INTRODUCTION
Kidney transplantation (KT) is the preferred treatment for patients with end-stage kidney disease (ESKD). It is associated with reduced mortality and improved quality of life when compared to dialysis therapy[1]. However, the number of ESKD patients awaiting KT far exceeds the number of organ donations globally and leads to a problem of organ shortage. This major barrier has led to a prolonged waiting time and subsequently excess mortality of patients in the waiting list pool[2]. There are several proposed rationales to solve the problem of organ shortage[3]. One possible solution is to expand the donor pool by utilizing “extended donor criteria organs”. Such organs include those from donors with hepatitis B virus (HBV) infection.
The prevalence of chronic HBV infection varies greatly by geographical region, ranging from 0.4% to 1.6% in the region of the Americas, 1.2% to 2.6% in Europe, 1.5% to 4.0% in Southeast Asia, 2.6% to 4.3% in the Eastern Mediterranean, 5.1 to 7.6 % in the Western Pacific, and 4.6% to 8.5% in Africa[4]. Discarding all kidneys from donors with markers of HBV infections may substantially harm the donor pool in endemic areas since the prevalence in donors is similar to that of the general population. Thus, one challenge is determining the optimal use of kidneys from such donors. The best utilization may involve allocating such kidneys to transplant candidates at low risk of acquiring a donor-transmitted hepatitis B infection. Prophylactic therapy and appropriate monitoring will further eliminate the risk of HBV transmission.
According to current guidelines, there is an increasing trend of accepting non-liver organs from total hepatitis B core antibody-positive [anti-HBc (+)] donors to be used in any recipient regardless of HBV immune status without prophylaxis due to the negligible risk of de novo infection. However, utilizing kidneys from donors with positive hepatitis B surface antigen (HBsAg) [HBsAg (+)] remains controversial, and it is generally suggested that such organs be discarded[5-7]. In this review, we aim to summarize the current evidence regarding the use kidneys from HBsAg (+) donors with an emphasis on the risk of HBV transmission, liver related morbidities, and the outcomes of KT.
SCREENING TEST FOR HBV INFECTION IN ORGAN DONORS
Screening for HBV infection usually relies on a panel of serologic tests. The test for HBsAg is widely distributed. However, it can fail to detect disease during a 35-44 d window period after inoculation or occult infection defined as detectable viral DNA in absence of HBsAg[8]. Another importance serologic screening test for previous HBV exposure is anti-HBc. In acute hepatitis B infection, immunoglobulin M (IgM) antibody to hepatitis B core antigen (IgM anti-HBc) becomes positive after 4 wk to 6 wk of exposure indicating recent infection and active viral replication whereas total hepatitis B core antibody (anti-HBc) appear at the onset of symptoms and persists for life. Hepatitis B e antigen (HBeAg) and hepatitis B e antibody (anti-HBe) are additional tests to identify viral replicative activity as HBeAg positivity which indicates active viral replication (i.e., usually a viral load > 10000 IU/mL). In contrast, anti-HBe positivity indicates the presence of the non-replication phase (i.e., a viral load < 10000 IU/mL). Lastly, hepatitis B antibody (anti-HBs) is a marker of immune status due to either naturally- or vaccine-acquired immunity[9].
In the situation of deceased kidney donation, HBs Ag and anti-HBc are generally accepted as cost-effective screening tools. The results should be integrated with additional essential information of the donors to assess the risk of donor-derived infection as previously described[10-12]. Some transplant centers in endemic areas routinely add on anti-HBe and HBeAg to the donor screening platform as biomarkers of high viral replication and infectivity activity relating to a high viral burden[13-15]. However, serologic testing alone still has limitations due to the long window period and the lack of sensitivity to detect occult infections, raising concerns over risk misclassification[16,17]. In clinical practice, isolated anti-HBc is commonly observed. This may occur in several clinical settings. First, the early window period of acute hepatitis B. Second, a resolved HBV infection with waning of anti-HBs titer. Third, a false positive anti-HBc. This setting is commonly found in an area with a low prevalence of HBV infection. Fourth, an occult chronic HBV infection with low viremia and undetectable HBsAg. The latter can occur with a poor test quality or when there is a mutation of HBsAg[18,19].
To improve the sensitivity of screening tests, the nucleic acid test (NAT), which is usually in the form of an HIV/HCV/HBV multiplex, has been proposed as an optional donor screening test. This test is advantageous, because it shortens the windows period to 20-22 d compared to 35-44 d by conventional serology[8]. Although NAT seems a promising solution, obstacles to its implementation include whether it is cost-effective in a particular healthcare setting, the logistic challenges, the long turn-around times (i.e., as much as 8 h), the technical proficiency required, and the reliability of an in-house developed test[8,20]. In low prevalence settings, such as the United States , the concern is that the benefit may not outweigh the disadvantage that can lead to loss of organ donor, and have suggested that routine use of NAT screening was unnecessary[8]. However, a look-back study demonstrated adding NAT to routine screening by serologic testing enhanced the physician’s confidence in using organ with discordant results [i.e., anti-HBc(+)/NAT(-)], and adding NAT led to a gain in overall organ utilization after policy implementation[21]. Currently, this test is gradually becoming accepted in national policies in several countries. For example, the US Public Health Service 2020 guideline revision suggests performing NAT for HIV, HBV, and HCV in organ donors in all donor transplants[22]. While guidelines in the Transplantation Society of Australia and New Zealand suggests performing NAT in donors with HBsAg positivity, anti-HBc positivity, or HBsAg and anti-HBc negativity with increasing behavioral risk for HBV infection[6]. However, the decision to use NAT in individuals depends on the context of each setting or country. For practical purposes, we suggest that all serologic tests for HBV (HBsAg, anti-HBc, HBeAg, anti-HBe) as well as other essential infectious markers are done at the donor hospitals. In parallel, a universal NAT test for HBV (usually in combination with HIV and HCV) should be conducted at the central or regional organ allocation center and the result should come back before or at the time of organ retrieval.
RISK OF HBV TRANSMISSION AND INFECTION AFTER KT
Donor factors and the role of HBV DNA
The risk of donor-transmitted HBV infection is lower in kidney transplant recipients compared with liver transplant recipients with similar serologic marker positivity[23]. Specific HBV receptor recognition may play important roles in this hepatotropism phenomenon[24]. The demonstration of persistent HBV viral genome in the liver and peripheral blood mononuclear cells of patients with acute and chronic HBV infection after the clearance of HBsAg in the blood has led to an awareness of possible HBV reactivation in the immunocompromised host. This notion was supported by previous studies that showed the presence of HBV covalently closed circular DNA and total DNA in the serum of patients with negative HBsAg[25,26].
Important behavioral risk factors to acquire HBV (and other coincidental infections such as HIV and HCV) should be carefully reviewed when assessing the risk of HBV transmission from the donors. Patients who have strong risk factors for HBV/ HCV/HIV combination should be tested for HBsAg, HBV NAT, and then HBV DNA by a test with the highest sensitivity and specificity. A previous study had suggested a test with a lower detection limit of less than < 0.1 ng/mL for HBsAg and 10 IU/mL for HBV DNA[27]. Donors with HBV infections are generally categorized into two groups according to their serologic status. The first donor group is the anti-HBc positive group in which the rate of transmission appears to be negligible according to the recipient's protective immunity status. The overall seroconversion rate was 3.24% (mostly anti-HBc seroconversion). HBsAg seroconversion rate from this study was shown to be 0.28% with no symptoms of hepatitis and no excess mortality[28]. The second donor group is the HBsAg positive group where the HBV transmission remains a challenging problem[5]. In the current era, interesting information regarding the use of kidneys from HBsAg (+) donors is increasing. Previously, it was generally believed to discard the use of these kidneys. However, several recent studies and guidelines suggested that kidneys from HBsAg (+) donors can be carefully considered to be transplanted to appropriate recipients after careful consideration of the risk and benefit with informed consent[5,29]. The role of NAT in reducing the window period of serological test in combination with a careful evaluation of the donor behavioral risk factors has been increasingly emphasized[30]. KT from living HBsAg (+) donors can be donated to anti-HBs (+) recipients with protection who have no abnormalities of liver function test, no history of liver disease within the previous 28 days, and who are not living in the area of possible mutation strain of HBV[31].
It is important to note that fulminant hepatitis B infection had been reported in a naïve recipient who received kidneys from donors with HBsAg (+)/HBeAg (+) donors[32]. Since this report, HBeAg and anti-HBe were routinely checked in HBsAg (+) donors to ensure a low infectivity rate of HBV before performing KT[14,15,33]. Use of antiviral medications to treat HBV add benefit to the treatment plan to use organs from HBsAg (+) donors. Unlike liver transplantation, KT from this type of donor can be associated with a functional cure of HBV. The functional cure was defined by a state of sustained loss of HBsAg with or without anti-HBs seroconversion which was usually associated with good clinical outcomes[34]. A recent study performed 83 living KTs from HBsAg (+) donors to HBsAg (-) recipients. Before the transplant, 28% of the donor in the latter study were HBV DNA (+) and 24% of the recipients had no anti-HBs. All recipients in the latter study received hepatitis B immunoglobulin (HBIG) and antiviral medication as prophylaxis treatment. The results showed that this treatment was associated with excellent graft and patient survival without excess HBV transmission when compared with the control group[35]. In recent years, tests for HBV DNA have increasingly become popular. Several studies revealed that the prevalence of hepatitis B viremia in HBsAg (+)/HBeAg (-) donors ranged from 2.3%-28.3%[14,15,35]. Chancharoenthana et al[14] reported that kidney transplants from HBsAg (+)/HBV DNA (-) (< 20 IU/mL) donors to 20 immune recipients (anti-HBs > 100 mIU/mL) was safe and was not associated with any HBV viremia, hepatitis or death despite the absence of antiviral prophylaxis. The other two studies reported excellent outcomes of transplanting kidneys from HBsAg (+)/HBV DNA (-) donors to a total of 146 recipients with anti-HBs > 10 mIU/mL. Those studies have also shown excellent outcomes with no evidence of HBV transmission[36,37]. It was interesting to note that there was one out of 58 recipients of HBsAg (+)/HBV DNA (-) donor who developed HBsAg seroconversion one month after transplantation. That patient had received HBV vaccination, but with low (non-protective) anti-HBs titer (4.6 mIU/ml). However, this patient did not develop clinical evidence of hepatitis and has acquired anti-HBs seroconversion which may be due to prophylactic therapy lamivudine and HBIG in the study protocol[15].
Recipient factors and the role of protective immunity
In principle, the recipients who received kidneys from donors with hepatitis B should have protective anti-HBs. Several guidelines and studies have suggested that an anti-HBs > 10 mIU/mL was protective[5,33,36,37]. It is important to note that HBV transmission may not necessarily lead to clinical evidence of HBV infection. This was clearly shown in one study that performed transplantation of HBsAg (+) kidney to four immunized patients with an anti-HBs ranged from 63 mIU/mL to > 1000 mIU/mL. The results showed that there was no HBsAg seroconversion, although the anti-HBc IgG was positive in all 4 cases at six months despite the presence of anti-HBs positivity. This study showed evidence of HBV transmission by the kidney grafts without any clinical manifestations of HBV infection[38].
For KT, it was unclear whether a higher level of anti-HBs concentration was associated with a higher level of protection of HBV transmission as was shown in liver transplantation. Immunity to hepatitis B was crucial to prevent donor-derived infection. However, it was suggested that an anti-HBs concentration of > 10 mIU/mL was protective[39]. It was shown in studies of transplanting kidneys from anti HBc (+) donors to 50 recipients with anti-HBs > 10 mIU/mL, that there was no anti-HBc IgM or HBsAg seroconversion[40]. Tuncer et al[36] and Asuman et al[37] reported that kidney transplants from HBsAg (+) donors to 146 recipients with anti-HBs > 10 mIU/mL were not associated with any de novo HBV infection or active liver diseases. A study in 43 recipients of HBsAg (+)/HBV DNA (-) donor with patients with higher anti-HBs level (> 100 mIU/mL) found that there was neither anti-HBc nor HBsAg seroconversion and there was no evidence of HBV DNAemia[14]. However, a recent study of kidney transplants from HBsAg (+) donors to 83 HBsAg (-) recipients with varying degrees of anti-HBs did not support the importance of high anti-HBs concentration[35].
There was variation in the definitions of HBV transmission via transplantation of non-liver organs[5]. In the setting of kidney transplants from HBsAg (-) donors to immune protective recipients (Anti-HBs > 10 mIU/mL), definitions of HBV transmission may include anti-HBc IgM seroconversion, HBsAg seroconversion, and HBV DNAemia. De novo HBV infection can occur as a consequence of HBV transmission with clinical evidence of acute or chronic liver disease associated with HBV.
Differences in the reported rate of HBV transmission and/or infection after kidney transplant may be related to the different targets of protective anti-HBs concentration. Subclinical infection presenting with anti-HBc seroconversion was observed with kidney transplants from both anti-HBc (+) and HBsAg (+) donors[14,15,35,41]. In addition, the need for higher levels of immunity is related to global variation in HBV genotypes. The genotype predominance by region is A in North America, B in Europe, C in Asia and Australia, and D in the middle east and central Asia[42,43]. Most commercially available HBV vaccines were developed using genotype A2. Although cross-protection against other genotypes is observed, it has been suggested that a higher antibody concentration (> 50 mIU/mL) might be required[43]. However, the immune benefit may be lost in cases of HBV antigenic variation due to mutation in the ‘a’ determinant region of HBsAg[43,44]. In this case, the protective effect of HBIG is also lost. One case of fulminant hepatitis B in a kidney transplant recipient with vaccine-acquired immunity and an HBV infection of the D2 genotype with an escape mutation at G145R (glycine to arginine, G145R) was reported after the recipient had received a kidney from an HBsAg (+) donor, despite the recipient having received HBIG and NA prophylaxis[45]. Although such cases are rare, they may lead to fatal complications.
MONITORING OF HBV INFECTION AFTER TRANSPLANTATION
For kidney transplant recipients, The American Association for the Study of Liver Diseases (AASDL) suggested periodic assessment of serum ALT, HBV DNA, and HBsAg during immunosuppressive therapy. Reactivation of HBV infection was defined by detectable HBV DNAemia or positive HBsAg seroconversion. In addition, hepatitis flare was defined by rising of serum ALT more than 3 times the baseline level and > 100 U/L with evidence of hepatitis B reactivation[19].
The optimal frequency of monitoring for HBV infection in a susceptible individual is still varied. The Infectious Disease Community of Practice of the American Society of Transplantation advised monitoring liver enzymes, HBsAg, and HBV DNA every 3 mo for at least 12 mo post-transplantation. Subsequent management was based on the evolution of test results over the first year[46]. In the case of naïve recipient receiving Anti-HBc (+) kidney without antiviral prophylaxis, the European guidelines recommend monitoring for HBsAg, and HBV DNA at least during the first year. Also, most of the recipients from donors with HBV infection were suggested to receive life-long monitoring[47].
Besides, all kidney transplant recipients who have a resolved infection of HBV (defined by positive anti-HBc serology) should be aware of a possibility of HBV reactivation during a course of intensive immunosuppression particularly rituximab[48]. Kim et al[49] studied HBV reactivation in a cohort of 499 kidney transplant recipients. 86.6 % of those recipients were anti-HBs (+) and 29.6% received kidneys from donors with positive anti-HBc IgG. No recipients received kidneys from donors with positive HBsAg. The authors reported that the incidence rate of hepatitis B reactivation was 2% during a follow-up period of 6.7 years. HBV reactivation was observed at the median time of 2.8 years (range 1.4-11.5). A high incidence of reactivation was observed in recipients with ABO incompatibility, who received plasmapheresis, received acute rejection therapy, and received induction therapy with rituximab[49]. These findings provided evidence that HBV reactivation can occur at any time after KT. As such, HBV reactivation may be the consequence of either donor-derived infection or the resolved recipient infection.
THE ROLE OF PROPHYLAXIS THERAPY
Vaccination and revaccination protocol
Anti-HBs play a key role to minimize the risk of HBV transmission. Hepatitis B vaccination should be given to naïve recipients or previously immune recipients who have anti-HBs concentration below 10 mIU/mL[39]. Also, the KDIGO guideline suggested a concentration of Anti-HBs below 100 mIU/mL can be rapidly lowered down to a non-protective level and may require a booster dose at this step[50]. Differences in suggestions may be due to a concern that patients with chronic kidney disease may have impaired anamnestic response to viral infection. This can lead to an insufficient immune response to HBV, and suppression of memory T and B cells that may result in a low or absence of antibody titer[44]. As an antibody concentration is likely to wane over time, monitoring of anti-HBs concentration should be done at least yearly. A further booster dose of vaccine may be required. This can be prescribed by either a single-shot high dose (40 µg) or a total complete course with a follow-up level at 4-wk after a complete course of treatment[39,51]. One study found that 95% of immune recipients with waning titer can be successfully boosted with a full course of hepatitis B. However, 10% of patients might have delay response of titer up to 6 mo after treatment completion. Higher antibody concentration was observed in patients who had a shorter duration of dialysis and positive anti-HBc status[52].
A high dose of HBV vaccine was suggested to patients with ESKD who were receiving hemodialysis therapy. A protocol of three or four high-dose (40 µg) hepatitis B vaccine series with a target level of 10 mIU/mL at 4 wk post-treatment was suggested. Also, a second three doses of vaccination were suggested if the anti-HBs could not reach the desired level[51]. Similarly, the CDC recommended RecombivaxTM vaccine at 0, 1, and 6 mo or Engerix BTM at 0, 1, 2, and 6 mo[53]. Despite this approach, at least 30% of hemodialysis patients were still not successfully immunized[54].
The strategies to improve vaccine efficacy may be related to the type, dose, and route of administration. Besides the use of commercially available hepatitis B vaccine derived from genotype A2, a vaccine specifically derived from common genotype in the specific geographical area will add a layer of protection. This practice has been investigated in Korea and Japan where Type-C derived vaccine (BimmugenTM) was being given. The proof of this concept will take up to a decade[43,55]. To those who were not responding to conventional vaccine protocol, a subcutaneous injection route was reported to be associated with increased responsiveness (70 by intramuscular, 74 by subcutaneous)[54]. Also, a third-generation vaccine containing pre s/s epitope vaccine has been reported to be associated with good immunogenicity and responsiveness in a healthy individual[56]. The results of this third-generation vaccine when administered to patients with ESKD are further required to fill the practice gap.
Despite a debate, there was a suggestion to keep anti-HBs concentration more than 100 mIU/mL. Reactivation after KT has been reported in patients with antibody titer less than 100 mIU/mL[57]. In another study, no anti-HBc or HBsAg seroconversion was developed in patients who had received a booster vaccine to keep levels above 100 mIU/mL[52]. Due to the low-risk nature of the interventions, KDIGO suggested re-evaluating anti-HBs annually and administering re-vaccination if anti-HBs were found to be below 10 mIU/mL[50].
Antiviral medications (nucleos(t)ide analogues) and HBIG
Another modality to prevent HBV transmission via kidney transplant organs was the use of antiviral medications and HBIG. HBIG provides passive immunity for a high concentration of anti-HBs that are aimed to act as neutralizing antibodies to HBV[58]. Most prescriptions of HBIG were used in combination with antiviral nucleos(t)ide analogs (NAs) that aim to prevent recurrent infection of HBV after liver transplantation. This regimen was found superior to HBIG or NA alone[59]. However, the optimal dose of HBIG to be used for kidney transplant recipients from donors with HBV was not clearly known.
NAs are a group of antiviral medications that directly suppress HBV virus replication. Lamivudine was the most popular prophylaxis agent being used globally[5]. However, its efficacy was hampered by small number of lamivudine-resistant hepatitis B. Therefore, other drugs with a high genetic barrier such as entecavir were considered as a better alternative[19]. This was especially noteworthy in selected patients who were at risk of exposure to a lamivudine-resistant strain of HBV, including those who received kidneys from the donors previously treated by lamivudine. From a meta-analysis of 12212 chronic naïve hepatitis B patients, the prevalence of lamivudine-resistant HBV was 8% in China, 0-6.6% in other Asian regions, 0%-4.5% in South America, 1%-3% in Europe, and 0.71% in the United State[60]. The incidence of lamivudine-resistance can be increased with longer durations of exposure (as high as a fifty percent increase after 2 years)[61]. In chronic hepatitis B liver transplant recipients, high genetic barrier nucleos(t)ide analog combined with HBIG was superior to lamivudine combined with HBIG in the prevention of recurrent HBV infection (disease recurrent rate 1.0% compare to 6.1%)[62].
Serologic markers of HBV infection may have some impact on the choice to prescribe antiviral medications (NA). Anti HBs (> 10 mIU/mL) and positive recipients can receive kidney transplants from anti-HBc (+) donors without a need for prophylaxis antiviral medications due to the negligible risk of HBV transmission. In contrast, naïve recipients who received kidneys from anti-HBc (+) donors should receive lamivudine prophylaxis without HBIG for at least 1 year[5]. In the setting of HBs Ag (+) donors, recipients with protective anti-HBs (> 10 mIU/mL) were considered suitable to receive the allocation of kidney grafts. Further risk should be assessed by the result of the NAT test and HBV DNA measurement. If the result of nucleic acid for HBV was negative and HBV DNA was undetectable (by a method with a detection limit as low as 20 copies per mL), preventive strategies varied from no NA prophylaxis (in the setting of no potent induction therapy), or prescription of NA alone without HBIG. If the anti-HBs is > 100 mIU/mL, one can proceed to KT without NA prophylaxis[14]. However, if HBV DNA was not measured by a method of optimum low detection limit or the result of HBV DNA cannot be obtained due to any reason, NA may be prescribed to make the risk of HBV transmission as low as possible.
In the setting of HBsAg (+)/HBV DNA (+) donor, most authors prescribed universal NA prophylaxis with or without HBIG as a prophylaxis regimen among patients with different levels of anti-HBs concentration[14,15,35]. However, the optimal dose of HBIG in this setting was not clearly known. One important issue in this setting was the use of potent induction therapy. A study in 24 immunized recipients (mean anti-HBS 452 ± 384 mIU/mL), 89% who received induction therapy including anti-thymocyte globulin, found that three of them had detectable HBV DNAemia. This HBV DNAemia occurred although all patients received six months of lamivudine therapy. Fortunately, none of those patients developed liver failure[13]. Thus we have suggested that the use of HBsAg (+)/HBV DNA (+) donors to patients with immunized anti-HBs should be exercised with due caution as this group still carries a significant risk of de novo HBV infection particularly in the recipients who receive potent induction therapy[13].
Use of HBsAg (+)/DNA (+) donors to recipients with naïve or anti-HBs < 10 mIU/mL was the group with the highest risk being reported. A study in 20 naïve recipients who received prophylactic NA or HBIG or combination showed that the incidence of acute liver injury, anti-HBc seroconversion, and HBV DNAemia was 20 %, 10%, and 10% respectively[35]. Thus the use of this treatment option should be restricted to patients with an urgent need for KT (exhausted multiple vascular access, with ongoing uremia despite adequate hemodialysis prescription).
Another interesting issue is the use of HBsAg positive donors to recipients with HBsAg positive serology. A few studies[63-66] have reported favorable outcomes of this treatment option provided that the recipients received antiviral treatment before transplantation. Also, there is a suggestion that the recipients with positive HBsAg should have the result of liver biopsy that did not show evidence of cirrhosis. However, it should be noted that the number of patients being reported with this option was small. Additional information for this setting may be required.
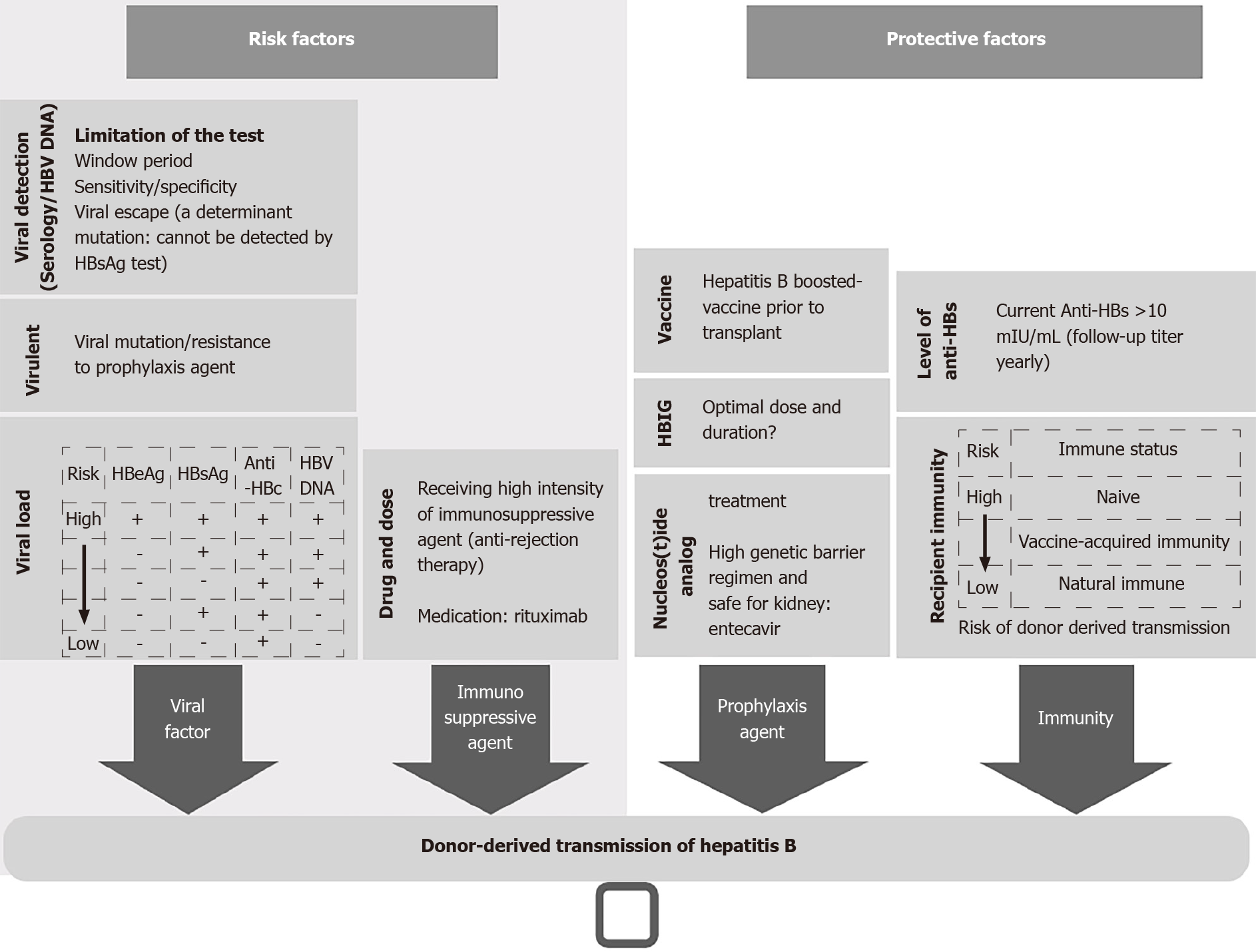
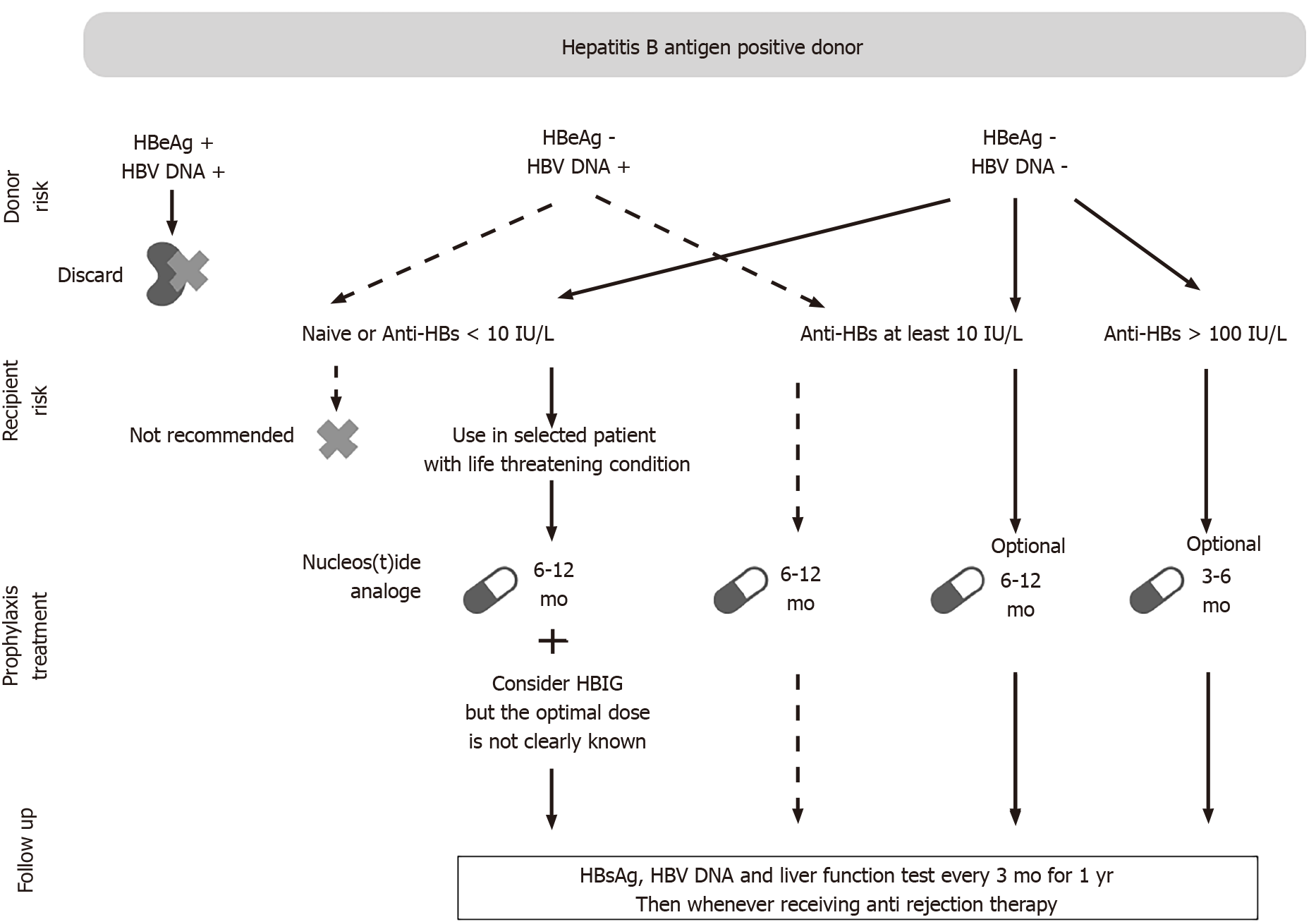
Figure 1 showed the important factors associated with the risk of HBV transmission in the setting of KT from donors with HBV. Figure 2 showed a practical approach to the use of kidneys from donors with positive HBsAg.
Figure 1 Risk factors of donor derived transmission of hepatitis B virus and proposed protective factors.
HBV: Hepatitis B virus; HBsAg: Hepatitis B surface antigen; HBeAg: Hepatitis B e antigen; Anti-HBc: Hepatitis B core antibody; Anti-HBs: Hepatitis B surface antibody.
Figure 2 Proposed management for several types of donor and recipient pairs according to the results of hepatitis B virus serology test and DNA markers.
HBV: Hepatitis B virus; HBeAg: Hepatitis B e antigen; Anti-HBs: Hepatitis B surface antibody; HBIG: Hepatitis B immunoglobulin; HBsAg: Hepatitis B surface antigen.
One important use of NAs was in the setting of treatment with rituximab. This monoclonal antibody acted directly against CD 20 which can lead to impaired immunoglobulin production[67]. There was a study that showed HBV reactivation in kidney transplant recipients with resolving hepatitis B infection[48]. We believe that NA should be prescribed to kidney transplant recipients, who receive kidney allograft from donors with HBV, who have been treated with rituximab either as anti-rejection therapy or induction therapy in ABO-incompatible recipients.
LONG TERM OUTCOMES AND SURVIVAL
Regarding HBsAg (-) anti-HBc (+) donors, historic studies found that there were no HBsAg seroconversion and no excess risk of morbidity and graft failure[68]. Subsequent studies that examined the outcomes in children have shown a similar result in terms of patient survival and graft survival[69]. A quantitative review of nine studies found the seroconversion rates of HBsAg, anti-HBc, anti-HBs were 4/1385, 32/1385, and 5/1385 recipients. Those numbers were considered to be very low and the authors conclude that HBsAg (-) anti-HBc (+) kidneys can be transplanted safely to patients with ESKD[28].
The amount of information on KT from HBsAg (+) donors is much less than anti-HBc (+) donors. However, the results of long-term outcomes being reported showed favorable outcomes when compared with donors with no markers of HBV with proper prophylaxis regimen[13-15,37]. Our result of HBsAg (+) donor to anti-HBs (> 10 mIU/mL) recipient reported a ten-year actuarial graft survival rate of 84.6% and patient survival rate of 92.8% (with no hepatitis and hepatoma) provided that the recipients received no induction therapy[33].
The previous report of fulminant hepatitis B infection in the setting of HBsAg (+) /HBeAg (+) donor to anti-HBs (-) recipients has been a major concern. However, our review of published articles from 2005 onwards (Supplementary Table 1) has shown there were a total of at least 412 KTs from HBsAg (+) donors to HBsAg (-) recipients. This treatment option was associated with good outcomes. First, in 20 HBsAg (+) donors to anti-HBs (-) recipients, there was 1 death from liver disease, and there were 2 HBV transmissions (2 HBsAg seroconversion). Next, in 392 HBsAg (+) donors to anti-HBs (+) recipients, there were two deaths and four HBV DNAemias. One death occurred in a patient with HBV mutation that escaped from the protective effect of anti-HBs. Another death was associated with liver failure which was reported to be due to nonadherence to lamivudine. There was one HBsAg seroconversion (with HBV DNAemia) associated with lamivudine resistance. The final three HBV DNAemias were reported from a single study. This study reported that the mean anti-HBs of the recipients was 452 ± 384 mIU/ml. However, all of the latter three patients could be successfully treated with lamivudine therapy. No excess risk of liver failure was reported[13,15,70]. It was important to note that most HBV DNAemias being observed were usually observed with lamivudine resistance or non-adherence. These HBV DNAemias occurred despite the presence of anti-HBs > 100 mIU/ml. These results suggested that kidney transplants from HBsAg positive donors to appropriate recipients was a cost-effective option when compared with keeping the potential recipient in the waiting list pool[71].
RISK BENEFIT OF TRANSPLANTATION AND PROPOSED CRITERIA FOR HBsAg (+) DONOR UTILIZATION
As has been mentioned earlier, organs from HBsAg (+) donors are generally suggested to be discarded[72]. However, with careful individual risk and benefit assessment, these organs may be utilized safely and serve as an alternative treatment to shorten waiting time rather than stay on a usual transplant waiting list. Shortened waiting time was also beneficial in improving 10-year graft survival in both living and deceased donor KTs[73]. Moreover, recipients can benefit from excellent graft survival without excess risk of liver disease as aforementioned[33,35].
It has long been shown and recently confirmed that kidney transplants promoted both longer life expectancy and better quality of life at a lower cost relative to staying on dialysis treatment[74,75]. In order to gain comparable survival benefit to kidney transplant, an intensive home hemodialysis has to be attained which would be a much higher effort than an in-center standard hemodialysis and this option is not feasible in some countries[76]. A recent economic study using data from USRDS showed that kidney transplants using standard donors were a cost saving procedure compared to remaining on dialysis. The same study also showed that kidney transplant using high risk donors were cost-effective[77]. All of the above studies have highlighted the benefit of expanding the donor pool by using kidneys from donors with HBsAg positivity.
Utilized kidneys from HBsAg (+) donors not only direct benefits to the potential recipients, but also the national society. However, the criteria for utilization of kidneys from donors with HBsAg positivity has not been well described. We would like to describe our proposed criteria to define three groups of potential recipients. The first group is patients with urgent need to receive KT. Urgent condition included patients with exhausted vascular access for hemodialysis, patients with ongoing uremia despite adequate dialysis prescription, and patients who cannot remain in the dialysis treatment (hemodialysis or CAPD) due to any reason. The second group is the recipients with positive HBsAg[6,30,46]. The third group is patients being registered as active waiting list who have waiting time longer than the median time to receive a kidney in each national society. The potential recipients should be discussed about the willingness to receive a kidney from donors with HBsAg positivity. They may choose not to take this opportunity and continue to wait for HBsAg negative donors. Examples of the use of kidneys with increased risk of blood borne viral infection has been previously described[78]. A short summary of prophylaxis regimen and special requirements for recipients of kidneys from HBsAg (+) donors is discussed below.
Our rationale for proposal of the third criteria is related to the following information. Data from OPTN (organ procurement and transplantation network) showed that the median time to receive a kidney for a new transplant candidate in waiting list is 4.5 years[77,79]. In US, waiting-listed patients were associated with 5%-7% increase in mortality which continues to increase in older waiting-listed patients. As reported in Matas et al[80], there was 2% mortality rate in those aged between 18-34 years which increased to 8% for patients over 65. Utilizing kidneys from HBV infection donors can be one strategy to shorten the recipients’ waiting time. This can help to decrease the mortality rate of waiting list candidates and downsize the waiting list pool.
Due to the risk of infection transmission before undergoing KT, recipients should be fully informed and consent must be obtained from each individual. In addition, all potential recipients should be vaccinated that aim to achieve anti-HBs at least > 10 mIU/mL. The potential recipients should not have HCV coinfection nor other cause of chronic liver disease which may worsen after KT. All recipients of HBsAg (+) donors should receive anti-viral medication, especially in the situation when the result of HBV DNA cannot be obtained before actual transplantation. HBIG may be considered for recipients with non-protective anti HBsAb level and/or in the situation of unknown HBV DNAemia of the donor. A protocol for close surveillance of viral reactivation and liver disease must be implemented. For HBsAg (+) recipient candidates, they must be treated with NA and evaluated by a specialist in liver disease. Untreated patients result in a higher mortality rate, with liver-related complications[19]. The AASDL recommends further evaluation of HBV DNA, ALT and to undergo staging with biopsy or elastography to determine whether advanced fibrosis or cirrhosis is present in order to assess the need for simultaneous liver KT[22,72].
It is an ethical challenge to allocate kidneys from donors with positive HBsAg to potential recipients with anti-HBs < 10 IU/ml. In our opinion, this treatment option should be limited to recipients with urgent criteria under a careful management that includes HBIG, antiviral medication and a careful protocol. Wang et al[35], demonstrated the possibility of this option (see section: Antiviral medications). However, these transplants should be performed by experienced teams.
CHALLENGING PERSPECTIVE
KT from donors with HBsAg (+) donors is not a risk-free procedure. A careful allocation to appropriate recipients can be successfully performed. NAT for HBV is now accepted to be a useful screening test. The result of a sensitive HBV DNA test is of prime importance in the organ allocation and the design of the prophylactic protocol. The rate of HBV transmission from this treatment option was reported to be low and manageable. HBV reactivation can occur in resolved HBV infection. Thus, a regular monitoring schedule for HBV is an essential part of post-transplant care. Differentiation between donor-derived HBV infection and reactivation of recipient strain HBV infection may be difficult. We believe that the use of kidney organs from donors with HBV infection in the area where the national organ donation rate is less than the rate of endemic HBV infection is a better alternative than discarding the organs.
CONCLUSION
Within this era of several newer antiviral medications, the presence of positive HBsAg in potential organ donors should not preclude the use of kidney organs. Several additional steps and experienced transplant teams are specifically required to prepare waiting list candidates who are willing to receive a kidney from such donors. These steps should be regularly assessed for each individual during his or her registration as active waiting list to receive KT from deceased donors. However, the criteria that we have described in this review, can also be applied to patients who are planning to receive living (related) KT as well.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pham TTT S-Editor: Gao CC L-Editor: A P-Editor: Guo X