Published online Jul 27, 2021. doi: 10.4254/wjh.v13.i7.781
Peer-review started: February 22, 2021
First decision: May 3, 2021
Revised: May 9, 2021
Accepted: June 23, 2021
Article in press: June 23, 2021
Published online: July 27, 2021
Processing time: 150 Days and 16.6 Hours
The coronavirus disease 2019 (COVID-19) pandemic has resulted in significant morbidity and mortality since its first case was discovered in December 2019. Since then, multiple countries have witnessed a healthcare system collapse due to the overwhelming demand for COVID-19 care. Drastic measures have been taken globally in order to curb the spread of the virus. However, those measures have led to the disruption of other aspects of healthcare, increasing the burden due to other medical conditions. We have also stepped back in achieving the ambitious goal set in place by World Health Organization to eliminate viral hepatitis as a public threat by 2030. Hepatitis B and C are chronic conditions with a significant worldwide burden, and COVID-19 has resulted in many hepatitis elimination programs slowing or stopping altogether. In this review, we elucidate the impact of the ongoing COVID-19 pandemic on the interventions targeted towards the elimination of hepatitis B virus and hepatitis C virus. Some of the salient features that we have covered in this review include hindrance to screening and diagnostic tests, neonatal vaccinations, the transmission dynamics affecting hepatitis B virus and hepatitis C virus, role of limited awareness, restrictions to treatment accessibility, and disparity in healthcare services. We have highlighted the major issues and provided recommendations in order to tackle those challenges.
Core Tip: There has been a multi-fold impact of the pandemic on viral hepatitis elimination strategies. Due to supply chain disruptions, hepatitis B virus vaccination campaigns have been halted. Increased preference for home deliveries, poor antenatal care, and unavailability of at-birth hepatitis B virus vaccine has increased the risk of vertical transmission. With needle-sharing activities on the rise and closure of harm reduction centers, the spread of blood-borne infections including the hepatitis C virus has risen. Hospitals are either being avoided due to the fear of contracting severe acute respiratory syndrome coronavirus 2 or are being converted into coronavirus treatment wards, resulting in poor management of patients.
- Citation: Rehman ST, Rehman H, Abid S. Impact of coronavirus disease 2019 on prevention and elimination strategies for hepatitis B and hepatitis C. World J Hepatol 2021; 13(7): 781-789
- URL: https://www.wjgnet.com/1948-5182/full/v13/i7/781.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i7.781
In December 2019 the first case of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was isolated and identified in Wuhan, China[1]. The coronavirus disease 2019 (COVID-19) pandemic that ensued, has led to 2.47 million deaths as of February 21, 2021[2].
This medical emergency shed light upon our fragile healthcare system worldwide and its vulnerabilities including the immense vacuum questioning our preparedness for the next pandemic[3]. Although we were able to achieve making vaccines in record time[4], the impact on human life and our economies are yet to be quantified.
On the other hand, hepatitis B virus (HBV) and hepatitis C virus (HCV) have had their impact quantified and have been studied for decades. In 2016, the World Health Organization (WHO) estimated the prevalence of chronic hepatitis B to be 257 million worldwide[5], while it was 71 million for chronic hepatitis C. Chronic hepatitis has a worldwide burden that is mostly clinically silent, as it goes undiagnosed in most low to middle-income countries (LMICs)[6,7].
We evaluated the sustainable development goals (SDGs) set in place by the WHO for the task of eliminating hepatitis B and C as a public health threat by 2030[8]. The SDGs include goals such as coverage of three-dose HBV neonatal vaccine, prevention of mother-to-child transmission, and harm reduction services such as sterile syringe set distribution for people injecting drugs. The efforts done to achieve these sustainable goals have been severely compromised due to the current pandemic.
Although it is debatable that having chronic viral hepatitis influences the outcomes of having the COVID-19[9-12], worse outcomes with acute respiratory distress syndrome in COVID-19 can be expected due to impaired immunity[1,13].
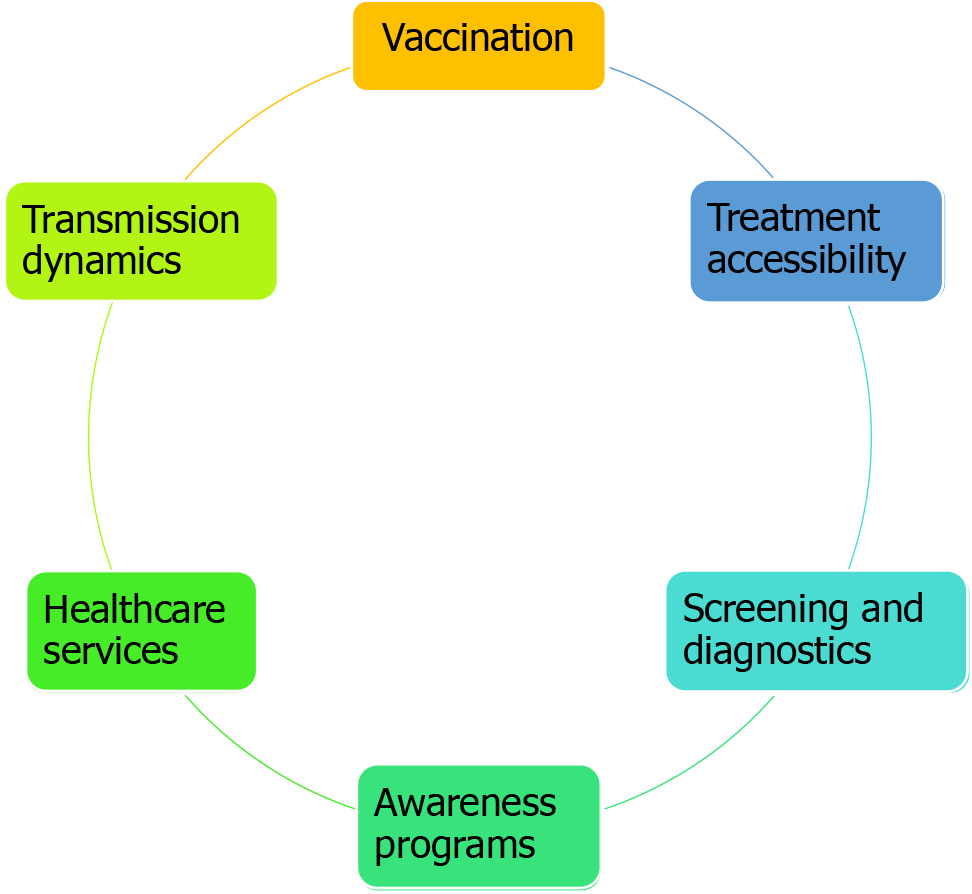
This review elucidates the impact of the COVID-19 pandemic on chronic viral hepatitis B and C; since hepatitis A and E contribute relatively less significantly to morbidity, mortality, and long-term impact[8]. We evaluated SDGs and current existing data in light of them. Some of the salient features, as shown in Figure 1, can be identified as a hindrance to screening tests and neonatal vaccinations, the transmission dynamics affecting HBV and HCV, the role of limited awareness, restrictions to treatment availability, and disparity in healthcare services.
The COVID-19 pandemic brought in conditions and circumstances that were unusual for countries and the world as a whole with factors not previously anticipated. Although the rate of hepatitis B vaccinations has been steadily on the rise since the 1990s, we have learned that geopolitical factors, financial priorities, the image of the government, and the health sector have played a huge role in their success or failure[3]. A recent example within an epidemic can be found in the Ebola outbreak in 2013 in West Africa. Due to disrupted vaccination services, limited availability, and allocation of funds, a sharp rise in the incidence of measles was reported during the epidemic and in the months that followed[14].
The Institute for Health Metrics and Evaluation at the University of Washington showcased an overall drop in global vaccination coverage in 2020 to levels as low as those seen in the 1990s with words depicting its severity as “… we have been set back 25 years in 25 wk”[15]. High-income countries like the United States had a drop in pediatric vaccinations being ordered and administered after an emergency was declared on March 13, 2020[16]. Between February and April of 2020, the United Kingdom also saw a drop of almost 20% in the administration of measles, mumps, and rubella vaccines, as compared to 2019[17].
Reduced availability and provision of HBV vaccines during this COVID-19 pandemic will have detrimental effects on the incidence of HBV during infancy, childhood, and in later years, thus increasing the chances of chronicity in the generation to come. This severely impedes our progress to the 2030 elimination goals set in place by WHO[8].
Vaccination rates are not in line with the target goals set in SDGs in the LMICs[18], and poor screening in the case of viral hepatitis might pose a greater threat in the long run compared to the pandemic.
Despite being a high-value investment, vaccines are the most cost-effective way of avoiding disease[19]. The decline in measles, mumps, polio, and yellow fever can be credited to this. Nothing can truly represent the effectiveness of vaccines other than the global eradication of the smallpox virus. This disfiguring disease that had infected over 11 million people from 1920 to 1977, was eradicated in 1978 following a world
Although the HBV vaccine is an effective modality, this modality does not exist for HCV. Progress has been made on HCV over the past few decades with the year 2020 being its limelight when Drs. Michael Houghton, Harvey Alter, and Charles Rice were awarded the Nobel Prize in Physiology/Medicine for their discovery of the HCV[20]. This raises hopes for a cure and even so a vaccine that will be beneficial for the years to come. Eliminating HCV as a global threat should be a priority as the disease is present actively in 71 million people and accounts for 500000 deaths annually[21,22].
Abbas et al[23] conducted a benefit-risk analysis study in Sub-Saharan Africa during the pandemic. The study compared the SARS-CoV-2 pandemic and its impact on routine childhood vaccination programs, encompassing several preventable diseases including hepatitis B as well as others such as diphtheria, tetanus, pertussis, Haemophilus influenzae type b, Streptococcus pneumoniae, rotavirus, measles, meningitis A, rubella, and yellow fever. The model found that in a high-impact scenario, for every one excess COVID-19 death attributable to SARS-CoV-2 infections acquired during routine vaccination clinic visits, 84 deaths in children could be prevented by sustaining routine childhood immunization in Africa[23].
HBV vaccination campaigns have also been halted due to disruptions in the supply chain. LMICs regions like Pakistan and Sub-Saharan Africa were faced with a shortage of HBV vaccines during the pandemic[24,25]. The latter had breakdowns in the cold chain and limited financial support from the government[25]. Despite healthcare services being ramped up, changes in healthcare-seeking behavior led to a change in attitude resulting in reluctance for availing vaccinations[25]. The acceptance and readiness of vaccinations are closely linked to the fear of the linked disease and the trust placed in the government and its practices[26-28]. Due to heightened misin
The actual numbers to quantify the effects on transmission dynamics in viral hepatitis spread are limited[3]. Even though as a result of movement restrictions and worldwide lockdowns, the physical spread is expected to decrease, such limiting behaviors give rise to risky attitudes on the part of undiagnosed and stable hepatitis. Alcohol consumption and unprotected sexual intercourse have increased. Drug abuse has been on the rise during the pandemic[30]. Disruption of needle exchange programs and harm-reducing services are already scarce in LMICs and with lockdowns in place and financial constraints, such limitations would result in cross-contamination of blood-borne viruses via needles especially HCV[31]. Stowe et al[32] reported the closing down of numerous harm reduction service centers in South Africa leading to rising in overdose cases in street-based heroin-using individuals. In general, the incidence of viral hepatitis will increase by the closing of harm reduction centers[33].
In Sub-Saharan Africa, liver diseases are highly prevalent although extremely underdiagnosed[25]. Being unaware of their viral hepatitis status creates ground for increased transmission dynamics in the population that already has limited funding for screening, vaccinations, and treatment as a whole. Government efforts will need a clear pragmatic strategy as the pandemic progresses to counter such transmission dynamics.
The chances of vertical transmission have also increased as the preference for home deliveries has surged during the pandemic[29]. There is an increased likelihood of missing out on routine HBV and HCV antenatal screening tests. The initial dose of HBV vaccine usually administered at birth could either be delayed or skipped. The intrapartum administration of hepatitis B immunoglobulin to decrease the vertical transmission has also been affected due to home deliveries. These above-mentioned limitations all increase the chances of vertical transmission, which will affect a generation that is to come, making them highly susceptible to chronic hepatitis due to early exposure.
Lack of awareness is an issue faced by multiple LMICs. Increasing the awareness amongst the general population about modes of transmission of viral hepatitis, symptoms, screening and diagnosis, management, and follow-up plays an important role in elimination programs[34]. Measures taken during the pandemic have led to the closure of community-based education and screening programs and in-person events. A decrease in voluntary activities such as the NoHep program seems to have decreased the diagnosis rate[35].
A lack of information dispersal has been noticed during the pandemic in regards to people suffering from viral hepatitis. According to a study conducted by the World Hepatitis Alliance, 99 countries were sent a survey to access viral hepatitis services during the pandemic. Only 39 (30%) of 131 analyzable responses indicated adequate information on COVID-19 had been provided to people living with viral hepatitis in their country. One participant from Ukraine said that no specific information had been provided for people living with viral hepatitis, although information had been provided for people living with human immunodeficiency virus[36].
In low-income countries like Pakistan, new and known cases of HBV and HCV patients were compared between January to June of 2020 to the corresponding months in 2019. These 23 centers were mostly government-run with free of cost hepatitis treatment being provided. All the centers remained open, with no shortage of staff. Despite this, the centers still recognized a lesser number of new people coming in for treatment; for example, in January 2020 a mean number of 45 new patients registered in these centers when there were no cases, while in June 2020, the number has fallen by 84%[37]. This highlights the lack of awareness amongst individuals regarding the seriousness of viral hepatitis.
One of the most important steps in eliminating viral hepatitis is to screen and diagnose in a timely fashion in order to start treatment and prevent transmission. Underdiagnosis is a key hurdle in eliminating viral hepatitis, as it can have a long-term impact on transmission dynamics.
In 2017, it was estimated that 91% of patients with chronic HBV and 80% of patients with chronic HCV had not been diagnosed. In a World Health Alliance survey conducted across 32 LMICs, only 36% of the respondents reported that testing services were accessible to people. The key issues identified in the survey were either the closures or avoidance of testing services[31]. A study revealed that within Sub-Saharan Africa, there was a reduction of 71%, 95%, and 83% in the number of patients in the hepatitis clinics of Burkina Faso, Tanzania, and the Gambia, respectively, from January to April 2020[38]. The primary reason for such a striking decline in the use of outpatient services was attributed to the fear of contracting the severe acute respiratory syndrome coronavirus 2. Similarly, a decline of 84% in HBV and 74% in HCV positive patients coming for a follow-up visit in district hepatitis clinics were recorded in Pakistan from January to June 2020[37].
In order to control the pandemic, multiple aggressive measures have been taken worldwide, leading to financial disruption of hospitals and healthcare services, often resulting in their closures[39]. There have also been shortages in the testing reagents of HBV and HCV due to global supply chain disruption. In Italy, a law was enacted in February 2020 to conduct graduated birth cohort screening for hepatitis, however, it had not been put into action as of May 2020. In Egypt, all the ongoing screening programs were also suspended in March 2020, as reported by Blach et al[40] to reserve polymerase chain reaction tests for COVID-19; all polymerase chain reaction testing for viral hepatitis was halted in Pakistan[37].
In most countries, travel bans have been enforced, making access to critical care difficult. In multiple high-income countries, continuity of care is being maintained by utilizing telemedicine services. This has made it convenient for patients to have access to remote healthcare. However, in LMICs including Sub-Saharan Africa, telemedicine is impractical due to a lack of resources including cell-phones, internet services, and modes of payment[25]. The task of generating dedicated phone numbers for gastroenterology and hepatology services and spreading awareness regarding telemedicine amongst the population is not easily established in communities with a low literacy rate. Furthermore, it is difficult for the patients to understand or perform the investigations that the doctor asks them to do.
Even though all the LMICs are not facing or responding to the pandemic in the same way, there has been a global negative impact on access to treatment and care. For instance, even though a strict lockdown was not imposed in Egypt, HCV management centers had a 50% reduction in new patients and follow-ups[40]. A study conducted across three clinical sites in the United States, Japan, and Singapore reported a significantly decreasing trend in the number of patients who visited liver clinics across the three clinical sites during February, March, and April in 2018, 2019, and 2020[41]. Although most Spanish harm reduction centers continued to operate during the pandemic, there was a reduction in the number of clients using them, which resulted in decreased testing and increased discontinuation of ongoing hepatitis C treatment[42]. A web-based survey conducted in Italy revealed that initiation of HBV and HCV treatment was deferred in 23% of the centers, and even in patients considered at high risk for serious complications, treatment had been started in only 20%-28% of the cases[43].
In many countries including Egypt, medications are not manufactured and are imported from other countries. Interruption of the supply chain and necessary reallocation of healthcare resources has resulted in a remarkable shortage of medications for viral hepatitis, as reported by studies conducted in Egypt[44], Sub-Saharan Africa[38], and Pakistan[37]. In Italy, 26% of the hepatology wards had been converted to COVID wards, and 33% had bed reductions[43].
As a result of interrupted and substandard treatment of viral hepatitis, there is an increased risk of disease flares that could promote transmission and also increase resistance to viral drugs. Routine monitoring of laboratory investigations including liver function tests and complete blood counts were also significantly reduced because of increased priority given to COVID tests, as reported by Mustafa et al[37]. This is likely going to result in higher rates of severe worse outcomes such as decompensated liver disease and hepatocellular carcinoma. Certain reports have suggested that medications such as tocilizumab and corticosteroids, which are commonly being used to treat COVID-19 infections, can result in the reactivation of dormant HBV infection[45,46]. This may be an important cause of increased morbidity and mortality in patients with a prior HBV infection as a rapid rise in alanine aminotransferase levels following viral reactivation can in some cases lead to a fulminant hepatic failure. Hence, antiviral prophylaxis against HBV reactivation should be considered[47]. Furthermore, it is also recommended that liver tests should be performed routinely in all COVID-19 patients, particularly the ones receiving remdesivir and tocilizumab, regardless of their baseline values[48].
The pandemic is causing health care and socioeconomic inequalities between regions and countries. The communities most underserved by the healthcare systems have an increased risk of contracting the SARS-COV-2 virus and are more likely to have non-communicable comorbidities, which further increases the chances of COVID associated complications[3,49].
The WHO survey reported that in LMICs, treatment access has been hampered due to movement restrictions and suspension of clinical services. Fifty-two percent of the frontline health workers from the 32 LMICs reported that treatment was not accessible by patients[31]. However, only 8% of the respondents from the United States reported an issue with access to treatment. This highlights the discrepancy between high-income countries and LMICs, the latter suffering from more severe consequences as a result of the pandemic[36].
National economies are crumbling and most giants in the varied sectors are downsizing to get through the pandemic. This increases the risk for people living in countries where universally accessible health care systems are not present, especially in rural areas of LMICs like India and Nigeria where daily wage earners are limited to healthcare by access and out-of-pocket expenditure for medical facilities[36]. Similar cases have also been accounted for in the United States, a high-income country where almost 6.2 million people have lost their jobs, thus losing the medical insurance linked to their jobs, during the pandemic[50]. Health disparity has affected almost everyone in one way or the other but the basic difference lies in access to basic medical help.
Primary care settings and general practitioners, which have an essential role in hepatitis elimination, are now focusing on the COVID-19 pandemic and this change can further reduce both diagnosis and treatment rates of hepatitis patients. Countries with a low number of doctors to population ratio will be affected more[51,52].
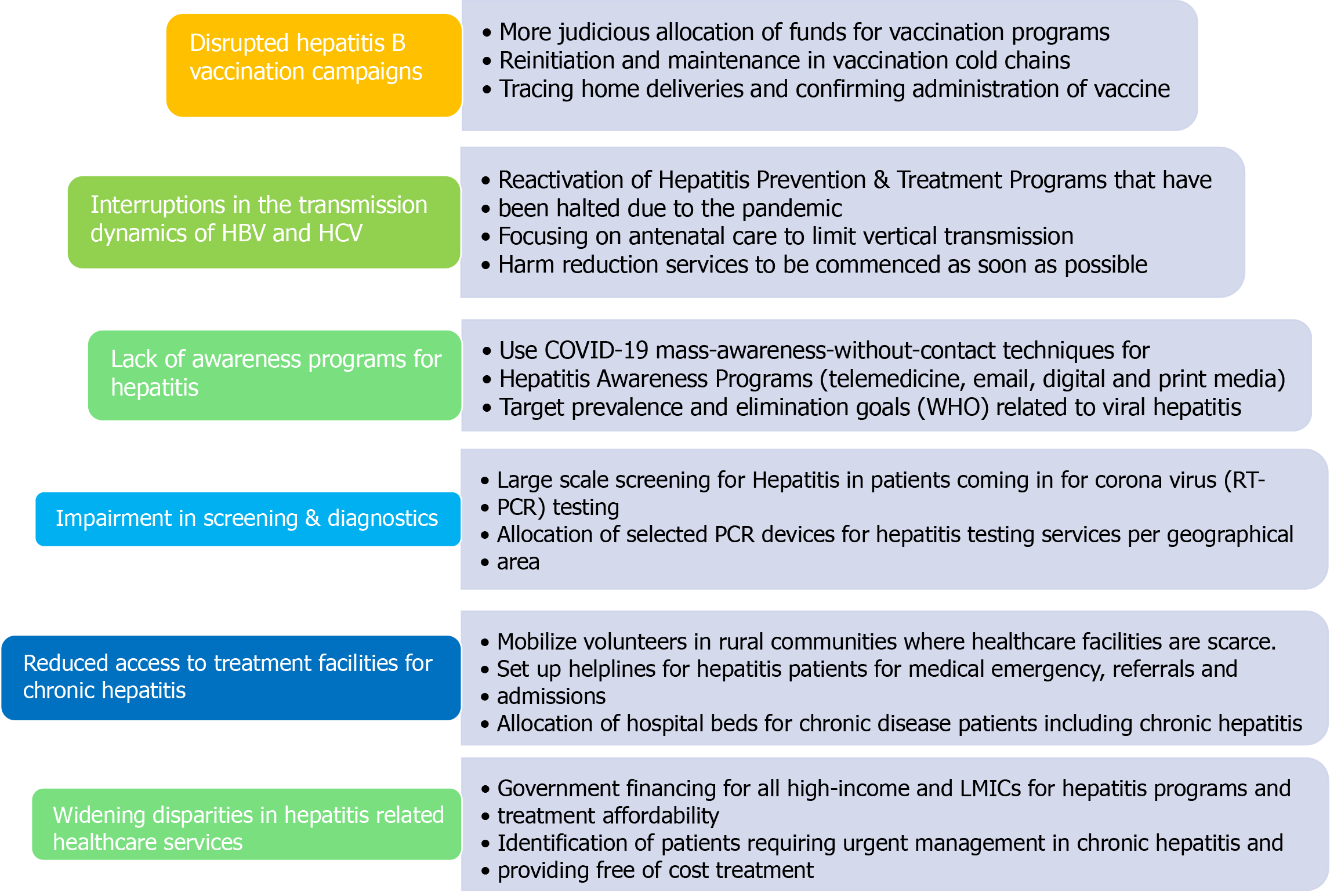
A pulse survey conducted across 100 countries of five different WHO regions not only provided an insight on the extent of healthcare disruption but also listed a few strategies that have been adopted by those regions to mitigate the impact of COVID-19 on essential health services during the pandemic[53]. Based on the approaches that the responding countries had implemented to overcome the healthcare disruptions, we have come up with a list of recommendations that can be utilized by researchers and policymakers to prevent transmission, increase screening and diagnosis, and provide prompt management of patients with HBV and HCV, to counter the impact of COVID-19 pandemic (refer to Figure 2). We can use this crisis as an opportunity to develop a healthcare system that is sustainable and does not collapse in case of continued morbidity and mortality due to the pandemic.
There is no doubt that drastic measures needed to be taken in order to curb the pandemic, but as a result of those measures, we might be stepping backward in achieving the goal of eliminating viral hepatitis by 2030. There is a dire need to come up with guidelines that guarantee consistent care of patients with viral hepatitis, in case there is another wave of the pandemic. The impact of COVID-19 is going to extend beyond just the morbidity and mortality related to that disease. Hence, elimination efforts for viral hepatitis must be resumed as soon as possible.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: de Melo FF, Liu Y S-Editor: Zhang H L-Editor: Filipodia P-Editor: Wang LL
| 1. | Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18987] [Cited by in RCA: 17630] [Article Influence: 3526.0] [Reference Citation Analysis (0)] |
| 2. | WHO. WHO Coronavirus Disease (COVID-19) Dashboard [cited 22 February 2021]. Available from: https://covid19.who.int/. |
| 3. | Pley CM, McNaughton AL, Matthews PC, Lourenço J. The global impact of the COVID-19 pandemic on the prevention, diagnosis and treatment of hepatitis B virus (HBV) infection. BMJ Glob Health. 2021;6:e004275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 4. | de Lange C. Vaccines made in record time. New Sci. 2020;248:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Tan M, Bhadoria AS, Cui F, Tan A, Van Holten J, Easterbrook P, Ford N, Han Q, Lu Y, Bulterys M, Hutin Y. Estimating the proportion of people with chronic hepatitis B virus infection eligible for hepatitis B antiviral treatment worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:106-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 6. | Wong WWL, Pechivanoglou P, Wong J, Bielecki JM, Haines A, Erman A, Saeed Y, Phoon A, Tadrous M, Younis M, Rayad NZ, Rac V, Janssen HLA, Krahn MD. Antiviral treatment for treatment-naïve chronic hepatitis B: systematic review and network meta-analysis of randomized controlled trials. Syst Rev. 2019;8:207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Ray RB, Ray R. Hepatitis C Virus Manipulates Humans as its Favorite Host for a Long-Term Relationship. Hepatology. 2019;69:889-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | WHO. Combating hepatitis B and C to reach elimination by 2030. [cited 22 February 2021]. Available from: http://www.who.int/hepatitis/publications/hep-elimination-by-2030-brief/en/. |
| 9. | Wang Q, Davis PB, Xu R. COVID-19 risk, disparities and outcomes in patients with chronic liver disease in the United States. EClinicalMedicine. 2021;31:100688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 10. | Verma N, Duseja A, Singh V. Impact of Preexisting Chronic Liver Disease on the Outcome of Patients With COVID-19 Disease. Gastroenterology. 2021;160:1893-1894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Pawlotsky JM. COVID-19 and the liver-related deaths to come. Nat Rev Gastroenterol Hepatol. 2020;17:523-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Sahin TT, Akbulut S, Yilmaz S. COVID-19 pandemic: Its impact on liver disease and liver transplantation. World J Gastroenterol. 2020;26:2987-2999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 13. | MK. COVID-19 and coronaviral hepatitis: Evidence of collateral damage. Future Virol. 2020;15. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Masresha BG, Luce R Jr, Weldegebriel G, Katsande R, Gasasira A, Mihigo R. The impact of a prolonged ebola outbreak on measles elimination activities in Guinea, Liberia and Sierra Leone, 2014-2015. Pan Afr Med J. 2020;35 (Suppl 1):8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | We can end COVID-19. Together. [cited 22 February 2021]. Available from: https://www.gatesfoundation.org/goalkeepers/report/2020-report/. |
| 16. | Santoli JM, Lindley MC, DeSilva MB, Kharbanda EO, Daley MF, Galloway L, Gee J, Glover M, Herring B, Kang Y, Lucas P, Noblit C, Tropper J, Vogt T, Weintraub E. Effects of the COVID-19 Pandemic on Routine Pediatric Vaccine Ordering and Administration - United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:591-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 485] [Article Influence: 97.0] [Reference Citation Analysis (0)] |
| 17. | McDonald HI, Tessier E, White JM, Woodruff M, Knowles C, Bates C, Parry J, Walker JL, Scott JA, Smeeth L, Yarwood J, Ramsay M, Edelstein M. Early impact of the coronavirus disease (COVID-19) pandemic and physical distancing measures on routine childhood vaccinations in England, January to April 2020. Euro Surveill. 2020;25: 2000848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 177] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 18. | Mohanty P, Jena P, Patnaik L. Vaccination against Hepatitis B: A Scoping Review. Asian Pac J Cancer Prev. 2020;21:3453-3459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Ratzan SC, Bloom BR, El-Mohandes A, Fielding J, Gostin LO, Hodge JG, Hotez P, Kurth A, Larson HJ, Nurse J, Omer SB, Orenstein WA, Salmon D, Rabin K. The Salzburg Statement on Vaccination Acceptance. J Health Commun. 2019;24:581-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | The Nobel Prize in Physiology or Medicine 2020. NobelPrize.org. [cited 22 February 2021]. Available from: https://www.nobelprize.org/prizes/medicine/2020/press-release/. |
| 21. | Ward JW, Hinman AR, Alter HJ. Time for the Elimination of Hepatitis C Virus as a Global Health Threat. In: The Liver. John Wiley & Sons, Ltd, 2020: 935–952. |
| 22. | WHO. Progress report on HIV, viral hepatitis and sexually transmitted infections 2019. [cited 22 February 2021]. Available from: http://www.who.int/hiv/strategy2016-2021/progress-report-2019/en/. |
| 23. | Abbas K, Procter SR, van Zandvoort K, Clark A, Funk S, Mengistu T, Hogan D, Dansereau E, Jit M, Flasche S; LSHTM CMMID COVID-19 Working Group. Routine childhood immunisation during the COVID-19 pandemic in Africa: a benefit-risk analysis of health benefits versus excess risk of SARS-CoV-2 infection. Lancet Glob Health. 2020;8:e1264-e1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 234] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 24. | Medicine shortage - Newspaper - Dawn.com. [cited 22 February 2021]. Available from: https://www.dawn.com/news/1551772. |
| 25. | Gupta N, Desalegn H, Ocama P, Lacombe K, Njouom R, Afihene M, Cunha L, Spearman CW, Sonderup MW, Kateera F. Converging pandemics: implications of COVID-19 for the viral hepatitis response in sub-Saharan Africa. Lancet Gastroenterol Hepatol. 2020;5:634-636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Mesch GS, Schwirian KP. Vaccination hesitancy: fear, trust, and exposure expectancy of an Ebola outbreak. Heliyon. 2019;5:e02016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Denis F, Cohen R, Martinot A, Stahl JP, Lery T, Le Danvic M, Gaudelus J. Evolution of hepatitis B vaccine coverage rates in France between 2008 and 2011. Med Mal Infect. 2013;43:272-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Assessment of the viral hepatitis response in Ukraine. Mission Report 6-9 June 2017 (2018). [cited 2021 February 22]. Available from: https://www.euro.who.int/en/countries/ukraine/publications/assessment-of-the-viral-hepatitis-response-in-ukraine.-mission-report-6-9-june-2017-2018. |
| 29. | Interest in Home Births Rises During the COVID-19 Pandemic. Healthline. 2020. [cited 22 February 2021]. Available from: https://www.healthline.com/health/pregnancy/home-births-rise-with-covid-19. |
| 30. | Zaami S, Marinelli E, Varì MR. New Trends of Substance Abuse During COVID-19 Pandemic: An International Perspective. Front Psychiatry. 2020;11:700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 31. | The Lancet Gastroenterology Hepatology. Eliminating viral hepatitis in the COVID-19 era: weighing challenge and opportunity. Lancet Gastroenterol Hepatol. 2020;5:789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Stowe MJ, Scheibe A, Shelly S, Marks M. COVID-19 restrictions and increased risk of overdose for street-based people with opioid dependence in South Africa. S Afr Med J. 2020;110:12939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Alexander GC, Stoller KB, Haffajee RL, Saloner B. An Epidemic in the Midst of a Pandemic: Opioid Use Disorder and COVID-19. Ann Intern Med. 2020;173:57-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 262] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 34. | Karimi-Sari H, Tajik M, Bayatpoor ME, Alavian SM. Increasing the Awareness of the General Population: An Important Step in Elimination Programs of Viral Hepatitis. Am J Gastroenterol. 2017;112:393-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Karimi-Sari H, Rezaee-Zavareh MS. COVID-19 and viral hepatitis elimination programs: Are we stepping backward? Liver Int. 2020;40:2042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Wingrove C, Ferrier L, James C, Wang S. The impact of COVID-19 on hepatitis elimination. Lancet Gastroenterol Hepatol. 2020;5:792-794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 37. | Mustafa ZU, Kow CS, Hasan SS. Effect of COVID-19 on viral hepatitis services in Pakistan. Lancet Gastroenterol Hepatol. 2021;6:163-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Lemoine M, Kim JU, Ndow G, Bah S, Forrest K, Rwegasha J, Bouyou M, Napon D, Somda S, Sawadogo A, Sombie R, Shimakawa Y. Effect of the COVID-19 pandemic on viral hepatitis services in sub-Saharan Africa. Lancet Gastroenterol Hepatol. 2020;5:966-967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Ioannidis JPA. Global perspective of COVID-19 epidemiology for a full-cycle pandemic. Eur J Clin Invest. 2020;50:e13423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 40. | Blach S, Kondili LA, Aghemo A, Cai Z, Dugan E, Estes C, Gamkrelidze I, Ma S, Pawlotsky JM, Razavi-Shearer D, Razavi H, Waked I, Zeuzem S, Craxi A. Impact of COVID-19 on global HCV elimination efforts. J Hepatol. 2021;74:31-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 195] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 41. | Toyoda H, Huang DQ, Le MH, Nguyen MH. Liver Care and Surveillance: The Global Impact of the COVID-19 Pandemic. Hepatol Commun. 2020;4:1751-1757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 42. | Picchio CA, Valencia J, Doran J, Swan T, Pastor M, Martró E, Colom J, Lazarus JV. The impact of the COVID-19 pandemic on harm reduction services in Spain. Harm Reduct J. 2020;17:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 43. | Aghemo A, Masarone M, Montagnese S, Petta S, Ponziani FR, Russo FP; Associazione Italiana Studio Fegato (AISF). Assessing the impact of COVID-19 on the management of patients with liver diseases: A national survey by the Italian association for the study of the Liver. Dig Liver Dis. 2020;52:937-941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 44. | El Kassas M, Abdelkader H, Medhat MA. COVID-19 in Egypt: Through crisis to adaptation; a gastroenterologist's perspective. Arab J Gastroenterol. 2020;21:207-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Rodríguez-Tajes S, Miralpeix A, Costa J, López-Suñé E, Laguno M, Pocurull A, Lens S, Mariño Z, Forns X. Low risk of hepatitis B reactivation in patients with severe COVID-19 who receive immunosuppressive therapy. J Viral Hepat. 2021;28:89-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 46. | Reddy KR. SARS-CoV-2 and the Liver: Considerations in Hepatitis B and Hepatitis C Infections. Clin Liver Dis (Hoboken). 2020;15:191-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 47. | Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck-Ytter YT; American Gastroenterological Association Institute. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:215-9; quiz e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 451] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 48. | Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, Pratt DS, Russo MW, Schilsky ML, Verna EC, Loomba R, Cohen DE, Bezerra JA, Reddy KR, Chung RT. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72:287-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 422] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 49. | HIMSS. COVID-19 risks amplified for underserved communities. 2020. [cited 22 February 2021]. Available from: https://www.mobihealthnews.com/news/covid-19-risks-amplified-underserved-communities. |
| 50. | Econ. Policy Inst. Health insurance and the COVID-19 shock: What we know so far about health insurance losses and what it means for policy. [cited 22 February 2021]. Available from: https://www.epi.org/publication/health-insurance-and-the-covid-19-shock/. |
| 51. | Guss D, Sherigar J, Rosen P, Mohanty SR. Diagnosis and Management of Hepatitis C Infection in Primary Care Settings. J Gen Intern Med. 2018;33:551-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 52. | Mendlowitz AB, Naimark D, Wong WWL, Capraru C, Feld JJ, Isaranuwatchai W, Krahn M. The emergency department as a setting-specific opportunity for population-based hepatitis C screening: An economic evaluation. Liver Int. 2020;40:1282-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 53. | WHO. Pulse survey on continuity of essential health services during the COVID-19 pandemic: interim report, 27 August 2020. [cited 22 February 2021]. Available from: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-EHS_continuity-survey-2020.1. |