Published online Sep 27, 2021. doi: 10.4254/wjh.v13.i9.1107
Peer-review started: March 14, 2021
First decision: April 6, 2021
Revised: April 21, 2021
Accepted: July 26, 2021
Article in press: July 26, 2021
Published online: September 27, 2021
Processing time: 191 Days and 15.9 Hours
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of liver disease worldwide, and its prevalence increases continuously. As it predisposes to hepatocellular carcinoma both in the presence and in the absence of cirrhosis, it is not surprising that the incidence of NAFLD-related hepatocellular carcinoma would also rise. Some of the mechanisms involved in hepatocarcinogenesis are particular to individuals with fatty liver, and they help explain why liver cancer develops even in patients without cirrhosis. Genetic and immune-mediated mechanisms seem to play an important role in the development of hepatocellular carcinoma in this population. Currently, it is consensual that patients with NAFLD-related cirrhosis should be surveilled with ultrasonography every 6 mo (with or without alpha-fetoprotein), but it is known that they are less likely to follow this recommendation than individuals with other kinds of liver disease. Moreover, the performance of the methods of surveillance are lower in NAFLD than they are in other liver diseases. Furthermore, it is not clear which subgroups of patients without cirrhosis should undergo surveillance. Understanding the mechanisms of hepatocarcinogenesis in NAFLD could hopefully lead to the identification of biomarkers to be used in the surveillance for liver cancer in these individuals. By improving surveillance, tumors could be detected in earlier stages, amenable to curative treatments.
Core Tip: Nonalcoholic fatty liver disease (NAFLD) is a growing cause of hepatocellular carcinoma, and liver cancer is one of the leading causes of cancer-related death worldwide. There are particular genetic and immune-mediated mechanisms for hepatocarcinogenesis in NAFLD. Moreover, hepatocellular carcinoma can develop in NAFLD in the absence of cirrhosis. Finally, the characteristics of NAFLD and its high prevalence lead to important challenges regarding surveillance for liver cancer in this population. This review will approach the most important issues concerning NAFLD-related hepatocellular carcinoma.
- Citation: Mattos ÂZ, Debes JD, Dhanasekaran R, Benhammou JN, Arrese M, Patrício ALV, Zilio AC, Mattos AA. Hepatocellular carcinoma in nonalcoholic fatty liver disease: A growing challenge. World J Hepatol 2021; 13(9): 1107-1121
- URL: https://www.wjgnet.com/1948-5182/full/v13/i9/1107.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i9.1107
Nonalcoholic fatty liver disease (NAFLD) is rapidly becoming one of the most common causes of liver disease worldwide[1]. According to a meta-analytic assessment of 86 studies, the global prevalence of NAFLD is 25.24%[2]. Therefore, its association with hepatocellular carcinoma (HCC) also becomes increasingly important[3]. The relevance of this association is demonstrated by the fact that NAFLD was responsible for 36300 incident cases of HCC and 34700 HCC-related deaths in 2019[4].
Although cirrhosis is considered a predisposing condition for HCC in general, diverse disease-specific mechanisms are involved in the development of NAFLD-related HCC[3,5,6]. Moreover, the observation that HCC can occur in patients with NAFLD even in the absence of cirrhosis suggests that, as in the case of hepatitis B virus infection, NAFLD itself could be etiologically linked to HCC development[7]. Over the last few years, an array of studies has shed light on the diverse genetic and immune-related mechanisms that link NAFLD to the process of hepatocarcinogenesis. Nonetheless, much work is still needed to further understand this inter-relation.
Considering the association between NAFLD and HCC, surveillance for liver cancer among patients with fatty liver has become an important topic of discussion. However, the extremely high prevalence of NAFLD and the distinct risk levels for HCC in different patients make defining the target population for surveillance quite challenging[8].
The aim of this article is to review the epidemiology of NAFLD-related HCC, the genetic and immune mechanisms involved in hepatocarcinogenesis in individuals with NAFLD, the current knowledge related to HCC in patients with NAFLD without cirrhosis, and key aspects to consider for HCC surveillance in NAFLD.
In the last few decades, HCC-related mortality has steadily increased and since the 1980s has almost tripled in the United States, where it is the fastest-rising cause of cancer-related death[9]. Notably, this increase parallels the growth in NAFLD prevalence, which increased 2 to 3-fold in a similar period of time[10], turning it into a leading etiology of cirrhosis worldwide[11]. These coinciding trends and the fact that NAFLD has been noted as an increasingly common cause of HCC in several series[12] as well as the fastest-growing cause of HCC in liver transplant candidates and recipients in the United States[13] suggest that NAFLD is a prominent contributor to HCC burden worldwide and that the prevalence of HCC will likely increase concomitantly with the global obesity epidemic[12,14]. In this context, a recent study used Bayesian models to estimate that the age-standardized incidence rate of NAFLD-related liver cancer would increase from 0.92/100000 inhabitants in 2018 to 1.18/100000 inhabitants in 2030[15].
Estimates regarding the annual incidence of HCC in patients with NAFLD-related cirrhosis in the western hemisphere range from 0.5% to 2.6%[14,16]. With regard to data from eastern hemisphere countries, a prospective study from Japan reported similar figures, with an annual incidence of 2.26% in a cohort followed for more than 15 years[17]. Another study from India reported lower figures (annual incidence of HCC of 0.5% in patients with biopsy-proven NAFLD-related cirrhosis)[18]. It is worth mentioning, though, that most of these estimates originate from cohorts followed in tertiary centers or from liver transplant registries and that population-based cohort studies are not available. Importantly, existing data suggest that older age, male sex, alcohol intake, and especially diabetes are factors that may increase HCC incidence in NAFLD-related cirrhosis[19]. The annual incidence of HCC among individuals with NAFLD who do not have cirrhosis is much lower than that reported for patients with cirrhosis, as it will be reviewed later in this article.
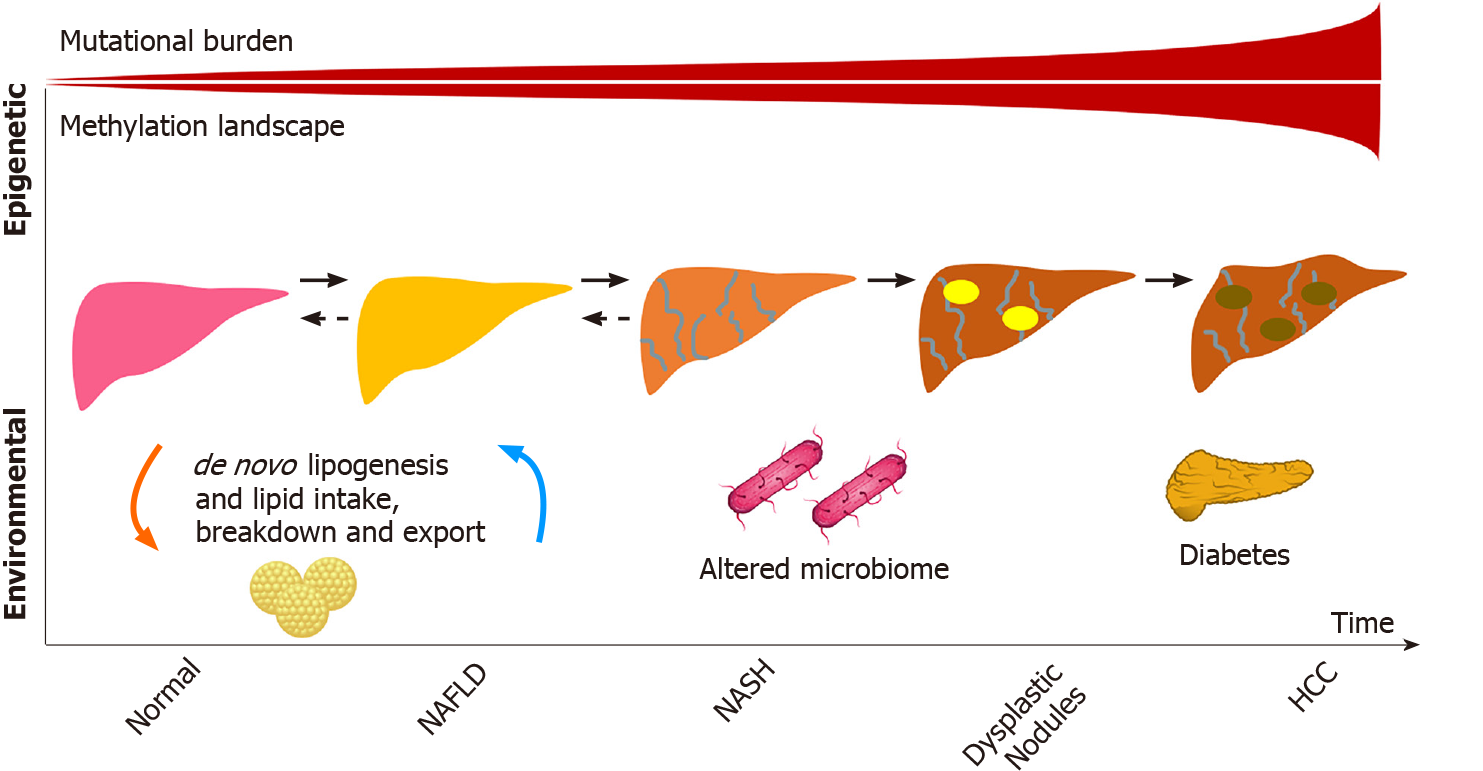
Considering the particular characteristics of NAFLD and NAFLD-related HCC as well as the fact that liver cancer also develops in individuals with NAFLD who do not have cirrhosis, the study of the genetic aspects of hepatocarcinogenesis in NAFLD has drawn substantial attention. The main genetic mechanisms involved in the development of NAFLD-related HCC will be discussed in this section and are summarized in Figure 1.
Early NAFLD studies have identified ethnic differences and evidence of familial clustering suggestive of a hereditary/genetic component to the disease[20]. The first study to demonstrate an association between genetic variants and NAFLD was published by Romeo et al[21] who conducted a genome wide association analysis using quantitative proton magnetic resonance spectroscopy to measure hepatic steatosis. The genome wide association analysis showed that carriers of the rs738409 variant of the patatin-like phospholipase domain containing protein 3 (PNPLA3) gene, most commonly found among Hispanics, had over a 2-fold increase in intrahepatic triglycerides[21]. Subsequent studies demonstrated the same variant to be associated with NAFLD-related HCC[22,23].
Following studies described conflicting evidence of an association between the transmembrane 6 superfamily member 2 (TM6SF2) rs58542926 polymorphism and NAFLD-related HCC, potentially from its low minor allele frequency[24,25]. The membrane bound O-acetyltransferase domain containing 7 rs641738 variant was posteriorly identified in a European cohort to be associated with NAFLD-related HCC[25-30]. Another European study focusing on the identification of rare variants in NAFLD-related HCC cases found, aside from PNPLA3 and TM6SF2, pathogenic variants in apolipoprotein B gene, among others[31]. As genetic association studies have mostly included patients of European ancestry, larger and more diverse cohorts are needed given the clinical observation that Hispanics are at higher risk for NAFLD-related HCC[32].
Association studies have provided a plethora of information regarding NAFLD-related hepatocarcinogenesis, although mechanistic studies have yet to elucidate how these variants cause disease. The observation that many of the polymorphisms involve lipid regulation raises the possibility that a lipid-rich dysregulated microenvironment may be key to HCC development. Although NAFLD-specific HCC studies are lacking, parallel mutations exist between NAFLD and other etiologies demonstrating a potential convergence in pathways that have previously been described in viral etiologies[33]. For instance, mutations in telomerase reverse transcriptase are known to play a role in the progression of dysplastic nodules and in the development of early HCC[34,35].
As hepatocyte damage increases from cirrhosis to dysplasia and eventually HCC, the mutational burden leading to cancer exponentially grows. This was well illustrated in a study by Brunner et al[36] who conducted whole genome sequencing of 100-500 hepatocytes from 5 healthy controls and 9 patients with cirrhosis. Structural variants and copy number variations were more commonly identified in those with cirrhosis compared to the normal controls, including in activin receptor type 2A, cyclin-dependent kinase inhibitor 2A, and AT-rich interaction domain 5A. Interestingly, similar signatures of somatic copy number variations were identified in a pilot study of 10 HCC cases in circulating tumor cells, raising the possibility of their use as biomarkers[37]. Other well described pathways include mutations in β-catenin, tumor antigen p53, and AKT/mechanistic target of rapamycin/mitogen-activated protein kinase signaling pathway, which includes tuberous sclerosis complex subunits 1 and 2, phosphatase and tensin homolog, and fibroblast growth factor 19[34].
Given the clinical and genetic heterogeneity in human HCCs, animal models have provided the pre-clinical tools to understand these pathways in NAFLD-related HCC[38]. Although NAFLD and nonalcoholic steatohepatitis (NASH) mouse models have limitations in recapitulating the human NAFLD phenotype, these animal models have proven especially relevant when comparing “obese” and “lean” NAFLD-related HCCs. Using whole exome sequencing, Shen et al[39] demonstrated that obese and lean NAFLD-related HCCs in mice had a different mutational burden. For instance, they identified mutations in the carboxyl ester lipase gene that caused an increase in cholesterol esters mostly in the obese mice. Similarly, Grohmann et al[40] studied obese and lean mouse models to show that HCC and NASH development were dependent on divergent pathways, raising the possibility of variable mechanisms in non-cirrhotic HCC development. The non-fibrotic pathway contributions were also demonstrated in European cohorts (from Germany and the United Kingdom), in which polygenic risk scores (including PNPLA3, TM6SF2, membrane bound O-acetyltransferase domain containing 7, and glucokinase regulator) predicted the risk of HCC in patients with NAFLD. This risk was associated with hepatic steatosis (adjusted hazard ratio of 1.35, P < 0.01), even after correcting for hepatic fibrosis (P < 0.05)[41].
The advent of single cell RNA sequencing has allowed for further understanding of the cell type proportions in HCC, which was a limitation of bulk RNA sequencing given tumor heterogeneity[42], including the understanding of the inflammatory microenvironment that may have effects on treatment responses[43]. Whether similar cell type proportions and mutational signatures will be identified in NAFLD-related HCC remains to be seen in populations with and without cirrhosis.
A summary of the genetic variants and mutations described in NAFLD-related HCC is presented in Tables 1 and 2.
| Ref. | SNP | Associated gene | Population/cohort |
| Sookoian et al[22]; Shen et al[23] | rs738409 C>G | PNPLA3 | American cohort; Swedish cohort; Italian cohort; British, Swiss cohort |
| Liu et al[24]; Donati et al[25] | rs58542926 C>T | TM6SF2 | American cohort |
| Donati et al[25]; Kozlitina et al[26]; Falleti et al[27]; Vespasiani-Gentilucci et al[28]; Luukkonen et al[29]; Mancina et al[30] | rs641738 C>T | MBOAT7 | Italian cohort |
| Ref. | Gene | Mechanism /pathway |
| Llovet et al[34]; Zucman-Rossi et al[35] | TERT | Telomere maintenance |
| Brunner et al[36] | ACVR2A | Transforming growth factor-β superfamily |
| Llovet et al[34]; Zucman-Rossi et al[35] | ARID5A | Chromatin remodeling |
| Llovet et al[34] | CDKN2A | Cell cycle |
| Llovet et al[34]; Zucman-Rossi et al[35] | CTNNB1 | β-catenin and WNT pathway activation |
| Llovet et al[34]; Zucman-Rossi et al[35] | TP53 | Cellular tumor antigen, cell cycle |
| Llovet et al[34]; Zucman-Rossi et al[35] | FGF19 | AKT/mTOR |
| Shen et al[39] | Cel | Cholesterol and lipids ester hydrolysis and absorption |
| Llovet et al[34] | TSC | mTOR, Hippo pathway |
Epigenetic modifiers also play a role in HCC development and account for approximately 32% of mutations found in HCC[44,45]. Many of the genes involved in structural chromosomal changes (AT-rich interaction domain 1A, AT-rich interaction domain 2, histone-lysine N-methyltransferase 2A) may not be directly involved in the pathogenesis of the disease but could be proxies to mutational changes in other genes linked by chromosomal looping captured by assay of transposase-accessible chromatin[46,47], an avenue that has not been yet explored in HCC related to NAFLD or to other etiologies of liver disease. Methylation aberrations also play a role. Recent work by Hernandez-Meza et al[48] demonstrated the extensive methylation landscape of different etiologies of HCC in a European cohort, with a minority represented by NAFLD. Similar to the increase in mutational burden seen from normal liver to cirrhosis, the study demonstrated that patients with HCC were more likely to have hypermethylation patterns compared to controls. Interestingly, some of these differential methylation patterns involved key lipid genes, including the transcription factor, sterol regulatory element-binding protein 1.
Serum metabolomic and microbiome studies have also identified signatures for poor NAFLD-related outcomes[49-51], although it remains to be seen whether these are surrogates for NASH progression or if they are involved in the pathways. The role of lipopolysaccharides has been studied in this context. The increase in lipopolysaccharides in NAFLD patients, as a surrogate for oxidative stress, is likely multifactorial and linked to the gut (bacterial overgrowth, increased permeability, among other factors), nutrients (including lipids), immune response, and hepatic injury, which adds another complexity to the NAFLD-related HCC spectrum of disease and potentially partly explains disease heterogeneity[52].
The use of metabolomics to identify signatures that are pathogenic in NAFLD-related HCC is also a novelty in the field. A recent study by Buchard et al[53] aimed to identify differences in metabolomics in tissues of patients with NAFLD-related HCC by stratifying the cohort according to the degree of liver fibrosis. Using 1H-nuclear magnetic resonance-based assays of 52 paired samples of HCC and adjacent non-tumoral tissue, the authors identified that, independently of fibrosis stage, glucose metabolism was increased in tumors as were branched chain amino acids, potentially reflecting the activation of mechanistic target of rapamycin pathways, which parallels the genetic alternations of HCC discussed previously. This study also demonstrated that HCCs had lower levels of monounsaturated fatty acids, suggesting a lipid reprogramming in HCC. Similarly, HCCs developing in the setting of advanced fibrosis also had lower monounsaturated fatty acids compared to HCCs that originated in livers with no or mild fibrosis[53]. The differences observed in tumoral vs non-tumoral tissues as well as in no or mild fibrosis vs advanced fibrosis illustrate that tumorigenesis in NAFLD may have fibrosis-independent mechanisms as suggested by Grohmann et al[40]. On the other hand, most patients with NAFLD who develop HCC in the absence of cirrhosis have NASH and advanced liver fibrosis instead of simple fatty liver with no or mild fibrosis, which could imply an association between fibrosis and hepatocarcinogenesis as well as common mechanisms for NASH and NAFLD-related HCC[12]. In this regard, the lipotoxicity and the metabolic reprogramming associated with steatosis are examples of pathogenic factors involved in the development of both NASH and HCC, and the inflammatory microenvironment of NASH also favors hepatocarcinogenesis[3].
Other genetic alterations that are a focus of current interest in NAFLD-related HCC are non-coding RNAs. Depending on further studies, they may provide an additional layer of complexity in epigenetic changes[45].
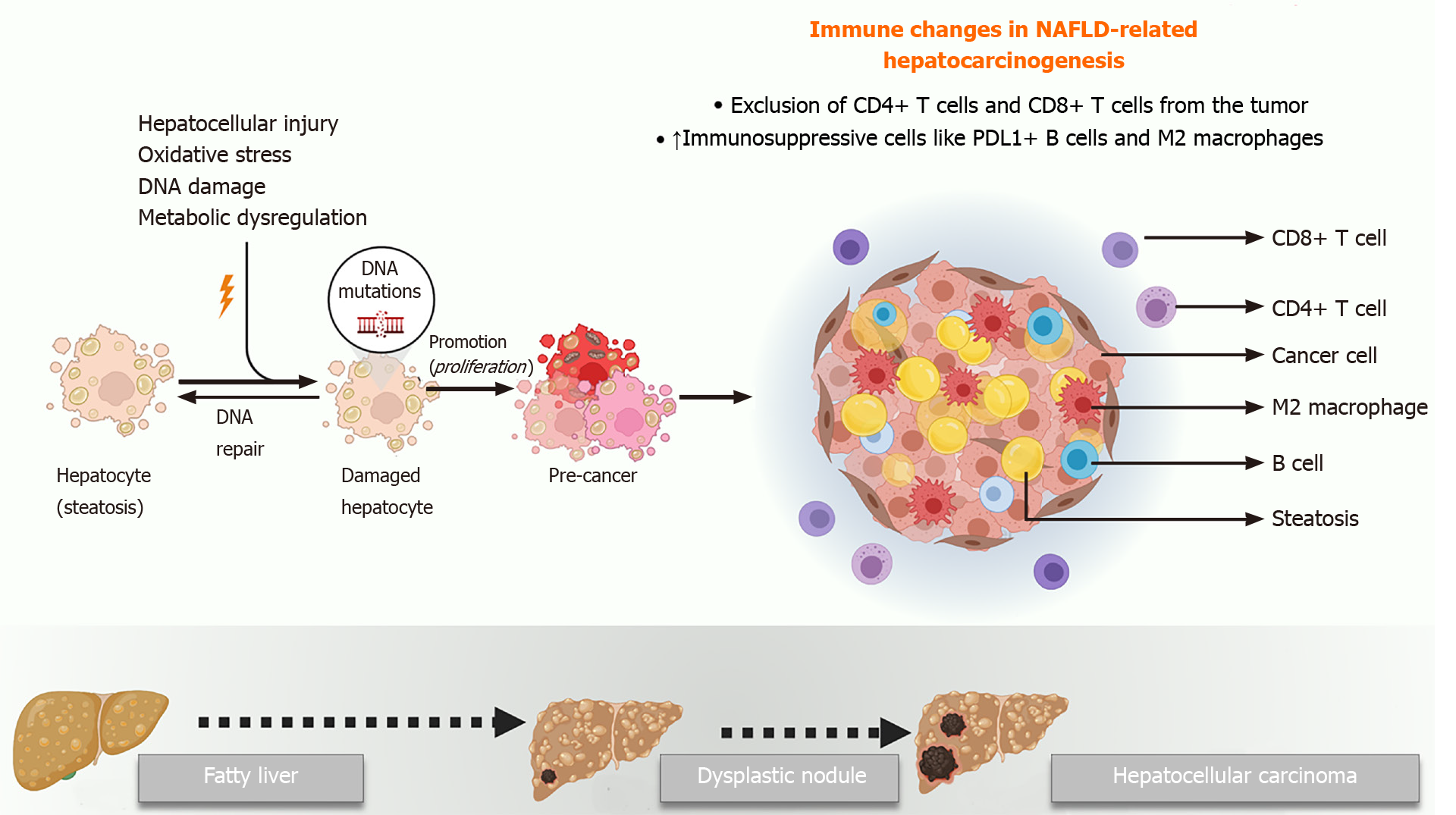
The mechanisms underlying the initiation and progression of HCC in the background of NAFLD are not fully understood. A number of factors including hepatic lipotoxicity, chronic inflammation, progressive fibrosis, and changes in the microbiome have all been implicated in NAFLD-related hepatocarcinogenesis. Recent studies have elegantly elucidated the role of the tumor microenvironment in this scenario[3,54-57]. Moreover, other authors have comprehensively discussed the role of cancer cell intrinsic factors that drive HCC in NAFLD[3,54,58,59]. Nevertheless, the role of the host immune system in NAFLD-related hepatocarcinogenesis must also be highlighted.
The liver is considered an immunologically privileged organ. It is constantly exposed to metabolites, toxins, and microbial products from the intestine since it derives a large part of its blood supply from the portal vein. However, there are several immune mechanisms within the liver that prevent an inflammatory hyper-response to this physiological antigenic load, including reduced expression of major histocompatibility class proteins, suppressed antigen presentation by Kupffer cells and dendritic cells, and enrichment of immunosuppressive cells like the regulatory T cells[60-62]. These mechanisms are overwhelmed in the context of NAFLD, where progressive steatosis leads to lipotoxicity, mitochondrial dysfunction, oxidative stress, and activation of cell death pathways, all of which trigger a state of chronic sterile inflammation. Unfortunately, a combination of the same factors that drive NASH progression also play mechanistic roles in the initiation of HCC in the background of this inflammatory milieu.
Progressive NASH influences both the innate and adaptive arms of the immune system, which together can enable cancer initiation and progression. The complex crosstalk among hepatocytes, adaptive immune cells, and cancer cells has been demonstrated by several studies. Wolf et al[54] found that infiltrating CD8+ T cells and natural killer cells contribute to NASH development and the subsequent transition to HCC. However, another study using a different mouse model of NASH showed that CD8+ T cells prevented HCC development and that a specific subset of immunosuppressive IgA+ plasma cells expressing programmed cell death ligand-1 and interleukin-10, which were abundant in NASH livers, directly suppressed liver cytotoxic CD8+ T cells, leading to HCC development[56]. Subsequently, Ma et al[55] showed that the metabolic dysregulation in NAFLD causes selective loss of CD4+ T lymphocytes, thus contributing to accelerated hepatocarcinogenesis. Meanwhile, Gomes et al[57] have shown that T helper 17 cells are activated upon hepatocyte DNA damage in NASH and can promote HCC.
Innate immune cells like macrophages, dendritic cells and natural killer cells are also important in the pathogenesis of NAFLD-related HCC. Kupffer cells are resident macrophages that play a significant proinflammatory and profibrotic role during NASH progression. However, their role in HCC is not clear yet. Wu et al[63] showed that the activation of Kupffer cells positive for triggering receptor expressed on myeloid cells-1 led to secretion of proinflammatory cytokines like interleukin-6, interleukin-1β, tumor necrosis factor, C-C motif chemokine ligand 2, and C-X-C motif chemokine ligand 10, which in turn promoted HCC. In general, though, protumorigenic M2-like macrophages that drive tumor progression via suppressing cytotoxic T cells and inducing angiogenesis appear to be recruited from circulating bone marrow derived monocytes rather than resident macrophages[64,65]. Other immune cells like neutrophils[66-68], monocytes[69], dendritic cells[70], and natural killer cells[71,72] have also been implicated in HCC progression in NASH, highlighting the complexity of the immune mechanisms of NAFLD-related hepatocarcinogenesis (Figure 2).
Given some of the specificities involved in NAFLD-related hepatocarcinogenesis, HCC in the setting of NAFLD is known to occur even in the absence of liver cirrhosis, an event previously related mostly to hepatitis B virus infection[12]. The prevalence of NAFLD-related HCC in the absence of cirrhosis varies dramatically according to the geographic location of the study and even among different studies performed in a similar region of the world. Most experts estimate that between 14% and 54% of NAFLD-related HCC cases occur in patients without cirrhosis. A study from the Veterans Affairs (VA) Health System in the United States by Mittal et al[73] found that 42% of veterans with NAFLD-related HCC had no evidence of cirrhosis. Interestingly, a similar study by the same group the following year found the prevalence of non-cirrhotic HCC related to NAFLD to be 13%[74]. In the latter study, however, the estimation of cirrhosis was separated by different levels of confidence. Small studies from Italy and Japan have also found that 50% and 48% of NAFLD-related HCC cases, respectively, occurred in the absence of cirrhosis, suggesting that the burden of non-cirrhotic HCC in NAFLD is also significant in other parts of the world[75,76]. Finally, a meta-analysis of 19 studies found the prevalence of non-cirrhotic HCC among NAFLD-related HCC to be approximately 38%[77].
Several issues help explain the variable results from multiple studies: (1) classifying patients as to whether or not they have cirrhosis through liver biopsy is possible mainly in small studies, while this classification is much less precise in larger studies that look at International Classification of Diseases codes or large commercial clinical databases; (2) most studies in the United States have been performed in the VA System, which is inevitably biased towards a large presence of male gender among the evaluated cohorts (> 90% in most studies[32,73,74,78]); and (3) the distinction between NAFLD and NASH is not completely clear in all the studies. In this regard, a study from the Netherlands looking at almost 100 non-cirrhotic NAFLD-related HCC cases found that most individuals had a low degree of or no steatohepatitis at all, suggesting a non-inflammatory carcinogenesis path towards HCC in this setting[79].
The lack of clarity on mechanisms leading to non-cirrhotic HCC with underlying NAFLD presents a difficult dilemma for practicing providers, as it is unclear who to screen for HCC. A retrospective cohort study of 271906 patients from the VA System (mean body mass index of 31.6 kg/m2, 28.7% with diabetes, 70.3% with hypertension, 62.3% with hyperlipidemia) suggested that diabetes and hyperlipidemia increase the risk of HCC in NAFLD[80]. However, the overall proportion of people with diabetes and NAFLD is still elevated as a total number of individuals to screen. Indeed, between 40% to 70% of individuals with diabetes have evidence of NAFLD[81]. Furthermore, it is unclear if the correlation between diabetes and HCC in patients without cirrhosis applies to other populations, as a recent study from Europe, characterizing the differences between cirrhotic and non-cirrhotic HCC in NAFLD, found an inverse association between diabetes and HCC in the non-cirrhotic group. Interest
Surveillance programs aim at allowing for early detection of HCC among high-risk patients so that they have higher odds of being candidates for curative treatments. In fact, when HCC is diagnosed during surveillance, it is diagnosed in earlier stages[83-86], and patients have significantly higher survival rates[85,87]. Thus, it is of utmost importance to define which patients should be submitted to surveillance.
For individuals with an estimated annual incidence of HCC ≥ 1.5%, surveillance is considered cost-effective[8], but it is not always clear which subgroups of patients reach such a cutoff. The main risk factor for HCC in patients with NAFLD is cirrhosis, and therefore the most important international guidelines are consensual that individuals with NAFLD and cirrhosis should be surveilled for HCC with ultrasonography (US) every 6 mo[88-91]. It should be highlighted, though, that obesity and steatosis might impair the performance of US[8], and the American Gastroenterological Association recommends using either computed tomography scan or magnetic resonance imaging in cases in which US quality is deemed unacceptable[91]. Regarding the use of biomarkers, some guidelines make it optional to add alpha-fetoprotein to the surveillance program[89-91], but its performance is suboptimal, especially in NAFLD-related HCC[8], and new biomarkers should be pursued, such as those currently under study by the European-South American Consortium to Assess Liver-Originated Neoplasia.
Despite these recommendations, patients with NAFLD-related cirrhosis seem to be less likely to undergo surveillance than those with other underlying liver diseases[86,92]. In order to overcome the low adherence to surveillance, screening tools to identify individuals at higher risk for HCC could be useful. The GALAD score (gender, age, lectin-binding alpha-fetoprotein-3, alpha-fetoprotein, and des-gamma-carboxyprothrombin) has been studied in this context, and it has been recently validated in patients with NASH. In such patients, the GALAD score had sensitivity and specificity over 90% to identify individuals who would develop HCC as early as 1.5 years before the diagnosis[93].
However, some authors believe that in order to stratify patients according to their risk of developing HCC, different tools might be necessary depending on the underlying liver disease. Using data from the VA Health System database, a study evaluated 7068 patients with NAFLD and cirrhosis, with an annual incidence of HCC of 1.56%. A predictive model based on age, sex, platelet count, albumin levels, aspartate aminotransferase/alanine aminotransferase ratio, diabetes, and body mass index was developed, and it had an area under the receiver operating characteristic curve of 0.775 and 0.721 for predicting HCC in the derivation- and in the validation-cohorts, respectively. This model was able to classify patients as low-risk (< 1%/year), medium-risk (1%-3%/year), and high-risk (> 3%/year) for HCC. A classification such as this could be used, if further validated, to define subgroups that might spare surveillance[78].
As discussed above, there are subgroups of patients with NAFLD who do not have cirrhosis but are at risk of developing HCC. In a large retrospective cohort study including 296707 individuals with NAFLD and a similar number of matched controls from the VA Health System database, patients with NAFLD had 7.6-fold higher risk of developing HCC than their counterparts, and the risk was greater among men, older people, and Hispanics. However, in the NAFLD-group, the annual incidence of HCC was 10.6/1000 person-years for individuals with cirrhosis and 0.08/1000 person-years for those without it, which was considered insufficient for a general recommendation of surveillance to be made for patients without cirrhosis. The FIB-4 score was also evaluated, and, despite its association with the development of HCC, individuals with high FIB-4 scores (> 2.67) but without a diagnosis of cirrhosis were still considered to have a low risk of developing HCC[32].
Another large study evaluated four European primary care databases including over 18 million individuals and verified an incidence of HCC of 0.3/1000 person-years among patients with NAFLD, which was much higher than that of controls (hazard ratio of 3.51). When the NAFLD group was classified according to the FIB-4 score, it was possible to identify which patients were under higher risks. When compared to individuals with a FIB-4 score < 1.30, those with scores between 1.30 and 2.67 had a hazard ratio for HCC of 3.74, and the ones with scores > 2.67 had a hazard ratio of 25.2[94]. Therefore, despite conflicting evidence, it is possible that the FIB-4 score could be used in order to select patients for surveillance.
Currently, guidelines are vague regarding surveillance for HCC in patients with NAFLD who do not have cirrhosis. The American Gastroenterological Association, in its position paper on surveillance for HCC in patients with NAFLD, recommends considering patients with NAFLD and advanced fibrosis for surveillance but recommends against routinely surveilling individuals with earlier stages of fibrosis[91]. While the position of the European Association for the Study of the Liver is similar to that[88], the American Association for the Study of Liver Diseases considers the benefit of surveillance in individuals with NAFLD who do not have cirrhosis to be uncertain and does not support it[90].
NAFLD currently affects one fourth of the global population[2]. Its increasing prevalence and the fact that it is associated with the development of liver cancer, both in the setting of cirrhosis and in its absence, make NAFLD-related HCC a growing challenge[12]. It is likely that the growth in NAFLD-related HCC will offset a decrease in viral hepatitis-related liver cancer, which is expected for the near future due to vaccination against hepatitis B virus and to the highly effective treatments for hepatitis B and C[95]. NAFLD-related HCC is already responsible for an important burden on public health, being associated with 796000 disability-adjusted life years in 2019, an increase of 33.6%in comparison to 2010[4].
This article has highlighted important genetic and immune-mediated mechanisms involved in NAFLD-related hepatocarcinogenesis. Understanding the role of certain genetic variants (especially those associated with genes such as PNPLA3[22,23], TM6SF2[24,25], and membrane bound O-acetyltransferase domain containing 7[25-30]) as well as the importance of epigenetic modifiers[44,45], the microenvironment of NAFLD, and the influences that this disease has on the innate and adaptive immune systems[54-57] will hopefully allow for a better knowledge of the clinical characteristics of NAFLD-related HCC, including the possibility of the development of liver cancer in the absence of cirrhosis. Moreover, this knowledge may help define more appropriate surveillance strategies, focusing not only in individuals with cirrhosis, since over one third of NAFLD-related HCC cases are diagnosed in patients without this condition[77]. At present, surveillance with US every 6 mo is recommended for individuals with advanced liver fibrosis[91].
This review has limitations associated especially with the incomplete understanding of NAFLD-related HCC by the scientific community. The pathophysiology of this condition must be further studied, particularly the mechanisms leading to non-cirrhotic HCC. Moreover, there is a profound necessity for the identification of better biomarkers to detect subgroups of patients that could benefit from surveillance aside from those with cirrhosis[96].
The worldwide growing prevalence of NAFLD and its association with the development of HCC in patients either with or without cirrhosis make NAFLD-related HCC a growing challenge. Improving surveillance strategies is of the utmost importance in order for the early detection of HCC and for patients to have higher chances of being cured. Further understanding of the mechanisms leading to HCC in the setting of NAFLD will likely lead to novel molecular candidates that could be used as biomarkers to identify patients who will progress to develop a liver malignancy even in the absence of cirrhosis.
Manuscript source: Invited manuscript
Corresponding Author’s Membership in Professional Societies: Federação Brasileira de Gastroenterologia
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Demidem A, Menichelli D, Saha S, Tajiri K S-Editor: Liu M L-Editor: Filipodia P-Editor: Li X
| 1. | Cotter TG, Rinella M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology. 2020;158:1851-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 841] [Article Influence: 168.2] [Reference Citation Analysis (2)] |
| 2. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7536] [Article Influence: 837.3] [Reference Citation Analysis (0)] |
| 3. | Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019;16:411-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 965] [Article Influence: 160.8] [Reference Citation Analysis (0)] |
| 4. | GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11327] [Cited by in RCA: 9637] [Article Influence: 1927.4] [Reference Citation Analysis (35)] |
| 5. | Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. 2016;64:S84-S101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 710] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 6. | Geh D, Manas DM, Reeves HL. Hepatocellular carcinoma in non-alcoholic fatty liver disease-a review of an emerging challenge facing clinicians. Hepatobiliary Surg Nutr. 2021;10:59-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 7. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3173] [Article Influence: 528.8] [Reference Citation Analysis (37)] |
| 8. | Plaz Torres MC, Bodini G, Furnari M, Marabotto E, Zentilin P, Strazzabosco M, Giannini EG. Surveillance for Hepatocellular Carcinoma in Patients with Non-Alcoholic Fatty Liver Disease: Universal or Selective? Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55823] [Article Influence: 7974.7] [Reference Citation Analysis (132)] |
| 10. | Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, Koteish A, Brancati FL, Clark JM. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013;178:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 625] [Article Influence: 52.1] [Reference Citation Analysis (1)] |
| 11. | Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin Gastroenterol Hepatol. 2020;18:2650-2666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 805] [Cited by in RCA: 720] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 12. | Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 1210] [Article Influence: 302.5] [Reference Citation Analysis (0)] |
| 13. | Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, Eguchi Y, Wong VW, Negro F, Yilmaz Y, Romero-Gomez M, George J, Ahmed A, Wong R, Younossi I, Ziayee M, Afendy A; Global Nonalcoholic Steatohepatitis Council. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin Gastroenterol Hepatol. 2019;17:748-755.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 575] [Article Influence: 95.8] [Reference Citation Analysis (0)] |
| 14. | Banini BA, Sanyal AJ. NAFLD-related HCC. Adv Cancer Res. 2021;149:143-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Liu Z, Xu K, Jiang Y, Cai N, Fan J, Mao X, Suo C, Jin L, Zhang T, Chen X. Global trend of aetiology-based primary liver cancer incidence from 1990 to 2030: a modelling study. Int J Epidemiol. 2021;50:128-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 16. | Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 965] [Article Influence: 64.3] [Reference Citation Analysis (1)] |
| 17. | Yatsuji S, Hashimoto E, Tobari M, Taniai M, Tokushige K, Shiratori K. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol. 2009;24:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 239] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 18. | Amarapurkar DN, Dharod M, Gautam S, Patel N. Risk of development of hepatocellular carcinoma in patients with NASH-related cirrhosis. Trop Gastroenterol. 2013;34:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 601] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 20. | Schwimmer JB, Celedon MA, Lavine JE, Salem R, Campbell N, Schork NJ, Shiehmorteza M, Yokoo T, Chavez A, Middleton MS, Sirlin CB. Heritability of nonalcoholic fatty liver disease. Gastroenterology. 2009;136:1585-1592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 358] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 21. | Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461-1465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2701] [Cited by in RCA: 2597] [Article Influence: 152.8] [Reference Citation Analysis (0)] |
| 22. | Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 739] [Article Influence: 52.8] [Reference Citation Analysis (1)] |
| 23. | Shen JH, Li YL, Li D, Wang NN, Jing L, Huang YH. The rs738409 (I148M) variant of the PNPLA3 gene and cirrhosis: a meta-analysis. J Lipid Res. 2015;56:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Liu YL, Reeves HL, Burt AD, Tiniakos D, McPherson S, Leathart JB, Allison ME, Alexander GJ, Piguet AC, Anty R, Donaldson P, Aithal GP, Francque S, Van Gaal L, Clement K, Ratziu V, Dufour JF, Day CP, Daly AK, Anstee QM. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat Commun. 2014;5:4309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 461] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 25. | Donati B, Dongiovanni P, Romeo S, Meroni M, McCain M, Miele L, Petta S, Maier S, Rosso C, De Luca L, Vanni E, Grimaudo S, Romagnoli R, Colli F, Ferri F, Mancina RM, Iruzubieta P, Craxi A, Fracanzani AL, Grieco A, Corradini SG, Aghemo A, Colombo M, Soardo G, Bugianesi E, Reeves H, Anstee QM, Fargion S, Valenti L. MBOAT7 rs641738 variant and hepatocellular carcinoma in non-cirrhotic individuals. Sci Rep. 2017;7:4492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 196] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 26. | Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjærg-Hansen A, Vogt TF, Hobbs HH, Cohen JC. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46:352-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 724] [Cited by in RCA: 930] [Article Influence: 84.5] [Reference Citation Analysis (0)] |
| 27. | Falleti E, Cussigh A, Cmet S, Fabris C, Toniutto P. PNPLA3 rs738409 and TM6SF2 rs58542926 variants increase the risk of hepatocellular carcinoma in alcoholic cirrhosis. Dig Liver Dis. 2016;48:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Vespasiani-Gentilucci U, Gallo P, Dell'Unto C, Volpentesta M, Antonelli-Incalzi R, Picardi A. Promoting genetics in non-alcoholic fatty liver disease: Combined risk score through polymorphisms and clinical variables. World J Gastroenterol. 2018;24:4835-4845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Luukkonen PK, Zhou Y, Hyötyläinen T, Leivonen M, Arola J, Orho-Melander M, Orešič M, Yki-Järvinen H. The MBOAT7 variant rs641738 alters hepatic phosphatidylinositols and increases severity of non-alcoholic fatty liver disease in humans. J Hepatol. 2016;65:1263-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 30. | Mancina RM, Dongiovanni P, Petta S, Pingitore P, Meroni M, Rametta R, Borén J, Montalcini T, Pujia A, Wiklund O, Hindy G, Spagnuolo R, Motta BM, Pipitone RM, Craxì A, Fargion S, Nobili V, Käkelä P, Kärjä V, Männistö V, Pihlajamäki J, Reilly DF, Castro-Perez J, Kozlitina J, Valenti L, Romeo S. The MBOAT7-TMC4 Variant rs641738 Increases Risk of Nonalcoholic Fatty Liver Disease in Individuals of European Descent. Gastroenterology. 2016;150:1219-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 514] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 31. | Pelusi S, Baselli G, Pietrelli A, Dongiovanni P, Donati B, McCain MV, Meroni M, Fracanzani AL, Romagnoli R, Petta S, Grieco A, Miele L, Soardo G, Bugianesi E, Fargion S, Aghemo A, D'Ambrosio R, Xing C, Romeo S, De Francesco R, Reeves HL, Valenti LVC. Rare Pathogenic Variants Predispose to Hepatocellular Carcinoma in Nonalcoholic Fatty Liver Disease. Sci Rep. 2019;9:3682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 32. | Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, Li L, Desiderio R, Thrift AP, Asch SM, Chu J, El-Serag HB. Risk of Hepatocellular Cancer in Patients With Non-Alcoholic Fatty Liver Disease. Gastroenterology. 2018;155:1828-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 570] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 33. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 3879] [Article Influence: 969.8] [Reference Citation Analysis (3)] |
| 34. | Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1933] [Cited by in RCA: 1873] [Article Influence: 208.1] [Reference Citation Analysis (4)] |
| 35. | Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology. 2015;149:1226-1239.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 951] [Article Influence: 95.1] [Reference Citation Analysis (0)] |
| 36. | Brunner SF, Roberts ND, Wylie LA, Moore L, Aitken SJ, Davies SE, Sanders MA, Ellis P, Alder C, Hooks Y, Abascal F, Stratton MR, Martincorena I, Hoare M, Campbell PJ. Somatic mutations and clonal dynamics in healthy and cirrhotic human liver. Nature. 2019;574:538-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 250] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 37. | Court CM, Hou S, Liu L, Winograd P, DiPardo BJ, Liu SX, Chen PJ, Zhu Y, Smalley M, Zhang R, Sadeghi S, Finn RS, Kaldas FM, Busuttil RW, Zhou XJ, Tseng HR, Tomlinson JS, Graeber TG, Agopian VG. Somatic copy number profiling from hepatocellular carcinoma circulating tumor cells. NPJ Precis Oncol. 2020;4:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Febbraio MA, Reibe S, Shalapour S, Ooi GJ, Watt MJ, Karin M. Preclinical Models for Studying NASH-Driven HCC: How Useful Are They? Cell Metab. 2019;29:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 194] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 39. | Shen J, Tsoi H, Liang Q, Chu ES, Liu D, Yu AC, Chan TF, Li X, Sung JJ, Wong VW, Yu J. Oncogenic mutations and dysregulated pathways in obesity-associated hepatocellular carcinoma. Oncogene. 2016;35:6271-6280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Grohmann M, Wiede F, Dodd GT, Gurzov EN, Ooi GJ, Butt T, Rasmiena AA, Kaur S, Gulati T, Goh PK, Treloar AE, Archer S, Brown WA, Muller M, Watt MJ, Ohara O, McLean CA, Tiganis T. Obesity Drives STAT-1-Dependent NASH and STAT-3-Dependent HCC. Cell. 2018;175:1289-1306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 285] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 41. | Bianco C, Jamialahmadi O, Pelusi S, Baselli G, Dongiovanni P, Zanoni I, Santoro L, Maier S, Liguori A, Meroni M, Borroni V, D'Ambrosio R, Spagnuolo R, Alisi A, Federico A, Bugianesi E, Petta S, Miele L, Vespasiani-Gentilucci U, Anstee QM, Stickel F, Hampe J, Fischer J, Berg T, Fracanzani AL, Soardo G, Reeves H, Prati D, Romeo S, Valenti L. Non-invasive stratification of hepatocellular carcinoma risk in non-alcoholic fatty liver using polygenic risk scores. J Hepatol. 2021;74:775-782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 254] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 42. | Craig AJ, von Felden J, Garcia-Lezana T, Sarcognato S, Villanueva A. Tumour evolution in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2020;17:139-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 557] [Article Influence: 111.4] [Reference Citation Analysis (1)] |
| 43. | Winograd P, Hou S, Court CM, Lee YT, Chen PJ, Zhu Y, Sadeghi S, Finn RS, Teng PC, Wang JJ, Zhang Z, Liu H, Busuttil RW, Tomlinson JS, Tseng HR, Agopian VG. Hepatocellular Carcinoma-Circulating Tumor Cells Expressing PD-L1 Are Prognostic and Potentially Associated With Response to Checkpoint Inhibitors. Hepatol Commun. 2020;4:1527-1540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 44. | Schulze K, Imbeaud S, Letouzé E, Alexandrov LB, Calderaro J, Rebouissou S, Couchy G, Meiller C, Shinde J, Soysouvanh F, Calatayud AL, Pinyol R, Pelletier L, Balabaud C, Laurent A, Blanc JF, Mazzaferro V, Calvo F, Villanueva A, Nault JC, Bioulac-Sage P, Stratton MR, Llovet JM, Zucman-Rossi J. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1006] [Cited by in RCA: 1343] [Article Influence: 134.3] [Reference Citation Analysis (0)] |
| 45. | Fujimoto A, Furuta M, Totoki Y, Tsunoda T, Kato M, Shiraishi Y, Tanaka H, Taniguchi H, Kawakami Y, Ueno M, Gotoh K, Ariizumi S, Wardell CP, Hayami S, Nakamura T, Aikata H, Arihiro K, Boroevich KA, Abe T, Nakano K, Maejima K, Sasaki-Oku A, Ohsawa A, Shibuya T, Nakamura H, Hama N, Hosoda F, Arai Y, Ohashi S, Urushidate T, Nagae G, Yamamoto S, Ueda H, Tatsuno K, Ojima H, Hiraoka N, Okusaka T, Kubo M, Marubashi S, Yamada T, Hirano S, Yamamoto M, Ohdan H, Shimada K, Ishikawa O, Yamaue H, Chayama K, Miyano S, Aburatani H, Shibata T, Nakagawa H. Whole-genome mutational landscape and characterization of noncoding and structural mutations in liver cancer. Nat Genet. 2016;48:500-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 537] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 46. | Claussnitzer M, Dankel SN, Kim KH, Quon G, Meuleman W, Haugen C, Glunk V, Sousa IS, Beaudry JL, Puviindran V, Abdennur NA, Liu J, Svensson PA, Hsu YH, Drucker DJ, Mellgren G, Hui CC, Hauner H, Kellis M. FTO Obesity Variant Circuitry and Adipocyte Browning in Humans. N Engl J Med. 2015;373:895-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 978] [Article Influence: 97.8] [Reference Citation Analysis (0)] |
| 47. | Benhammou JN, Ko A, Alvarez M, Kaikkonen MU, Rankin C, Garske KM, Padua D, Bhagat Y, Kaminska D, Kärjä V, Pihlajamäki J, Pisegna JR, Pajukanta P. Novel Lipid Long Intervening Noncoding RNA, Oligodendrocyte Maturation-Associated Long Intergenic Noncoding RNA, Regulates the Liver Steatosis Gene Stearoyl-Coenzyme A Desaturase As an Enhancer RNA. Hepatol Commun. 2019;3:1356-1372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 48. | Hernandez-Meza G, von Felden J, Gonzalez-Kozlova EE, Garcia-Lezana T, Peix J, Portela A, Craig AJ, Sayols S, Schwartz M, Losic B, Mazzaferro V, Esteller M, Llovet JM, Villanueva A. DNA methylation profiling of human hepatocarcinogenesis. Hepatology. 2020;epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 49. | Jacobs JP, Dong TS, Agopian V, Lagishetty V, Sundaram V, Noureddin M, Ayoub WS, Durazo F, Benhammou J, Enayati P, Elashoff D, Goodman MT, Pisegna J, Hussain S. Microbiome and bile acid profiles in duodenal aspirates from patients with liver cirrhosis: The Microbiome, Microbial Markers and Liver Disease Study. Hepatol Res. 2018;48:1108-1117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 50. | Muir K, Hazim A, He Y, Peyressatre M, Kim DY, Song X, Beretta L. Proteomic and lipidomic signatures of lipid metabolism in NASH-associated hepatocellular carcinoma. Cancer Res. 2013;73:4722-4731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 51. | Wong CM, Tsang FH, Ng IO. Non-coding RNAs in hepatocellular carcinoma: molecular functions and pathological implications. Nat Rev Gastroenterol Hepatol. 2018;15:137-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 341] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 52. | Ferro D, Baratta F, Pastori D, Cocomello N, Colantoni A, Angelico F, Del Ben M. New Insights into the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Gut-Derived Lipopolysaccharides and Oxidative Stress. Nutrients. 2020;12:2762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 53. | Buchard B, Teilhet C, Abeywickrama Samarakoon N, Massoulier S, Joubert-Zakeyh J, Blouin C, Reynes C, Sabatier R, Biesse-Martin AS, Vasson MP, Abergel A, Demidem A. Two Metabolomics Phenotypes of Human Hepatocellular Carcinoma in Non-Alcoholic Fatty Liver Disease According to Fibrosis Severity. Metabolites. 2021;11:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 54. | Wolf MJ, Adili A, Piotrowitz K, Abdullah Z, Boege Y, Stemmer K, Ringelhan M, Simonavicius N, Egger M, Wohlleber D, Lorentzen A, Einer C, Schulz S, Clavel T, Protzer U, Thiele C, Zischka H, Moch H, Tschöp M, Tumanov AV, Haller D, Unger K, Karin M, Kopf M, Knolle P, Weber A, Heikenwalder M. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell. 2014;26:549-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 547] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 55. | Ma C, Kesarwala AH, Eggert T, Medina-Echeverz J, Kleiner DE, Jin P, Stroncek DF, Terabe M, Kapoor V, ElGindi M, Han M, Thornton AM, Zhang H, Egger M, Luo J, Felsher DW, McVicar DW, Weber A, Heikenwalder M, Greten TF. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531:253-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 589] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 56. | Shalapour S, Lin XJ, Bastian IN, Brain J, Burt AD, Aksenov AA, Vrbanac AF, Li W, Perkins A, Matsutani T, Zhong Z, Dhar D, Navas-Molina JA, Xu J, Loomba R, Downes M, Yu RT, Evans RM, Dorrestein PC, Knight R, Benner C, Anstee QM, Karin M. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature. 2017;551:340-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 425] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 57. | Gomes AL, Teijeiro A, Burén S, Tummala KS, Yilmaz M, Waisman A, Theurillat JP, Perna C, Djouder N. Metabolic Inflammation-Associated IL-17A Causes Non-alcoholic Steatohepatitis and Hepatocellular Carcinoma. Cancer Cell. 2016;30:161-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 311] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 58. | Takakura K, Oikawa T, Nakano M, Saeki C, Torisu Y, Kajihara M, Saruta M. Recent Insights Into the Multiple Pathways Driving Non-alcoholic Steatohepatitis-Derived Hepatocellular Carcinoma. Front Oncol. 2019;9:762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 59. | Kutlu O, Kaleli HN, Ozer E. Molecular Pathogenesis of Nonalcoholic Steatohepatitis- (NASH-) Related Hepatocellular Carcinoma. Can J Gastroenterol Hepatol. 2018;2018:8543763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 60. | Li F, Tian Z. The liver works as a school to educate regulatory immune cells. Cell Mol Immunol. 2013;10:292-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 61. | Knolle PA. Staying local-antigen presentation in the liver. Curr Opin Immunol. 2016;40:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 62. | Doherty DG. Immunity, tolerance and autoimmunity in the liver: A comprehensive review. J Autoimmun. 2016;66:60-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 229] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 63. | Wu J, Li J, Salcedo R, Mivechi NF, Trinchieri G, Horuzsko A. The proinflammatory myeloid cell receptor TREM-1 controls Kupffer cell activation and development of hepatocellular carcinoma. Cancer Res. 2012;72:3977-3986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 198] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 64. | Glavind E, Gotthardt DN, Pfeiffenberger J, Sandahl TD, Bashlekova T, Willemoe GL, Hasselby JP, Weiss KH, Møller HJ, Vilstrup H, Lee WM, Schilsky ML, Ott P, Grønbæk H. The macrophage activation marker soluble CD163 is elevated and associated with liver disease phenotype in patients with Wilson's disease. Orphanet J Rare Dis. 2020;15:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Dhanasekaran R, Baylot V, Kim M, Kuruvilla S, Bellovin DI, Adeniji N, Rajan Kd A, Lai I, Gabay M, Tong L, Krishnan M, Park J, Hu T, Barbhuiya MA, Gentles AJ, Kannan K, Tran PT, Felsher DW. MYC and Twist1 cooperate to drive metastasis by eliciting crosstalk between cancer and innate immunity. Elife. 2020;9:e50731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 66. | van der Windt DJ, Sud V, Zhang H, Varley PR, Goswami J, Yazdani HO, Tohme S, Loughran P, O'Doherty RM, Minervini MI, Huang H, Simmons RL, Tsung A. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology. 2018;68:1347-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 358] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 67. | Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu Z, Yin XY, Zheng L. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol. 2011;54:948-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 391] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 68. | Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z, Chen EB, Fan J, Cao Y, Dai Z, Zhou J. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology. 2016;150:1646-1658.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 618] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 69. | Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206:1327-1337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 591] [Cited by in RCA: 724] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 70. | Henning JR, Graffeo CS, Rehman A, Fallon NC, Zambirinis CP, Ochi A, Barilla R, Jamal M, Deutsch M, Greco S, Ego-Osuala M, Bin-Saeed U, Rao RS, Badar S, Quesada JP, Acehan D, Miller G. Dendritic cells limit fibroinflammatory injury in nonalcoholic steatohepatitis in mice. Hepatology. 2013;58:589-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 71. | Cai L, Zhang Z, Zhou L, Wang H, Fu J, Zhang S, Shi M, Zhang H, Yang Y, Wu H, Tien P, Wang FS. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol. 2008;129:428-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 229] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 72. | Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, Manns MP, Greten TF, Korangy F. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50:799-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 533] [Cited by in RCA: 527] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 73. | Mittal S, Sada YH, El-Serag HB, Kanwal F, Duan Z, Temple S, May SB, Kramer JR, Richardson PA, Davila JA. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin Gastroenterol Hepatol. 2015;13:594-601.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 210] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 74. | Mittal S, El-Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, May SB, Kramer JR, Richardson PA, Davila JA. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2016;14:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 492] [Article Influence: 54.7] [Reference Citation Analysis (1)] |
| 75. | Piscaglia F, Svegliati-Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C, Bellentani S; HCC-NAFLD Italian Study Group. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology. 2016;63:827-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 465] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 76. | Yasui K, Hashimoto E, Komorizono Y, Koike K, Arii S, Imai Y, Shima T, Kanbara Y, Saibara T, Mori T, Kawata S, Uto H, Takami S, Sumida Y, Takamura T, Kawanaka M, Okanoue T; Japan NASH Study Group, Ministry of Health, Labour, and Welfare of Japan. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:428-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 322] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 77. | Stine JG, Wentworth BJ, Zimmet A, Rinella ME, Loomba R, Caldwell SH, Argo CK. Systematic review with meta-analysis: risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment Pharmacol Ther. 2018;48:696-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 260] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 78. | Ioannou GN, Green P, Kerr KF, Berry K. Models estimating risk of hepatocellular carcinoma in patients with alcohol or NAFLD-related cirrhosis for risk stratification. J Hepatol. 2019;71:523-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 79. | van Meer S, van Erpecum KJ, Sprengers D, Klümpen HJ, Jansen PL, Ijzermans JN, Siersema PD, de Man RA, Verheij J. Hepatocellular carcinoma in noncirrhotic livers is associated with steatosis rather than steatohepatitis: potential implications for pathogenesis. Eur J Gastroenterol Hepatol. 2016;28:955-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 80. | Kanwal F, Kramer JR, Li L, Dai J, Natarajan Y, Yu X, Asch SM, El-Serag HB. Effect of Metabolic Traits on the Risk of Cirrhosis and Hepatocellular Cancer in Nonalcoholic Fatty Liver Disease. Hepatology. 2020;71:808-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 215] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 81. | Debes JD, Boonstra A, de Knegt RJ. NAFLD-Related Hepatocellular Carcinoma and the Four Horsemen of the Apocalypse. Hepatology. 2020;71:774-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 82. | Bengtsson B, Stål P, Wahlin S, Björkström NK, Hagström H. Characteristics and outcome of hepatocellular carcinoma in patients with NAFLD without cirrhosis. Liver Int. 2019;39:1098-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 83. | Fassio E, Díaz S, Santa C, Reig ME, Martínez Artola Y, Alves de Mattos A, Míguez C, Galizzi J, Zapata R, Ridruejo E, de Souza FC, Hernández N, Pinchuk L; Multicenter Group for Study of Hepatocarcinoma in Latin America; Asociación Latinoamericana para el Estudio del Hígado (ALEH). Etiology of hepatocellular carcinoma in Latin America: a prospective, multicenter, international study. Ann Hepatol. 2010;9:63-69. [PubMed] |
| 84. | Appel-da-Silva MC, Miozzo SA, Dossin IA, Tovo CV, Branco F, de Mattos AA. Incidence of hepatocellular carcinoma in outpatients with cirrhosis in Brazil: A 10-year retrospective cohort study. World J Gastroenterol. 2016;22:10219-10225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 85. | Piñero F, Rubinstein F, Marciano S, Fernández N, Silva J, Zambelo Y, Anders M, Zerega A, Ridruejo E, Miguez C, Ameigeiras B, D'Amico C, Gaite L, Bermúdez C, Rosales C, Romero G, McCormack L, Reggiardo V, Colombato L, Gadano A, Silva M. Surveillance for Hepatocellular Carcinoma: Does the Place Where Ultrasound Is Performed Impact Its Effectiveness? Dig Dis Sci. 2019;64:718-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 86. | Aby E, Phan J, Truong E, Grotts J, Saab S. Inadequate Hepatocellular Carcinoma Screening in Patients With Nonalcoholic Steatohepatitis Cirrhosis. J Clin Gastroenterol. 2019;53:142-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 87. | Debes JD, Chan AJ, Balderramo D, Kikuchi L, Gonzalez Ballerga E, Prieto JE, Tapias M, Idrovo V, Davalos MB, Cairo F, Barreyro FJ, Paredes S, Hernandez N, Avendaño K, Diaz Ferrer J, Yang JD, Carrera E, Garcia JA, Mattos AZ, Hirsch BS, Gonçalves PT, Carrilho FJ, Roberts LR. Hepatocellular carcinoma in South America: Evaluation of risk factors, demographics and therapy. Liver Int. 2018;38:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 88. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6059] [Article Influence: 865.6] [Reference Citation Analysis (3)] |
| 89. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3028] [Article Influence: 432.6] [Reference Citation Analysis (3)] |
| 90. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3241] [Article Influence: 463.0] [Reference Citation Analysis (1)] |
| 91. | Loomba R, Lim JK, Patton H, El-Serag HB. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology. 2020;158:1822-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 262] [Article Influence: 52.4] [Reference Citation Analysis (1)] |
| 92. | Wolf E, Rich NE, Marrero JA, Parikh ND, Singal AG. Use of Hepatocellular Carcinoma Surveillance in Patients With Cirrhosis: A Systematic Review and Meta-Analysis. Hepatology. 2021;73:713-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 218] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 93. | Best J, Bechmann LP, Sowa JP, Sydor S, Dechêne A, Pflanz K, Bedreli S, Schotten C, Geier A, Berg T, Fischer J, Vogel A, Bantel H, Weinmann A, Schattenberg JM, Huber Y, Wege H, von Felden J, Schulze K, Bettinger D, Thimme R, Sinner F, Schütte K, Weiss KH, Toyoda H, Yasuda S, Kumada T, Berhane S, Wichert M, Heider D, Gerken G, Johnson P, Canbay A. GALAD Score Detects Early Hepatocellular Carcinoma in an International Cohort of Patients With Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol. 2020;18:728-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 187] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 94. | Alexander M, Loomis AK, van der Lei J, Duarte-Salles T, Prieto-Alhambra D, Ansell D, Pasqua A, Lapi F, Rijnbeek P, Mosseveld M, Waterworth DM, Kendrick S, Sattar N, Alazawi W. Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed NAFLD: real-world study of 18 million patients in four European cohorts. BMC Med. 2019;17:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 220] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 95. | McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73 Suppl 1:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 1342] [Article Influence: 335.5] [Reference Citation Analysis (1)] |
| 96. | Debes JD, Carrera E, Mattos AZ, Prieto JE, Boonstra A; ESCALON investigators. Hepatocellular carcinoma, a unique tumor with a lack of biomarkers. Ann Hepatol. 2019;18:786-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |