] [Full Text] |
| 9. | Adler DD, Glazer GM, Silver TM. Computed tomography of liver infarction. AJR Am J Roentgenol. 1984;142:315-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Giovine S, Pinto A, Crispano S, Lassandro F, Romano L. Retrospective study of 23 cases of hepatic infarction: CT findings and pathological correlations. Radiol Med. 2006;111:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Tiwari HA, Khan AS. Magnetic resonance imaging of non-occlusive hepatic infarction associated with diabetic ketoacidosis. Open J Case Rep. 2021;2:140. |
| 12. | Xu W, Dong D, Tong L, Chi B, Gong F. A round-shaped hepatic infarction detected in a diabetes patient: MRI findings and literature review. Int J Clin Exp Med. 2017;10:12726-12729. |
| 13. | Klein SH, Klein ED, Ackerman Z, Hiller N. Liver infarction: to treat or not to treat? Intern Med J. 2018;5:28-31. [DOI] [Full Text] |