Published online Sep 27, 2021. doi: 10.4254/wjh.v13.i9.1019
Peer-review started: May 4, 2021
First decision: June 4, 2021
Revised: June 8, 2021
Accepted: August 9, 2021
Article in press: August 9, 2021
Published online: September 27, 2021
Processing time: 141 Days and 7.2 Hours
Herbal-induced liver injury (HILI) is an important and increasingly concerning cause of liver toxicity, and this study presents recent updates to the literature. An extensive literature review was conducted encompassing September 2019 through March 2021. Studies with clinically significant findings were analyzed and included in this review. We emphasized those studies that provided a causality assessment methodology, such as Roussel Uclaf Causality Assessment Method scores. Our review includes reports of individual herbals, including Garcinia cambogia, green tea extract, kratom as well as classes such as performance enhancing supplements, Traditional Chinese medicine, Ayurvedic medicine and herbal contamination. Newly described herbals include ashwagandha, boldo, skyfruit, and ‘Thermo gun’. Several studies discussing data from national registries, including the United States Drug-Induced Liver Injury (DILI) Network, Spanish DILI Registry, and Latin American DILI Network were incorporated. There has also been a continued interest in hepatoprotection, with promising use of herbals to counter hepatotoxicity from anti-tubercular medications. We also elucidated the current legal conversation surrounding use of herbals by presenting updates from the Federal Drug Administration. The highlights of the literature over the past year indicate interest in HILI that will continue as the supplement industry in the United States grows.
Core Tip: Herbal-induced liver injury is a growing concern worldwide with increasing rates of reported cases. Here we provide an encompassing review of reported new cases of well-established herbals along with newly described herbals causing liver injury over the past year. Causality assessment was emphasized. New studies addressing the hepatocytoprotective effects in human studies are also emphasized.
- Citation: Woo SM, Davis WD, Aggarwal S, Clinton JW, Kiparizoska S, Lewis JH. Herbal and dietary supplement induced liver injury: Highlights from the recent literature. World J Hepatol 2021; 13(9): 1019-1041
- URL: https://www.wjgnet.com/1948-5182/full/v13/i9/1019.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i9.1019
Reports of herbal-induced liver injury (HILI) and dietary and weight loss supplement liver injury (DSLI) continue to be published at an increasing rate, highlighting the growing interest in the field, as well as enhanced recognition of HILI by clinicians. For example, a routine PubMed search revealed eight systematic reviews and meta-analyses on HILI published in 2020 and four in 2019, compared to none published earlier than 2002[1-8]. In this review, we discuss the highlights chosen from the recent literature regarding HILI and DSLI liver injury since our last review period[9]. New information on the incidence of HILI and DSLI, reports of new herbal hepatotoxins and updates on previously described HILI are included, along with the current regulatory status of kratom and other agents.
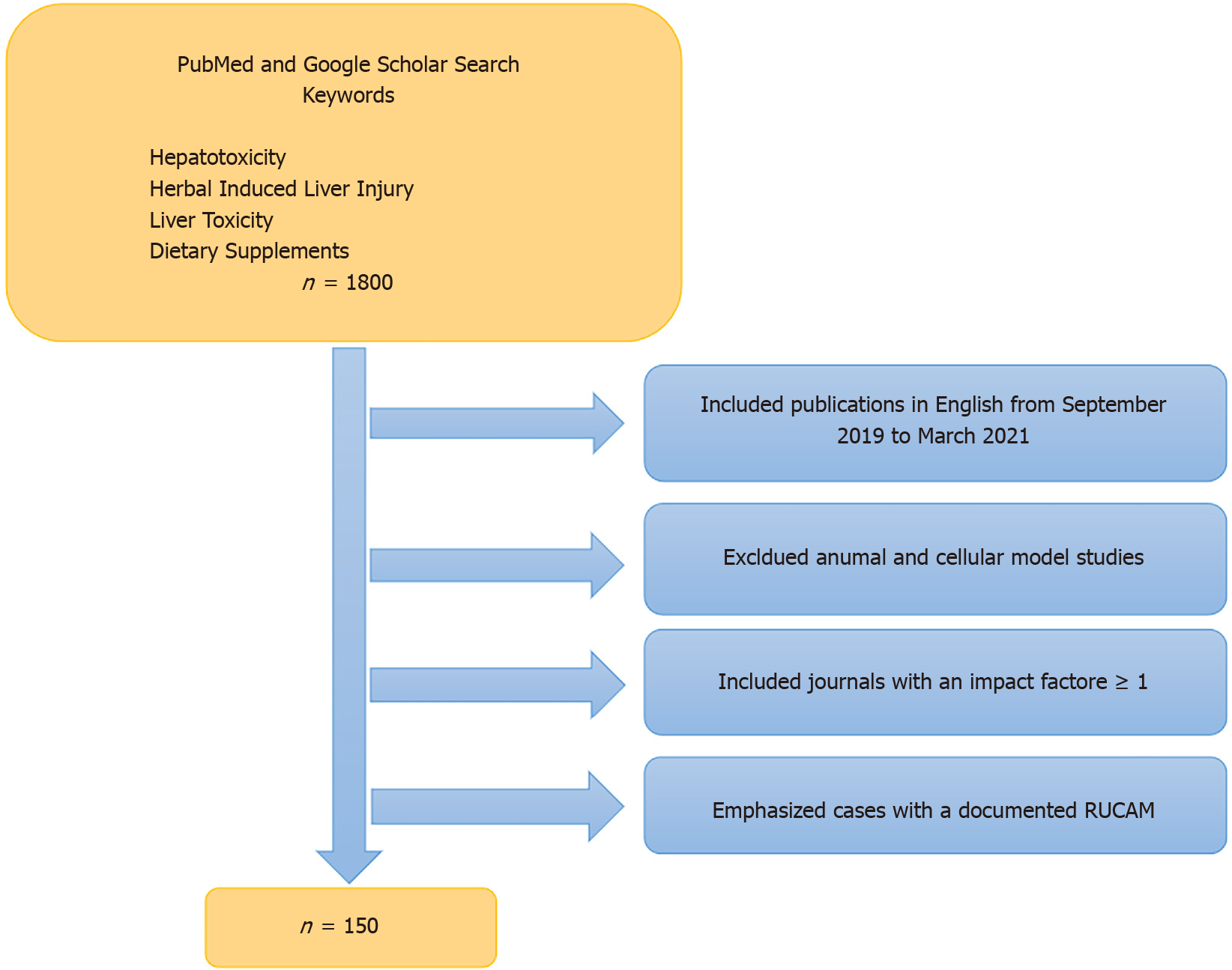
A literature review for this paper was performed utilizing PubMed and Google Scholar search engines spanning September 1, 2019 through March 31, 2021. Keywords utilized included “hepatotoxicity,” “hepatic toxicity,” “liver toxicity,” “herbal induced liver injury,” “HILI” and “dietary supplements.” Using both search engines, we came across approximately 1800 publications. In order to narrow down this extensive search we focused on case reports, case series, review articles and original research that were published in journals with an impact factor ≥ 1 based on listings contained in Scholar One[10]. Of note, seven of the 85 discrete journals that we reviewed had an impact factor < 1 or none at all. However, we felt the information within those particular articles was important enough to include in our review. The range of journal impact factors (IF) was 0.28-60 (Zhongguo Zhong Yao Za Zhi and Lancet respectively), mean IF was 5.46, and median was 3.37[10]. Additionally, we focused on recent literature reporting new cases of HILI/DSLI along with particular herbal agents of interest such as green tea extract (GTE) and kratom along with performance enhancing supplements (PES), traditional Chinese medicines (TCM) and Ayurvedic medicines. Many reports described the cytoprotective effects of herbal compounds, and we focused on those utilized in human studies. Legal and regulatory ramifications were also addressed in particular with regard to kratom. As in past years, we emphasized those studies that provided a causality assessment methodology, such as RUCAM scores, believing that this enhanced their validity[11]. Through this selection process we narrowed our review to approximately 150 publications (Figure 1). Given the number of publications reviewed, the omission of any specific article should not be viewed as lacking importance or significance.
Data on the true frequency of HILI/DSLI are generally lacking, in part due to under-diagnosis and under-reporting[12]. The incidence of HILI in mainland China, which we would expect to be among the highest worldwide, is estimated to be 6.38 per 100000 based on the large retrospective study by Shen et al[13], who described DILI incidence to be 23.8 per 100000 of which 26.8% of single agents were TCM. In the United States, the estimated incidence of HILI was 1.16 per 100000 based on a small prospective study conducted in Delaware[14]. Perhaps a better estimation for a Western country comes from a prospective population-based study from Iceland that found an incidence of 3 per 100000[15]. While these estimates are lower than China’s, HILI cases reported to the United States DILI Network have increased from 7% of all drug-induced liver injury cases in 2005 to 20% in 2014, with herbal and dietary supplements (HDS) representing the second leading class of compounds causing liver injury after antibiotics[16]. The most recent update of the United States DILI Network contained 404 cases of HILI enrolled between 2003 to 2019[17].
Registry-based frequency data demonstrates HDS responsible for 8% and 4% of DILI cases reported by the Latin DILI and Spanish DILI Networks, respectively[18]. Data from a single German hospital dedicated to TCM indicates a HILI frequency of 0.12% over a 20-year period[19]. The increasing number of reports of HILI are likely explained by the combination of more widespread HDS use as well as clinician awareness[13].
An ongoing difficulty with assessing a true incidence of HILI relates to the fact that herbal supplements commonly contain multiple ingredients, and several products are often used concurrently. As a result, it is challenging, if not impossible, to determine which specific HDS component might be responsible for the hepatotoxicity[16]. Frequent mislabeling of supplements, patient non-disclosure, and physician lack of awareness further complicate the diagnosis of HILI[16,20]. Nevertheless, it is crucial that clinicians maintain awareness of HILI, as it may have a greater potential for acute liver failure than DILI[16].
In contrast to the United States, herbal supplements undergo much more regulatory scrutiny in member states of the European Union (EU), where according to Directive 2004/24/EC, herbal medicinal products are required to not only register with the EU, but also comply with specific manufacturing and quality standards[21]. Herbal supplements are widely accessible to Americans both online and in nutrition stores and pharmacies, and their appeal is heightened by marketers portraying them as natural and healthy[22]. In 2019, Americans spent $9.6 billion on herbal supplements alone, (exclusive of vitamins and other complementary and alternative therapies, which represented an 8.6% increase from the previous year[23]. As the use of HDS continues to climb, clinicians and patients alike will be faced with the challenge of recognizing and managing potential hepatic injury. The relative lack of regulatory control over HDS in the US compared to conventional medications, means there are fewer protections available to the consumer, such as quality control.
Despite the general lack of regulation of the herbal and supplement industry, the Food and Drug Administration (FDA) does maintain a role in providing for their safety. Herbal medications and vitamin supplements have long been categorized as food supplements and thus have a lower threshold required to maintain evidence for safety[24]. This was changed when the Dietary Supplement Health and Education Act of 1994 (DSHEA) was passed that named the FDA as responsible for safety concerns and for taking action against dietary supplements if needed[25]. Unfortunately, the provision only takes effect after supplements reach the market and supplement companies are not required to register themselves or their products with the FDA before offering them for sale. Until recently, the FDA mainly monitored product information through a voluntary dietary supplement adverse reporting system and took action retroactively against companies when necessary[25,26].
In recent years however, there has been an outcry regarding the sheer number of herbal supplements that have come to market with little to no consumer protection regarding their claims[27,28]. In 2019, then FDA Commissioner, Dr. Scott Gottlieb, issued a statement announcing plans for major policy changes toward the oversight of the dietary supplement industry. This included improvements in the adverse reporting system and a proposal to require the listing of the ingredients of dietary supplements with the FDA[29]. Since then, the proposal to register ingredients of various supplements has been a primary objective of the FDA with both the 2020 and 2021 budget proposals to Congress including a provision for this. In addition, they have asked for a mandate to allow them to act against products and manufacturers providing misleading information to the FDA[30]. However, both proposals have been met with significant resistance from the industry and have yet to become enacted into law.
Diagnosing HILI remains a challenge and while there are several assessment tools used to determine causality, ultimately it is a diagnosis of exclusion[31]. Currently, Roussel Uclaf Causality Assessment Method (RUCAM), designed in 1993, is the most widely used assessment tool for determining causality[32]. Indeed, Teschke et al[33] identified 12068 HILI cases reported in the recent literature in which RUCAM was used as the basis of causality.
In another retrospective review, Teschke et al[34] analyzed 11,160 HILI cases from Asian countries - mainland China, Hong Kong and Taiwan, Korea, Singapore, and Japan - collected from 1964 to 2019. They identified China and Korea as being exemplary in their use of RUCAM to evaluate HILI cases. They suggest that RUCAM will be a particularly valuable tool when assessing causality of liver injury occurring during the COVID-19 pandemic, which may confound findings given the high incidence of liver test abnormalities associated with the infection[34,35]. Anirvan et al[36] described the effects of COVID-19 on the liver, concluding it has both direct viral cytopathic mechanisms and also acts indirectly, through immune-mediated, drug-induced, and other pathways. These investigators suggest that acute non-icteric hepatitis may precede pulmonary symptoms in COVID-19 infection[36].
RUCAM, however, is an imperfect tool, and some authors argue that it should be developed further as some of its criteria are not evidence-based[31]. For example, RUCAM does not accommodate evaluation of the several individual hepatotoxins that may comprise a single HDS[4]. Other assessment tools include the Clinical Diagnostic Scale (CDS) and Digestive Disease Week Japan 2004 Scale (DDW-J). Liu et al[37] compared RUCAM, CDS, and DDW-J in a cohort of 458 DILI patients at a hospital in Tianjin, China and found the CDS to be the most accurate in diagnosing DILI. The six variables that CDS employs are comparable to RUCAM’s seven, though the former allocates different point values for timing of drug administration to onset of symptoms in addition to assigning points for extrahepatic manifestations including rash, fevers, eosinophilia, arthralgia, and cytopenia[38]. Of note, the most common causative agents for liver injury in this cohort were TCM, used in 52.41% of patients.
This past year showed continued interest in innovative tools for diagnosing HILI. Liu et al[37] investigated the potential role of an in vitro monocyte-derived hepatocyte-like (MH) cell test in diagnosing HILI. Investigators identified 47 patients in Munich and Hong Kong who were determined by RUCAM to have had HILI. Among these patients, the MH cell test exhibited sensitivity and specificity of 90.6% and 86.7%, respectively. In a prior study, the MH cell test was shown to have higher specificity than RUCAM[39]. Thus, the MH cell test may be a valuable test in diagnosing HILI in the future.
Studies have investigated potential biomarkers for specific agents. Pyrrolizidine alkaloids (PA) are hepatotoxins commonly found in food items and herbs used in TCM, including Gynura japonica (G. japonica). Pyrrole-hemoglobin adducts and three miRNAs - has-miR-148a-3p, has-miR-362-5p, and hs-miR-194-5p - have been shown to increase diagnostic accuracy of PA-induced liver injury. Similarly, Polygonum multiflorum (P. multiflorum) is an herbal popular in TCM. Metabolomics profiling has shown to successfully differentiate between DILI caused by P. multiflorum and autoimmune hepatitis (AIH) as well as hepatitis B virus[40]. Given the widespread ingestion of PA and P. multiflorum, these pioneering diagnostic tests may help guide clinicians in managing liver injury caused by these herbals.
The United States DILI Network (DILIN) examined the association between GTE and the proinflammatory allele HLA-B*35:01 (see “Green Tea Extract”)[41]. The other update focused on ashwagandha, a popular Ayurvedic medicine using data from the United States DILIN and Iceland (see “Ayurveda”)[42].
Two updates from the Spanish DILI registry were published in 2020. While mainly focused on DILI, there is also comment on HILI. In a study of liver injury in the elderly, Weersink et al[43] found herbal products accounted for 4% of cases in younger patients, with a decreasing overall incidence with increased age. Similarly, in their comprehensive review of DILI over the span of 20 years up until 2018, Stephens et al[44] identified 843 cases of liver injury, 29 (3.4%) of which were attributable to HDS and an additional 22 (2.6%) were caused by selective androgen receptor modulators (SARMs).
The Latin DILI Network (LATINDILI) comprises a group of seven countries that collect DILI cases prospectively, using RUCAM to determine causality. Bessone et al[18] published an analysis of HDS in Latin America from 2011 to 2019, and found that, similar to the findings from the prospective Spanish DILI and United States DILIN, HILI was more common among young women attempting to lose weight[18,45,46]. Rates of acute liver failure were 17%, 16%, and 6%, respectively for the LATINDILI, DILIN, and Spanish DILI networks. In another study using LATINDILI data, Santos et al[5] reviewed 17 records of HILI and found Centiella asiatica, Carthamus tinctorius, and the weight loss supplement ‘HerbaLife’ (that previously contained GTE and ephedra), as the most common causes[47]. They also found weight loss to be the most common reason for supplement use, which was also the most common indication reported by Bessone et al[18] Interestingly, while Garcinia cambogia (G. cambogia) is the third most frequent cause of liver injury in Latin America, as reported by Bessone et al[18], it was not present in the Spanish DILI registry. The authors suspected this was due to native cultural influences and surrounding geography, as well as the growing potential of different regions.
In Malaysia, a national centralized database of hepatic adverse drug reactions sponsored by the Ministry of Health was used to collect cases retrospectively and Lee et al[47] presented data from 2000 to 2017. They presented 2090 cases of DILI, 11.24% of which were attributable to HDS. Causality was determined using WHO-Uppsala Monitoring Center criteria employed by physician and pharmacist members of the Malaysian Adverse Drug Reactions Advisory Committee (MADRAC). Of note, only 27.1% of products causing liver injury in this study were registered with the Ministry of Health, meaning the vast majority were unregulated. This highlights the similar regulatory challenges faced by authorities in Asia and in the West.
There are no significant updates to the China registry since Shen et al[13] extrapolated data from the National Health and Family Planning Commission to conduct the first nationwide study on HILI in mainland China, published in 2019. However, given the surge in literature investigating the impact of COVID-19 on liver injury, a potential confounder, we expect updates to Chinese HILI and DILI registries will be forthcoming.
Livertox is a database founded and maintained by the National Institute of Health. At present, it lists 1095 drugs, including 66 herbal and dietary supplements, and their potential for hepatotoxicity[48]. Likelihood scores are attributed to each herbal or supplement, ranging A-E, as designed by the United States DILIN to determine causality. In the LiverTox compendium, 24 (36.4%) of listed herbs or supplements have an A, B, or C rating, meaning a drug has “well known or more than 50 cases described”, “known or highly likely or 12-50 cases described”, or “probable or less than 12 cases described” to cause liver injury, respectively, based on published reports. In 2020, entries for 11 (16.6%) herbal and dietary supplements were updated on the website (Table 1).
| Herbal or supplement | Likelihood score | Last updated | Most recent citation |
| Aloe vera | B | 2016 | 2015 |
| Ashwagandha | C | 2020 | 2019 |
| Black cohosh | A | 2020 | 2019 |
| Butterbur | C | 2019 | 2018 |
| Polygonum multiflorum | A | 2020 | 2020 |
| Sho Saiko to and Dai Saiko to | B | 2020 | 2019 |
| Eugenol | C | 2019 | 2018 |
| Flavocoxid | C | 2018 | 2013 |
| Garcinia cambogia | C | 2018 | 2013 |
| Germander | A | 2018 | 2017 |
| Green tea | A | 2020 | 2020 |
| Kava | A | 2018 | 2017 |
| Kratom | B | 2020 | 2020 |
| Margosa oil | C | 2020 | 2019 |
| Noni | C | 2020 | 2017 |
| Pennyroyal oil | B | 2020 | 2017 |
| Red yeast rice | C | 2018 | 2017 |
| Skullcap | B | 2020 | 2019 |
| Usnic acid | B | 2018 | 2017 |
| Valerian | C | 2020 | 2018 |
| Move free | C | 2020 | 2018 |
| OxyELITE pro | C | 2020 | 2018 |
Peumus boldus (P. boldus) has been implicated as a cause of hepatotoxicity when consumed orally as an infusion, like a tea, especially in elderly patients[49]. The compound Epigallocatechin gallate (EGCG) has been identified as the underlying cause of hepatotoxicity[49]. Oliveira et al[49] describe a case report of an 87-year-old male patient who presented with weakness, anorexia, and jaundice. He was found to have a hepatocellular injury pattern. It was later discovered that the patient had been orally ingesting infusions of P. boldus over the past month as a treatment for dyspepsia. After exclusion of other causes of liver injury, the authors determined P. boldus was the probable cause of HILI, although they did not include a causality assessment score[49]. The patient’s liver tests returned to baseline with conservative management.
Skyfruit, also known as Xiang-tian-guo, is used to treat diabetes and hypertension, and was first reported to be hepatotoxic in 2018[50]. Since then, fewer than five case reports are documented in the literature. A 67-year-old woman with skyfruit exposure for six months and presenting with jaundice received a RUCAM score of 7, indicating ‘probable’ causality, described by Shao et al[51]. Xia et al[52] describe another case of a 63-year-old woman with a three day history of skyfruit use, who developed epigastric pain, nausea, and fever, and was given a RUCAM score of 10, indicating ‘highly probable’ causality. As diabetes and hypertension are common afflictions and clinicians become more aware of skyfruit’s hepatotoxic potential, the incidence of skyfruit-induced liver injury may increase.
Ashwagandha, from the roots of Withania somnifera, is an Ayurvedic medication used to treat anxiety, depression, and erectile dysfunction. Björnsson et al[42] published a case series, drawing from an Icelandic registry and the United States DILIN, of five patients with Ashwagandha-induced liver injury. The authors used DILI expert opinion to determine causality in these patients who developed jaundice and pruritus after a latency period ranging from two to twelve weeks. The pattern of liver injury was cholestatic or mixed and liver enzyme abnormalities self-resolved within one to five months in four of the five patients; the fifth patient was lost to follow up. Prior to this paper, only one case report had been published on the topic.
Ferreira et al[53] described a case of a 36-year-old male who presented to the hospital with jaundice one week after taking the dietary supplement ‘Thermo gun’. The authors reported no previous reports of a HILI association, but noted that oxilofrine, white willow, and caffeine could all play a possible role. Laboratory exams showed a cholestatic liver injury pattern. The drug was discontinued but the patient's liver function continued to deteriorate and he eventually developed acute liver failure. He successfully underwent liver transplantation and continued to do well at long term follow up. The authors assigned this case a RUCAM score of 7, indicating ‘probable’ causality by ‘Thermo gun’.
Kratom is a controversial herbal compound derived from Mitragyona speciosa and originating in Southeast Asia. It has dominated headlines in recent years because of its popularity as a stimulant and the associated legal ramifications of its use to reduce opiate withdrawal symptoms[54,55]. The active components are believed to be Mitragyona and 7-Hydroxymitragynine (7-HMG)[56]. Kratom has been used as a stimulant at lower doses or to treat pain and precipitate euphoria at higher doses. At even higher doses, it acts as a sedative. Although it has found use in people who suffer from opioid addiction to prevent withdrawal, at present there are no medical indications for kratom use in the United States, and the FDA has labeled it a ‘drug of concern’. Despite being banned in countries including Thailand and Malaysia, it remains widely available in the United States over the internet - although it is banned in Alabama, Arkansas, Indiana, Rhode Island, Vermont, and Wisconsin.
Despite these admonitions, it is becoming more mainstream with over $207 million in annual sales[57]. Of note, in 2021, Schimmel et al[58] published the first national survey of kratom use in the United States. Using data from the Non-Medical Use of Prescription Drugs (NMURx) Program, these investigators conducted a cross-sectional study of kratom users in the United States from 2018 to 2019, and found kratom users were more likely to be young, male, and have more severe substance abuse profiles, as measured using DAST-10, than cannabis, alcohol, or cigarette users[58]. They also estimated a prevalence of kratom use of 0.8%.
Cultural differences may influence the use of kratom, and subsequently its adverse effects. Ramanathan and McCurdy argue that kratom has been more harmful in the west as compared to Southeast Asia. These authors propose this is because western users are more likely to ingest kratom recreationally[59]. To further delineate the motivations for using kratom in their Malaysian cohort, they found that current opioid users were more likely to use kratom to ameliorate withdrawal symptoms as compared to former opioid users, who used kratom recreationally (OR 1.9, P < 0.035)[60].
In 2016, the Drug Enforcement Agency (DEA) attempted to classify kratom as a Schedule I drug, meaning it has no medical indication and high potential for abuse, alongside heroin, lysergic acid diethylamide, and methylenedioxymethamphetamine (ecstasy). However, this effort was met with pushback from lobbying groups, members of congress, and the public. A bipartisan group of senators, including Bernie Sanders and Orrin Hatch, signed a letter protesting the FDA’s immediate scheduling of kratom, and encouraged a lengthier investigation into the safety of kratom given its long history of use in other countries and growing popularity in the United States[54]. Moreover, some researchers believe that restricting kratom as a Schedule I drug would prevent advancement of research because of increased bureaucratic processes previously illustrated by studies on marijuana and psychedelic-assisted therapies[61]. Thus, kratom remains legal at the federal level, despite its known hepatotoxic potential.
Polypharmacy plays a significant role in kratom’s potential for hepatotoxicity. Mitragynine inhibits glucuronidation by UDP-glucuronosyltransferases (UGT), which may explain kratom’s increased toxicity when co-administered with other substances, such as UGT substrates including buprenorphine and ketamine[56]. Polysubstance abuse with kratom furthermore increases rates of death. The CDC collects data on death from substance abuse in the State Unintentional Drug Overdose Reporting System (SUDORS), and has investigated kratom, most recently publishing updated data in 2019[62]. Of the 27338 deaths due to overdose reported to SUDORS from July 2016 to December 2017, kratom was implicated in 152 (0.56%) cases. Among the 152 cases, medical examiners determined kratom to be a cause of death in 91 (59.9%), with kratom identified as the only substance in seven cases. Eggleston et al[63] conducted a retrospective review using kratom exposures reported to the National Poison Data System and New York State’s county medical examiner’s office records, and found 2312 cases of kratom exposure, of which 935 reported kratom as the only substance used.
The potential lethality of kratom is heightened by issues with product contamination, with both heavy metals and organisms that may cause illness. Most recently, in 2018, the FDA/DEA completed an investigation of kratom products contaminated with salmonella resulting in an outbreak affecting 199 individuals across 41 states[64]. Contaminants in kratom products were most recently found in a survey of kratom use in a Chicago suburb, which also revealed the presence of heavy metals, fungi, and bacteria[65,66].
Despite the safety concerns surrounding kratom, its popularity is continuing to rise. According to data from the System to Retrieve Information from Drug Evidence/ST
Schimmel and Dart published a review of 85 kratom cases that nicely summarizes its clinical signature with respect to liver injury[68]. Using published case reports and abstracts, cases in the United States DILIN, FDA databases, and online user forum, they found most patients presented with abdominal discomfort, jaundice, pruritus, and dark urine. While liver tests revealed a mixed injury pattern, histology often showed cholestasis. The authors were only able to calculate a RUCAM score for 20 cases, with a median modified RUCAM score of 5 and mean of 4.5 (range 1-8), indicating ‘possible’ causality.
A newly reported form of kratom-induced injury is cholestasis resembling primary biliary cholangitis. A case report by Gandhi et al[69] from India, is only the second in the literature, reported. Causality in this case was determined by clinical judgment using symptoms of nausea, decreased appetite, fatigue, and jaundice with associated elevated bilirubin levels in the setting of kratom use two weeks prior to presentation. Cholestatic liver injury consistent with primary biliary cholangitis, was confirmed by histology revealing centrilobular cholestasis, moderate chronic portal tract inflammation, and brisk lymphocytic-predominant bile duct injury. Symptoms resolved with supportive care and steroids.
Green tea is one of the most widely consumed drinks worldwide, and is not considered a hepatotoxin[70]. In contrast, GTE has gained significant popularity for its weight loss enhancing potential and can be found in over a 100 herbal preparations in varying concentrations[70]. and have been associated with the potential for hepatotoxicity[71]. A systematic review of GTE performed by the United States Pharmacopeial Convention (USP) in 2008 and revisited in 2019 urged the use of cautionary labels to warn the general public of such causal relationships[71].
The USP reviewed both human case reports and animal studies to establish the role of GTE in hepatotoxicity. EGCG, a highly bioactive phytochemical, is felt to be the main compound implicated in liver injury and is seen in approximately 10% of GTE formulations at varying concentrations[70-72]. Indeed, the concentration of EGCG has been directly correlated to risk of liver injury[71]. The review conducted by the USP of human cases determined the median intake of 720 mg/d of EGCG for at least two weeks was related to liver injury[71]. Notably, the average over-the-counter GTE supplement contains an EGCG concentration from 45-1575 mg/d[71]. In addition, the bioavailability of EGCG increases in a fasting state, increasing serum concentrations at lower consumed dosages[71].
GTE-related hepatotoxicity almost always presents as an acute hepatitis with a hepatocellular injury pattern[70,71]. While the exact pathogenesis of injury is unclear, proposed mechanisms include the interaction of cytochrome P450 and EGCG, direct mitochondrial toxicity from reactive oxygen species produced by EGCG, or possibly, bactericidal effects of EGCG causing endotoxic induced liver injury[72,73]. Additionally, there is believed to be an idiosyncratic, dose-independent cause in genetically susceptible individuals related to individual HLA phenotype[41,72].
Hoofnagle et al[41] performed a retrospective review of 1414 cases of drug induced liver injury, of which 40 were attributed to GTE. 95% of these patients had the typically hepatocellular injury pattern with 3 ultimately requiring liver transplant. Notably, an HLA analysis on these 40 patients found that 72% had HLA-B*35:01[41]. There have been reports of other drugs causing idiosyncratic liver injury related to HLA-B *35:01, including trimethoprim-sulfamethoxazole and P. multiflorum[74,75]. This pharmacogenetic association suggests there may be a possible immunologic susceptibility in GTE-related HILI.
G. cambogia is derived from the fruit of the Malabar tamarind tree found in South East Asia[76-78]. This herb continues to be an increasingly popular over the counter herbal supplement for its potential for enhancing weight loss[78,79]. Its weight loss potential stems from the active agent within G. cambogia, hydroxyl citric acid (HCA). HCA is thought to be an appetite suppressant which has demonstrated weight loss in rat models[78,80]. Additionally, HCA prohibits cholesterol and fatty acid synthesis in tissue through inhibition of adenosine triphosphate-dependent citrate lyase enzyme helping in weight reduction[78,81]. Although it is an OTC supplement, caution must be taken as there have been rare, but serious cases of serotonin syndrome, rhabdomyolysis and hepatotoxicity[78].
It has been estimated that approximately 1 in 10000 individuals using G. cambogia experience significant liver-related injury[76,78]. Onset of injury generally occurs over one week to a few months after initiation[77]. The pattern of liver injury is typically hepatocellular. This year, cases of G. cambogia -induced liver injury with a pattern similar to autoimmune hepatitis appeared[76-79]. Injury and subsequent recovery is frequently managed with abstinence from offending supplements and supportive care[77-79]. However, there have been instances of individuals requiring liver transplant or even death related to such liver injury[78]. Although the pathogenesis of liver injury is unclear, proposed mechanisms through rat models include excessive production of reactive oxygen radicals from lipid peroxidation resulting in increased oxidative stress and cytoplasmic vacuolization signaling hepatocyte injury[77,79]. Nonetheless, there is thought to be two broader mechanisms; a dose-dependent mechanism through HCA consumption and an idiosyncratic, dose-independent etiology[78].
One of the most well-known examples of G. cambogia associated hepatotoxicity was seen in the weight loss supplement, “Hydroxycut™”[81]. This product was recalled in 2009 after the FDA issued a warning of its potential hepatotoxic effects based on numerous case reports reporting severe hepatotoxicity[81,82]. Andueza et al[82] summarized 21 cases of G. cambogia related liver injury of which seven were attributed to the use of Hydroxycut. RUCAM was utilized in two of seven cases and was deemed ‘highly probable’ in both cases with a score of 9. Hydroxycut™ has been newly formulated in the absence of G. cambogia and continues to be marketed. Despite the new formulations however, new cases of Hydroxycut-related liver injury continue to be reported. Yousaf et al[77] described a tabulated summary of eight reported cases of non-G. cambogia containing Hydroxycut induced hepatotoxicity cases from found in 2010-2018. Of the eight reported cases, RUCAM was used in six, with scores ≥ 6[77]. An additional case associated with the use of “Proclinical Hydroxycut™” over a twelve weeks period presented with tremor, progressive fatigue, chest pain and hepatocellular liver injury on laboratory tests. RUCAM was 9, indicating a ‘highly probable’ causality with this new formulation of Hydroxycut[81].
In addition to the cases of liver injury from Hydroxycut, there have been other notable cases of G. cambogia-induced liver injury from other G. cambogia containing products this year[77,80,83]. Three recent cases described liver injury related to GC-containing products occurring four weeks to seven months after ingestion[77,80]. Both were in young patients and presented with hepatocellular injury patterns[77,80,83]. It is important to note, however, that the patient presenting seven months after ingestion was also taking GTE[80]. Two of the three patients ultimately required liver transplant due to failed conservative management[80,83]. RUCAM scoring was used in one of three cases, who did not require liver transplant and recovered with conservative management. The RUCAM score in this case was 9, deeming causality ‘highly probable’[77].
An additional noteworthy case was the first presentation of G. cambogia-induced liver injury with a pattern of AIH. A 39-year-old female presented with jaundice, hepatomegaly and fatigue five weeks after using “slimming tea” containing G. cambogia[76]. Liver tests demonstrated a hepatocellular injury pattern with positive ANA and anti-smooth muscle antibodies. A liver biopsy was suggestive of DILI with superimposed AIH[76]. Given these findings, the patient was treated with high-dose prednisone but relapsed after a steroid taper, and was eventually transitioned to chronic immunosuppressive agents. No causality score was presented for this patient.
The use of PES has become a billion dollar industry[84]. Usage of multiple different PES is commonplace, confounding the ability to determine causality in many cases of liver injury[84].
SARMs have become increasingly popular outside the fields of bodybuilding and professional athletics[85]. Their selective tissue effects on muscle and bone allow for the benefit of building muscle mass without unwanted side effects[86,87]. SARMs act intracellularly through the binding of androgen receptors that subsequently regulate the production of androgen genes within the cell's nucleus[87]. Due to these effects, SARMs are being actively investigated in the management of sarcopenia, osteoporosis and profound nutritional deficiency. However, they are not approved by the United States FDA for such uses[87].
In fact, the FDA warns users of such supplements due to their hepatotoxic effects[87]. Several recent reports have described both SARM-induced cholestatic as well as hepatocellular injury, all starting within two weeks to four months after ingestion[86-89]. The SARMs described in these cases were Ligandrol (Alpha Elite), RAD-140 (Alpha Bolic) and enobosarm[86-89]. Liver enzymes improved with conservative management in all cases. In the cases described by Flores et al[89], liver injury was related to Ligandrol and RAD-140, presenting five weeks and four months respectively after initial ingestion. Laboratory findings were consistent with hepatocellular and hepatocellular-cholestatic injury respectively. RUCAM scoring deemed both cases as ‘probable’. RUCAM was not used in the other cases, with causality determined simply by ruling out viral, autoimmune and possible other medication-induced liver injuries[86-88].
Stimulant workout supplements have also been implicated in DSLI[90-92]. These mixtures may vary in concentrations of ingredients or contain undeclared active ingredients that can result in harm[90]. Eiswerth et al[91] described a case of hepatocellular liver injury in a previously healthy 38-year-old male after using a popular pre-workout brand “Bucked Up.” It is thought the component “deer antler extract,” which contains insulin-like growth factor, was the culprit for such injury[8]. Liver enzymes were shown to downtrend with supportive care[90]. A RUCAM causality score was deemed ‘probable’ with a score of 7[91].
Two additional cases of pre-workout PES-induced liver injury were reported in previously healthy young adults[90,92]. In one case, the patient was found to have a cholestatic injury pattern[92]. He admitted to taking creatine, whey protein powder and “Mr. Hyde” pre-workout, containing the ingredient theacrine which was thought to be the cause of liver injury[92]. Indeed, rats exposed to theacrine in high concentrations demonstrated centrilobular hepatocellular necrosis[92]. Additionally, the co-ingestion of caffeine, which is also found in “Mr. Hyde” pre-workout, has been shown to increase the bioavailability of theacrine, potentially raising serum concentrations to hepatotoxic levels[92]. The other case described hepatocellular liver injury after ingesting of “Dust V2” pre-workout consistently for four months [90]. While the patient’s liver enzymes declined with conservative management, his clinical course was further complicated by severe aplastic anemia two months after the initial presentation requiring hematopoietic stem cell transplant[90]. RUCAM was not used to assess causality in either of these two cases.
Additional brief reports of DSLI noted on our literature review included usage of creatine and glutamine powder[84,93].
TCM aims to establish and maintain balance in patients through acupuncture, massage, tai chi, and herbals, and its influence continues to grow[94]. In 2019, TCM was officially recognized by the World Health Organization[95]. TCM is included in Chapter 26 of the 11th ICD, set to roll out in 2022, which will broaden its reach worldwide[96]. However, controversy exists over this decision, as some clinicians argue it is dangerous to perpetuate practices that are not evidence based[97].
Our search yielded 264 results on TCM published during 2020. The interested reader is referred to a comprehensive review by Pan et al[98] emphasizing the complexity of TCM agents and their mechanisms for hepatotoxicity. We will highlight a few examples of TCM liver injury.
P. multiflorum is a commonly used and widely researched herbal within TCM, with its major active ingredients being stilbene glucosides and anthroquinones[99]. Although believed to have therapeutic effects on the liver, it is also a known hepatotoxin and is the only TCM listed on LiverTox with a likelihood score of A[49]. Much of the current literature on P. multiflorum induced liver injury is focused on its mechanism of toxicity. Li et al[99] argue that P. multiflorum liver toxicity is idiosyncratic and immune-mediated, rather than direct as previously proposed in the literature. Zhang et al[100] conducted a prospective study using metabolomics to examine serum samples, and identified 25 metabolites that could distinguish between groups susceptible to or tolerant of P. multiflorum induced liver injury. In another study investigating risk factors for P. multiflorum-induced hepatotoxicity, Yang et al[101] identified HLA-B35:01 as a potential susceptibility factor.
San-Qi is a TCM that is used for hemostasis and to treat trauma and ischemic cardiovascular disease, with the main component being Panax notoginseng[102]. Two other herbals, both called Tu-San-Qi, one of which contains the pyrrolizydine alkaloid (PA)-producing G. Japonica and the other is Sedum aizoon (S. Aizoon), which does not produce PAs[102]. G. Japonica and S. Aizoon are also known to induce blood flow and detumescence as well as treat pain. The similarity of the names has led to confusion with regard to usage which has led to cases of liver injury, as PAs are known hepatotoxic agents, specifically causing hepatic sinusoidal obstruction syndrome (HSOS). A review by Zhu et al[102] identified 2156 incidences of Tu-San-Qi induced HSOS. While the authors used the ‘Nanjing Criteria’, developed by the Hepatobiliary Diseases Committee of the Chinese Society of Gastroenterology, to evaluate PA-induced HSOS, it is unclear how causality was determined for the patients identified in this study. Furthermore, the authors conceded that in many of the cases, they did not specify which agent was included in the specific formulation of Tu-San-Qi.
Bu Gu Zhi (BGZ) is a TCM used to treat osteoporosis, and the main ingredient is Psoralea corylifolia (P. corylifolia). In a retrospective review conducted by Wang et al[103], 40 cases of BGZ-induced liver injury were identified at a single hospital in Beijing. Causality was determined using presence of clinical symptoms, namely decreased appetite, dark urine, and fatigue, as well as liver enzyme abnormalities, 92% of which were consistent with hepatocellular injury. Zero patients died or required liver transplantation. This is the first study of this size examining BGZ-induced liver injury.
Rhubarb, also known as dahuang in TCM, possesses anti-inflammatory properties through its anthraquinones, specifically rhein, emodin, aloe-emodin[104]. Zhuang et al[104] reviewed the literature on the dual protective and toxic properties of rhubarb on the liver, and concluded rhubarb’s hepatotoxicity increases with higher doses and less processing of the product. More studies are required to make definitive conclusions regarding rhubarb’s effect on the liver.
Ayurveda is another form of ancient medicine, and while not as mainstream in the United States, interest in the field is growing. The practice of Ayurveda comes from India and is based on balancing the five elements to optimize bodily humors[105].
Assessing HILI due to Ayurvedic medication is difficult because labeling of ingredients is often incomplete or incorrect. In one case series, a woman is described to have developed acute liver injury after consumption of a combination powder medication called “puriyas” prescribed by a local healer[106]. When Ayurvedic ingredients are identified, the literature commonly describes ashwagandha, brahmi/gotu kola, turmeric, guggul, bakuchi, Indian senna, aloe vera, Indian mulberry, pyrrolizidine alkaloids[107].
In their case series, Karousatos et al[108] present three patients with HILI from three different Ayurvedic preparations. The medications presented were Giloy kwarth containing the hepatotoxic Tinospora cordifolia, followed by a combination of Manjishthadi kwatham and Aragwadhi kwatham, containing 52 and 10 individual plant extracts with 23 and nine known hepatotoxins, respectively, and finally Kanchnar guggulu, comprised of 10 individual plant extracts of which nine are known hepatotoxins. The individual RUCAM scores for each product ranged from 7 to 8, indicating ‘probable’ HILI. The complexity of these preparations highlights the need for clinician awareness with regard to HILI from Ayurveda.
Turmeric has been suggested to have hepatotoxic effects through its active ingredient of curcumin. Lombardi et al[109] published a series of cases of acute liver injury in Tuscany following ingestion of turmeric, using RUCAM to establish a causal relationship that was supported by a positive de-challenge response in six of seven ‘possible’ and ‘probable cases, although the actual RUCAM scores were not provided. A systematic review identified 23 cases of ‘possible’ to ‘probable’ turmeric-induced liver injury, but the majority of patients had a concomitant exposure to another medication. A case reported by Lee et al[110] described a patient who developed AIH following turmeric ingestion, established using a RUCAM of score 9, indicating turmeric was ‘highly probable’. This patient also was using piperine, and the authors propose the combined use of turmeric and piperine increased the absorption of turmeric and increased the risk for liver injury.
Ayurvedic medicine has also been shown to worsen liver injury in patients with existing liver disease. In their single-center case-control study, Philips et al[111] found that in patients diagnosed with AIH who are treated with Ayurvedic and herbal medicines, defined in this study broadly as complementary alternative medicine, had significantly worse biomarkers and changes on pathology, leading to reduced short-term survival compared to those who were treated with conventional medicine. Specifically, patients treated with polyherbal Ayurvedic compounds, which comprised the majority of complementary and alternative medicine (CAM) therapy employed, displayed significantly higher Child-Pugh, chronic liver failure, and discriminant function scores. When comparing the two groups at the end of one-, three-, and six-month follow-up periods, authors found a significantly higher mortality among CAM patients, with sepsis the most common cause of death in both groups. Authors also identified the contamination of the CAM compounds with heavy metals, antibiotics, chemotherapy agents, nonsteroidal anti-inflammatory drugs, alcohols, antidepressants, anxiolytics, and recreational drugs.
Khat is an herbal stimulant originating in Ethiopia and used in Eastern Africa, Somalia, and Yemen that can be chewed, ingested, or smoked[112]. The main active components are cathine and cathinone. A number of case reports depicting khat-induced liver injury have been published, but Argueta et al[113] present the first case of hepatotoxicity due to khat in the United States. The patient was a 28-year-old man from Yemen who presented with hepatotoxicity in the setting of regular recreational khat use until one week prior to presentation. The authors identified abnormal liver enzymes consistent with hepatocellular injury. Cessation of Khat resulted in clinical improvement, indicated a positive de-challenge response which was the basis of causality as RUCAM scoring was not mentioned. Of note, American clinicians are likely unfamiliar with the presentation of Khat, as it is illegal in the United States.
Skullcap comes from the root of Scutellaria baicalensis and is commonly used in TCM. There are previously published case reports of skullcap causing liver injury through the active ingredient wogonin. Skullcap has a designated LiverTox likelihood score of B[114]. Puri et al[115] imply that these case reports may have overstated the hepatotoxic potential of skullcap, as patients were all concurrently at least one other HDS with established association with hepatotoxicity, and conducted their own prospective study to test their hypothesis. They found that skullcap ingestion did not result in significant liver enzyme abnormalities or hepatic dysfunction.
Black cohosh, from Cimicifuga racemose, is a well-established hepatotoxin with greater than 50 cases reported cases[116]. It is native to North America and is used to treat menopausal symptoms[117]. Recent studies have investigated the effect of adding black cohosh to clomiphene to treat infertility[118,119]. Black cohosh’s main active ingredients are glycoside and terpene. Arborvitae or white cedar, from Thuja occidentalis (T. occidentalis), is a tree native to North America and is used to treat respiratory infections, uterine malignancy, amenorrhea[120]. Unlike black cohosh, arborvitae has not been described in the literature as a hepatotoxin. Arborvitae’s main active ingredient is thujone. Caruntu et al[120] present a case of a 40-year-old female from Bangladesh and living in the United States who concomitantly used black cohosh and arborvitae to increase her fertility. The combination of these herbal supplements was given a RUCAM score of 6, indicating ’probable‘ HILI. Both agents were discontinued at the same time, neither were re-challenged, and the patient showed clinical improvement. Thus, it is impossible to determine if the liver injury was caused entirely by black cohosh or if arborvitae also contributed. As such, clinicians should remain aware of the possibility of hepatotoxicity from arborvitae use.
A significant number of review articles were identified in the past year dealing with the potential protective effects of herbals on the liver. The majority of reports found however, were conducted either using in vitro or in vivo rat models. In order to provide the most relevant information to clinical practice we focused only of those herbals utilized in human studies, in particular, silibinin (milk thistle) and N-acetylcysteine (NAC) to prevent anti-tuberculosis medication liver injury and vitamin E to protect against methotrexate DILI.
Mycobacterium tuberculosis continues to be the leading cause of infection related mortality amongst adults worldwide[1]. The mainstay of treatment consists of quadruple therapy [isoniazid (INH), pyrazinamide (PZA), ethambutol and rifampin] for two months followed by rifampin and isoniazid for the remaining four months [121]. Despite adjustments in duration of treatment, the hepatotoxic effects of PZA, INH and rifampin limit their use, leading to therapy discontinuation in approximately 11% of patients[122,123]. The mechanism of hepatotoxicity of PZA and INH is thought to stem from oxidative injury and production of toxic metabolites[122]. Rifampin upregulates hepatic microsomal enzymes accelerating INH metabolism, increasing toxic metabolites thus increasing risk of liver injury[123].
Silibinin (milk thistle) is a TCM flavonoid derived from the extract of the plant Silybum marianum[123]. Goh et al[122] investigated silibinin’s hepatoprotective role against INH, PZA and combination regimen with in vitro assays as a prophylactic agent (prior to anti-TB treatment), rescue agent (given with anti-TB treatment), and as a salvage agent (given after onset of hepatotoxicity). They found that silibinin was most effective as a rescue agent by way of reducing intracellular levels of oxidative stress and oxidative damage to intracellular targets and mitochondria, leading to decreased apoptotic activity[122]. Silibinin was not effective as a prophylactic or salvage agent. Additionally, it was found that silibinin was more protective against INH alone compared to PZA or combination regimens suggesting that silibinin does not protect against PZA-induced hepatotoxicity[122].
Additionally, Singh et al[2] performed a systematic review of randomized control trials of chemoprophylaxis in the setting of four-drug regimen anti-tuberculosis treatment. They identified four trials utilizing silymarin/silibinin and three trials utilizing NAC[2]. Only one of four trials demonstrated clinically significant cytoprotection. The study in question, however, was shown to have insufficient power and was stopped prematurely for safety concerns[2]. These findings are concordant with the study performed by Goh et al[122] in which silibinin showed protection against INH, but not PZA. NAC however, showed clinically significant cytoprotection in all three studies reviewed. Its hepatoprotective effect is thought to stem from the increase in glutathione, protecting the liver against oxidative stress[2].
Sanjay et al[123] studied gallic acid, an Ayurvedic herbal medicine that is present in various fruits and vegetables in the setting of INH and rifampin DILI in Wistar rat models. Gallic acid was co-administered with INH and rifampin and was compared to both negative control and positive control (silymarin treated) models[123]. Gallic acid demonstrated a hepatic protective effect with co-administration and was comparable to the protective effect of the silymarin treated group[123]. Mechanism of action was attributed to gallic acid’s antioxidant properties by increasing expression and activation of Nrf2[123].
Methotrexate is one of the main treatments used in rheumatoid arthritis[124]. However, long term use has been associated with the development of fatty liver disease, fibrosis and cirrhosis[125]. As a result, it is often discontinued when aminotransferases reach 3× upper limit of normal (ULN) or remain persistently above 2× ULN[124]. Vitamin E has been studied for its beneficial effects in patients with non-alcoholic fatty liver disease (NAFLD), and a systematic review and meta-analysis performed by Amanullah et al[126] looked at five randomized controlled trials of adult patients with NAFLD treated with vitamin E that demonstrated biochemical and histological improvement.
Vaidya et al[124] performed a prospective open-label case-control study over a six month span evaluating the hepatoprotective effects of vitamin E in the setting of methotrexate use. Prior animal studies have demonstrated vitamin E hepatoprotection against methotrexate[124]. The groups were randomized such that each consisted of their individualized methotrexate regimen, folate 1mg/daily along with dietary and exercise advice to minimize lifestyle induced fatty liver disease. The treatment group received vitamin E 400 mg twice a day while the control group did not. This study also included a crossover design in which the control group individuals that were shown to have ≥ 1-fold but less than 3-fold rise in aminotransferase levels at the three month follow up visit were then treated with vitamin E. The study found that the vitamin E treated group had a statistically significant reduction in AST/ALT levels compared to controls. Additionally, those individuals who were crossed over to receive vitamin E also demonstrated a statistically significant decrease in AST/ALT. The authors concluded that vitamin E attenuates methotrexate-induced liver injury. A limitation of this study is not knowing if these patients had underlying fatty liver disease prior to methotrexate initiation.
Numerous additional studies were identified that investigated the hepatoprotective effects of many other herbal medications for their antioxidant, anti-inflammatory and anti-apoptotic roles. These studies were largely conducted through in vitro or in vivo rat models as mentioned above. The individual studies that may be of interest to the reader include polyphenols and acetaminophen (APAP)[127,128], Gamisou-san and APAP[129], Lycopene and tamoxifen[130], and licorice and cisplatin[131]. Additional herbal agents were identified as cytoprotective after induction of liver injury by carbon tetrachloride or APAP[132-142].
Psoralen, an organic compound found in the seeds of P. corylifolia, is known for its photosynthesizing properties used to treat psoriasis and vitiligo. Unfortunately, it has also been implicated in hepatotoxicity and is one of the key ingredients responsible for liver injury in the popular TCM, buguzhi. Britza et al[143] conducted an in vitro study using a line of liver carcinoma cells and showed that psoralen exacerbates APAP hepatotoxicity. Interestingly, when non-toxic doses of psoralen and APAP were concurrently applied to the cell cultures, they synergistically induced liver injury. These findings have yet to be applied to in vivo animal models.
Selenium is a trace element abundant in brazil nuts and fish and believed to protect against oxidative stress and infection[144]. In a cross-sectional study conducted by Aktary et al[145], a negative association was observed between selenium intake and the presence of NAFLD in a Canadian cohort. Similar findings suggesting selenium’s hepatoprotective effect was seen in multiple rat models[146,147]. However, in a population-based study in China, Wu et al[148] found a significant association between dietary selenium intake and the presence of NAFLD, consistent with a dose-response relationship. Our understanding of selenium’s effect on the liver therefore remains inconclusive.
Usnic acid derived from lichens is a well-documented agent of liver injury with first reported cases dating to 2000 in relation to the dietary supplement, LipoKinetix[149}. Approximately 21 cases of LipoKinetix-induced liver toxicity were reported leading to one death and one liver transplant[149]. This dietary supplement has since been removed from the market[150]. Usnic acid’s known mechanism of liver injury is a dose- and time-dependent manner through decoupling oxidative phosphorylation along with inducing oxidative stress through glutathione depletion[149].
Herbal products are not subjected to the same quality control measures as prescription drugs and such can lead to contaminations and subsequent liver injury. Quan et al[4] describes contaminates of herbal products as nonphyto-hepatoxins. These contaminates can be divided into heavy metals, biologic factors, pesticide and herbicidal residue[4]. Of the heavy metal arsenic, mercury, cadmium, nickel and lead are most commonly detected[4]. A study performed by Abualhasan et al[151] analyzed 18 green and herbal tea samples. Seven of 18 samples were detected to contain chromium and lead at concentrations above set limits set by WHO. In this study microbial contamination were also detected in six of these seven metal containing samples[151]. These microbial contaminations have been shown to be hepatotoxic through decreasing antioxidation, increasing lipid peroxidation and upregulating apoptotic genes.[4] Additionally, the use of pesticides and herbicides have been shown to cause hepatotoxicity through hepatic mitochondrial toxicity and obstructive cholestasis[4].
HILI continues to be a growing concern for clinicians both in the United States and worldwide. While currently considered a subtype of DILI, differences in composition, application, and outcomes of HDS compared to conventional medications indicate that HILI may deserve to be considered independently.
The lack of HDS regulation in the United States limits our understanding of their potential for hepatotoxicity. Even an accurate estimate of the incidence of HILI is difficult to ascertain, and the frequencies that are reported using registries, single center hospitals, and population-based cohorts, make them difficult to compare.
Moreover, the diagnosis of HILI remains a challenge, and while assessment tools are valuable in determining causality, even the widely applied RUCAM scale - designed to evaluate DILI - falls short of adequately evaluating HILI[31]. Complete data are required for proper utilization of RUCAM, thus highlighting the importance of prospective registries. While imperfect, the RUCAM scale is currently the most widely used tool available, and until a better alternative is developed, we encourage its continued use and refinement to help identify verifiable HILI cases[31]. Development of prospective HILI/DSLI registries in Asia would also improve the overall utility of RUCAM and provide a more reliable and standardized causality scoring system. Future studies in HILI should examine (1) causality assessment scores; (2) clinical significance of using multiple herbal ingredients simultaneously; and (3) prospective studies to better understand incidence in Western countries. By improving assessment tools and expanding the data, advocates may be able to make stronger arguments to regulatory boards in support of consumer protection laws with regard to HDS.
The use of pharmacogenetics has identified susceptibility factors to HILI in the case of GTE and HLA-B *35:01. The search for other associations showing a strong correlation to idiosyncratic HILI is ongoing[41].
The use of herbals in hepato-protection continue to show promising outcomes in preventing and/or attenuating DILI from anti-TB liver injury. Further human clinical trials are still required in order to assess the true therapeutic benefit of cytoprotective herbals in other settings.
Kratom and its legal status will undoubtedly remain a hotly debated topic in coming years, as the opioid epidemic continues. At present, kratom is legal at the federal level, but banned in several states and countries. The literature indicates kratom is potentially lethal, not only through overdose but also by contaminated products, and some degree of regulation certainly seems warranted[63-65,152]. However, it has yet to be determined which end of the spectrum, through a ban or legalization, would best serve consumers.
The highlights of the updated literature over the past year indicate interest in HILI that we expect will continue to increase as the multi-billion-dollar supplement industry in the United States grows.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: American Association for the Study of Liver Diseases, No. 251372; and American College of Gastroenterology, No. 53664.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Becker MW S-Editor: Wu YXJ L-Editor: A P-Editor: Guo X
| 1. | Shi Z, Wu J, Yang Q, Xia H, Deng M, Yang Y. Efficacy and safety of milk thistle preventive treatment of anti-tuberculosis drug-induced liver injury: A protocol for systematic review and meta-analysis. Medicine (Baltimore). 2020;99:e23674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Singh AK, Verma S, Kumar-M P, Soni H, Sharma S, Patil A, Sharma V. Appropriate chemopreventive strategy for anti-tubercular therapy related liver injury is unsettled: Results from a systematic review and network meta-analysis. Expert Rev Clin Pharmacol. 2020;13:1253-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 3. | Zi-Yu K, Feng J, Jia-Xu WU, Hui-Min L. [Network Meta-analysis of traditional Chinese medicine in treating drug-induced liver injury]. Zhongguo Zhong Yao Za Zhi. 2020;45:4746-4755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Quan NV, Dang Xuan T, Teschke R. Potential Hepatotoxins Found in Herbal Medicinal Products: A Systematic Review. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Santos G, Gasca J, Parana R, Nunes V, Schinnoni M, Medina-Caliz I, Cabello MR, Lucena MI, Andrade RJ. Profile of herbal and dietary supplements induced liver injury in Latin America: A systematic review of published reports. Phytother Res. 2021;35:6-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Xiao Z, Jiang Y, Chen XF, Wang CQ, Xu WH, Liu Y, Hu SS, Huang XR, Shan LJ, Tang YH, Yang YB, Feng JH, Xiao X, Li XF. The Hepatorenal Toxicity and Tumor Response of Chemotherapy With or Without Aidi Injection in Advanced Lung Cancer: A Meta-Analysis of 80 Randomized Controlled Trials. Clin Ther. 2020;42:515-543.e31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Zhu J, Chen M, Borlak J, Tong W. The landscape of hepatobiliary adverse reactions across 53 herbal and dietary supplements reveals immune-mediated injury as a common cause of hepatitis. Arch Toxicol. 2020;94:273-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Low EXS, Zheng Q, Chan E, Lim SG. Drug induced liver injury: East vs West – a systematic review and meta-analysis. Clin Mol Hepatol. 2020;26:142-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Rosenberg J, Higley C, Shabazi S, Lewis J. Selected Highlights and Controversies of Drug-Induced Liver Injury from the Recent Literature. World J Gastroenterol Hepatol Endosc Res. 2020;1:1-16. |
| 10. | Professor E. Journal Impact Factor List. 2020. Available from: https://www.scopusjournals.com/2020/07/journal-impact-factor.html#1. [DOI] [Full Text] |
| 11. | Real M, Barnhill MS, Higley C, Rosenberg J, Lewis JH. Drug-Induced Liver Injury: Highlights of the Recent Literature. Drug Saf. 2019;42:365-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 12. | Kennedy J. Herb and supplement use in the US adult population. Clin Ther. 2005;27:1847-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 205] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 13. | Shen T, Liu Y, Shang J, Xie Q, Li J, Yan M, Xu J, Niu J, Liu J, Watkins PB, Aithal GP, Andrade RJ, Dou X, Yao L, Lv F, Wang Q, Li Y, Zhou X, Zhang Y, Zong P, Wan B, Zou Z, Yang D, Nie Y, Li D, Wang Y, Han X, Zhuang H, Mao Y, Chen C. Incidence and Etiology of Drug-Induced Liver Injury in Mainland China. Gastroenterology. 2019;156:2230-2241.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 384] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 14. | Vega M, Verma M, Beswick D, Bey S, Hossack J, Merriman N, Shah A, Navarro V; Drug Induced Liver Injury Network (DILIN). The Incidence of Drug- and Herbal and Dietary Supplement-Induced Liver Injury: Preliminary Findings from Gastroenterologist-Based Surveillance in the Population of the State of Delaware. Drug Saf. 2017;40:783-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 15. | Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419-1425, 1425.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 581] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 16. | Zheng E, Sandhu N, Navarro V. Drug-induced Liver Injury Secondary to Herbal and Dietary Supplements. Clin Liver Dis. 2020;24:141-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | J, Odin JA, Hayashi PH, Fontana RJ, Conjeevaram H, Avula B, Khan IA, Barnhart H, Vuppalanchi R, Navarro VJ; Drug-Induced Liver Injury Network. Liver injury associated with kratom, a popular opioid-like product: Experience from the U.S. drug induced liver injury network and a review of the literature. Drug Alcohol Depend. 2021;218:108426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Bessone F, García-Cortés M, Medina-Caliz I, Hernandez N, Parana R, Mendizabal M, Schinoni MI, Ridruejo E, Nunes V, Peralta M, Santos G, Anders M, Chiodi D, Tagle M, Montes P, Carrera E, Arrese M, Lizarzabal MI, Alvarez-Alvarez I, Caballano-Infantes E, Niu H, Pinazo J, Cabello MR, Lucena MI, Andrade RJ. Herbal and Dietary Supplements-Induced Liver Injury in Latin America: Experience From the Latindili Network. Clin Gastroenterol Hepatol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 19. | Melchart D, Hager S, Albrecht S, Dai J, Weidenhammer W, Teschke R. Herbal Traditional Chinese Medicine and suspected liver injury: A prospective study. World J Hepatol. 2017;9:1141-1157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (2)] |
| 20. | Gardiner P, Sadikova E, Filippelli AC, White LF, Jack BW. Medical reconciliation of dietary supplements: don't ask, don't tell. Patient Educ Couns. 2015;98:512-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | European Commission. Herbal medicinal products. Medicinal Products. 2014. Available from: https://ec.europa.eu/health/human-use/herbal-medicines_en. |
| 22. | McGroarty B. Wellness Industry Statistics and Facts. Global Wellness Institute. October 2019. Available from: https://globalwellnessinstitute.org/press-room/statistics-and-facts/. |
| 23. | Smith T, May G, Eckl V, Morton C. MARKET REPORT US Sales of Herbal Supplements Increase by 8.6% in 2019 CBD, mushroom, and elderberry supplements continue to drive sales. Published online 2020. [cited 4 February 2021]. Available from: www.herbalgram.org. |
| 24. | Goldman P. Herbal medicines today and the roots of modern pharmacology. Ann Intern Med. 2001;135:594-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Navarro VJ. Herbal and dietary supplement hepatotoxicity. Semin Liver Dis. 2009;29:373-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | FDA. Dietary Supplements. FDA Food. 16 August 2019. Available from: https://www.fda.gov/food/dietary-supplements. |
| 27. | Himes DO, Clayton MF, Donaldson GW, Ellington L, Buys SS, Kinney AY. Breast Cancer Risk Perceptions among Relatives of Women with Uninformative Negative BRCA1/2 Test Results: The Moderating Effect of the Amount of Shared Information. J Genet Couns. 2016;25:258-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | McGinley L. FDA Launches Tougher Oversight of Supplements. The Washington Post. 11 February 2019. Available from: https://www.washingtonpost.com/health/2019/02/11/fda-launches-tougher-oversight-supplements/. |
| 29. | Gottlieb S. Statement from FDA Commissioner Scott Gottlieb, M.D., on the agency’s new efforts to strengthen regulation of dietary supplements by modernizing and reforming FDA’s oversight. FDA Press Announcements. 11 February 2019. Available from: https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-agencys-new-efforts-strengthen-regulation-dietary. |
| 30. | United States Environment Protection Agency. FY 2020 Justification of Appropriation Estimates for the Committee on Appropriations. Available from: https://www.epa.gov/planandbudget/fy-2020-justification-appropriation-estimates-committee-appropriations. |
| 31. | Garcia-Cortes M, Robles-Diaz M, Stephens C, Ortega-Alonso A, Lucena MI, Andrade RJ. Drug induced liver injury: an update. Arch Toxicol. 2020;94:3381-3407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 155] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 32. | Teschke R, Danan G. Worldwide Use of RUCAM for Causality Assessment in 81,856 Idiosyncratic DILI and 14,029 HILI Cases Published 1993-Mid 2020: A Comprehensive Analysis. Medicines (Basel). 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 33. | Teschke R, Schulze J, Eickhoff A, Danan G. Drug Induced Liver Injury: Can Biomarkers Assist RUCAM in Causality Assessment? Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Teschke R, Zhu Y, Jing J. Herb-induced Liver Injury in Asia and Current Role of RUCAM for Causality Assessment in 11,160 Published Cases. J Clin Transl Hepatol. 2020;8:200-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Chen F, Chen W, Chen J, Xu D, Xie W, Wang X, Xie Y. Clinical features and risk factors of COVID-19-associated liver injury and function: A retrospective analysis of 830 cases. Ann Hepatol. 2021;21:100267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 36. | Anirvan P, Bharali P, Gogoi M, Thuluvath PJ, Singh SP, Satapathy SK. Liver injury in COVID-19: The hepatic aspect of the respiratory syndrome - what we know so far. World J Hepatol. 2020;12:1182-1197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 37. | Liu Y, Li P, Wang F, Liu L, Zhang Y, Liu Y, Shi R. Comparison of diagnostic accuracy of 3 diagnostic criteria combined with refined pathological scoring system for drug-induced liver injury. Medicine (Baltimore). 2020;99:e22259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Tillmann HL, Suzuki A, Barnhart HX, Serrano J, Rockey DC. Tools for causality assessment in drug-induced liver disease. Curr Opin Gastroenterol. 2019;35:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Benesic A, Leitl A, Gerbes AL. Monocyte-derived hepatocyte-like cells for causality assessment of idiosyncratic drug-induced liver injury. Gut. 2016;65:1555-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 40. | Huang Y, Zhao X, Zhang ZT, Chen SS, Li SS, Shi Z, Jing J, Huang A, Guo YM, Bai ZF, Zou ZS, Xiao XH, Wang JB, Niu M. Metabolomics Profiling and Diagnosis Biomarkers Searching for Drug-Induced Liver Injury Implicated to Polygonum multiflorum: A Cross-Sectional Cohort Study. Front Med (Lausanne). 2020;7:592434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Hoofnagle JH, Bonkovsky HL, Phillips EJ, Li YJ, Ahmad J, Barnhart H, Durazo F, Fontana RJ, Gu J, Khan I, Kleiner DE, Koh C, Rockey DC, Seeff LB, Serrano J, Stolz A, Tillmann HL, Vuppalanchi R, Navarro VJ; Drug-Induced Liver Injury Network. HLA‐B*35:01 and Green Tea Induced Liver Injury. Hepatol. 2021;73:2484-2493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 42. | Björnsson HK, Björnsson ES, Avula B, Khan IA, Jonasson JG, Ghabril M, Hayashi PH, Navarro V. Ashwagandha-induced liver injury: A case series from Iceland and the US Drug-Induced Liver Injury Network. Liver Int. 2020;40:825-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (2)] |
| 43. | Weersink RA, Alvarez-Alvarez I, Medina-Cáliz I, Sanabria-Cabrera J, Robles-Díaz M, Ortega-Alonso A, García-Cortés M, Bonilla E, Niu H, Soriano G, Jimenez-Perez M, Hallal H, Blanco S, Kaplowitz N, Lucena MI, Andrade RJ. Clinical Characteristics and Outcome of Drug-Induced Liver Injury in the Older Patients: From the Young-Old to the Oldest-Old. Clin Pharmacol Ther. 2021;109:1147-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | Stephens C, Robles-Diaz M, Medina-Caliz I, Grove JI, Ortega-Alonso A, Lucena MI, Aithal GP. Comprehensive analysis and insights gained from long-term experience of the Spanish DILI registry. J Hepatol. 2021;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | Navarro VJ, Barnhart H, Bonkovsky HL, Davern T, Fontana RJ, Grant L, Reddy KR, Seeff LB, Serrano J, Sherker AH, Stolz A, Talwalkar J, Vega M, Vuppalanchi R. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014;60:1399-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 284] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 46. | Medina-Caliz I, Garcia-Cortes M, Gonzalez-Jimenez A, Cabello MR, Robles-Diaz M, Sanabria-Cabrera J, Sanjuan-Jimenez R, Ortega-Alonso A, García-Muñoz B, Moreno I, Jimenez-Perez M, Fernandez MC, Ginés P, Prieto M, Conde I, Hallal H, Soriano G, Roman E, Castiella A, Blanco-Reina E, Montes MR, Quiros-Cano M, Martin-Reyes F, Lucena MI, Andrade RJ; Spanish DILI Registry. Herbal and Dietary Supplement-Induced Liver Injuries in the Spanish DILI Registry. Clin Gastroenterol Hepatol. 2018;16:1495-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 47. | Lee FY, Wong HS, Chan HK, Mohamed Ali N, Abu Hassan MR, Omar H, Abdul Mutalib NA. Hepatic adverse drug reactions in Malaysia: An 18-year review of the national centralized reporting system. Pharmacoepidemiol Drug Saf. 2020;29:1669-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012 . [PubMed] |
| 49. | Oliveira Sá A, Pimentel T, Oliveira N. Boldo-Induced Hepatotoxicity: A Case of Unexplained Jaundice. Eur J Case Rep Intern Med. 2020;7:002116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 50. | Yeap V, Tan TJY, Loh T, Kumar R. Liver failure associated with mahogany seed extract consumption. BMJ Case Rep. 2018;bcr-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 51. | Shao YM, Zhang Y, Yin X, Qin TT, Jin QL, Wen XY. Herb-induced autoimmune-like hepatitis associated with Xiang-tian-guo (Swietenia macrophylla seeds): A case report and literature review. Medicine (Baltimore). 2021;100:e24045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Xia C, Liu Y, Yao H, Zhu W, Ding J, Jin J. Causality assessment of skyfruit-induced liver injury using the updated RUCAM: a case report and review of the literature. J Int Med Res. 2020;48:300060520917569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 53. | Ferreira GSA, Watanabe ALC, Trevizoli NC, Jorge FMF, Diaz LGG, Couto CF, Lima LV, Raupp DRL, Araujo BE. Acute Liver Failure Caused by Use of Fat Burner: A Case Report. Transplant Proc. 2020;52:1409-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 54. | Oppel RJ, Kovaleski SF. Opioid Users Call Kratom a Godsend. The F.D.A. Says It’s a Menace. The New York Times. 17 April 2019. Available from: https://www.nytimes.com/2019/04/17/us/kratom-overdose-deaths.html. |
| 55. | Marshall E. The Rise and Decline of Temik: Once hailed as a miracle pest killer and still warmly regarded by farmers, it is being investigated, denounced, and threatened with federal controls. Science. 1985;229:1369-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Eastlack SC, Cornett EM, Kaye AD. Kratom-Pharmacology, Clinical Implications, and Outlook: A Comprehensive Review. Pain Ther. 2020;9:55-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 57. | Han C, Schmitt J, Gilliland KM. DARK Classics in Chemical Neuroscience: Kratom. ACS Chem Neurosci. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 58. | Schimmel J, Amioka E, Rockhill K, Haynes CM, Black JC, Dart RC, Iwanicki JL. Prevalence and description of kratom (Mitragyna speciosa) use in the United States: a cross-sectional study. Addiction. 2021;116:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 59. | Ramanathan S, McCurdy CR. Kratom (Mitragyna speciosa): worldwide issues. Curr Opin Psychiatry. 2020;33:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 60. | Singh D, Yeou Chear NJ, Narayanan S, Leon F, Sharma A, McCurdy CR, Avery BA, Balasingam V. Patterns and reasons for kratom (Mitragyna speciosa) use among current and former opioid poly-drug users. J Ethnopharmacol. 2020;249:112462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 61. | Prozialeck WC, Avery BA, Boyer EW, Grundmann O, Henningfield JE, Kruegel AC, McMahon LR, McCurdy CR, Swogger MT, Veltri CA, Singh D. Kratom policy: The challenge of balancing therapeutic potential with public safety. Int J Drug Policy. 2019;70:70-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 62. | Olsen EO, O'Donnell J, Mattson CL, Schier JG, Wilson N. Notes from the Field: Unintentional Drug Overdose Deaths with Kratom Detected - 27 States, July 2016-December 2017. MMWR Morb Mortal Wkly Rep. 2019;68:326-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 63. | Eggleston W, Stoppacher R, Suen K, Marraffa JM, Nelson LS. Kratom Use and Toxicities in the United States. Pharmacotherapy. 2019;39:775-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 64. | FDA. FDA Investigated Multistate Outbreak of Salmonella Infections Linked to Products Reported to Contain Kratom. FDA Outbreaks of Foodborne Illnesses. 29 June 2018. Available from: https://www.fda.gov/food/outbreaks-foodborne-illness/fda-investigated-multistate-outbreak-salmonella-infections-linked-products-reported-contain-kratom. |
| 65. | Prozialeck WC, Edwards JR, Lamar PC, Plotkin BJ, Sigar IM, Grundmann O, Veltri CA. Evaluation of the Mitragynine Content, Levels of Toxic Metals and the Presence of Microbes in Kratom Products Purchased in the Western Suburbs of Chicago. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 66. | Kuehn B. Kratom-Related Deaths. JAMA. 2019;321:1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 67. | DEA. KRATOM. DEA Diversion Control Division. November 2019. Available from: https://www.deadiversion.usdoj.gov/drug_chem_info/kratom.pdf. |
| 68. | Schimmel J, Dart RC. Kratom (Mitragyna Speciosa) Liver Injury: A Comprehensive Review. Drugs. 2020;80:263-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 69. | Gandhi D, Ahuja K, Quade A, Batts KP, Patel L. Kratom induced severe cholestatic liver injury histologically mimicking primary biliary cholangitis: A case report. World J Hepatol. 2020;12:863-869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 70. | LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Disease; 2012-Green Tea. 2020 . [PubMed] |
| 71. | Oketch-Rabah HA, Roe AL, Rider CV, Bonkovsky HL, Giancaspro GI, Navarro V, Paine MF, Betz JM, Marles RJ, Casper S, Gurley B, Jordan SA, He K, Kapoor MP, Rao TP, Sherker AH, Fontana RJ, Rossi S, Vuppalanchi R, Seeff LB, Stolz A, Ahmad J, Koh C, Serrano J, Low Dog T, Ko R. United States Pharmacopeia (USP) comprehensive review of the hepatotoxicity of green tea extracts. Toxicol Rep. 2020;7:386-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 72. | Teschke R, Xuan TD. Suspected herb induced liver injury by green tea extracts: Critical review and case analysis applying RUCAM for causality assessment. Japanese J Gastroenterol Hepatol. 2019;6:1-16. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 73. | European Symposium on Animal, Plant and Microbial Toxins. Basel, Switzerland, 25-28 August 1996. Abstracts. Toxicon. 1997;35:801-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 74. | Li YJ, Phillips EJ, Dellinger A, Nicoletti P, Schutte R, Li D, Ostrov DA, Fontana RJ, Watkins PB, Stolz A, Daly AK, Aithal GP, Barnhart H, Chalasani N; Drug-induced Liver Injury Network. Human Leukocyte Antigen B*14:01 and B*35:01 Are Associated With Trimethoprim-Sulfamethoxazole Induced Liver Injury. Hepatology. 2021;73:268-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 75. | Li C, Rao T, Chen X, Zou Z, Wei A, Tang J, Xiong P, Li P, Jing J, He T, Bai Z, Yin J, Tan Z, Yu P, Zhou H, Wang J, Xiao X, Ouyang D. HLA‐B*35:01 Allele Is a Potential Biomarker for Predicting Polygonum multiflorum –Induced Liver Injury in Humans. Hepatology. 2019;70:346-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 76. | Malik N, Brandt L, Timsar AK. First Case of Garcinia Camogio (GC)-Induced Autoimmune Hepatitis (AIH). Am J Gastroenterol. 2019;s1043. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 77. | Yousaf MN, Chaudhary FS, Hodanazari SM, Sittambalam CD. Hepatotoxicity associated with Garcinia cambogia: A case report. World J Hepatol. 2019;11:735-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 78. | LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestion and Kidney Disease; 2012-. Garcinia Cambogia. 2019 February 13. |
| 79. | Erratum for the Research Article: "A rapid triage test for active pulmonary tuberculosis in adult patients with persistent cough" by R. Ahmad, L. Xie, M. Pyle, M. F. Suarez, T. Broger, D. Steinberg, S. M. Ame, M. G. Lucero, M. J. Szucs, M. MacMullan, F. S. Berven, A. Dutta, D. M. Sanvictores, V. L. Tallo, R. Bencher, D. P. Eisinger, U. Dhingra, S. Deb, S. M. Ali, S. Mehta, W. W. Fawzi, I. D. Riley, S. Sazawal, Z. Premji, R. Black, C. J. L. Murray, B. Rodriguez, S. A. Carr, D. R. Walt, M. A. Gillette. Sci Transl Med. 2019;11: eaaz9925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 80. | Ferreira V, Mathieu A, Soucy G, Giard JM, Erard-Poinsot D. Acute Severe Liver Injury Related to Long-Term Garcinia cambogia Intake. ACG Case Rep J. 2020;7:e00429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 81. | Khetpal N, Mandzhieva B, Shahid S, Khetpal A, Jain AG. Not All Herbals are Benign: A Case of Hydroxycut-induced Acute Liver Injury. Cureus. 2020;12:e6870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 82. | Andueza N, Giner RM, Portillo MP. Risks Associated with the Use of Garcinia as a Nutritional Complement to Lose Weight. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 83. | McCarthy RE, Bowen DG, Strasser SI, McKenzie C. The dangers of herbal weight loss supplements: a case report of drug-induced liver injury secondary to Garcinia cambogia ingestion. Pathology. 2021;53:545-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 84. | Villavicencio Kim J, Wu GY. Body Building and Aminotransferase Elevations: A Review. J Clin Transl Hepatol. 2020;8:161-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 85. | Richards JR, Scheerlinck PH, Owen KP, Colby DK. Bodybuilding supplements leading to copper toxicity, encephalopathy, fulminant hepatic failure and rhabdomyolysis. Am J Emerg Med. 2020;38:2487.e1-2487.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 86. | Barbara M, Dhingra S, Mindikoglu AL. Drug-Induced Liver Injury Associated With Alpha Bolic (RAD-140) and Alpha Elite (RAD-140 and LGD-4033). ACG Case Rep J. 2020;7:e00409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 87. | Barbara M, Dhingra S, Mindikoglu AL. Ligandrol (LGD-4033)-Induced Liver Injury. ACG Case Rep J. 2020;7:e00370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 88. | Bedi H, Hammond C, Lond M. Drug-Induced Liver Injury From Enobosarm (Ostarine), a Selective Androgen Receptor Modulator. ACG Case Rep J. 2021;8:7-9. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 89. | Flores JE, Chitturi S, Walker S. Drug-Induced Liver Injury by Selective Androgenic Receptor Modulators. Hepatol Commun. 2020;4:450-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 90. | Bastola S, Kc O, Khanal S, Halalau A. Hepatitis-associated aplastic anemia from workout supplement: Rare but potentially fatal entity. SAGE Open Med Case Rep. 2020;8:2050313X20901937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 91. | Eiswerth M, Heckroth MA, Yacyshyn BR. The Buck Stops Here: A Case of Deer Antler Extract-Induced Liver Injury. Ghady Moafa. 2020;115:1308-1309. [DOI] [Full Text] |
| 92. | Romick J, Viswanathan L, Ramos B, Grant D, Badal BD, Wellner M. Pre-Workout Peril: Drug-Induced Liver Injury in a Young Deployed Service Member. Am J Gastroenterol. 2020;115:S1365-S1366. [DOI] [Full Text] |
| 93. | Hatami B, Saffaei A, Jamali F, Abbasinazari M. Glutamine powder-induced hepatotoxicity: it is time to understand the side effects of sports nutritional supplements. Gastroenterol Hepatol Bed Bench. 2020;13:86-89. [PubMed] |
| 94. | Hopp C, Schutleff D. Traditional Chinese Medicine: What You Need To Know. Available from: https://www.nccih.nih.gov/health/traditional-chinese-medicine-what-you-need-to-know. |
| 95. | Sung WS, Jeon SR, Hong YJ, Kim TH, Shin S, Lee HJ, Seo BK, Park YC, Kim EJ, Nam DW. Efficacy, safety, and cost-effectiveness analysis of adjuvant herbal medicine treatment, Palmijihwang-hwan, for chronic low back pain: a study protocol for randomized, controlled, assessor-blinded, multicenter clinical trial. Trials. 2019;20:778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 96. | Cyranoski D. Why Chinese medicine is heading for clinics around the world. Nature News Feature. 26 December 2018. Available from: https://www.nature.com/articles/d41586-018-06782-7. |
| 97. | Eigenschink M, Dearing L, Dablander TE, Maier J, Sitte HH. A critical examination of the main premises of Traditional Chinese Medicine. Wien Klin Wochenschr. 2020;132:260-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 98. | Pan X, Zhou J, Chen Y, Xie X, Rao C, Liang J, Zhang Y, Peng C. Classification, hepatotoxic mechanisms, and targets of the risk ingredients in traditional Chinese medicine-induced liver injury. Toxicol Lett. 2020;323:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 99. | Li D, Yang M, Zuo Z. Overview of Pharmacokinetics and Liver Toxicities of Radix Polygoni Multiflori. Toxins (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 100. | Zhang L, Niu M, Wei AW, Tang JF, Tu C, Bai ZF, Zou ZS, Xiao XH, Liu YP, Wang JB. Risk profiling using metabolomic characteristics for susceptible individuals of drug-induced liver injury caused by Polygonum multiflorum. Arch Toxicol. 2020;94:245-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 101. | Yang WN, Pang LL, Zhou JY, Qiu YW, Miao L, Wang SY, Liu XZ, Tan KA, Shi WW, Wang GQ, Hou FQ. Single-nucleotide polymorphisms of HLA and Polygonum multiflorum -induced liver injury in the Han Chinese population. World J Gastroenterol. 2020;26:1329-1339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 102. | Zhu L, Zhang CY, Li DP, Chen HB, Ma J, Gao H, Ye Y, Wang JY, Fu PP, Lin G. Tu-San-Qi (Gynura japonica): The culprit behind pyrrolizidine alkaloid-induced liver injury in China. Acta Pharmacol Sin. 2021;42:1212-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 103. | Wang L, Wang Y, Wee A, Soon G, Gouw ASH, Yang R, Tian Q, Liu L, Ma H, Zhao X. Clinicopathological features of Bu Gu Zhi-induced liver injury, a long-term follow-up cohort study. Liver Int. 2020;40:571-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 104. | Zhuang T, Gu X, Zhou N, Ding L, Yang L, Zhou M. Hepatoprotection and hepatotoxicity of Chinese herb Rhubarb (Dahuang): How to properly control the "General (Jiang Jun)" in Chinese medical herb. Biomed Pharmacother. 2020;127:110224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 105. | Mukherjee PK, Harwansh RK, Bahadur S, Banerjee S, Kar A, Chanda J, Biswas S, Ahmmed SM, Katiyar CK. Development of Ayurveda – Tradition to trend. J Ethnopharmacol. 2017;197:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 106. | Paudyal B, Thapa A, Sigdel KR, Adhikari S, Basnyat B. Adverse events with ayurvedic medicines- possible adulteration and some inherent toxicities. Wellcome Open Res. 2019;4:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 107. | Philips CA, Ahamed R, Rajesh S, George T, Mohanan M, Augustine P. Comprehensive review of hepatotoxicity associated with traditional Indian Ayurvedic herbs. World J Hepatol. 2020;12:574-595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (3)] |
| 108. | Karousatos CM, Lee JK, Braxton DR, Fong TL. Case series and review of Ayurvedic medication induced liver injury. BMC Complement Med Ther. 2021;21:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 109. | Lombardi N, Crescioli G, Maggini V, Ippoliti I, Menniti-Ippolito F, Gallo E, Brilli V, Lanzi C, Mannaioni G, Firenzuoli F, Vannacci A. Acute liver injury following turmeric use in Tuscany: An analysis of the Italian Phytovigilance database and systematic review of case reports. Br J Clin Pharmacol. 2021;87:741-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 110. | Lee BS, Bhatia T, Chaya CT, Wen R, Taira MT, Lim BS. Autoimmune Hepatitis Associated With Turmeric Consumption. ACG Case Rep J. 2020;7:e00320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 111. | Philips CA, Rajesh S, George T, Ahamed R, Kumbar S, Augustine P. Outcomes and Toxicology of Herbal Drugs in Alcoholic Hepatitis - A Single Center Experience from India. J Clin Transl Hepatol. 2019;7:329-340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 112. | DEA. Khat. DEA Drug Fact Sheet. April 2020. Available from: https://www.dea.gov/factsheets/khat. |
| 113. | Palacios Argueta P, Attar B, Sikavi C, Alagiozian-Angelova V, Mishra S. Drug-Induced Liver Injury Caused by "Khat," an Herbal Stimulant. ACG Case Rep J. 2020;7:e00480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 114. | LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestion and Kidney Disease; 2012-. Skullcap. 2020 March 28. |
| 115. | Puri BK, White N, Monro JA. The effect of supplementation with Scutellaria baicalensis on hepatic function. Med Hypotheses. 2019;133:109402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 116. | LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestion and Kidney Disease; 2012-. Black Cohosh. 2020 November 4. |
| 117. | Black Cohosh. Available from: https://ods.od.nih.gov/factsheets/BlackCohosh-HealthProfessional/. |
| 118. | Shahin AY, Mohammed SA. Adding the phytoestrogen Cimicifugae Racemosae to clomiphene induction cycles with timed intercourse in polycystic ovary syndrome improves cycle outcomes and pregnancy rates - a randomized trial. Gynecol Endocrinol. 2014;30:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 119. | Shahin AY, Ismail AM, Shaaban OM. Supplementation of clomiphene citrate cycles with Cimicifuga racemosa or ethinyl oestradiol--a randomized trial. Reprod Biomed Online. 2009;19:501-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 120. | Caruntu S, Ciceu A, Olah NK, Don I, Hermenean A, Cotoraci C. Thuja occidentalis L. (Cupressaceae): Ethnobotany, Phytochemistry and Biological Activity. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 121. | Zha BS, Nahid P. Treatment of Drug- Susceptible Tuberculosis. Clin Chest Med. 2019;40:763-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 122. | Goh ZH, Tee JK, Ho HK. An Evaluation of the In Vitro Roles and Mechanisms of Silibinin in Reducing Pyrazinamide- and Isoniazid-Induced Hepatocellular Damage. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 123. | Sanjay S, Girish C, Toi PC, Bobby Z. Gallic acid attenuates isoniazid and rifampicin-induced liver injury by improving hepatic redox homeostasis through influence on Nrf2 and NF-κB signalling cascades in Wistar Rats. J Pharm Pharmacol. 2021;73:473-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 124. | Vaidya B, Bhochhibhoya M, Nakarmi S. Efficacy of Vitamin E in Methotrexate-Induced Hepatotoxicity in Rheumatoid Arthritis : An Open-Label Case-Control Study. Int J Rheumatol. 2020;2020:5723485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 125. | LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestion and Kidney Disease; 2012-. Methotrexate. 2020 February 19. |
| 126. | Amanullah I, Khan YH, Anwar I, Gulzar A, Mallhi TH, Raja AA. Effect of vitamin E in non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomised controlled trials. Postgrad Med J. 2019;95:601-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 127. | Liu FC, Yu HP, Chou AH, Lee HC, Liao CC. Corilagin reduces acetaminophen-induced hepatotoxicity through MAPK and NF- κ B signaling pathway in a mouse model. Am J Transl Res. 2020;12:5597-5607. [PubMed] |
| 128. | Hu F, Guo Q, Wei M, Huang Z, Shi L, Sheng Y, Ji L. Chlorogenic acid alleviates acetaminophen-induced liver injury in mice via regulating Nrf2-mediated HSP60-initiated liver inflammation. Eur J Pharmacol 2020; 883: 173286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 129. | Jin SE, Shin HK, Ha H. Hepatoprotective effects of Gamisoyo-san against acetaminophen-induced liver injuries. Integr Med Res. 2021;10:100466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 130. | Adikwu E, Ebinyo NC, Benalayefa O. Protective effect of lycopene against tamoxifen-induced hepatotoxicity in albino rats. Biomed Biotechnol Res J. 2020;4:69-75. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 131. | Man Q, Deng Y, Li P, Ma J, Yang Z, Yang X, Zhou Y, Yan X. Licorice Ameliorates Cisplatin-Induced Hepatotoxicity Through Antiapoptosis, Antioxidative Stress, Anti-Inflammation, and Acceleration of Metabolism. Front Pharmacol. 2020;11:563750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 132. | Lin W, Wu Y, Wang J, Lin H, Xu X, He G, He B, Ma X. Network Pharmacology Study of the Hepatoprotective Effects of Quercetin- Containing Traditional Chinese Medicine, Anoectochilus roxburghii, and Validation of Quercetin as an Anti-Liver Injury Agent in a Mouse Model of Liver Injury. Med Sci Monit. 2020;26:e923533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 133. | Al-Sayed E, Korinek M, Esmat A, Chen GY, Cheng YB, Hsieh PW, Chen BH, Hwang TL. Anti-inflammatory, hepatoprotective and antioxidant activity of ellagitannin isolated from Melaleuca styphelioides. Phytochemistry. 2020;177:112429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 134. | Shakya AK. Drug-induced Hepatotoxicity and Hepatoprotective Medicinal Plants: A Review. Indian J Pharmaceut Educat Res. 2020;54:234-250. [DOI] [Full Text] |
| 135. | Fan X, Lin L, Cui B, Zhao T, Mao L, Song Y, Wang X, Feng H, Qingxiang Y, Zhang J, Jiang K, Cao X, Wang B, Sun C. Therapeutic potential of genipin in various acute liver injury, fulminant hepatitis, NAFLD and other non-cancer liver diseases: More friend than foe. Pharmacol Res. 2020;159:104945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 136. | Shen Y, Shen X, Cheng Y, Liu Y. Myricitrin pretreatment ameliorates mouse liver ischemia reperfusion injury. Int Immunopharmacol. 2020;89:107005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 137. | Wang Z, Zhao P, Zhang Y, Shi S, Chen X. The hepatoprotective effect and mechanism of lotus leaf on liver injury induced by Genkwa Flos. J Pharm Pharmacol. 2020;72:1909-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 138. | Gao Y, Tian Y, Zhang X, Duan Z, Ren F, Chen Y. Magnesium isoglycyrrhizinate ameliorates concanavalin A-induced liver injury via the p38 and JNK MAPK pathway. Immunopharmacol Immunotoxicol. 2020;42:445-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 139. | Dong Y, Qiu P, Zhao L, Zhang P, Huang X, Li C, Chai K, Shou D. Metabolomics study of the hepatoprotective effect of Phellinus igniarius in chronic ethanol-induced liver injury mice using UPLC-Q/TOF-MS combined with ingenuity pathway analysis. Phytomedicine. 2020;74:152697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 140. | Zhao Y, Zhang Y, Kong H, Zhang M, Cheng J, Wu J, Qu H, Zhao Y. Carbon Dots from Paeoniae Radix Alba Carbonisata: Hepatoprotective Effect. Int J Nanomedicine. 2020;15:9049-9059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 141. | Ezzat MI, Okba MM, Ahmed SH, El-Banna HA, Prince A, Mohamed SO, Ezzat SM. In-depth hepatoprotective mechanistic study of Phyllanthus niruri: In vitro and in vivo studies and its chemical characterization. PLoS One. 2020;15:e0226185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 142. | Zou J, Li W, Wang G, Fang S, Cai J, Wang T, Zhang H, Liu P, Wu J, Ma Y. Hepatoprotective effects of Huangqi decoction (Astragali Radix and Glycyrrhizae Radix et Rhizoma) on cholestatic liver injury in mice: Involvement of alleviating intestinal microbiota dysbiosis. J Ethnopharmacol. 2021;267:113544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 143. | Britza SM, Musgrave IF, Byard RW. Paracetamol (acetaminophen) hepatotoxicity increases in the presence of an added herbal compound. Leg Med (Tokyo). 2020;47:101740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 144. | National Institute of Health Office of Dietary Supplements: Selenium. Available from: https://ods.od.nih.gov/factsheets/Selenium-HealthProfessional/. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 145. | Aktary ML, Eller LK, Nicolucci AC, Reimer RA. Cross-sectional analysis of the health profile and dietary intake of a sample of Canadian adults diagnosed with non-alcoholic fatty liver disease. Food Nutr Res. 2020;64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 146. | Malyar RM, Li H, Liu D, Abdulrahim Y, Farid RA, Gan F, Ali W, Enayatullah H, Banuree SAH, Huang K, Chen X. Selenium/Zinc-Enriched probiotics improve serum enzyme activity, antioxidant ability, inflammatory factors and related gene expression of Wistar rats inflated under heat stress. Life Sci. 2020;248:117464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 147. | Malyar RM, Naseri E, Li H, Ali I, Farid RA, Liu D, Maroof K, Nasim M, Banuree SAH, Huang K, Waldron KJ, Chen X. Hepatoprotective Effects of Selenium-Enriched Probiotics Supplementation on Heat-Stressed Wistar Rat Through Anti-Inflammatory and Antioxidant Effects. Biol Trace Elem Res. 2021;199:3445-3456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 148. | Wu J, Zeng C, Yang Z, Li X, Lei G, Xie D, Wang Y, Wei J, Yang T. Association Between Dietary Selenium Intake and the Prevalence of Nonalcoholic Fatty Liver Disease: A Cross-Sectional Study. J Am Coll Nutr. 2020;39:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 149. | Kwong SP, Wang C. Review: Usnic acid-induced hepatotoxicity and cell death. Environ Toxicol Pharmacol. 2020;80:103493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 150. | Sanchez W, Maple JT, Burgart LJ, Kamath PS. Severe hepatotoxicity associated with use of a dietary supplement containing usnic acid. Mayo Clin Proc. 2006;81:541-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 151. | Abualhasan MN, Nidal Jaradat, Hawash M, Khayat R, Khatatbeh E, Ehmidan M, Al-Atrash M. Evaluation of Heavy Metal and Microbial Contamination in Green Tea and Herbal Tea Used for Weight Loss in the Palestinian Market. Evid Based Complement Alternat Med. 2020;2020:7631562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 152. | Stenson J. What is kratom? The popular herbal supplement has caught flak from the FDA. NBC Health News. 16 October 2019. Available from: https://www.nbcnews.com/health/health-news/what-kratom-popular-herbal-supplement-has-caught-flak-fda-n1066526. |