Published online Jul 27, 2021. doi: 10.4254/wjh.v13.i7.790
Peer-review started: May 4, 2021
First decision: June 4, 2021
Revised: June 10, 2021
Accepted: July 9, 2021
Article in press: July 9, 2021
Published online: July 27, 2021
Processing time: 79 Days and 16.6 Hours
Non-alcoholic fatty liver disease (NAFLD) is the leading cause of chronic liver disease in children and adolescents.
To determine the prevalence and risk factors of steatosis and advanced fibrosis using transient elastography (TE) in the United States’ adolescent population.
Using the National Health and Nutrition Examination Survey 2017-2018, adolescent participants aged 13 to 17 years who underwent TE and controlled attenuation parameter (CAP) were included in this study. Forty-one factors associated with liver steatosis and fibrosis were collected. Univariate and multivariate linear regression analysis were used to identify statistically significant predictors.
Seven hundred and forty participants met inclusion criteria. Steatosis (S1-S3), based on CAP, and advanced fibrosis (F3-F4), based on TE, were present in 27% and 2.84% of the study population, respectively. Independent predictors of steatosis grade included log of alanine aminotransferase, insulin resistance, waist-to-height ratio, and body mass index. Independent predictors of fibrosis grade included steatosis grade, non-Hispanic black race, smoking history, and systolic blood pressure.
This study demonstrated a high prevalence of steatosis in the United States’ adolescent population. Almost 3% of United States’ adolescents had advanced fibrosis. These findings are concerning because a younger age of onset of NAFLD can lead to an earlier development of severe disease, including steatohepatitis, cirrhosis, and liver decompensation.
Core Tip: Adolescents in the United States were found to have a high prevalence of non-alcoholic fatty liver disease, which was estimated to be 27%. Nearly 3% were found to have advanced fibrosis diagnosed by transient elastography. The severity of steatosis was associated with alanine aminotransferase, insulin resistance, waist-to-height ratio, and body mass index. Risk factors of fibrosis included steatosis grade, non-Hispanic black race, smoking history, and systolic blood pressure.
- Citation: Atsawarungruangkit A, Elfanagely Y, Pan J, Anderson K, Scharfen J, Promrat K. Prevalence and risk factors of steatosis and advanced fibrosis using transient elastography in the United States’ adolescent population. World J Hepatol 2021; 13(7): 790-803
- URL: https://www.wjgnet.com/1948-5182/full/v13/i7/790.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i7.790
With the rise of obesity and metabolic syndrome among younger populations, non-alcoholic fatty liver disease (NAFLD) is a growing concern in adolescents. NAFLD has become the most common cause of chronic liver disease in children and adolescents, with a prevalence previously estimated to be 3%-10% in the global pediatric population[1,2]. The prevalence of NAFLD in children with obesity is exceedingly high at 40%-70%[3]. Unsurprisingly, the rates of NAFLD have grown with the rise of childhood obesity over recent decades. Other established risk factors include insulin resistance, metabolic syndrome, and dyslipidemia. The development of NAFLD in childhood is clinically important because of the progressive nature of the disease. Earlier development of NAFLD increases the risk of earlier-onset fibrosis and frank cirrhosis[4].
Liver biopsy is the gold-standard diagnostic test for NAFLD. It not only confirms the diagnosis of NAFLD, but can also grade the level of inflammation and stage the liver fibrosis. However, this invasive procedure is ill-suited to serve as a general screening tool. Non-invasive alternatives which include a physical exam, biochemical tests, and serum biomarkers for fibrosis are not reliable predictors of fibrosis[5,6]. Because fibrosis is the single most important predictor of long-term mortality in NAFLD, transient elastography (TE) has emerged as a non-invasive, reproducible modality in the assessment of patients with NAFLD. Using ultrasound, TE measures the liver stiffness as a proxy for fibrosis stage. Its accuracy has been demonstrated in adult patients with fibrosis secondary to chronic hepatitis B and C, alcoholic and non-alcoholic liver disease, and biliary disease[7-9]. TE’s accuracy however is reduced by active hepatitis, increased waist circumference, recent eating, and liver congestion. In adults with NAFLD, TE has an area under the receiver operating characteristic for detecting advanced fibrosis (bridging fibrosis or cirrhosis) of 0.88[10]. In children and adolescents, TE has been validated for chronic liver disease, including NAFLD with similar accuracy, but the data are limited[11-14]. Further research is needed to confirm the liver stiffness thresholds for fibrosis used in the pediatric population.
In addition to liver stiffness, modern TE is also able to calculate the controlled attenuation parameter (CAP). CAP is a quantitative measurement for steatosis. In adults, significant steatosis is defined by having more than 33% of the hepatocytes on a liver biopsy contain steatotic architecture. This correlates to CAP scores greater than 250 db/m[7]. Cut-offs for CAP of 248 db/m, 268 db/m, and 280 db/m were proposed to correspond with steatosis ≥ 11%, ≥ 33%, and ≥ 66%, respectively[15]. CAP cut-offs in children are suspected to be similar[16,17], but require additional validation.
In the present study, we reported the prevalence of NAFLD characterized by TE and CAP in United States adolescents. Our study employed novel data from the unselected, general cohort of the 2017-2018 National Health and Nutrition Examina
NHANES is a program of studies designed to assess the health and nutritional status of adults and children in the United States, conducted by the National Center for Health Statistics (NCHS)[18]. The survey collected multiple data sets, including demographic, interviews, physical examinations, and laboratory testing of biologic samples. NHANES protocol was approved by the NCHS Research Ethics Review Board.
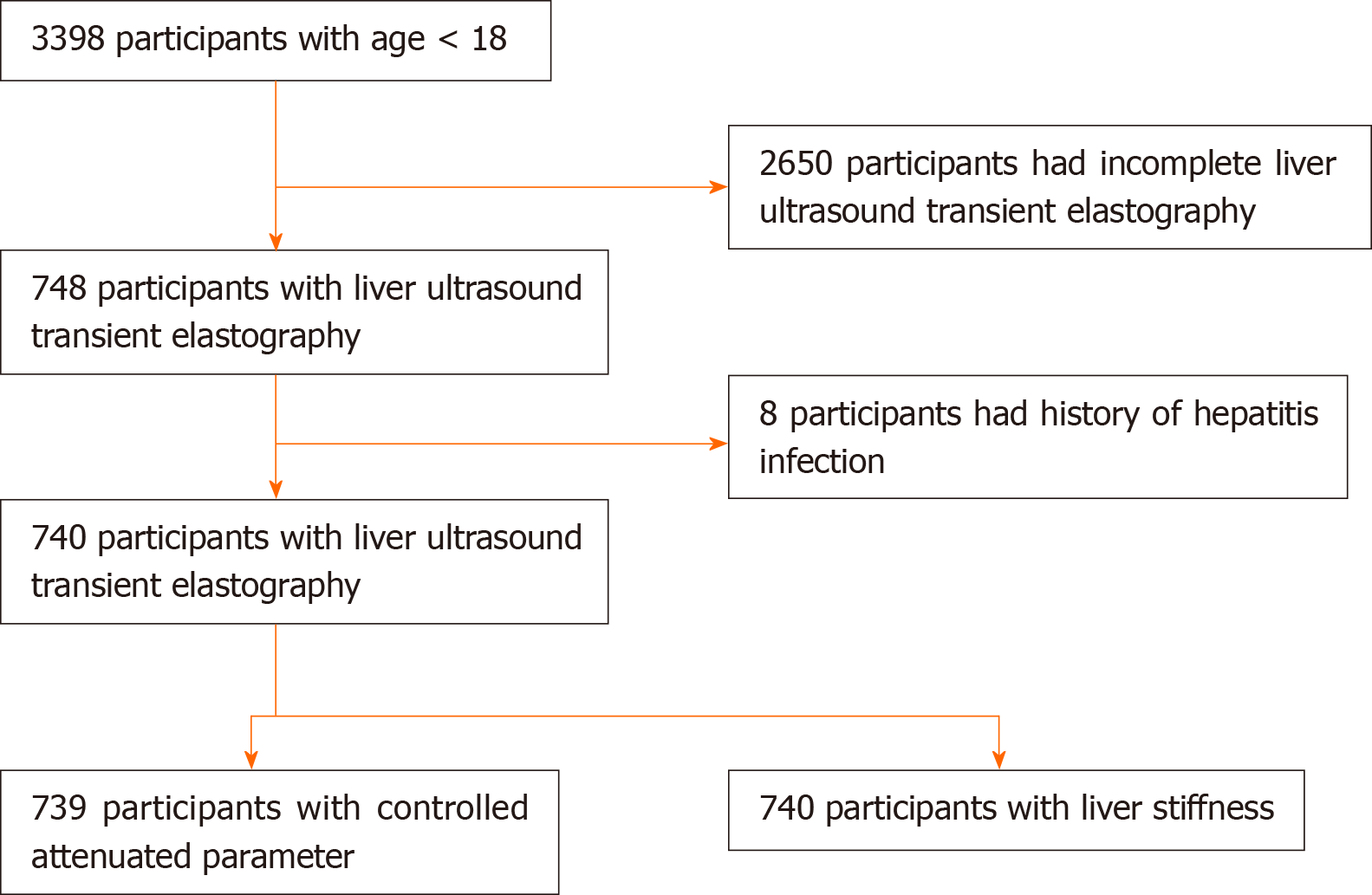
Currently, NHANES has been collecting data in a 2-year cycle. The liver ultrasound transient elastography examination was first introduced in NHANES 2017-2018, which has been released in March 2020 along with other data files. Out of 9254 participants in NHANES 2017-2018, there were 740 participants aged younger than 18 years that met inclusion criteria for this study. The exclusion criteria included: (1) Incomplete TE exam status; and (2) Hepatitis B, hepatitis C, or hepatitis E infection. It is worth noting that alcohol consumption data in participants younger than 18 years is not publicly accessible and has not been published by the time of writing this article.
We included 41 factors associated with liver steatosis and fibrosis in this study: demographic (i.e., age , gender, race/ethnicity, and smoking), body measurement (i.e., body mass index (BMI), waist-to-height ratio, and waist-to-hip ratio), physical activities (days of physical active, hours of TV/videos watching, and hours of computer usage), diet (i.e., energy, protein, carbohydrate, sugars, dietary fiber, fat, saturated fatty acids, and cholesterol), blood pressure (i.e., systolic and diastolic), laboratory tests [i.e., triglycerides, uric acid, aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyl transferase, alkaline phosphatase, total bilirubin, total protein, albumin, iron, total iron binding capacity, transferrin saturation, ferritin, total cholesterol, direct HDL-Cholesterol, high-sensitivity C-reactive protein, platelet count, HbA1c, fasting glucose, and insulin)]. Additionally, we manually calculated LDL-cholesterol and homeostatic model assessment of insulin resistance (HOMA-IR) from the existing variables.
The above variables were chosen based on the availability of data in NHANES 2017-2018, the usage in clinical practice, and the supporting evidence that demonstrated an association with NAFLD. Additionally, we compared the predictive performance of liver fibrosis indices with the steatosis grade and fibrosis stage. Three liver fibrosis indices used in this study included (1) AST to platelet ratio index (APRI)[19]; (2) Fibrosis-4 (FIB-4) index[20]; and (3) Pediatric NAFLD fibrosis index (PNFI)[21].
Assessment by liver ultrasound TE examination resulted in measurement of CAP. CAP is a standardized non-invasive measure for assessment of fibrosis and quantification of steatosis in NAFLD[22]. Cut-off values for median CAP score for different grades of steatosis (S0-S3) were derived from a meta-analysis on CAP technology. S0 was defined as a score of less than 248 dB/m (< 10% steatosis). S1 was defined as a score of 248 to less than 268 dB/m [10% to < 33% steatosis (mild)]. S2 was a defined as a score of 268 to less than 280 dB/m [33% to < 66% steatosis (moderate)]. S3 was defined as a score of 280 dB/m or more [≥ 66% steatosis (severe)][15]. Median CAP scores of 248 dB/m or greater (≥S1) were considered as suspected steatosis.
Participants were also categorized according to stage of hepatic fibrosis. The METAVIR scoring system was used for fibrosis staging (F0-F4)[23]. Stages of hepatic fibrosis ranged from no fibrosis (F0) through intermediate stages of hepatic fibrosis (F1-F3) to end-stage cirrhosis (F4)[24]. The degree of fibrosis was equivalent to the liver stiffness measured in kPa as calculated by liver ultrasound transient elastrography[25]. Stage F0-F1 were defined as a median stiffness < 7 kPa. Stage F2 was defined as a median stiffness of 7 to < 8.6 kPa. Stage F3 was defined as a median stiffness of 8.6 to < 11.5 kPa. Stage F4 was defined as a median stiffness ≥ 11.5 kPa. Participants with a median stiffness of 8.6 kPa or greater (≥F3) were considered to have advanced fibrosis[26].
BMI was discretized into four classes (1) Underweight, BMI < 5th percentile; (2) Normal, 5th percentile ≤ BMI < 85th percentile; (3) Risk of overweight, 85th percentile ≤ BMI < 95th percentile; and (4) Overweight BMI ≥ 85th percentile[27]. Participants who smoked during the past 30 d or had ever smoked ≥ 100 cigarettes in their entire lives were classified as smokers in this study.
Statistical analyses were performed using STATA Release 16 (StataCorp LP, TX, United States). Categorical and ordinal factors were presented in frequency (%). Continuous factors were presented in median (interquartile range). All continuous factors were first tested for skewness; if the distributions were extremely skewed to the right (herein defined as skewness > 3), the factors were log transformed before using them as predictors in regression models. Since the response variables evaluated in this study are the steatosis grade (0 to 3) and the fibrosis score (0 to 4), linear regression model is an appropriate model for determining if predictors are significantly associated with each response variable. The significant factors in univariate level were included as predictors in stepwise regression to determine the significant predictors in multivariate level. The significance level was 0.05.
A total of 740 participants were included in the data analysis as shown in Figure 1. General characteristics of the study population are shown in Table 1. The median age was 15 years old with male comprising greater than 50% of the study population (n = 386, 52.16%). The largest race was Non-Hispanic White (n = 229, 30.39%), followed by Non-Hispanic Black (n = 171, 23.11%) and Mexican American (n = 130, 17.57%) respectively. The majority of the study population had a steatosis grade of S0 (n = 538, 72.8%) and fibrosis stages of F0 and F1 (n = 693, 93.65%). Steatosis (S1-S3) was present in 27% of the study population. Advanced fibrosis (F3-F4) was present in 2.84% of the study population. 53.33% (n = 392) of the study population had a normal BMI, while 28.71% (n = 211) were overweight and 0.54% (n = 4) were underweight.
| All participants (n = 740) | |
| Age | 15 (13-16) |
| Sex, n (%) | |
| Male | 386 (52.16) |
| Female | 354 (47.84) |
| Race, n (%) | |
| Mexican American | 130 (17.57) |
| Other Hispanic | 55 (7.43) |
| Non-Hispanic White | 229 (30.95) |
| Non-Hispanic Black | 171 (23.11) |
| Non-Hispanic Asian | 83 (11.22) |
| Other race-including multi-racial | 72 (9.73) |
| Smoking, n (%) | 6 (0.84) |
| Steatosis grade, n (%) | |
| S0 | 538 (72.8) |
| S1 | 63 (8.53) |
| S2 | 39 (5.28) |
| S3 | 99 (13.4) |
| Fibrosis result, n (%) | |
| F0-F1 | 693 (93.65) |
| F2 | 26 (3.51) |
| F3 | 12 (1.62) |
| F4 | 9 (1.22) |
| Waist-to-height ratio | 0.48 (0.43-0.55) |
| Waist-to-hip ratio | 0.57 (0.53-0.63) |
| Body mass index, n (%) | |
| Underweight | 4 (0.54) |
| Normal | 392 (53.33) |
| Risk of overweight | 128 (17.41) |
| Overweight | 211 (28.71) |
| Days physically active at least 60 min | 4 (2-5) |
| Hours/day watch TV or videos past 30 d, n (%) | |
| Less than 1 h | 107 (14.72) |
| 1 h | 121 (16.64) |
| 2 h | 166 (22.83) |
| 3 h | 105 (14.44) |
| 4 h | 78 (10.73) |
| 5 h or more | 150 (20.63) |
| Hours/day use computer past 30 d, n (%) | |
| Less than 1 h | 68 (9.34) |
| 1 h | 85 (11.68) |
| 2 h | 131 (17.99) |
| 3 h | 83 (11.4) |
| 4 h | 100 (13.74) |
| 5 h or more | 261 (35.85) |
Data concerning social history and physical activity were also analyzed. A smoking history was endorsed by 6 participants (0.84%). The percent of study participants who spent ≥ 5 h per day of watching TV in the past 30 d was 20.63% (n = 150). Similarly, 35.85% (n = 261) of study participants reported spending ≥ 5 h per day on the computer for the past 30 d.
Table 2 is a univariate analysis of participant characteristics stratified according to steatosis grade. Out of the 47 variables, there were 28 significant predictors. Statistically significant variables that were positively associated with steatosis grade in the multivariate analysis were log of ALT (P = 0.001), HOMA-IR (P = 0.006), waist-to-height ratio (P = 0.001), and BMI (P = 0.011) (Table 3).
| Steatosis grade | Coefficient | P value | ||||
| S0 (n = 538) | S1 (n = 63) | S2 (n = 39) | S3 (n = 99) | |||
| Age | 14 (13-16) | 14 (13-16) | 15 (14-16) | 15 (14-16) | 0.0562 | 0.016a |
| Sex | ||||||
| Male | 273 (50.74%) | 29 (46.03%) | 20 (51.28%) | 64 (64.65%) | 0.1747 | 0.027 a |
| Female | 265 (49.26%) | 34 (53.97%) | 19 (48.72%) | 35 (35.35%) | ||
| Race | ||||||
| Mexican American | 82 (15.24%) | 11 (17.46%) | 7 (17.95%) | 30 (30.3%) | 0.3542 | < 0.001a |
| Other Hispanic | 42 (7.81%) | 5 (7.94%) | 1 (2.56%) | 7 (7.07%) | -0.0903 | 0.549 |
| Non-Hispanic White | 178 (33.09%) | 18 (28.57%) | 13 (33.33%) | 20 (20.2%) | -0.2008 | 0.019 a |
| Non-Hispanic Black | 128 (23.79%) | 10 (15.87%) | 11 (28.21%) | 21 (21.21%) | -0.0440 | 0.640 |
| Non-Hispanic Asian | 60 (11.15%) | 8 (12.7%) | 4 (10.26%) | 11 (11.11%) | -0.0026 | 0.983 |
| Other Race-Including Multi-Racial | 48 (8.92%) | 11 (17.46%) | 3 (7.69%) | 10 (10.1%) | 0.0666 | 0.618 |
| Smoking | 4 (0.77%) | 1 (1.59%) | 0 (0%) | 1 (1.04%) | 0.0732 | 0.868 |
| Waist-to-height ratio | 0.45 (0.42-0.51) | 0.54 (0.47-0.59) | 0.57 (0.48-0.62) | 0.6 (0.55-0.66) | 6.5565 | < 0.001a |
| Waist-to-hip ratio | 0.56 (0.52-0.6) | 0.6 (0.57-0.65) | 0.63 (0.57-0.68) | 0.64 (0.61-0.69) | 6.6835 | < 0.001a |
| Body mass index | 0.6128 | < 0.001a | ||||
| Underweight | 4 (0.75%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Normal | 349 (65.48%) | 22 (34.92%) | 10 (25.64%) | 10 (10.1%) | ||
| Risk of overweight | 97 (18.2%) | 14 (22.22%) | 8 (20.51%) | 9 (9.09%) | ||
| Overweight | 83 (15.57%) | 27 (42.86%) | 21 (53.85%) | 80 (80.81%) | ||
| Days physically active at least 60 min | 4 (2-6) | 3.5 (1-5) | 4 (2-5) | 4 (2-5) | -0.0167 | 0.348 |
| Hours/day watch TV or videos past 30 d | 2 (1-4) | 2 (1-4) | 3 (1.75-5) | 2 (1-4.25) | 0.0421 | 0.070 |
| Hours/day use computer past 30 d | 3 (2-5) | 4 (2-5) | 5 (2-5) | 4 (2-5) | 0.0560 | 0.014a |
| Diet | ||||||
| Energy (1000 kcal) | 1.82 (1.43-2.45) | 1.75 (1.26-2.28) | 1.62 (1.33-2) | 1.71 (1.34-2.38) | -0.0732 | 0.145 |
| Protein (mg) | 63.59 (48.32-86.06) | 63.15 (40.29-77.27) | 53.38 (37.92-80.78) | 64.1 (49.36-87.48) | -0.0732 | 0.145 |
| Carbohydrate (mg) | 230.94 (180.54-301.73) | 233.36 (154.21-296.51) | 213.08 (169.63-253.71) | 219.06 (173.09-290.58) | -0.0005 | 0.178 |
| Total sugars (mg) | 94.74 (67.04-133.77) | 89.84 (54.32-140.86) | 75.37 (51.43-97.67) | 90.5 (63.35-127.21) | -0.0008 | 0.224 |
| Dietary fiber (mg) | 12.85 (9.25-17.36) | 12.2 (8.77-18.3) | 12.5 (9.24-16.79) | 12.2 (8.8-16.4) | -0.0051 | 0.368 |
| Total fat (mg) | 75.09 (55.41-97.52) | 66.13 (43.61-93.44) | 62.68 (45.53-83.34) | 71.58 (47.42-95.31) | -0.0016 | 0.143 |
| Total saturated fatty acids (mg) | 25.07 (17.39-35.43) | 24.43 (11.47-32.25) | 18.7 (11.7-30.89) | 22.91 (14.97-31.11) | -0.0044 | 0.122 |
| Cholesterol (mg) | 197 (132.88-320.5) | 165 (90.25-305.5) | 162 (72.38-283.63) | 199 (134-279.25) | -0.0003 | 0.254 |
| Systolic blood pressure (mm Hg) | 106 (100-114) | 108 (103.5-114.5) | 112 (104-120) | 112 (104-120) | 0.0254 | < 0.001a |
| Diastolic blood pressure (mm Hg) | 62 (54-68) | 60 (51.5-68) | 62 (55.5-70) | 60 (54-66) | -0.0038 | 0.222 |
| Triglycerides, refrig serum (mg/dL)1 | 74 (57-98) | 79 (62-103) | 78.5 (70-105.5) | 98 (68-159) | 0.0051 | < 0.001a |
| Uric acid (mg/dL) | 4.7 (4-5.6) | 5.1 (4.15-6.05) | 5.45 (4.65-6.15) | 5.75 (4.7-6.7) | 0.1984 | < 0.001a |
| Aspartate aminotransferase (IU/L)1 | 18 (16-22) | 18 (15.25-21.75) | 16.5 (15-21) | 20.5 (18-27) | 0.0151 | 0.002a |
| Alanine aminotransferase (IU/L)1 | 12 (10-15) | 15 (11.25-19) | 14 (10-17.5) | 20.5 (14-34) | 0.0372 | < 0.001a |
| Gamma glutamyl transferase (IU/L)1 | 12 (10-15) | 12 (10-18.75) | 15.5 (10-19) | 18 (12-24) | 0.0375 | < 0.001a |
| Alkaline phosphatase (ALP) (IU/L) | 130 (87-225.75) | 121 (86.75-235) | 135 (75.5-188) | 126.5 (99-188) | -0.0003 | 0.537 |
| Total bilirubin (mg/dL)1 | 0.4 (0.3-0.6) | 0.3 (0.23-0.48) | 0.4 (0.3-0.5) | 0.4 (0.3-0.4) | -0.3419 | 0.009a |
| Total protein (g/dL) | 7.3 (7-7.5) | 7.3 (7-7.5) | 7.35 (7.15-7.6) | 7.35 (7.2-7.6) | 0.3620 | 0.002a |
| Albumin, refrigerated serum (g/dL) | 4.3 (4.1-4.5) | 4.3 (4.1-4.5) | 4.25 (4.05-4.45) | 4.2 (4-4.4) | -0.5553 | < 0.001a |
| Iron frozen, serum (μg/dL) | 85 (61-113) | 86 (58.25-105.75) | 69 (49.5-85.75) | 75 (56-103) | -0.0027 | 0.013a |
| Total iron binding capacity (μg/dL) | 348 (317.5-382) | 366 (342-392.25) | 360 (326.25-406.5) | 356 (322-385) | 0.0015 | 0.092 |
| Transferrin Saturation (%) | 24 (17-33) | 23 (15.25-30.75) | 19 (13.5-26) | 22.5 (15-30) | -0.0104 | 0.004a |
| Ferritin (ng/mL) | 39.2 (24.85-59.85) | 35.25 (18.75-57.5) | 30.85 (14.65-60.15) | 59.2 (35-93.12) | 0.0038 | < 0.001a |
| Total cholesterol (mg/dL) | 150 (134-168) | 158 (132.75-174) | 152 (139.5-166.25) | 157 (139.25-178.75) | 0.0032 | 0.035a |
| Low-density lipoprotein cholesterol (mg/dL) | 78.8 (64.8-94.6) | 85.8 (69.15-107.45) | 82.5 (70.1-97.8) | 87 (70.6-103.6) | 0.0041 | 0.019a |
| Direct high-density lipoprotein cholesterol (mg/dL) | 53 (46-61) | 50 (46-56) | 48 (41.5-55) | 44 (39-51) | -0.0238 | < 0.001a |
| HS C-reactive protein (mg/L)1 | 0.49 (0.32-1.01) | 0.72 (0.35-1.51) | 0.95 (0.43-1.89) | 1.76 (0.87-3.74) | 0.0448 | < 0.001a |
| Platelet count (1000 cells/uL) | 258 (228-292) | 269 (228.5-318.5) | 273 (239-307) | 282 (248-313) | 0.0026 | < 0.001a |
| Hemoglobin A1c (%)1 | 5.3 (5.1-5.5) | 5.3 (5.1-5.45) | 5.3 (5.1-5.6) | 5.4 (5.2-5.5) | 0.2280 | 0.054 |
| Fasting glucose (mg/dL) | 97 (93-101) | 98 (93.25-101.75) | 101 (94-103) | 99.5 (96-103) | 0.0219 | 0.017a |
| Insulin (pmol/L) | 54.96 (39.84-79.38) | 101.1 (71.58-130.8) | 88.32 (62.28-118.14) | 129.63 (75.66-185.46) | 0.0086 | < 0.001a |
| Homeostatic model assessment for insulin resistance | 2.23 (1.58-3.32) | 4.08 (2.96-5.47) | 3.56 (2.64-4.96) | 5.34 (3.08-7.78) | 0.1976 | < 0.001a |
| Predictors | Coefficient (standard error) | P value |
| Alanine aminotransferase (IU/L)1 | 0.3912 (0.1159) | 0.001 |
| Homestatic model assessment for insulin resistance | 0.0684 (0.0247) | 0.006 |
| Waist-to-height ratio | 3.2299 (0.0912) | 0.001 |
| Body mass index | 0.2335 (0.0912) | 0.011 |
Similarly, Table 4 is a univariate analysis of participant characteristics stratified according to fibrosis stage. Out of the 48 variables, there were only 9 significant predictors. In the multivariate analysis (Table 5), steatosis grade (P < 0.001), non-Hispanic black race (P = 0.002), a smoking history (P = 0.028), and systolic blood pressure (P = 0.035) were predictors of fibrosis stage that were statistically significant and positively associated with fibrosis stage.
| Fibrosis stage | Coefficient | P value | ||||
| F0 - F1 (n = 693) | F2 (n = 26) | F3 (n = 12) | F4 (n = 9) | |||
| Age | 15 (13-16) | 15 (13-17) | 14 (13-15) | 15 (14.75-17) | 0.0106 | 0.276 |
| Sex | ||||||
| Male | 356 (51.37%) | 17 (65.38%) | 6 (50%) | 7 (77.78%) | 0.0533 | 0.105 |
| Female | 337 (48.63%) | 9 (34.62%) | 6 (50%) | 2 (22.22%) | ||
| Race | ||||||
| Mexican American | 123 (17.75%) | 4 (15.38%) | 0 (0%) | 3 (33.33%) | -0.0049 | 0.909 |
| Other Hispanic | 53 (7.65%) | 1 (3.85%) | 1 (8.33%) | 0 (0%) | -0.0535 | 0.393 |
| Non-Hispanic White | 222 (32.03%) | 3 (11.54%) | 2 (16.67%) | 2 (22.22%) | -0.0685 | 0.054 |
| Non-Hispanic Black | 148 (21.36%) | 13 (50%) | 8 (66.67%) | 2 (22.22%) | 0.1309 | < 0.001a |
| Non-Hispanic Asian | 78 (11.26%) | 4 (15.38%) | 0 (0%) | 1 (11.11%) | -0.0222 | 0.669 |
| Other Race-Including Multi-Racial | 69 (9.96%) | 1 (3.85%) | 1 (8.33%) | 1 (11.11%) | -0.0230 | 0.679 |
| Smoking | 5 (0.75%) | 0 (0%) | 0 (0%) | 1 (11.11%) | 0.3967 | 0.032a |
| Waist-to-height ratio | 0.48 (0.43-0.55) | 0.49 (0.44-0.61) | 0.49 (0.4-0.61) | 0.5 (0.42-0.68) | 0.3746 | 0.042a |
| Waist-to-hip ratio | 0.57 (0.53-0.62) | 0.59 (0.54-0.69) | 0.6 (0.52-0.64) | 0.59 (0.5-0.7) | 0.2804 | 0.215 |
| Body mass index | 0.0330 | 0.079 | ||||
| Underweight | 4 (0.58%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Normal | 370 (53.78%) | 11 (42.31%) | 6 (50%) | 5 (55.56%) | ||
| Risk of overweight | 126 (18.31%) | 2 (7.69%) | 0 (0%) | 0 (0%) | ||
| Overweight | 188 (27.33%) | 13 (50%) | 6 (50%) | 4 (44.44%) | ||
| Days physically active at least 60 min | 4 (2-5) | 4 (2-5) | 2.5 (0.5-6) | 5 (2.5-6) | -0.0039 | 0.597 |
| Hours/day watch TV or videos past 30 d | 2 (1-4) | 2 (1-4) | 2.5 (2-4.5) | 2 (0-3.5) | -0.0027 | 0.779 |
| Hours/day use computer past 30 d | 3 (2-5) | 5 (3-5) | 4 (2.5-5) | 3 (0.75-5) | 0.0062 | 0.519 |
| Steatosis grade | 0.0757 | < 0.001a | ||||
| S0 | 518 (74.86%) | 13 (50%) | 3 (25%) | 4 (44.44%) | ||
| S1 | 57 (8.24%) | 2 (7.69%) | 3 (25%) | 1 (11.11%) | ||
| S2 | 35 (5.06%) | 2 (7.69%) | 2 (16.67%) | 0 (0%) | ||
| S3 | 82 (11.85%) | 9 (34.62%) | 4 (33.33%) | 4 (44.44%) | ||
| Diet | ||||||
| Energy (1000 kcal) | 1.8 (1.4-2.42) | 1.5 (1.37-2.11) | 1.62 (1.4-1.75) | 1.75 (1.32-2.4) | -0.0225 | 0.282 |
| Protein (mg) | 63.69 (46.81-85.41) | 50.66 (42.74-94.29) | 59.33 (45.9-76.55) | 68.03 (49.61-73.78) | -0.0004 | 0.405 |
| Carbohydrate (mg) | 230.55 (174.26-299.84) | 202.56 (152.11-255.23) | 204.26 (177.87-238.7) | 242.27 (186.12-305.52) | -0.0001 | 0.671 |
| Total sugars (mg) | 92.59 (64.25-133.63) | 87.43 (58.07-120.32) | 75.76 (62.31-94.74) | 94.74 (85.8-123.02) | -0.0001 | 0.697 |
| Dietary fiber (mg) | 12.7 (9.25-17.1) | 10.8 (7.62-17.64) | 10.4 (9.02-14.29) | 12.85 (10.8-16.96) | -0.0018 | 0.461 |
| Total fat (mg) | 74.07 (52.04-97.34) | 55.7 (45.07-79.29) | 65.98 (50.43-78.07) | 69.09 (45.95-97.94) | -0.0007 | 0.091 |
| Total saturated fatty acids (mg) | 24.72 (16.8-34.81) | 21.06 (14.84-29.88) | 23.04 (18.87-27.64) | 22.84 (18.35-28.35) | -0.0020 | 0.083 |
| Cholesterol (mg) | 197 (129.13-317.63) | 150.5 (85-213.25) | 162.5 (118.38-228.88) | 146.5 (124.75-310.25) | -0.0002 | 0.065 |
| Systolic blood pressure (mmHg) | 106 (102-114) | 108 (103.5-126.5) | 108 (100.5-120) | 116 (113-122) | 0.0066 | < 0.001a |
| Diastolic blood pressure (mmHg) | 62 (54-68) | 64 (53-71) | 56 (50.5-65.5) | 60 (56-68) | -0.0001 | 0.967 |
| Triglycerides, refrig serum (mg/dL)1 | 78 (61-104) | 67.5 (50-111) | 88 (61.75-161) | 88.5 (56.5-121.5) | 0.0276 | 0.471 |
| Uric acid (mg/dL) | 4.9 (4.1-5.8) | 5 (3.7-6) | 4.7 (3.3-5.98) | 5.75 (4.2-7.45) | 0.0079 | 0.560 |
| Aspartate aminotransferase (IU/L)1 | 18 (16-22) | 18 (15-25) | 15 (14-23) | 29 (20-32) | 0.0882 | 0.137 |
| Alanine aminotransferase (IU/L)1 | 13 (10-17) | 14 (9-20) | 12 (9.5-15) | 20.5 (15-37.5) | 0.0738 | 0.046a |
| Gamma glutamyl transferase (IU/L)1 | 13 (10-17) | 12 (9-16) | 12 (10-19.5) | 20.5 (14-32.5) | 0.0047 | 0.018a |
| Alkaline phosphatase (IU/L) | 129 (88-222.5) | 121.5 (81-205) | 187 (127.75-242.75) | 113 (105-129.5) | -0.0001 | 0.470 |
| Total bilirubin (mg/dL)1 | 0.4 (0.3-0.5) | 0.3 (0.2-0.6) | 0.4 (0.3-0.5) | 0.45 (0.35-0.7) | 0.0178 | 0.577 |
| Total protein (g/dL) | 7.3 (7-7.5) | 7.15 (6.8-7.3) | 7.3 (7-7.63) | 7.2 (7.15-7.45) | -0.0156 | 0.745 |
| Albumin, refrigerated serum (g/dL) | 4.3 (4.1-4.5) | 4.05 (3.8-4.4) | 4.2 (4.03-4.3) | 4.3 (4.1-4.65) | -0.0744 | 0.229 |
| Iron frozen, Serum (μg/dL) | 83 (59-112) | 80.5 (47-88) | 88 (68-106) | 67.5 (58-120.5) | 0.0001 | 0.777 |
| Total iron binding capacity (μg/dL) | 352 (322-387) | 346 (314-378) | 355 (315.25-375.25) | 314 (310-327.5) | -0.0009 | 0.018a |
| Transferrin saturation (%) | 23 (17-32) | 22 (15-26) | 28 (19.25-31.5) | 20 (18-39) | 0.0013 | 0.377 |
| Ferritin (ng/mL) | 39.3 (24.5-62.1) | 45.55 (24.45-61.85) | 56.25 (29-71) | 102.35 (35.75-141) | 0.0009 | 0.030a |
| Total cholesterol (mg/dL) | 151 (134-171) | 140.5 (136-156) | 161 (143-175) | 131 (119-147.5) | -0.0010 | 0.102 |
| Low-density lipoprotein cholesterol (mg/dL) | 81 (66.2-97.6) | 77.5 (64.2-92.6) | 88.2 (71.6-91.4) | 57.2 (55.5-80.1) | -0.0013 | 0.082 |
| Direct high-density lipoprotein cholesterol (mg/dL) | 51 (44-59) | 49.5 (44-58) | 50 (46-62) | 49.5 (39-56) | -0.0012 | 0.426 |
| HS C-reactive protein (mg/L)1 | 0.57 (0.35-1.39) | 0.83 (0.34-1.34) | 0.72 (0.37-1.12) | 0.97 (0.53-7.09) | 0.0240 | 0.134 |
| Platelet count (1000 cells/uL) | 262 (230-297.5) | 275.5 (242-302.5) | 262.5 (226-277) | 262.5 (234-275) | -0.0001 | 0.769 |
| Hemoglobin A1c (%)1 | 5.3 (5.1-5.5) | 5.3 (5.25-5.6) | 5.45 (5.25-5.65) | 5.35 (5.15-5.6) | 0.4629 | 0.098 |
| Fasting glucose (mg/dL) | 98 (94-102) | 99 (94-103) | 101 (95.5-104.25) | 92 (89.75-95.75) | -0.0031 | 0.490 |
| Insulin (pmol/L) | 64.83 (43.38-99) | 70.26 (45.87-183.17) | 87.06 (59.28-160.28) | 51.42 (27.29-127.14) | 0.0005 | 0.291 |
| Homeostatic model assessment for insulin resistance | 2.61 (1.71-3.96) | 2.66 (1.96-7.9) | 4.08 (2.34-6.66) | 1.95 (1.1-4.95) | 0.0101 | 0.383 |
| Predictors | Coefficient (standard error) | P value |
| Steatosis grade | 0.0730 (0.0172) | < 0.001 |
| Race: Non-Hispanic Black | 0.1352 (0.0430) | 0.002 |
| Smoke | 0.4065 (0.1845) | 0.028 |
| Systolic blood pressure (mmHg) | 0.0040 (0.0019) | 0.035 |
The performance of liver fibrosis indices (APRI, FIB4, and PNFI) were summarized in Table 6. PNFI was the only significant predictor of steatosis grade. However, all liver fibrosis indices had very low positive predictive values (0%-3.26%) for predicting cirrhosis (F4).
| Liver fibrosis indices (Predictor) | Outcome | Predictive performance | |||||
| Index | Cutoff | Accuracy | PPV | NPV | Sensitivity | Specificity | |
| APRI | 0.7 | F4 | 98.45% | 0% | 98.8% | 0.0% | 99.7% |
| FIB4 | 1.3 | F4 | 98.61% | 0% | 98.8% | 0.0% | 99.8% |
| PNFI | 9 | F4 | 85.31% | 3.26% | 99.1% | 37.5% | 85.9% |
| PNFI | 3 | S1-S3 | 85.60% | 83.33% | 86.2% | 59.7% | 95.5% |
This study reported the prevalence of steatosis and fibrosis in United States adolescents who participated in NHANES 2017-2018 as diagnosed by TE and CAP. We also identified predictors of steatosis grade and fibrosis stage in this study population. Although there was a recent study on a similar topic that utilized the same database from Ciardullo et al[28], the study designs were distinct as follows: (1) The maximum age in this study is 17 since the age 18 and above was used as a cut-off for many adult questionnaires in NHANES (e.g., alcohol use, physical activity, and smoking); (2) We discretized the steatosis grades and fibrosis levels into 4 Levels each; (3) Advanced fibrosis was defined as ≥F3 (≥ 8.6 kPa) rather than ≥F2 (≥ 7.4 kPa); (4) We included more risk factors that were widely known to be associated with NAFLD (e.g., smoking, physical activity, diet, and insulin resistance); and (5) Linear regression was used instead of logistic regression. For this reason, our results on prevalence and significant predictors are different from the previous study even though we used the same database.
We found that significant steatosis was present in over a fifth of the adolescents studied as indicated by a median CAP ≥ 248 dB/m and that advanced fibrosis (F3-F4) was present in 2.84% of the adolescents studied. Log of ALT, waist-to-height ratio, HOMA-IR, and BMI were significant predictors of steatosis in multivariate level. These four factors can be categorized into three groups that are commonly known as risk factors of NAFLD: liver chemistry (ALT), insulin resistance (HOMA-IR), and body fat (BMI and waist-to-height ratio). North American Society of Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) guidelines suggested using ALT as a screening test for NAFLD with the cutoff levels of 22 mg/dL for girls and 26 mg/dL for boys[29]. BMI, waist-to-height ratio, and insulin resistance have been heavily documented as risk factors for hepatic steatosis in obese children[30,31]. In fact, insulin resistance plays a central role in the pathogenesis of non-alcoholic fatty liver disease[32].
Identifying predictors of fibrosis in adolescents is important because fibrosis has been shown to be a strong predictor of liver related complications and overall mortality[33]. Having sensitive and specific predictors of fibrosis allows us to effectively prevent and manage associated liver-related complications such as hepatocellular carcinoma and cirrhosis. In our study, multivariate stepwise regression revealed that the independent predictors of fibrosis were steatosis grade, non-Hispanic black race, smoking, and systolic blood pressure.
Non-Hispanic black race as an independent predictor of fibrosis that may be a proxy for other socioeconomic and environmental factors not collected in the research effort. Although the pathogenesis of NAFLD is not fully understood, NAFLD is widely accepted to be a genetic-environment-metabolism-related disease[34]. Consumption of refined carbohydrates and sugar-sweetened beverages have been associated with NAFLD[35]. In a study that documented self-reported sugar-sweetened beverage intake among college students, black undergraduates were found to have a higher intake of sugared beverages than compared to their contemporaries[36]. Additionally, non-Hispanic blacks are reported to have suboptimal diet quality and to not meet national dietary recommendations with lower intakes of total vegetables, milk, and whole grains than whites[37]. Our findings may reflect the dietary and environmental differences among black adolescents and requires further investigation.
Smoking has been identified as an independent risk factor of NAFLD in adult patients[38,39]. The presumed pathogenesis is through the consumption of toxins in cigarettes that affect the antioxidant system, which includes cytochrome P450 and inflammatory cytokines[35]. Our smoking sub-group was adolescents and underpowered with a sample size of 6, so further investigation is needed to confirm smoking as a specific predictor for fibrosis.
Previous animal model study showed that the steatosis of any cause was associated with hepatic inflammatory changes and fibrosis by causing oxidative stress and mitochondrial dysfunction[40]. However, there were limited clinical evidence on the association between steatosis and fibrosis in general pediatric or adolescent population. Systolic hypertension is known as a primary clinical feature of metabolic syndrome, which were previously reported as independence risk factor of NAFLD[41].
Additionally, we compared the performance of three liver fibrosis indices for predicting steatosis (S1-S3) and cirrhosis (F4). PNFI was the only liver fibrosis index having a PPV and sensitivity greater than zero. Although it was only index that can be used to predict NAFLD, the performance on this dataset was moderately high with an accuracy of 85.6%. The superior performance of PNFI could derive from the fact that it is the only index developed by using the liver biopsy in the pediatric population[21] while other two indices (APRI and FIB4) were originally developed from the adult population[19,20], which could perform poorly in pediatric or adolescent population.
There are several limitations of this study. Our study population is of United States adolescents and may not be reflective of non-American populations. Alcohol was not measured in the study population and also presumed to be zero because the population was United States adolescents. The legal age to drink in the United States is 21 but for some people drinking alcohol begins in adolescence[42]. Another limitation is subgroup sample size which was seen subgroups such as smoking, F3, and F4. Low statistical power reduces the chance of detecting a true effect[43]. Some variables not available in the NHANES include hormonal levels and Tanner stages of the participants. Hypogonadism and low testosterone level are associated with an increased risk for NAFLD and NASH[44]. Additionally, low sex hormone binding globulin (SHBG) can be viewed as a marker for NAFLD in women with oligomenorrhea and/or hirsutism[45]. Since these variables were not included in the NHANES database, they were not accounted for. Lastly, though seeing increasing utility in diagnostic value, TE has not been traditionally studied in adolescents.
In conclusion, this study showed steatosis and advanced liver fibrosis in 27.2% and 2.7% of United States adolescents, respectively. ALT, BMI, HOMA-IR, and waist-to-height ratio were predictors of steatosis, while steatosis grade, smoking, non-Hispanic black race, systolic blood pressure were predictors of fibrosis. Environmental, dietary, and social history are important information to gather from adolescents as these factors can contribute to a risk of steatosis and fibrosis. Given the progressive nature of chronic liver disease, the evidence of steatosis or advanced fibrosis in younger age could lead to increased steatohepatitis and cirrhosis in young adults.
Non-alcoholic fatty liver disease (NAFLD) is the leading cause of chronic liver disease in children and adolescents.
With the rise of obesity and metabolic syndrome among younger populations, NAFLD is a growing concern in adolescents.
The authors aimed to determine the prevalence and risk factors of steatosis and advanced fibrosis using transient elastography in the United States’ adolescent population.
The authors studied adolescent participants aged 13 to 17 years who underwent TE and controlled attenuation parameter using the National Health and Nutrition Examination Survey 2017-2018.
There is a high prevalence of steatosis (27.2%) in the United States’ adolescent population, with 2.84% having advanced fibrosis. Risk factors of steatosis grade included alanine aminotransferase, insulin resistance, waist-to-height ratio, and body mass index. Steatosis grade, non-Hispanic black race, smoking history, and systolic blood pressure were significant predictors of fibrosis.
Adolescents with steatosis or advanced fibrosis could progress to increased steatohepatitis and cirrhosis in young adults.
Environmental, dietary, and social history are important information to gather from adolescents as these factors can contribute to a risk of steatosis and fibrosis. Given the progressive nature of chronic liver disease, the evidence of steatosis or advanced fibrosis in younger age could lead to increased steatohepatitis and cirrhosis in young adults.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vignozzi L S-Editor: Ma YJ L-Editor: A P-Editor: Wang LL
| 1. | Nobili V, Alisi A, Valenti L, Miele L, Feldstein AE, Alkhouri N. NAFLD in children: new genes, new diagnostic modalities and new drugs. Nat Rev Gastroenterol Hepatol. 2019;16:517-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 218] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 2. | Bellentani S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017;37 Suppl 1:81-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 438] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 3. | Do A, Lim JK. Epidemiology of nonalcoholic fatty liver disease: A primer. Clin Liver Dis (Hoboken). 2016;7:106-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Conjeevaram Selvakumar PK, Kabbany MN, Alkhouri N. Nonalcoholic Fatty Liver Disease in Children: Not a Small Matter. Paediatr Drugs. 2018;20:315-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Adams LA. Biomarkers of liver fibrosis. J Gastroenterol Hepatol. 2011;26:802-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Zelber-Sagi S, Nitzan-Kaluski D, Halpern Z, Oren R. Prevalence of primary non-alcoholic fatty liver disease in a population-based study and its association with biochemical and anthropometric measures. Liver Int. 2006;26:856-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 203] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 7. | Castera L, Friedrich-Rust M, Loomba R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019; 156: 1264-1281. e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 1035] [Article Influence: 172.5] [Reference Citation Analysis (0)] |
| 8. | Corpechot C, El Naggar A, Poujol-Robert A, Ziol M, Wendum D, Chazouillères O, de Lédinghen V, Dhumeaux D, Marcellin P, Beaugrand M, Poupon R. Assessment of biliary fibrosis by transient elastography in patients with PBC and PSC. Hepatology. 2006;43:1118-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 322] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 9. | Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011;54:650-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 524] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 10. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4939] [Article Influence: 705.6] [Reference Citation Analysis (9)] |
| 11. | de Lédinghen V, Le Bail B, Rebouissoux L, Fournier C, Foucher J, Miette V, Castéra L, Sandrin L, Merrouche W, Lavrand F, Lamireau T. Liver stiffness measurement in children using FibroScan: feasibility study and comparison with Fibrotest, aspartate transaminase to platelets ratio index, and liver biopsy. J Pediatr Gastroenterol Nutr. 2007;45:443-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 196] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 12. | Lee CK, Perez-Atayde AR, Mitchell PD, Raza R, Afdhal NH, Jonas MM. Serum biomarkers and transient elastography as predictors of advanced liver fibrosis in a United States cohort: the Boston children's hospital experience. J Pediatr. 2013;163:1058-64.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Alkhouri N, Sedki E, Alisi A, Lopez R, Pinzani M, Feldstein AE, Nobili V. Combined paediatric NAFLD fibrosis index and transient elastography to predict clinically significant fibrosis in children with fatty liver disease. Liver Int. 2013;33:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Nobili V, Vizzutti F, Arena U, Abraldes JG, Marra F, Pietrobattista A, Fruhwirth R, Marcellini M, Pinzani M. Accuracy and reproducibility of transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. Hepatology. 2008;48:442-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 309] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 15. | Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, Kumar M, Lupsor-Platon M, Han KH, Cardoso AC, Ferraioli G, Chan WK, Wong VW, Myers RP, Chayama K, Friedrich-Rust M, Beaugrand M, Shen F, Hiriart JB, Sarin SK, Badea R, Jung KS, Marcellin P, Filice C, Mahadeva S, Wong GL, Crotty P, Masaki K, Bojunga J, Bedossa P, Keim V, Wiegand J. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 836] [Article Influence: 104.5] [Reference Citation Analysis (0)] |
| 16. | Desai NK, Harney S, Raza R, Al-Ibraheemi A, Shillingford N, Mitchell PD, Jonas MM. Comparison of Controlled Attenuation Parameter and Liver Biopsy to Assess Hepatic Steatosis in Pediatric Patients. J Pediatr. 2016;173:160-164.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 17. | Ferraioli G, Calcaterra V, Lissandrin R, Guazzotti M, Maiocchi L, Tinelli C, De Silvestri A, Regalbuto C, Pelizzo G, Larizza D, Filice C. Noninvasive assessment of liver steatosis in children: the clinical value of controlled attenuation parameter. BMC Gastroenterol. 2017;17:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 18. | Moore JX, Chaudhary N, Akinyemiju T. Metabolic Syndrome Prevalence by Race/Ethnicity and Sex in the United States, National Health and Nutrition Examination Survey, 1988-2012. Prev Chronic Dis. 2017;14:E24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 425] [Cited by in RCA: 631] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 19. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3242] [Article Influence: 147.4] [Reference Citation Analysis (0)] |
| 20. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3555] [Article Influence: 187.1] [Reference Citation Analysis (0)] |
| 21. | Nobili V, Alisi A, Vania A, Tiribelli C, Pietrobattista A, Bedogni G. The pediatric NAFLD fibrosis index: a predictor of liver fibrosis in children with non-alcoholic fatty liver disease. BMC Med. 2009;7:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W, Sung JJ, de Lédinghen V. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 970] [Article Influence: 64.7] [Reference Citation Analysis (1)] |
| 23. | Pagliaro L. Lebrec D, Poynard T, Hillon P, Benhamou J-P. Propranolol for prevention of recurrent gastrointestinal bleeding in patients with cirrhosis. A controlled study [N Engl J Med 1981;305:1371-1374]. J Hepatol. 2002;36:148-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Shiffler K, Lee D, Rowan M, Aghaloo T, Pi-Anfruns J, Moy PK. Effect of length, diameter, intraoral location on implant stability. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:e193-e198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Engelmann G, Gebhardt C, Wenning D, Wühl E, Hoffmann GF, Selmi B, Grulich-Henn J, Schenk JP, Teufel U. Feasibility study and control values of transient elastography in healthy children. Eur J Pediatr. 2012;171:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 26. | Staub F, Tournoux-Facon C, Roumy J, Chaigneau C, Morichaut-Beauchant M, Levillain P, Prevost C, Aubé C, Lebigot J, Oberti F, Galtier JB, Laumonier H, Trillaud H, Bernard PH, Blanc JF, Sironneau S, Machet F, Drouillard J, de Ledinghen V, Couzigou P, Foucher P, Castéra L, Tranquard F, Bacq Y, d'Altéroche L, Ingrand P, Tasu JP. Liver fibrosis staging with contrast-enhanced ultrasonography: prospective multicenter study compared with METAVIR scoring. Eur Radiol. 2009;19:1991-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Barlow SE; Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120 Suppl 4:S164-S192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3035] [Cited by in RCA: 3063] [Article Influence: 170.2] [Reference Citation Analysis (0)] |
| 28. | Ciardullo S, Monti T, Perseghin G. Prevalence of Liver Steatosis and Fibrosis Detected by Transient Elastography in Adolescents in the 2017-2018 National Health and Nutrition Examination Survey. Clin Gastroenterol Hepatol. 2021;19:384-390.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 29. | Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R, Mouzaki M, Sathya P, Schwimmer JB, Sundaram SS, Xanthakos SA. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr. 2017;64:319-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 724] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 30. | Chan DF, Li AM, Chu WC, Chan MH, Wong EM, Liu EK, Chan IH, Yin J, Lam CW, Fok TF, Nelson EA. Hepatic steatosis in obese Chinese children. Int J Obes Relat Metab Disord. 2004;28:1257-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 197] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 31. | Tominaga K, Kurata JH, Chen YK, Fujimoto E, Miyagawa S, Abe I, Kusano Y. Prevalence of fatty liver in Japanese children and relationship to obesity. An epidemiological ultrasonographic survey. Dig Dis Sci. 1995;40:2002-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 274] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 32. | Polyzos SA, Kountouras J, Zavos C. Nonalcoholic fatty liver disease: the pathogenetic roles of insulin resistance and adipocytokines. Curr Mol Med. 2009;9:299-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 245] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 33. | Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1353] [Cited by in RCA: 1701] [Article Influence: 170.1] [Reference Citation Analysis (1)] |
| 34. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. Obes Facts. 2016;9:65-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 367] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 35. | Ou H, Fu Y, Liao W, Zheng C, Wu X. Association between Smoking and Liver Fibrosis among Patients with Nonalcoholic Fatty Liver Disease. Can J Gastroenterol Hepatol. 2019;2019:6028952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | West DS, Bursac Z, Quimby D, Prewitt TE, Spatz T, Nash C, Mays G, Eddings K. Self-reported sugar-sweetened beverage intake among college students. Obesity (Silver Spring). 2006;14:1825-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Hiza HA, Casavale KO, Guenther PM, Davis CA. Diet quality of Americans differs by age, sex, race/ethnicity, income, and education level. J Acad Nutr Diet. 2013;113:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 569] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 38. | Zein CO, Unalp A, Colvin R, Liu YC, McCullough AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Smoking and severity of hepatic fibrosis in nonalcoholic fatty liver disease. J Hepatol. 2011;54:753-759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 39. | Jung HS, Chang Y, Kwon MJ, Sung E, Yun KE, Cho YK, Shin H, Ryu S. Smoking and the Risk of Non-Alcoholic Fatty Liver Disease: A Cohort Study. Am J Gastroenterol. 2019;114:453-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 40. | Berson A, De Beco V, Lettéron P, Robin MA, Moreau C, El Kahwaji J, Verthier N, Feldmann G, Fromenty B, Pessayre D. Steatohepatitis-inducing drugs cause mitochondrial dysfunction and lipid peroxidation in rat hepatocytes. Gastroenterology. 1998;114:764-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 259] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 41. | López-Suárez A, Guerrero JM, Elvira-González J, Beltrán-Robles M, Cañas-Hormigo F, Bascuñana-Quirell A. Nonalcoholic fatty liver disease is associated with blood pressure in hypertensive and nonhypertensive individuals from the general population with normal levels of alanine aminotransferase. Eur J Gastroenterol Hepatol. 2011;23:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 42. | Chassin L, DeLucia C. Drinking During Adolescence. Alcohol Health Res World. 1996;20:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafò MR. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4056] [Cited by in RCA: 4255] [Article Influence: 354.6] [Reference Citation Analysis (0)] |
| 44. | Sarkar M, Yates K, Suzuki A, Lavine J, Gill R, Ziegler T, Terrault N, Dhindsa S. Low Testosterone Is Associated With Nonalcoholic Steatohepatitis and Fibrosis Severity in Men. Clin Gastroenterol Hepatol. 2021;19:400-402.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 45. | Di Stasi V, Maseroli E, Rastrelli G, Scavello I, Cipriani S, Todisco T, Marchiani S, Sorbi F, Fambrini M, Petraglia F, Maggi M, Vignozzi L. SHBG as a Marker of NAFLD and Metabolic Impairments in Women Referred for Oligomenorrhea and/or Hirsutism and in Women With Sexual Dysfunction. Front Endocrinol (Lausanne). 2021;12:641446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |