Published online Jan 27, 2022. doi: 10.4254/wjh.v14.i1.45
Peer-review started: February 24, 2021
First decision: October 17, 2021
Revised: October 24, 2021
Accepted: January 6, 2022
Article in press: January 6, 2022
Published online: January 27, 2022
Processing time: 330 Days and 10.9 Hours
The development of chronic kidney disease (CKD) after liver transplantation (LT) exerts a severe effect on the survival of patients. The widespread adoption of the model for end-stage liver disease score strongly impacted CKD incidence after the procedure, as several patients are transplanted with previously deteriorated renal function. Due to its multifactorial nature, encompassing pre-transplantation conditions, perioperative events, and nephrotoxic immunosuppressor therapies, the accurate identification of patients under risk of renal disease, and the implementation of preventive approaches, are extremely important. Methods for the evaluation of renal function in this setting range from formulas that estimate the glomerular filtration rate, to non-invasive markers, although no option has yet proved efficient in early detection of kidney injury. Considering the nephrotoxicity of calcineurin inhibitors (CNI) as a factor of utmost importance after LT, early nephroprotective strategies are highly recommended. They are based mainly on delaying the application of CNI during the immediate postoperative-period, reducing their dosage, and associating them with other less nephrotoxic drugs, such as mycophenolate mofetil and everolimus. This review provides a critical assessment of the causes of renal dysfunction after LT, the methods of its evaluation, and the interventions aimed at preserving renal function early and belatedly after LT.
Core Tip: Post-liver transplantation renal dysfunction is a frequent complication that has a major impact on the survival rate of the graft and the patient. Due to the multifactorial nature of post-transplantation chronic kidney disease, the ability to accurately identify patients under risk and the development of preventive approaches are paramount. This review presents the state-of-the-art on the topic: Its causes, renal function assessment methods, and the most studied nephroprotective strategies.
- Citation: Pacheco MP, Carneiro-D'Albuquerque LA, Mazo DF. Current aspects of renal dysfunction after liver transplantation. World J Hepatol 2022; 14(1): 45-61
- URL: https://www.wjgnet.com/1948-5182/full/v14/i1/45.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i1.45
Liver transplantation (LT) changed the natural history of cirrhotic patients. It is considered the gold standard treatment for liver diseases on terminal stages, including hepatocellular carcinoma[1,2]. Significant advances were achieved in immunosuppression, in the treatment of acute and chronic cellular rejection, in the prevention of infections, and in preoperative preparation, organ preservation, surgical procedure, and anesthesiologic techniques[1]. Therefore, short-term mortality, which was due mainly to intraoperative causes, infection, and acute rejection, has considerably decreased[1]. On the other hand, long-term mortality has not been altered for the past few years[3]. Longer survival rates have, in turn, increased the so-considered late complications of LT, such as diabetes mellitus, cardiovascular diseases, malignance, and renal dysfunction[1,4].
Chronic kidney disease (CKD) develops in the majority of patients who survive the first 6 postoperative months[5,6]. The cumulative incidence of post-LT CKD is signi-ficantly higher than those following cardiac and lung transplants[5]. The presence of CKD post-LT, defined by the Chronic Kidney Foundation as the reduction of the glomerular filtration rate (GFR) to values lower than 60 mL/min/1.73 m² for 3 mo or longer, is a frequent complication and has a negative impact on the graft’s and patient’s survival rates[7]. A recent study from the United States assessed 602 liver transplanted patients between 2010 and 2016 and reported a prevalence of CKD in its distinct stages in 41.5% of recipients[8]. In addition, renal failure was responsible for 6% of deaths of patients who survived the first 6 post-transplantation months[8].
Prevalence and incidence studies of post-LT renal dysfunction show wide variations, attributable mainly to different criteria used for CKD definition, and to the various follow-up periods evaluated[9]. The first consensus of the International Liver Transplantation Society reported that the prevalence of post-LT CKD ranged between 30% and 90%, and terminal CKD that required renal replacement therapy (RRT) was described in 2% to 5% of patients per year[10]. According to the Scientific Registry of Transplantation Recipients, the incidence of stage 4 or 5 of CKD after 1, 3, and 5 years of transplantation was 8%, 14%, and 18%, respectively, reaching up to a quarter of recipients within 10 years after the transplant[5,7]. An extensive prospective study evaluated the prevalence of CKD through measurement of GFR by iothalamate clearance and the associated mortality in 1211 patients over 25 years[9]. The authors reported that after 4 mo of LT, 40% of the patients already had CKD stage ≥ 3, and the risk of death increased when the GFR decreased to values below 30 mL/min/1.73 m2 or worse[9]. Only 18% of the subjects had normal renal function after 25 years, in opposition to 39% of age-group matched individuals from the general population[9].
When the whole spectrum of renal dysfunction after LT is evaluated, few studies exhibit data of its occurrence in the early stages after the procedure. A Spanish study with 230 patients revealed that 30.8%, 28.8%, and 26.4% of patients had stage 3 CKD after 12, 24, and 30 mo of transplantation, respectively[11]. It is interesting to highlight that, despite a mild reduction in GFR (60-89 mL/min/1.73 m2) is not considered CKD, this same Spanish study observed that a significant percentage of patients had this GFR range (46.2%, 41.9%, and 46.2% within 12, 24, and 30 mo after the transplant, respectively), even with normal GFR prior to LT[11]. In the American cohort, this mild reduction in GFR occurred in 21.7% of patients who had controlled blood pressure, and in 24.9% of patients with uncontrolled blood pressure within 1 year after LT[8].
Renal dysfunction etiologies in LT are multifactorial and are related to the period of its occurrence. Therefore, the main factors leading to renal dysfunction can be grouped into pre-LT, intraoperative, and post-LT periods (Table 1). Though efforts have been made to reduce or avoid such predisposing factors of renal dysfunction, many of them are not modifiable, such as, for instance, pre-existing conditions.
| Pre-transplantation | Perioperative | After transplantation |
| Hypovolemia; Infections; Nephrotoxic drugs; Hepatorenal syndrome; High MELD; NASH/MAFLD; Renal parenchymal diseases associated with hepatitis B, C and alcohol | Hemodynamic instability; Reperfusion injury; Nephrotoxic drugs | Calcineurin inhibitors; Diabetic nephropathy; Hypertensive nephropathy |
Acute kidney injury (AKI) is a common and increasing clinical event in subjects with cirrhosis[12]. It is estimated to occur in 20% to 57% of hospitalized subjects with decompensated liver disease, with a significant impact on survival[12-14]. AKI in this setting is an underestimated problem because the main assessed parameter is the serum creatinine (Scr). Scr overestimates GFR for several reasons, such as muscle mass, frequently reduced in patients with cirrhosis[15]. In advanced liver disease, the presence of AKI is common, often secondary to infection, hypovolemia, use of vasodilators, and other nephrotoxic drugs, such as non-steroidal anti-inflammatory agents and contrasts. Nonetheless, particularly in those with advanced decompensated liver disease, one of the main causes of loss of renal function is the circulatory dysfunction induced by portal hypertension[16]. Activation of the renin-angiotensin-aldosterone system (RAAS) leads to kidney vasoconstriction, which may be reverted with the resolution of the portal hypertension, as seen in cases of hepatorenal syndrome (HRS)[16]. The understanding of the pathophysiology of HRS-AKI has evolved and currently encompasses, in addition to circulatory dysfunction, systemic inflammation, microvascular dysfunction, and direct tubular damage[17-19]. The combination of albumin and terlipressin can restore renal function in 40% to 73% of patients with HRS-AKI[20-23]. Moreover, response to terlipressin and albumin was associated with a reduction in the need for RRT after LT and reduced the risk of CKD at 1 year after LT, as recently reported by Piano et al[24]. However, LT remains the definitive treatment for this condition. On the other hand, renal vasoconstriction for extended periods, associated with the intrinsic kidney damage caused by the surgical procedure, may lead to organic and less reversible renal injury, which explains why some patients with HRS develop worse renal function after LT[16]. Indeed, non-recovery of renal function is associated with the duration of pre-LT dialysis in HRS patients[25]. In addition, renal recovery and patient survival post-LT are better in those with HRS than in those with acute tubular necrosis[26].
It is noteworthy that AKI is an increasingly recognized risk factor for CKD development and progression[27]. A recent Spanish study reported that in a cohort of patients with cirrhosis who survive an episode of AKI, 25% of them developed CKD, and this passage from AKI to CKD was associated with an increased risk of AKI, complications of cirrhosis, and hospital re-admissions[28]. According to Francoz[29] (2020), cirrhosis, in addition to being a risk factor for AKI, would also be a predisposing condition for the development of CKD, with an impact on those needing LT.
Some renal parenchymal chronic diseases relatively specific to cirrhosis, such as immune complex glomerulonephritis (seen in hepatitis B and C) or immunoglobulin A nephropathy (seen in cirrhosis due to alcohol), are increasingly found in candidates for LT[16]. However, non-specific causes of CKD, mainly secondary to metabolic syndrome, are also increasingly seen in this population[30]. Special attention must be paid to subjects with non-alcoholic steatohepatitis (NASH)/metabolic dysfunction-associated fatty liver disease (MAFLD), which represents a growing LT indication worldwide[31-33]. These patients have additional risk factors for renal injury, such as diabetes, hypertension, and obesity, which in turn are associated to some degree with kidney injury in the pre-LT period[34]. In addition, it has already been demonstrated that NASH is an independent predictor of stage 3 renal injury post-LT[35].
Finally, the model for end-stage liver disease (MELD) score itself, used for the allocation of organs, which includes Scr in its estimation, favors candidates with renal function impairment for LT. Therefore, its widespread use may increase the number of patients who require RRT and simultaneous liver and kidney transplants, impacting the long-term renal function of the recipients[16,36]. The risk of post-LT end-stage renal disease, which is related to post-LT mortality, was 15% higher in the MELD era, as shown by Sharma et al in 2011[37]. Interestingly, the proportion of MELD sodium score attributable to creatinine ≥ 50% was associated with advanced renal dysfunction at 1 year post-LT in a recent United States retrospective study using the United Network for Organ Sharing (UNOS) database[38].
Altogether, these factors in the pre-LT period contribute to the increased finding of renal dysfunction after LT.
The development of renal injury in the perioperative period leads to extended hospitalization, increases the risk of acute rejection and infection, and impacts global mortality[39]. Renal dysfunction during this period has a reported incidence of 11% to 94%, depending upon the definition and the assessment method applied[40], with acute tubular necrosis as the most frequent etiology[6,16]. Risk factors for perioperative AKI include sepsis, nephrotoxic drugs, impairment of renal perfusion associated with hemodynamic instability during surgery, and the harmful effect of the ischemia-reperfusion injury[6,41].
High perioperative aminotransferase aspartate peak is independently correlated with the risk of renal injury after LT[6,42]. The use of blood transfusion in the intraoperative period, especially above 10 units, increased the risk of renal dysfunction when combined with diuresis lower than 100 mL/h[43]. The excessive use of blood products may be related to large blood losses and, consequently, hypotension, but could also induce a pro-inflammatory state that impairs oxygen supply to tissues and increases the concentration of free hemoglobin and iron, both nephrotoxic[44].
In addition, the lack of grafts forces surgeons to use more marginal grafts (of older patients, with steatosis, and organ donors with circulatory causes of death), which is directly related to reperfusion-ischemia injury[45]. This may result in more renal dysfunction posteriorly[46]. Predictive models for renal dysfunction have already been assessed, but none of the candidates was capable of adequately predicting the outcome within a time frame suitable for appropriate intervention[40].
Up to 1 year: The most common histopathological findings in subjects with CKD 1 year after the LT included calcineurin inhibitors (CNI) toxicity, diabetic nephropathy, and thrombotic microangiopathy[7].
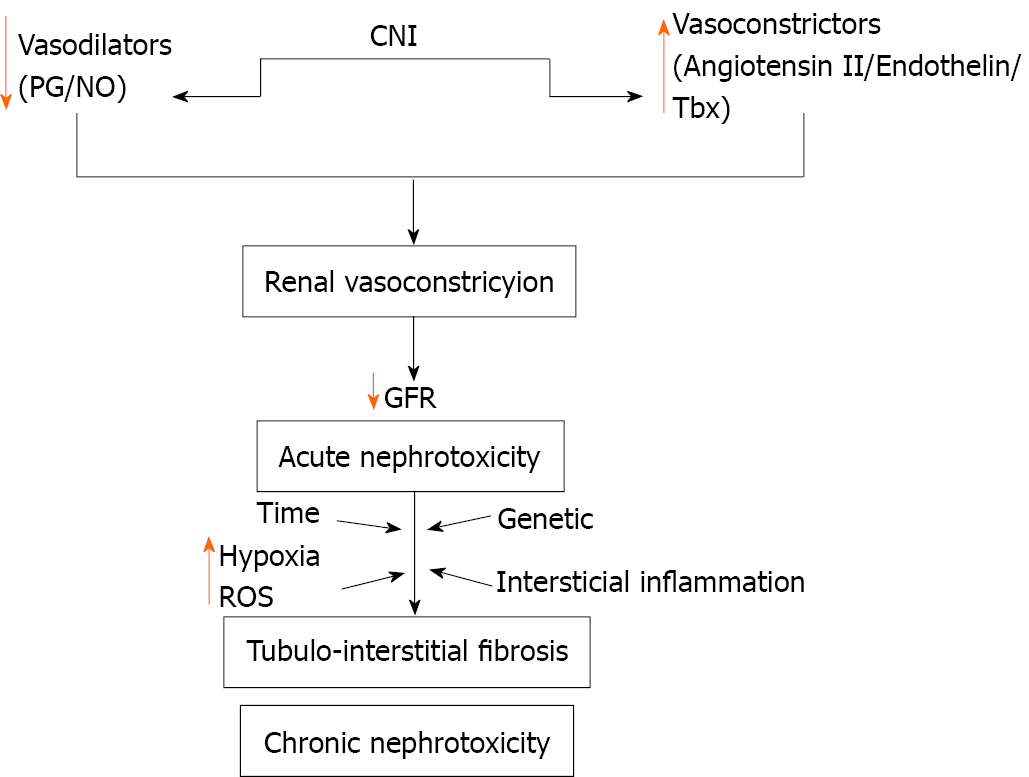
Early nephrotoxicity by CNI is in most part functional, and a dose-dependent mechanism. CNI induce vasoconstriction of afferent and efferent arterioles, with reduction of renal perfusion and of the ultrafiltration coefficient and, consequently, reduction of the glomerular filtration[10,47]. Therefore, early renal alteration may be reversible with the reduction of the CNI dose[5]. The accurate vasoconstriction mechanism is still unclear, but it is known that there is a disequilibrium of vasoactive substances that lead to the increase of vasoconstrictors, for instance, endothelin, angiotensin II, and thromboxane, and the decrease in the generation of vasodilators, such as prostaglandins and nitric oxide[10,48] (Figure 1).
A comprehensive study using the UNOS database evaluated 1720 patients with pre-LT renal dysfunction and demonstrated that the most important independent predictor of recovery of renal function, defined as creatinine < 1.5 mg/dL and survival rate greater than 29 d, was the absence of liver graft dysfunction[49]. Induction with anti-thymocyte globulin, decreasing the use of CNI, was also considered a protective factor[49]. Interestingly, the authors showed that the need for RRT for up to 8 wk was not a contributing factor to CKD evolution[49]. In Taiwan, Lin et al[50] (2012) reported that the Scr in the 4th wk after LT was a good predictive variable for CKD over 5 years, which implies that the aggressive management of early kidney injury may avoid the development of CKD. Ye et al[51] (2020) described that the estimated GFR at 1 year after LT, beyond the stage at which postoperative complications may occur and with greater immunosuppression stabilization, had a good correlation with the estimated GFR in 5 years.
After 1 year: Unquestionably, the main mechanism of CKD evolution is CNI nephrotoxicity. It is estimated that in about 50% of patients who develop renal dysfunction in the postoperative period, CNI nephrotoxicity is the root cause[6]. Gonwa et al[52] (2001) evaluated 843 liver-transplanted patients for up to 13 years, and the presumed etiologies of end-stage renal disease that occurred in 45 patients were CNI toxicity (73.3%), progression of subjacent renal disease (11.1%), focal segmental glomerulosclerosis (6.66%), non-recovered HRS (6.66%), and acute tubular necrosis/toxicity of amphotericin (2.22%). In the longer term, diabetes mellitus and high blood pressure worsen renal damage even further[16]. As standard immunosuppressive therapy is based upon CNI [tacrolimus (FK) and cyclosporine] monotherapy or is associated with other agents (for instance, mycophenolate)[53], handling such complications is one of the core challenges of physicians who manage liver-transplanted patients.
The chronic renal damage that CNI causes is characterized by the development of irreversible structural injury and may culminate in terminal stages of kidney disease[10]. Upon histology evaluation, obliterative arteriopathy, glomerular ischemic collapse, tubular vacuolization, and focal areas of tubular atrophy and interstitial fibrosis may occur[10]. The development of chronic nephropathy induced by CNI is also influenced by genetic variability[54]. The factors responsible for chronic injury by CNI are complex and not completely understood, and involve interstitial inflammation and renal vasoconstriction, with activation of the RAAS in a relevant manner (Figure 1). Consequently, there is an imbalance between vasodilator and vasoconstrictor factors, leading to renal damage[10]. Another possible mechanism generated by CNI nephrotoxicity is the direct injury of tubular epithelial cells, derived from the blocking of mitochondrial permeability and inhibition of prolyl isomerase, the enzyme responsible for the interconversion of the cis- and trans-isomers of peptide bonds, which can speed up or slow down protein cleavage[55].
The previously mentioned study from Ye et al[51] (2020) also evaluated several predictive factors of the evolution to CKD in post-LT patients under the FK regimen[51]. The authors reported that the GFR found 1 or 2 years after LT showed a good correlation with the one found after 5 years, and demonstrated that those subjects with GFR < 60 mL/min/1.73 m² are those who will probably develop an irreversible renal injury in the following years. It is important to highlight that while statistical significance was not found in the annual reduction of the GFR between FK-using and FK-free groups, the serum concentration of FK influenced the progression to CKD within 1 and 2 years, with receiver operating characteristic curves of 0.73 and 0.78, respectively[51].
The development of post-LT metabolic syndrome is frequent[56]. In addition to lingering pre-LT risk factors, there is an increase in post-LT risks due to immunosuppression with corticosteroids and CNI[57]. Moreover, it is expected that metabolic syndrome components after LT will continue to rise due to the increase of NASH/MAFLD as an indication for LT[31-33,58]. VanWagner et al[8] (2020) reported that hypertension was observed in up to 92% of patients and diabetes in 53%, adding risk to the development of CKD in such patients. Moreover, there is the possibility of an evolution to advanced liver fibrosis/cirrhosis, which adds renal dysfunction components of cirrhotic patients to those associated with immunosuppression.
Due to the multifactorial nature of CKD in the post-LT period, the ability to accurately identify patients under risk and the development of preventive strategies are crucial[59]. The discovery of a more sensitive biomarker would make it feasible to quickly detect renal damage factors and implement early therapeutic interventions. To assess renal function after LT, the GFR measurement is the most used laboratory tool[60]. For such, the most commonly used test in clinical practice is the dosage of Scr, which supplies information about GFR and is widely available and inexpensive[7,60]. Nevertheless, besides considerations concerning analytical aspects of the test, there are individual characteristics that may interfere with the results. Reference Scr values are influenced by non-renal factors, such as body weight, muscle mass, race, age, gender, and protein intake[61]. In this way, Scr values differ among children and adult women and men[61]. Scr is also considered a late renal dysfunction marker, requiring a reduction above 50% of glomerular ultrafiltration before an increase in Scr is observed[60]. Accuracy can be improved through measurement of 24 h creatinine clearance, but it also brings limitations: Higher costs, the need to store urine for 24 h (subject to errors in sample collection and incomplete bladder emptying), and the effect of tubular secretion of creatinine[61]. As it is a small molecule and does not bind to serum proteins, creatinine is freely filtrated by glomeruli; however, about 10% to 20% of the creatinine excreted in urine comes from its secretion by the proximal tubular cell. Tubular secretion is the main determinant of the overestimation of renal function when creatinine clearance is used[62]. This secretion by the tubular cell is variable in the same individual and increases with the reduction of the glomerular filtration[60-62].
Equations specifically developed for the estimation of creatinine clearance, such as Cockcroft-Gault[63] or the Modification of Diet in Renal Disease (MDRD)[64], have been widely used in clinical practice[60]. MDRD-4 (simplified MDRD) is the equation usually employed to compute GFR because it is considered to be as accurate as MDRD-6, the original equation[65]. Indeed, there are undeniable advantages to its use, but despite its generalized use in clinical practice, the measurement of GFR through formulae is not accurate, particularly in patients with uncommon biotypes or diet alterations, in the presence of rapid deterioration of renal function, or when the GFR values are above 60 mL/min/1.73 m²[62].
The 2012 joint guideline of the American Society of Transplantation along with the American Association for the Study of Liver Diseases (AASLD) recommended the use of the MDRD equation in any of its four variations as superior to the use of the isolated Scr and of the 24 h creatinine clearance[66]. Nevertheless, in 2016 the American Society of Transplantation developed a document that specifically endorsed the use of MDRD-4 and CKD-epidemiology-creatinine[67] as the formula that yields the most accurate results of GFR in this population[68] (Table 2). The 2019 British guideline for post-LT management advised that close monitoring of renal dysfunction after LT is necessary, but did not cite which method to use in the evaluation[69].
| Formulas | |
| Cockcroft Gault | [(140 – age) × weight]/[(72 × Scr) × (0.85 if female)] |
| MDRD 4 | 175 × (Scr)-1.154 × (age)-0.203 × (0.742 if female) × (1.212 if black) |
| MDRD 6 | 198 × (Scr)-0.858 × (age)-0.1678 × (0.822 if female) × (1.178 if black) × (Ur)-0.293 × (urine urea nitrogen excretion g/d)0.249 |
| CKD-EPI creatinine equation | 141 × min (creat/κ, 1)α × max (creat/κ, 1)-1.209 × 0.993age × (1.018 if female) × (1.159 if black) |
Due to the antiproteinuric effect of CNI, proteinuria may be absent even in advanced stages of CKD[66]. The AASLD guideline recommends its measurement in isolated samples at least once per year[66].
Cystatin-C is placed as an alternative glomerular filtration marker because, as it can be completely eliminated in the circulation, its serum concentration could properly reflect the GFR[70]. Unlike creatinine, it is not influenced by muscle mass, diet, or the presence of infection or malignance[60,70]. However, other factors, such as age, male gender, weight, height, tobacco use, steroid use, and thyroid disease, are indepen-dently associated with elevated cystatin-C levels, suggesting low specificity in detecting renal impairment[60]. The 2016 guideline of the American Society of Transplantation states that among all blood-based estimates of GFR, the cystatin-C equations are the most accurate in post-LT patients[68].
Renal clearance of inulin is the gold standard of GFR measurement, but the necessity of performing a test in standardized conditions, with continuous intravenous injection of the marker, its elevated cost, and peculiar aspects of laboratory dosage limit its use in clinical practice, restricting it to research settings[71]. The use of renal and plasma clearance of radioactive isotopes, such as 51Cr-ethylenediamine tetraacetic acid (EDTA), is growing in clinical practice, as they are safer and simpler methods - and were sufficiently accurate - to measure GFR[71]. Despite the ongoing discussion about the underestimation of renal clearance of 51Cr-EDTA in comparison to renal clearance of inulin, the determination of GFR by 51Cr-EDTA and by inulin had comparable results in kidney-transplanted patients[72]. The authors have shown elevated correlation coefficients between both methods (0.9516)[72]. Only a few studies used 51Cr-EDTA clearance measurement to evaluate GFR in children and adults post-LT[73-76].
Neutrophil gelatinase-associated lipocalin (NGAL), a protein expressed in the renal tubular cells, has been gaining attention as an early marker of AKI, including the immediate post-LT period, though there is still considerable variation among studies[77]. In a Japanese study, Tsuchimoto et al[78] (2014) reported that NGAL was the best urinary marker in comparison to 5 other assessed candidates [liver-type fatty acid binding protein, monocyte chemoattractant protein-1, interleukin (IL)-18, cystatin-C, and osteopontin], with its values in the 1st and 7th postoperative days being helpful to predict AKI by FK in liver-transplanted patients. In 2019, Lima et al[79] evaluated the urine and plasma NGAL elevation pattern in the LT perioperative period of 100 patients and showed that these measurements were able to predict AKI diagnosis earlier in this setting. Urinary NGAL levels evaluated just after the LT procedure could accurately predict AKI development in 27 subjects in the United Kingdom, as Robertson et al[80] reported in 2019. NGAL could also be useful in the context of chronic renal injury, not only to predict its progressions but also to monitor the response to treatment aimed at protecting renal function[60,81,82]. However, changes in urinary NGAL are not specific to CKD, and more studies are required to further explore its potential in the context of LT[83]. The utility of NGAL and other urinary and serum biomarkers for the prediction of AKI in patients undergoing LT has yet to be defined because AKI pathogenesis in this context is complex[84]. Moreover, optimal cut-off values and source of confounding factors must be addressed prior to routine clinical use in LT[60,77].
The use of imaging tests for kidney evaluation, under several clinical situations, is a well-established method. In adults, the ultrasound exam finding of a more echogenic renal cortex as compared to liver echogenicity clearly suggests renal disease[85-87]. It is a very sensitive marker of renal parenchymal disease and correlates well with some glomerular and tubular-interstitial injuries[87]. Recent studies displayed the role of magnetic resonance imaging (MRI) in evaluating hypoxia and fibrosis of the renal parenchyma through 2 techniques (blood oxygen level-dependent MRI and diffusion-weighted MRI). Both provided information on the progression of kidney disease[88]. The standardization of acquisition and processing protocols is required, as current methodological differences exist across studies and pose difficulties in comparing the results[88]. In addition, kidney evaluation data in liver-transplanted patients are awaited, as the non-invasive assessment of renal changes by magnetic resonance diffusion imaging has so far been evaluated only after lung transplantation[89].
Acoustic radiation force impulse (ARFI) is a recently developed noninvasive technique. It is safe and convenient to assess the elasticity of tissues[90]. The technique is capable of identifying the parenchymal elasticity by measuring the speed of the shear wave, and it is integrated into conventional ultrasound devices[91]. It has been used mainly in the determination of hepatic fibrosis and cirrhosis in chronic viral hepatitis and displays a good correlation with the degree of liver fibrosis[92,93]. In recent years, the ARFI technique has also been applied to other organs, such as the muscles[94], prostate[95], and breast[96]. Fibrosis is the core process of the progression of CKD and this method has also been evaluated in this scenario. Despite the inability to predict pathological alterations, ARFI results were significantly correlated with GFR and the stage of CKD in several studies[97]. However, kidney hemodynamical alterations may affect the renal parenchymatic elasticity during CKD progression[97]. In a pilot study, Bob et al[98] (2015) reported that ARFI measurements diminished with the decrease in GFR, suggesting a cutoff at 2.26 m/s or less as a predictor of stage 4 or 5 CKD. Structurally, the final via of post-LT CKD culminates in kidney fibrosis and, similar to what happens in other organs such as the liver, the collagen deposition may culminate in an increase in tissue stiffness. Therefore, elastography techniques could play a role in this setting.
Patients developing CKD, besides having limitations regarding the use of immunosuppressing drugs, exhibit an increased risk of hospitalization, infectious complications, and graft dysfunction. Moreover, they have a 2 to 4 times greater risk of death[5,99]. Thus, preventive strategies to preserve kidney function after LT are paramount. The management of comorbidities and other general factors leading to CKD must be remembered. Therefore, it is possible to extrapolate non-transplanted CKD orientations to these patients[100]. It is advisable to, at least once per year, measure or calculate GFR via formulae, besides performing albuminuria or proteinuria tests and, if necessary, referring the patient to a nephrologist (Table 3).
| Indication |
| AKI or abrupt sustained fall in GFR |
| GFR < 30 mL/min/1.73 m² |
| Consistent significant albuminuria (albumin/creatinine ratio ≥ 300 mg/g or albumin excretion rate ≥ 300 mg/24 h, equivalent to protein/creatinine ratio ≥ 500 mg/g or protein excretion rate ≥ 500 mg/24 h) |
| Progression of CKD (a drop in GFR from baseline by 25% or a sustained decline in GFR of more than 5 mL/min/1.73 m2/yr) |
Regarding arterial hypertension, the pressure target should be below 140/90 mmHg in the absence of proteinuria, and below 130/80 mmHg when it is present[66,68]. These objectives must be reached with a combination of lifestyle changes and pharmacological options. The choice of the anti-hypertensive must be based upon safety and drug interaction[69]. Dihydropyridine calcium channel blockers, such as amlodipine, are considered first-choice agents, as they reduce systemic vascular resistance and improve renal blood flow, thus blocking CNI’s vasoconstrictor action[68,69]. Drugs that block the RAAS, such as angiotensin converting enzyme inhibitors and angio-tensin receptor blockers, must be avoided in the immediate postoperative period because, in this period, the activity of plasmatic renin is decreased and their use may worsen the hyperkalemia observed with FK use[69]. After this period, these drugs are of choice for patients with diabetes, significant proteinuria, and CKD[66,68,69]. Beta blockers are safe, but diuretics must be employed with caution, as they may further affect renal function[69]. Cardiovascular complications are frequent non-graft-related causes of mortality after LT, and it has already been shown that the mean GFR is inversely proportional to the time of the first cardiovascular event[6,101]. In these cases, immunosuppression based on everolimus, with the withdrawal or reduction of FK, improved both renal function and the risk of major cardiac events in comparison to standard therapy, as shown by Saliba et al[101].
Post-LT diabetes treatment must target an Hb1AC below 7.0%[66]. Though there is no consensus about which is the best antidiabetic, it is advisable to decrease or interrupt corticosteroids as soon as possible[66,69]. When corticosteroids are administered at higher doses, the use of insulin is safer and more efficient[66].
Dietary interventions may help to slow down CKD’s progress. Salt intake should be restricted to less than 2 g of sodium per day to better control blood pressure and proteinuria[69,102]. Other interventions with less evidence would be to avoid high protein intake (less than 1.3 g/kg/d) in subjects at risk of CKD and to further reduce it to 0.8 g/kg/d in those with GFR < 30 mL/min/1.73 m²[102]. Post-LT weight gain is also associated with the development of metabolic syndrome, cardiovascular events, and renal dysfunction[103,104]. Therefore, weight gain should be avoided. Charlton et al[105] (2017) demonstrated that the introduction of everolimus as an attempt to reduce the FK dosage decreased the weight gain of patients within 1-2 years after LT.
Liver-transplanted patients are particularly vulnerable to hemodynamic insults and present an increased risk of developing AKI after exposure to nephrotoxins, such as non-steroidal anti-inflammatory drugs, amphotericin B, aminoglycosides, and contrast agents. Whenever possible, therapy with CNI before and after exposure to potential nephrotoxins should be reduced or suspended, and a temporary switch to other non-nephrotoxic immunosuppressors should be considered, due to rejection risks, in addition to other nephroprotective measures established for other patients[100].
CNI-induced nephrotoxicity contributes to the worsening of renal function in both the short and long term; the greatest challenge is the choice of a strategy that minimizes renal dysfunction without simultaneously affecting the survival rate of the liver graft. In the immediate postoperative stage (< 1 mo), a strategy to spare the renal function has been the administration of short-term induction therapy (mono- or polyclonal antibodies), with delayed introduction of CNI[6,69]. Several clinical trials have shown that in individuals with preoperative renal dysfunction, this approach resulted in a better renal outcome, as it avoided the vasoconstrictor risks of CNI in synergy with other perioperative risk factors associated with AKI[6,69,106]. Basiliximab and daclizumab, which have a selective target upon activated T-cells blocking CD25, the IL-2 receptor, are the most used. The use of belatacept has also been studied, in addition to other standard strategies of induction, but the study had to be terminated due to a higher mortality rate of the belatacept group[107].
Early usage of mycophenolate mofetil (MMF) has also been assessed in subjects without preoperative kidney dysfunction, with an improvement of GFR without disadvantages in terms of graft rejection[108]. Thus, reduced FK doses in combination with MMF are capable of protecting renal function more efficiently than isolated FK use[109].
The use of the mammalian target of rapamycin inhibitors (mTor-I) everolimus has also been demonstrated to be a protective renal strategy[110]. Everolimus was capable of promoting an early decrease of FK dosage with similar efficiency and safety, and with preservation of renal function[111,112]. A continued effect was observed after 1, 2, and 3 years of LT[112]. In these studies, in which everolimus was introduced 4 wk after LT, thrombosis of the hepatic artery and impaired wound healing were not observed[111,112]. In the PROTECT randomized trial, it was shown that the monotherapy with everolimus displayed better results regarding GFR within 12 mo after LT, with similar mortality, graft rejection, and therapeutic failure rates[111]. In addition, maintenance of the GFR benefit in the extension of the study of 24 and 36 mo was observed[113,114]. However, infections, leukopenia, dyslipidemia, and treatment discontinuation were higher in the everolimus group[111].
The use of sirolimus, another mTor-I, was also evaluated as a nephroprotective option after LT. A large randomized prospective trial assessed the conversion of CNI to sirolimus in 607 liver-transplanted patients, and a higher rate of acute rejection and discontinuations was observed in the sirolimus group, with no gains regarding the GFR[115]. Also, the early use of sirolimus in de novo LT in a phase II trial did not exhibit nephroprotection and showed higher graft loss rates, mortality, and sepsis as compared to the use of tacrolimus at standard doses alone[116]. The FDA added a black box warning for the use of sirolimus and belatacept in LT recipients[68].
Therefore, strategies for the primary preservation of long-term renal function are based upon precocious post-operative CNI reduction in combination with non-nephrotoxic immunosuppressing drugs, such as MMF or everolimus, and the use of induction therapy in selected patients[6,66,68,69].
Despite the evidence that early MMF and mTor-I usage minimizes renal dysfunction, this strategy does not seem to be as effective when performed after 1 year from the LT[68]. The significant reduction of the CNI dose (below 50% of the original dosage) with the addition of MMF resulted in an improvement of GFR without negatively affecting the graft’s survival rate, and it did not increase the incidence of adverse events, even when it was implemented after 1 year of LT, but with a weaker effect[117-120]. Complete withdrawal of CNI increased the risk of rejection and graft loss, without adding gains to the GFR[120,121]. Unfortunately, these studies demonstrated that once the renal function is markedly affected (GFR < 60 mL/min/1.73 m²), changing to a kidney preservation approach is less efficient to improve GFR, possibly due to irreversible kidney structural damage[6]. Regarding everolimus, the studies revealed no increase in the rate of liver rejection, but they also reported little to no improvement in the GFR (about 4 mL/min)[122-125], with a discontinuity rate above 10% and the development of proteinuria in some recipients[125]. Therefore, there is little evidence that the substitution of CNI for mTor-I after 1 year has some benefit for the improvement of renal function[68]. Indeed, early rather than late conversion of CNI to everolimus after LT was shown to be a safe approach to preserve long-term renal function, as recently reported by Saliba et al[126] (2020) in the EVEROLIVER Registry.
To minimize the use of CNI, new drugs are currently being tested, such as CFZ533, an IgG1 anti-CD40 antibody, which blocks the signaling pathways implicated in rejection; however, the majority of such studies were in renal transplantation[127,128]. Finally, for those patients who develop end-stage renal disease with a need for dialysis, there is a benefit from renal transplantation from either living or deceased donors, with a mortality reduction of 44% to 60% in comparison to those patients who stay in RRT[66,129,130].
Post-LT renal dysfunction is a frequent and severe problem, impacting patients’ morbimortality. Its etiology is multifactorial, with pre-, intra-, and post-LT factors. Its incidence is increasing, mainly after the changes in organ allocation by MELD score. Early diagnosis is paramount, but the most conventional methods of estimating GFR have limitations, and there is currently no accurate, non-invasive marker ready to use in clinical practice. Taking into consideration that CNI’s toxicity is an important post-LT cause of renal dysfunction, strategies to minimize its use, such as induction therapy followed by a reduction in CNI levels, and the introduction of less nephrotoxic drugs, such as MMF and everolimus, are still the best options to preserve renal function. Also, aggressive treatment of other comorbidities that can negatively impact GFR is important. Nonetheless, once the renal function is significantly compromised, the adoption of a nephroprotective immunosuppression approach is less efficient. New immunosuppressing drugs that do not lead to GFR impairment and do not increase liver rejection rates are eagerly awaited.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tanwandee T S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Weber ML, Ibrahim HN, Lake JR. Renal dysfunction in liver transplant recipients: evaluation of the critical issues. Liver Transpl. 2012;18:1290-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 2. | Hughes CB, Humar A. Liver transplantation: current and future. Abdom Radiol (NY). 2021;46:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Durand F. How to improve long-term outcome after liver transplantation? Liver Int. 2018;38 Suppl 1:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Becchetti C, Dirchwolf M, Banz V, Dufour JF. Medical management of metabolic and cardiovascular complications after liver transplantation. World J Gastroenterol. 2020;26:2138-2154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1703] [Cited by in RCA: 1637] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 6. | Nevens F, Pirenne J. Renal disease in the allograft recipient. Best Pract Res Clin Gastroenterol. 2020;46-47:101690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Bahirwani R, Reddy KR. Outcomes after liver transplantation: chronic kidney disease. Liver Transpl. 2009;15 Suppl 2:S70-S74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | VanWagner LB, Holl JL, Montag S, Gregory D, Connolly S, Kosirog M, Campbell P, Pine S, Daud A, Finn D, Ladner D, Skaro AI, Levitsky J, Lloyd-Jones DM. Blood pressure control according to clinical practice guidelines is associated with decreased mortality and cardiovascular events among liver transplant recipients. Am J Transplant. 2020;20:797-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Allen AM, Kim WR, Therneau TM, Larson JJ, Heimbach JK, Rule AD. Chronic kidney disease and associated mortality after liver transplantation--a time-dependent analysis using measured glomerular filtration rate. J Hepatol. 2014;61:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 10. | Charlton MR, Wall WJ, Ojo AO, Ginès P, Textor S, Shihab FS, Marotta P, Cantarovich M, Eason JD, Wiesner RH, Ramsay MA, Garcia-Valdecasas JC, Neuberger JM, Feng S, Davis CL, Gonwa TA; International Liver Transplantation Society Expert Panel. Report of the first international liver transplantation society expert panel consensus conference on renal insufficiency in liver transplantation. Liver Transpl. 2009;15:S1-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 11. | Herrero JI, Cuervas-Mons V, Gómez-Bravo MÁ, Fabregat J, Otero A, Bilbao I, Salcedo MM, González-Diéguez ML, Fernández JR, Serrano MT, Jiménez M, Rodrigo JM, Narváez I, Sánchez G. Prevalence and progression of chronic kidney disease after liver transplant: a prospective, real-life, observational, two-year multicenter study. Rev Esp Enferm Dig. 2018;110:538-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Desai AP, Knapp SM, Orman ES, Ghabril MS, Nephew LD, Anderson M, Ginès P, Chalasani NP, Patidar KR. Changing epidemiology and outcomes of acute kidney injury in hospitalized patients with cirrhosis - a US population-based study. J Hepatol. 2020;73:1092-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 13. | Khatua CR, Sahu SK, Barik RK, Pradhan S, Panigrahi S, Mishra D, Singh SP. Validation of International Club of Ascites subclassification of stage 1 acute kidney injury in chronic liver disease. JGH Open. 2019;3:290-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Vaz NF, da Cunha VNR, Cunha-Silva M, Sevá-Pereira T, de Souza Almeida JR, Mazo DF. Evolution of diagnostic criteria for acute kidney injury in patients with decompensated cirrhosis: A prospective study in a tertiary university hospital. Clin Res Hepatol Gastroenterol. 2020;44:551-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Piano S, Brocca A, Angeli P. Renal Function in Cirrhosis: A Critical Review of Available Tools. Semin Liver Dis. 2018;38:230-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Nevens F, Pirenne J. Role of Immunosuppression in causing renal failure after liver transplantation. In EASL Postgraduate Course: Transplantation & the Liver. Amsterdam, 2013: 94-99. |
| 17. | Angeli P, Garcia-Tsao G, Nadim MK, Parikh CR. News in pathophysiology, definition and classification of hepatorenal syndrome: A step beyond the International Club of Ascites (ICA) consensus document. J Hepatol. 2019;71:811-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 280] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 18. | Ginès P, Solà E, Angeli P, Wong F, Nadim MK, Kamath PS. Hepatorenal syndrome. Nat Rev Dis Primers. 2018;4:23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (3)] |
| 19. | Solé C, Solà E, Huelin P, Carol M, Moreira R, Cereijo U, Mas JM, Graupera I, Pose E, Napoleone L, dePrada G, Juanola A, Fabrellas N, Torres F, Morales-Ruiz M, Farrés J, Jiménez W, Ginès P. Characterization of inflammatory response in hepatorenal syndrome: Relationship with kidney outcome and survival. Liver Int. 2019;39:1246-1255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 20. | Cavallin M, Piano S, Romano A, Fasolato S, Frigo AC, Benetti G, Gola E, Morando F, Stanco M, Rosi S, Sticca A, Cillo U, Angeli P. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: A randomized controlled study. Hepatology. 2016;63:983-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 21. | Allegretti AS, Israelsen M, Krag A, Jovani M, Goldin AH, Schulman AR, Winter RW, Gluud LL. Terlipressin versus placebo or no intervention for people with cirrhosis and hepatorenal syndrome. Cochrane Database Syst Rev. 2017;6:CD005162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Arora V, Maiwall R, Rajan V, Jindal A, Muralikrishna Shasthry S, Kumar G, Jain P, Sarin SK. Terlipressin Is Superior to Noradrenaline in the Management of Acute Kidney Injury in Acute on Chronic Liver Failure. Hepatology. 2020;71:600-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 23. | Moore K, Jamil K, Verleger K, Luo L, Kebede N, Heisen M, Corman S, Leonardi R, Bakker R, Maï C, Shamseddine N, Huang X, Allegretti AS. Real-world treatment patterns and outcomes using terlipressin in 203 patients with the hepatorenal syndrome. Aliment Pharmacol Ther. 2020;52:351-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 24. | Piano S, Gambino C, Vettore E, Calvino V, Tonon M, Boccagni P, Gringeri E, Germani G, Burra P, Cillo U, Angeli P. Response to Terlipressin and Albumin Is Associated With Improved Liver Transplant Outcomes in Patients With Hepatorenal Syndrome. Hepatology. 2021;73:1909-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 25. | Wong F, Leung W, Al Beshir M, Marquez M, Renner EL. Outcomes of patients with cirrhosis and hepatorenal syndrome type 1 treated with liver transplantation. Liver Transpl. 2015;21:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 26. | Nadim MK, Genyk YS, Tokin C, Fieber J, Ananthapanyasut W, Ye W, Selby R. Impact of the etiology of acute kidney injury on outcomes following liver transplantation: acute tubular necrosis versus hepatorenal syndrome. Liver Transpl. 2012;18:539-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 27. | Kurzhagen JT, Dellepiane S, Cantaluppi V, Rabb H. AKI: an increasingly recognized risk factor for CKD development and progression. J Nephrol. 2020;33:1171-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 28. | Bassegoda O, Huelin P, Ariza X, Solé C, Juanola A, Gratacós-Ginès J, Carol M, Graupera I, Pose E, Napoleone L, Albertos S, de Prada G, Cervera M, Fernández J, Fabrellas N, Poch E, Solà E, Ginès P. Development of chronic kidney disease after acute kidney injury in patients with cirrhosis is common and impairs clinical outcomes. J Hepatol. 2020;72:1132-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 29. | Francoz C. Acute kidney injury in cirrhosis: An immediate threat but also a ticking time bomb. J Hepatol. 2020;72:1043-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Francoz C, Nadim MK, Durand F. Kidney biomarkers in cirrhosis. J Hepatol. 2016;65:809-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 31. | Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188-2195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 585] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 32. | Sayiner M, Younossi ZM. Nonalcoholic Steatohepatitis Is Becoming a Top Indication for Liver Transplantation Worldwide. Liver Transpl. 2019;25:10-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Piñero F, Costa P, Boteon YL, Duque SH, Marciano S, Anders M, Varón A, Zerega A, Poniachik J, Soza A, Padilla Machaca M, Menéndez J, Zapata R, Vilatoba M, Muñoz L, Maraschio M, Podestá LG, McCormack L, Gadano A, Boin ISFF, García P, Silva M; Latin American Liver Research, Education, Awareness Network (LALREAN). A changing etiologic scenario in liver transplantation for hepatocellular carcinoma in a multicenter cohort study from Latin America. Clin Res Hepatol Gastroenterol. 2018;42:443-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 34. | Burra P, Becchetti C, Germani G. NAFLD and liver transplantation: Disease burden, current management and future challenges. JHEP Rep. 2020;2:100192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 35. | Fussner LA, Charlton MR, Heimbach JK, Fan C, Dierkhising R, Coss E, Watt KD. The impact of gender and NASH on chronic kidney disease before and after liver transplantation. Liver Int. 2014;34:1259-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Sethi A, Estrella MM, Ugarte R, Atta MG. Kidney function and mortality post-liver transplant in the Model for End-Stage Liver Disease era. Int J Nephrol Renovasc Dis. 2011;4:139-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Sharma P, Schaubel DE, Guidinger MK, Goodrich NP, Ojo AO, Merion RM. Impact of MELD-based allocation on end-stage renal disease after liver transplantation. Am J Transplant. 2011;11:2372-2378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 38. | Bittermann T, Hubbard RA, Lewis JD, Goldberg DS. The proportion of Model for End-stage Liver Disease Sodium score attributable to creatinine independently predicts post-transplant survival and renal complications. Clin Transplant. 2020;34:e13817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 39. | Biancofiore G, Davis CL. Renal dysfunction in the perioperative liver transplant period. Curr Opin Organ Transplant. 2008;13:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Caragata R, Wyssusek KH, Kruger P. Acute kidney injury following liver transplantation: a systematic review of published predictive models. Anaesth Intensive Care. 2016;44:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Jochmans I, Meurisse N, Neyrinck A, Verhaegen M, Monbaliu D, Pirenne J. Hepatic ischemia/reperfusion injury associates with acute kidney injury in liver transplantation: Prospective cohort study. Liver Transpl. 2017;23:634-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 42. | Leithead JA, Rajoriya N, Gunson BK, Muiesan P, Ferguson JW. The evolving use of higher risk grafts is associated with an increased incidence of acute kidney injury after liver transplantation. J Hepatol. 2014;60:1180-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 43. | Guitard J, Cointault O, Kamar N, Muscari F, Lavayssière L, Suc B, Ribes D, Esposito L, Barange K, Durand D, Rostaing L. Acute renal failure following liver transplantation with induction therapy. Clin Nephrol. 2006;65:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | de Haan JE, Hoorn EJ, de Geus HRH. Acute kidney injury after liver transplantation: Recent insights and future perspectives. Best Pract Res Clin Gastroenterol. 2017;31:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 45. | Dar WA, Sullivan E, Bynon JS, Eltzschig H, Ju C. Ischaemia reperfusion injury in liver transplantation: Cellular and molecular mechanisms. Liver Int. 2019;39:788-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 268] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 46. | Tokodai K, Lannsjö C, Kjaernet F, Romano A, Januszkiewicz A, Ericzon BG, Nowak G. Association of post-reperfusion syndrome and ischemia-reperfusion injury with acute kidney injury after liver transplantation. Acta Anaesthesiol Scand. 2020;64:742-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 47. | Duvoux C, Pageaux GP. Immunosuppression in liver transplant recipients with renal impairment. J Hepatol. 2011;54:1041-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 48. | Beckebaum S, Cicinnati VR, Radtke A, Kabar I. Calcineurin inhibitors in liver transplantation - still champions or threatened by serious competitors? Liver Int. 2013;33:656-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 49. | Iglesias J, Frank E, Mehandru S, Davis JM, Levine JS. Predictors of renal recovery in patients with pre-orthotopic liver transplant (OLT) renal dysfunction. BMC Nephrol. 2013;14:147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Lin YH, Lin CC, Wang CC, Wang SH, Liu YW, Yong CC, Lin TL, Li WF, Concejero AM, Chen CL. The 4-week serum creatinine level predicts long-term renal dysfunction after adult living donor liver transplantation. Transplant Proc. 2012;44:772-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Ye L, Zhang Y, Tang H, Yao J, Wang G, Yang Y, Chen G. Prediction of chronic kidney disease progression used by calcineurin inhibitor concentration and estimated glomerular filtration rate early after liver transplantation. Niger J Clin Pract. 2020;23:1387-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 52. | Gonwa TA, Mai ML, Melton LB, Hays SR, Goldstein RM, Levy MF, Klintmalm GB. End-stage renal disease (ESRD) after orthotopic liver transplantation (OLTX) using calcineurin-based immunotherapy: risk of development and treatment. Transplantation. 2001;72:1934-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 379] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 53. | Di Maira T, Little EC, Berenguer M. Immunosuppression in liver transplant. Best Pract Res Clin Gastroenterol. 2020;46-47:101681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 54. | Gijsen VM, Madadi P, Dube MP, Hesselink DA, Koren G, de Wildt SN. Tacrolimus-induced nephrotoxicity and genetic variability: a review. Ann Transplant. 2012;17:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 55. | Karam S, Wali RK. Current State of Immunosuppression: Past, Present, and Future. Crit Rev Eukaryot Gene Expr. 2015;25:113-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 56. | Gitto S, Falcini M, Marra F; MEDITRA Research Group. Metabolic Disorders After Liver Transplantation. Metab Syndr Relat Disord. 2021;19:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Sharma P, Arora A. Approach to prevention of non-alcoholic fatty liver disease after liver transplantation. Transl Gastroenterol Hepatol. 2020;5:51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 58. | Gill MG, Majumdar A. Metabolic associated fatty liver disease: Addressing a new era in liver transplantation. World J Hepatol. 2020;12:1168-1181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 59. | Weismüller TJ, Lerch C, Evangelidou E, Strassburg CP, Lehner F, Schrem H, Klempnauer J, Manns MP, Haller H, Schiffer M. A pocket guide to identify patients at risk for chronic kidney disease after liver transplantation. Transpl Int. 2015;28:519-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 60. | Sandilands EA, Dhaun N, Dear JW, Webb DJ. Measurement of renal function in patients with chronic kidney disease. Br J Clin Pharmacol. 2013;76:504-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 61. | Kashani K, Rosner MH, Ostermann M. Creatinine: From physiology to clinical application. Eur J Intern Med. 2020;72:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 221] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 62. | Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473-2483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2001] [Cited by in RCA: 2074] [Article Influence: 109.2] [Reference Citation Analysis (0)] |
| 63. | Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10672] [Cited by in RCA: 11003] [Article Influence: 224.6] [Reference Citation Analysis (1)] |
| 64. | Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11183] [Cited by in RCA: 11816] [Article Influence: 454.5] [Reference Citation Analysis (0)] |
| 65. | Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3805] [Cited by in RCA: 4221] [Article Influence: 222.2] [Reference Citation Analysis (0)] |
| 66. | Lucey MR, Terrault N, Ojo L, Hay JE, Neuberger J, Blumberg E, Teperman LW. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 2013;19:3-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 362] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 67. | Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20319] [Cited by in RCA: 20093] [Article Influence: 1255.8] [Reference Citation Analysis (0)] |
| 68. | Levitsky J, O'Leary JG, Asrani S, Sharma P, Fung J, Wiseman A, Niemann CU. Protecting the Kidney in Liver Transplant Recipients: Practice-Based Recommendations From the American Society of Transplantation Liver and Intestine Community of Practice. Am J Transplant. 2016;16:2532-2544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 69. | Millson C, Considine A, Cramp ME, Holt A, Hubscher S, Hutchinson J, Jones K, Leithead J, Masson S, Menon K, Mirza D, Neuberger J, Prasad R, Pratt A, Prentice W, Shepherd L, Simpson K, Thorburn D, Westbrook R, Tripathi D. Adult liver transplantation: UK clinical guideline - part 2: surgery and post-operation. Frontline Gastroenterol. 2020;11:385-396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 70. | Newman DJ, Thakkar H, Edwards RG, Wilkie M, White T, Grubb AO, Price CP. Serum cystatin C measured by automated immunoassay: a more sensitive marker of changes in GFR than serum creatinine. Kidney Int. 1995;47:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 440] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 71. | Soveri I, Berg UB, Björk J, Elinder CG, Grubb A, Mejare I, Sterner G, Bäck SE; SBU GFR Review Group. Measuring GFR: a systematic review. Am J Kidney Dis. 2014;64:411-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 383] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 72. | Medeiros FS, Sapienza MT, Prado ES, Agena F, Shimizu MH, Lemos FB, Buchpiguel CA, Ianhez LE, David-Neto E. Validation of plasma clearance of 51Cr-EDTA in adult renal transplant recipients: comparison with inulin renal clearance. Transpl Int. 2009;22:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 73. | Berding G, Geisler S, Melter M, Marquardt P, Lühr A, Scheller F, Knoop BO, Pfister ED, Pape L, Bischoff L, Knapp WH, Ehrich JH. Estimation of glomerular filtration rate in liver-transplanted children: comparison of simplified procedures using 51Cr-EDTA and endogenous markers with Sapirstein's method as a reference standard. Pediatr Transplant. 2010;14:786-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 74. | Skytte Larsson J, Bragadottir G, Redfors B, Ricksten SE. Renal function and oxygenation are impaired early after liver transplantation despite hyperdynamic systemic circulation. Crit Care. 2017;21:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 75. | Kivelä JM, Lempinen M, Holmberg C, Jalanko H, Pakarinen MP, Isoniemi H, Lauronen J. Renal function after combined liver-kidney transplantation: A longitudinal study of pediatric and adult patients. Pediatr Transplant. 2019;23:e13400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 76. | Åberg F, Berntsson J, Herlenius G, Castedal M, Bennet W. Everolimus and long-term decline in renal function after liver transplantation: real-life experience with measured GFR. Scand J Gastroenterol. 2020;55:718-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 77. | Yeung ACY, Morozov A, Robertson FP, Fuller BJ, Davidson BR. Neutrophil Gelatinase-Associated Lipocalin (NGAL) in predicting acute kidney injury following orthotopic liver transplantation: A systematic review. Int J Surg. 2018;59:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 78. | Tsuchimoto A, Shinke H, Uesugi M, Kikuchi M, Hashimoto E, Sato T, Ogura Y, Hata K, Fujimoto Y, Kaido T, Kishimoto J, Yanagita M, Matsubara K, Uemoto S, Masuda S. Urinary neutrophil gelatinase-associated lipocalin: a useful biomarker for tacrolimus-induced acute kidney injury in liver transplant patients. PLoS One. 2014;9:e110527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 79. | Lima C, de Paiva Haddad LB, de Melo PDV, Malbouisson LM, do Carmo LPF, D'Albuquerque LAC, Macedo E. Early detection of acute kidney injury in the perioperative period of liver transplant with neutrophil gelatinase-associated lipocalin. BMC Nephrol. 2019;20:367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 80. | Robertson FP, Yeung AC, Male V, Rahman S, Mallett S, Fuller BJ, Davidson BR. Urinary Neutrophil Gelatinase Associated Lipocalins (NGALs) predict acute kidney injury post liver transplant. HPB (Oxford). 2019;21:473-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 81. | Yoon KC, Lee KW, Oh SC, Kim H, Kim HS, Hong SK, Ahn SW, Yi NJ, Suh KS. Urinary Neutrophil Gelatinase-Associated Lipocalin as a Biomarker for Renal Injury in Liver Transplant Recipients Using Calcineurin Inhibitors. Transplant Proc. 2018;50:3667-3672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 82. | Tsai KF, Li LC, Hsu CN, Lin CC, Lin YH, Cheng YF, Wang CC, Chen CL. Effects of Conversion From Calcineurin Inhibitors to Sirolimus or Everolimus on Renal Function and Possible Mechanisms in Liver Transplant Recipients. J Clin Pharmacol. 2019;59:326-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 83. | Fukuda M, Suetsugu K, Tajima S, Katsube Y, Watanabe H, Harada N, Yoshizumi T, Egashira N, Mori M, Masuda S. Neutrophil Gelatinase-Associated Lipocalin Is Not Associated with Tacrolimus-Induced Acute Kidney Injury in Liver Transplant Patients Who Received Mycophenolate Mofetil with Delayed Introduction of Tacrolimus. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 84. | Lewandowska L, Małyszko J, Joanna Matuszkiewicz-Rowińska J. Urinary and Serum Biomarkers for Prediction of Acute Kidney Injury in Patients Undergoing Liver Transplantation. Ann Transplant. 2019;24:291-297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 85. | Buturović-Ponikvar J, Visnar-Perovic A. Ultrasonography in chronic renal failure. Eur J Radiol. 2003;46:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 86. | Siddappa JK, Singla S, Al Ameen M, Rakshith SC, Kumar N. Correlation of ultrasonographic parameters with serum creatinine in chronic kidney disease. J Clin Imaging Sci. 2013;3:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 87. | Araújo NC, Rebelo MAP, da Silveira Rioja L, Suassuna JHR. Sonographically determined kidney measurements are better able to predict histological changes and a low CKD-EPI eGFR when weighted towards cortical echogenicity. BMC Nephrol. 2020;21:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 88. | Caroli A, Schneider M, Friedli I, Ljimani A, De Seigneux S, Boor P, Gullapudi L, Kazmi I, Mendichovszky IA, Notohamiprodjo M, Selby NM, Thoeny HC, Grenier N, Vallée JP. Diffusion-weighted magnetic resonance imaging to assess diffuse renal pathology: a systematic review and statement paper. Nephrol Dial Transplant. 2018;33:ii29-ii40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 89. | Derlin K, Hellms S, Gutberlet M, Peperhove M, Jang MS, Greite R, Hartung D, Derlin T, Fegbeutel C, Tudorache I, Jüttner B, Wiese B, Lichtinghagen R, Haller H, Haverich A, Wacker F, Warnecke G, Gueler F. Application of MR diffusion imaging for non-invasive assessment of acute kidney injury after lung transplantation. Medicine (Baltimore). 2020;99:e22445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 90. | Guo LH, Xu HX, Fu HJ, Peng A, Zhang YF, Liu LN. Acoustic radiation force impulse imaging for noninvasive evaluation of renal parenchyma elasticity: preliminary findings. PLoS One. 2013;8:e68925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 91. | Palmeri ML, Wang MH, Dahl JJ, Frinkley KD, Nightingale KR. Quantifying hepatic shear modulus in vivo using acoustic radiation force. Ultrasound Med Biol. 2008;34:546-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 561] [Cited by in RCA: 434] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 92. | Paranaguá-Vezozzo DC, Andrade A, Mazo DF, Nunes V, Guedes AL, Ragazzo TG, Moutinho R, Nacif LS, Ono SK, Alves VA, Carrilho FJ. Concordance of non-invasive mechanical and serum tests for liver fibrosis evaluation in chronic hepatitis C. World J Hepatol. 2017;9:436-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 93. | Ragazzo TG, Paranagua-Vezozzo D, Lima FR, de Campos Mazo DF, Pessoa MG, Oliveira CP, Alves VAF, Carrilho FJ. Accuracy of transient elastography-FibroScan®, acoustic radiation force impulse (ARFI) imaging, the enhanced liver fibrosis (ELF) test, APRI, and the FIB-4 index compared with liver biopsy in patients with chronic hepatitis C. Clinics (Sao Paulo). 2017;72:516-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 94. | Bekci T, Bilgici MC, Tekcan D, Ulus Y, Akyuz B. Quantitative Assessment of Muscular Stiffness in Children With Chronic Kidney Disease Using Acoustic Radiation Force Impulse Ultrasound Elastography. Ultrasound Q. 2019;37:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 95. | Alan B, Utangaç M, Göya C, Dağgülli M. Role of Acoustic Radiation Force Impulse (ARFI) Elastography in Determination of Severity of Benign Prostate Hyperplasia. Med Sci Monit. 2016;22:4523-4528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 96. | Zhang S, Wan J, Liu H, Yao M, Xiang L, Fang Y, Jia L, Wu R. Value of conventional ultrasound, ultrasound elasticity imaging, and acoustic radiation force impulse elastography for prediction of malignancy in breast lesions. Clin Hemorheol Microcirc. 2020;74:241-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 97. | Wang L. Applications of acoustic radiation force impulse quantification in chronic kidney disease: a review. Ultrasonography. 2016;35:302-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 98. | Bob F, Bota S, Sporea I, Sirli R, Popescu A, Schiller A. Relationship between the estimated glomerular filtration rate and kidney shear wave speed values assessed by acoustic radiation force impulse elastography: a pilot study. J Ultrasound Med. 2015;34:649-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 99. | Pawarode A, Fine DM, Thuluvath PJ. Independent risk factors and natural history of renal dysfunction in liver transplant recipients. Liver Transpl. 2003;9:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 223] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 100. | Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1707] [Cited by in RCA: 2474] [Article Influence: 206.2] [Reference Citation Analysis (0)] |
| 101. | Saliba F, Fischer L, de Simone P, Bernhardt P, Bader G, Fung J. Association Between Renal Dysfunction and Major Adverse Cardiac Events After Liver Transplantation: Evidence from an International Randomized Trial of Everolimus-Based Immunosuppression. Ann Transplant. 2018;23:751-757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 102. | Jones-Burton C, Mishra SI, Fink JC, Brown J, Gossa W, Bakris GL, Weir MR. An in-depth review of the evidence linking dietary salt intake and progression of chronic kidney disease. Am J Nephrol. 2006;26:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 103. | Laryea M, Watt KD, Molinari M, Walsh MJ, McAlister VC, Marotta PJ, Nashan B, Peltekian KM. Metabolic syndrome in liver transplant recipients: prevalence and association with major vascular events. Liver Transpl. 2007;13:1109-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 237] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 104. | Fussner LA, Heimbach JK, Fan C, Dierkhising R, Coss E, Leise MD, Watt KD. Cardiovascular disease after liver transplantation: When, What, and Who Is at Risk. Liver Transpl. 2015;21:889-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 148] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 105. | Charlton M, Rinella M, Patel D, McCague K, Heimbach J, Watt K. Everolimus Is Associated With Less Weight Gain Than Tacrolimus 2 Years After Liver Transplantation: Results of a Randomized Multicenter Study. Transplantation. 2017;101:2873-2882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 106. | Utsumi M, Umeda Y, Sadamori H, Nagasaka T, Takaki A, Matsuda H, Shinoura S, Yoshida R, Nobuoka D, Satoh D, Fuji T, Yagi T, Fujiwara T. Risk factors for acute renal injury in living donor liver transplantation: evaluation of the RIFLE criteria. Transpl Int. 2013;26:842-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 107. | Klintmalm GB, Feng S, Lake JR, Vargas HE, Wekerle T, Agnes S, Brown KA, Nashan B, Rostaing L, Meadows-Shropshire S, Agarwal M, Harler MB, Garcia-Valdecasas JC. Belatacept-based immunosuppression in de novo liver transplant recipients: 1-year experience from a phase II randomized study. Am J Transplant. 2014;14:1817-1827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 108. | Yoshida EM, Marotta PJ, Greig PD, Kneteman NM, Marleau D, Cantarovich M, Peltekian KM, Lilly LB, Scudamore CH, Bain VG, Wall WJ, Roy A, Balshaw RF, Barkun JS. Evaluation of renal function in liver transplant recipients receiving daclizumab (Zenapax), mycophenolate mofetil, and a delayed, low-dose tacrolimus regimen vs. a standard-dose tacrolimus and mycophenolate mofetil regimen: a multicenter randomized clinical trial. Liver Transpl. 2005;11:1064-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 109. | Boudjema K, Camus C, Saliba F, Calmus Y, Salamé E, Pageaux G, Ducerf C, Duvoux C, Mouchel C, Renault A, Compagnon P, Lorho R, Bellissant E. Reduced-dose tacrolimus with mycophenolate mofetil vs. standard-dose tacrolimus in liver transplantation: a randomized study. Am J Transplant. 2011;11:965-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 110. | De Simone P, Fagiuoli S, Cescon M, De Carlis L, Tisone G, Volpes R, Cillo U; Consensus Panel. Use of Everolimus in Liver Transplantation: Recommendations From a Working Group. Transplantation. 2017;101:239-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 111. | Fischer L, Klempnauer J, Beckebaum S, Metselaar HJ, Neuhaus P, Schemmer P, Settmacher U, Heyne N, Clavien PA, Muehlbacher F, Morard I, Wolters H, Vogel W, Becker T, Sterneck M, Lehner F, Klein C, Kazemier G, Pascher A, Schmidt J, Rauchfuss F, Schnitzbauer A, Nadalin S, Hack M, Ladenburger S, Schlitt HJ. A randomized, controlled study to assess the conversion from calcineurin-inhibitors to everolimus after liver transplantation--PROTECT. Am J Transplant. 2012;12:1855-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 112. | Saliba F, De Simone P, Nevens F, De Carlis L, Metselaar HJ, Beckebaum S, Jonas S, Sudan D, Fischer L, Duvoux C, Chavin KD, Koneru B, Huang MA, Chapman WC, Foltys D, Dong G, Lopez PM, Fung J, Junge G; H2304 Study Group. Renal function at two years in liver transplant patients receiving everolimus: results of a randomized, multicenter study. Am J Transplant. 2013;13:1734-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 113. | Sterneck M, Kaiser GM, Heyne N, Richter N, Rauchfuss F, Pascher A, Schemmer P, Fischer L, Klein CG, Nadalin S, Lehner F, Settmacher U, Neuhaus P, Gotthardt D, Loss M, Ladenburger S, Paulus EM, Mertens M, Schlitt HJ. Everolimus and early calcineurin inhibitor withdrawal: 3-year results from a randomized trial in liver transplantation. Am J Transplant. 2014;14:701-710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 114. | Sterneck M, Kaiser GM, Heyne N, Richter N, Rauchfuss F, Pascher A, Schemmer P, Fischer L, Klein CG, Nadalin S, Lehner F, Settmacher U, Gotthardt D, Loss M, Ladenburger S, Wimmer P, Dworak M, Schlitt HJ. Long-term follow-up of five yr shows superior renal function with everolimus plus early calcineurin inhibitor withdrawal in the PROTECT randomized liver transplantation study. Clin Transplant. 2016;30:741-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 115. | Abdelmalek MF, Humar A, Stickel F, Andreone P, Pascher A, Barroso E, Neff GW, Ranjan D, Toselli LT, Gane EJ, Scarola J, Alberts RG, Maller ES, Lo CM; Sirolimus Liver Conversion Trial Study Group. Sirolimus conversion regimen versus continued calcineurin inhibitors in liver allograft recipients: a randomized trial. Am J Transplant. 2012;12:694-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 116. | Asrani SK, Wiesner RH, Trotter JF, Klintmalm G, Katz E, Maller E, Roberts J, Kneteman N, Teperman L, Fung JJ, Millis JM. De novo sirolimus and reduced-dose tacrolimus versus standard-dose tacrolimus after liver transplantation: the 2000-2003 phase II prospective randomized trial. Am J Transplant. 2014;14:356-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 117. | Pageaux GP, Rostaing L, Calmus Y, Duvoux C, Vanlemmens C, Hardgwissen J, Bernard PH, Barbotte E, Vercambre L, Bismuth M, Puche P, Navarro F, Larrey D. Mycophenolate mofetil in combination with reduction of calcineurin inhibitors for chronic renal dysfunction after liver transplantation. Liver Transpl. 2006;12:1755-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 118. | Kornberg A, Küpper B, Thrum K, Krause B, Büchler P, Kornberg J, Sappler A, Altendorf-Hofmann A, Wilberg J, Friess H. Sustained renal response to mycophenolate mofetil and CNI taper promotes survival in liver transplant patients with CNI-related renal dysfunction. Dig Dis Sci. 2011;56:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 119. | Goralczyk AD, Bari N, Abu-Ajaj W, Lorf T, Ramadori G, Friede T, Obed A. Calcineurin inhibitor sparing with mycophenolate mofetil in liver transplantion: a systematic review of randomized controlled trials. Am J Transplant. 2012;12:2601-2607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 120. | Beckebaum S, Klein CG, Sotiropoulos GC, Saner FH, Gerken G, Paul A, Cicinnati VR. Combined mycophenolate mofetil and minimal dose calcineurin inhibitor therapy in liver transplant patients: clinical results of a prospective randomized study. Transplant Proc. 2009;41:2567-2569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 121. | De Simone P, Nevens F, De Carlis L, Metselaar HJ, Beckebaum S, Saliba F, Jonas S, Sudan D, Fung J, Fischer L, Duvoux C, Chavin KD, Koneru B, Huang MA, Chapman WC, Foltys D, Witte S, Jiang H, Hexham JM, Junge G; H2304 Study Group. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: a randomized controlled trial. Am J Transplant. 2012;12:3008-3020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 253] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 122. | De Simone P, Metselaar HJ, Fischer L, Dumortier J, Boudjema K, Hardwigsen J, Rostaing L, De Carlis L, Saliba F, Nevens F. Conversion from a calcineurin inhibitor to everolimus therapy in maintenance liver transplant recipients: a prospective, randomized, multicenter trial. Liver Transpl. 2009;15:1262-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 123. | Saliba F, Dharancy S, Lorho R, Conti F, Radenne S, Neau-Cransac M, Hurtova M, Hardwigsen J, Calmus Y, Dumortier J. Conversion to everolimus in maintenance liver transplant patients: a multicenter, retrospective analysis. Liver Transpl. 2011;17:905-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 124. | Bilbao I, Salcedo M, Gómez MA, Jimenez C, Castroagudín J, Fabregat J, Almohalla C, Herrero I, Cuervas-Mons V, Otero A, Rubín A, Miras M, Rodrigo J, Serrano T, Crespo G, De la Mata M, Bustamante J, Gonzalez-Dieguez ML, Moreno A, Narvaez I, Guilera M; EVEROLIVER study group. Renal function improvement in liver transplant recipients after early everolimus conversion: A clinical practice cohort study in Spain. Liver Transpl. 2015;21:1056-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 125. | Castroagudín JF, Molina E, Romero R, Otero E, Tomé S, Varo E. Improvement of renal function after the switch from a calcineurin inhibitor to everolimus in liver transplant recipients with chronic renal dysfunction. Liver Transpl. 2009;15:1792-1797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 126. | Saliba F, Dharancy S, Salamé E, Conti F, Eyraud D, Radenne S, Antonini T, Guillaud O, Guguenheim J, Neau-Cransac M, Demartin E, Lasailly G, Duvoux C, Sobesky R, Coilly A, Tresson S, Cailliez V, Boillot O, Pageaux GP, Samuel D, Calmus Y, Dumortier J. Time to Conversion to an Everolimus-Based Regimen: Renal Outcomes in Liver Transplant Recipients From the EVEROLIVER Registry. Liver Transpl. 2020;26:1465-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 127. | Viklicky O, Slatinska J, Novotny M, Hruba P. Developments in immunosuppression. Curr Opin Organ Transplantation. 2021;26:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 128. | Cordoba F, Wieczorek G, Audet M, Roth L, Schneider MA, Kunkler A, Stuber N, Erard M, Ceci M, Baumgartner R, Apolloni R, Cattini A, Robert G, Ristig D, Munz J, Haeberli L, Grau R, Sickert D, Heusser C, Espie P, Bruns C, Patel D, Rush JS. A novel, blocking, Fc-silent anti-CD40 monoclonal antibody prolongs nonhuman primate renal allograft survival in the absence of B cell depletion. Am J Transplant. 2015;15:2825-2836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 129. | Fabrizi F, Dixit V, Martin P, Messa P. Chronic kidney disease after liver transplantation: Recent evidence. Int J Artif Organs. 2010;33:803-811. [PubMed] |
| 130. | Demirci G, Becker T, Nyibata M, Lueck R, Bektas H, Lehner F, Tusch G, Strassburg C, Schwarz A, Klempnauer J, Nashan B. Results of combined and sequential liver-kidney transplantation. Liver Transpl. 2003;9:1067-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |