Published online Jan 27, 2022. doi: 10.4254/wjh.v14.i1.62
Peer-review started: March 6, 2021
First decision: July 27, 2021
Revised: August 2, 2021
Accepted: December 9, 2021
Article in press: December 9, 2021
Published online: January 27, 2022
Processing time: 320 Days and 13.6 Hours
Loss of follow-up or reinfections hinder the expectations of hepatitis C eradication despite the existence of highly effective treatments. Moreover, the elimination of the infection does not imply the reversion of those chronic alterations derived from the previous infection by hepatitis C virus (HCV). This review analyzes the risk factors associated with loss to follow-up in diagnosis or treatment, and the possibility of reinfection. Likewise, it assesses the residual alterations induced by chronic HCV infection considering the liver alterations (inflammation, fibrosis, risk of decompensation, hepatocellular carcinoma, liver transplantation) and, on the other hand, the comorbidities and extrahepatic manifestations (cryoglobulinemia, non-Hodgkin lymphoma, peripheral insulin resistance, and lipid, bone and cognitive alterations). Peculiarities present in subjects coinfected with human immunodeficiency virus are analyzed in each section.
Core Tip: The excellent hepatitis C virus (HCV) response to direct acting agents should not obviate certain obstacles to eradicate this pathology, especially the loss to follow-up and the possibility of reinfections. Chronic hepatitis C determines persistent alterations despite the elimination of HCV, such as liver dysfunction and continued risk of decompensation and hepatocarcinoma, especially in subjects treated in advanced stages of the disease. Weight gain after sustained virological response (SVR) may favor liver steatosis, increasing the risk of progression of hepatic disease. The probability of complications after SVR in human immunodeficiency virus coinfected patients is similar to that of those HCV-monoinfected.
- Citation: Cuesta-Sancho S, Márquez-Coello M, Illanes-Álvarez F, Márquez-Ruiz D, Arizcorreta A, Galán-Sánchez F, Montiel N, Rodriguez-Iglesias M, Girón-González JA. Hepatitis C: Problems to extinction and residual hepatic and extrahepatic lesions after sustained virological response. World J Hepatol 2022; 14(1): 62-79
- URL: https://www.wjgnet.com/1948-5182/full/v14/i1/62.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i1.62
In 2015, 71 million people were estimated to be infected by hepatitis C virus (HCV) worldwide[1]. Based on the release of curative treatment for chronic hepatitis C infection[2], the World Health Assembly set the target of a 90% reduction in new infections and a 65% reduction in viral hepatitis related mortality by 2030 as compared to 2015[3].
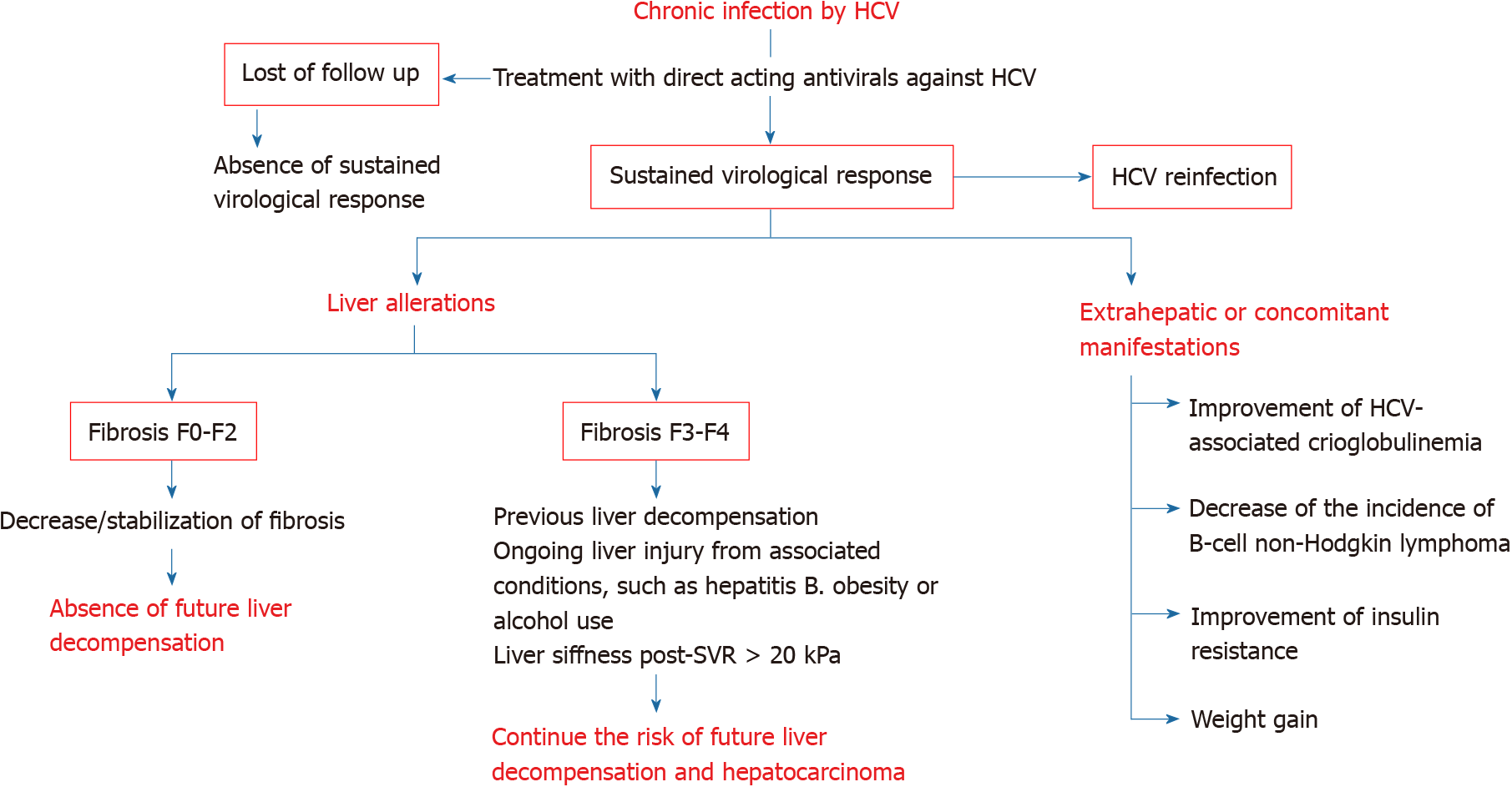
These expectations should not let us forget some problems that underlie the current situation and that could be classified into the following (Figure 1): (1) Patients whose HCV infection has not been eradicated or those who have been reinfected; and (2) Organic injuries associated with chronic hepatitis C, whether hepatic or extrahepatic, whose normalization is not reached by the elimination of the virus.
Both American[4] and European[5] Associations for the Study of Liver Diseases (AASLD and EASL, respectively) recommend combinations of direct acting agents (DAAs) against HCV, such as an NS5A inhibitor with either an NS3/4 protease inhibitor (grazoprevir/elbasvir or glecaprevir/pibrentasvir), or a nucleotide analogue plus an NS5A inhibitor (sofosbuvir/velpatasvir), for eight to twelve weeks. The preferred regimens to simplify HCV therapy are pangenotypics combinations (sofosbuvir/velpatasvir or glecaprevir/pibrentasvir)[6].
Treatment should be offered to all HCV RNA-positive patients. The efficacy of these combinations has been higher than 90%[6].
The proportion of patients who do not achieve a sustained virological response (SVR) is lower than 10%, considering those who have virologic relapse and those who are lost during the follow-up[7,8]. The combined therapy with sofosbuvir, velpatasvir and voxilaprevir is recommended for retreating patients with previously failing DAAs regimens[9,10].
There are few contraindications to therapy with DAAs. The use of certain cytochrome P450/P-glycoprotein inducing agents are contraindicated with all regimens, because of the risk of reducing DAAs concentrations. In patients with Child-Pugh B or C decompensated cirrhosis, NS3/4a protease inhibitors are contraindicated due to the increased concentrations of protease inhibitor in these patients and its associated toxicity risk. In patients with a glomerular filtration rate lower than 30 mL/min/1.73 m2, increased serum levels of sofosbuvir are detected[8]. Interactions between DAAs and other drugs need to be addressed in patients, mainly in those with human immunodeficiency virus (HIV)/HCV-coinfection and those with central nervous system-acting drugs[11].
A major barrier to HCV elimination is the loss to follow-up, defined as nonattendance to any appointment in the care cascade at any time[12]. A review about the loss to follow-up in HCV care has been recently published[13]. Factors associated with the loss to follow-up are younger age (< 45 years old)[14], treatment in hospital[15], a history of homelessness[15,16], mental illness[16,17] and injecting drug use, either past[18] or ongoing[17]. In contrast, factors associated with retention in care are older age (≥ 60 years old) and HIV coinfection[19].
Several strategies have been proposed to overcome the loss to follow-up in HCV care[20]: (1) Enhancing HCV identification and linkage to care for vulnerable populations (injecting drug users) through intensified outreach screening. A large-scale intensified screening initiative across Europa (Hep-Check and Hep-Link) has been started up[21,22]; (2) HCV micro-elimination strategies, focused on collectives with a high prevalence and/or increased risk of loss of follow-up and/or in patients with worst short-term prognosis[23,24]; (3) Reflex testing, a strategy of hepatitis C diagnosis in a single step, based on the detection of HCV RNA or HCV core antigen when the anti-HCV antibody test proves to be positive[25] and referral to an HCV specialist for further evaluation[26]; (4) Use of pan-genotypic HCV drug regimens; and (5) Inclusion of HCV-infected patients who use drugs on opioid agonist therapy programs can reach elevated HCV elimination rates with current DAAs[27].
Reinfection following SVR has been documented in several studies in drug users[28], prisoners[29], and men who have sex with men (MSM)[30]. After SVR, the incidence of reinfection is 2 to 6/100 person-years in subjects who inject drugs and 10 to 15/100 person-years in HIV-infected MSM[30-33]. Elevated rates of reinfection may compromise the benefits of treatment.
The analysis of the changes in the hepatitis C evolution after SVR will be considered in several sections: (1) Overall survival; (2) Changes in liver disease (liver fibrosis, liver function, decompensation of chronic liver disease, hepatocarcinoma); and (3) Modifications of extrahepatic alterations. In each section and whenever evidence is available, the changes in HIV/HCV-coinfected patients will be discussed.
Chronic HCV infection is associated with a substantially impaired overall survival, both by liver-related and extrahepatic causes[34].
Individuals with compensated cirrhosis who reach SVR with interferon-based treatments have an improved long-term outcome[35-37]. Real-world cohorts have confirmed this significant reduction in the liver-related death risk after DAAs: In the HEPATHER study, the annual incidence of liver-related mortality in subjects with SVR was 0.36% vs 0.96% in non-SVRs; and in individuals with cirrhosis, the respective incidence was 0.64% vs 1.57%[38]. Even though in other studies[39-41], the risk reduction was more pronounced, the risk still existed. Therefore, a proportion of subjects (small but worrisome) dies because of liver-related disease after viral clearance.
Symptoms and mortality from severe extrahepatic manifestations, such as cryoglobulinemic vasculitis, renal-related effects[42,43] and some lymphoproliferative disorders[44,45] decrease with HCV eradication as well. Moreover, subjects with SVR have a better physical and emotional health and an improved life quality[46].
In HIV/HCV coinfected patients with compensated cirrhosis, benefits from SVR due to interferon-based regimens in the incidence of liver-related decompensation, some extrahepatic manifestations and the overall mortality, have been demonstrated[47,48]. In addition, decreases of HIV reservoirs[49] and HIV progression[48] have been observed after HCV eradication.
Inflammation, fibrosis, and liver function previous to treatment against HCV affect the prognosis of chronic liver disease[50].
Liver inflammation and fibrosis: Several factors contribute to liver inflammation, acting on macrophage receptors. They include viral particles; pathogen-associated molecular patterns, such as lipopolysaccharides, which can translocate from the intestine into the circulation because of increased intestinal permeability; and damage-associated molecular patterns released by hepatocytes[51]. These factors induce and amplify hepatic inflammation by activating macrophages[52]. Macrophage activation promotes hepatic stellate cell activation and extracellular matrix accumulation[53]. After antiviral therapy of chronic hepatitis C, macrophage activation is diminished, as indicated by biomarkers[54] or aminotransferase levels[55], in parallel with the amelioration of hepatic inflammation observed in liver biopsies[56]. Only patients who achieve a SVR solve inflammation in liver biopsies[57].
Biopsy-proven fibrosis regression is developed when SVR is achieved[58]. In patients treated with interferon-based regimens, a 39%-73% of subjects who reached SVR had decreased liver fibrosis and necrosis, as assessed by liver biopsy[55]. Likewise, several studies have reported a significant diminution in liver stiffness after treatment, either by interferon- or DAAs-based schemes[59-64].
HCV-induced fibrosis progresses more rapidly in HIV-coinfected patients than in monoinfected individuals[65]. The impact of SVR on liver fibrosis (biopsy-proven) or liver stiffness within HIV/HCV patients treated with interferon- or DAAs-based regimens has been also proved[66].
Liver function: In routine clinical practice, Child-Pugh and Model for End-stage Liver Disease (MELD) scores are often used for the evaluation of liver function. Although improvement of liver function is not uniformly demonstrated in studies with HCV-induced liver cirrhosis, a Child-Pugh decrease ≥ 1 and/or MELD decrease ≥ 2 between baseline and SVR has been demonstrated in a 56%-57% of DAAs-treated patients. Factors independently related with liver function improvement are male gender, bilirubin < 1.2 mg/dL and international normalized rate < 1.3 at baseline[67].
Short-term outcomes after DAAs in individuals with decompensated cirrhosis showed a decrease of the MELD score in the majority, while it did not change in 17%, and worsened in 25% of them[68-73]. Subjects with low MELD scores can even be removed from liver transplantation lists[74-76], although the clinical improvement may not necessarily persist, and they may be still in risk of relisting on the transplant list or even death[76-78].
A more detailed analysis of liver function changes can be obtained by using other methods[79]. Thus, it has been demonstrated that amelioration of inflammation improves the ureagenesis[80].
Decompensation of liver cirrhosis: Decompensated cirrhosis is characterized by the development of new ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, bleeding gastro-esophageal varices, hepato-renal syndrome or hepato-pulmonary syndrome[81].
With DAAs, subjects with compensated cirrhosis achieve SVR rates over 95%[82,83]. Among subject with ongoing or previous decompensation, SVR rates of approximately 80% are reached with DAAs treatment[69,73,84].
Hepatic venous pressure gradient (HVPG) improves shortly after DAAs therapy in patients with HCV-related cirrhosis[85-87]. However, an HVPG more elevated at baseline is linked with smaller reductions in portal pressure and practically all of those with an HVPG ≥ 16 mmHg remain with clinically significant portal hypertension after SVR[85,88,89]. These findings contribute to explain the persistence of risk of decompensations due to portal hypertension in patients with decompensated liver cirrhosis after SVR.
The severity of liver disease prior to the therapy is a predictor of liver decompen
Attending to these contradictory findings in patients with decompensated cirrhosis, in a retrospective study of patients with Child-Pugh class B or C, El-Sherif et al[77] analyzed those factors related with the reduction of Child-Pugh score to class A (implicating the absence of new decompensations) after DAAs. During a follow-up of 255 d, 31.6% of subjects with baseline Child-Pugh class B cirrhosis and 12.3% of subjects with Child-Pugh class C cirrhosis met the primary study end point. The presence of complications such as ascites or encephalopathy, serum concentration of albumin < 3.5 g/dL or alanine aminotransferase < 60 U/L, and body mass index (BMI) > 25 kg/m2 were related to a higher risk of not achieving a decrease in Child-Pugh to class A, regardless of SVR[77].
Bleeding from esophageal varices is uncommon after SVR[91]. However, subjects with compensated cirrhosis who achieve SVR should follow on receiving endoscopic surveillance for esophageal varices, according to the AASLD guidance[92]: (1) In those without known varices, surveillance endoscopy is indicated every 2 years if there are associated conditions, such as obesity or alcohol use; and every 3 years if liver injury is suppressed, such as after alcohol abstinence; and (2) In those with known varices, surveillance endoscopy is indicated every 12 mo if there is proof of present liver injury from associated conditions and every 24 mo if liver injury is quiescent[92]. However, in our opinion, these recommendations could be modulated by the knowledge of liver stiffness, as will be analyzed later on.
In individuals with compensated cirrhosis, HIV coinfection was not related with an increased probability of liver complications after viral eradication than those HCV-monoinfected[93,94]. In the series of Corma-Gómez et al[95], the likelihood of staying free of hepatic complications or transplant at 12 and 24 mo was 99% and 96% in HCV-monoinfected patients and 99 and 98% in HIV/HCV coinfected patients with predominantly (> 95% of individuals) Child-Pugh class A cirrhosis (P = 0.648). In the multivariate analysis of the overall population, liver decompensation before SVR, drug use as the risk factor for HCV infection –reduced healthcare adherence and ongoing use of toxics may underlie this finding– and liver stiffness at SVR were independently linked to the presence of a hepatic complication or requiring a liver transplant[96].
The importance of the liver stiffness at SVR has been remarked. Post-treatment liver stiffness > 20 kPa is significantly associated with developing cirrhosis decom
Furthermore, liver stiffness–based strategies recognize subjects with reduced risk of developing esophageal variceal bleeding episodes, in whom esophagogastroduodenoscopy screening can be unnecessary[95,99-102]. Corma-Gómez et al[95] demonstrated that subjects with a liver stiffness < 30 kPa and a platelet count > 110000/mm3 after SVR are not at risk of variceal bleeding[102].
Hepatocellular carcinoma: Hepatocellular carcinoma (HCC) incidence has been growing over the last two decades and is expected to rise until 2030 in several countries[103]. HCV-infected patients have a lifetime risk of approximately 5%. HCC is expected to occur 30 years after infection. In patients with hepatitis C, HCC is almost invariably present in the setting of cirrhosis[104].
Antiviral treatment of chronic HCV infections significantly reduces the risk of HCC[104-109]. Meta-analyses have shown that DAAs therapy is associated with a decrease of de novo HCC incidence close to 80%, similar to that achieved with interferon-based therapies[108,109]. However, it is the most frequent liver-related event after SVR[98,106]. The incidence rate of HCC after SVR is 1.1-1.9/100 patient-years[94,98].
A Spanish series including 1035 HCV-infected patients, of which 667 (64%) were coinfected with HIV, has demonstrated that HIV-coinfection appears to be associated with an inferior risk of HCC occurrence among patients with HCV and advanced fibrosis who reach SVR due to DAA[110], although these data are controversial[94]. HIV/HCV coinfected patients have an earlier onset and aggressive HCC, with associated higher mortality risk[93].
Post-SVR surveillance by liver imaging and alpha-fetoprotein (AFP) tests every six months after SVR is recommended in cirrhotic population by international guidelines[111,112]. This recommendation has also been extended to patients with advanced fibrosis (F3) by EASL guidelines[113]. Unless they are affected by liver comorbidities, patients with lower stages of fibrosis may be discharged from specialized care. Age, male sex, lower baseline albumin or higher bilirrubin levels, a FIB-4 score > 3.25, hepatitis B coinfection or a liver stiffness post-SVR ≥ 20 kPa have been associated with a higher risk of developing HCC[94,98,114]. A scheme of the factors that could influence the need of surveillance of HCC is shown in Table 1.
| Factors existing previous to sustained virological response | Factors existing after sustained virological response |
| Comorbidities (steatosis, diabetes mellitus, excessive alcohol consumption) | Persistently elevated ALT, AST, GGT, alpha-fetoprotein, liver stiffness, FIB-4, APRI, or VITRO |
| Male gender | Hypoalbuminemia |
| Age > 64 years | Increasing body weight (?) |
| F4 | |
| Portal hypertension | |
| Elevated FIB-4, APRI, alpha-fetoprotein | |
| History of decompensation | |
| History of IFN therapy (?) | |
| HCV genotypes 1 and 3 (?) |
A low sensitivity of usual screening methods of HCC occurrence has been observed. Ultrasonography has been reported to have 60% sensitivity and 97% specificity as a screening method of HCC in cirrhotic patients; it has been proved to be cost-effective[115]. The performance of ultrasound surveillance of HCC is even worse in HIV-coinfected patients with cirrhosis[116]. AFP by itself is not adequate for screening purposes: A low sensitivity (40%-75%), as well as a high false positive rate in active hepatitis, precludes its use as screening method[117].
Liver transplantation: Liver transplantation is an appropriate treatment option for individuals with acute liver failure, end-stage liver disease, and primary hepatic malignancy. Patients with cirrhosis are typically candidates for liver transplantation once MELD score is ≥ 15[118].
A decrease of MELD score is expected in a proportion of patients with cirrhosis after SVR[90]. However, although a clinically significant decrease in MELD score is achieved by a 25% of DAAs-treated patients across a short follow-up, after a longer period (median follow-up of 4 years), the average MELD variations are not significant[78]. Yet, these data suggest that certain patients with decompensated cirrhosis may benefit from therapy with DAAs.
Both International Liver Transplantation Society Consensus Statement[113] and EASL guidelines[114] only advise against antivirals when MELD score > 20, based on the ELITA study[74]. Treatment of subjects with more elevated scores could make MELD score improves at such point that they could no longer be eligible for liver transplantation, but they would still be at risk of fatal complications and/or low life quality. Below this threshold, the recommendations suggest offering antiviral therapy with the hope of a stable improvement in liver function.
Nowadays, the 5-year overall survival after liver transplantation in individuals with chronic HCV infection is expected to be approximately 75%, since HCV recurrence does not limit anymore the liver transplantation outcome because of possibility of SVR after DAAs therapies[119,120].
Two-thirds of patients with chronic hepatitis C present extrahepatic manifestations[75]. These include autoimmune and lymphoproliferative disorders, ranging from cryoglobulinemia vasculitis to malignant B-cell lymphoma[42-45], cutaneous, meta
Cryoglobulinemia and B cell non-Hodgkin lymphoma: There is evidence that SVR after treatment with peginterferon α and ribavirin is related to improvements in cryoglobulinemia associated to HCV infection and possible regression of B-cell non-Hodgkin lymphoma. In Cacoub’s meta-analysis, SVR was confirmed to be linked to notably more elevated proportion of complete remissions in subjects with cryoglobulinemia vasculitis [odds ratio (OR) 20.76] and objective response in those with malignant B-cell lymphoproliferative diseases (OR 6.49)[121].
Some data with DAAs therapy in the scenario of vasculitis end-organ disease related to cryoglobulinemia, including renal disease, have demonstrated responses in 20% to 90% of subjects[122,123]. Notwithstanding, subjects with severe end-organ disease are likely to still need plasmapheresis and/or rituximab[123].
Regression of marginal zone lymphomas in HCV-infected individuals after interferon-based therapies has been noticed[124]. In addition, HCV infection treatment diminishes the incidence of lymphomas in HCV-monoinfected individuals[45]. HCV treatment with interferon does not change the incidence of lymphomas in patients coinfected with HIV[125].
There are few data about the effects of an SVR achieved with DAAs therapy on extrahepatic diseases apart from potential regression of cryoglobulinemia and B-cell non-Hodgkin lymphoma[126,127].
Extrahepatic dermatologic manifestations: Approximately 50% of individuals with porphyria cutanea tarda present HCV infection[128]. Amelioration of this metabolic condition during interferon-based therapy has been described repeatedly[129]. Currently, there are not enough data to ascertain the effect of DAA therapy on porphyria cutanea tarda.
Between 10% and 40% of patients with lichen planus present HCV antibodies[130,131]. Contradictory data have been reported about resolution of lichen planus with interferon-based regimens[130,131], but promising perspectives with DAAs are present[131].
Comorbidities: Liver steatosis: Metabolic dysfunction-associated fatty liver disease (MAFLD)[132] is the main chronic liver disorder[133]. Subjects with chronic HCV infection and MAFLD display accelerated liver fibrosis progression, and a higher risk of developing HCC[134,135]. HCV clearance can lead to amelioration (and even to regression) of liver steatosis, at least when directly related to HCV genotype 3 infection[135,136]. However, a meaningful percentage of patients with SVR may still have continued to have steatosis not related to HCV, but to other factors associated with it, especially overweight/obesity[135].
An additional problem in patients with hepatitis C is the weight gain after SVR. In a prospective study on more than 11000 patients, 52.6% gained weight and 19.8% gained excess weight (defined as at least 9 kg gain after 24 mo). SVR was an independent weight gain predictor[137,138]. The mechanisms behind body weight modifications could involve neuropsychiatric alterations or diminished circulating levels of inflammatory cytokines[137]. Increased BMI after viral clearance has clinical impact on fibrosis evolution of HCV-infected patients. The long-term evaluation of the German HCV-contaminated anti-D cohort demonstrated that a 6% of patients with SVR after DAAs developed advanced liver fibrosis after 35 years from infection; BMI and viral clearance independently predicted the evolution to cirrhosis[138].
Comorbidities: Insulin resistance and diabetes mellitus, lipid alterations: HCV perturbs glucose metabolism inducing insulin resistance, which may progress to type 2 diabetes[135]. Furthermore, type 2 diabetes is one of the main risk factors of progression to chronic hepatitis C[134,135].
SVR has been shown to improve insulin resistance, as measured by the Homeostatic Model Assessment of Insulin Resistance score; it provides a significant protective effect on the incidence of diabetes[122,139]. However, patients with diabetes mellitus type 2 diagnosed previously to DAAs remain diabetic despite to SVR, although the doses of antidiabetic drugs could be smaller[139]. Moreover, antiviral therapy may reduce renal and cardiovascular (ischemic stroke, acute coronary syndrome) complications in HCV-infected patients with established diabetes, as has been demonstrated in a prospective cohort[140].
Type 2 diabetes mellitus could continue affecting progression of hepatitis C after SVR[134]. Pre-treatment diabetes has been linked to a higher risk of cirrhosis, liver decompensation and HCC in a population of 33000 patients without baseline cirrhosis, treated with DAA and followed up for 3 years. The effect of diabetes mellitus was independent of the attainment of SVR[141].
DAAs increase triglyceride and cholesterol release through very low-density lipoproteins, thus normalizing hepatic lipid homeostasis[142]. An increase in total cholesterol and low- and high-density lipoproteins is observed during treatment and after treatment completion[143].
Comorbidities: Osteoporosis: Viral hepatitis has been linked to decreased bone mineral density (BMD). Diverse factors have been hypothesized to contribute to it: elevated serum levels of inflammatory cytokines, decreased hepatic hydroxylation of vitamin D, altered hepatic production of insulin-like growth factor 1 and osteoprotegerin, and hypogonadism[144]. Osteoporosis and bone fractures are usual among individuals with liver cirrhosis, especially in those with other risk factors[145].
The knowledge about the effects of SVR on BMD in HCV-infected subjects is limited. Studies have included usually samples of less than 50 patients treated with peginterferon plus ribavirin regimens. Although limitations of these studies are evident, they have demonstrated that BMD values at the lumbar spine and the femoral neck improve after the treatment[146,147], but controversial data have been also reported[148].
In HIV coinfected patients, a higher risk of osteoporosis and bone fractures has been communicated. Meaningful modifications in BMD and bone remodeling biomarkers plasma levels have not been observed after HCV eradication[149].
Comorbidities: Cognitive alterations: Improvements of neurocognitive dysfunction are observed after interferon-based SVR[150,151], including self-reported mood outcomes[152]. This finding is corroborated by DAAs-induced improvements in brain magnetic resonance spectroscopy[153]. However, controversial data have been published[154]. These discrepancies could be due to the distinct methods used to assess cognitive dysfunction and the timing of the tests.
The excellent HCV response to DAAs treatment should not obviate certain obstacles to eradicate this pathology, especially the loss of follow-up and the possibility of reinfections.
Apart from the above, chronic hepatitis C determines several alterations whose normalization is not expected despite the elimination of HCV, especially in subjects treated in advanced stages of the disease. These include persistent liver dysfunction and continued risk of decompensation and HCC, although certainly less frequently than in individuals without SVR. Furthermore, weight gain after SVR may favor MAFLD in these patients increasing the risk of progression of liver disease. In HIV coinfected patients, SVR is not associated with a higher probability of liver complications than that of those HCV-monoinfected.
To summarize, DAAs administration reduces the death risk by cirrhosis and HCC and also reduces common comorbidities among people with HCV. Nevertheless, we are still far from eradicating the disease, but the World Health Organization goal by 2030 (a 90% reduction in new infections and a 65% reduction in viral hepatitis related mortality as compared to 2015)[3] is hopefully feasible.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shahini E S-Editor: Liu M L-Editor: A P-Editor: Liu M
| 1. | Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol Hepatol. 2017;2:161-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1493] [Cited by in RCA: 1472] [Article Influence: 184.0] [Reference Citation Analysis (0)] |
| 2. | Gottwein JM, Pham LV, Mikkelsen LS, Ghanem L, Ramirez S, Scheel TKH, Carlsen THR, Bukh J. Efficacy of NS5A inhibitors against hepatitis c virus genotypes 1-7 and escape variants. Gastroenterology. 2018;154:1435-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection 2018. [cited 30 December 2020]. Available from: https://www.who.int/hepatitis/publications/hepatitis-c-guidelines-2018/en/. |
| 4. | AASLD/IDSA HCV guidance: Recommendations for testing, managing, and treating hepatitis C. Clin Liver Dis. 2018;12:117-117. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol. 2018;69:461-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1281] [Cited by in RCA: 1211] [Article Influence: 173.0] [Reference Citation Analysis (0)] |
| 6. | Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral Direct-Acting Agent Therapy for Hepatitis C Virus Infection: A Systematic Review. Ann Intern Med. 2017;166:637-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 551] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 7. | Pawlotsky JM. Hepatitis C Virus Resistance to Direct-Acting Antiviral Drugs in Interferon-Free Regimens. Gastroenterology. 2016;151:70-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 413] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 8. | Naggie S, Muir AJ. Oral Combination Therapies for Hepatitis C Virus Infection: Successes, Challenges, and Unmet Needs. Annu Rev Med. 2017;68:345-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Bourlière M, Gordon SC, Flamm SL, Cooper CL, Ramji A, Tong M, Ravendhran N, Vierling JM, Tran TT, Pianko S, Bansal MB, de Lédinghen V, Hyland RH, Stamm LM, Dvory-Sobol H, Svarovskaia E, Zhang J, Huang KC, Subramanian GM, Brainard DM, McHutchison JG, Verna EC, Buggisch P, Landis CS, Younes ZH, Curry MP, Strasser SI, Schiff ER, Reddy KR, Manns MP, Kowdley KV, Zeuzem S; POLARIS-1 and POLARIS-4 Investigators. Sofosbuvir, Velpatasvir, and Voxilaprevir for Previously Treated HCV Infection. N Engl J Med. 2017;376:2134-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 418] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 10. | Llaneras J, Riveiro-Barciela M, Lens S, Diago M, Cachero A, García-Samaniego J, Conde I, Arencibia A, Arenas J, Gea F, Torras X, Luis Calleja J, Antonio Carrión J, Fernández I, María Morillas R, Rosales JM, Carmona I, Fernández-Rodríguez C, Hernández-Guerra M, Llerena S, Bernal V, Turnes J, González-Santiago JM, Montoliu S, Figueruela B, Badia E, Delgado M, Fernández-Bermejo M, Iñarrairaegui M, Pascasio JM, Esteban R, Mariño Z, Buti M. Effectiveness and safety of sofosbuvir/velpatasvir/voxilaprevir in patients with chronic hepatitis C previously treated with DAAs. J Hepatol. 2019;71:666-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 11. | Macías J, Monge P, Mancebo M, Merchante N, Neukam K, Real LM, Pineda JA. High frequency of potential interactions between direct-acting antivirals and concomitant therapy in HIV/hepatitis C virus-coinfected patients in clinical practice. HIV Med. 2017;18:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Rivero-Juarez A, Lopez-Cortes LF, Castaño M, Merino D, Marquez M, Mancebo M, Cuenca-Lopez F, Jimenez-Aguilar P, Lopez-Montesinos I, Lopez-Cardenas S, Collado A, Lopez-Ruz MA, Omar M, Tellez F, Perez-Stachowski X, Hernandez-Quero J, Girón-Gonzalez JA, Fernandez-Fuertes E, Rivero A; HERACLES cohort study team of the Grupo de Estudio de Hepatitis Virales (HEPAVIR) of the Sociedad Andaluza de Enfermedades Infecciosas (SAEI). Impact of universal access to hepatitis C therapy on HIV-infected patients: implementation of the Spanish national hepatitis C strategy. Eur J Clin Microbiol Infect Dis. 2017;36:487-494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | van Dijk M, Drenth JPH; HepNed study group. Loss to follow-up in the hepatitis C care cascade: A substantial problem but opportunity for micro-elimination. J Viral Hepat. 2020;27:1270-1283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Haridy J, Wigg A, Muller K, Ramachandran J, Tilley E, Waddell V, Gordon D, Shaw D, Huynh D, Stewart J, Nelson R, Warner M, Boyd M, Chinnaratha MA, Harding D, Ralton L, Colman A, Liew D, Iyngkaran G, Tse E; Adelaide Liver Group. Real-world outcomes of unrestricted direct-acting antiviral treatment for hepatitis C in Australia: The South Australian statewide experience. J Viral Hepat. 2018;25:1287-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Sherbuk JE, McManus KA, Kemp Knick T, Canan CE, Flickinger T, Dillingham R. Disparities in Hepatitis C Linkage to Care in the Direct Acting Antiviral Era: Findings From a Referral Clinic With an Embedded Nurse Navigator Model. Front Public Health. 2019;7:362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Adamson PC, Miceli J, Shiferaw B, Villanueva MS, Canterino JE. A Colocalized Hepatitis C Virus Clinic in a Primary Care Practice Improves Linkage to Care in a High Prevalence Population. Am J Med. 2020;133:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Read P, Lothian R, Chronister K, Gilliver R, Kearley J, Dore GJ, van Beek I. Delivering direct acting antiviral therapy for hepatitis C to highly marginalised and current drug injecting populations in a targeted primary health care setting. Int J Drug Policy. 2017;47:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 18. | Cachay ER, Mena A, Morano L, Benitez L, Maida I, Ballard C, Hill L, Torriani F, Castro A, Dore E, Castro S, de Mendoza Fernández C, Soriano V, Mathews WC; HCV-TREN Cohort. Predictors of Hepatitis C Treatment Failure After Using Direct-Acting Antivirals in People Living With Human Immunodeficiency Virus. Open Forum Infect Dis. 2019;6:ofz070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Darvishian M, Wong S, Binka M, Yu A, Ramji A, Yoshida EM, Wong J, Rossi C, Butt ZA, Bartlett S, Pearce ME, Samji H, Cook D, Alvarez M, Chong M, Tyndall M, Krajden M, Janjua NZ. Loss to follow-up: A significant barrier in the treatment cascade with direct-acting therapies. J Viral Hepat. 2020;27:243-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | AASLD. The American Association for the Study of Liver Diseases and the Infectious Diseases Society of America. HCV Testing and Linkage to Care. [cited 30 December 2020]. Available from: https://www.hcvguidelines.org/evaluate/testing-and-linkage. |
| 21. | Nic An Riogh E, Swan D, McCombe G, O'Connor E, Avramovic G, Macías J, Oprea C, Story A, Surey J, Vickerman P, Ward Z, Lambert JS, Tinago W, Ianache I, Iglesias M, Cullen W. Integrating hepatitis C care for at-risk groups (HepLink): baseline data from a multicentre feasibility study in primary and community care. J Antimicrob Chemother. 2019;74:v31-v38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Barror S, Avramovic G, Oprea C, Surey J, Story A, Macías J, Cullen W, Crowley D, Horan A, Naughton AM, Iglesias M, Ianache I, Lazar S, Popa I, McHugh T, Menezes D, Tinago W, Lambert JS. HepCare Europe: a service innovation project. HepCheck: enhancing HCV identification and linkage to care for vulnerable populations through intensified outreach screening. A prospective multisite feasibility study. J Antimicrob Chemother. 2019;74:v39-v46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Rivero-Juarez A, Tellez F, Mayorga MI, Merino D, Espinosa N, Macias J, Palacios R, Paniagua M, Collado A, Mohamed O, Perez-Stachowski J, Hernandez-Quero J, Fernandez-Fuertes E, Rivero A; Grupo de estudio de Hepatitis virales (HEPAVIR) of the Sociedad Andaluza de Enfermedades Infecciosas (SAEI). Progression to hepatitis C virus micro-elimination in people living with HIV in Spain. Clin Microbiol Infect. 2020; epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Hollande C, Parlati L, Pol S. Micro-elimination of hepatitis C virus. Liver Int. 2020;40 Suppl 1:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | López-Martínez R, Arias-García A, Rodríguez-Algarra F, Castellote-Bellés L, Rando-Segura A, Tarraso G, Vargas-Accarino E, Montserrat-Lloan I, Blanco-Grau A, Caballero-Garralda A, Ferrer-Costa R, Pumarola-Sunye T, Buti-Ferret M, Esteban-Mur R, Quer J, Casis-Saez E, Rodríguez-Frías F. Significant Improvement in Diagnosis of Hepatitis C Virus Infection by a One-Step Strategy in a Central Laboratory: an Optimal Tool for Hepatitis C Elimination? J Clin Microbiol. 2019;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Coyle C, Moorman AC, Bartholomew T, Klein G, Kwakwa H, Mehta SH, Holtzman D. The Hepatitis C Virus Care Continuum: Linkage to Hepatitis C Virus Care and Treatment Among Patients at an Urban Health Network, Philadelphia, PA. Hepatology. 2019;70:476-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 27. | Macías J, Morano LE, Téllez F, Granados R, Rivero-Juárez A, Palacios R, Ríos M, Merino D, Pérez-Pérez M, Collado A, Figueruela B, Morano A, Freyre-Carrillo C, Martín JM, Rivero A, García F, Pineda JA; HEPAVIR group from the Sociedad Andaluza de Enfermedades Infecciosas (SAEI) and the GEHEP group from the Sociedad Española de Enfermedades Infecciosas y Microbiología (SEIMC). Response to direct-acting antiviral therapy among ongoing drug users and people receiving opioid substitution therapy. J Hepatol. 2019;71:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Pineda JA, Núñez-Torres R, Téllez F, Mancebo M, García F, Merchante N, Pérez-Pérez M, Neukam K, Macías J, Real LM; HEPAVIR Group of The Andalusian Society of Infectious Diseases (SAEI). Hepatitis C virus reinfection after sustained virological response in HIV-infected patients with chronic hepatitis C. J Infect. 2015;71:571-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Marco A, Esteban JI, Solé C, da Silva A, Ortiz J, Roget M, Sarriera C, Teixidó N, Guerrero RA, Caylà JA. Hepatitis C virus reinfection among prisoners with sustained virological response after treatment for chronic hepatitis C. J Hepatol. 2013;59:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Vanhommerig JW, Thomas XV, van der Meer JT, Geskus RB, Bruisten SM, Molenkamp R, Prins M, Schinkel J; MOSAIC (MSM Observational Study for Acute Infection with hepatitis C) Study Group. Hepatitis C virus (HCV) antibody dynamics following acute HCV infection and reinfection among HIV-infected men who have sex with men. Clin Infect Dis. 2014;59:1678-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Aspinall EJ, Corson S, Doyle JS, Grebely J, Hutchinson SJ, Dore GJ, Goldberg DJ, Hellard ME. Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta-analysis. Clin Infect Dis. 2013;57 Suppl 2:S80-S89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 261] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 32. | Midgard H, Weir A, Palmateer N, Lo Re V 3rd, Pineda JA, Macías J, Dalgard O. HCV epidemiology in high-risk groups and the risk of reinfection. J Hepatol. 2016;65:S33-S45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 33. | Gonzalez-Serna A, Macias J, Palacios R, Gómez-Ayerbe C, Tellez F, Rivero-Juárez A, Fernandez M, Santos J, Real LM, Gonzalez-Domenech CM, Gomez-Mateos J, Pineda JA; HEPAVIR study group. Incidence of recently acquired hepatitis C virus infection among HIV-infected patients in southern Spain. HIV Med. 2021;22:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Lee MH, Yang HI, Lu SN, Jen CL, You SL, Wang LY, Wang CH, Chen WJ, Chen CJ; R. E.V.E.A.L.-HCV Study Group. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 426] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 35. | van der Meer AJ, Berenguer M. Reversion of disease manifestations after HCV eradication. J Hepatol. 2016;65:S95-S108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 36. | Singal AG, Volk ML, Jensen D, Di Bisceglie AM, Schoenfeld PS. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol. 2010;8:280-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 260] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 37. | Simmons B, Saleem J, Heath K, Cooke GS, Hill A. Long-Term Treatment Outcomes of Patients Infected With Hepatitis C Virus: A Systematic Review and Meta-analysis of the Survival Benefit of Achieving a Sustained Virological Response. Clin Infect Dis. 2015;61:730-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 212] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 38. | Carrat F, Fontaine H, Dorival C, Simony M, Diallo A, Hezode C, De Ledinghen V, Larrey D, Haour G, Bronowicki JP, Zoulim F, Asselah T, Marcellin P, Thabut D, Leroy V, Tran A, Habersetzer F, Samuel D, Guyader D, Chazouilleres O, Mathurin P, Metivier S, Alric L, Riachi G, Gournay J, Abergel A, Cales P, Ganne N, Loustaud-Ratti V, D'Alteroche L, Causse X, Geist C, Minello A, Rosa I, Gelu-Simeon M, Portal I, Raffi F, Bourliere M, Pol S; French ANRS CO22 Hepather cohort. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet. 2019;393:1453-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 462] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 39. | McDonald SA, Pollock KG, Barclay ST, Goldberg DJ, Bathgate A, Bramley P, Dillon JF, Fraser A, Innes HA, Kennedy N, Morris J, Went A, Hayes PC, Hutchinson SJ. Real-world impact following initiation of interferon-free hepatitis C regimens on liver-related outcomes and all-cause mortality among patients with compensated cirrhosis. J Viral Hepat. 2020;27:270-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 40. | Butt AA, Yan P, Shaikh OS, Lo Re V 3rd, Abou-Samra AB, Sherman KE. Treatment of HCV reduces viral hepatitis-associated liver-related mortality in patients: An ERCHIVES study. J Hepatol. 2020;73:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 41. | Backus LI, Belperio PS, Shahoumian TA, Mole LA. Impact of Sustained Virologic Response with Direct-Acting Antiviral Treatment on Mortality in Patients with Advanced Liver Disease. Hepatology. 2019;69:487-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 161] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 42. | Fabrizi F, Dixit V, Messa P. Antiviral therapy of symptomatic HCV-associated mixed cryoglobulinemia: meta-analysis of clinical studies. J Med Virol. 2013;85:1019-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 43. | Sise ME, Bloom AK, Wisocky J, Lin MV, Gustafson JL, Lundquist AL, Steele D, Thiim M, Williams WW, Hashemi N, Kim AY, Thadhani R, Chung RT. Treatment of hepatitis C virus-associated mixed cryoglobulinemia with direct-acting antiviral agents. Hepatology. 2016;63:408-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 190] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 44. | Gisbert JP, García-Buey L, Pajares JM, Moreno-Otero R. Systematic review: regression of lymphoproliferative disorders after treatment for hepatitis C infection. Aliment Pharmacol Ther. 2005;21:653-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Kawamura Y, Ikeda K, Arase Y, Yatsuji H, Sezaki H, Hosaka T, Akuta N, Kobayashi M, Suzuki F, Suzuki Y, Kumada H. Viral elimination reduces incidence of malignant lymphoma in patients with hepatitis C. Am J Med. 2007;120:1034-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 46. | Boscarino JA, Lu M, Moorman AC, Gordon SC, Rupp LB, Spradling PR, Teshale EH, Schmidt MA, Vijayadeva V, Holmberg SD; Chronic Hepatitis Cohort Study (CHeCS) Investigators. Predictors of poor mental and physical health status among patients with chronic hepatitis C infection: the Chronic Hepatitis Cohort Study (CHeCS). Hepatology. 2015;61:802-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 47. | Mira JA, Rivero-Juárez A, López-Cortés LF, Girón-González JA, Téllez F, de los Santos-Gil I, Macías J, Merino D, Márquez M, Ríos-Villegas MJ, Gea I, Merchante N, Rivero A, Torres-Cornejo A, Pineda JA; Grupo Andaluz para el Estudio de las Hepatitis Víricas de la Sociedad Andaluza de Enfermedades Infecciosas. Benefits from sustained virologic response to pegylated interferon plus ribavirin in HIV/hepatitis C virus-coinfected patients with compensated cirrhosis. Clin Infect Dis. 2013;56:1646-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 48. | Berenguer J, Rodríguez-Castellano E, Carrero A, Von Wichmann MA, Montero M, Galindo MJ, Mallolas J, Crespo M, Téllez MJ, Quereda C, Sanz J, Barros C, Tural C, Santos I, Pulido F, Guardiola JM, Rubio R, Ortega E, Montes ML, Jusdado JJ, Gaspar G, Esteban H, Bellón JM, González-García J; GESIDA HIV/HCV Cohort Study Group. Eradication of hepatitis C virus and non-liver-related non-acquired immune deficiency syndrome-related events in human immunodeficiency virus/hepatitis C virus coinfection. Hepatology. 2017;66:344-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 49. | Arca-Suarez J, Corrales-Cuevas M, Pascual-Pérez S, Trujillo-Soto T, Fernández-Gutiérrez Del Álamo C, Cuesta-Sancho S, Rodríguez-Iglesias M, Girón-González JA. HIV antibodies level as a marker of HIV persistence: The role of hepatitis C virus coinfection. Eur J Clin Microbiol Infect Dis. 2020;39:1503-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 50. | Marcellin P, Asselah T, Boyer N. Fibrosis and disease progression in hepatitis C. Hepatology. 2002;36:S47-S56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 51. | Heydtmann M. Macrophages in hepatitis B and hepatitis C virus infections. J Virol. 2009;83:2796-2802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 52. | Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 751] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 53. | Lidofsky A, Holmes JA, Feeney ER, Kruger AJ, Salloum S, Zheng H, Seguin IS, Altinbas A, Masia R, Corey KE, Gustafson JL, Schaefer EA, Hunt PW, Deeks S, Somsouk M, Chew KW, Chung RT, Alatrakchi N. Macrophage Activation Marker Soluble CD163 Is a Dynamic Marker of Liver Fibrogenesis in Human Immunodeficiency Virus/Hepatitis C Virus Coinfection. J Infect Dis. 2018;218:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 54. | Laursen TL, Wong GL, Kazankov K, Sandahl T, Møller HJ, Hamilton-Dutoit S, George J, Chan HL, Grønbaek H. Soluble CD163 and mannose receptor associate with chronic hepatitis B activity and fibrosis and decline with treatment. J Gastroenterol Hepatol. 2018;33:484-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, Ling MH, Albrecht J. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 803] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 56. | Schwabl P, Mandorfer M, Steiner S, Scheiner B, Chromy D, Herac M, Bucsics T, Hayden H, Grabmeier-Pfistershammer K, Ferlitsch A, Oberhuber G, Trauner M, Peck-Radosavljevic M, Reiberger T. Interferon-free regimens improve portal hypertension and histological necroinflammation in HIV/HCV patients with advanced liver disease. Aliment Pharmacol Ther. 2017;45:139-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 57. | Shiffman ML, Sterling RK, Contos M, Hubbard S, Long A, Luketic VA, Stravitz RT, Fuchs M, Sanyal AJ. Long term changes in liver histology following treatment of chronic hepatitis C virus. Ann Hepatol. 2014;13:340-349. [PubMed] |
| 58. | Akhtar E, Manne V, Saab S. Cirrhosis regression in hepatitis C patients with sustained virological response after antiviral therapy: a meta-analysis. Liver Int. 2015;35:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 59. | European Association for Study of Liver. Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1177] [Cited by in RCA: 1332] [Article Influence: 133.2] [Reference Citation Analysis (0)] |
| 60. | Macías J, Girón-González JA, González-Serrano M, Merino D, Cano P, Mira JA, Arizcorreta-Yarza A, Ruíz-Morales J, Lomas-Cabeza JM, García-García JA, Corzo JE, Pineda JA. Prediction of liver fibrosis in human immunodeficiency virus/hepatitis C virus coinfected patients by simple non-invasive indexes. Gut. 2006;55:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 61. | Castéra L, Sebastiani G, Le Bail B, de Lédinghen V, Couzigou P, Alberti A. Prospective comparison of two algorithms combining non-invasive methods for staging liver fibrosis in chronic hepatitis C. J Hepatol. 2010;52:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 62. | Vergniol J, Foucher J, Castéra L, Bernard PH, Tournan R, Terrebonne E, Chanteloup E, Merrouche W, Couzigou P, de Lédinghen V. Changes of non-invasive markers and FibroScan values during HCV treatment. J Viral Hepat. 2009;16:132-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 63. | Hézode C, Castéra L, Roudot-Thoraval F, Bouvier-Alias M, Rosa I, Roulot D, Leroy V, Mallat A, Pawlotsky JM. Liver stiffness diminishes with antiviral response in chronic hepatitis C. Aliment Pharmacol Ther. 2011;34:656-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 64. | Pons M, Santos B, Simón-Talero M, Ventura-Cots M, Riveiro-Barciela M, Esteban R, Augustin S, Genescà J. Rapid liver and spleen stiffness improvement in compensated advanced chronic liver disease patients treated with oral antivirals. Therap Adv Gastroenterol. 2017;10:619-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 65. | Martinez-Sierra C, Arizcorreta A, Díaz F, Roldán R, Martín-Herrera L, Pérez-Guzmán E, Girón-González JA. Progression of chronic hepatitis C to liver fibrosis and cirrhosis in patients coinfected with hepatitis C virus and human immunodeficiency virus. Clin Infect Dis. 2003;36:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 183] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 66. | Lledó GM, Carrasco I, Benítez-Gutiérrez LM, Arias A, Royuela A, Requena S, Cuervas-Mons V, de Mendoza C. Regression of liver fibrosis after curing chronic hepatitis C with oral antivirals in patients with and without HIV coinfection. AIDS. 2018;32:2347-2352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 67. | Macías J, Granados R, Téllez F, Merino D, Pérez M, Morano LE, Palacios R, Paniagua M, Frías M, Merchante N, Pineda JA; HEPAVIR GEHEP, RIS-HEP07 study groups. Similar recovery of liver function after response to all-oral HCV therapy in patients with cirrhosis with and without HIV coinfection. J Viral Hepat. 2019;26:16-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 68. | Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS Jr, Fried MW, Terrault NA, O'Leary JG, Vargas HE, Kuo A, Schiff E, Sulkowski MS, Gilroy R, Watt KD, Brown K, Kwo P, Pungpapong S, Korenblat KM, Muir AJ, Teperman L, Fontana RJ, Denning J, Arterburn S, Dvory-Sobol H, Brandt-Sarif T, Pang PS, McHutchison JG, Reddy KR, Afdhal N; SOLAR-1 Investigators. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients With Advanced Liver Disease. Gastroenterology. 2015;149:649-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 633] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 69. | Curry MP, O'Leary JG, Bzowej N, Muir AJ, Korenblat KM, Fenkel JM, Reddy KR, Lawitz E, Flamm SL, Schiano T, Teperman L, Fontana R, Schiff E, Fried M, Doehle B, An D, McNally J, Osinusi A, Brainard DM, McHutchison JG, Brown RS Jr, Charlton M; ASTRAL-4 Investigators. Sofosbuvir and Velpatasvir for HCV in Patients with Decompensated Cirrhosis. N Engl J Med. 2015;373:2618-2628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 613] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 70. | Maan R, van Tilborg M, Deterding K, Ramji A, van der Meer AJ, Wong F, Fung S, Sherman M, Manns MP, Cornberg M, Hansen BE, Wedemeyer H, Janssen HL, de Knegt RJ, Feld JJ. Safety and Effectiveness of Direct-Acting Antiviral Agents for Treatment of Patients With Chronic Hepatitis C Virus Infection and Cirrhosis. Clin Gastroenterol Hepatol. 2016;14:1821-1830.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 71. | Poordad F, Schiff ER, Vierling JM, Landis C, Fontana RJ, Yang R, McPhee F, Hughes EA, Noviello S, Swenson ES. Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post-liver transplantation recurrence. Hepatology. 2016;63:1493-1505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 349] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 72. | Manns M, Samuel D, Gane EJ, Mutimer D, McCaughan G, Buti M, Prieto M, Calleja JL, Peck-Radosavljevic M, Müllhaupt B, Agarwal K, Angus P, Yoshida EM, Colombo M, Rizzetto M, Dvory-Sobol H, Denning J, Arterburn S, Pang PS, Brainard D, McHutchison JG, Dufour JF, Van Vlierberghe H, van Hoek B, Forns X; SOLAR-2 investigators. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis. 2016;16:685-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 354] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 73. | Cheung MCM, Walker AJ, Hudson BE, Verma S, McLauchlan J, Mutimer DJ, Brown A, Gelson WTH, MacDonald DC, Agarwal K, Foster GR, Irving WL; HCV Research UK. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;65:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 317] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 74. | Belli LS, Berenguer M, Cortesi PA, Strazzabosco M, Rockenschaub SR, Martini S, Morelli C, Donato F, Volpes R, Pageaux GP, Coilly A, Fagiuoli S, Amaddeo G, Perricone G, Vinaixa C, Berlakovich G, Facchetti R, Polak W, Muiesan P, Duvoux C; European Liver and Intestine Association (ELITA). Delisting of liver transplant candidates with chronic hepatitis C after viral eradication: A European study. J Hepatol. 2016;65:524-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 253] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 75. | Pascasio JM, Vinaixa C, Ferrer MT, Colmenero J, Rubin A, Castells L, Manzano ML, Lorente S, Testillano M, Xiol X, Molina E, González-Diéguez L, Otón E, Pascual S, Santos B, Herrero JI, Salcedo M, Montero JL, Sánchez-Antolín G, Narváez I, Nogueras F, Giráldez Á, Prieto M, Forns X, Londoño MC. Clinical outcomes of patients undergoing antiviral therapy while awaiting liver transplantation. J Hepatol. 2017;67:1168-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 76. | Perricone G, Duvoux C, Berenguer M, Cortesi PA, Vinaixa C, Facchetti R, Mazzarelli C, Rockenschaub SR, Martini S, Morelli C, Monico S, Volpes R, Pageaux GP, Fagiuoli S, Belli LS; European Liver and Intestine Transplant Association (ELITA). Delisting HCV-infected liver transplant candidates who improved after viral eradication: Outcome 2 years after delisting. Liver Int. 2018;38:2170-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 77. | El-Sherif O, Jiang ZG, Tapper EB, Huang KC, Zhong A, Osinusi A, Charlton M, Manns M, Afdhal NH, Mukamal K, McHutchison J, Brainard DM, Terrault N, Curry MP. Baseline Factors Associated With Improvements in Decompensated Cirrhosis After Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection. Gastroenterology. 2018;154:2111-2121.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 78. | Verna EC, Morelli G, Terrault NA, Lok AS, Lim JK, Di Bisceglie AM, Zeuzem S, Landis CS, Kwo P, Hassan M, Manns MP, Vainorius M, Akushevich L, Nelson DR, Fried MW, Reddy KR. DAA therapy and long-term hepatic function in advanced/decompensated cirrhosis: Real-world experience from HCV-TARGET cohort. J Hepatol. 2020;73:540-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 79. | Laursen TL, Sandahl TD, Kazankov K, George J, Grønbæk H. Liver-related effects of chronic hepatitis C antiviral treatment. World J Gastroenterol. 2020;26:2931-2947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 80. | Chang ML, Cheng ML, Chang SW, Tang HY, Chiu CT, Yeh CT, Shiao MS. Recovery of pan-genotypic and genotype-specific amino acid alterations in chronic hepatitis C after viral clearance: transition at the crossroad of metabolism and immunity. Amino Acids. 2017;49:291-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 81. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2293] [Article Influence: 229.3] [Reference Citation Analysis (3)] |
| 82. | Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, Ghalib R, Gitlin N, Herring R, Lalezari J, Younes ZH, Pockros PJ, Di Bisceglie AM, Arora S, Subramanian GM, Zhu Y, Dvory-Sobol H, Yang JC, Pang PS, Symonds WT, McHutchison JG, Muir AJ, Sulkowski M, Kwo P; ION-2 Investigators. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1065] [Cited by in RCA: 1064] [Article Influence: 96.7] [Reference Citation Analysis (0)] |
| 83. | Feld JJ, Ramji A, Shafran SD, Willems B, Marotta P, Huchet E, Vachon ML, Svarovskaia ES, Huang KC, Hyland RH, Yun C, Massetto B, Brainard DM, McHutchison JG, Tam E, Bailey R, Cooper C, Yoshida EM, Greenbloom S, Elkhashab M, Borgia S, Swain MG. Ledipasvir-Sofosbuvir Plus Ribavirin in Treatment-Naive Patients With Hepatitis C Virus Genotype 3 Infection: An Open-Label Study. Clin Infect Dis. 2017;65:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 84. | Foster GR, Irving WL, Cheung MC, Walker AJ, Hudson BE, Verma S, McLauchlan J, Mutimer DJ, Brown A, Gelson WT, MacDonald DC, Agarwal K; HCV Research, UK. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64:1224-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 360] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 85. | Mandorfer M, Kozbial K, Schwabl P, Freissmuth C, Schwarzer R, Stern R, Chromy D, Stättermayer AF, Reiberger T, Beinhardt S, Sieghart W, Trauner M, Hofer H, Ferlitsch A, Ferenci P, Peck-Radosavljevic M. Sustained virologic response to interferon-free therapies ameliorates HCV-induced portal hypertension. J Hepatol. 2016;65:692-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 241] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 86. | Afdhal N, Everson GT, Calleja JL, McCaughan GW, Bosch J, Brainard DM, McHutchison JG, De-Oertel S, An D, Charlton M, Reddy KR, Asselah T, Gane E, Curry MP, Forns X. Effect of viral suppression on hepatic venous pressure gradient in hepatitis C with cirrhosis and portal hypertension. J Viral Hepat. 2017;24:823-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 87. | Díez C, Berenguer J, Ibañez-Samaniego L, Llop E, Pérez-Latorre L, Catalina MV, Hontañón V, Jiménez-Sousa MA, Aldámiz-Echevarría T, Martínez J, Calleja JL, Albillos A, Bellón JM, Resino S, González-García J, Bañares R. Persistence of Clinically Significant Portal Hypertension After Eradication of Hepatitis C Virus in Patients With Advanced Cirrhosis. Clin Infect Dis. 2020;71:2726-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 88. | Lens S, Alvarado-Tapias E, Mariño Z, Londoño MC, LLop E, Martinez J, Fortea JI, Ibañez L, Ariza X, Baiges A, Gallego A, Bañares R, Puente A, Albillos A, Calleja JL, Torras X, Hernández-Gea V, Bosch J, Villanueva C, Forns X, García-Pagán JC. Effects of All-Oral Anti-Viral Therapy on HVPG and Systemic Hemodynamics in Patients With Hepatitis C Virus-Associated Cirrhosis. Gastroenterology. 2017;153:1273-1283.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 206] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 89. | Lens S, Baiges A, Alvarado-Tapias E, LLop E, Martinez J, Fortea JI, Ibáñez-Samaniego L, Mariño Z, Rodríguez-Tajes S, Gallego A, Bañares R, Puente Á, Albillos A, Calleja JL, Torras X, Hernández-Gea V, Bosch J, Villanueva C, García-Pagán JC, Forns X. Clinical outcome and hemodynamic changes following HCV eradication with oral antiviral therapy in patients with clinically significant portal hypertension. J Hepatol. 2020;73:1415-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 90. | Krassenburg LAP, Maan R, Ramji A, Manns MP, Cornberg M, Wedemeyer H, de Knegt RJ, Hansen BE, Janssen HLA, de Man RA, Feld JJ, van der Meer AJ. Clinical outcomes following DAA therapy in patients with HCV-related cirrhosis depend on disease severity. J Hepatol. 2021;74:1053-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 91. | Morisco F, Granata R, Stroffolini T, Guarino M, Donnarumma L, Gaeta L, Loperto I, Gentile I, Auriemma F, Caporaso N. Sustained virological response: a milestone in the treatment of chronic hepatitis C. World J Gastroenterol. 2013;19:2793-2798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 92. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1441] [Article Influence: 180.1] [Reference Citation Analysis (3)] |
| 93. | Salmon-Ceron D, Nahon P, Layese R, Bourcier V, Sogni P, Bani-Sadr F, Audureau E, Merchadou L, Dabis F, Wittkop L, Roudot-Thoraval F; ANRS CO12 CirVir and ANRS CO13 HEPAVIH study groups. Human Immunodeficiency Virus/Hepatitis C Virus (HCV) Co-infected Patients With Cirrhosis Are No Longer at Higher Risk for Hepatocellular Carcinoma or End-Stage Liver Disease as Compared to HCV Mono-infected Patients. Hepatology. 2019;70:939-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 94. | Quaranta MG, Ferrigno L, Monti M, Filomia R, Biliotti E, Iannone A, Migliorino G, Coco B, Morisco F, Vinci M, D'Ambrosio R, Chemello L, Massari M, Ieluzzi D, Russo FP, Blanc P, Verucchi G, Puoti M, Rumi MG, Barbaro F, Santantonio TA, Federico A, Chessa L, Gentile I, Zuin M, Parruti G, Morsica G, Kondili LA; PITER Collaborating Group. Advanced liver disease outcomes after hepatitis C eradication by human immunodeficiency virus infection in PITER cohort. Hepatol Int. 2020;14:362-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 95. | Corma-Gómez A, Macías J, Morano L, Rivero A, Téllez F, Ríos MJ, Santos M, Serrano M, Palacios R, Merino D, Real LM, De Los Santos I, Vera-Méndez FJ, Galindo MJ, Pineda JA; RIS-HEP13 and GEHEP 011 Study Groups. Liver Stiffness-Based Strategies Predict Absence of Variceal Bleeding in Cirrhotic Hepatitis C Virus-Infected Patients With and Without Human Immunodeficiency Virus Coinfection After Sustained Virological Response. Clin Infect Dis. 2021;72:e96-e102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 96. | Corma-Gómez A, Morano L, Téllez F, Rivero-Juárez A, Real LM, Alados JC, Ríos-Villegas MJ, Vera-Méndez FJ, Muñoz RP, Geijo P, Macías J, Pineda JA; RIS-HEP13 and GEHEP 011 study groups. HIV infection does not increase the risk of liver complications in hepatitis C virus-infected patient with advanced fibrosis, after sustained virological response with direct-acting antivirals. AIDS. 2019;33:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 97. | Vutien P, Kim NJ, Moon AM, Pearson M, Su F, Berry K, Gelman H, Ioannou GN. Fibroscan liver stiffness after anti-viral treatment for hepatitis C is independently associated with adverse outcomes. Aliment Pharmacol Ther. 2020;52:1717-1727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 98. | Pons M, Rodríguez-Tajes S, Esteban JI, Mariño Z, Vargas V, Lens S, Buti M, Augustin S, Forns X, Mínguez B, Genescà J. Non-invasive prediction of liver-related events in patients with HCV-associated compensated advanced chronic liver disease after oral antivirals. J Hepatol. 2020;72:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 99. | Pineda JA, Recio E, Camacho A, Macías J, Almodóvar C, González-Serrano M, Merino D, Tellez F, Ríos MJ, Rivero A; Grupo Andaluz de Hepatitis Vírica (HEPAVIR) de la Sociedad Andaluza de Enfermedades Infecciosas (SAEI). Liver stiffness as a predictor of esophageal varices requiring therapy in HIV/hepatitis C virus-coinfected patients with cirrhosis. J Acquir Immune Defic Syndr. 2009;51:445-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 100. | Merchante N, Rivero-Juárez A, Téllez F, Merino D, Ríos-Villegas MJ, Ojeda-Burgos G, Omar M, Macías J, Rivero A, Pérez-Pérez M, Raffo M, López-Montesinos I, Márquez-Solero M, Gómez-Vidal MA, Pineda JA; Grupo Andaluz para el Estudio de las Hepatitis Víricas (HEPAVIR) de la Sociedad Andaluza de Enfermedades Infecciosas (SAEI). Liver stiffness predicts variceal bleeding in HIV/HCV-coinfected patients with compensated cirrhosis. AIDS. 2017;31:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 101. | Augustin S, Pons M, Maurice JB, Bureau C, Stefanescu H, Ney M, Blasco H, Procopet B, Tsochatzis E, Westbrook RH, Bosch J, Berzigotti A, Abraldes JG, Genescà J. Expanding the Baveno VI criteria for the screening of varices in patients with compensated advanced chronic liver disease. Hepatology. 2017;66:1980-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 204] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 102. | Merchante N, Saroli Palumbo C, Mazzola G, Pineda JA, Téllez F, Rivero-Juárez A, Ríos-Villegas MJ, Maurice JB, Westbrook RH, Judge R, Guaraldi G, Schepis F, Perazzo H, Rockstroh J, Boesecke C, Klein MB, Cervo A, Ghali P, Wong P, Petta S, De Ledinghen V, Macías J, Sebastiani G. Prediction of Esophageal Varices by Liver Stiffness and Platelets in Persons With Human Immunodeficiency Virus Infection and Compensated Advanced Chronic Liver Disease. Clin Infect Dis. 2020;71:2810-2817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 103. | World Health Organization. International Agency for Research on Cancer. Liver. [cited 30 December 2020]. Available from: http://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf. |
| 104. | Trinchet JC, Ganne-Carrié N, Nahon P, N'kontchou G, Beaugrand M. Hepatocellular carcinoma in patients with hepatitis C virus-related chronic liver disease. World J Gastroenterol. 2007;13:2455-2460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 105. | Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 376] [Article Influence: 47.0] [Reference Citation Analysis (1)] |
| 106. | Ogasawara N, Saitoh S, Akuta N, Fujiyama S, Kawamura Y, Sezaki H, Hosaka T, Kobayashi M, Suzuki F, Suzuki Y, Arase Y, Ikeda K, Kumada H. Long-term outcome of hepatocellular carcinoma occurrence, esophageal varices exacerbation, and mortality in hepatitis C virus-related liver cirrhosis after interferon-based therapy. Hepatol Res. 2019;49:1441-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 107. | El-Serag HB, Kanwal F, Richardson P, Kramer J. Risk of hepatocellular carcinoma after sustained virological response in Veterans with hepatitis C virus infection. Hepatology. 2016;64:130-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 313] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 108. | Waziry R, Hajarizadeh B, Grebely J, Amin J, Law M, Danta M, George J, Dore GJ. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J Hepatol. 2017;67:1204-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 381] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 109. | Saraiya N, Yopp AC, Rich NE, Odewole M, Parikh ND, Singal AG. Systematic review with meta-analysis: recurrence of hepatocellular carcinoma following direct-acting antiviral therapy. Aliment Pharmacol Ther. 2018;48:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 110. | Corma-Gómez A, Macías J, Lacalle-Remigio JR, Téllez F, Morano L, Rivero A, Serrano M, Ríos MJ, Vera-Méndez FJ, Alados JC, Real LM, Palacios R, Santos IL, Imatz A, Pineda JA; RIS-HEP13 and GEHEP 011 study groups. Human Immunodeficiency Virus (HIV) Infection Is Associated With Lower Risk of Hepatocellular Carcinoma After Sustained Virological Response to Direct-acting Antivirals in Hepatitis C Infected Patients With Advanced Fibrosis. Clin Infect Dis. 2021;73:e2109-e2116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 111. | Ghany MG, Morgan TR; AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology. 2020;71:686-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 529] [Article Influence: 105.8] [Reference Citation Analysis (0)] |
| 112. | Terrault NA, McCaughan GW, Curry MP, Gane E, Fagiuoli S, Fung JYY, Agarwal K, Lilly L, Strasser SI, Brown KA, Gadano A, Kwo PY, Burra P, Samuel D, Charlton M, Pessoa MG, Berenguer M. International Liver Transplantation Society Consensus Statement on Hepatitis C Management in Liver Transplant Candidates. Transplantation. 2017;101:945-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 113. | European Association for the Study of the Liver. Clinical Practice Guidelines Panel: Chair; EASL Governing Board representative; Panel members. EASL recommendations on treatment of hepatitis C: Final update of the series. J Hepatol. 2020;73:1170-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 779] [Article Influence: 155.8] [Reference Citation Analysis (0)] |
| 114. | Nabatchikova E, Abdurakhmanov D, Rozina T, Nikulkina E, Tanaschuk E, Moiseev S. Hepatocellular carcinoma surveillance after hepatitis C virus eradication: Is liver stiffness measurement more useful than laboratory fibrosis markers? J Hepatol. 2020;73:469-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 115. | Colli A, Fraquelli M, Casazza G, Massironi S, Colucci A, Conte D, Duca P. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol. 2006;101:513-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 387] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 116. | Merchante N, Figueruela B, Rodríguez-Fernández M, Rodríguez-Arrondo F, Revollo B, Ibarra S, Galindo MJ, Merino E, Montero M, Téllez F, García-Deltoro M, Rivero-Juárez A, Delgado-Fernández M, Ríos-Villegas MJ, Aguirrebengoa K, García MA, Portu J, Vera-Méndez FJ, Villalobos M, Mínguez C, De Los Santos I, López-Ruz MA, Omar M, Galera C, Macias J, Pineda JA; GEHEP-002 Study Group. Low performance of ultrasound surveillance for the diagnosis of hepatocellular carcinoma in HIV-infected patients. AIDS. 2019;33:269-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 117. | Peng SY, Chen WJ, Lai PL, Jeng YM, Sheu JC, Hsu HC. High alpha-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: significance of hepatitis virus infection, age, p53 and beta-catenin mutations. Int J Cancer. 2004;112:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 253] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 118. | Martin P, DiMartini A, Feng S, Brown R Jr, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59:1144-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 700] [Article Influence: 63.6] [Reference Citation Analysis (1)] |
| 119. | Neuberger JM, Bechstein WO, Kuypers DR, Burra P, Citterio F, De Geest S, Duvoux C, Jardine AG, Kamar N, Krämer BK, Metselaar HJ, Nevens F, Pirenne J, Rodríguez-Perálvarez ML, Samuel D, Schneeberger S, Serón D, Trunečka P, Tisone G, van Gelder T. Practical Recommendations for Long-term Management of Modifiable Risks in Kidney and Liver Transplant Recipients: A Guidance Report and Clinical Checklist by the Consensus on Managing Modifiable Risk in Transplantation (COMMIT) Group. Transplantation. 2017;101:S1-S56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 221] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 120. | Cotter TG, Paul S, Sandıkçı B, Couri T, Bodzin AS, Little EC, Sundaram V, Charlton M. Improved Graft Survival After Liver Transplantation for Recipients With Hepatitis C Virus in the Direct-Acting Antiviral Era. Liver Transpl. 2019;25:598-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 121. | Cacoub P, Desbois AC, Comarmond C, Saadoun D. Impact of sustained virological response on the extrahepatic manifestations of chronic hepatitis C: a meta-analysis. Gut. 2018;67:2025-2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 122. | Comarmond C, Garrido M, Pol S, Desbois AC, Costopoulos M, Le Garff-Tavernier M, Si Ahmed SN, Alric L, Fontaine H, Bellier B, Maciejewski A, Rosenzwajg M, Klatzmann D, Musset L, Poynard T, Cacoub P, Saadoun D. Direct-Acting Antiviral Therapy Restores Immune Tolerance to Patients With Hepatitis C Virus-Induced Cryoglobulinemia Vasculitis. Gastroenterology. 2017;152:2052-2062.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 123. | Emery JS, Kuczynski M, La D, Almarzooqi S, Kowgier M, Shah H, Wong D, Janssen HLA, Feld JJ. Efficacy and Safety of Direct Acting Antivirals for the Treatment of Mixed Cryoglobulinemia. Am J Gastroenterol. 2017;112:1298-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 124. | Arcaini L, Vallisa D, Rattotti S, Ferretti VV, Ferreri AJM, Bernuzzi P, Merli M, Varettoni M, Chiappella A, Ambrosetti A, Tucci A, Rusconi C, Visco C, Spina M, Cabras G, Luminari S, Tucci M, Musto P, Ladetto M, Merli F, Stelitano C, d'Arco A, Rigacci L, Levis A, Rossi D, Spedini P, Mancuso S, Marino D, Bruno R, Baldini L, Pulsoni A. Antiviral treatment in patients with indolent B-cell lymphomas associated with HCV infection: a study of the Fondazione Italiana Linfomi. Ann Oncol. 2014;25:1404-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 125. | Gutiérrez-Saborido D, Gutiérrez-Valencia A, González Domenech CM, López Ruz MÁ, Raffo Márquez M, Omar M, Girón-González JA; Grupo de Estudio de Hepatitis Virales (HEPAVIR) of the Sociedad Andaluza de Enfermedades Infecciosas (SAEI). Incidence of lymphoma in HIV-HCV-infected patients. Modifications in function of the anti-hepatitis C virus therapy. Ann Hematol. 2019;98:1953-1959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 126. | Sherman AC, Sherman KE. Extrahepatic manifestations of hepatitis C infection: navigating CHASM. Curr HIV/AIDS Rep. 2015;12:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 127. | Negro F, Forton D, Craxì A, Sulkowski MS, Feld JJ, Manns MP. Extrahepatic morbidity and mortality of chronic hepatitis C. Gastroenterology. 2015;149:1345-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 274] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 128. | Gisbert JP, García-Buey L, Pajares JM, Moreno-Otero R. Prevalence of hepatitis C virus infection in porphyria cutanea tarda: systematic review and meta-analysis. J Hepatol. 2003;39:620-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 129. | Takikawa H, Yamazaki R, Shoji S, Miyake K, Yamanaka M. Normalization of urinary porphyrin level and disappearance of skin lesions after successful interferon therapy in a case of chronic hepatitis C complicated with porphyria cutanea tarda. J Hepatol. 1995;22:249-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 130. | Gumber SC, Chopra S. Hepatitis C: a multifaceted disease. Review of extrahepatic manifestations. Ann Intern Med. 1995;123:615-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 299] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 131. | Sayiner M, Golabi P, Farhat F, Younossi ZM. Dermatologic Manifestations of Chronic Hepatitis C Infection. Clin Liver Dis. 2017;21:555-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 132. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 2830] [Article Influence: 566.0] [Reference Citation Analysis (1)] |
| 133. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 3792] [Article Influence: 541.7] [Reference Citation Analysis (2)] |
| 134. | Negro F. Residual risk of liver disease after hepatitis C virus eradication. J Hepatol. 2021;74:952-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 135. | Bugianesi E, Salamone F, Negro F. The interaction of metabolic factors with HCV infection: does it matter? J Hepatol. 2012;56 Suppl 1:S56-S65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 136. | Tada T, Kumada T, Toyoda H, Sone Y, Takeshima K, Ogawa S, Goto T, Wakahata A, Nakashima M, Nakamuta M, Tanaka J. Viral eradication reduces both liver stiffness and steatosis in patients with chronic hepatitis C virus infection who received direct-acting anti-viral therapy. Aliment Pharmacol Ther. 2018;47:1012-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 137. | Musialik J, Suchecka W, Klimacka-Nawrot E, Petelenz M, Hartman M, Błońska-Fajfrowska B. Taste and appetite disorders of chronic hepatitis C patients. Eur J Gastroenterol Hepatol. 2012;24:1400-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 138. | Wiese M, Fischer J, Löbermann M, Göbel U, Grüngreiff K, Güthoff W, Kullig U, Richter F, Schiefke I, Tenckhoff H, Zipprich A, Berg T, Müller T; East German HCV Study Group. Evaluation of liver disease progression in the German hepatitis C virus (1b)-contaminated anti-D cohort at 35 years after infection. Hepatology. 2014;59:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 139. | Dawood AA, Nooh MZ, Elgamal AA. Factors Associated with Improved Glycemic Control by Direct-Acting Antiviral Agent Treatment in Egyptian Type 2 Diabetes Mellitus Patients with Chronic Hepatitis C Genotype 4. Diabetes Metab J. 2017;41:316-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 140. | Hsu YC, Lin JT, Ho HJ, Kao YH, Huang YT, Hsiao NW, Wu MS, Liu YY, Wu CY. Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology. 2014;59:1293-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 227] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 141. | Benhammou JN, Moon AM, Pisegna JR, Su F, Vutien P, Moylan CA, Ioannou GN. Nonalcoholic Fatty Liver Disease Risk Factors Affect Liver-Related Outcomes After Direct-Acting Antiviral Treatment for Hepatitis C. Dig Dis Sci. 2021;66:2394-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 142. | Sun HY, Cheng PN, Tseng CY, Tsai WJ, Chiu YC, Young KC. Favouring modulation of circulating lipoproteins and lipid loading capacity by direct antiviral agents grazoprevir/elbasvir or ledipasvir/sofosbuvir treatment against chronic HCV infection. Gut. 2018;67:1342-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 143. | Villani R, Di Cosimo F, Romano AD, Sangineto M, Serviddio G. Serum lipid profile in HCV patients treated with direct-acting antivirals: a systematic review and meta-analysis. Sci Rep. 2021;11:13944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 144. | Lai JC, Shoback DM, Zipperstein J, Lizaola B, Tseng S, Terrault NA. Bone Mineral Density, Bone Turnover, and Systemic Inflammation in Non-cirrhotics with Chronic Hepatitis C. Dig Dis Sci. 2015;60:1813-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 145. | Patel N, Muñoz SJ. Bone disease in cirrhosis. Clin Liver Dis (Hoboken). 2015;6:96-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 146. | Redondo-Cerezo E, Casado-Caballero F, Martin-Rodriguez JL, Hernandez-Quero J, Escobar-Jimenez F, Gonzalez-Calvin JL. Bone mineral density and bone turnover in non-cirrhotic patients with chronic hepatitis C and sustained virological response to antiviral therapy with peginterferon-alfa and ribavirin. Osteoporos Int. 2014;25:1709-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 147. | Megahed A, Salem N, Fathy A, Barakat T, Alsayed MAEL, Mabood SAE, Zalata KR, Abdalla AF. Pegylated interferon α/ribavirin therapy enhances bone mineral density in children with chronic genotype 4 HCV infection. World J Pediatr. 2017;13:346-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 148. | Hofmann WP, Kronenberger B, Bojunga J, Stamm B, Herrmann E, Bücker A, Mihm U, von Wagner M, Zeuzem S, Sarrazin C. Prospective study of bone mineral density and metabolism in patients with chronic hepatitis C during pegylated interferon alpha and ribavirin therapy. J Viral Hepat. 2008;15:790-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 149. | Carrero A, Berenguer J, Hontañón V, Guardiola JM, Navarro J, von Wichmann MA, Téllez MJ, Quereda C, Santos I, Sanz J, Galindo MJ, Hernández-Quero J, Jiménez-Sousa MA, Pérez-Latorre L, Bellón JM, Resino S, Esteban H, Martínez E, González-García J; Grupo de Estudio del Sida (GESIDA) 3603B Study Group. Effects of Hepatitis C Virus (HCV) Eradication on Bone Mineral Density in Human Immunodeficiency Virus/HCV-Coinfected Patients. Clin Infect Dis. 2021;73:e2026-e2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 150. | Byrnes V, Miller A, Lowry D, Hill E, Weinstein C, Alsop D, Lenkinski R, Afdhal NH. Effects of anti-viral therapy and HCV clearance on cerebral metabolism and cognition. J Hepatol. 2012;56:549-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 151. | Laursen TL, Siggaard CB, Kazankov K, Sandahl TD, Møller HJ, Tarp B, Kristensen LH, Laursen AL, Leutscher P, Grønbaek H. Time-dependent improvement of liver inflammation, fibrosis and metabolic liver function after successful direct-acting antiviral therapy of chronic hepatitis C. J Viral Hepat. 2020;27:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 152. | Dusheiko G. The impact of antiviral therapy for hepatitis C on the quality of life: a perspective. Liver Int. 2017;37 Suppl 1:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 153. | Alsop D, Younossi Z, Stepanova M, Afdhal NH. Cerebral MR spectroscopy and patient-reported mental health outcomes in hepatitis C genotype 1 naive patients treated with ledipasvir and sofosbuvir. Hepatology. 2014;60:221a. |
| 154. | Curry MP, Moczynski NP, Liu L, Stamm L, Yun C, Brainard DM, McHutchison JG, Alsop D, Afdhal NH. The Effect of Sustained Virologic Response on Cerebral Metabolism and Neurocognition in Patients with Chronic Genotype 1 HCV Infection. J Hepatol. 2016;64:S797. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |