Published online Oct 27, 2022. doi: 10.4254/wjh.v14.i10.1884
Peer-review started: July 26, 2022
First decision: August 18, 2022
Revised: September 2, 2022
Accepted: October 2, 2022
Article in press: October 2, 2022
Published online: October 27, 2022
Processing time: 93 Days and 2 Hours
Chronic liver diseases (CLD) are the major public health burden due to the con
To evaluate the potential applicability of DiD dye as a cell labeling agent for long-term, and non-invasive in vivo tracking of transplanted cells in the liver.
Magnetically sorted, epithelial cell adhesion molecule positive (1 × 106 cells/mL) fetal hepatic progenitor cells were labeled with DiD dye and transplanted into the livers of CLD-severe combined immunodeficiency (SCID) mice. Near-infrared (NIR) imaging was performed for in vivo tracking of the DiD-labeled transplanted cells along with colocalization of hepatic markers for up to 80 d. The existence of human cells within mouse livers was identified using Alu polymerase chain reaction and sequencing.
NIR fluorescence imaging of CLD-SCID mice showed a positive fluorescence signal of DiD at days 7, 15, 30, 45, 60, and 80 post-transplantation. Furthermore, positive staining of cytokeratin, c-Met, and albumin colocalizing with DiD flu
DiD-labeling is promising for long-term and non-invasive in vivo cell tracking, and understanding the regenerative mechanisms incurred by the transplanted cells.
Core Tip: Non-invasive tracking of transplanted cells is crucial to understand the homing, distribution, and differentiation into the desired cell types contributing to organ regeneration. Lipophilic fluorescent dye DiD-labeled fetal hepatic progenitor cells (fHPCs) were transplanted into the livers of mice with chronic liver diseases. DiD labeling of cells enabled long-term and non-invasive tracking of transplanted cells in vivo up to 80 d. Immunostaining and colocalization using liver-specific markers with DiD confirmed the persistence of transplanted cells in mice liver post-transplantation. Transplanted fHPCs supported liver function recovery, while identification of the Alu gene sequence revealed survival and engraftment of human cells within the mouse liver.
- Citation: Tripura C, Gunda S, Vishwakarma SK, Thatipalli AR, Jose J, Jerald MK, Khan AA, Pande G. Long-term and non-invasive in vivo tracking of DiD dye-labeled human hepatic progenitors in chronic liver disease models. World J Hepatol 2022; 14(10): 1884-1898
- URL: https://www.wjgnet.com/1948-5182/full/v14/i10/1884.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i10.1884
Chronic liver diseases (CLD) represent one of the leading causes of morbidity and mortality worldwide, specifically in developing countries. They result from the progressive deterioration of liver functions which is caused by the continuous process of inflammation, destruction, and inadequate repair of the liver parenchyma leading to cirrhosis. Liver cirrhosis is characterized by irreversible distortion of the liver architecture in the form of fibrosis, scar formation, the occurrence of several regenerative nodules, vascular reorganization, and immense liver failure. The major aim of the current treatment approaches is to halt the progression of CLD into more severe forms, and further reduce the associated complications representing the need for an appropriate multidisciplinary approach. The clinical signs and symptoms of CLD can be nonspecific; hence the major management perceptions are the elimination of underlying causes, management of portal hypertension, and personalized therapy for each associated condition. Although such strategies provide temporary support to the failing liver; they cannot prevent long-term disease progression. Hence, more effective management approaches are required to overcome such hurdles and bridge the gap of a long-term therapeutic window to improve health-related quality of life.
Cell therapy is an emerging technology and has shown significant promise in addressing the current demand for alternative options to liver transplantation to improve liver functions and act as a supportive bridge therapy in CLD[1,2]. However, it is necessary to ascertain appropriate cell types from widely acceptable sources and resolve several unanswered questions before utilizing cell-based technology in the clinical setting. Since the last two decades, transplantation of different types of cells from various tissue sources such as autologous bone marrow-derived mononuclear cells and mesenchymal stem cells (MSCs), and allogenic fetal hepatic progenitor cells (fHPCs) have shown promising outcomes in clinical studies of CLD[3-7]. In addition, several preclinical studies have also demonstrated the potential applicability of such approaches in immune-compromised/deficient mice to improve the current understanding of the safety, efficacy, and functionality of human stem/progenitor cells post-transplantation[8-11]. While several of these studies have proved the safety and involvement of the transplanted cells in liver recovery, the availability of methods for easy and long-term tracking of infused cells would be extremely beneficial in determining their viability, bio-distribution, homing, and differentiation which represents a major roadblock for cell-based therapies in clinical settings.
The majority of existing strategies employ radioisotopes, magnetic particles, fluorescent tags, or reporter genes as cell labeling agents prior to transplantation in preclinical settings[12]. Furthermore, non-invasive radionuclide imaging methods such as single-photon emission tomography and positron emission tomography using radionuclides [Technetium (99mTc) and 111In-oxine] are currently employed in clinical settings[4,6,13]. However, the short life of radionuclides limits their wider applicability due to monitoring of the immediate cellular behavior for only a few hours. Magnetic resonance imaging (MRI) is another non-invasive imaging method that has been explored for cell tracing in preclinical CLD models for up to 1-2 wk[14,15]. While MRI offers good spatial resolution and contrast, it is less sensitive and is not effective for follow-up studies due to the gradual loss of signal intensity[12]. Hence, several other tracking methods based on reporter gene expressions, such as fluorescence imaging and bioluminescence imaging are being successfully employed for monitoring the fate of transplanted cells in animal models of liver injury[16,17]. Although this method enables long-term cell tracking, the safety concerns owing to genetic manipulation represent a major hurdle for clinical translation. Hence, direct labeling of the cells without involving genetic manipulation represents a crucial need for a sensitive, relatively safer, and less cumbersome process for tracking transplanted cells in both preclinical and clinical settings.
In the present study, long-term and non-invasive tracking of transplanted fHPCs was evaluated in an experimental severe combined immunodeficiency (SCID) mouse model of CLD using DiD. DiD is a carbocyanine dye having good photochemical properties of strong fluorescence, and stability[18-20]. It is a cationic dye and belongs to the family of lipid intercalating long alkyl side-chain carbocyanine derivatives that have a long-range (540-780 nm) emission. Due to the long-range emission, tissue autofluorescence of DiD is minimum, permitting the use of other fluorochromes such as fluorescein isothiocyanate (FITC) for co-localization studies to evaluate the expression of other essential markers specific to transplanted cells in the recipient tissue. Moreover, the process of labeling the cells using DiD is easy due to its excellent efficiency for integration and diffusion into the cell membranes[18,19,21,22]. Although DiD is insoluble in water, its fluorescence is readily detected when incorporated into the cell membranes. Therefore, it has been classified as one of the most appropriate carbocyanine families of dyes in cell labeling and tracking. After incorporation into cell membranes, it diffuses laterally within the plasma membranes, resulting in staining of the entire cell. Structural similarity with the cell membrane phospholipids and prolonged dye retention within the cells are among the major advantages of DiD for live organisms. Hence, DiD has been used for labeling different types of cells without interfering with cellular differentiation; however, the effects of DiD labeling on human liver cells and its effect on the in vivo retention of labeled human liver cells remain to be investigated.
We specifically utilized magnetically sorted fHPCs using epithelial cell adhesion molecule (EpCAM) as a surface marker due to its associated crucial functions such as cell-to-cell adhesion, proliferation, maintenance of pluripotent state, and regulation of differentiation and migration[23]. It has also been demonstrated that EpCAM-positive HPCs are highly proliferative and have diminished class II MHC presentation, and are classified as immature cells suitable for regenerative applications[24]. In our earlier study, EpCAM-positive fHPCs revealed a significant improvement in liver functions and increased disease-free life span in patients with end-stage CLD[6]. Thus, using EpCAM-positive fHPCs could highlight how good DiD is for long-term, non-invasive, and real-time monitoring of cell survival, and structural and functional improvements in preclinical models of CLD post-transplantation. Accordingly, the present study aimed to shed light on the fate of DiD-labeled human liver cells in CLD-SCID mice using live imaging up to 80 d post-transplantation.
Experimental animals were obtained from inbred colonies of SCID mice (strain: NOD.CB17-Prkdcscid/J) and maintained at the animal facility of the Centre for Cellular and Molecular Biology (CCMB), Hyderabad, India. This study was approved by the Institutional Animal Ethics Committee (Animal trial registration number 20/1999/CPCSEA dated 10/03/1999) of CCMB. All the animal experimental procedures were performed in accordance with the approved ethical guidelines of CCMB for the care and use of animals. All the animals were maintained in standard ventilated cages with a 12 h light-dark cycle and were fed ad libitum.
CLD mouse model was generated using 25% carbon tetrachloride (CCl4, Rankem, India) diluted with mineral oil (Sigma, United States). A sub-lethal dose of diluted CCl4 was administered according to 125 μL/kg body weight in each animal. A total of 26 mice (either sex) at eight weeks of age were randomly assigned to the vehicle control group (n = 4) and the CCl4 group (n = 22). The CCl4 group received intraperitoneal injections of diluted CCl4 twice a week for 4 wk and the vehicle control mice received only mineral oil. After 4 wk, liver damage was confirmed by changes in liver enzymes and liver tissue histology. For the biochemical evaluation of liver damage, 100-150 μL of blood was collected by orbital sinus puncture. Serum levels of total bilirubin, glutamate-pyruvate transaminase (SGPT), and glutamic-oxaloacetic transaminase (SGOT) were measured by the Jendrassik-Grof and Reitman-Frankel’s methods, respectively, using kits (Coral Clinical Systems, India). The mice after 4 wk of CCl4 (referred to as CLD-SCID mice) were ready for cell transplantation (TX).
The total fetal liver cells (tFLCs) isolation protocol was approved by the Institutional Ethics Committee of Deccan College of Medical Sciences, Hyderabad. Informed consent was obtained prior to sample collection, and cell processing was performed according to the ethical guidelines for the use of human cells. Briefly, the whole liver was dissected from spontaneously aborted fetuses (n = 3, 10-12 wk gestation), and perfused twice with ice-cold phosphate-buffered saline (PBS) for 5 min to eliminate circulating peripheral blood cells, followed by digestion with 0.025% collagenase in 1× PBS for 5 min at room temperature. Then the liver tissue was minced with a scalpel blade and disintegrated into a single-cell suspension by passing through 40 μm cell strainers (BD Biosciences, United States). Cell viability and counting were performed using the trypan blue dye exclusion test.
For flow cytometry analysis, a single cell suspension of 2 × 106 tFLCs was fixed in 4% paraformaldehyde for 15 min at room temperature. Following fixation, the cells were washed twice with 1× PBS and stained with anti-human EpCAM (CD326) antibody conjugated with FITC (Miltenyi Biotech, Germany) for 30 min. Cells were washed once with 1× PBS before analyzing on FACS CaliburTM (BD Biosciences, United States) using 488 nm argon laser emission at 530/30 BP filter. The data were analyzed and plotted using Kaluza software 1.5a (Beckman Coulter Inc., United States).
To isolate EpCAM-positive cells, a 5 × 107 tFLC suspension in 500 μL buffer containing FCR blocking reagent was incubated with anti-human EpCAM antibody (Miltenyi Biotech, Germany) conjugated with magnetic beads at 4°C for 30 min and sorted using the magnetic cell sorter, AutoMACS according to the manufacturer’s instructions (Miltenyi Biotech, Germany). Magnetically activated cell sorting (MACS) enriched EpCAM-positive cells were collected and resuspended in RPMI 1640 medium (Sigma, United States) supplemented with 10% fetal bovine serum (FBS, Sigma, United States). Cell isolation and MACS sorting procedures were carried out under sterile conditions in a Class 100 biosafety cabinet.
MACS-sorted EpCAM-positive cells were fixed in 4% paraformaldehyde, and cytospin preparations were conducted on “Probe-on-Plus” slides (Fisher Scientific, United States). Cells were blocked with 10% goat serum, and stained with mouse monoclonal anti-human EpCAM antibodies directly conjugated with FITC (Miltenyi Biotech, Germany), and co-stained with either anti-cytokeratin (CK) 8+18+19 or anti-c-Met (Abcam Inc., MA, United States) primary antibodies. Alexa 594 (Molecular Probes, United States) was used as the secondary antibody. Images were captured using a confocal laser scanning microscope (Leica, Germany, SP2 AOBS).
MACS sorted EpCAM-positive cells (1 × 106 cells/mL) in Hank’s buffered salt solution (HBSS) were labeled with DiD dye by adding 5 μL of DiD cell labeling solution (Life Technologies, Eugene, United States) to the cell suspension and incubating for 20 min at 37°C. DiD-labeled cells were washed thrice with HBSS, and resuspended in the same buffer. 1 × 105 cells (100 μL) were injected directly into the liver lobes of the CLD mice (n = 14) at a single site using a 26-gauge needle. CLD mice receiving plain HBSS buffer served as non-transplanted (non-TX) controls (n = 5). Post-transplantation, mice were maintained in different cages, imaged, and sacrificed at different time points.
DiD-specific fluorescence from the transplanted animals was detected on the Multispectral FXPRO Fluorescence Imager (Carestream-KODAK, United States) using 630 nm excitation and 670/30 BP emission filters. Highly sensitive fluorescence images were combined with the high resolution X-ray images to precisely locate the DiD-labeled cells. Animals were imaged prior to TX (0 d) and after 7, 15, 30, 45, 60, and 80 d, post-TX. For each imaging experiment, the animals were anaesthetized with Xylazine (5 mg/kg body weight) and Ketamine (25 mg/kg body weight), the abdominal hair was shaved, placed within the chamber, and imaged. After in vivo imaging at days 15, 30, and 80 post-TX, mice were sacrificed, liver tissues were excised, and imaged (ex vivo imaging) to locate the fluorescing lobe for further processing. The fluorescing area of the liver lobe at day 15 and day 80 was immersed in OCT mounting solution and stored at -80°C. Liver lobes of the non-TX mice were randomly selected and processed similarly. The liver lobes at day 30 post-TX were processed for paraffin embedding and histology.
The excised liver lobes were fixed in 10% buffered formalin and processed for paraffin embedding. Paraffin-embedded liver tissues were sectioned at 4.0 μm thickness using a rotatory microtome (Leica RM2135, Germany), and stained with Hematoxylin and Eosin, and Sirius Red (Sigma-Aldrich, United States) using standard protocols. Bright-field images were acquired from both control and CLD mice (n = 3) at 10× and 40× using an Olympus inverted microscope (IX3-SSU, Tokyo, Japan). A total of 20-25 random fields captured with the 10× objective was used to calculate the total collagen area using Image J (1.5 2q) software, and expressed as total collagen percent area (% CPA).
Serial cryosections (7.0 μm thick) of transplanted and non-transplanted mouse liver lobes at 15 and 80 d were stained to determine the expression of hepatic markers using anti-CK, anti-c-Met, and anti-human albumin (MP Biomedicals) primary antibodies, and detected with Alexa 488 (Molecular Probes, United States) secondary antibody. Images were captured using either a confocal laser scanning microscope (Leica, Germany, SP2 AOBS), or the Axioimager Z2 Fluorescence microscope (Zeiss, Germany).
DNA was isolated from the fHPCs prior to transplantation and from the liver tissues of transplanted and non-transplanted mice at day 80. Isolated DNA was analyzed using human-specific primers for Alu sequence (Forward primer 5’-GGCGCGGTGGCTCACG-3’, Reverse primer 5’-TTTTTTGAGACGGAGTCTCGCTC-3’). Polymerase chain reaction (PCR) was performed in a 25 μL reaction mixture containing 1 μL DNA, 2.5 μL 10× complete PCR Buffer with MgCl2, 1 μL of 10 mmol/L dNTPs, 0.5 μL forward and 0.5 μL reverse primers, and 0.2 μL Taq DNA Polymerase (5 U/mL) with an initial denaturation step of 94°C for 5 min, followed by 35 cycles of a three-step program of 94°C for 30 s, 54°C for 30 s and 72°C for 45 s, followed by a final extension step at 72°C for 5 min. The PCR products were electrophoresed on a 2% agarose gel and observed under UV with ethidium bromide staining. The images were captured using the Gel Documentation System (BIO-RAD, United States). The PCR product was cleaned using QIAquick Gel Extraction Kit (Qiagen, United States), and sequenced. The amplicon sequences were analyzed using the ClustalW2 online tool (https://www.ebi.ac.uk/Tools/msa/clustalw2/).
All statistical analyses were performed using GraphPad Prism software (ver. 5.0). Data are presented as mean ± standard error of the mean. Paired Student t-test at 95% confidence interval for a P value of ≤ 0.05 was considered statistically significant.
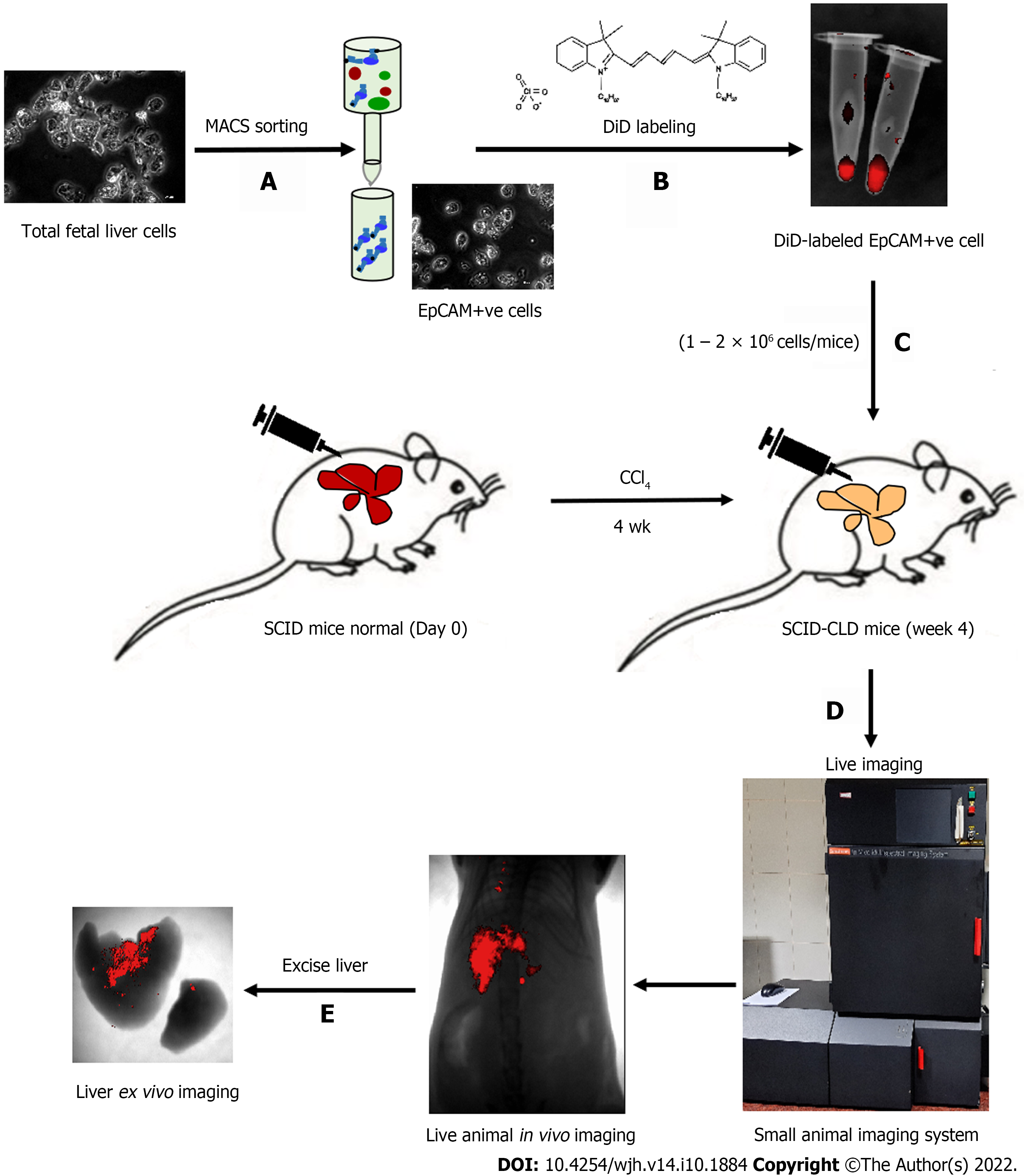
The schematic representation in Figure 1 shows the different steps involved in measuring different outcomes throughout the study process. Isolated tFLCs were enriched for EpCAM-positive cells by MACS in step 1 (Figure 1A), labeled with DiD dye in step 2 (Figure 1B), and then transplanted into CLD mouse livers (Figure 1C). Furthermore, live non-invasive near-infrared (NIR) imaging was performed at regular intervals using a small animal imaging system to detect the fluorescence signal (Figure 1D). However, ex vivo imaging of the excised liver was performed to confirm the localization of the fluorescence in mice liver post-TX (Figure 1E).
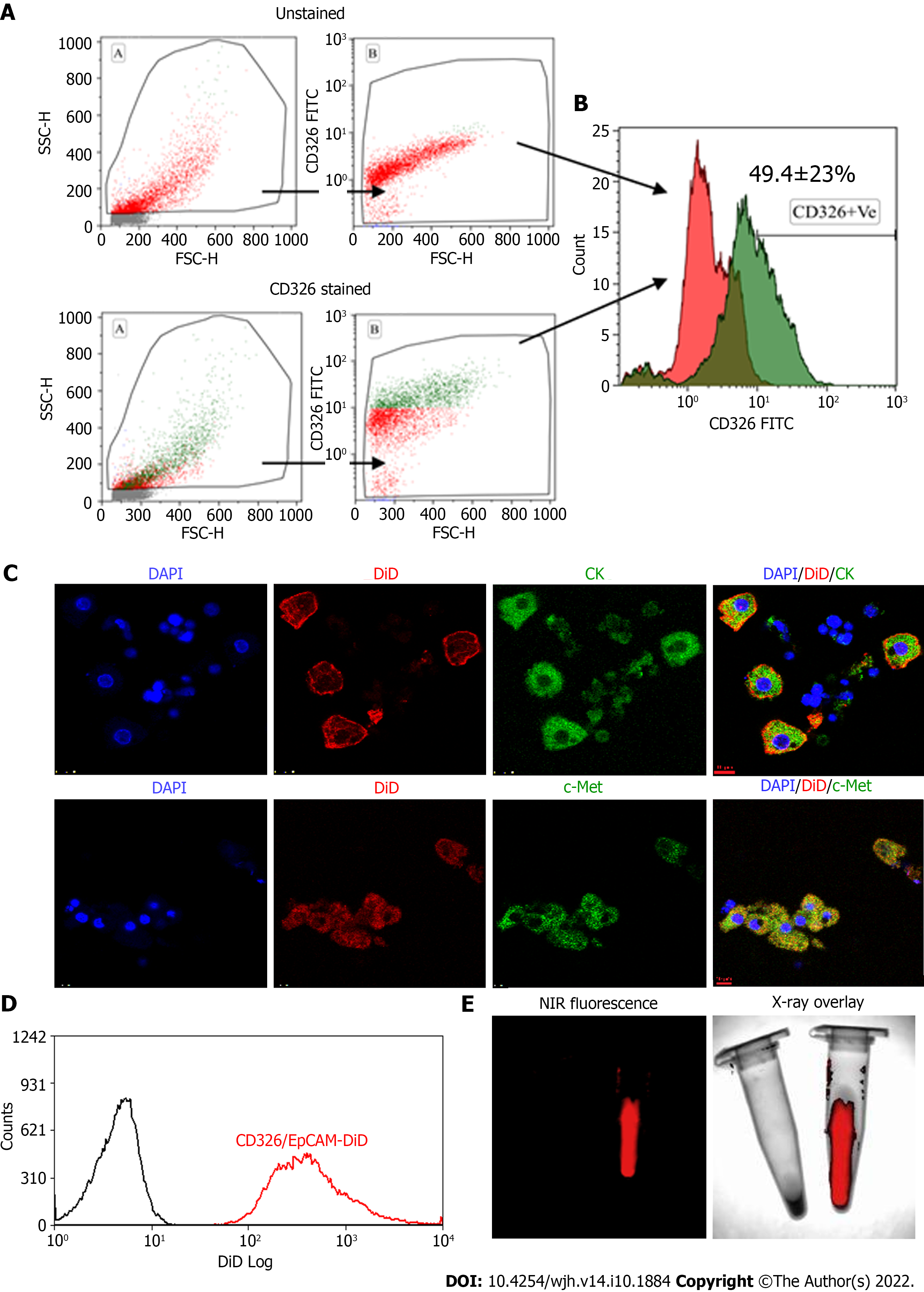
Human tFLCs stained with FITC-tagged anti-CD326 (EpCAM) antibodies were analyzed using flow cytometry. The gating strategy and the analysis of cell fluorescence vs cell size, and the overlaid histogram of the unstained and EpCAM-stained cells were acquired (Figure 2A and B). Of these tFLCs, 49 ± 23% of cells were found to be EpCAM positive and designated as fHPCs.
MACS-sorted EpCAM-positive fHPCs were characterized for the expression of specific hepatic cell markers such as CK and c-Met together with EpCAM (Figure 2C). Magnetically sorted EpCAM-positive cells which were labeled with DiD dye showed a separate peak corresponding to the DiD fluorescence (Figure 2D). Furthermore, before transplantation, the DiD-labeled EpCAM-positive cells in the tube were visualized for fluorescence in the multispectral imaging system. The overlay image of the NIR fluorescence and X-ray showed a positive signal only in the tube containing DiD-labeled cells, while the tube with non-labeled cells was devoid of any such fluorescence (Figure 2E).
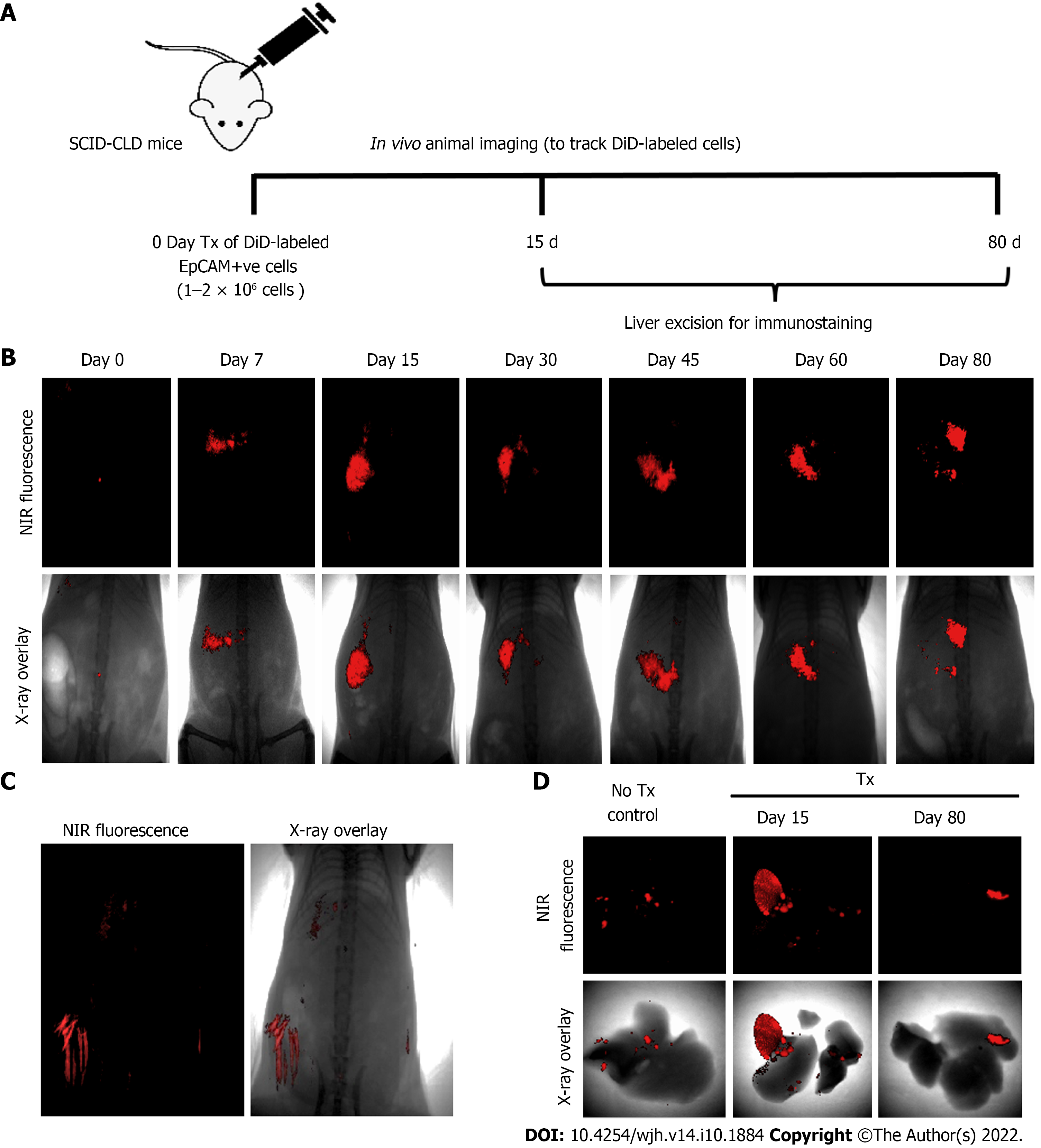
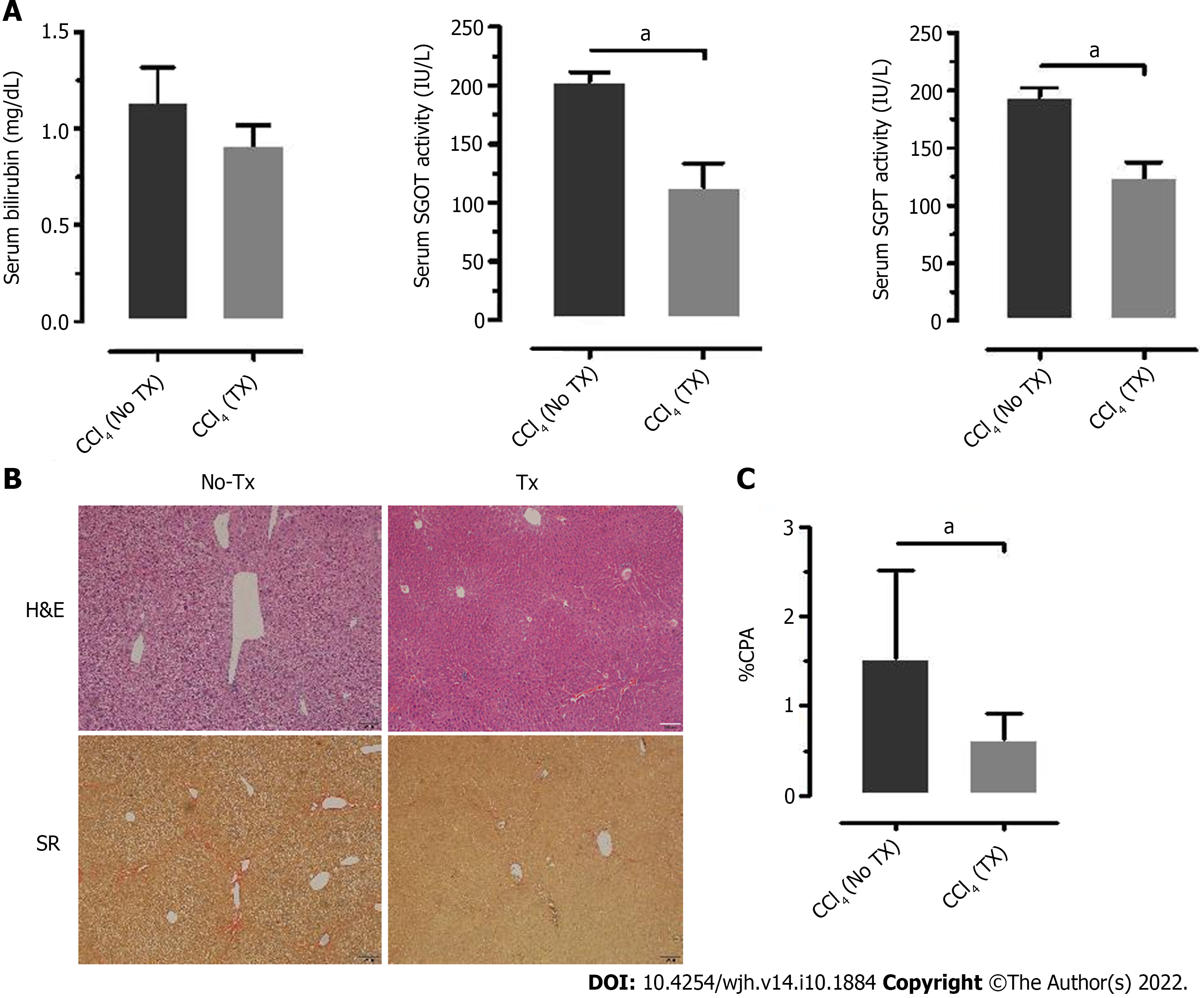
Assessment of the biochemical and histological changes in CLD-SCID mice showed an increase in serum parameters (SGOT, SGPT, and bilirubin), and collagen accumulation compared to the control animals, confirming liver injury after 4 wk of CCl4 injection (Supplementary Figure 1). These CLD mice were utilized for cell transplantation (at day 0), and tracking through in vivo imaging on different days from day 0 to day 80 (Figure 3A). NIR fluorescence imaging of CLD-SCID mice before intra-hepatic transplantation of DiD-labeled fHPCs cells at day 0 did not show fluorescence, while mice at days 7, 15, 30, 45, 60, and 80 post-TX showed a positive fluorescence signal of DiD (Figure 3B). The overlay images showed DiD fluorescence in the upper part of the abdominal cavity near the rib cage, suggesting that the cells continue to localize in the liver lobes until day 80 post-TX. The non-TX mouse abdomen lacked such fluorescence signals (Figure 3C). To further confirm the localization, the liver was excised on day 15 and day 80 post-TX, and ex vivo imaging was performed which confirmed the localization of DiD-labeled cells within the mice livers (Figure 3D). These results indicated that DiD-labeled cells can be efficiently visualized and tracked for a longer duration post-TX both in vivo and ex vivo.
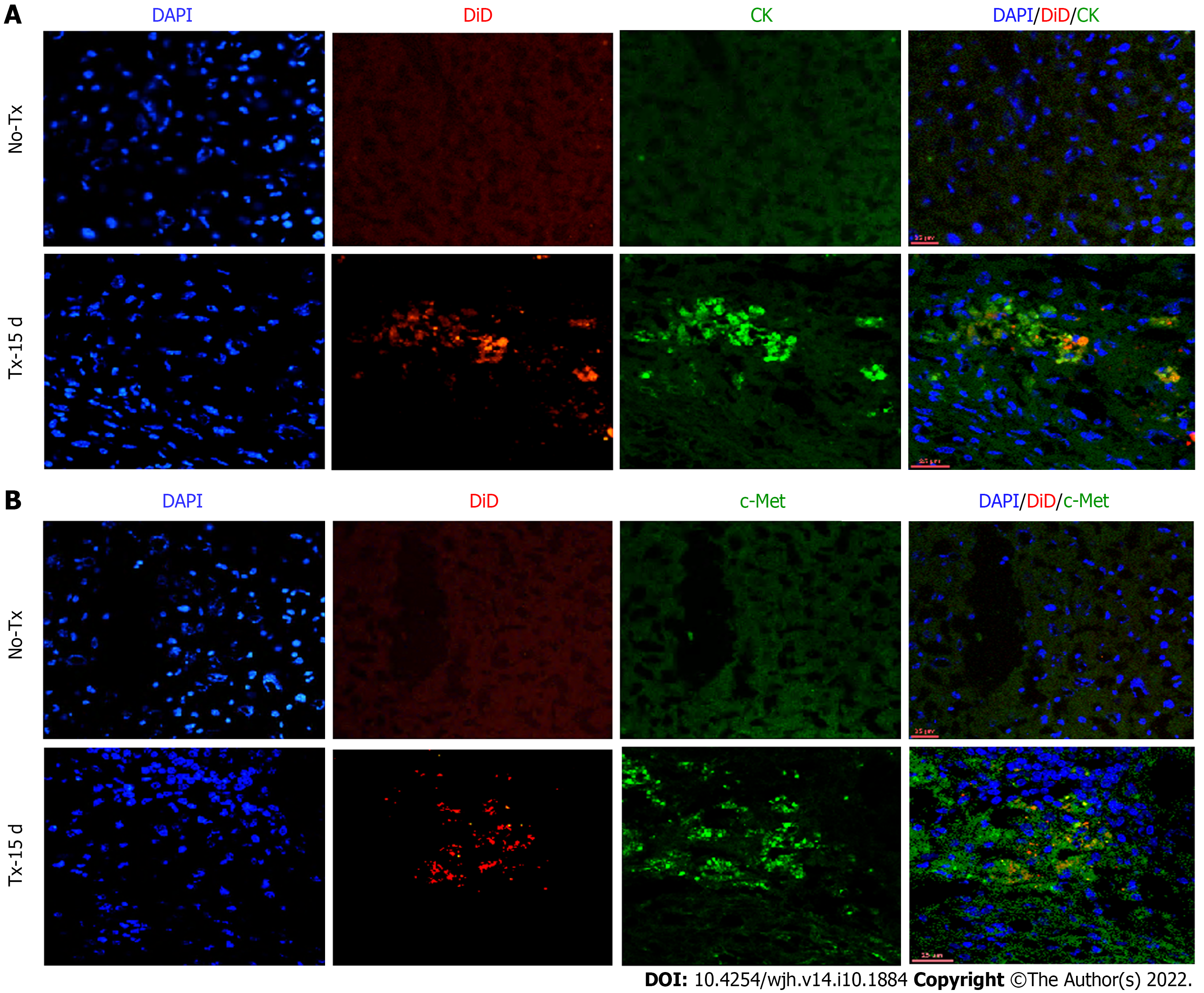
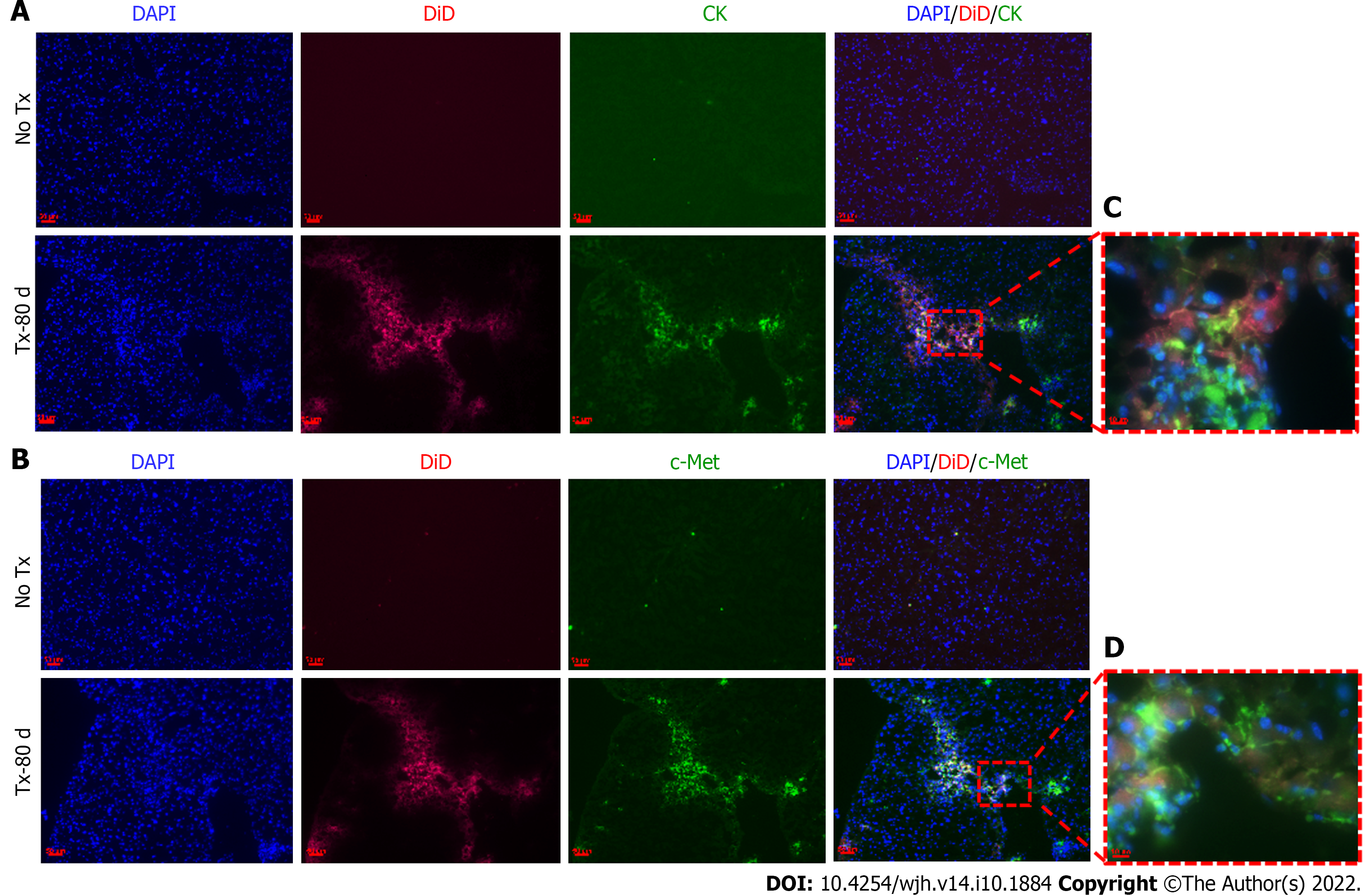
To evaluate the expression of hepatic markers in transplanted cells, frozen mouse liver sections were obtained from the portion of the liver that displayed a DiD-positive fluorescence signal at day 15 and day 80 post-TX. Serial sections of the liver transplanted with DiD-labeled fHPCs and non-transplanted control mice were obtained and stained for CK and c-Met (Figures 4 and 5). The presence of DiD fluorescence only in the transplanted mice confirmed the existence of transplanted cells, while non-transplanted mice did not show DiD fluorescence in liver tissue sections. In addition, positive staining for both CK (Figures 4A, 5A and 5C) and c-Met (Figures 4B, 5B and 5D) colocalization with DiD fluorescence clearly demonstrated that the fluorescent signal of the hepatic markers, CK and c-Met emerged from the DiD-labeled transplanted cells.
After 15 d of transplantation, recovery of mouse liver function was analyzed by assaying liver enzymes SGOT, SGPT, and bilirubin. Serum SGOT and SGPT levels were significantly reduced in the transplanted mice compared to the non-transplanted mice (Figure 6A), while serum bilirubin was reduced to normal levels in both groups. Thus, the serum enzyme parameters suggested improved liver function in the transplanted mice compared to the non-transplanted mice. Furthermore, the lower collagen percentage area in transplanted mice compared to the non-transplanted mice suggested that this recovery may be attributed to the transplanted fHPCs (Figure 6B and C).
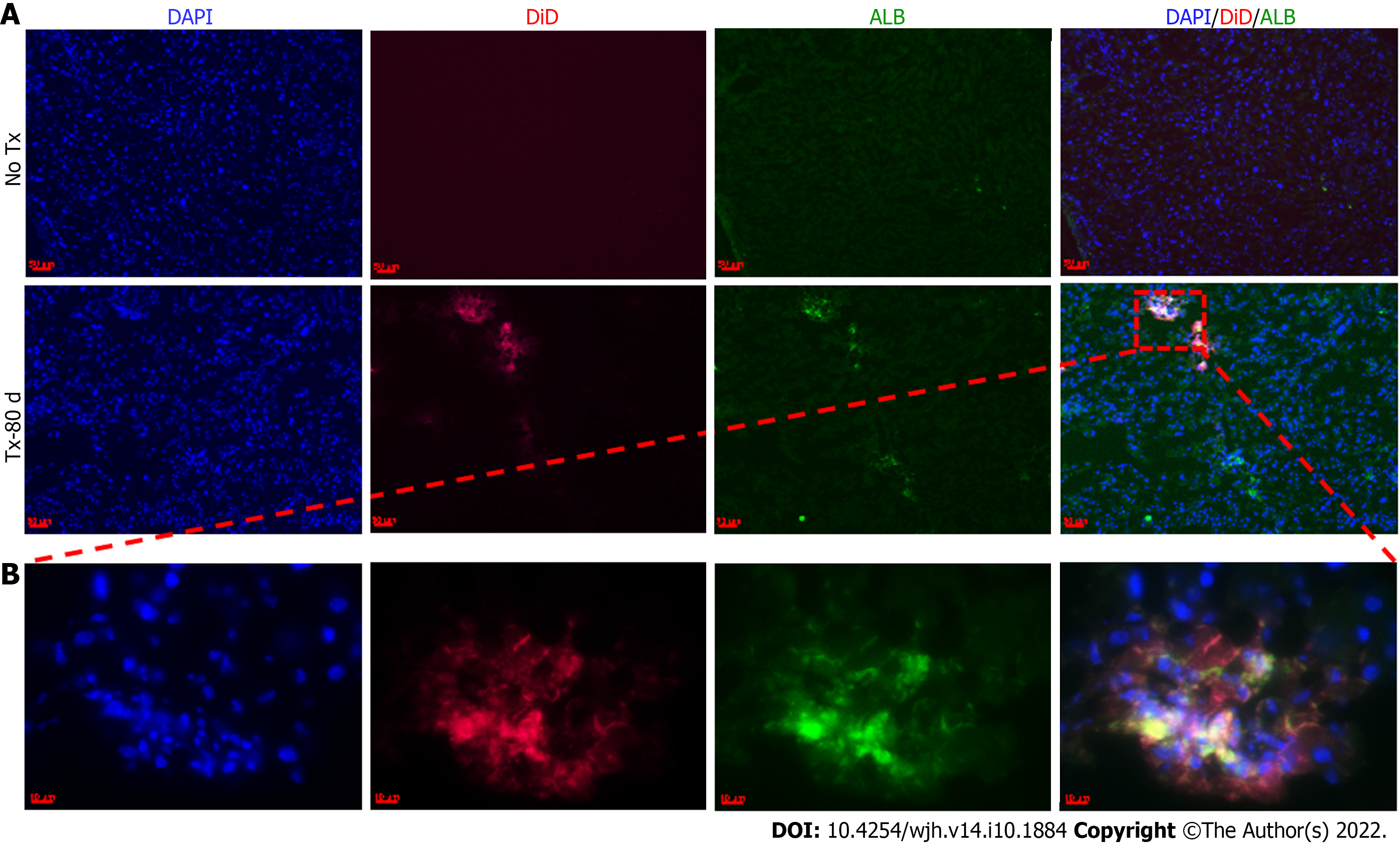
The above assumption was further confirmed by the positive expression of hu-ALB in CLD-SCID mouse liver cells at day 80 post-TX (Figure 7). ALB-positive cells were also tested for their colocalization with DiD to confirm the effect was only in labeled cells. The non-transplanted mouse livers tested negative for ALB staining. The presence of hu-ALB only in the livers of transplanted mice, but not in the non-transplanted mice further confirmed the differentiation of fHPCs into functional hepatocytes (Figure 7). In addition, the amplification and detection of human-specific Alu-sequence from the transplanted mouse livers provided molecular evidence for the continued presence of human fetal liver cells in mouse liver at day 80. Sequencing analysis of the amplified Alu gene confirmed the presence of human Alu gene sequence in mouse liver tissues (Supplementary Figure 2). These results suggest that the transplanted human EpCAM-positive DiD-labeled cells continue to survive long-term without eliciting serious unfavorable effects in CLD-SCID mice.
This study reports a new application of the lipophilic fluorescent dye DiD for non-invasive in vivo imaging to monitor transplanted fHPCs in SCID mice with CLD. In our earlier clinical study, transplanted fHPCs in end-stage CLD patients were tracked for only 24 h using radio-labeled 99mTc-HM-PAO post-TX[6]. In our current study, labeling of fHPCs with DiD enabled long-term in vivo monitoring of the transplanted cells, which was further confirmed by ex vivo imaging and immunohistological analysis. Using DiD as a cell label, the transplanted cells could be tracked for 80 d non-invasively in CLD-SCID mouse liver. Our findings revealed that DiD labeling is a relatively simple, safe, and effective approach for long-term and non-invasive tracking of fHPCs post-TX in mouse liver. In addition, our results also showed the efficacy of transplanted human fHPCs as a suitable cell type for improving liver functions similar to earlier studies[11,25].
Long-term in vivo tracking of transplanted cells is currently required to answer several concerns and to reduce existing controversies in cell-based therapies. Now, it has been identified as an essential component of cell-based therapeutic strategies for optimizing the cell number, route of delivery, biodistribution, cell viability post-transplantation, and evaluating the regenerative capabilities, which would aid in accelerating their clinical applications[26]. The depth of penetration, sensitivity, spatial and temporal resolution, ease of availability of the molecular probe, and cost of imaging are some of the important factors to be considered for a suitable imaging approach[26]. Direct labeling of the tran
The carbocyanine dyes, CM-DiI, and DiR have been used to label transplanted cells in animal models of liver injury. Although both CM-DiI and DiR proved to be safe for cell tracking applications, monitoring has only been demonstrated in ex vivo settings[30], or if in vivo, the signal was reported to have faded within 5 d after infusion[22]. Hence, the above carbocyanine dyes have not provided sufficient evidence of their long-term applicability for cell tracking in vivo. In contrast, DiD has been demonstrated to be effective for long-term monitoring of labeled neuronal and cancer cells in vitro for up to 4 wk[31,32]. Moreover, DiD at higher concentrations of up to 2 μM also does not affect cell growth, proliferation, migration, and apoptosis, and does not cross-transfer to neighboring cells[33]. In line with this, DiD-labeled MSCs have demonstrated the absence of cytotoxicity and signs of altered functional performance in terms of cytokine production or trilineage differentiation[34], thus assuring the safety of DiD for potential applicability in stem cell tracking. DiD-labeled neural stem cells have also shown promising results following direct injection into the cerebrospinal fluid in vivo[35]. Also, among the Vybrant® dye series (DiR, DiI, CM-DiI, DiD), DiD has demonstrated comparatively high intense fluorescence[32]. Thus, these observations support our choice of DiD for cell labeling and long-term in vivo imaging.
In the present study, DiD did not show any interference with tissue autofluorescence and FITC due to fluorescence emission close to the NIR region. The quick and easy methodology of staining, non-toxicity, no interference with the functionality of the labeled cells, non-diffusion to adjacent cells, and lack of photo-bleaching were identified as the major advantages of DiD labeling. Furthermore, co-localization of CK and c-Met hepatic markers with the DiD-labeled transplanted cells in the recipient mouse livers showed the long-term persistence of transplanted cells. These observations support the involvement of selected markers in supporting the proliferation, survival, and differentiation of transplanted cells in the recipient’s liver regeneration and functional improvements[36-38]. In addition, the detection of hu-ALB-specific expression within the transplanted mouse livers further confirmed that the transplanted fHPCs not only persisted for the longer duration but also differentiated into functional hepatocytes contributing to liver regeneration and functional recovery. In addition, fHPCs successfully attenuated liver fibrosis in mouse liver. However, future studies could aid in deciphering the detailed molecular mechanisms by which fHPCs contribute to liver repair and regeneration.
Overall, the results from our study were supportive of the use of DiD in long-term, non-invasive, in vivo tracking of fHPCs in the recipient’s liver, but there are certain limitations. Although the results indicate the suitability of DiD for monitoring transplanted fHPCs in the liver, quantification of the signal to correlate the cell number or survival was not reported. Future studies using a larger cohort of animals, varying cell numbers, and quantification of the signal intensity in relation to cell doses would be very useful to understand the efficacy and survival. Moreover, optimizing the route of cell delivery, and assessing the dynamic changes in the expression of EpCAM over time will help in addressing the questions on engraftment and differentiation of fHPCs. Lastly, DiD has been proven to be safe and non-toxic with no effect on the metabolic functioning of cells in vitro. However, for regular in vivo imaging applications of DiD, it is essential to evaluate the metabolic cycle of the dye for long-term use. Addressing such issues would make DiD labeling more valuable for wider in vivo cell tracking applications.
Monitoring the fate of transplanted cells through in vivo tracking or imaging can help in understanding the homing, engraftment, long-term survival, and function of the transplanted cells. In this preclinical study, DiD-labeled fHPCs showed efficient long-term cell tracking for up to 80 d. The ease of handling, non-toxicity, and long-term signal retention proved to be major advantages in using DiD as a cell labeling agent for non-invasive, long-term tracking of cells both in vivo and ex vivo. These findings could pave the way to unravel the underlying regenerative mechanisms and contribution of exogenously transplanted cells in restoring the structural and functional deficits of the liver in CLD.
Determining the fate of transplanted cells in vivo through long-term cell tracking remains a crucial field of investigation. Long-term live cell tracking in vivo has always been challenging due to the absence of a safe cell-labeling agent.
DiD is a carbocyanine dye having good photochemical properties of strong fluorescence, and stability. Due to the long-range emission of DiD (670 nm), tissue autofluorescence is minimum, permitting the use of other fluorochromes such as fluorescein isothiocyanate, for colocalization studies to evaluate the expression of other essential markers specific to the transplanted cells in the recipient tissue. Moreover, the process for labeling the cells using DiD is easy due to its excellent efficiency for integration and diffusion into the cell membranes. The effects of DiD labeling on in vivo retention of labeled human liver cells remain to be investigated.
The present study aimed to shed light on the fate of DiD-labeled human liver cells in chronic liver diseases (CLD)-severe combined immunodeficiency (SCID) mice using live imaging up to 80 d post-transplantation.
A chronic liver disease SCID mouse model was developed, which received DiD-labeled EpCAM-positive human hepatic progenitor cells by intra-hepatic infusion. The long-term survival and functional response of transplanted DiD-labeled cells were investigated up to 80 d.
This study showed that DiD labeling of human liver cells is easy and efficient for long-term and non-invasive tracking in vivo up to 80 d post-transplantation. Using DiD, the fate of transplanted cells was determined. Transplanted human fetal liver cells were able to provide structural and functional improvement in CLD-SCID mice.
Monitoring the fate of transplanted cells through DiD-based in vivo live cell imaging can help in understanding the homing, engraftment, long-term survival, and function of the transplanted cells.
The findings of the current study may pave the way to unravel the underlying regenerative mechanisms and contribution of exogenously transplanted cells in restoring the structural and functional deficits of the liver in CLD.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu L, China; Sintusek P, Thailand S-Editor: Fan JR L-Editor: Webster JR P-Editor: Cai YX
| 1. | Tripura C, Khan A, Pande G. Cell Based Therapy for Chronic Liver Disease: Role of Fetal Liver Cells in Restoration of the Liver Cell Functions. In (Ed.), Liver Regeneration. Chapter 12, IntechOpen 2012: 217-240. |
| 2. | Kwak KA, Cho HJ, Yang JY, Park YS. Current Perspectives Regarding Stem Cell-Based Therapy for Liver Cirrhosis. Can J Gastroenterol Hepatol. 2018;2018:4197857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 3. | Mohamadnejad M, Vosough M, Moossavi S, Nikfam S, Mardpour S, Akhlaghpoor S, Ashrafi M, Azimian V, Jarughi N, Hosseini SE, Moeininia F, Bagheri M, Sharafkhah M, Aghdami N, Malekzadeh R, Baharvand H. Intraportal Infusion of Bone Marrow Mononuclear or CD133+ Cells in Patients With Decompensated Cirrhosis: A Double-Blind Randomized Controlled Trial. Stem Cells Transl Med. 2016;5:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Gholamrezanezhad A, Mirpour S, Bagheri M, Mohamadnejad M, Alimoghaddam K, Abdolahzadeh L, Saghari M, Malekzadeh R. In vivo tracking of 111In-oxine labeled mesenchymal stem cells following infusion in patients with advanced cirrhosis. Nucl Med Biol. 2011;38:961-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 196] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 5. | Rajaram R, Subramani B, Abdullah BJJ, Mahadeva S. Mesenchymal stem cell therapy for advanced liver cirrhosis: A case report. JGH Open. 2017;1:153-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Khan AA, Shaik MV, Parveen N, Rajendraprasad A, Aleem MA, Habeeb MA, Srinivas G, Raj TA, Tiwari SK, Kumaresan K, Venkateswarlu J, Pande G, Habibullah CM. Human fetal liver-derived stem cell transplantation as supportive modality in the management of end-stage decompensated liver cirrhosis. Cell Transplant. 2010;19:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Khan AA, Parveen N, Mahaboob VS, Rajendraprasad A, Ravindraprakash HR, Venkateswarlu J, Rao SG, Narusu ML, Khaja MN, Pramila R, Habeeb A, Habibullah CM. Safety and efficacy of autologous bone marrow stem cell transplantation through hepatic artery for the treatment of chronic liver failure: a preliminary study. Transplant Proc. 2008;40:1140-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Zhang RR, Zheng YW, Li B, Tsuchida T, Ueno Y, Nie YZ, Taniguchi H. Human hepatic stem cells transplanted into a fulminant hepatic failure Alb-TRECK/SCID mouse model exhibit liver reconstitution and drug metabolism capabilities. Stem Cell Res Ther. 2015;6:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Tolosa L, Caron J, Hannoun Z, Antoni M, López S, Burks D, Castell JV, Weber A, Gomez-Lechon MJ, Dubart-Kupperschmitt A. Transplantation of hESC-derived hepatocytes protects mice from liver injury. Stem Cell Res Ther. 2015;6:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 10. | Nagamoto Y, Takayama K, Ohashi K, Okamoto R, Sakurai F, Tachibana M, Kawabata K, Mizuguchi H. Transplantation of a human iPSC-derived hepatocyte sheet increases survival in mice with acute liver failure. J Hepatol. 2016;64:1068-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 11. | Irudayaswamy A, Muthiah M, Zhou L, Hung H, Jumat NHB, Haque J, Teoh N, Farrell G, Riehle KJ, Lin JS, Su LL, Chan JK, Choolani M, Wong PC, Wee A, Lim SG, Campbell J, Fausto N, Dan YY. Long-Term Fate of Human Fetal Liver Progenitor Cells Transplanted in Injured Mouse Livers. Stem Cells. 2018;36:103-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Cen P, Chen J, Hu C, Fan L, Wang J, Li L. Noninvasive in-vivo tracing and imaging of transplanted stem cells for liver regeneration. Stem Cell Res Ther. 2016;7:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Couto BG, Goldenberg RC, da Fonseca LM, Thomas J, Gutfilen B, Resende CM, Azevedo F, Mercante DR, Torres AL, Coelho HS, Maiolino A, Alves AL, Dias JV, Moreira MC, Sampaio AL, Sousa MA, Kasai-Brunswick TH, Souza SA, Campos-de-Carvalho AC, Rezende GF. Bone marrow mononuclear cell therapy for patients with cirrhosis: a Phase 1 study. Liver Int. 2011;31:391-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Pang P, Wu C, Gong F, Zhu K, Meng X, Cheng D, Hu X, Shan H, Shuai X. Nanovector for Gene Transfection and MR Imaging of Mesenchymal Stem Cells. J Biomed Nanotechnol. 2015;11:644-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Zhao J, Vykoukal J, Abdelsalam M, Recio-Boiles A, Huang Q, Qiao Y, Singhana B, Wallace M, Avritscher R, Melancon MP. Stem cell-mediated delivery of SPIO-loaded gold nanoparticles for the theranosis of liver injury and hepatocellular carcinoma. Nanotechnology. 2014;25:405101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Li C, Kong Y, Wang H, Wang S, Yu H, Liu X, Yang L, Jiang X, Li L. Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. J Hepatol. 2009;50:1174-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 17. | Di Rocco G, Gentile A, Antonini A, Truffa S, Piaggio G, Capogrossi MC, Toietta G. Analysis of biodistribution and engraftment into the liver of genetically modified mesenchymal stromal cells derived from adipose tissue. Cell Transplant. 2012;21:1997-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Progatzky F, Dallman MJ, Lo Celso C. From seeing to believing: labelling strategies for in vivo cell-tracking experiments. Interface Focus. 2013;3:20130001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 172] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 19. | Johnson I, Spence M. The Molecular Probes Handbook. A Guide to Fluorescent Probes and Labeling Technologies, 11th Edn. Life Technologies Corporation, 2010. [cited 10 June 2022]. Available from: https://www.thermofisher.com/us/en/home/references/molecular-probes-the-handbook.html. |
| 20. | Konno A, Matsumoto N, Tomono Y, Okazaki S. Pathological application of carbocyanine dye-based multicolour imaging of vasculature and associated structures. Sci Rep. 2020;10:12613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Ezzat T, Dhar DK, Malago M, Olde Damink SW. Dynamic tracking of stem cells in an acute liver failure model. World J Gastroenterol. 2012;18:507-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Ma HC, Shi XL, Ren HZ, Yuan XW, Ding YT. Targeted migration of mesenchymal stem cells modified with CXCR4 to acute failing liver improves liver regeneration. World J Gastroenterol. 2014;20:14884-14894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Dollé L, Theise ND, Schmelzer E, Boulter L, Gires O, van Grunsven LA. EpCAM and the biology of hepatic stem/progenitor cells. Am J Physiol Gastrointest Liver Physiol. 2015;308:G233-G250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 24. | Rao MS, Khan AA, Parveen N, Habeeb MA, Habibullah CM, Pande G. Characterization of hepatic progenitors from human fetal liver during second trimester. World J Gastroenterol. 2008;14:5730-5737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Aizarani N, Saviano A, Sagar, Mailly L, Durand S, Herman JS, Pessaux P, Baumert TF, Grün D. A human liver cell atlas reveals heterogeneity and epithelial progenitors. Nature. 2019;572:199-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 480] [Cited by in RCA: 745] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 26. | Chikate TR, Tang L. Tracking and Imaging of Transplanted Stem Cells in Animals. Methods Mol Biol. 2020;2150:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Ikeda E, Yagi K, Kojima M, Yagyuu T, Ohshima A, Sobajima S, Tadokoro M, Katsube Y, Isoda K, Kondoh M, Kawase M, Go MJ, Adachi H, Yokota Y, Kirita T, Ohgushi H. Multipotent cells from the human third molar: feasibility of cell-based therapy for liver disease. Differentiation. 2008;76:495-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Zhan Y, Wang Y, Wei L, Chen H, Cong X, Fei R, Gao Y, Liu F. Differentiation of hematopoietic stem cells into hepatocytes in liver fibrosis in rats. Transplant Proc. 2006;38:3082-3085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Li P, Zhang R, Sun H, Chen L, Liu F, Yao C, Du M, Jiang X. PKH26 can transfer to host cells in vitro and vivo. Stem Cells Dev. 2013;22:340-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Sun S, Chen G, Xu M, Qiao Y, Zheng S. Differentiation and migration of bone marrow mesenchymal stem cells transplanted through the spleen in rats with portal hypertension. PLoS One. 2013;8:e83523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Mäkinen M, Joki T, Ylä-Outinen L, Skottman H, Narkilahti S, Aänismaa R. Fluorescent probes as a tool for cell population tracking in spontaneously active neural networks derived from human pluripotent stem cells. J Neurosci Methods. 2013;215:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Quayle LA, Ottewell PD, Holen I. Chemotherapy resistance and stemness in mitotically quiescent human breast cancer cells identified by fluorescent dye retention. Clin Exp Metastasis. 2018;35:831-846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Yumoto K, Berry JE, Taichman RS, Shiozawa Y. A novel method for monitoring tumor proliferation in vivo using fluorescent dye DiD. Cytometry A. 2014;85:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Mohtasebi MS, Nasri F, Kamali Sarvestani E. Effect of DiD Carbocyanine Dye Labeling on Immunoregulatory Function and Differentiation of Mice Mesenchymal Stem Cells. Stem Cells Int. 2014;2014:457614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Carradori D, Barreau K, Eyer J. The carbocyanine dye DiD labels in vitro and in vivo neural stem cells of the subventricular zone as well as myelinated structures following in vivo injection in the lateral ventricle. J Neurosci Res. 2016;94:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Kawai M, Saegusa Y, Kemmochi S, Harada T, Shimamoto K, Shibutani M, Mitsumori K. Cytokeratin 8/18 is a useful immunohistochemical marker for hepatocellular proliferative lesions in mice. J Vet Med Sci. 2010;72:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Huh CG, Factor VM, Sánchez A, Uchida K, Conner EA, Thorgeirsson SS. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci U S A. 2004;101:4477-4482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 615] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 38. | Suárez-Causado A, Caballero-Díaz D, Bertrán E, Roncero C, Addante A, García-Álvaro M, Fernández M, Herrera B, Porras A, Fabregat I, Sánchez A. HGF/c-Met signaling promotes liver progenitor cell migration and invasion by an epithelial-mesenchymal transition-independent, phosphatidyl inositol-3 kinase-dependent pathway in an in vitro model. Biochim Biophys Acta. 2015;1853:2453-2463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |