Published online Sep 27, 2021. doi: 10.4254/wjh.v13.i9.1181
Peer-review started: April 20, 2021
First decision: June 17, 2021
Revised: June 25, 2021
Accepted: August 24, 2021
Article in press: August 24, 2021
Published online: September 27, 2021
Processing time: 154 Days and 14.5 Hours
Severe acute respiratory syndrome coronavirus 2, or coronavirus disease-2019 (COVID-19), has infected millions worldwide since its discovery in Wuhan, China in December 2019, but little is still known about the disease process. Preliminary research in China notes liver function tests (LFTs) abnormalities are common in COVID-19 patients, suggesting decreased hepatic function, and that abnormalities in LFTs are related to complicated disease course and negative outcomes. However, there has been limited large-scale data assessing COVID-19’s association with liver dysfunction and negative outcomes.
To investigate how COVID-19 affects the liver function and disease course in patients infected with the virus treated at Henry Ford Hospital from March to September 2020.
A total of 8028 patients infected with COVID-19 were identified and included in the study at a single academic center. Data from medical charts on laboratory testing including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (AP), and bilirubin levels, past history of liver disease, and disease course indicators including hospital admission, intensive care unit (ICU) admission, intubation, and death were recorded and analyzed. Elevated liver enzymes were defined as ALT/AST greater than 60, AP greater than 150, or bilirubin greater than 1.5, super-elevated liver enzymes were defined as ALT/AST greater than 120, AP greater than 300, or bilirubin greater than 3.0.
A total of 8028 COVID-19 patients were identified and included in the study. Data from medical charts on LFTs (namely, AST, ALT, AP, and bilirubin levels), past history of liver disease, and disease course indicators (hospital/ICU admission, intubation, death) were recorded and analyzed. LFTs from 3937 patients were available for interpretation. 45% were found to have elevated or super-elevated LFT. When compared to COVID-19 patients without elevated LFTs, this cohort was found to have significantly higher odds of hospital admittance, ICU admission, intubation, and death (all P < 0.001). 248 (3.1%) had a history of liver disease. Those with elevated and super elevated LFTS had significantly higher odds of having a past history of liver disease (P < 0.001).
The findings from this study suggest that in patients who have tested positive for COVID-19, those with elevated and super elevated liver enzyme levels have significantly higher odds of hospital admittance, ICU admittance, intubation and death in comparison to those COVID-19 patients without elevated liver enzyme levels.
Core Tip: This study suggests that in coronavirus disease-2019 (COVID-19) positive patients, those with elevated and super elevated liver function tests (LFTs) have significantly higher odds of hospital admittance, intensive care unit admittance, intubation, and death in comparison to those COVID-19 patients without elevated LFTs (all P < 0.001). LFT elevations may serve as an indicator for medical professionals in the treatment of COVID-19 patients and may allow for proactive treatment of those patients at increased risk of complications.
- Citation: Currier EE, Dabaja M, Jafri SM. Elevated liver enzymes portends a higher rate of complication and death in SARS-CoV-2. World J Hepatol 2021; 13(9): 1181-1189
- URL: https://www.wjgnet.com/1948-5182/full/v13/i9/1181.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i9.1181
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), or coronavirus disease-2019 (COVID-19), was first reported in Wuhan, China in December 2019, and as of March 2020, the World Health Organization declared COVID-19 a global pandemic[1]. While millions of people have been infected and have died from COVID-19 worldwide, still much is unknown about COVID-19’s disease process and the systemic effects of the disease[1]. However, preliminary research on COVID-19 shows that the disease may have a significant impact on the gastrointestinal and hepatic systems.
Early studies have shown that gastrointestinal (GI) symptoms are common in COVID-19 patients and symptoms such as nausea, diarrhea, etc, are present in approximately 10% of cases[2,3]. It has been noted that liver function test (LFT) abnormalities are common, however, the incidence has ranged widely from preliminary data, from 14.8% to 78%[2-5]. Abnormal LFTs, namely increases in aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, and alkaline phosphatase (AP), have been reported, which indicates decreased hepatic functions[2-11]. Thus, these noted LFT abnormalities in COVID-19 patients suggest that COVID-19 may negatively impact liver function[4-6,8]. Furthermore, three meta-analyses have both shown that patients presenting with abnormal LFTs had a significant association with an increased risk of complication risk course [i.e. intensive care unit (ICU) admission, intubation, death][2,8,10]. Little is still known about the impact of pre-existing hepatic conditions on COVID-19 outcomes (i.e. cirrhosis, post-liver transplant, etc)[4].
The current hypothesis behind COVID-19’s involvement of the hepatic system is multifactorial liver damage, secondary to systemic inflammatory response syndrome, hypoxia-reperfusion injury, cytokine-storm induced damage, drug-induced liver damage, sepsis-mediated damage, and/or multiorgan failure[2,4,5,11,12]. However, little is known about the mechanism behind hepatic damage.
The current available research is limited in that almost all of the data was obtained from China, as few studies, especially large-scale studies, outside China have been published[2,3,10]. Furthermore, most of the current research published is limited in the study sample sizes, leading to current meta-analyses receiving data from a large number of hospitals. In these studies patients were held to different clinical cutoffs when advancing medical interventions, which could have negatively impacted the accuracy of the data and determination of the significance of abnormal LFTs and its impact on disease complications. To date, there has been no published large-scale research investigating the relationship between COVID-19 patient’s LFTs and their relationship to a complicated disease course in the United States. Additionally, epidemiologic studies of COVID-19’s impact have shown that Black and minority populations are disproportionally represented in the number of cases, complications, and deaths due to the virus[13,14]. While this is postulated to be due to increased incidence of comorbidities, increase odds of living in high-density areas, and lack of access to healthcare, more studies with populations that reflect demographics of COVID infection are needed to assess COVID-19’s association with liver dysfunction across a diverse population[15].
The significance of this research is to investigate how SARS-CoV-2/COVID-19 affects liver function and disease course in patients infected with the virus treated at Henry Ford Hospital from March to September 2020. As studies have linked liver dysfunction with severe disease and negative disease outcomes, it is important to confirm the preliminary research currently available. If COVID-19 is continued to be linked to liver dysfunction, this information can help clinicians determine the level of care patients need and proactively treat potentially complicated disease processes.
We hypothesize that COVID-19 patients with elevations in LFTs will have higher chances of a complicated and severe disease process.
With approval from the institutional review board at Henry Ford Health System (HFHS), the study used the medical records of patients treated at HFHS to identify patients who tested positive for COVID-19. Medical records from individuals who had tested positive from the beginning of the pandemic until September 2020 were isolated and included in the study. No individuals were excluded from the study. For this type of study formal consent is not required.
After isolating the patient population, all records of liver enzyme levels (AST, ALT, AP, bilirubin), medical history of liver disease (defined as medical documentation of alcoholic liver disease, toxic liver disease, hepatic failure, hepatitis, inflammatory liver disease, hepatic fibrosis, liver transplant, and other liver diseases- not elsewhere classified), and complicated disease course (designated by a hospital admission, ICU admission, intubation, and death) were recorded. Individuals with a past medical history of liver disease were screened through retrospective chart review and identified by a prior diagnosis of one of the above conditions; details on disease severity, length, etc were not recorded. However, those with history of liver disease were separated into another cohort due to the possibility of liver enzyme elevation secondary to liver disease and not the COVID-19 disease process.
Descriptive statistics of demographic variables and hospital-related outcomes are provided. Continuous data are reported as mean ± SD, while categorical data are reported as counts and column percentages [n (%)]. Prevalence rates for elevated and super elevated liver enzymes are computed using binary indicator variables. Logistic regression is used to calculate odds ratios and their 95%CIs for the outcomes of interest. Statistical significance is set at P < 0.05. All analyses are performed using SAS 9.4 (SAS Institute Inc., Cary, NC, United States).
There is a total of 8028 unique patient medical record numbers used in this descriptive analysis. Table 1 displays the descriptive statistics of these patients. Of the 8028 patients included, 4638 (57%) are female, 3389 (42%) are male, and 1 (0%) is unknown. Additionally, 4268 (53%) are Black, 2541 (32%) are White, and 1219 (15%) are another race; 6921 (86%) are not Hispanic, 339 (4%) are Hispanic, and 768 (10%) are unknown. Patients were classified by Hispanic vs non-Hispanic to identify those who are Central or South American/Latino who are considered “White” on this hospital’s demographic information but are of Hispanic descent.
| Variable | Response | All patients (n = 8028) |
| Sex | Female | 4638 (58%) |
| Male | 3389 (42%) | |
| Unknown | 1 (0%) | |
| Race | Black | 4268 (53%) |
| Other | 1219 (15%) | |
| White | 2541 (32%) | |
| Hispanic | No | 6921 (86%) |
| Unknown | 768 (10%) | |
| Yes | 339 (4%) |
Binary indicator variables for history of liver disease, death, hospital admission, ICU admission, and intubation were created. Table 2 displays the counts, percentages, and 95%CIs for these hospital-related outcomes. ICU admission and intubation are recorded for only those patients who were admitted to the hospital, noted by the change of n. Of the 8028 patients, 245 (3.1%) had a history of liver disease, 673 (8.4%) died, and 5199 (64.8%) were admitted to the hospital. Of the 5199 admitted to the hospital, 807 (15.5%) were admitted to the ICU, and 637 (12.3%) were intubated.
| Outcome | Count (%) (95%CI) |
| n = 8028 | |
| History of liver disease | 245 (3.1) (2.7, 3.5) |
| Death | 673 (8.4) (7.8, 9.0) |
| Hospital admission | 5199 (64.8) (63.7, 65.8) |
| Outcome | Count (%) (95%CI) |
| n = 5199 | |
| ICU admit | 807 (15.5) (14.6, 16.5) |
| Intubation | 637 (12.3) (11.4, 13.2) |
| Outcome | Count (%) (95%CI) |
| Any elevated liver enzyme | 1722 (44.9) (43.3, 46.5) |
| Elevated AST | 1297 (33.5) (32.0, 35.0) |
| Elevated ALT | 1052 (26.7) (25.4, 28.2) |
| Elevated AP | 392 (10.1) (9.2, 11.1) |
| Elevated bilirubin | 468 (12.0) (11.0, 13.1) |
| Any super elevated liver enzyme | 714 (18.6) (17.4, 19.9) |
| Super elevated AST | 480 (12.4) (11.4, 13.5) |
| Super elevated ALT | 468 (11.9) (10.9, 13.0) |
| Super elevated AP | 94 (2.4) (1.9, 3.0) |
Table 2 displays the descriptive statistics for elevated liver enzymes. There was a total of 115846 lab values from 3937 patients. When we assessed elevated liver enzymes, we looked at this at the patient level – if they have ever had elevated liver enzymes. Binary indicator variables were created for ever having any elevated liver enzyme, and this was further broken down by specific enzymes (AST, ALT, AP, and bilirubin). Elevated liver enzymes are defined as an AST greater than 60, ALT greater than 60, AP greater than 150, or a bilirubin greater than 1.5.
There are 1722 patients who had elevated liver enzymes, 2114 who never had an elevated liver enzyme, and 101 patients who were indeterminable. Approximately 45% of patients had an elevated liver enzyme level, 34% of patients had an elevated AST, 27% of patients had an elevated ALT, 10% of patients had an elevated AP, and 12% had an elevated bilirubin.
In Table 2, we looked at super elevated liver enzyme levels, which is double the elevated threshold (AST greater than 120, ALT greater than 120, AP greater than 300, or a bilirubin greater than 3). There were 714 patients who had super elevated liver enzymes, 3116 who never had super elevated liver enzymes, and 107 patients who were indeterminable. Approximately 19% of patients had a super elevated enzyme level, 12% with AST, 12% with ALT, 2% with AP, and 3% with bilirubin.
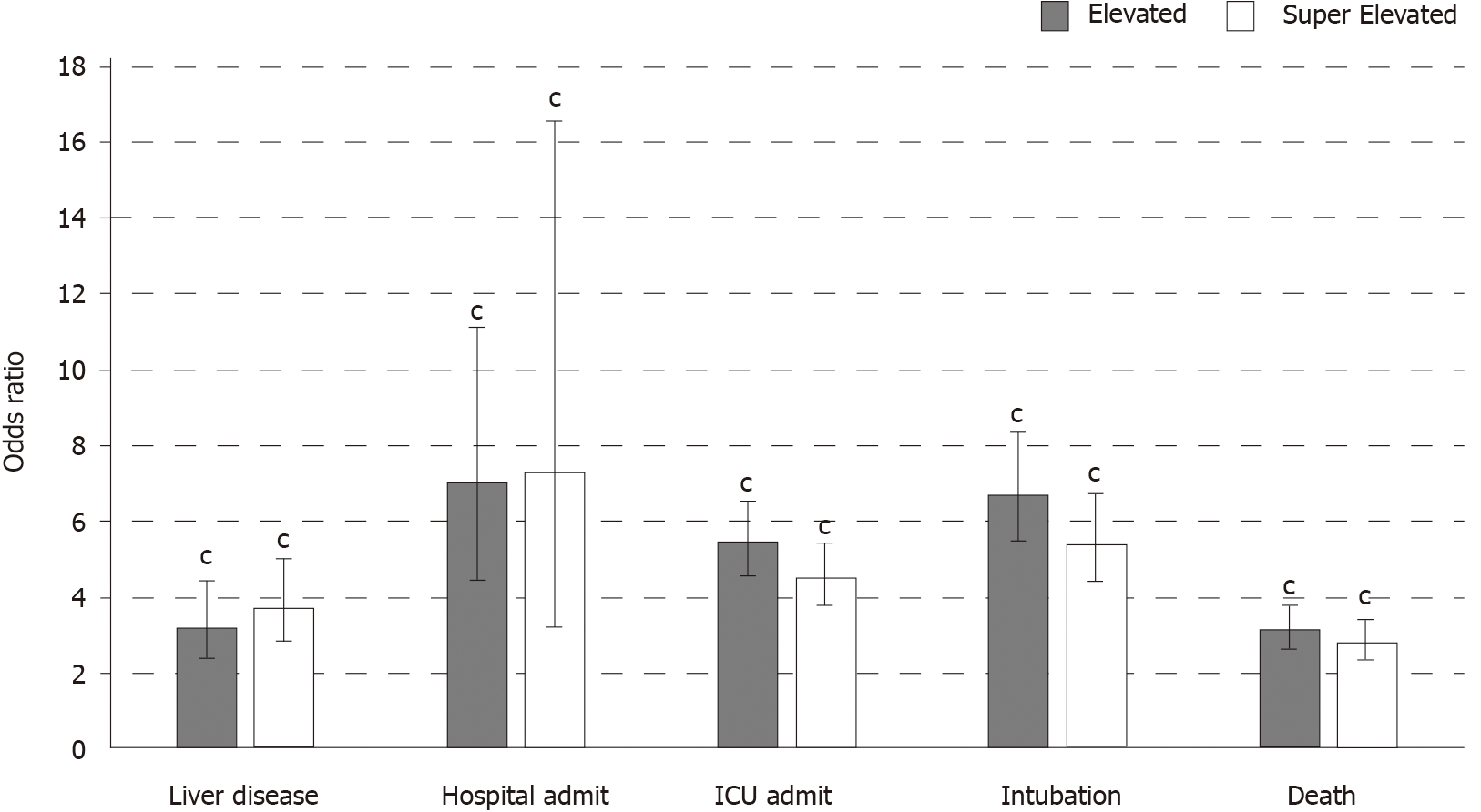
Lastly, Figure 1 displays the logistic regression models examining the effect of elevated and super elevated liver enzymes on each outcome. Presence of elevated liver enzymes and super elevated liver enzymes are associated with increased odds of liver disease, hospital admittance, death, intubation and ICU admittance (all P < 0.001).
The findings from this study suggest that in patients with a positive COVID-19 test, those who have elevated and super elevated liver enzyme levels have significantly higher odds of hospital admittance, ICU admittance, intubation and death in comparison to those COVID-19 patients without elevated liver enzyme levels. While little is known about COVID-19’s effect on organ systems during infection and recovery, the link between elevated LFTs and poor outcomes is important and suggests that COVID-19 negatively impacts liver function; this is also consistent with early data from other studies[2-11].
Of the 8028 patients identified in this study, LFTs from 3937 patients were available for statistical interpretation. Of this cohort, 45% were found to have elevated or super-elevated LFTs and when compared to COVID-19 patients without elevated LFTs, this cohort was found to have significantly higher odds of hospital admittance, ICU admission, intubation, and death (all P < 0.001). The data suggest that the risk of hospital admission and the necessity for more aggressive medical interventions (i.e. intubation, ICU admission) are more common in those with elevated LFTs. Thus, elevations in LFTs may serve as an indicator for medical professionals in the preventative treatment of complicated COVID-19 patients. By identifying those patients who have poor liver function and are thus linked to a more complicated disease course, providers may be able to monitor, proactively treat patients at increased risk, and mitigate disease complications.
Interestingly, however, this data does not show that elevation in LFTs is linearly correlated with outcomes, as seen by the odds ratio of ICU admission, intubation, and death in patients with super elevated enzyme levels being less than those with elevated enzyme levels (Figure 1). The cause of this relationship is unknown; however, we hypothesize that those with super elevated liver enzymes may have been clinically identified as severe COVID-19 cases earlier and been treated more aggressively. Retrospective research has shown that those with LFT elevations at time of admission were more likely to receive aggressive mediation interventions than those with normal LFTs (58% compared to 31%)[15]. Therefore, this lack of linear relationship may be related to early clinical treatment of patients who present with LFT abnormalities, compared to those who develop elevations throughout their hospital stay or who have moderate elevations.
Little is still known about COVID-19’s effect on liver function, however, the findings from this study indicating COVID’s negative impact on liver function is consistent with the limited preliminary COVID studies in China on outcomes and predictive markers of disease[16]. As noted in the previous studies, abnormal LFTs are seen as predictive markers of a complicated disease process, thus indicating hepatic dysfunction. A weakness in previously available research is the homogeneity of the population studied, with most research being derived from almost solely Asian and South Asian populations. This study, however, consisted of 53% Black, 32% white, 15% other, and 4% Hispanic persons. Therefore, this cohort is more closely representative of the current demographics affected by COVID-19 in the United States, where Black people are more likely to be infected and die from COVID-19[17,18]. Thus, these findings suggest that the relationship between LFT elevations and disease complications is not limited to race and can be applied to populations outside of the Asian community and countries.
Of the 8028 patients identified in the study, 248 (3.1%) had a history of liver disease. Those with elevated and super elevated LFTs had significantly higher odds of having a past history of liver disease (P < 0.001). This is important as previous research on underlying liver disease and COVID-19 infection has been limited due to sparse data on persons with underlying liver disease[19]. This data indicates that LFT abnormalities are consistent with complicated disease process in those who have a history of liver dysfunction, as seen in those without liver disease. While it is unclear if LFT elevations were due to the effects of the COVID-19 disease process or is secondary to their underlying liver disease, we do hypothesize the COVID’s negative impact on liver function exacerbates already lowered liver function in these patients, thus increasing their odds for complications.
This study does have several weaknesses. While over 8000 patients were treated for COVID-19 at the hospital, liver enzymes were only available for about half of those included in the study. This discrepancy may be due to a high number of ambulatory patients who were tested for COVID-19, but whose disease process was self-limited and did not require medical intervention beyond diagnoses and supportive care. Furthermore, this research did not investigate the medications patients in the study received and as some medications used to treat COVID-19 have been linked to elevation in LFTs, this may confound some of the elevations seen in this study[20]. Additionally, as the study was retrospective, there were a variable number of lab tests available to analyze for each patient (i.e. some had multiple LFTs available while others had a single test). Thus, some patients may have had high LFTs during the disease course, but this was not captured on the available lab results. In research going forward, an area for improvement would be to find consistent lab values to compare and limit the possibility of missed LFT fluctuations. In addition, capturing and assessing LFTs from ambulatory patients not requiring hospitalization.
In conclusion, abnormal liver biochemistry, namely AST, ALT, AP, and bilirubin, is very common in COVID-19 patients, noted in 45% of our patient population. Abnormal LFTs are closely linked to disease complications and the prognosis for COVID-19 patients. These findings are consistent with other early research and support that COVID-19 is related to hepatic dysfunction. Importantly, as LFT elevation has been linked to severe disease outcomes, patients with elevations should be monitored closely and treated prophylactically to mitigate disease complications. Going forward, chronic effects of COVID-19 infection of hepatic function will be important to monitor as indicators of acute liver dysfunction is common in COVID-19 patients.
Preliminary research on coronavirus disease-2019 (COVID-19) shows that the disease may have a significant impact on the gastrointestinal and hepatic systems. Namely, early research shows that liver function test (LFT) abnormalities are common, however, the incidence has ranged widely from preliminary data, from 14.8% to 78%. Furthermore, three meta-analyses have both shown that patients presenting with abnormal LFTs had a significant association with an increased risk of complication risk course [i.e. intensive care unit (ICU) admission, intubation, death], but there is currently limited single-site, large scale research on the link between LFT abnormalities and COVID outcomes.
The motivation of this research is to identify a link between LFT abnormalities and COVID-19 outcomes.
The objective of this research was to identify if there was a link between LFT elevation and outcomes in COVID-19 patients. This study did support the hypothesis that those with LFT abnormalities are at increased risk of complicated disease processes and death. Clinically, this is very important as LFT abnormalities may identify patients at risk for disease complications and may lead to early medical intervention.
Of 8028 patients infected with COVID-19 were identified and included in the study at a single academic center. Data from medical charts on laboratory testing including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (AP), and bilirubin levels, past history of liver disease, and disease course indicators including hospital admission, ICU admission, intubation, and death were recorded and analyzed. Elevated liver enzymes were defined as ALT/AST greater than 60, AP greater than 150, or bilirubin greater than 1.5, super-elevated liver enzymes were defined as ALT/AST greater than 120, AP greater than 300, or bilirubin greater than 3.0.
Of 8028 COVID-19 patients were identified and included in the study. Data from medical charts on LFTs (namely, AST, ALT, AP, and bilirubin levels), past history of liver disease, and disease course indicators (hospital/ICU admission, intubation, death) were recorded and analyzed. LFTs from 3937 patients were available for interpretation. 45% were found to have elevated or super-elevated LFT. When compared to COVID-19 patients without elevated LFTs, this cohort was found to have significantly higher odds of hospital admittance, ICU admission, intubation, and death (all P < 0.001). 248 (3.1%) had a history of liver disease. Those with elevated and super elevated LFTS had significantly higher odds of having a past history of liver disease (P < 0.001).
The findings from this study suggest that in patients who have tested positive for COVID-19, those with elevated and super elevated liver enzyme levels have significantly higher odds of hospital admittance, ICU admittance, intubation and death in comparison to those COVID-19 patients without elevated liver enzyme levels. While this research is unsure of the cause of this relationship, this research supports that LFT changes could serve as an indicator of COVID-19 outcomes and serve as a metric for evaluating those at risk for severe complications.
In research going forward, an area for improvement would be to find consistent lab values to compare and limit the possibility of missed LFT fluctuations. In addition, capturing and assessing LFTs from ambulatory patients not requiring hospitalization would increase the validity of the link between LFTs and outcomes.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Watanabe A S-Editor: Zhang H L-Editor: A P-Editor: Guo X
| 1. | Sun P, Lu X, Xu C, Sun W, Pan B. Understanding of COVID-19 based on current evidence. J Med Virol. 2020;92:548-551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 514] [Cited by in RCA: 559] [Article Influence: 111.8] [Reference Citation Analysis (0)] |
| 2. | Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, Singla V, Khare S, Srivastava A. Gastrointestinal and hepatic manifestations of Corona Virus Disease-19 and their relationship to severe clinical course: A systematic review and meta-analysis. Indian J Gastroenterol. 2020;39:268-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Cha MH, Regueiro M, Sandhu DS. Gastrointestinal and hepatic manifestations of COVID-19: A comprehensive review. World J Gastroenterol. 2020;26:2323-2332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 124] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (2)] |
| 4. | Musa S. Hepatic and gastrointestinal involvement in coronavirus disease 2019 (COVID-19): What do we know till now? Arab J Gastroenterol. 2020;21:3-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 5. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 556] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 6. | Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Ye P, Xiao B, Mao W, Liu L, Yan Y, Chen G, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 309] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 7. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1295] [Article Influence: 259.0] [Reference Citation Analysis (4)] |
| 8. | Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 622] [Cited by in RCA: 575] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 9. | Wu Y, Li H, Guo X, Yoshida EM, Mendez-Sanchez N, Levi Sandri GB, Teschke R, Romeiro FG, Shukla A, Qi X. Incidence, risk factors, and prognosis of abnormal liver biochemical tests in COVID-19 patients: a systematic review and meta-analysis. Hepatol Int. 2020;14:621-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 10. | Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, Singla V, Khare S, Srivastava A. Clinical Features of COVID-19 and Factors Associated with Severe Clinical Course: A Systematic Review and Meta-Analysis. SSRN. 2020;3566166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Polakos NK, Cornejo JC, Murray DA, Wright KO, Treanor JJ, Crispe IN, Topham DJ, Pierce RH. Kupffer cell-dependent hepatitis occurs during influenza infection. Am J Pathol. 2006;168:1169-78; quiz 1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, Choi KW, Tso YK, Lau T, Lai ST, Lai CL. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 307] [Cited by in RCA: 305] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 13. | Abuelgasim E, Saw LJ, Shirke M, Zeinah M, Harky A. COVID-19: Unique public health issues facing Black, Asian and minority ethnic communities. Curr Probl Cardiol. 2020;45:100621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 14. | Golestaneh L, Neugarten J, Fisher M, Billett HH, Gil MR, Johns T, Yunes M, Mokrzycki MH, Coco M, Norris KC, Perez HR, Scott S, Kim RS, Bellin E. The association of race and COVID-19 mortality. EClinicalMedicine. 2020;25:100455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 158] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 15. | Gu T, Mack JA, Salvatore M, Prabhu Sankar S, Valley TS, Singh K, Nallamothu BK, Kheterpal S, Lisabeth L, Fritsche LG, Mukherjee B. Characteristics Associated With Racial/Ethnic Disparities in COVID-19 Outcomes in an Academic Health Care System. JAMA Netw Open. 2020;3:e2025197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 162] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 16. | Paliogiannis P, Zinellu A. Bilirubin levels in patients with mild and severe Covid-19: A pooled analysis. Liver Int. 2020;40:1787-1788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 17. | Holmes L Jr, Enwere M, Williams J, Ogundele B, Chavan P, Piccoli T, Chinacherem C, Comeaux C, Pelaez L, Okundaye O, Stalnaker L, Kalle F, Deepika K, Philipcien G, Poleon M, Ogungbade G, Elmi H, John V, Dabney KW. Black-White Risk Differentials in COVID-19 (SARS-COV2) Transmission, Mortality and Case Fatality in the United States: Translational Epidemiologic Perspective and Challenges. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 134] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 18. | Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and Mortality among Black Patients and White Patients with Covid-19. N Engl J Med. 2020;382:2534-2543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1214] [Cited by in RCA: 1257] [Article Influence: 251.4] [Reference Citation Analysis (0)] |
| 19. | Sun J, Aghemo A, Forner A, Valenti L. COVID-19 and liver disease. Liver Int. 2020;40:1278-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 219] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 20. | Olry A, Meunier L, Délire B, Larrey D, Horsmans Y, Le Louët H. Drug-Induced Liver Injury and COVID-19 Infection: The Rules Remain the Same. Drug Saf. 2020;43:615-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |