Published online Sep 27, 2021. doi: 10.4254/wjh.v13.i9.1190
Peer-review started: February 24, 2021
First decision: June 15, 2021
Revised: June 29, 2021
Accepted: August 3, 2021
Article in press: August 3, 2021
Published online: September 27, 2021
Processing time: 209 Days and 20.1 Hours
The hepatitis B virus (HBV) infection is a global public health concern that affects about 2 billion people and causes 1 million people deaths yearly. HBV is a blood-borne disease and healthcare workers (HCWs) are a high-risk group because of occupational hazard to patients’ blood. Different regions of the world show a highly variable proportion of HCWs infected and/or immunized against HBV. Global data on serologic markers of HBV infection and immunization in HCWs are very important to improve strategies for HBV control.
To determine the worldwide prevalence of HBV serological markers among HCWs.
In this systematic review and meta–analyses, we searched PubMed and Excerpta Medica Database (Embase) to identify studies published between 1970 and 2019 on the prevalence of HBV serological markers in HCWs worldwide. We also manually searched for references of relevant articles. Four independent investigators selected studies and included those on the prevalence of each of the HBV serological markers including hepatitis B surface antigen (HBsAg), hepatitis e antigen (HBeAg), immunoglobulin M anti-HBc, and anti-HBs. Methodological quality of eligible studies was assessed and random-effect model meta-analysis resulted in the pooled prevalence of HBV serological markers HBV infection in HCWs. Heterogeneity (I²) was assessed using the χ² test on Cochran’s Q statistic and H parameters. Heterogeneity’ sources were explored through subgroup and metaregression analyses. This study is registered with PROSPERO, number CRD42019137144.
We reviewed 14059 references, out of which 227 studies corresponding to 448 prevalence data among HCWs (224936 HCWs recruited from 1964 to 2019 in 71 countries) were included in this meta-analysis. The pooled seroprevalences of current HBsAg, current HBeAg, and acute HBV infection among HCWs were 2.3% [95% confidence interval (CI): 1.9-2.7], 0.2% (95%CI: 0.0-1.7), and 5.3% (95%CI: 1.4-11.2), respectively. The pooled seroprevalences of total immunity against HBV and immunity acquired by natural HBV infection in HCWs were 56.6% (95%CI: 48.7-63.4) and 9.2% (95%CI: 6.8-11.8), respectively. HBV infection was more prevalent in HCWs in low-income countries, particularly in Africa. The highest immunization rates against HBV in HCWs were recorded in urban areas and in high-income countries including Europe, the Eastern Mediterranean and the Western Pacific.
New strategies are needed to improve awareness, training, screening, vaccination, post-exposure management and treatment of HBV infection in HCWs, and particularly in low-income regions.
Core Tip: This study showed that healthcare workers (HCWs) are at an intermediate level (2%-8%) of hepatitis B virus (HBV) infection worldwide. The study also shows that globally, about half of HCWs are immune to HBV. Resource-limited areas with the lowest HBV immunization levels also have the highest HBV infection levels. To achieve the goal of HBV eradication by 2030, new strategies are needed to improve awareness, training, screening, vaccination, post-exposure management and treatment of HBV-infected HCWs, and especially in low-income regions.
- Citation: Mahamat G, Kenmoe S, Akazong EW, Ebogo-Belobo JT, Mbaga DS, Bowo-Ngandji A, Foe-Essomba JR, Amougou-Atsama M, Monamele CG, Mbongue Mikangue CA, Kame-Ngasse GI, Magoudjou-Pekam JN, Zemnou-Tepap C, Meta-Djomsi D, Maïdadi-Foudi M, Touangnou-Chamda SA, Daha-Tchoffo AG, Selly-Ngaloumo AA, Nayang-Mundo RA, Yéngué JF, Taya-Fokou JB, Fokou LKM, Kenfack-Momo R, Tchami Ngongang D, Atembeh Noura E, Tazokong HR, Demeni Emoh CP, Kengne-Ndé C, Bigna JJ, Boyomo O, Njouom R. Global prevalence of hepatitis B virus serological markers among healthcare workers: A systematic review and meta-analysis. World J Hepatol 2021; 13(9): 1190-1202
- URL: https://www.wjgnet.com/1948-5182/full/v13/i9/1190.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i9.1190
Hepatitis B virus (HBV) is one of the main causes of liver disease. HBV infection remains asymptomatic in most infected people but also causes acute or chronic infections which can progress to liver failure, fulminant hepatitis, cirrhosis, hepatocellular carcinoma, and death[1-3]. Globally, hepatitis B is a major public health concern, with approximately a third of the world's population infected, including about 360 million chronic infections and 1 million deaths per year[4]. The HBV infection prevalence varies widely across World Health Organization (WHO) regions, with the African and Western Pacific regions bearing the highest burden (6.1% and 6.2% in the general population, respectively)[5,6].
HBV is transmitted parenterally through the blood and other body fluids of infected people. Several HBV transmission pathways have been identified, such as transmissions from mother to child, through unprotected sexual intercourse, during blood transfusions, via organ transplants, or through splashes and wounds caused by cuts and pricks of contaminated objects[7]. HBV, being a blood-borne pathogen, represents a significant occupational risk among healthcare workers (HCWs). The frequencies of infection in HCWs are up to 4-times greater than in individuals who do not work in hospitals[8-10]. Among the 35 million HCWs working globally, approximately 3 million each year have occupational exposure to HBV infection, leading to up to 66 thousand HBV infections (261 deaths)[9,11]. The chain of transmission of HBV is thus maintained from patients to HCWs and vice versa as well as to HCW relatives[12]. Vaccination against HBV is recommended in most countries for newborns and high-risk individuals, such as HCWs. Vaccination policies targeting HCWs vary widely according to geographic regions, including the absence of a policy, systematic vaccination, confirmation of vaccine protection, and adherence to maintenance of immunity[10,13-16].
According to high heterogeneity across regions regarding HBV routes of transmission, risk factors of infection, interventions for prevention and immunization among HCWs as well as clinical practice, the global epidemiology of HBV infection in HCWs need to be described. Understanding the seroprevalence, immunization rate, and risk factors for HBV infection in HCWs can provide useful information for decision-making and context-specific interventions to curtail the burden of disease of HBV infection. Therefore, the objective of this systematic review with meta-analysis was to determine the seroprevalence and factors associated with HBV infection and rate of HBV immunization in HCWs.
This review was reported following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (Supplementary Table 1)[17]. The protocol for this review was registered in the International Prospective Register of Systematic Reviews (PROSPERO, No. CRD42019137144).
This review included cross-sectional, case-control and cohort (baseline data) studies. Studies in English or French, without geographic restriction, were selected. We included studies using any assay for detecting serological markers of hepatitis B infection. This review considered the following different markers of HBV infection: anti-HBs > 10 IU/mL (total immunity against HBV); anti-HBs (+) and anti-HBc (+) (immunity due to natural infection); hepatitis B surface antigen (HBsAg) (+) and immunoglobulin (Ig) M anti-HBc (+) (acute hepatitis B infection); HBsAg (+) (current HBV infection); and hepatitis e antigen (HBeAg) (+) (current HBV infectivity)[18]. Studies for which the abstract or full text were not available, duplicates, comments, case reports, case series, and studies with less than 10 participants were excluded.
A search was conducted for articles published from 1970 to 2020 at PubMed and Excerpta Medica Database (Embase). The search terms were related to hepatitis B and HCWs (Supplementary Table 2). To supplement the bibliographic database searches and identify potential additional data sources, we scrutinized the reference list of all relevant articles.
Duplicates identified from the complete list of studies were removed. Titles and abstracts of articles retrieved from electronic literature search were independently screened by four investigators (Mahamat G, Kenmoe S, Ebogo-Belobo JT, and Amougou-Atsama M), and the full texts of those potentially eligible were obtained and further assessed for final inclusion. Data from the included studies was extracted using a Google form by 18 of the study’s authors and verified by Kenmoe S. The extracted data were the name of the first author, year of publication, study design, country, country income level, sampling method, timing of data collection, study period, study participant age, male percentage, recruitment setting, HCW category, HBV detection assay, HBV detected markers (HBsAg, HBeAg, anti-HBs, and anti-HBc IgM and IgG), type of sample used for HBV detection, sample size, and number of HBV-positive for each marker. Disagreements observed during study selection and data extraction were resolved by discussion and consensus.
The tool developed by Hoy and collaborators[19] for cross-sectional studies was used to assess the methodological quality of the included studies (Supplementary Table 3). Discussion and consensus were used to resolve disagreements.
The review included HCWs grouped according to WHO guidelines[20]. This classification includes the following as major categories: Health professionals; health associate professionals; personal care workers in health services; health management and support personnel; and other health service providers not classified elsewhere. Prevalence of pooled data was conducted using a random-effects meta-analysis with a Freeman-Tukey double arcsine transformation[21,22]. The I² (> 50%), H (> 1) parameters and the Q test P value (< 0.05) were used to indicate significant heterogeneity[21,23]. Subgroup and meta-regression analyses were used to determine sources of heterogeneity. Egger’s test (P value < 0.1) and asymmetry of funnel plot were used to indicate publication bias and sensitivity analyses were performed on studies with low risk of bias and cross-sectional studies[24]. R version 3.6.2. statistical software was used to conduct all meta-analyses[25,26].
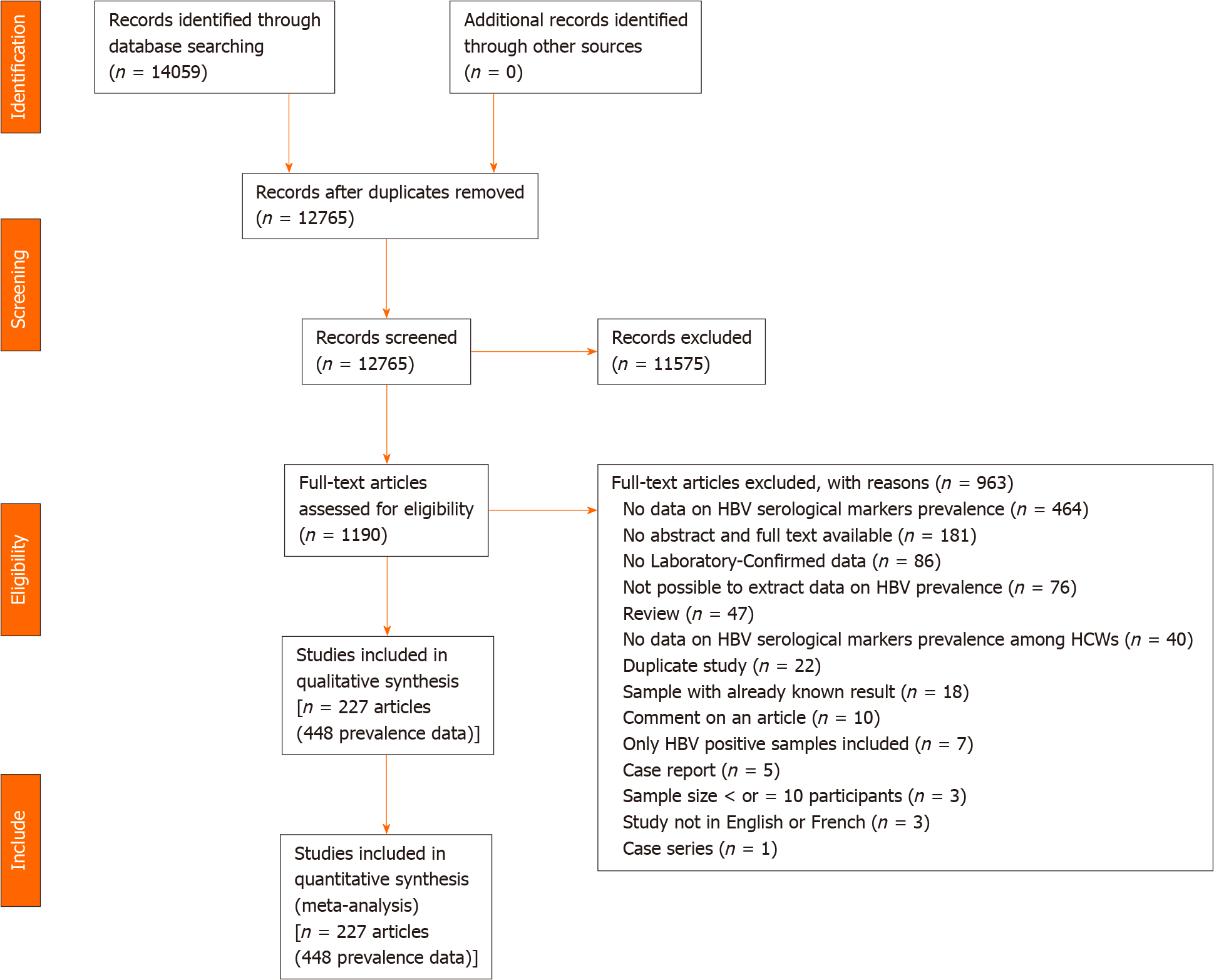
The database search yielded a total of 14059 articles (Figure 1). After removing duplicates, 11575 articles were excluded due to irrelevant titles and abstracts. Of the 1190 articles fully screened 963 were excluded for multiple reasons (Supple
Most of the prevalence data were at moderate risk of bias (n = 279 prevalence data) (Supplementary Tables 6 and 7). Most of the participants were health professionals. Most prevalence data were reported in high (n = 176) and lower-middle (n = 125) income countries. Most of the prevalence data were from cross-sectional studies (n = 439) with non-probabilistic sampling methods (386), with prospective data collection and analysis (420), and in urban setting (212). The most widely used detection assay was direct ELISA (n = 126) for the detection of HBsAg (n = 292). Almost all the prevalence data reported serological markers of hepatitis in serum (n = 435).
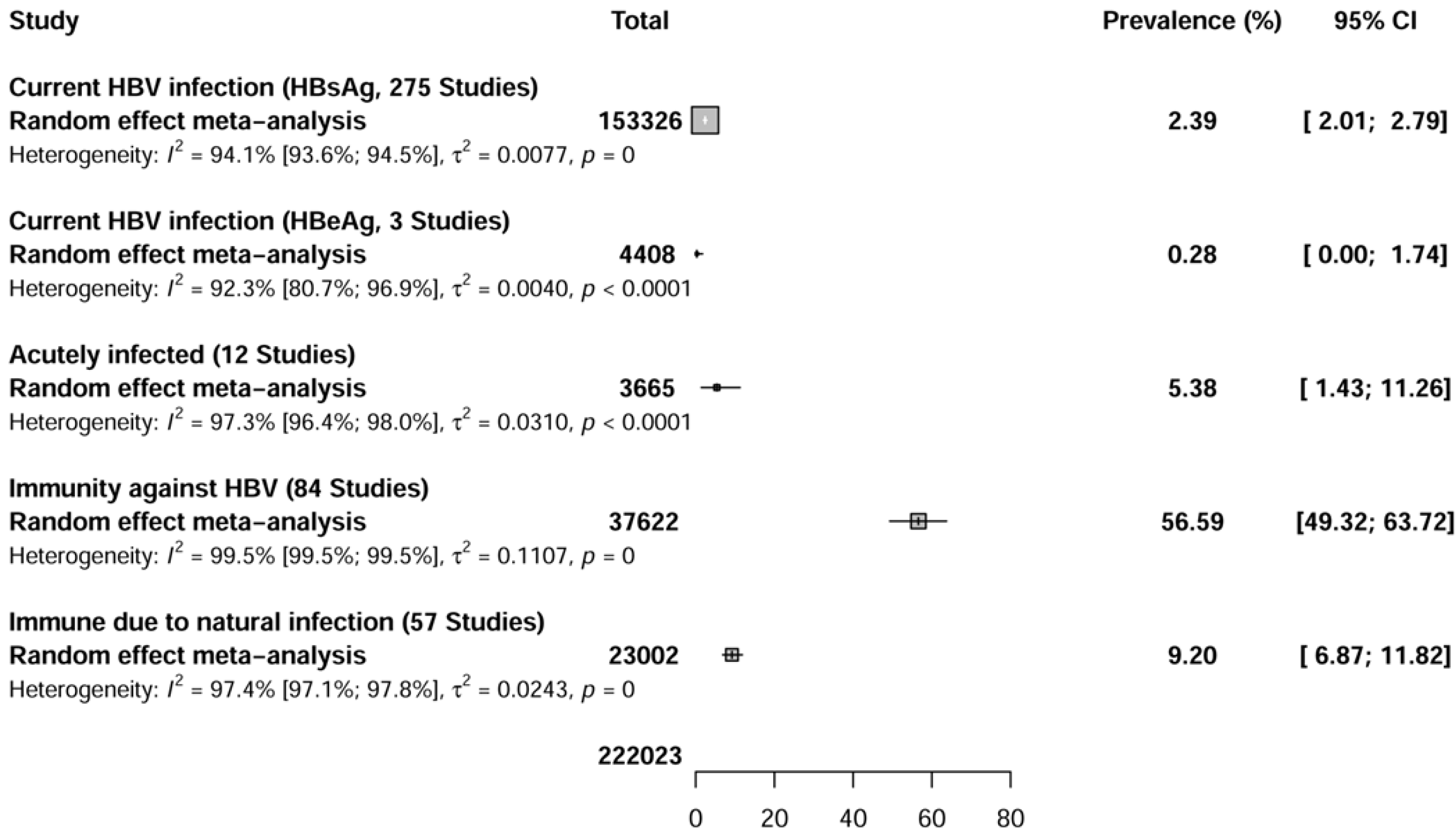
The seroprevalence of current hepatitis B infection (HBsAg) was assessed in 275 seroprevalence data conducted in 62 countries (Figure 2 and Supplementary Figure 1). The overall seroprevalence of current hepatitis B infections (HBsAg) among HCWs was 2.3% [95% confidence interval (CI): 2.0-2.7].
The seroprevalence of current hepatitis B infectivity (HBeAg) positivity was assessed in three seroprevalence data conducted in three countries (Figure 2 and Supple
The seroprevalence of acute VHB (IgM anti-HBs + HBsAg) infection was assessed in 12 seroprevalence data conducted in seven countries (Figure 2 and Supplemen
The seroprevalence of hepatitis B immunity (due to natural infection or vaccination) was assessed in 84 seroprevalence data conducted in 29 countries (Figure 2 and Supplementary Figure 4). The overall seroprevalence of total immunity against HBV among HCWs was 56.6% (95%CI: 48.7-63.4).
The seroprevalence of immunity against hepatitis B acquired through natural infection was assessed in 41 studies (57 seroprevalence data) conducted in 22 countries (Figure 2 and Supplementary Figure 5). The overall seroprevalence of immunity to hepatitis B acquired through natural infection among HCWs was 9.2% (95%CI: 6.8-11.8).
The estimate of these seroprevalence data was associated with substantial heterogeneity current HBV infection (I2 = 94.1%; 95%CI: 93.6-94.5), current HBV infectivity (I2 = 92.3%; 95%CI: 80.7-96.9), HBV acute infection (I2 = 97.9%; 95%CI: 96.9-98.5), total HBV immunity (I2 = 99.5%; 95%CI: 99.5-99.6), and HBV immunity due to natural infection (I2 = 96.9%; 95%CI: 96.4-97.3). Egger's test was significant (P < 0.001) for the seroprevalence of current HBV infection (HBsAg) among HCWs, suggesting the presence of publication bias (Table 1). Egger's tests were not significant for the seroprevalence in HCWs of current HBV infection due to HBeAg positivity (P = 0.577), acute HBV infection (P = 0.256), total immunity against hepatitis B (P = 0.509), and immunity due to natural infection (P = 0.853), suggesting the absence of publication bias. Funnel plots confirmed the results of publication bias obtained by Egger's test (Supplementary Figures 6-10).
| Prevalence % (95%CI) | 95% Prediction interval | Studies, n | Participants, n | 1H (95%CI) | 2I² (95%CI) | P value (heterogeneity) | P value (Egger test) | |
| Current HBV infection (HBsAg) | ||||||||
| Overall | 2.4 (2-2.8) | 0-11 | 275 | 153326 | 4.1 (4-4.3) | 94.1 (93.6-94.5) | < 0.001 | < 0.001 |
| Cross-sectional | 2.4 (2-2.9) | 0-11.1 | 271 | 150516 | 4.1 (4-4.3) | 94.2 (93.7-94.6) | < 0.001 | < 0.001 |
| Low risk of bias | 1.8 (1.4-2.3) | 0-8.2 | 107 | 40212 | 3 (2.8-3.2) | 88.8 (87-90.3) | < 0.001 | < 0.001 |
| Current HBV infection (HBeAg) | ||||||||
| Overall | 0.3 (0-1.7) | 0-70.6 | 3 | 4408 | 3.6 (2.3-5.7) | 92.3 (80.7-96.9) | < 0.001 | 0.577 |
| Cross-sectional | 0.3 (0-1.7) | 0-70.6 | 3 | 4408 | 3.6 (2.3-5.7) | 92.3 (80.7-96.9) | < 0.001 | 0.577 |
| Low risk of bias | 0 (0-0.1) | NA-NA | 1 | 3513 | NA (NA-NA) | NA (NA-NA) | 1 | NA |
| HBV acute infection | ||||||||
| Overall | 5.4 (1.4-11.3) | 0-37 | 12 | 3665 | 6.1 (5.3-7.1) | 97.3 (96.4-98) | < 0.001 | 0.256 |
| Cross-sectional | 5.4 (1.4-11.3) | 0-37 | 12 | 3665 | 6.1 (5.3-7.1) | 97.3 (96.4-98) | < 0.001 | 0.256 |
| Low risk of bias | 1.9 (0-8.7) | 0-48.1 | 5 | 1639 | 6.5 (5.1-8.2) | 97.6 (96.2-98.5) | < 0.001 | 0.824 |
| Immunity against HBV | ||||||||
| Overall | 56.6 (49.3-63.7) | 2.8-100 | 84 | 37622 | 14 (13.5-14.4) | 99.5 (99.5-99.5) | < 0.001 | 0.763 |
| Cross-sectional | 56.3 (48.8-63.7) | 2.4-100 | 80 | 36311 | 14.2 (13.8-14.7) | 99.5 (99.5-99.5) | < 0.001 | 0.811 |
| Low risk of bias | 65.9 (56.1-75.1) | 10.3-100 | 35 | 22401 | 14.7 (14.1-15.4) | 99.5 (99.5-99.6) | < 0.001 | 0.974 |
| Immunity due to natural HBV infection | ||||||||
| Overall | 9.2 (6.9-11.8) | 0-34.5 | 57 | 23002 | 6.3 (5.9-6.7) | 97.4 (97.1-97.8) | < 0.001 | 0.853 |
| Cross-sectional | 9.2 (6.9-11.9) | 0-34.6 | 56 | 22867 | 6.3 (5.9-6.7) | 97.5 (97.1-97.8) | < 0.001 | 0.851 |
| Low risk of bias | 7 (4-10.8) | 0-30.3 | 20 | 10408 | 6.4 (5.7-7.1) | 97.6 (97-98) | < 0.001 | 0.463 |
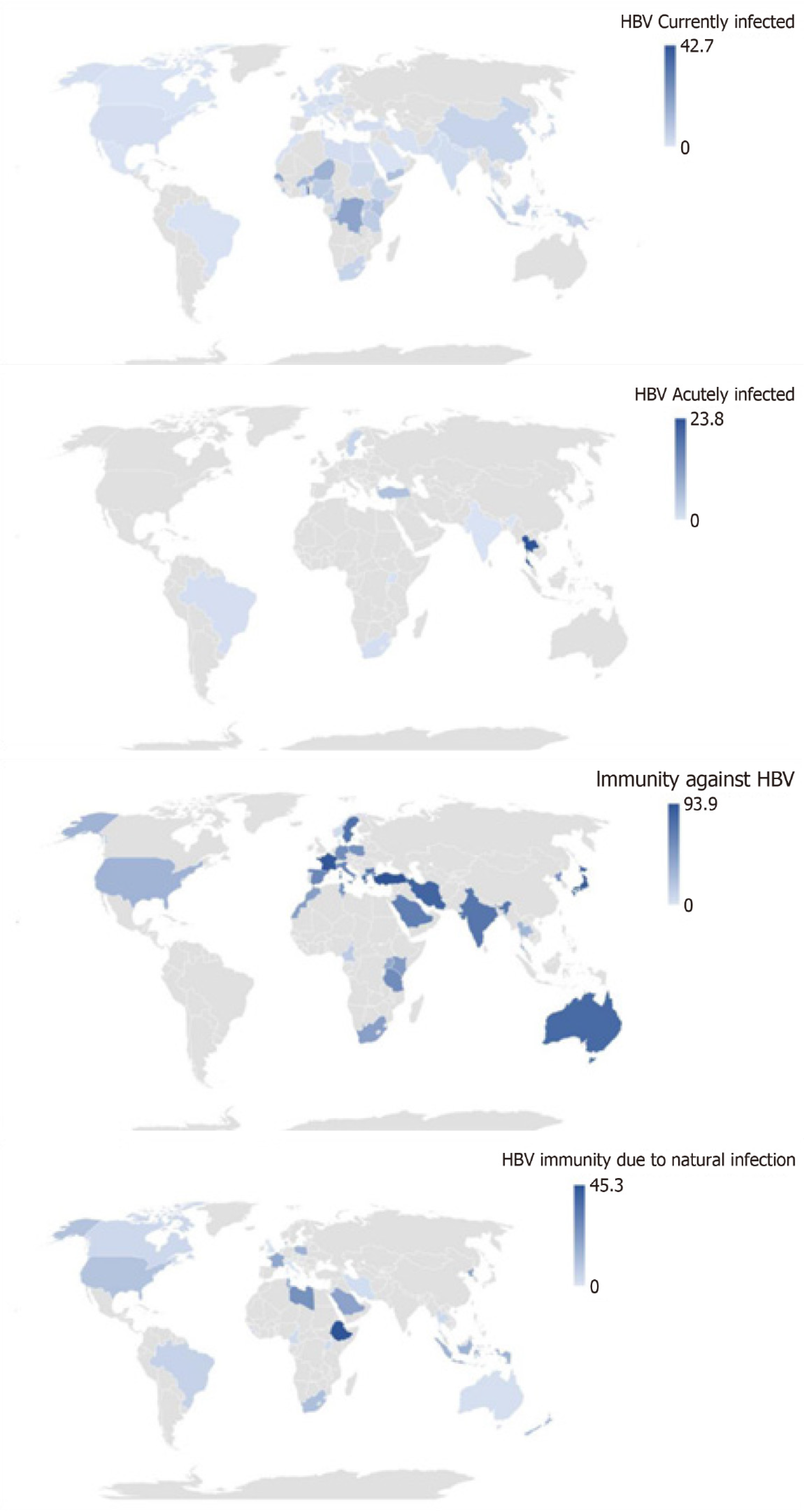
Subgroup analysis of current HBV infection in HCWs showed that seroprevalence was higher in cross-sectional studies, low-income countries, WHO Africa region, health management and support personnel, and personal care workers in health services (Figure 3 and Supplementary Table 8). Subgroup analysis of acute HBV infection in HCWs showed that seroprevalence was higher in non-probabilistic studies, prospective studies, upper-middle-income countries, the WHO South-East Asia region, urban areas and health associate professionals. Subgroup analysis of total immunity against HBV in HCWs showed that seroprevalence was higher in retrospective studies, the European, Eastern Mediterranean, and Western Pacific WHO regions, urban areas, and among personal care workers in health services and health associate professionals. Subgroup analysis of immunity against HBV due to natural infection in HCWs showed that the seroprevalence was higher in non-probabilistic studies, retrospective studies, urban areas, and health management and support personnel.
The univariate metaregression allowed the selection of the relevant covariates (Supplementary Table 9). Only the WHO region variable significantly explained the variance observed in estimating the prevalence of current HBV infection and immunity due to natural infection. The variables sampling approach and the HCWs classification significantly explained the variance observed for the estimation of the prevalence of acute HBV infection. No covariates explained the variance observed in the estimate of the prevalence of total immunity to HBV.
Our findings showed that the pooled prevalence rates of HBV serological markers among HCWs with current (HBsAg and HBeAg) and acute HBV infections were 2.3%, 0.2% and 5.3, respectively. Our findings also showed that the pooled prevalence rates of total immunity against HBV and immunity due to natural HBV infection were 56.5% and 9.2%, respectively. HBV serological markers varied considerably among categories of HCWs. In the subgroup analysis, the pooled seroprevalence of HBV in HCWs with current infection was highest in low-income countries and particularly in Africa. The pooled seroprevalence of HBV in HCWs with acute infection was higher in upper-middle-income countries, in the South-East Asia and in urban areas. The pooled seroprevalence of total immunity against HBV was higher in the Europe, Eastern Mediterranean, Western Pacific, and urban areas. The pooled seroprevalence of immunity against HBV due to natural infection was higher in urban areas.
A previous meta-analysis reported a pooled seroprevalence of current HBV infection (HBsAg) in HCWs of 2.3% in Eastern Mediterranean and Middle Eastern Countries (EMRO) and in the European Union/European Economic regions[27,28]. Our estimated HBV infection seroprevalence, however, presented a strong disparity according to geographic and socioeconomic regions in favor of African regions, South-East Asia and urban areas. These differences may be linked to several factors, including socio-demographic, ethnic, cultural factors, risk factors for transmission, protective factors (heterogeneous vaccination policies, levels of education, availability of preventive measures, and the practice of barrier measures against occupational exposure to blood)[29]. HBV vaccination policies are applied with strong temporal, socio-cultural and economic disparities around the world. Low-resource countries for example are prone to imperfect vaccine policies, including partial coverage of eligible individuals and without any catch-up strategy for adults including HCWs[10,13-16]. This aspect could well explain the high seroprevalence of HBV infections observed in low-income setting in the present review. It is also conceivable that the various detection tests used to search for the serological HBV markers in the present review could be associated with the significant heterogeneity observed. The various occupational categories considered in this review could also be at the origin of the great variability in the observed seroprevalence rates. It has in fact been shown that inexperienced people at the start of training, such as medical students and nurses, were more at risk of occupational contraction of HBV[30]. Nurses who are closer to patients and who are responsible for collecting blood from patients are also at high risk of contracting HBV[31,32]. It should also be noted that dentists and surgeons present a very worrying risk of occupational contamination by HBV, due to their use of sharp objects and procedures that generate aerosols[33,34]. The age and number of years of service (> 5 years) of the health workers have also been associated with a greater risk of contracting HBV infections[35,36]. The number of HCWs per patient as well as the number of hematogenous exposure by HCWs is very variable across the world and could also account for this great heterogeneity observed in the estimates of this review[37]. In resource-limited countries, unlike developed countries, high infection rates are linked to high immunization coverage and the application of the post-exposure prophylaxis policy[38]. The varying dates in countries of immunization policies can also pay dividends. Due to the lack of time restriction in the inclusion criteria for this review, it is highly likely that some participants benefited from universal childhood immunization policies, suggesting different vaccine coverage and hence variable infection rates. In addition, vaccination coverage rates among HCWs vary widely between countries, ranging from 18% in Africa to 77% in Australia[38]. In this review over half of HCWs had full immunity to HBV and this immunity was highest in urban areas and developed countries, including those in Europe, the Western Pacific, and the Eastern Mediterranean. Recently, a review showed that only a quarter of African HCWs had received the three doses of vaccines recommended for HBV immunization[39]. It is also noted that among this quarter of vaccinated HCWs in Africa, there is still a significant proportion of non-responders who remain at occupational risk of contracting HBV, as reported by other authors[40,41].
Some limitations should be noted for this review. One of the major difficulties of this review was the high variability of the professional categories of HCWs and the difficulty of having an easily applicable definition to group them together in a coherent way. Secondly, we did not consider the contribution of other major risk factors for HBV transmission in assessing the risk of HBV transmission in these HCWs, including sexual behavior or a history of parenteral injections. Also, the prevalence of current HBV infection in this study did not discriminate those with chronic infection (HBsAg ≥ 6 mo) from those with acute infection. Despite these limitations, one of the strengths of this review lies in the representativeness of all regions of the world. An added value in this review is the concomitant consideration of several serological markers of HBV infection and immunity.
In order to hope to achieve the 2030 goal of eliminating HBV infections, decision-makers should implement training, vaccination and care policies for HCWs who represent a high-risk group of occupational HBV infections. These programs should ideally be subsidized or free to ensure universal access to these measures. Vaccination coverage rates remain low in some regions (Turkey) where the vaccine is free for HCWs[30]. Continuous training of HCWs on the importance of vaccination against HBV, the appropriate use of personal protective equipment, barrier measures against occupational exposure to blood and associated diseases, as well as on proper disposal of sharp objects would be of great benefit in reducing occupational exposure. Training on barrier measures for occupational percutaneous injuries should incorporate safety behaviors, such as the use of puncture-resistant trash cans. In countries with limited resources that bear the heaviest burden of HBV infections, expanded routine immunization programs at birth should also include catch-up vaccinations for high-risk people, such as HCWs. For medical students, to implement systematic vaccination of all HCWs at the start of the professional training or before commencement of duty and verify effective immunization before starting could be more cost effective. For HCWs already in service, an initial phase would be the search for unvaccinated HCWs. For a rational integration of the vaccination program in HCWs, anonymized pre-vaccination anti-HBc screening tests should be carried out beforehand to avoid unnecessary vaccinations. The anti-HBc test should be followed by the HBsAg screening in anti-HBc-positive HCWs. Costly conventional enzyme-linked immunosorbent assay (ELISA) techniques are often unavailable in resource-limited areas, although they bear the heaviest burden of HBV infections[42]. Low cost and easy to use alternative assays with comparable performance to conventional ELISA assays should be made available to resource-limited areas[42-44]. The HCWs eligible to receive the three doses should be those susceptible to HBV infection, negative for the anti-HBc marker. Checks for anti-HBs levels should follow 2 mo to 3 mo after complete vaccination to ensure that the protective titer is achieved (anti-HBs ≥ 10 IU/mL). HCWs not responding to full vaccination should receive additional doses of vaccine. Booster doses could be given periodically (like 10 years if anti-HBs titer is below 10 IU/mL). HBsAg-positive HCWs would benefit from expert guidance for their orientation, rational and appropriate treatment to avoid wastage. Positive HBsAg tests should not disqualify HCWs from their daily practice, although urgent measures should be taken to control their viral load to minimize their risk of transmitting HBV to their patients and to those around them.
This systematic review highlights an important burden of HBV infections among HCWs around the world. It also reveals that around half of HCWs are protected against HBV infections worldwide. This protection is mainly attributed to vaccination compared to immunization due to natural infection. The burden of HBV infection is mainly borne by resource-limited countries, particularly Africa, which in parallel also reveals the lowest levels of immunization against HBV.
Hepatitis B infection is a deadly disease that affects and kills more than 1 million people a year. During their work, healthcare workers (HCWs) are exposed to certain direct or indirect risk factors that could lead to hepatitis B virus (HBV) infection. Existing data have shown that HBV infection, depending on markers, is widespread and heterogeneously distributed worldwide among HCWs. Therefore, there is a need to quantify the global proportion of HBV serological markers among HCWs.
HCWs are one of the most vulnerable groups to HBV infection during their routine work, which exposes them to a variety of accidents, e.g., needle stick injuries, exposure to blood and fluids of HBV-infected patients, etc. However, these groups are under-diagnosed in many parts of the world, especially in low-income countries. It remains to be seen how the burden of each marker of hepatitis B infection is distributed worldwide in order to guide future research. We therefore sought to quantify the burden of several serological markers of HBV infection in HCWs. This will enable the development of new strategies to better manage HBV infection in HCWs.
In this review, we aimed to quantify the pooled prevalence rates of serological markers of HBV infection among HCWs. We were able to report these prevalence data among HCWs based on world regions, country income levels, and categories of HCWs. Quantifying these prevalence rates in each region of the world is crucial to improving and/or implementing new strategies for managing HBV infection, as well as guiding future research that will contribute to the elimination of HBV by 2030 and the achievement of Sustainable Development Goal 3.3 related to well-being and good health, specifically ending the AIDS epidemic, tuberculosis, malaria and neglected tropical diseases and combating hepatitis, water-borne and other communicable diseases.
To synthesize data from the existing literature on the prevalence of HBV serological markers in HCWs, we followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guideline. We registered the study in Prospero and the search strategy was applied in PubMed and Embase to retrieve observational studies, including cross-sectional, cohort (baseline data) and case-control studies. These studies were selected for eligibility on the Rayyan platform by four investigators (Mahamat G, Kenmoe S, Ebogo-Belobo JT and Amougou-Atsama M) and data extraction was performed by 18 extractors using a Google form questionnaire. The quality of the included studies was assessed by the tool of Hoy et al. A random-effects meta-analysis model was used to pool the prevalence of each serological marker in HCWs. Meta-regression and subgroup analyses were used to determine the source of heterogeneity. The statistical software R version 3.6.2. was used to perform all meta-analyses.
In all, we reported prevalence rates of current infection [hepatitis B surface antigen (HBsAg) and hepatitis e antigen], acute infection (anti-HBs immunoglobulin M + HBsAg), full immunity (anti-HBs > 10 IU/mL), and acquired immunity by natural infection (anti-HBS + anti-HBc) among HCWs of 2.3% and 0.2%, 5.3%, 56.6%, and 9.2%, respectively. Low-income countries, particularly African countries, bear the greatest burden of current infection and have low immunization rates. High-income countries and urban areas are more protected from HBV infection. These results suggest that attention should increasingly be focused on low-income countries and in particular African countries where future research should be directed.
There is a need to improve awareness, training, screening, vaccination, post-test management and treatment of HBV infection worldwide in order to achieve the World Health Organization goal of eliminating hepatitis B infection by 2030.
Future research should be directed towards low-income countries, including African countries, where the highest burden of current infection with low vaccination coverage among HCWs has been reported.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Cameroon
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Trevisan A S-Editor: Gao CC L-Editor: Filipodia P-Editor: Li JH
| 1. | Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000--summary of a workshop. Gastroenterology. 2001;120:1828-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 513] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 2. | Lok AS, McMahon BJ; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Chronic hepatitis B. Hepatology. 2001;34:1225-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 642] [Cited by in RCA: 639] [Article Influence: 26.6] [Reference Citation Analysis (1)] |
| 3. | Seeger C, Mason WS. Hepatitis B virus biology. Microbiol Mol Biol Rev. 2000;64:51-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1094] [Cited by in RCA: 1111] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 4. | World Health Organization. Hepatitis B. 2020. [cited 29 June 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. |
| 5. | André F. Hepatitis B epidemiology in Asia, the Middle East and Africa. Vaccine. 2000;18 Suppl 1:S20-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 174] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Lesmana LA, Leung NW-Y, Mahachai V, Phiet PH, Suh DJ, Yao G, Zhuang H. Hepatitis B: overview of the burden of disease in the Asia-Pacific region. Liver Int. 2006;26:3-10. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Kammerlander R, Zimmermann H. Transmission de l’hépatite B. Soz Präventivmed. 1998;43:S105-S107. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 8. | Byrne EB. Viral hepatitis: an occupational hazard of medical personnel. Experience of the Yalenew Haven Hospital, 1952 to 1965. JAMA. 1966;195:362-364. [PubMed] |
| 9. | Prüss-Ustün A, Rapiti E, Hutin Y. Estimation of the global burden of disease attributable to contaminated sharps injuries among health-care workers. Am J Ind Med. 2005;48:482-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 414] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 10. | Schillie S, Murphy TV, Sawyer M, Ly K, Hughes E, Jiles R, de Perio MA, Reilly M, Byrd K, Ward JW; Centers for Disease Control and Prevention (CDC). CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm Rep. 2013;62:1-19. [PubMed] |
| 11. | Wicker S, Cinatl J, Berger A, Doerr HW, Gottschalk R, Rabenau HF. Determination of risk of infection with blood-borne pathogens following a needlestick injury in hospital workers. Ann Occup Hyg. 2008;52:615-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Lewis JD, Enfield KB, Sifri CD. Hepatitis B in healthcare workers: Transmission events and guidance for management. World J Hepatol. 2015;7:488-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Noubiap JJN, Nansseu JRN, Kengne KK, Wonkam A, Wiysonge CS. Low hepatitis B vaccine uptake among surgical residents in Cameroon. Int Arch Med. 2014;7:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | De Schryver A, Claesen B, Meheus A, van Sprundel M, François G. European survey of hepatitis B vaccination policies for healthcare workers. Eur J Public Health. 2011;21:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Bonanni P, Bonaccorsi G. Vaccination against hepatitis B in health care workers. Vaccine. 2001;19:2389-2394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Prüss-Üstün A, Rapiti E, Hutin YJF. Sharps injuries: global burden of disease from sharps injuries to health-care workers / Annette Prüss-Üstun, Elisabetta Rapiti, Yvan Hutin. [cited 29 June 2021]. In: World Health Organization [Internet]. Available from: https://apps.who.int/iris/handle/10665/42743. |
| 17. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 46994] [Article Influence: 2937.1] [Reference Citation Analysis (0)] |
| 18. | Song JE, Kim DY. Diagnosis of hepatitis B. Ann Transl Med. 2016;4:338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, Baker P, Smith E, Buchbinder R. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65:934-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1821] [Article Influence: 140.1] [Reference Citation Analysis (0)] |
| 20. | World Health Organization. Classifying health workers: Mapping occupations to the international standard classification. [cited 29 June 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/hrh/statistics/Health_workers_classification.pdf. |
| 21. | Cochran WG. The Combination of Estimates from Different Experiments. Biometrics. 1954;10:101-129. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3226] [Cited by in RCA: 3276] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 22. | Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67:974-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 1491] [Article Influence: 124.3] [Reference Citation Analysis (0)] |
| 23. | Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, Kuss O, Higgins JP, Langan D, Salanti G. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods. 2016;7:55-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 786] [Cited by in RCA: 901] [Article Influence: 100.1] [Reference Citation Analysis (0)] |
| 24. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 40323] [Article Influence: 1440.1] [Reference Citation Analysis (2)] |
| 25. | R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [cited 29 June 2021]. In: R Core Team [Internet]. Available from: https://www.R-project.org/. |
| 27. | Babanejad M, Izadi N, Alavian SM. A Systematic Review and Meta-analysis on the Prevalence of HBsAg in Health Care Workers from Eastern Mediterranean and Middle Eastern Countries. Int J Prev Med. 2019;10:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Tavoschi L, Mason L, Petriti U, Bunge E, Veldhuijzen I, Duffell E. Hepatitis B and C among healthcare workers and patient groups at increased risk of iatrogenic transmission in the European Union/European Economic Area. J Hosp Infect. 2019;102:359-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Bwogi J, Braka F, Makumbi I, Mishra V, Bakamutumaho B, Nanyunja M, Opio A, Downing R, Biryahwaho B, Lewis RF. Hepatitis B infection is highly endemic in Uganda: findings from a national serosurvey. Afr Health Sci. 2009;9:98-108. [PubMed] |
| 30. | Irmak Z, Ekinci B, Akgul AF. Hepatitis B and C seropositivity among nursing students at a Turkish university. Int Nurs Rev. 2010;57:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Baldo V, Floreani A, Dal Vecchio L, Cristofoletti M, Carletti M, Majori S, Di Tommaso A, Trivello R. Occupational risk of blood-borne viruses in healthcare workers: a 5-year surveillance program. Infect Control Hosp Epidemiol. 2002;23:325-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Tarantola A, Golliot F, Astagneau P, Fleury L, Brücker G, Bouvet E; CCLIN Paris-Nord Blood and Body Fluids (BBF) Exposure Surveillance Taskforce. Occupational blood and body fluids exposures in health care workers: four-year surveillance from the Northern France network. Am J Infect Control. 2003;31:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Prospero E, Savini S, Annino I. Microbial aerosol contamination of dental healthcare workers' faces and other surfaces in dental practice. Infect Control Hosp Epidemiol. 2003;24:139-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Ahmad Akhoundi MS, Momeni N, Norouzi M, Ghalichi L, Shamshiri AR, Alavian SM, Poortahmasebi V, Jazayeri SM. Prevalence of blood-borne viruses among Iranian dentists: Results of a national survey. Int J Occup Med Environ Health. 2015;28:593-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Mueller A, Stoetter L, Kalluvya S, Stich A, Majinge C, Weissbrich B, Kasang C. Prevalence of hepatitis B virus infection among health care workers in a tertiary hospital in Tanzania. BMC Infect Dis. 2015;15:386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 36. | Ziraba AK, Bwogi J, Namale A, Wainaina CW, Mayanja-Kizza H. Sero-prevalence and risk factors for hepatitis B virus infection among health care workers in a tertiary hospital in Uganda. BMC Infect Dis. 2010;10:191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | devices. Health Devices. 1999;28:381-408. [PubMed] |
| 38. | Hutin Y, Hauri A, Chiarello L, Catlin M, Stilwell B, Ghebrehiwet T, Garner J; Injection Safety Best Practices Development Group. Best infection control practices for intradermal, subcutaneous, and intramuscular needle injections. Bull World Health Organ. 2003;81:491-500. [PubMed] |
| 39. | Auta A, Adewuyi EO, Kureh GT, Onoviran N, Adeloye D. Hepatitis B vaccination coverage among health-care workers in Africa: A systematic review and meta-analysis. Vaccine. 2018;36:4851-4860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 40. | Halpern SD, Asch DA, Shaked A, Stock P, Blumberg EA. Inadequate hepatitis B vaccination and post-exposure evaluation among transplant surgeons: prevalence, correlates, and implications. Ann Surg. 2006;244:305-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Smith ER, Banatvala JE, Tilzey AJ. Hepatitis B vaccine uptake among surgeons at a London teaching hospital: how well are we doing? Ann R Coll Surg Engl. 1996;78:447-449. [PubMed] |
| 42. | Xiao Y, Thompson AJ, Howell J. Point-of-Care Tests for Hepatitis B: An Overview. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 43. | Cooke GS, Andrieux-Meyer I, Applegate TL, Atun R, Burry JR, Cheinquer H, Dusheiko G, Feld JJ, Gore C, Griswold MG, Hamid S, Hellard ME, Hou J, Howell J, Jia J, Kravchenko N, Lazarus JV, Lemoine M, Lesi OA, Maistat L, McMahon BJ, Razavi H, Roberts T, Simmons B, Sonderup MW, Spearman CW, Taylor BE, Thomas DL, Waked I, Ward JW, Wiktor SZ; Lancet Gastroenterology & Hepatology Commissioners. Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol. 2019;4:135-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 391] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 44. | Peeling RW, Boeras DI, Marinucci F, Easterbrook P. The future of viral hepatitis testing: innovations in testing technologies and approaches. BMC Infect Dis. 2017;17:699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |