Published online Aug 27, 2021. doi: 10.4254/wjh.v13.i8.896
Peer-review started: March 2, 2021
First decision: May 2, 2021
Revised: May 16, 2021
Accepted: August 3, 2021
Article in press: August 3, 2021
Published online: August 27, 2021
Processing time: 170 Days and 16.9 Hours
Solid pseudopapillary neoplasms are rare. This article reviews the clinical and pathologic features of solid pseudopapillary neoplasm of the pancreas, including the epidemiology, cytology, molecular pathology, differential diagnosis, treatment, and prognosis. Solid pseudopapillary neoplasms are low-grade malignant tumours of the pancreas characterized by poorly cohesive epithelial cells with solid and pseudopapillary patterns. Solid pseudopapillary neoplasms occur predominantly in young women. Although solid pseudopapillary neoplasms can occur throughout the pancreas, they arise slightly more frequently in the tail of the pancreas. The aetiology is unknown. Extremely rare cases have been reported in the setting of familial adenomatous polyposis. There are no symptoms unique to solid pseudopapillary neoplasms, however, the most common symptom is abdominal pain or discomfort. The features of solid pseudopapillary neoplasms on computed tomography imaging are indicative of the pathologic changes within the tumour. Typically, well-demarcated masses with variably solid and cystic appearances. Microscopically, these tumours are composed of epithelial cells forming solid and pseudopapillary structures, frequently undergoing haemorrhagic cystic degeneration. Typically, these tumours express nuclear and/or cytoplasmic β-catenin. Almost all solid pseudopapillary neoplasms harbour mutations in exon 3 of CTNNB1, the gene encoding β-catenin. The overall prognosis is excellent, and most patients are cured by complete surgical resection.
Core Tip: Solid pseudopapillary neoplasms are low-grade malignant tumours that mimic other solid cellular neoplasms of the pancreas. This article summarizes the clinical and pathologic features of solid pseudopapillary neoplasm of the pancreas including the epidemiology, molecular pathology, differential diagnosis, treatment, and prognosis.
- Citation: Omiyale AO. Solid pseudopapillary neoplasm of the pancreas. World J Hepatol 2021; 13(8): 896-903
- URL: https://www.wjgnet.com/1948-5182/full/v13/i8/896.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i8.896
First described by Frantz in 1959[1], solid pseudopapillary neoplasms are low-grade malignant tumours composed of poorly cohesive uniform epithelial cells forming solid and pseudopapillary structures[2]. Several names have been used to describe these tumours including solid cystic tumour, papillary cystic tumour, solid and papillary epithelial neoplasm, papillary cystic carcinoma, Hamoudi’s tumour, and Frantz’s tumour[3-5]. Solid pseudopapillary neoplasms are rare tumours of uncertain histogenesis. In certain cases, distinguishing between solid pseudopapillary neoplasms and other solid cellular neoplasms of the pancreas may pose a diagnostic dilemma.
This article reviews state-of-the-art knowledge on the clinical and pathologic features of solid pseudopapillary neoplasm of the pancreas, including the epi
Solid pseudopapillary neoplasms are exceptionally rare. They account for approximately 0.9%-2.7% of all exocrine pancreatic neoplasms and 5% of cystic pancreatic neoplasms[2,3]. Although these tumours occur in a wide age range from 2 to 85 years[3], the mean age at presentation is 28.5 years[5]. They occur predominantly in young women with a female-male ratio of 9.8:1[3]. There is no known ethnic predilection. An increased number of cases have been reported in the literature since 2000, most likely because of rising awareness of these tumours and advances in imaging and other diagnostic techniques[5].
The aetiology is currently unknown. Although rare cases have been reported in the setting of familial adenomatous polyposis[6,7], there are no well-established risk factors for solid pseudopapillary neoplasms. There is no association with functional endocrine syndromes[2].
The clinical symptoms are non-specific. A large number of patients are asymptomatic (38.1%)[5], however most patients are symptomatic, with the most common presenting symptom being abdominal pain or discomfort[3,5,8]. Other symptoms include abdominal mass, weight loss, jaundice, anorexia, fever, fatigue, abdominal discomfort, nausea, and vomiting[3,5,8]. Rarely, patients may present with spontaneous[9,10] or traumatic[11] rupture of the tumour leading to haemoperitoneum.
These tumours may involve any portion of the pancreas but are slightly more common in the tail of the pancreas[2,3,5,12]. Rarely, these tumours can arise in extra pancreatic sites including the omentum[13], mesentery[14], retroperitoneum[15], ovary[16], stomach, and duodenum[17].
Distant metastases occur in 7.7% of cases and lymph node metastases occur in approximately 1.6% of cases[5]. Other sites of metastases include the lung[18], small and large bowel mesentery, liver, and peritoneum[3,5,12,19]. Occasionally, these tumours directly infiltrate adjacent structures including the portal vein, duodenum, and spleen[2,3,5].
Solid pseudopapillary neoplasms on computed tomography (CT) imaging show features reflective of the pathologic changes within the tumour. Usually, well-demarcated large heterogeneous masses with variably solid and cystic appearances on CT. Enhancing solid areas are mostly peripheral, with cystic areas tending to be centrally located. Peripheral or central stippled calcifications may be identified in the tumour[20,21].
MRI shows a well-defined mass with heterogeneous signal intensity on T1- and T2-weighted images indicative of the variably solid and cystic nature of the tumour. High signal intensity on T1-weighted images correspond to areas of haemorrhagic necrosis or debris[21,22]. The signal intensity of these areas is variable on T2-weighted images because of the presence of multiple degradation products of haemoglobin. The solid component of the tumour may show iso- to low signal intensity on T1-weighted images and slightly high signal intensity on T2-weighted images[21,22].
Although endoscopic ultrasound-guided fine needle aspiration is operator dependent, it is a well-tolerated minimally invasive procedure that has become the method of choice for the diagnosis of solid and cystic pancreatic neoplasms. The sensitivity for malignant cytology is 85% and the specificity is about 98%[23].
Typically, these smears are very cellular, with neoplastic cells forming loose papillary clusters with central fibrovascular cores. The neoplastic cells are uniform with nuclear indentations. There are multinucleated giant cells, foamy macrophages, and haemorrhagic debris in the background[24,25].
Grossly, solid pseudopapillary neoplasms are round solitary masses with fibrous pseudocapsule. Multicentric tumours are exceptionally rare[26]. They are large tumours ranging from 0.5 cm to 25 cm (mean, 10 cm)[2]. These tumours are typically solid with varying proportion of cystic degeneration. They have a well-demarcated fleshy cut surface with haemorrhagic and necrotic areas[27]. In rare cases, extension into adjacent structures may occur[2].
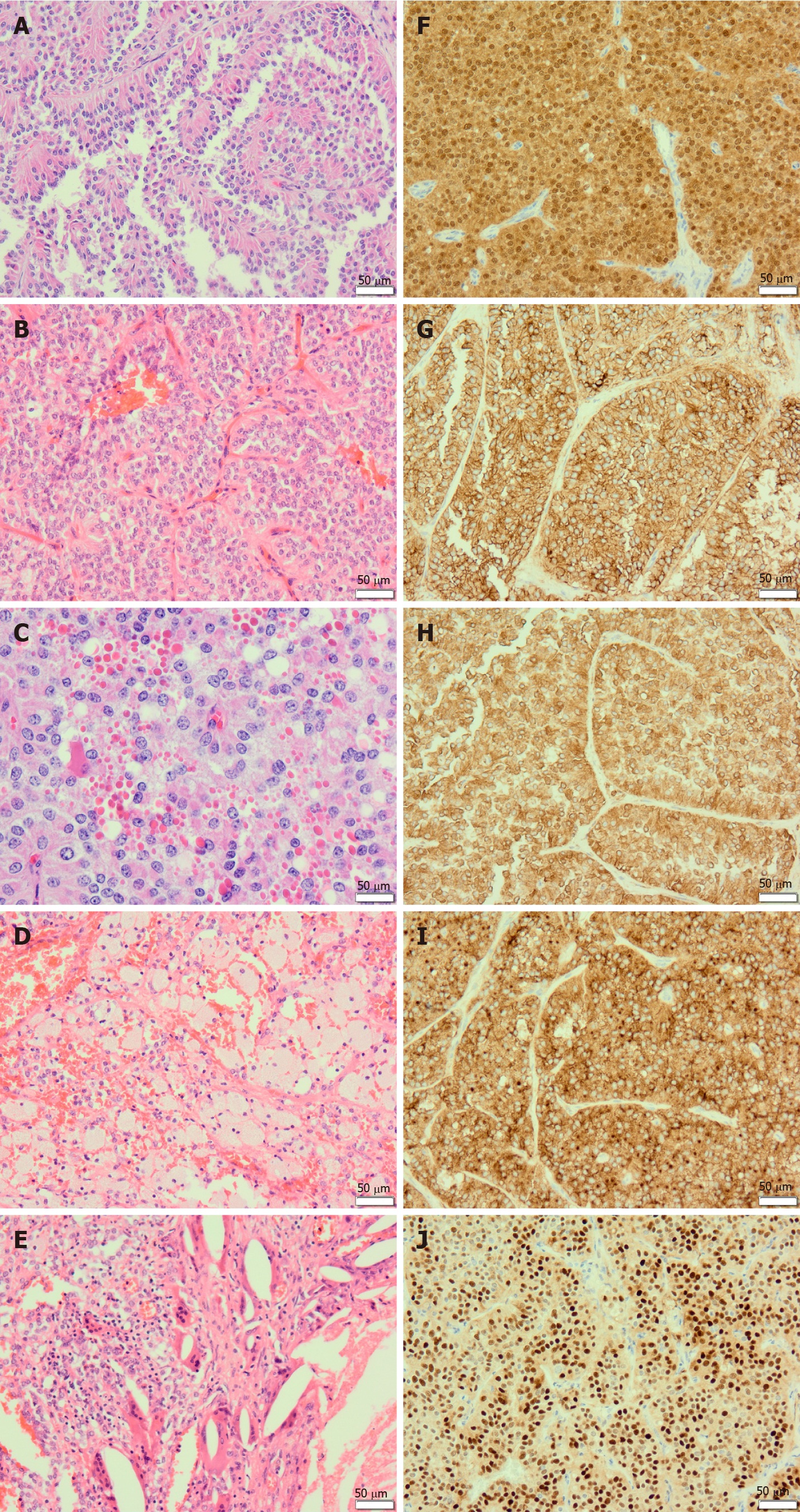
Microscopically, solid pseudopapillary neoplasms are composed of poorly cohesive epithelial cells forming solid and pseudopapillary structures (Figure 1A and B). The pseudopapillae are formed by epithelial cells loosely arranged around hyalinised stroma that contains thin-walled blood vessels (Figure 1A and B). The neoplastic cells are small and monomorphic. The cytoplasm of the neoplastic cells is eosinophilic or clear, and usually lacks mucin. The nuclei are round to oval and may show grooves, indentations, and clefts. The nuclei have fine chromatin pattern and absent or inconspicuous nucleoli. Mitotic figures are infrequent.
Although not specific, the presence of hyaline globules is a characteristic feature of solid pseudopapillary neoplasms. These globules are diastase-resistant, periodic acid-Schiff (PASD)-positive eosinophilic cytoplasmic inclusions (Figure 1C), corresponding to α-1-antitrypsin granules[2,27]. Most tumours contain foamy histiocytes (Figure 1D), cholesterol clefts, and foreign body giant cells (Figure 1E). Calcifications may be present. Perineural infiltration and vascular invasion is uncommon[28]. Rarely, undifferentiated carcinoma component may be seen[19].
Solid pseudopapillary neoplasms usually express nuclear and/or cytoplasmic β-catenin (Figure 1F). They are also positive for a wide range of antibodies including CD56 (Figure 1G), vimentin (Figure 1H), CD10 (Figure 1I), α-1-antitrypsin, α-1-antichymotrypsin, cyclin D1 (Figure 1J), CD99, claudin 5, claudin 7, and progesterone receptors[2,12,27]. Immunoreactivity for E-cadherin depends on the antibodies used. Antibodies to the intracellular domain of E-cadherin shows an abnormal cytoplasmic/nuclear expression while antibodies to the extracellular domain of the protein shows complete loss of expression[28].
Solid pseudopapillary neoplasms may be focally positive for synaptophysin and neurone-specific enolase. However, these tumours are negative for chromogranin A, trypsin, chymotrypsin, lipase, oestrogen receptors, and BCL10[2,27,28].
Solid pseudopapillary neoplasms harbour mutations in exon 3 of CTNNB1, the gene encoding β-catenin[2,27,28]. They lack the molecular alterations that have been described in pancreatic ductal adenocarcinoma such as KRAS, TP53, SMAD4/DPC4, and CDKN2A[27,28].
Β-catenin maintains cell-cell adhesion and regulates gene transcription in the canonical Wnt (β-catenin dependent) signalling pathway[29,30]. β-catenin is regulated by the β-catenin destruction complex composed of proteins including adenomatous polyposis coli, axin, protein phosphatase 2A, glycogen synthase kinase 3, and casein kinase-1[29,30]. In the absence of Wnt signalling, the β-catenin destruction complex targets β-catenin for ubiquitin-mediated proteasomal degradation. However, in the presence of Wnt signalling, the β-catenin destruction complex is inactivated, preventing β-catenin degradation. This leads to β-catenin accumulation in the cytoplasm and eventual translocation into the nucleus, where it acts as a co-transcriptional activator of lymphoid enhancer binding factor/T cell factor (LEF/TCF) family of transcription factors. Activated LEF/TCF family of transcription factors upregulates the expression of a variety of target genes involved in diverse cell functions such as cell proliferation, differentiation, and epithelial-mesenchymal transition. The CTNNB1 mutations observed in solid pseudopapillary neoplasms and other cancers lead to constitutive activation of the Wnt/β-catenin pathway and abnormal stabilization of cytoplasmic β-catenin[29,30].
Gene expression studies have identified solid pseudopapillary neoplasm-specific mRNA and microRNA expression profiles distinct from other pancreatic tumours[31]. Pathway enrichment analysis of differentially expressed genes in solid pseudopapillary neoplasms has shown that in addition to Wnt/β-catenin signalling pathway, Hedgehog and androgen receptor signaling pathways are also activated in these tumours[31].
Proteomic analyses of solid pseudopapillary neoplasms have confirmed that proteins involved in Wnt/β-catenin signaling (CTNNB1 and DKK4) and proteins that bind directly to β-catenin (FUS, hnRNPM, BGN, NONO, YWHAZ, DDX5, SELENBP1, and FN1) are upregulated in these tumours[32]. Furthermore, 9 proteins involved in metabolism including SLC25A13, GPI, PGK1, HK1, ENO2, PDHB, ALDH7A1, PKM2, and DLD are overexpressed in solid pseudopapillary neoplasms[32].
Distinguishing solid pseudopapillary neoplasm of the pancreas from the more common pancreatic ductal adenocarcinoma is not diagnostically challenging. The differential diagnosis of solid pseudopapillary neoplasms include pancreatoblastoma, acinar cell carcinoma and pancreatic neuroendocrine tumour.
Pancreatoblastoma is a malignant epithelial tumour composed of neoplastic cells showing predominantly acinar differentiation with characteristic squamoid nests. Although pancreatoblastomas frequently occur in childhood, they can be seen in adults[33,34,35]. Pancreatoblastomas exhibit malignant behaviour with local infiltration of adjacent structures and distant metastasis at the time of diagnosis or afterwards in the course of the disease[33,34,35]. Both pancreatoblastomas and solid pseudopapillary neoplasms are solid cellular tumours of the pancreas. The features that favour a diagnosis of pancreatoblastomas include predominant acinar units, squamoid nests, prominent central nucleoli, granular eosinophilic cytoplasm containing DPAS-positive zymogen granules, and immunolabelling for trypsin, chymotrypsin, BCL10, and lipase[2,33,35].
Acinar cell carcinomas are malignant neoplasms of the pancreas characterized by acinar differentiation but without squamoid nests. Unlike solid pseudopapillary neoplasms, acinar cell carcinomas frequently occur in men, lack pseudopapillary structures, and express trypsin, chymotrypsin, lipase, and BCL10. Acinar cell carcinomas are negative for β-catenin, CD56, and CD10. The prognosis of acinar cell carcinoma is poor with a 5-year survival rate of 25%[2].
Solid pseudopapillary neoplasms with a predominant solid pattern can be confused with pancreatic neuroendocrine tumours. In addition, both tumours express synaptophysin and CD56. Typically, pancreatic neuroendocrine tumours are composed of uniform cells with round to oval nuclei. The nuclei are centrally located with characteristic salt and pepper chromatin[2,33]. Features that favour a diagnosis of solid pseudopapillary neoplasms include the presence of solid and pseudopapillary structures, foamy histiocytes, cholesterol clefts, foreign body giant cells, scattered PASD-positive hyaline globules, nuclei with indentations, and expression of nuclear and/or cytoplasmic β-catenin, CD56, CD10, and vimentin.
Surgical resection is the treatment of choice for solid pseudopapillary neoplasms[3,5]. The type of operation will depend on the site and size of tumour. Common surgical procedures include distal pancreatectomy and splenectomy, spleen preserving distal pancreatectomy, central pancreatectomy, total pancreatectomy, pancreaticoduodenectomy, and pylorus-preserving pancreaticoduodenectomy[3,5]. A Cochrane systematic review comparing the effectiveness of classic Whipple procedure vs pylorus-preserving pancreaticoduodenectomy, showed no significant differences in overall survival, post-operative mortality, and morbidity between both procedures except for delayed gastric emptying, which significantly favoured classic Whipple procedure[36].
Although most of these tumours are treated by open surgery[5], a recent systematic review suggested that compared to a traditional open approach, minimally invasive pancreatectomy is associated with decreased intraoperative blood loss, lower blood transfusion requirements, and a shorter post-operative time to diet and hospital stay[37]. However, there were no significant differences in operating time, margin positivity, post-operative morbidity, and post-operative pancreatic fistula rates[37].
The overall prognosis is excellent with a cure rate of > 95% following complete surgical resection[2,3,5]. Of the 2158 patients with solid pseudopapillary neoplasm reported in a systematic review, outcome data were available in 1952 patients with a mean follow-up of 36.1 mo. Eighty-six patients (4.4%) had recurrent disease and twenty-nine patients (1.5%) died of the disease[5]. Long-term survival has been reported for patients with locally advanced, recurrent, and metastatic disease[3,12]. It is worth emphasizing that malignant behaviour cannot be predicted by vascular invasion, perineural invasion, and invasion of adjacent structures in these tumours[2]. However, it has been suggested that tumours with an undifferentiated carcinoma component have a dismal outcome[19], and large tumour size, high proliferation index, and lymph node metastasis may be risk factors for a poor prognosis[38].
In summary, solid pseudopapillary neoplasms are rare low-grade malignant tumours occurring predominantly in adolescent girls and young women with an excellent prognosis. It is therefore important to distinguish solid pseudopapillary neoplasms from morphological mimics.
The author would like to thank Prof. Robert Goldin for the critical reading of the manuscript and valuable suggestions. The author also wishes to acknowledge Dr Christopher Ross for his helpful comments.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu T S-Editor: Zhang H L-Editor: A P-Editor: Liu JH
| 1. | Frantz VK. Tumours of the Pancreas. In: Atlas of Tumor Pathology. Section VII, Fascicles 27 and 28. Washington, DC: US Armed Forces Institute of Pathology; 1959: 32–33. |
| 2. | Carneiro F, Chan JKC, Cheung NYA. (Eds): WHO Classification of Tumors of the Digestive System. 5th ed. IARC: Lyon 2019: 333-352. |
| 3. | Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 536] [Article Influence: 26.8] [Reference Citation Analysis (1)] |
| 4. | Hamoudi AB, Misugi K, Grosfeld JL, Reiner CB. Papillary epithelial neoplasm of pancreas in a child. Report of a case with electron microscopy. Cancer. 1970;26:1126-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Law JK, Ahmed A, Singh VK, Akshintala VS, Olson MT, Raman SP, Ali SZ, Fishman EK, Kamel I, Canto MI, Dal Molin M, Moran RA, Khashab MA, Ahuja N, Goggins M, Hruban RH, Wolfgang CL, Lennon AM. A systematic review of solid-pseudopapillary neoplasms: are these rare lesions? Pancreas. 2014;43:331-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 6. | Ruo L, Coit DG, Brennan MF, Guillem JG. Long-term follow-up of patients with familial adenomatous polyposis undergoing pancreaticoduodenal surgery. J Gastrointest Surg. 2002;6:671-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Inoue T, Nishi Y, Okumura F, Mizushima T, Nishie H, Iwasaki H, Anbe K, Ozeki T, Kachi K, Fukusada S, Suzuki Y, Mizuno A, Kajikawa M, Watanabe K, Sano H. Solid pseudopapillary neoplasm of the pancreas associated with familial adenomatous polyposis. Intern Med. 2015;54:1349-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Yu PF, Hu ZH, Wang XB, Guo JM, Cheng XD, Zhang YL, Xu Q. Solid pseudopapillary tumor of the pancreas: a review of 553 cases in Chinese literature. World J Gastroenterol. 2010;16:1209-1214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 189] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 9. | Huang SC, Wu TH, Chen CC, Chen TC. Spontaneous rupture of solid pseudopapillary neoplasm of the pancreas during pregnancy. Obstet Gynecol. 2013;121:486-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Takamatsu S, Nagano H, Ohtsukasa S, Kawachi Y, Maruyama H. A case of spontaneous ruptured solid pseudopapillary tumor of pancreas resected by laparoscopic surgery. Case Rep Med. 2013;2013:953240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Mirapoğlu SL, Aydogdu I, Gucin Z, Yilmaz TF, Umutoglu T, Kilincaslan H. Traumatic rupture of solid pseudopapillary tumors of the pancreas in children: A case report. Mol Clin Oncol. 2016;5:587-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Estrella JS, Li L, Rashid A, Wang H, Katz MH, Fleming JB, Abbruzzese JL. Solid pseudopapillary neoplasm of the pancreas: clinicopathologic and survival analyses of 64 cases from a single institution. Am J Surg Pathol. 2014;38:147-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | Hibi T, Ojima H, Sakamoto Y, Kosuge T, Shimada K, Sano T, Sakamoto M, Kitajima M, Yamasaki S. A solid pseudopapillary tumor arising from the greater omentum followed by multiple metastases with increasing malignant potential. J Gastroenterol. 2006;41:276-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Wu H, Huang YF, Liu XH, Xu MH. Extrapancreatic solid pseudopapillary neoplasm followed by multiple metastases: Case report. World J Gastrointest Oncol. 2017;9:497-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Miyazaki Y, Miyajima A, Maeda T, Yuge K, Hasegawa M, Kosaka T, Kikuchi E, Kameyama K, Jinzaki M, Nakagawa K, Oya M. Extrapancreatic solid pseudopapillary tumor: case report and review of the literature. Int J Clin Oncol. 2012;17:165-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Deshpande V, Oliva E, Young RH. Solid pseudopapillary neoplasm of the ovary: a report of 3 primary ovarian tumors resembling those of the pancreas. Am J Surg Pathol. 2010;34:1514-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Walter T, Hommell-Fontaine J, Hervieu V, Adham M, Poncet G, Dumortier J, Lombard-Bohas C, Scoazec JY. Primary malignant solid pseudopapillary tumors of the gastroduodenal area. Clin Res Hepatol Gastroenterol. 2011;35:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Takahashi Y, Fukusato T, Aita K, Toida S, Fukushima J, Imamura T, Tanaka F, Amano H, Takada T, Mori S. Solid pseudopapillary tumor of the pancreas with metastases to the lung and liver. Pathol Int. 2005;55:792-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 19. | Tang LH, Aydin H, Brennan MF, Klimstra DS. Clinically aggressive solid pseudopapillary tumors of the pancreas: a report of two cases with components of undifferentiated carcinoma and a comparative clinicopathologic analysis of 34 conventional cases. Am J Surg Pathol. 2005;29:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 224] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 20. | Kawamoto S, Scudiere J, Hruban RH, Wolfgang CL, Cameron JL, Fishman EK. Solid-pseudopapillary neoplasm of the pancreas: spectrum of findings on multidetector CT. Clin Imaging. 2011;35:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Buetow PC, Buck JL, Pantongrag-Brown L, Beck KG, Ros PR, Adair CF. Solid and papillary epithelial neoplasm of the pancreas: imaging-pathologic correlation on 56 cases. Radiology. 1996;199:707-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 193] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Chung EM, Travis MD, Conran RM. Pancreatic tumors in children: radiologic-pathologic correlation. Radiographics. 2006;26:1211-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 23. | Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75:319-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 510] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 24. | Mehta N, Modi L, Patel T, Shah M. Study of cytomorphology of solid pseudopapillary tumor of pancreas and its differential diagnosis. J Cytol. 2010;27:118-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Ardengh JC, Lopes CV, Venco FE, Machado MA. Diagnosis of pancreatic solid pseudopapillary neoplasms using cell-blocks and immunohistochemical evaluation of endoscopic ultrasound-guided fine needle aspiration biopsy specimens. Cytopathology. 2021;32:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Orlando CA, Bowman RL, Loose JH. Multicentric papillary-cystic neoplasm of the pancreas. Arch Pathol Lab Med. 1991;115:958-960. [PubMed] |
| 27. | Dinarvand P, Lai J. Solid Pseudopapillary Neoplasm of the Pancreas: A Rare Entity With Unique Features. Arch Pathol Lab Med. 2017;141:990-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Bosman FT, Carneiro F, Hruban RH. Theise ND (Eds.): WHO Classification of Tumours of the Digestive System. 4th ed. IARC: Lyon 2010: 327-330. |
| 29. | Gao C, Wang Y, Broaddus R, Sun L, Xue F, Zhang W. Exon 3 mutations of CTNNB1 drive tumorigenesis: a review. Oncotarget. 2018;9:5492-5508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 156] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 31. | Park M, Kim M, Hwang D, Park M, Kim WK, Kim SK, Shin J, Park ES, Kang CM, Paik YK, Kim H. Characterization of gene expression and activated signaling pathways in solid-pseudopapillary neoplasm of pancreas. Mod Pathol. 2014;27:580-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Park M, Lim JS, Lee HJ, Na K, Lee MJ, Kang CM, Paik YK, Kim H. Distinct Protein Expression Profiles of Solid-Pseudopapillary Neoplasms of the Pancreas. J Proteome Res. 2015;14:3007-3014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Klimstra DS. Nonductal neoplasms of the pancreas. Mod Pathol. 2007;20 Suppl 1:S94-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Omiyale AO. Clinicopathological review of pancreatoblastoma in adults. Gland Surg. 2015;4:322-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 35. | Omiyale AO. Adult pancreatoblastoma: Current concepts in pathology. World J Gastroenterol. 2021;27:4172-4181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 36. | Hüttner FJ, Fitzmaurice C, Schwarzer G, Seiler CM, Antes G, Büchler MW, Diener MK. Pylorus-preserving pancreaticoduodenectomy (pp Whipple) versus pancreaticoduodenectomy (classic Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochrane Database Syst Rev. 2016;2:CD006053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 37. | Tan HL, Syn N, Goh BKP. Systematic Review and Meta-analysis of Minimally Invasive Pancreatectomies for Solid Pseudopapillary Neoplasms of the Pancreas. Pancreas. 2019;48:1334-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Chen H, Huang Y, Yang N, Yan W, Yang R, Zhang S, Yang P, Li N, Feng Z. Solid-Pseudopapillary Neoplasm of the Pancreas: A 63-Case Analysis of Clinicopathologic and Immunohistochemical Features and Risk Factors of Malignancy. Cancer Manag Res. 2021;13:3335-3343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |