Published online Jan 27, 2022. doi: 10.4254/wjh.v14.i1.209
Peer-review started: October 7, 2021
First decision: December 2, 2021
Revised: December 3, 2021
Accepted: December 23, 2021
Article in press: December 23, 2021
Published online: January 27, 2022
Processing time: 105 Days and 19.8 Hours
Hepatic resection has become the preferred treatment of choice for colorectal liver metastasis (CLM) patients.
To identify the prognostic factors and to formulate a new scoring system for management of CLM.
Clinicopathologic and long-term survival data were analyzed to identify the significant predictors of survival by univariate and multivariate analyses with the Cox model. A clinical score was constructed based on the analysis results.
Three factors of worse overall survival were identified in the multivariate analysis. They were number of liver metastases ≥ 5, size of the largest liver lesion ≥ 4 cm, and the presence of nodal metastasis from the primary tumor. These three factors were chosen as criteria for a clinical risk score for overall survival. The clinical score highly correlated with median overall survival and 5-year survival (P = 0.002).
Priority over surgical resection should be given to the lowest score groups, and alternative oncological treatment should be considered in patients with the highest score.
Core Tip: Using multivariate analysis with the Cox model, we identified three criteria-number of liver metastases ≥ 5, size of the largest liver lesion ≥ 4 cm, and the presence of nodal metastasis from the primary tumor-for a new clinical scoring system. This new clinical score highly correlated with median overall survival and 5-year survival. We propose to use this score to formulate cancer-specific treatment for the patients. Priority over surgical resection should be given to the lowest score groups, and alternative oncological treatment should be considered in patients with the highest score.
- Citation: Cheng KC, Yip ASM. Prognostic factors of survival and a new scoring system for liver resection of colorectal liver metastasis. World J Hepatol 2022; 14(1): 209-223
- URL: https://www.wjgnet.com/1948-5182/full/v14/i1/209.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i1.209
Colorectal cancer (CRC) is the third leading cause of cancer-related death in developed countries[1]. About half of the cases will develop liver metastasis, and 25% of them will present synchronously[2]. Hepatic resection has become the standard management in selected patients, with a reported 5-year survival rate ranging from 36% to 60% after curative liver resection[2-4]. Yet, this is a heterogeneous group of patients with variable prognoses[2]. As such, many studies have been directed towards the investigation of factors that might influence the recurrence and survival of patients with colorectal liver metastasis (CLM), with a goal to differentiate patients that would best benefit from surgical resection from those who should be directed to palliative care[5-8]. The objectives of the present study were to identify the prognostic factors of survival in patients subjected to resection of CLM and to propose a risk score accordingly, to differentiate these patients.
Between June 1999 and June 2020, all resections of CLM in Kwong Wah Hospital were recorded prospectively in the institution’s database and retrospectively analyzed. Patients who underwent palliative resection or ablation treatment only were excluded from analysis.
All patients were followed according to a defined protocol including serum carcinoembryonic antigen level, chest X-ray, and computed tomography scan of the abdomen with contrast or ultrasonography of the liver if the patient was contraindicated for contrast injection. Patients were followed every 3 mo for the first 2 years after the operation and every 6 mo afterwards. Patients were actively called back for follow-up if they missed the appointment.
Patient demographics were extracted, including age at resection of liver metastasis and sex. Information on preoperative factors such as the site of the primary tumor, American Joint Committee on Cancer stage of primary tumor, primary tumor nodal stage, extrahepatic metastasis, disease-free interval from CRC resection to development of metastatic liver disease, carcinoembryonic antigen (CEA) level, and administration of systemic chemotherapy before liver resection was recorded. Regional lymph node metastasis of primary tumor was defined as mesenteric lymph node metastasis found histologically after resection of primary CRC. Synchronous metastases were defined as metastases detected by preoperative screening or during resection of the primary tumor or occurring within 6 mo of the initial diagnosis of CRC[9].
Data on operative details including the extent of liver resection (major vs minor hepatectomy), concomitant use of ablation and operative approach (laparoscopic vs open), volume of blood loss, and requirement of blood transfusion were collected; major hepatectomy was defined as a resection of at least three Couinaud liver segments. Perioperative outcomes, including 30-d mortality and complications, were reported. Pathologic details, including number of tumors, size of the largest tumor nodule, and resection margin, were extracted. Positive resection margin was defined as the presence of tumor cells within 1 mm of the transection line.
The primary endpoint was overall survival, which was defined as the time interval between primary surgical treatment of liver metastasis and the date of death or last follow-up. Secondary endpoint was disease-free survival, which was defined as the time interval between primary surgical treatment of liver metastasis and the date of radiological diagnosis of recurrence.
Continuous variables are summarized as the median with interquartile range (IQR) and categorical variables as frequencies with percentage. Overall and disease-free survival curves were plotted using Kaplan-Meier estimator. Variables affecting long-term survival were determined using the Cox proportional hazards regression model. In order to formulate a risk score, inclusion of variables into multivariable Cox models was based mainly on preoperative factors with clinical relevance, irrespective of the P value in the univariate analysis. This type of variable selection was appropriate because the bivariate selection method wrongly rejects potentially important variables when the relationship between an outcome and a risk factor is confounded by any confounder and when this confounder is not properly controlled[10]. Data were calculated for hazard ratio (HR). Continuous variables were discretized into categorical variables by clinical relevance. A clinical risk score for overall survival was formulated according to factors identified by the multivariate analysis. Statistical significance was defined as P value of the Wald test < 0.05. All the statistical analyses were carried out using SPSS software version 26 (IBM Corp., Chicago, IL, United States).
All 98 patients who underwent resection of CLM during the study period were included in this analytic cohort. Median follow-up period was 36 mo (IQR: 17.00-57.75). There were no missing data or patients lost to follow-up. The clinicopathological data are summarized in Table 1. The study population included 62 males (63.3%) and 36 females (36.7%). The median age of patients at liver resection was 65.5 years (IQR: 59-72). The location of the primary colorectal tumor was mostly in the left colon (n = 40, 40.8%) and rectum (n = 32, 32.7%), and 26 patients (26.5%) had a primary right-sided colon cancer. Regional lymph node metastases were present in 62 patients (63.3%). Fifty-nine patients (60.2%) had synchronous hepatic metastasis. Sixteen patients (16.3%) underwent combined liver and colorectal resection, and eleven (68.8%) of them were performed laparoscopically. Only four patients (4.1%) had a synchronous extrahepatic disease; all of them were pulmonary metastases. Two of the pulmonary metastasis patients underwent curative pulmonary metastasectomy. One patient did not have surgery because he was subsequently diagnosed with a brain metastasis before pulmonary resection.
| Characteristic | Total (n = 98) |
| Age in yr, median (IQR) | 65.5 (59-72) |
| Sex, n (%) | |
| Male | 62 (63.3) |
| Female | 36 (36.7) |
| Location of primary colorectal tumor, n (%) | |
| Right | 26 (26.5) |
| Left | 40 (40.8) |
| Rectum | 32 (32.7) |
| LN involvement in primary tumor, n (%) | |
| Yes | 62 (63.3) |
| No | 36 (36.7) |
| Time of diagnosis of liver metastasis, n (%) | |
| Synchronous | 59 (60.2) |
| Metachronous | |
| Disease-free interval < 12 mo | 9 (9.2) |
| Disease-free interval ≥ 12 mo | 30 (30.6) |
| Synchronous extrahepatic metastasis, n (%) | |
| Yes | 4 (4.1) |
| No | 94 (95.9) |
| Preoperative CEA level in ng/mL, n (%) | |
| < 200 | 90 (91.8) |
| ≥ 200 | 6 (6.1) |
| Systemic chemotherapy before liver resection, n (%) | |
| Yes | 9 (9.2) |
| No | 89 (90.8) |
| Number of liver metastases, n (%) | |
| < 5 lesions | 91 (92.9) |
| ≥ 5 lesions | 7 (7.1) |
| Size of largest liver metastasis, n (%) | |
| < 4 cm | 67 (68.4) |
| ≥ 4 cm | 28 (28.6) |
| Surgical margin, n (%) | |
| Positive | 19 (19.4) |
| Negative | 78 (79.6) |
| Concurrent ablation, n (%) | |
| No | 90 (91.8) |
| Yes | 8 (8.2) |
| Operative approach, n (%) | |
| Laparoscopic | 57 (58.2) |
| Open | 41 (41.8) |
| Type of hepatectomy, n (%) | |
| Minor | 61 (62.2) |
| Major | 37 (37.8) |
| Intraoperative blood loss, n (%) | |
| < 500 mL | 49 (50.0) |
| ≥ 500 mL | 47 (48.0) |
| Requirement of blood transfusion, n (%) | |
| No | 79 (80.6) |
| Yes | 19 (19.4) |
The median operative time was 270 min (IQR: 177.5-376.0). The median length of hospital stay was 7 d (IQR: 6-11). There was no 30-d postoperative mortality. Eight postoperative complications required interventional radiology. Bile leak (n = 4) was the most common cause, followed by intra-abdominal collection (n = 3), and there was one case of drainage of pleural effusion. There were three postoperative endoscopic retrograde cholangiopancreatographies, indicated for bile leakage, with a common bile duct stent inserted. There was one esophagogastroduodenoscopy performed for coffee-ground aspirate from the nasogastric tube, which only showed gastritis. There were three reoperations. One reoperation was due to adhesive intestinal obstruction and the other two because of intra-abdominal sepsis.
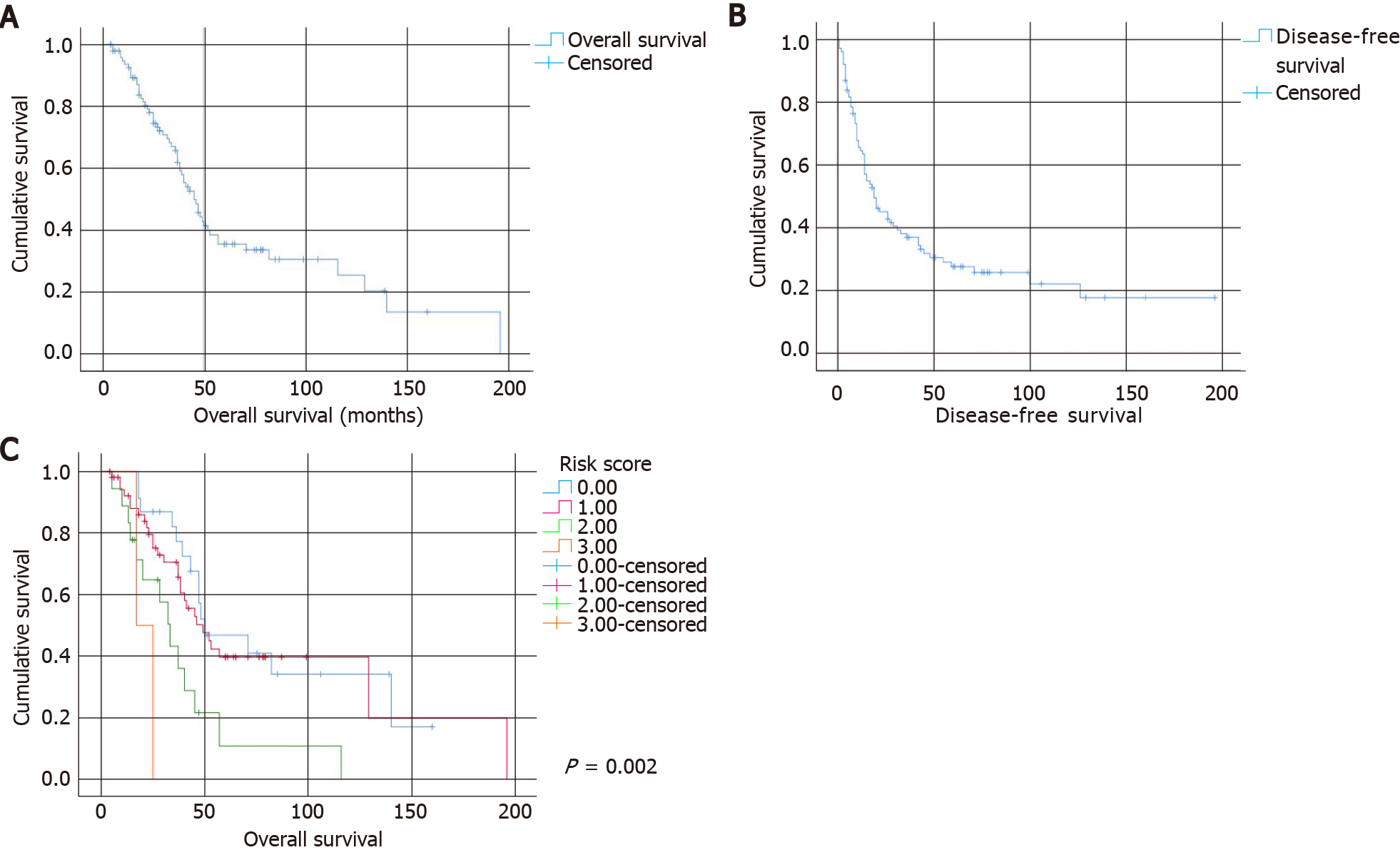
The median overall survival of the entire cohort was 45 mo. The 1-, 3-, and 5-year overall survival rates were 93.6%, 65.8%, and 35.5%, respectively. The overall survival curve is shown in Figure 1A. The median disease-free survival was 19 mo. The 1-, 3-, and 5-year disease-free survival rates were 64.4%, 36.8%, and 27.4%, respectively. The disease-free survival curve is shown in Figure 1B. Univariate analyses of factors affecting overall survival and disease-free survival are shown in Tables 2 and 3, respectively.
| Variable | HR | 95%CI | P value |
| Age | 1.015 | 0.984-1.047 | 0.350 |
| Sex | |||
| Male | Ref | ||
| Female | 1.259 | 0.733-1.162 | 0.405 |
| Location of primary tumor | |||
| Rectum | Ref | ||
| Right | 1.542 | 0.780-3.048 | 0.213 |
| Left | 1.370 | 0.737-2.545 | 0.319 |
| Regional LN metastasis | |||
| No | Ref | ||
| Yes | 1.444 | 0.836-2.492 | 0.187 |
| Time of diagnosis of liver metastasis, % | |||
| Synchronous | Ref | ||
| Metachronous | |||
| Disease-free interval < 12 mo | 0.814 | 0.317-2.094 | 0.670 |
| Disease-free interval ≥ 12 mo | 0.750 | 0.416-1.352 | 0.338 |
| Synchronous extrahepatic metastasis | |||
| No | Ref | ||
| Yes | 1.884 | 0.253-14.0 | 0.536 |
| Preoperative CEA level | |||
| < 200 ng/mL | Ref | ||
| ≥ 200 ng/mL | 1.104 | 0.392-3.111 | 0.851 |
| Systemic chemotherapy before liver resection | |||
| No | Ref | ||
| Yes | 1.104 | 0.439-2.776 | 0.833 |
| Number of liver metastases | |||
| < 5 lesions | Ref | ||
| ≥ 5 lesions | 2.506 | 1.124-5.585 | 0.025a |
| Size of the largest liver lesion | |||
| < 4 cm | Ref | ||
| ≥ 4 cm | 1.645 | 0.934-2.896 | 0.085 |
| Surgical margin | |||
| Clear | Ref | ||
| Involved | 0.965 | 0.509-1.829 | 0.912 |
| Concurrent ablation | |||
| No | Ref | ||
| Yes | 1.449 | 0.573-3.663 | 0.434 |
| Operative approach | |||
| Laparoscopic | Ref | ||
| Open | 1.069 | 0.624-1.832 | 0.808 |
| Intraoperative blood loss, % | |||
| < 500 mL | Ref | ||
| ≥ 500 mL | 1.845 | 0.985-3.457 | 0.056 |
| Requirement of blood transfusion, % | |||
| No | Ref | ||
| Yes | 1.326 | 0.712-2.472 | 0.374 |
| Variable | HR | 95%CI | P value |
| Age | 0.984 | 0.957-1.012 | 0.271 |
| Sex | |||
| Male | Ref | ||
| Female | 1.000 | 0.614-1.628 | 0.999 |
| Location of primary tumor | |||
| Rectum | Ref | ||
| Right | 0.892 | 0.499-1.593 | 0.698 |
| Left | 0.678 | 0.362-1.271 | 0.226 |
| Regional LN metastasis | |||
| No | Ref | ||
| Yes | 2.324 | 1.348-4.008 | 0.002a |
| Synchronous liver metastasis | |||
| No | Ref | ||
| Yes | 0.820 | 0.502-1.342 | 0.431 |
| Time of diagnosis of liver metastasis, % | |||
| Synchronous | Ref | ||
| Metachronous | |||
| Disease-free interval < 12 mo | 1.066 | 0.452-2.509 | 0.884 |
| Disease-free interval ≥ 12 mo | 0.765 | 0.446-1.312 | 0.330 |
| Preoperative CEA level | |||
| < 200 ng/mL | Ref | ||
| ≥ 200 ng/mL | 1.064 | 0.426-2.657 | 0.894 |
| Systemic chemotherapy before liver resection | |||
| No | Ref | ||
| Yes | 1.724 | 0.779-3.818 | 0.179 |
| Number of liver metastases | |||
| < 5 lesions | Ref | ||
| ≥ 5 lesions | 3.138 | 1.409-6.987 | 0.005a |
| Size of the largest liver lesion | |||
| < 4 cm | Ref | ||
| ≥ 4 cm | 1.272 | 0.763-2.121 | 0.355 |
| Surgical margin | |||
| Clear | Ref | ||
| Involved | 1.110 | 0.616-2.000 | 0.728 |
| Concurrent ablation | |||
| No | Ref | ||
| Yes | 1.705 | 0.777-3.739 | 0.183 |
| Operative approach | |||
| Laparoscopic | Ref | ||
| Open | 0.785 | 0.480-1.285 | 0.336 |
| Intraoperative blood loss, % | |||
| < 500 mL | Ref | ||
| ≥ 500 mL | 1.305 | 0.808-2.107 | 0.276 |
| Requirement of blood transfusion, % | |||
| No | Ref | ||
| Yes | 1.037 | 0.585-1.840 | 0.900 |
On multivariate analysis, the number of liver metastases ≥ 5 [HR: 2.962, 95% confidence interval (CI): 1.174-7.473, P = 0.022], the size of the largest liver lesion ≥ 4 cm (HR: 2.983, 95%CI: 1.343-6.625, P = 0.007), and the presence of nodal metastasis from the primary tumor (HR: 1.955, 95%CI: 1.031-3.707, P = 0.040) were associated with a worse overall survival (Table 4). On the other hand, the number of liver metastases ≥ 5 (HR: 2.753, 95%CI: 1.052-7.205, P = 0.039) and the presence of nodal metastasis (HR: 2.234, 95%CI: 1.219-4.093, P = 0.009) were associated with a worse disease-free survival on multivariate analysis (Table 5).
| Variable | Adjusted HR | 95%CI | P value |
| Age | 1.039 | 0.999-1.080 | 0.054 |
| Sex | |||
| Male | Ref | ||
| Female | 1.874 | 0.984-3.572 | 0.056 |
| Location of primary tumor | |||
| Rectum | Ref | ||
| Right | 1.180 | 0.572-2.435 | 0.654 |
| Left | 0.943 | 0.427-2.084 | 0.884 |
| Regional LN metastasis | |||
| No | Ref | ||
| Yes | 1.955 | 1.031-3.707 | 0.040a |
| Time of diagnosis of liver metastasis, % | |||
| Synchronous | Ref | ||
| Metachronous | |||
| Disease-free interval < 12 mo | 1.192 | 0.431-3.295 | 0.735 |
| Disease-free interval ≥ 12 mo | 0.668 | 0.324-1.378 | 0.275 |
| Synchronous extrahepatic metastasis | |||
| No | Ref | ||
| Yes | 2.454 | 0.308-19.572 | 0.397 |
| Preoperative CEA level | |||
| < 200 ng/mL | Ref | ||
| ≥ 200 ng/mL | 0.495 | 0.137-1.785 | 0.282 |
| Systemic chemotherapy before liver resection | |||
| No | Ref | ||
| Yes | 1.031 | 0.363-2.929 | 0.954 |
| Number of liver metastases | |||
| < 5 lesions | Ref | ||
| ≥ 5 lesions | 2.962 | 1.174-7.473 | 0.022a |
| Size of the largest liver lesion | |||
| < 4 cm | Ref | ||
| ≥ 4 cm | 2.983 | 1.343-6.625 | 0.007a |
| Concurrent ablation | |||
| No | Ref | ||
| Yes | 1.241 | 0.436-3.533 | 0.685 |
| Operative approach | |||
| Laparoscopic | Ref | ||
| Open | 1.655 | 0.873-3.137 | 0.123 |
| Requirement of blood transfusion, % | |||
| Yes | Ref | ||
| No | 0.681 | 0.320-1.451 | 0.320 |
| Variable | Adjusted HR | 95%CI | P value |
| Age | 0.988 | 0.955-1.021 | 0.467 |
| Sex | |||
| Male | Ref | ||
| Female | 1.022 | 0.579-1.805 | 0.941 |
| Location of primary tumor | |||
| Rectum | Ref | ||
| Right | 1.044 | 0.538-2.025 | 0.899 |
| Left | 0.635 | 0.302-1.337 | 0.232 |
| Regional LN metastasis | |||
| No | Ref | ||
| Yes | 2.234 | 1.219-4.093 | 0.009a |
| Time of diagnosis of liver metastasis, % | |||
| Synchronous | Ref | ||
| Metachronous | |||
| Disease-free interval < 12 mo | 1.392 | 0.536-3.615 | 0.496 |
| Disease-free interval ≥ 12 mo | 0.846 | 0.445-1.610 | 0.611 |
| Synchronous extrahepatic metastasis | |||
| No | Ref | ||
| Yes | 9.716 | 2.034-46.413 | 0.004a |
| Preoperative CEA level | |||
| < 200 ng/mL | Ref | ||
| ≥ 200 ng/mL | 0.734 | 0.238-2.263 | 0.591 |
| Systemic chemotherapy before liver resection | |||
| No | Ref | ||
| Yes | 1.878 | 0.774-4.557 | 0.163 |
| Number of liver metastases | |||
| < 5 lesions | Ref | ||
| ≥ 5 lesions | 2.753 | 1.052-7.205 | 0.039a |
| Size of the largest liver lesion | |||
| < 4 cm | Ref | ||
| ≥ 4 cm | 1.690 | 0.847-3.374 | 0.137 |
| Concurrent ablation | |||
| No | Ref | ||
| Yes | 0.788 | 0.267-2.324 | 0.666 |
| Operative approach | |||
| Laparoscopic | Ref | ||
| Open | 1.000 | 0.572-1.748 | 1.000 |
| Requirement of blood transfusion, % | |||
| Yes | Ref | ||
| No | 0.692 | 0.342-1.399 | 0.306 |
Three factors–the number of liver metastases ≥ 5, the size of the largest liver lesion ≥ 4 cm, and the presence of nodal metastasis from the primary tumor–were chosen as criteria for a clinical risk score for overall survival. As the HRs of these three factors were similar, for the sake of simplicity, each criterion was assigned 1 point. The total score was compared with overall survival using the log-rank test (Figure 1C). Although the survival of patients with score 0 (5-year survival: 46.8%, median survival of 50 mo) and score 1 was similar (5-year survival: 49.7%, median survival of 49 mo), overall survival clearly separated from those with score 2 (5-year survival: 10.8%, median survival of 33 mo) and score 3 (no 5-year survivors, median survival of 17 mo, P = 0.002).
The management of CLM has seen a marked change over the last decade, owing to the advancement of surgical techniques and perioperative treatments[3]. The achievement of curative resection of liver metastasis has transformed the 5-year survival from 11% to a range of 36%-60%[2-4]. The current study demonstrated a 5-year overall survival rate of 35.5%, slightly lower than the reported survival rate. This is probably due to the extended duration of the study period, which could be traced back to as early as 1999, in which management of CLM was less aggressive.
Many studies have investigated the prognostic factors of survival after resection of CLM. The most frequently cited prognostic factors are the number and the largest size of CLM, regional lymph node metastasis of the primary tumor, and preoperative CEA level[2]. Other proposed factors included disease-free interval from the treatment of primary CRC, location of primary CRC, and surgical resection margin[4,11,12]. The present study confirmed that a larger number of liver metastases, a larger size of the liver tumor, and the presence of regional lymph node metastasis of the primary tumor were associated with a poorer long-term survival. Among them, the number of liver lesions and the size of the largest liver tumor had the highest HRs (2.962 and 2.983, respectively).
Our study also identified that the largest tumor size 4 cm was the optimal cutoff value for prognostic purposes. Fong et al[5] and Nordlinger et al[6] were among the earliest groups of investigators to produce a clinical risk score, which utilized the size of the largest tumor > 5 cm as one of the criteria. This cutoff value has been used in subsequent studies as well[13,14]. Yet, this cutoff value was not universal; other size parameters (i.e., 2 cm, 3 cm, or 4 cm) have been adopted as well[4,15,16]. Hence, size parameter of liver metastasis is a generally accepted risk factor, and our study is consistent with previous studies.
The current study evaluated that number of liver metastases 5 was the cutoff value that predicted a negative survival. The number of liver metastases is another frequently reported prognostic factor[2,5,6,13,14,16-18]. Again, there was not a universally accepted cutoff value for the number of liver metastases. However, a Japanese group of researchers analyzed 727 patients who had undergone CLM resections and reported that 4–5 was the most reliable cutoff value (HR: 2.35)[19]. Some studies also demonstrated that solitary liver metastasis had a significantly better prognosis than multiple metastases[16,18,20]. The present study echoed the past studies and was able to demonstrate the prognostic significance of the number of liver metastases.
Our study failed to show that the preoperative CEA level had a significant impact on long-term survival. Half of the published data referred to preoperative CEA level as a poor prognostic factor[2]. One of the possible explanations is that the sample size of the current study was too small to detect a significant result for this factor.
Concerning the surgical approach, past studies suggested that laparoscopic surgery was a favorable alternative to open surgery in selected CLM patients[21,22]. The OSLO-COMET randomized controlled trial, which compared laparoscopic and open parenchyma-sparing liver resection for CLM, concluded that laparoscopic surgery was associated with significantly less postoperative complications[23,24]. Although the evidence of the benefit of laparoscopic surgery on long-term survival is limited, there was a meta-analysis published in 2020 that aimed to evaluate the long-term oncologic outcome of laparoscopic and open liver surgery for CLM patients[25]. The study included 13 propensity-score matched studies and two randomized controlled trials, with a total of 3148 patients. The study concluded that laparoscopic surgery had a restricted mean survival time 8.6 mo longer at 10 years (P < 0.0001) and 30.0 mo longer at 15 years (P < 0.0001) than the open surgery group. The current study concurred with previous findings of similar survival between laparoscopic and open liver resections. Further research on this subject using a case-matched cohort study would be helpful.
Elderly patients are bound to have less physiological reserve and suffer from more medical comorbidity than younger patients. These factors will cause older patients to be more prone to surgical risks and mortality from other non-cancer related causes. Yet, from our study, liver surgery in elderly patients appeared to be safe, with a comparable outcome to younger patients, and these patients should not be denied surgery due to the sole reason of advanced age[26,27]. As a result of this argument, age should not be used as a criterion in formulating management of CLM.
The first large-scale clinical scoring system was the Nordlinger score, which incorporated preoperative and postoperative factors[6]. Then, Fong et al[5] developed a frequently cited clinical score system in 1999. Recently, the Tumor Burden Score was developed based on the concept of the “Metro-Ticket” paradigm and utilized a continuum of liver tumor size and number. This score was developed and validated in studies where most patients received modern neoadjuvant chemotherapy[7]. It is a growing recognition that KRAS and BRAF mutation statuses are important prognostic biochemical markers[28]. Brudvik et al[8] and Beamish et al[29] created a clinical scoring system specifically examining the impact of KRAS mutational status on survival of CLM patients. Many studies had been conducted to validate these clinical prediction scores[30-32]. A recent study examined the validity of previous clinical risk scoring systems in the contemporary era where chemotherapeutic treatment for CLM patients had significant improvement. It was shown that previous systems were still relevant in modern clinical use[29].
Despite the emergence of numerous clinical scoring systems in keeping with the development of oncological treatment for CLM, the most frequently cited scoring system was still the Fong score due to its incorporation of clinical criteria available for all patients (size, number, nodal status, preoperative CEA level, and disease-free interval)[5]. This was also applicable to our clinical scoring system, which was basically a simplified version of the Fong score. Apart from its simplicity, the factors of the current scoring system are easily available and are available before resection of the liver tumor (except in cases of synchronous resection). This is of vital importance when clinicians are formulating the cancer-specific treatment for patients. The distinct difference in overall survival between the higher and lower score groups means that we can identify two groups of patients who are the most and the least likely to benefit from surgical treatment. A more reserved attitude should be given to the group of patients with the highest score (score = 3), in which there were no 5-year survivors, and the median survival was 17 mo, which was similar to patients without liver resection (15.5-21.3 mo)[33,34]. With the advancement in chemotherapeutic and radiological treatment, this group of patients may achieve a comparable life expectancy without the need to sustain surgical risks and discomforts. The lowest score groups (score = 0 or 1) are clearly the group of patients that can enjoy the benefit of extension of overall survival as a result of surgical treatment. Grey area existed for the average score (score = 2) group. In this group, additional factors, such as patient premorbid status, should be taken into consideration (Table 6).
| Factor | Score1 |
| Number of liver metastases ≥ 5 | 1 |
| Size of liver metastasis ≥ 4 cm | 1 |
| Presence of lymph node metastasis in the primary tumor | 1 |
Several limitations should be considered when interpreting the results of the current study. The retrospective design may limit its conclusions on associations over time. Second, it is a single-center study involving only a small study population with data recorded over 21 years. Perioperative management, including chemotherapy, changes over time, and consequently survival, may be influenced.
Nodal metastasis from the primary tumor, number of liver metastasis, and size of the largest liver tumor have a significant negative impact on overall survival of the patient after resection of CLM. In clinical practice, laparoscopic surgery should be an available option for a selected group of patients due to its potential benefits. When formulating cancer-specific treatment for patients with CLM, we proposed using a simplified clinical scoring system consisting of three significant prognostic factors. Priority over surgical resection should be given to the lowest score groups, and alternative oncological treatment should be considered in the group of patients with the highest score.
Colorectal cancer is the third leading cause of cancer-related death in developed countries. About half of the cases will develop liver metastasis. Hepatic resection has become the standard management in selected patients, with a reported 5-year survival rate ranging from 36% to 60% after curative liver resection.
Patients with colorectal liver metastasis (CLM) are a heterogeneous group, with variable prognoses even after liver resection. As such, many studies have investigated factors that might influence the recurrence and survival of this group of patients, with a hope to differentiate patients that would best benefit from surgical resection from those who should be directed to palliative care.
The objectives of the present study were to identify the prognostic factors of survival in patients subjected to resection of CLM and to propose a risk score accordingly, to differentiate these patients.
Between June 1999 and June 2020, all resections of CLM at Kwong Wah Hospital were recorded prospectively in the institution’s database and retrospectively analyzed. Variables affecting long-term survival were determined using the Cox proportional hazards regression model. A clinical risk score for overall survival was formulated according to factors identified by multivariate analysis.
On multivariate analysis, the number of liver metastases ≥ 5 [hazard ratio (HR): 2.962, 95% confidence interval (CI): 1.174-7.473, P = 0.022], the size of the largest liver lesion ≥ 4 cm (HR: 2.983, 95%CI: 1.343-6.625, P = 0.007), and the presence of nodal metastasis from the primary tumor (HR: 1.955, 95%CI: 1.031-3.707, P = 0.040) were associated with a worse overall survival. These three factors were chosen as criteria for a clinical risk score for overall survival, and the total risk score was compared with overall survival using the log-rank test. Lower total risk score groups had a significantly improved overall survival than the higher total risk score group.
The newly proposed clinical risk score consisting of three significant prognostic factors (nodal metastasis from the primary tumor, number of liver metastases, and size of the largest liver tumor) is simple and easy to use. Priority over surgical resection should be given to the lowest score groups, and alternative oncological treatment should be considered in the group of patients with the highest score.
Small study population (98 patients) and retrospective design limit the conclusions on associations over time. Future study with an expanded study population may allow weighting assignment to each component of the clinical risk score for a more accuracy in prognosis prediction. An external validation study is needed for the actual application of this clinical score in clinical use.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: International Hepato-Pancreato-Biliary Association, No. M02134.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Díez M, Elkady N S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, Lacy AM, Bemelman WA, Andersson J, Angenete E, Rosenberg J, Fuerst A, Haglind E; COLOR II Study Group. A randomized trial of laparoscopic vs open surgery for rectal cancer. N Engl J Med. 2015;372:1324-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 931] [Article Influence: 93.1] [Reference Citation Analysis (0)] |
| 2. | Coimbra FJF, Brandao PHM, Diniz AL, de Castro Ribeiro HS, da Costa Junior WL, de Godoy AL. Prognostic Factors of Colorectal Cancer Liver Metastasis. In: Correia M, Choti M, Rocha F, Wakabayashi G, editors. Colorectal Cancer Liver Metastases, Springer, 2020: 87. |
| 3. | Margonis GA, Sasaki K, Kim Y, Samaha M, Buettner S, Amini N, Antoniou E, Pawlik TM. Tumor Biology Rather Than Surgical Technique Dictates Prognosis in Colorectal Cancer Liver Metastases. J Gastrointest Surg. 2016;20:1821-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Acciuffi S, Meyer F, Bauschke A, Settmacher U, Lippert H, Croner R, Altendorf-Hofmann A. Analysis of prognostic factors after resection of solitary liver metastasis in colorectal cancer: a 22-year bicentre study. J Cancer Res Clin Oncol. 2018;144:593-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309-18; discussion 318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2799] [Article Influence: 107.7] [Reference Citation Analysis (1)] |
| 6. | Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, Jaeck D. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer. 1996;77:1254-1262. [PubMed] |
| 7. | Sasaki K, Morioka D, Conci S, Margonis GA, Sawada Y, Ruzzenente A, Kumamoto T, Iacono C, Andreatos N, Guglielmi A, Endo I, Pawlik TM. The Tumor Burden Score: A New "Metro-ticket" Prognostic Tool For Colorectal Liver Metastases Based on Tumor Size and Number of Tumors. Ann Surg. 2018;267:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 325] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 8. | Brudvik KW, Jones RP, Giuliante F, Shindoh J, Passot G, Chung MH, Song J, Li L, Dagenborg VJ, Fretland ÅA, Røsok B, De Rose AM, Ardito F, Edwin B, Panettieri E, Larocca LM, Yamashita S, Conrad C, Aloia TA, Poston GJ, Bjørnbeth BA, Vauthey JN. RAS Mutation Clinical Risk Score to Predict Survival After Resection of Colorectal Liver Metastases. Ann Surg. 2019;269:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 177] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 9. | Siriwardena AK, Mason JM, Mullamitha S, Hancock HC, Jegatheeswaran S. Management of colorectal cancer presenting with synchronous liver metastases. Nat Rev Clin Oncol. 2014;11:446-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 10. | Sun GW, Shook TL, Kay GL. Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. J Clin Epidemiol. 1996;49:907-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 699] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 11. | McVey JC, Sasaki K, Margonis GA, Nowacki AS, Firl DJ, He J, Berber E, Wolfgang C, Miller CC, Weiss M, Aucejo FN. The impact of resection margin on overall survival for patients with colon cancer liver metastasis varied according to the primary cancer location. HPB (Oxford). 2019;21:702-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Spelt L, Andersson B, Nilsson J, Andersson R. Prognostic models for outcome following liver resection for colorectal cancer metastases: A systematic review. Eur J Surg Oncol. 2012;38:16-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Partelli S, Mukherjee S, Mawire K, Hutchins RR, Abraham AT, Bhattacharya S, Kocher HM. Larger hepatic metastases are more frequent with N0 colorectal tumours and are associated with poor prognosis: implications for surveillance. Int J Surg. 2010;8:453-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Beppu T, Sakamoto Y, Hasegawa K, Honda G, Tanaka K, Kotera Y, Nitta H, Yoshidome H, Hatano E, Ueno M, Takamura H, Baba H, Kosuge T, Kokudo N, Takahashi K, Endo I, Wakabayashi G, Miyazaki M, Uemoto S, Ohta T, Kikuchi K, Yamaue H, Yamamoto M, Takada T. A nomogram predicting disease-free survival in patients with colorectal liver metastases treated with hepatic resection: multicenter data collection as a Project Study for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2012;19:72-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 15. | Moro A, Mehta R, Tsilimigras DI, Sahara K, Paredes AZ, Bagante F, Guglielmi A, Alexandrescu S, Poultsides GA, Sasaki K, Aucejo FN, Pawlik TM. Prognostic factors differ according to KRAS mutational status: A classification and regression tree model to define prognostic groups after hepatectomy for colorectal liver metastasis. Surgery. 2020;168:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Ren W, Sell NM, Ferrone CR, Tanabe KK, Lillemoe KD, Qadan M. Size of the Largest Colorectal Liver Metastasis Is an Independent Prognostic Factor in the Neoadjuvant Setting. J Surg Res. 2021;259:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Hokuto D, Nomi T, Yasuda S, Yoshikawa T, Ishioka K, Yamada T, Akahori T, Nakagawa K, Nagai M, Nakamura K, Obara S, Kanehiro H, Sho M. Risk Factors for Unresectable Recurrence After Up-Front Surgery for Colorectal Liver Metastasis. World J Surg. 2018;42:884-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Chan KM, Wu TH, Cheng CH, Lee WC, Chiang JM, Chen JS, Wang JY. Prognostic significance of the number of tumors and aggressive surgical approach in colorectal cancer hepatic metastasis. World J Surg Oncol. 2014;12:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Beppu T, Sakamoto Y, Hasegawa K, Honda G, Tanaka K, Kotera Y, Nitta H, Yoshidome H, Hatano E, Ueno M, Takamura H, Baba H, Kosuge T, Kokudo N, Takahashi K, Endo I, Wakabayashi G, Miyazaki M, Uemoto S, Ohta T, Kikuchi K, Takayama T, Yamaue H, Yamamoto M, Takada T. Optimal cut-off value for the number of colorectal liver metastases: a project study for hepatic surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2014;21:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Brouquet A, Andreou A, Vauthey JN. The management of solitary colorectal liver metastases. Surgeon. 2011;9:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Kazaryan AM, Marangos IP, Røsok BI, Rosseland AR, Villanger O, Fosse E, Mathisen O, Edwin B. Laparoscopic resection of colorectal liver metastases: surgical and long-term oncologic outcome. Ann Surg. 2010;252:1005-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Shim JR, Lee SD, Park HM, Lee EC, Park B, Han SS, Kim SH, Park SJ. Outcomes of liver resection in patients with colorectal liver metastases by laparoscopic or open surgery. Ann Hepatobiliary Pancreat Surg. 2018;22:223-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, Kazaryan AM, Kristiansen R, Fagerland MW, Hausken J, Tønnessen TI, Abildgaard A, Barkhatov L, Yaqub S, Røsok BI, Bjørnbeth BA, Andersen MH, Flatmark K, Aas E, Edwin B. Laparoscopic Versus Open Resection for Colorectal Liver Metastases: The OSLO-COMET Randomized Controlled Trial. Ann Surg. 2018;267:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 484] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 24. | Chan AKC, Jamdar S, Sheen AJ, Siriwardena AK. The OSLO-COMET Randomized Controlled Trial of Laparoscopic Versus Open Resection for Colorectal Liver Metastases. Ann Surg. 2018;268:e69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Syn NL, Kabir T, Koh YX, Tan HL, Wang LZ, Chin BZ, Wee I, Teo JY, Tai BC, Goh BKP. Survival Advantage of Laparoscopic Versus Open Resection For Colorectal Liver Metastases: A Meta-analysis of Individual Patient Data From Randomized Trials and Propensity-score Matched Studies. Ann Surg. 2020;272:253-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 26. | Schmidt T, Strowitzki MJ, Reissfelder C, Rahbari NN, Nienhueser H, Bruckner T, Rahäuser C, Keppler U, Schneider M, Büchler MW, Ulrich A. Influence of age on resection of colorectal liver metastases. J Surg Oncol. 2015;111:729-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | van Tuil T, Dhaif AA, Te Riele WW, van Ramshorst B, van Santvoort HC. Systematic Review and Meta-Analysis of Liver Resection for Colorectal Metastases in Elderly Patients. Dig Surg. 2019;36:111-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Passiglia F, Bronte G, Bazan V, Galvano A, Vincenzi B, Russo A. Can KRAS and BRAF mutations limit the benefit of liver resection in metastatic colorectal cancer patients? Crit Rev Oncol Hematol. 2016;99:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 29. | Beamish P, Lemke M, Li J, Dixon E, Abraham MT, Hernandez-Alejandro R, Bennett S, Martel G, Karanicolas PJ; HPB CONCEPT Team. Validation of clinical risk score for colorectal liver metastases resected in a contemporary multicenter cohort. HPB (Oxford). 2017;19:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Ribeiro HS, Costa WL Jr, Diniz AL, Godoy AL, Herman P, Coudry RA, Begnami MD, Mello CA, Silva MJ, Zurstrassen CE, Coimbra FJ. Extended preoperative chemotherapy, extent of liver resection and blood transfusion are predictive factors of liver failure following resection of colorectal liver metastasis. Eur J Surg Oncol. 2013;39:380-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 31. | Creasy JM, Sadot E, Koerkamp BG, Chou JF, Gonen M, Kemeny NE, Balachandran VP, Kingham TP, DeMatteo RP, Allen PJ, Blumgart LH, Jarnagin WR, D'Angelica MI. Actual 10-year survival after hepatic resection of colorectal liver metastases: what factors preclude cure? Surgery. 2018;163:1238-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 160] [Article Influence: 22.9] [Reference Citation Analysis (1)] |
| 32. | Araujo RL, Gönen M, Allen P, DeMatteo R, Kingham P, Jarnagin W, D'Angelica M, Fong Y. Positive postoperative CEA is a strong predictor of recurrence for patients after resection for colorectal liver metastases. Ann Surg Oncol. 2015;22:3087-3093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Beppu T, Miyamoto Y, Sakamoto Y, Imai K, Nitta H, Hayashi H, Chikamoto A, Watanabe M, Ishiko T, Baba H. Chemotherapy and targeted therapy for patients with initially unresectable colorectal liver metastases, focusing on conversion hepatectomy and long-term survival. Ann Surg Oncol. 2014;21 Suppl 3:S405-S413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 34. | Nagashima I, Takada T, Matsuda K, Adachi M, Nagawa H, Muto T, Okinaga K. A new scoring system to classify patients with colorectal liver metastases: proposal of criteria to select candidates for hepatic resection. J Hepatobiliary Pancreat Surg. 2004;11:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |