Published online Jul 27, 2021. doi: 10.4254/wjh.v13.i7.815
Peer-review started: February 14, 2021
First decision: April 6, 2021
Revised: April 12, 2021
Accepted: July 2, 2021
Article in press: July 2, 2021
Published online: July 27, 2021
Processing time: 158 Days and 16.8 Hours
Metabolic dysfunction-associated fatty liver disease corresponds to a clinical entity that affects liver function triggered by the accumulation of fat in the liver and is linked with metabolic dysregulation.
To evaluate the effects of the intragastric balloon (IGB) in patients with metabolic dysfunction-associated fatty liver disease through the assessment of liver enzymes, imaging and several metabolic markers.
A comprehensive search was done of multiple electronic databases (MEDLINE, EMBASE, LILACS, Cochrane and Google Scholar) and grey literature from their inception until February 2021. Inclusion criteria involved patients with a body mass index > 25 kg/m2 with evidence or previous diagnosis of hepatic steatosis. Outcomes analyzed before and after 6 mo of IGB removal were alanine amino
Ten retrospective cohort studies evaluating a total of 508 patients were included. After 6 mo of IGB placement, this significantly reduced alanine aminotransferase [mean difference (MD): 10.2, 95% confidence interval (CI): 8.12-12.3], gamma-glutamyltransferase (MD: 9.41, 95%CI: 6.94-11.88), glycated hemoglobin (MD: 0.17%, 95%CI: 0.03-0.31), triglycerides (MD: 38.58, 95%CI: 26.65-50.51), systolic pressure (MD: 7.27, 95%CI: 4.79-9.76), homeostatic model assessment (MD: 2.23%, 95%CI: 1.41-3.04), abdominal circumference (MD: 12.12, 95%CI: 9.82-14.41) and body mass index (MD: 5.07, 95%CI: 4.21-5.94).
IGB placement showed significant efficacy in improving alanine aminotransferase and gamma-glutamyltransferase levels in patients with metabolic dysfunction-associated fatty liver disease as well as improving metabolic markers related to disease progression.
Core Tip: Metabolic dysfunction-associated fatty liver disease corresponds to the accumulation of fat in the liver and is linked with metabolic dysregulation. We evaluated the effects of the intragastric balloon in patients with metabolic dysfunction-associated fatty liver disease through the assessment of liver enzymes, imaging and several metabolic markers. Outcomes analyzed before and after 6 mo of intragastric balloon placement were alanine aminotransferase (IU/L), gamma-glutamyltransferase (IU/L), glycated hemoglobin (%) and other parameters related to metabolic disorders. This is the first systematic review and meta-analysis to assess the role of the intragastric balloon in the new definition of metabolic dysfunction-associated fatty liver disease.
- Citation: de Freitas Júnior JR, Ribeiro IB, de Moura DTH, Sagae VMT, de Souza GMV, de Oliveira GHP, Sánchez-Luna SA, de Souza TF, de Moura ETH, de Oliveira CPMS, Bernardo WM, de Moura EGH. Effects of intragastric balloon placement in metabolic dysfunction-associated fatty liver disease: A systematic review and meta-analysis. World J Hepatol 2021; 13(7): 815-829
- URL: https://www.wjgnet.com/1948-5182/full/v13/i7/815.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i7.815
The term nonalcoholic fatty liver disease, first proposed by Ludwig and collaborators in 1980[1] corresponds to a clinical entity that affects the histological structure and liver function triggered by the accumulation of fat in the liver unrelated to alcohol intake with a risk of developing nonalcoholic steatohepatitis and cirrhosis. It is estimated that this condition affects a quarter of the adult world population[2], and it will be the main cause of liver transplantation by 2030[3].
Recently, an international consensus panel of experts[4] proposed metabolic dysfunction-associated fatty liver disease (MAFLD) as a change in nomenclature and more appropriate term to reflect the pathophysiology and current knowledge of the disease rather than the outdated terms of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. The new definition is based on current knowledge of the role of metabolic dysfunction in the pathophysiology of fatty liver disease related mainly to obesity, type 2 diabetes mellitus and other metabolic disorders. Also, they provided diagnostic criteria to facilitate stratification and the subsequent management of patients along with new horizons for translational research and new treatments.
The natural history of fatty liver disease navigates through the initial stages of hepatic steatosis with progression to steatohepatitis and liver cirrhosis in certain chronic cases[5]. The treatment of these patients still represents a challenge[6]. Lifestyle changes and control of metabolic disorders are the mainstays of the therapeutic approach. Pharmacological therapies are promising but have not yet evidenced efficacy in regressing the inflammation and liver fibrosis associated with the evolution of the disease[7]. Bariatric surgery has gained notoriety, but the expansion of its indication as a form of treatment for MAFLD has been discussed in view of the added morbidity and irreversibility of different surgical modalities.
Research for alternative therapies is relevant in the treatment of MAFLD, with endoscopic bariatric and metabolic therapies, especially with the intragastric balloon (IGB), seen as a safe and less invasive treatment option[8-12]. The IGB is a widespread therapy for short-term control of obesity and its mechanism of action is based on the occupation of the gastric chamber, causing a delay in gastric emptying, an increase in the feeling of satiety and consequently a reduction in caloric intake. Currently, several models of IGB are available for clinical use, with variations in its design, volume, fluid vs air filled-balloons, implantation duration and efficacy[13].
This study aims to evaluate the impact of IGB placement on MAFLD through the assessment of liver enzymes, certain metabolic markers and imaging parameters.
This study was performed in conformity with the PRISMA[14] guidelines, and it was registered in the PROSPERO[15] database under the file number (CRD42020204485). The study was approved by the Ethics Committee of Hospital das Clínicas, Faculty of Medicine at The University of São Paulo.
Data search was made without limitations of language or publication date. The eligibility criteria adopted were: (1) population: patients with a body mass index (BMI) > 25 kg/m2 with evidence or previous diagnosis of hepatic steatosis; (2) intervention: endoscopic IGB placement; (3) comparator: the outcomes in baseline and post IGB moments; and (4) outcomes: alanine aminotransferase (ALT), gamma-glutamyltransferase (GGT), glycated hemoglobin, triglycerides, systolic blood pressure, homeostatic model assessment (HOMA-IR), abdominal circumference and liver volume were analyzed.
Studies that did not involve the use of an IGB for at least 6 mo of duration were excluded.
We performed a search in electronic databases (MEDLINE, EMBASE, Cochrane, LILACS) and grey literature, from their inception until February 2021. As a search strategy, we used descriptors available from the United States National Library of Medicine Medical Subject Headings and other related terms that increased the sensitivity of search as described in Table 1. Two independent reviewers conducted the assessment of eligibility criteria. Disagreements were resolved by consensus or consultation with a third reviewer.
| Search strategy | |
| Medline | [(intragastric OR bariatric endoscopy OR balloon OR balloons OR bubble OR bubbles OR gastric balloon OR balloons)] AND [(mafld OR non alcoholic fatty liver disease OR nafld OR fatty liver OR nonalcoholic steatohepatitis OR nash OR nonalcoholic steatohepatitis OR alanine transaminase OR aspartate aminotransferase OR gamma-glutamyltransferase OR alkaline phosphatase OR fatty liver OR steatohepatitis OR steatohepatitis OR steatosis of liver OR visceral steatosis OR visceral] |
| MEDLINE, Embase, Cochrane, LILACS | [(intragastric OR balloon)] AND [(fatty liver)] |
| Grey literature | [(intragastric OR balloon)] AND [(fatty liver)] |
The data related to the analyzed outcomes were tabulated in an Excel table and included the IGB model used as well as the average volume of filling of the balloons and the number of calories in the diet associated with the treatment. In the comparison studies between IGB and diet, only data from the balloon intervention group were extracted, and not all outcomes were evaluated in all studies. When data of the published articles were insufficient, the corresponding authors were consulted by e-mail for further elucidation.
The risk of bias was assessed by the Risk of Bias in Non-randomized Studies-of Interventions tool[16]. The quality of evidence, expressed in high, moderate, low and very low, was assessed utilizing the objective criteria from Grading Recommendations Assessment, Development, and Evaluation (Table 2) using the GRADEpro-Guideline Development Tool software (McMaster University, 2015; Evidence Prime, Inc., Ontario, Canada)[17].
| Certainty evidence assessment | Study event rates (%) | |||||||||
| Participants (studies) follow up | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall certainty of evidence | With post-IGB | With pre-IGB | Risk difference with Pre-IGB | |
| ALT | 1114 (10 observational studies) | Not serious | Serious1 | Not serious2 | Not serious | Publication bias strongly suspected3 | ⨁⨁◯◯, Low | 557 | 557 | Mean 10.27 UI/L more (8.25 more to 12.29 more) |
| GGT | 1014 (8 observational studies) | Not serious | Not serious | Not serious2 | Not serious | None | ⨁⨁⨁⨁, High | 507 | 507 | Mean 9.23 UI/L more (6.88 more to 11.58 more) |
| Hb1Ac | 300 (6 observational studies) | Not serious | Not serious | Not serious4 | Not serious | Publication bias strongly suspected3 | ⨁⨁⨁◯, Moderate | 150 | 150 | Mean 0.17 % higher (0.03 higher to 0.31 higher) |
| Triglycerides | 564 (6 observational studies) | Not serious | Not serious | Not serious | Not serious | None | ⨁⨁⨁⨁, High | 282 | 282 | Mean 38.58 mg/dL higher (26.65 higher to 50.51 higher) |
| Systolic blood pressure | 468 (3 observational studies) | Not serious | Not serious | Not serious | Not serious | None | ⨁⨁⨁⨁, High | 234 | 234 | Mean 7.27 mmHg higher (4.79 higher to 9.76 higher) |
| HOMA-IR | 378 (5 observational studies) | Not serious | Serious1 | Not serious | Not serious | None | ⨁⨁⨁◯, Moderate | 189 | 189 | Mean 2.07 higher (1.64 higher to 2.49 higher) |
| BMI | 912 (8 observational studies) | Not serious | Not serious | Not serious | Not serious | Strong association | ⨁⨁⨁⨁, High | 456 | 456 | Mean 5.07 kg/m2 higher (4.21 higher to 5.94 higher) |
| Waist | 672 (7 observational studies) | Not serious | Not serious | Not serious | Not serious | None | ⨁⨁⨁⨁, High | 336 | 336 | Mean 12.12 cm higher (9.82 higher to 14.41 higher) |
| Liver volume | 32 (2 observational studies) | Not serious | Not serious | Not serious | Serious5 | None | ⨁⨁⨁◯, Moderate | 16 | 16 | MD 303.24 higher (56.66 lower to 663.15 higher) |
Our outcomes were continuous variables, and values of means and standard deviations were used for the statistical analysis. In studies that expressed the results in median and interquartile range, mathematical formulas were used for the data conversion[18].
The data of interest extracted from the selected studies were meta-analyzed using the RevMan software (Review Manager Software version 5.4-Cochrane Collaboration Copyright© 2020) using the inverse variance test. The mean values of each continuous outcome were calculated as well as the 95% confidence interval (CI). P < 0.05 were considered statistically significant, and the results were exposed through forest plots. Heterogeneity was calculated using the Higgins method (I2)[19]. When heterogeneity < 50% was found, the fixed-effect model was used. In cases of heterogeneity > 50%, the funnel plot analysis was performed, and outlier cases were removed to maintain the analysis by a fixed effect. In cases where no outlier was evidenced, the analysis by the random effect model was performed. The correlation between outcomes was performed using the meta-regression using the Comprehensive Meta-Analysis tool version 2.2.057.
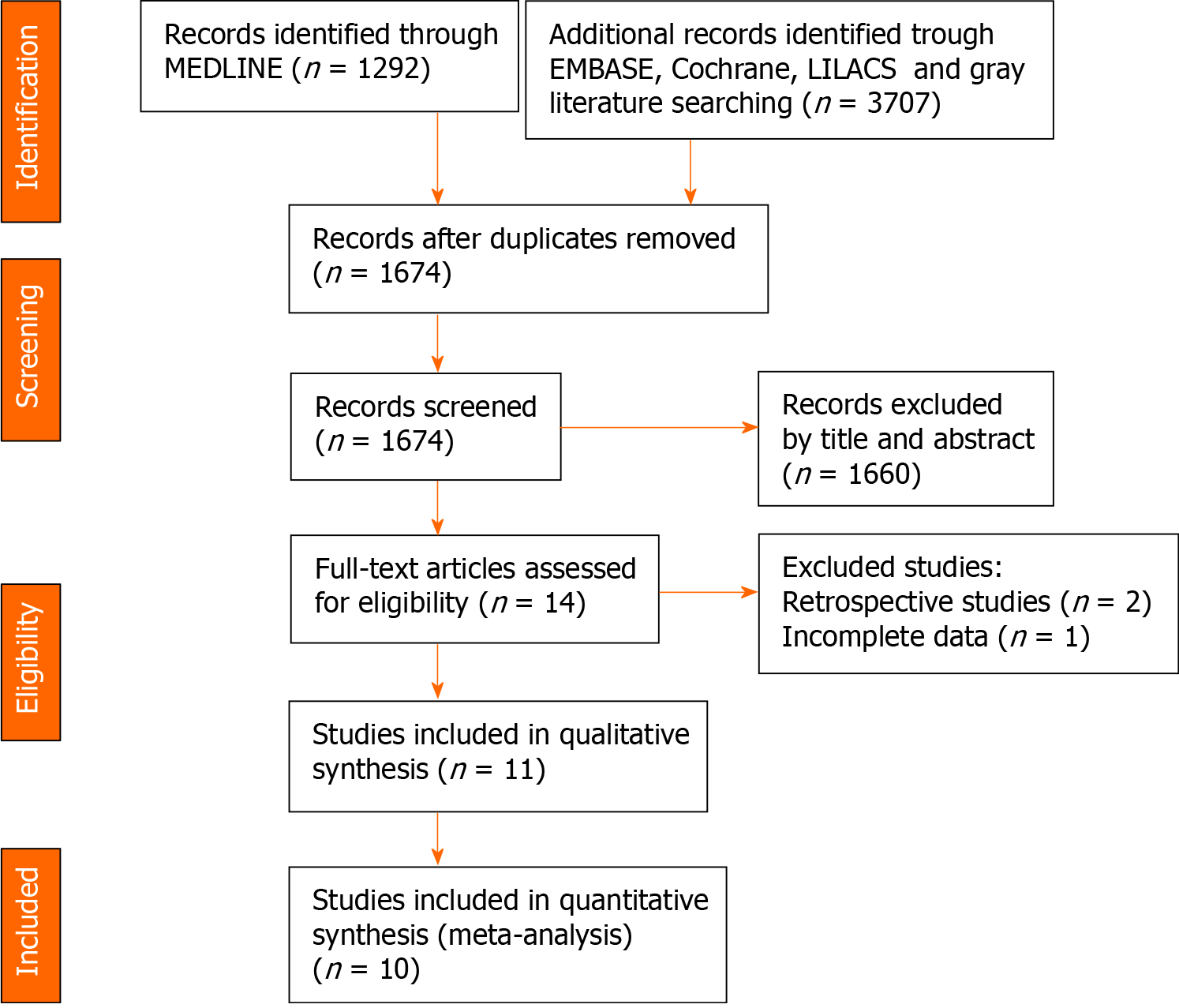
The article selection process is shown in Figure 1. After applying the eligibility criteria, eleven articles were included in the qualitative analysis. Ten articles were included in the quantitative analysis, considering that one of the studies was a randomized controlled clinical trial. The individual results of each study are described in Table 3.
| Ref. | n | Balloon volume (cm3) | ALT (UI/L) | GGT (UI/L) | HbA1c (%) | Triglycerides (mg/dL) | Waist (cm) | HOMA-IR | BMI (kg/m2) | SBP (mmHg) | ||||||||
| Pre-IGB | Post-IGB | Pre-IGB | Post-IGB | Pre-IGB | Post-IGB | Pre-IGB | Post-IGB | Pre-IGB | Post-IGB | Pre-IGB | Post-IGB | Pre-IGB | Post-IGB | Pre-IGB | Post-IGB | |||
| Forlano et al[25], 2010 | 120 | 500 | 39.3 (25.6) | 24.4 (10.0) | 37.5 (20.5) | 24.5 (17.1) | - | - | - | - | - | - | - | - | 43.1 (8.0) | 38.8 (8.0) | - | - |
| Bazerbachi et al[22], 2021 | 21 | - | 91.6 (59.9) | 39.4 (25.4) | - | - | 7.7 (1.6) | 6.5 (1.2) | - | - | 128.9 (15.4) | 119.7 (16.9) | - | - | 43.2 (6.8) | 37.9 (6.6) | - | - |
| Nikolic et al[21], 2011 | 33 | 600 | 30 (23.25) | 27 (16.75) | 31 (50.75) | 21 (36.75) | 4.7 (0.50) | 4.6 (0.45) | 124 (86.25) | 124 (124.75) | 122 (21.00) | 110 (14.25) | - | - | 41.4 (5.25) | 35.6 (5.25) | - | - |
| Donadio et al[23], 2009 | 40 | 500 | 30.7 (14.0) | 23.4 (9.3) | 29.8 (19.1) | 28.0 (28.1) | 5.4 (0.5) | 5.3 (0.4) | 134.1 (67.8) | 118.8 (66.5) | 125.9 (18.6) | 115.8 (17) | 4.1 (2.1) | 2.7 (1.6) | 44.8 (8.9) | 38.9 (6.8) | 129.3 (14.0) | 122.6 (10.4) |
| Stimac et al[29], 2011 | 166 | 600 | 34.7 (31.5) | 26.5 (23.1) | 33.3 (23.3) | 24.7 (16.9) | - | - | 118.6 (87.6) | 81.0 (66.4) | 127.8 (16.7) | 113.3 (18.9) | - | - | 41.6 (7.5) | 35.8 (7.9) | 130.9 (14.5) | 124.2 (14.1) |
| Takihata et al[20], 2014 | 8 | Variable | 57.1 (55.6) | 43.1 (48.8) | 53.0 (25.4) | 40.1 (19.3) | 6.70 (1.43) | 6.38 (1.49) | 223.2 (194.8) | 153.2 (80.6) | 129.2 (8.3) | 123.8 (12.3) | 12.3 (10.9) | 8.0 (7.3) | 45.2 (5.9) | 41.0 (6.2) | - | - |
| Folini et al[24], 2014 | 40 | - | 25.9 (10.31) | 18.1 (5.96) | 27.8 (27.57) | 17.9 (12.21) | 6.5 (1.17) | 6.0 (0.74) | - | - | 130.2 (13.96) | 118 (13.01) | 5.2 (2.23) | 2.3 (1.66) | 43.8 (6.62) | 38.2 (6.19) | - | - |
| Ricci et al[26], 2008 | 65 | - | 31.5 (19.33) | 24.0 (10.67) | 31.0 (16.05) | 23.5 (12.6) | - | - | - | - | - | - | 4.71 (2.11) | 3.10 (2.79) | - | - | - | - |
| Sekino et al[27], 2011 | 8 | 1000 | 74.2 (49.67) | 56.7 (42.40) | 57.00 (23.11) | 41.25 (14.74) | 6.30 (1.15) | 6.31 (1.29) | 251 (168.9) | 163 (62.0) | - | - | 6.74 (1.27) | 3.27 (1.18) | - | - | - | - |
| Tai et al[28], 2013 | 28 | 500 | 49 (45.25) | 22 (23.25) | - | - | - | - | 149.0 (49.00) | 88.5 (39.75) | 101.9 (8.9) | 90.6 (9.3) | - | - | 32.4 (3.7) | 28.5 (3.7) | 136.8 (14.30) | 125.9 (11.15) |
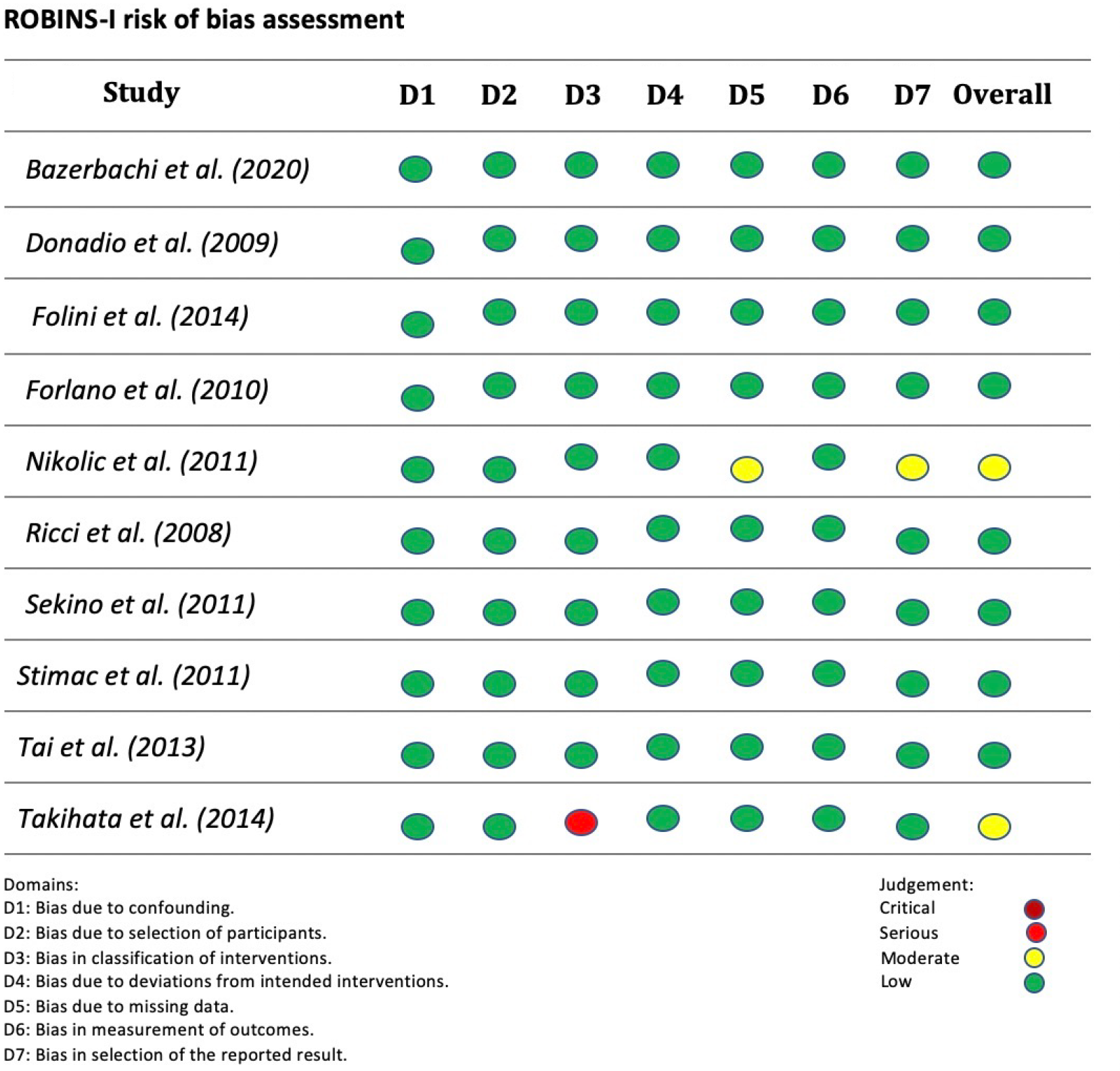
Two studies presented moderate risk and eight studies presented low risk in the global analysis according to the Risk of Bias in Non-randomized Studies-of Interventions criteria. The study by Takihata et al[20] had a risk of serious bias in the classification of interventions because the patients themselves chose whether to participate in the IGB intervention group or the lifestyle modification (diet/physical exercise) group. The study by Nikolic et al[21] presented a moderate risk of lack of data due to the exclusion of participants due to a loss of follow-up in the study. The overall risk of bias in each study is detailed in Figure 2.
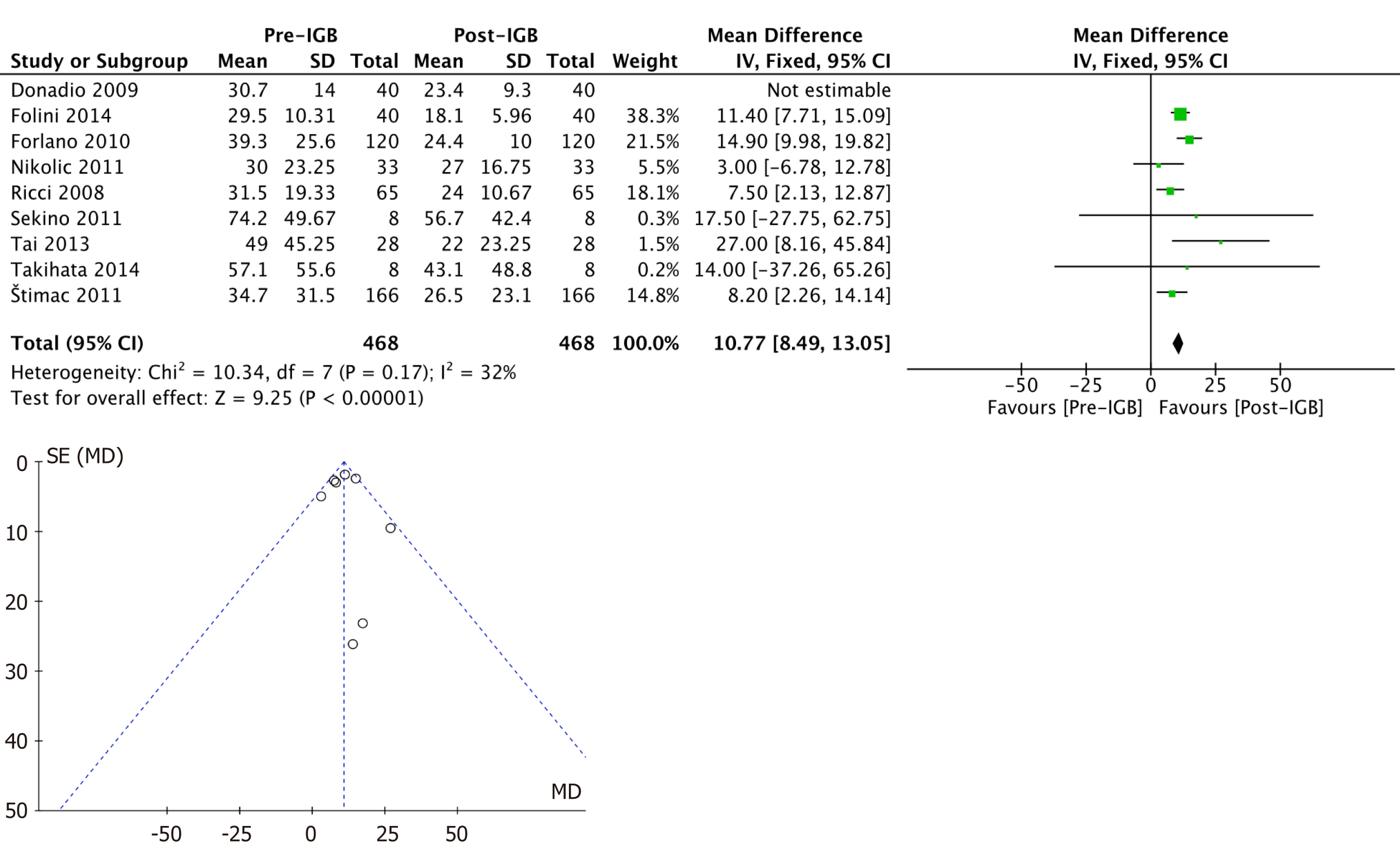
ALT (IU/L): Ten studies[20-29] with 508 patients were included in the meta-analysis of the outcome. The mean reduction in serum ALT values was 10.2 (95%CI: 8.12-12.3; P < 0.01) after 6 mo, favoring the use of the IGB. This analysis showed high heterogeneity (I2= 56%), and the study by Bazerbachi et al[22] was identified as an outlier in the funnel plot analysis. After removing this study from the analysis, the heterogeneity remained at < 50% (I2= 32%), maintaining the analysis by a fixed effect (Figure 3).
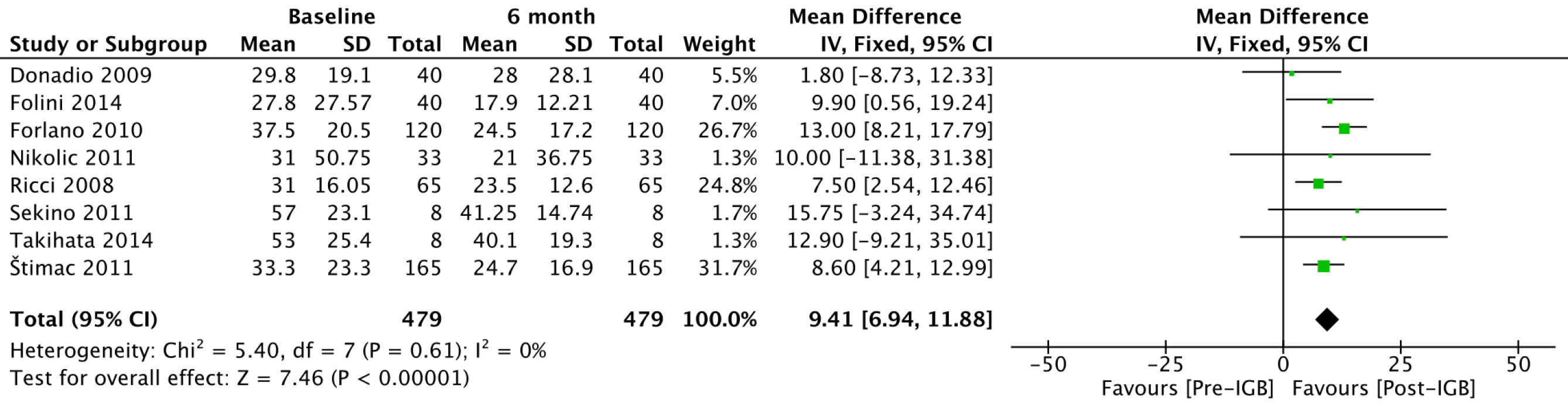
GGT (IU/L): Eight studies[20,21,23-27,29] with 479 patients were included in the outcome meta-analysis (Figure 4). The mean reduction in serum GGT levels was 9.41 (95%CI: 6.94-11.88; P < 0.01) after 6 mo of IGB use.
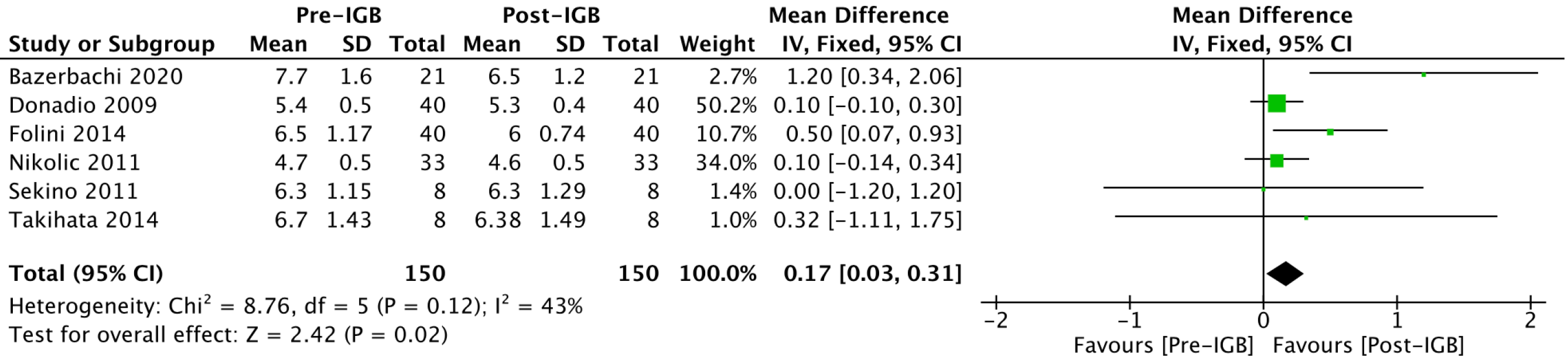
Glycated hemoglobin (%): Six studies[20-24,27] with 150 patients analyzed the effect of the IGB on glycated hemoglobin (Figure 5). The mean reduction in serum glycated hemoglobin values was 0.17% (95%CI: 0.03-0.31; P = 0.02) after 6 mo of IGB placement.
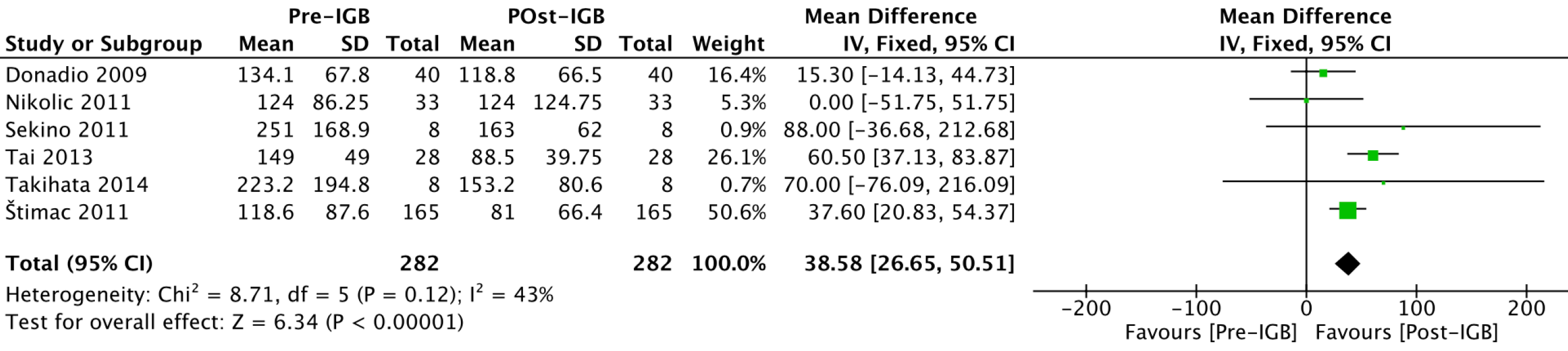
Triglycerides (mg/dL): Six studies[20,21,23,27-29] with 282 patients analyzed the effect of the IGB on serum triglyceride levels (Figure 6). The mean reduction in triglycerides was 38.58 (95%CI: 26.65-50.51; P < 0.01) after 6 mo of use of the balloon.
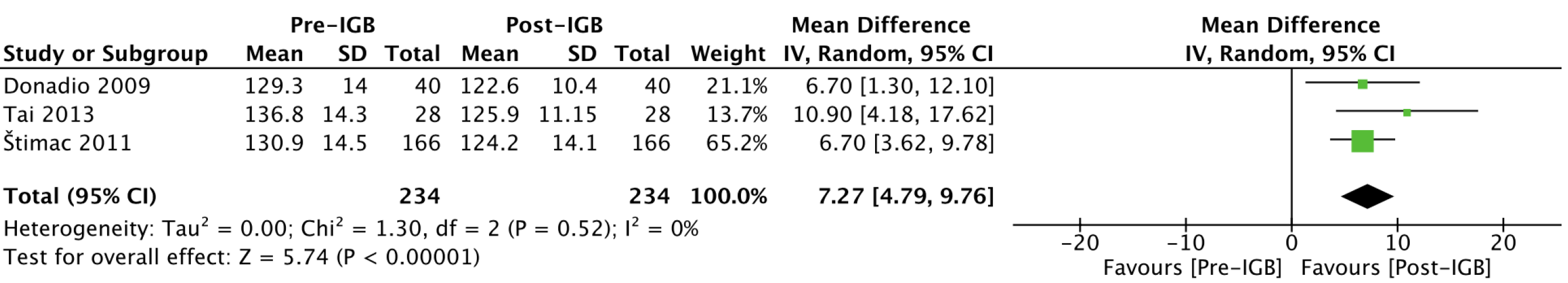
Systolic blood pressure (mmHg): Three studies[23,28,29] with 234 patients analyzed the effect of the IGB on blood pressure levels (Figure 7). After 6 mo of IGB placement, the mean reduction in systolic blood pressure was 7.27 (95%CI: 4.79-9.76; P < 0.01).
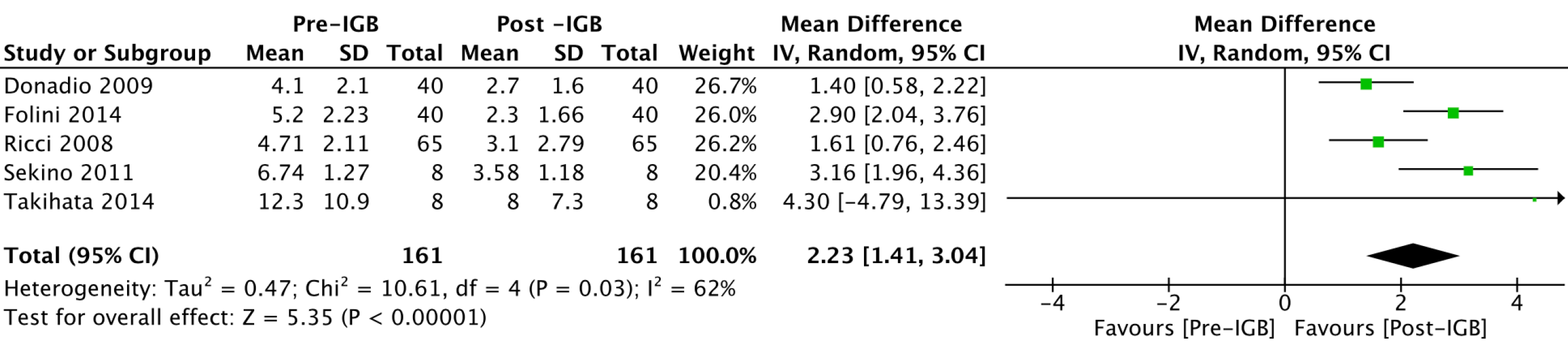
HOMA-IR: Five studies[20,23-25,27], with 161 patients, were included in the outcome meta-analysis. The mean reduction in HOMA-IR values was 2.23 (95%CI: 1.41-3.04; P < 0.01) after 6 mo using the IGB (Figure 8).
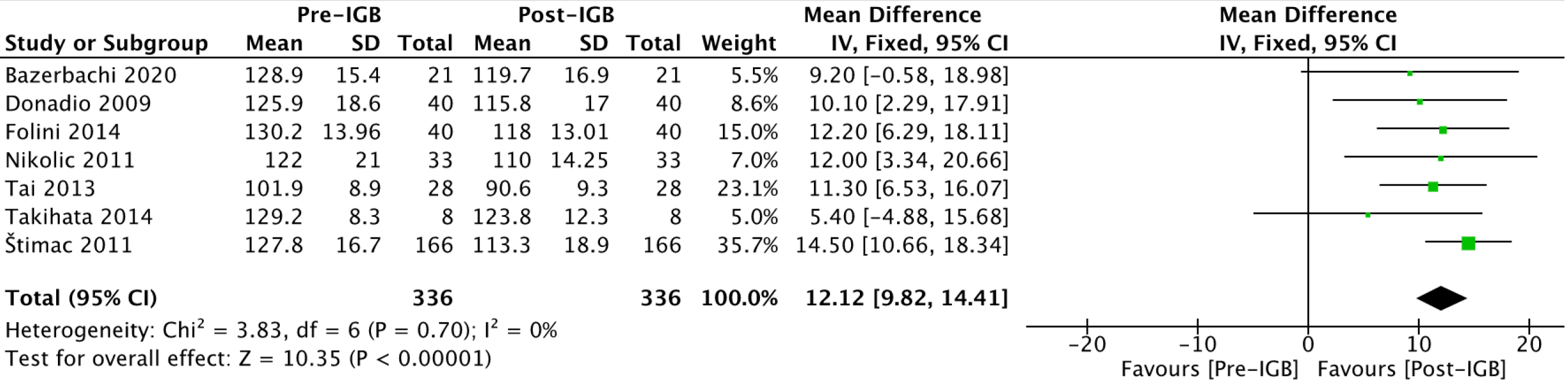
Abdominal circumference (cm): Seven studies[20-24,28,29], with 336 patients (Figure 9), were included in the outcome meta-analysis. The mean reduction in abdominal circumference was 12.12 (95%CI: 9.82-14.41; P < 0.01) after 6 mo of IGB use.
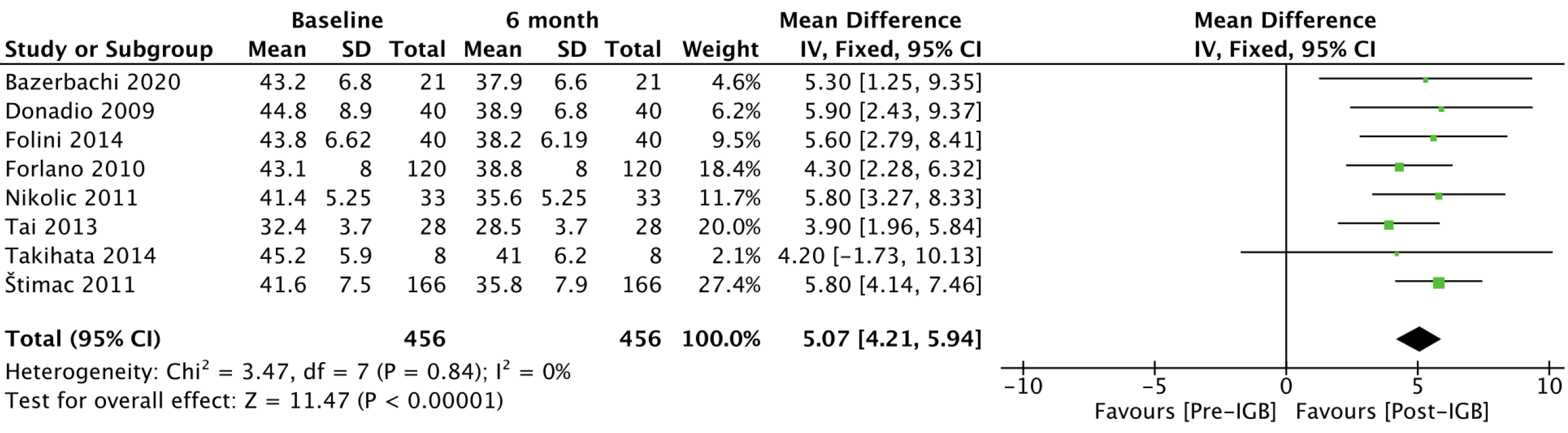
BMI (kg/m2): Eight studies[20-25,28,29], with 456 patients, were included in the outcome meta-analysis (Figure 10). The mean reduction in BMI was 5.07 (95%CI: 4.21-5.94; P < 0.01) after 6 mo of use of the IGB.
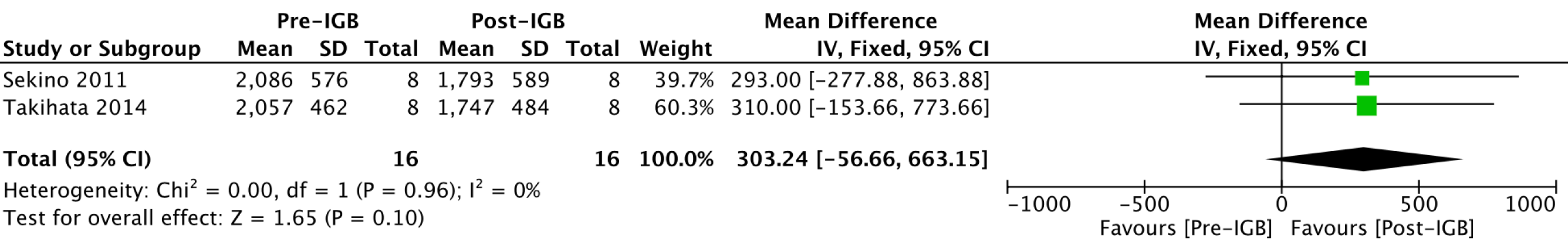
Liver volume (cm3): Two studies[20,27], with 16 patients, were included in the meta-analysis of the outcome (Figure 11). The mean reduction in liver volume was 303 cm3 (95%CI: -56.6-663.15; P = 0.1) after 6 mo of using the IGB but without statistical significance.
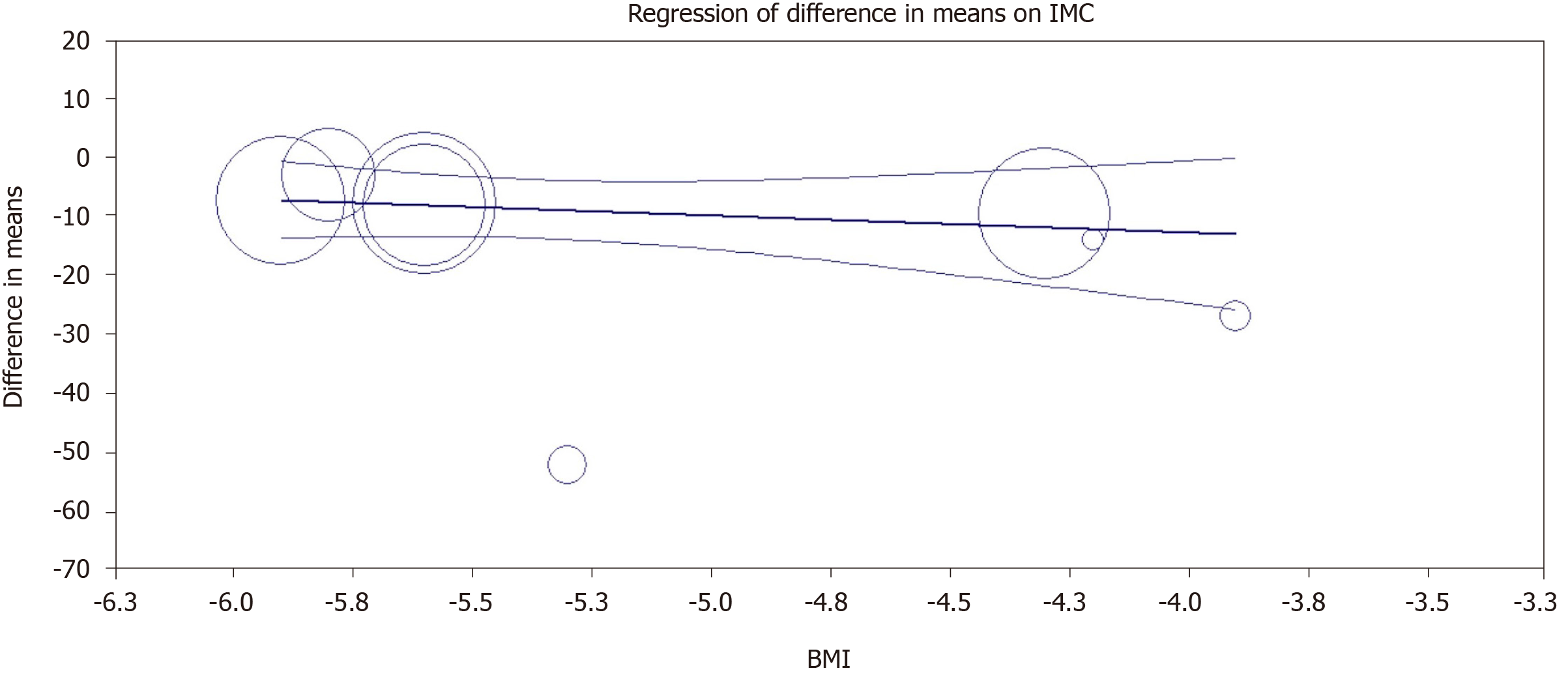
In the analysis by logistic meta-regression, there was no statistically significant correlation between the reduction in ALT and the reduction in BMI, with a P = 0.37. The graphical correlation between the outcomes is shown in Figure 12.
This is the first meta-analysis to assess the role of the IGB in the new definition of MAFLD. The IGB is an endoscopic bariatric and metabolic therapy for short-term management of obesity that has gained popularity due to its low rate of complications and reversibility[30]. Its mechanism of action is based on the occupation of space in the stomach causing a delay in gastric emptying, changes in gastric accommodation, neurohormonal effects, increased feelings of satiety and consequently a reduction in caloric intake[31]. A meta-analysis of randomized clinical trials published in 2020[13 evidenced that the IGB placement provided a loss of 17.98% of excess weight compared to the control group, showing to be an effective technique for weight loss. However, its metabolic effects were not evaluated.
The inclusion criteria for MAFLD showed that factors such as obesity, type 2 diabetes mellitus and metabolic disorders [increased waist circumference, increased blood pressure, lipidogram abnormalities, insulin resistance (IR) and increased C-reactive protein] were isolated variables related to progression to the most severe forms of liver disease under histopathological analysis[32,33]. Therefore, the control of progression factors is of fundamental importance in the management of these patients.
In the analysis of the metabolic parameters obtained by our study, we found results that show that IGB placement improves glycated hemoglobin, triglycerides, systolic blood pressure, abdominal circumference and HOMA-IR parameters. The improvement in such outcomes reflects a positive effect of IGB on metabolic dysfunction parameters, which are inclusion criteria in the new MAFLD classification and nomenclature.
The main relationship between obesity, fatty liver and metabolic syndrome appears to be in IR. IR is associated with a decrease in circulating adiponectin, a hormone secreted by adipocytes, that triggers fatty acid oxidation in the liver, favoring the increase and accumulation of visceral fat[34]. According to Bazerbachi et al[22], IGB has a weight-dependent pathway and a weight-independent pathway justifying the improvement in both the metabolic and inflammatory profiles of liver disease. The first is related to an improvement of IR in peripheral organs. The second, in turn, is linked to a downregulation in ghrelin and hunger control, a reduction of postprandial glycemia and an improvement of the action of Sirtuin 1[35]. In this sense, the improvement of IR, represented by the evaluation of HOMA-IR[36], a mathematical model that assesses IR and functional capacity of pancreatic beta cells, seems to have a fundamental role in the positive impact of IGB on MAFLD.
In the meta-regression correlating the reduction in BMI with the reduction in liver enzymes, no statistically significant relationship was found between the two variables, showing that the improvement in ALT levels was an independent outcome of weight loss after the use of the IGB.
As demonstrated in the results of our meta-analysis, there were a statistically significant reduction in ALT and GGT levels, inferring a significant positive response in the progression of MAFLD. Although the histological evaluation by percutaneous liver biopsy is the gold standard in the evaluation of the degree of steatosis and steatohepatitis and the presence of fibrosis, this still presents limitations regarding its availability and risk of adverse events (AEs). The main AEs range from transient hypotension and pain to more serious complications such as bleeding, pneumothorax and death. A case series of 847 patients described by Filingeri et al[37] reported an incidence of post-procedural bleeding of approximately 2.4%.
Considering the risk of AEs, the use of alternative methods to assess clinical evolution and improvement, such as biomarkers and certain imaging methods, is necessary. The use of liver enzymes as an indirect marker of liver steatosis is controversial. Studies have shown that elevated liver enzymes can be used as a predictor of liver inflammation in obese individuals regardless of metabolic syndrome[38]. In patients undergoing bariatric surgery, the reduction in ALT and GGT is a predictor of improvement in lobular inflammation and liver fibrosis assessed in biopsies[39]. However, patients with advanced fibrosis may have normal transaminase levels[40].
Two of the studies found in our data search[10,22] demonstrated histopathological improvement in liver biopsies 6 mo after placement of IGB. Because they are studies with different designs, they could not be correlated in this meta-analysis. According to a randomized clinical trial[10] that included 18 patients, there was a statistically significant reduction in the nonalcoholic fatty liver disease Activity Score in the comparison between the use of IGB and sham procedure (decrease from score 5 to 2 with P < 0.03). A similar endpoint was found in the uncontrolled study conducted by Bazerbachi et al[22], which included 21 patients demonstrating histological improvement through nonalcoholic fatty liver disease Activity Score (decrease from score 4 to 1 with P < 0.001), an improvement in liver fibrosis measured by nuclear magnetic resonance and a reduction in ALT levels after 6 mo of IGB use.
In the assessment of the impact of IGB on image parameters of hepatic steatosis, the studies analyzed did not show linearity in the assessment methods. Folini et al[24] found a positive correlation between the improvement in the fraction of liver fat, measured by magnetic resonance imaging, and a reduction in GGT, BMI and waist circumference 6 mo after IGB placement. Similar results were evidenced by Bazerbachi et al[22], which found a reduction in hepatic fibrosis, measured on nuclear magnetic resonance elastography, after IGB use. In the meta-analysis of liver volume by computed tomography, assessed by two studies involving 16 patients, a reduction of 330 cm3 was observed after 6 mo of IGB placement but without statistical significance.
Regarding adverse effects, five studies[21,25,27-29] evaluated reported some AEs. The main ones being nausea, vomiting and abdominal pain, which were mostly controlled with symptomatic medications. Only three studies[21,25,29] reported early balloon withdrawal due to refractory symptoms. No study reported deaths or serious AEs. In a meta-analysis[41] including 6101 patients, nausea/vomiting and abdominal pain in 23% and 19.9% of patients, respectively, was described. Serious complications such as perforation and death were reported in 0.1% and 0.05%, respectively[41].
This study has some limitations. The short follow-up time (the studied outcomes were analyzed 6 mo after the insertion of the IGB) and the heterogeneity of the patients included in the studies shows how obesity is a plural disease that makes long-term results difficult to assess. Another limitation of our study corresponds to the indirect analysis of the improvement of hepatic steatosis employing liver enzymes, without a significant sample of histopathological analysis, considered as the gold standard as well as the existence of only one randomized controlled study on the subject. This showed the difficulty in including the biopsy in controlled studies due to its risks, costs and availability.
Because MAFLD is a disease with a high prevalence and complex pathophysiology that involves a multidisciplinary approach of the patients with dietary, pharmacological and often surgical interventions, the IGB should be considered as another tool in the therapy of this population. Its positive effects in the control of metabolic disorders, biomarkers of hepatic metabolism and histology of patients with MAFLD may play an important role in controlling this new worldwide epidemic.
The IGB showed significant efficacy in reducing liver enzymes in patients with MAFLD as well as improving metabolic parameters related to disease progression such as systolic blood pressure, triglycerides, HOMA-IR, waist circumference and glycated hemoglobin.
Endoscopy has improved and has become the treatment of several diseases in recent decades. Bariatric endoscopy, through its various devices, helps in the treatment of obesity and its complications. Thus, the intragastric balloon (IGB) proves to be an effective and safe therapy for coping with this disease, and its indications have increased.
Metabolic dysfunction-associated fatty liver disease (MAFLD) corresponds to the accumulation of fat in the liver linked with metabolic dysregulation and has a high prevalence rate among the population. Unfortunately, no pharmacological therapy has yet shown efficacy in its treatment. In this sense, there is a need for new therapies to treat this new global epidemic.
We aimed to evaluate the effect of IGB in patients with MAFLD through the assessment of liver enzymes, imaging and metabolic markers in a systematic review of literature and meta-analysis.
This systematic review was conducted according to the PRISMA guidelines and registered in PROSPERO international database. The search was performed in the electronic databases (MEDLINE, Embase, Cochrane, LILACS) and grey literature. The quality of evidence was assessed utilizing criteria from Grading Recommendations Assessment, Development, and Evaluation. The risk of bias was assessed by the Risk of Bias in Non-randomized Studies-of Interventions tool and the data were meta-analyzed using the RevMan software (Review Manager Software version 5.4-Cochrane Collaboration Copyright© 2020) using the inverse variance test.
Ten studies (non-randomized studies-of interventions) with 508 patients were meta-analyzed from an initial search of 1674 articles. The outcomes analyzed before and after 6 mo of IGB removal were alanine aminotransferase (IU/L), gamma-glutamyltransferase (IU/L), glycated hemoglobin (%), triglycerides (mg/dL), systolic blood pressure (mmHg), homeostatic model assessment, abdominal circumference (cm), body mass index (kg/m2) and liver volume (cm3). After 6 mo of use, the IGB showed an improvement in alanine aminotransferase, gamma-glutamyltransferase, glycated hemoglobin, triglycerides, systolic blood pressure, homeostatic model assessment, abdominal circumference and body mass index. The liver volume analysis showed a non-statistically significant reduction.
Our findings suggest that IGB had a significant improvement in liver enzymes (alanine aminotransferase and gamma-glutamyltransferase) in patients with MAFLD as well as improved metabolic biomarkers related to disease progression.
Future studies should assess prolonged follow-up of patients after the intervention to analyze the long-term response to the improvements observed in the initial studies. A histological analysis using liver biopsies seems to be the best method of analyzing the effects of the IGB on the progression of MAFLD, and further studies should consider this method of evaluation.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Di Pasqua LG, Wu SZ S-Editor: Fan JR L-Editor: Filipodia P-Editor: Wang LL
| 1. | Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434-438. [PubMed] |
| 2. | Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:e1-2014.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2367] [Cited by in RCA: 2201] [Article Influence: 440.2] [Reference Citation Analysis (1)] |
| 3. | Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1516] [Cited by in RCA: 2138] [Article Influence: 213.8] [Reference Citation Analysis (0)] |
| 4. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 2819] [Article Influence: 563.8] [Reference Citation Analysis (1)] |
| 5. | Sanyal AJ. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2019;16:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 379] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 6. | Tahir M, Martinez A, Ravikumar NPG, Zhou K. A Systematic Review of the Non-Alcoholic Fatty Liver Disease (NAFLD) Treatment Strategies: 948. Off J Am Coll Gastroenterol|ACG. 2018;113 [cited 23 May 2021]. Available from: https://journals.lww.com/ajg/Fulltext/2018/10001/A_Systematic_Review_of_the_Non_Alcoholic_Fatty.948. |
| 7. | Jeong SW. Nonalcoholic Fatty Liver Disease: A Drug Revolution Is Coming. Diabetes Metab J. 2020;44:640-657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Sullivan S, Edmundowicz SA, Thompson CC. Endoscopic Bariatric and Metabolic Therapies: New and Emerging Technologies. Gastroenterology. 2017;152:1791-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 9. | Bazerbachi F, Vargas EJ, Abu Dayyeh BK. Endoscopic Bariatric Therapy: A Guide to the Intragastric Balloon. Am J Gastroenterol. 2019;114:1421-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Lee YM, Low HC, Lim LG, Dan YY, Aung MO, Cheng CL, Wee A, Lim SG, Ho KY. Intragastric balloon significantly improves nonalcoholic fatty liver disease activity score in obese patients with nonalcoholic steatohepatitis: a pilot study. Gastrointest Endosc. 2012;76:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | de Oliveira GHP, de Moura DTH, Funari MP, McCarty TR, Ribeiro IB, Bernardo WM, Sagae VMT, Freitas JR Jr, Souza GMV, de Moura EGH. Metabolic Effects of Endoscopic Duodenal Mucosal Resurfacing: a Systematic Review and Meta-analysis. Obes Surg. 2021;31:1304-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Ribeiro IB, Kotinda APST, Sánchez-Luna SA, de Moura DTH, Mancini FC, de Souza TF, Matuguma SE, Sakai CM, Rocha RSP, Luz GO, Lera Dos Santos ME, Chaves DM, Franzini TAP, de Moura ETH, de Moura EGH. Adverse Events and Complications with Intragastric Balloons: a Narrative Review (with Video). Obes Surg. 2021;31:2743-2752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Kotinda APST, de Moura DTH, Ribeiro IB, Singh S, da Ponte Neto AM, Proença IM, Flor MM, de Souza KL, Bernardo WM, de Moura EGH. Efficacy of Intragastric Balloons for Weight Loss in Overweight and Obese Adults: a Systematic Review and Meta-analysis of Randomized Controlled Trials. Obes Surg. 2020;30:2743-2753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 14. | Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15040] [Cited by in RCA: 15847] [Article Influence: 1584.7] [Reference Citation Analysis (1)] |
| 15. | Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7463] [Cited by in RCA: 8233] [Article Influence: 823.3] [Reference Citation Analysis (0)] |
| 16. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7683] [Cited by in RCA: 10804] [Article Influence: 1200.4] [Reference Citation Analysis (2)] |
| 17. | Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11058] [Cited by in RCA: 14870] [Article Influence: 874.7] [Reference Citation Analysis (0)] |
| 18. | Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4895] [Cited by in RCA: 6881] [Article Influence: 344.1] [Reference Citation Analysis (0)] |
| 19. | Patel MK, Kulkarni YS, Dhande GW. Dolichos biflorus L., a new host of Xanthomonas phaseoli sojense (Hedges) Dowson. Curr Sci. 1949;18:83. [PubMed] |
| 20. | Takihata M, Nakamura A, Aoki K, Kimura M, Sekino Y, Inamori M, Maeda S, Gotoh E, Nakajima A, Terauchi Y. Comparison of intragastric balloon therapy and intensive lifestyle modification therapy with respect to weight reduction and abdominal fat distribution in super-obese Japanese patients. Obes Res Clin Pract. 2014;8:e331-e338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Nikolic M, Mirosevic G, Ljubicic N, Boban M, Supanc V, Nikolic BP, Zjacic-Rotkvic V, Bekavac-Beslin M, Gacina P. Obesity treatment using a Bioenterics intragastric balloon (BIB)--preliminary Croatian results. Obes Surg. 2011;21:1305-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Bazerbachi F, Vargas EJ, Rizk M, Maselli DB, Mounajjed T, Venkatesh SK, Watt KD, Port JD, Basu R, Acosta A, Hanouneh I, Gara N, Shah M, Mundi M, Clark M, Grothe K, Storm AC, Levy MJ, Abu Dayyeh BK. Intragastric Balloon Placement Induces Significant Metabolic and Histologic Improvement in Patients With Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol. 2021;19:146-154.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 23. | Donadio F, Sburlati LF, Masserini B, Lunati EM, Lattuada E, Zappa MA, Mozzi E, Beck-Peccoz P, Orsi E. Metabolic parameters after BioEnterics Intragastric Balloon placement in obese patients. J Endocrinol Invest. 2009;32:165-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Folini L, Veronelli A, Benetti A, Pozzato C, Cappelletti M, Masci E, Micheletto G, Pontiroli AE. Liver steatosis (LS) evaluated through chemical-shift magnetic resonance imaging liver enzymes in morbid obesity; effect of weight loss obtained with intragastric balloon gastric banding. Acta Diabetol. 2014;51:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Forlano R, Ippolito AM, Iacobellis A, Merla A, Valvano MR, Niro G, Annese V, Andriulli A. Effect of the BioEnterics intragastric balloon on weight, insulin resistance, and liver steatosis in obese patients. Gastrointest Endosc. 2010;71:927-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Ricci G, Bersani G, Rossi A, Pigò F, De Fabritiis G, Alvisi V. Bariatric therapy with intragastric balloon improves liver dysfunction and insulin resistance in obese patients. Obes Surg. 2008;18:1438-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Sekino Y, Imajo K, Sakai E, Uchiyama T, Iida H, Endo H, Hosono K, Sakamoto Y, Fujita K, Yoneda M, Takahashi H, Koide T, Tokoro C, Abe Y, Saito S, Maeda S, Gotoh E, Takihata M, Terauchi Y, Nakajima A, Inamori M. Time-course of changes of visceral fat area, liver volume and liver fat area during intragastric balloon therapy in Japanese super-obese patients. Intern Med. 2011;50:2449-2455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Tai CM, Lin HY, Yen YC, Huang CK, Hsu WL, Huang YW, Chang CY, Wang HP, Mo LR. Effectiveness of intragastric balloon treatment for obese patients: one-year follow-up after balloon removal. Obes Surg. 2013;23:2068-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Stimac D, Majanović SK, Turk T, Kezele B, Licul V, Orlić ZC. Intragastric balloon treatment for obesity: results of a large single center prospective study. Obes Surg. 2011;21:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Moura D, Oliveira J, De Moura EG, Bernardo W, Galvão Neto M, Campos J, Popov VB, Thompson C. Effectiveness of intragastric balloon for obesity: A systematic review and meta-analysis based on randomized control trials. Surg Obes Relat Dis. 2016;12:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 31. | Gómez V, Woodman G, Abu Dayyeh BK. Delayed gastric emptying as a proposed mechanism of action during intragastric balloon therapy: Results of a prospective study. Obesity (Silver Spring). 2016;24:1849-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 32. | Rastogi A, Shasthry SM, Agarwal A, Bihari C, Jain P, Jindal A, Sarin S. Non-alcoholic fatty liver disease - histological scoring systems: a large cohort single-center, evaluation study. APMIS. 2017;125:962-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 33. | Arrese M, Barrera F, Triantafilo N, Arab JP. Concurrent nonalcoholic fatty liver disease and type 2 diabetes: diagnostic and therapeutic considerations. Expert Rev Gastroenterol Hepatol. 2019;13:849-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 2005;42:987-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 615] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 35. | Mariani S, di Giorgio MR, Martini P, Persichetti A, Barbaro G, Basciani S, Contini S, Poggiogalle E, Sarnicola A, Genco A, Lubrano C, Rosano A, Donini LM, Lenzi A, Gnessi L. Inverse Association of Circulating SIRT1 and Adiposity: A Study on Underweight, Normal Weight, and Obese Patients. Front Endocrinol (Lausanne). 2018;9:449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22373] [Cited by in RCA: 24499] [Article Influence: 612.5] [Reference Citation Analysis (0)] |
| 37. | Filingeri V, Sforza D, Tisone G. Complications and risk factors of a large series of percutaneous liver biopsies in patients with liver transplantation or liver disease. Eur Rev Med Pharmacol Sci. 2015;19:1621-1629. [PubMed] |
| 38. | Yamada J, Tomiyama H, Yambe M, Koji Y, Motobe K, Shiina K, Yamamoto Y, Yamashina A. Elevated serum levels of alanine aminotransferase and gamma glutamyltransferase are markers of inflammation and oxidative stress independent of the metabolic syndrome. Atherosclerosis. 2006;189:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 39. | Dixon JB, Bhathal PS, O'Brien PE. Weight loss and non-alcoholic fatty liver disease: falls in gamma-glutamyl transferase concentrations are associated with histologic improvement. Obes Surg. 2006;16:1278-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 40. | Ong JP, Elariny H, Collantes R, Younoszai A, Chandhoke V, Reines HD, Goodman Z, Younossi ZM. Predictors of nonalcoholic steatohepatitis and advanced fibrosis in morbidly obese patients. Obes Surg. 2005;15:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 209] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 41. | Yorke E, Switzer NJ, Reso A, Shi X, de Gara C, Birch D, Gill R, Karmali S. Intragastric Balloon for Management of Severe Obesity: a Systematic Review. Obes Surg. 2016;26:2248-2254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |