Published online Jun 27, 2021. doi: 10.4254/wjh.v13.i6.699
Peer-review started: February 4, 2021
First decision: February 24, 2021
Revised: March 9, 2021
Accepted: May 20, 2021
Article in press: May 20, 2021
Published online: June 27, 2021
Processing time: 138 Days and 22.9 Hours
In hepatocellular carcinoma (HCC), detection and treatment prior to growth beyond 2 cm are important as a larger tumor size is more frequently associated with microvascular invasion and/or satellites. In the surveillance of very small HCC nodules (≤ 2 cm in maximum diameter, Barcelona clinical stage 0), we demonstrated that the tumor markers alpha-fetoprotein and PIVKA-Ⅱ are not so useful. Therefore, we must survey with imaging modalities. The superiority of magnetic resonance imaging (MRI) over ultrasound (US) to detect HCC was confirmed in many studies. Although enhanced MRI is now performed to accurately diagnose HCC, in conventional clinical practice for HCC surveillance in liver diseases, unenhanced MRI is widely performed throughout the world. While, MRI has made marked improvements in recent years.
To make a comparison of unenhanced MRI and US in detecting very small HCC that was examined in the last ten years in patients in whom MRI and US examinations were performed nearly simultaneously.
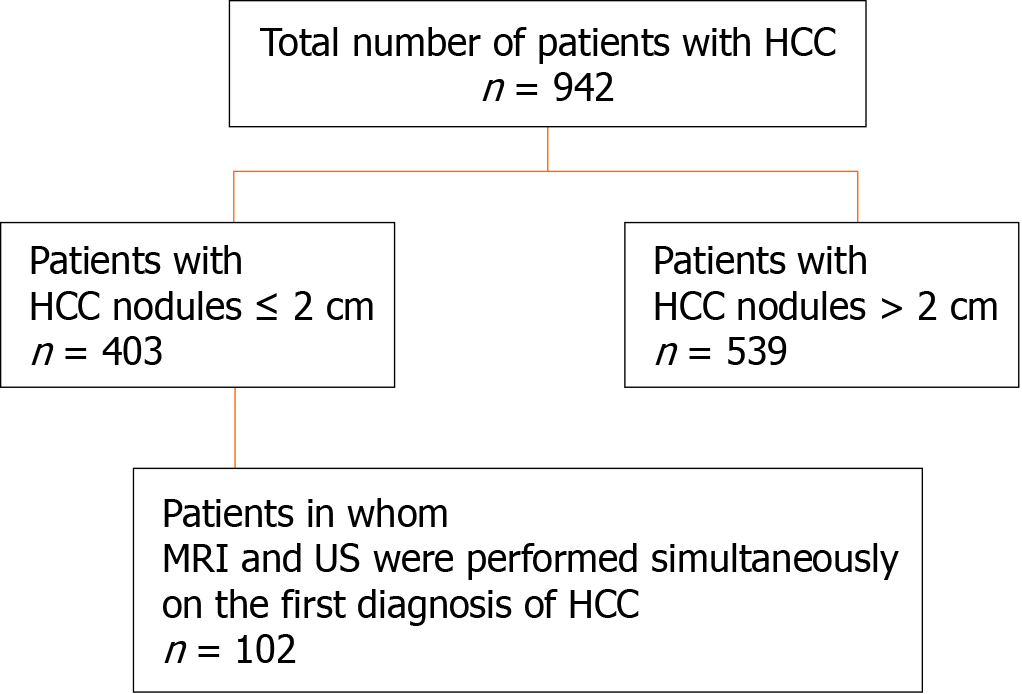
In 394 patients with very small HCC nodules, those who underwent MRI and US at nearly the same time (on the same day whenever possible or at least within 14 days of one another) at the first diagnosis of HCC were selected. The detection rate of HCC with unenhanced MRI was investigated and compared with that of unenhanced US.
The sensitivity of unenhanced MRI for detecting very small HCC was 95.1% (97/102, 95% confidence interval: 90.9-99.3) and that of unenhanced US was 69.6% (71/102, 95% confidence interval: 60.7-78.5). The sensitivity of unenhanced MRI for detecting very small HCC was significantly higher than that of unenhanced US (P < 0.001). Regarding the location of HCC in the liver in patients in whom detection by US was unsuccessful, S7-8 was identified in 51.7%.
Currently, unenhanced MRI is a very useful tool for the surveillance of very small HCC in conventional clinical follow-up practice.
Core Tip: Recent technological development of magnetic resonance imaging (MRI) scanners has been excellent. The 3.0-tesla (T) MR scanner with a higher field strength has been increasingly used because improved lesion detection can be expected as a result of the increased signal-to-noise ratio, which is theoretically twice with 3.0-T compared with 1.5-T. Another important improvement in MRI is the practical use of diffusion-weighted imaging. In this study, a comparison of unenhanced MRI and ultrasound in detecting very small hepatocellular carcinoma (2 cm in maximum diameter) was made. The sensitivity of unenhanced MRI for detecting very small hepatocellular carcinoma was as high as 95.1% as compared with 69.6% of unenhanced ultrasound (P < 0.001).
- Citation: Tarao K, Nozaki A, Komatsu H, Komatsu T, Taguri M, Tanaka K, Yoshida T, Koyasu H, Chuma M, Numata K, Maeda S. Comparison of unenhanced magnetic resonance imaging and ultrasound in detecting very small hepatocellular carcinoma. World J Hepatol 2021; 13(6): 699-708
- URL: https://www.wjgnet.com/1948-5182/full/v13/i6/699.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i6.699
If hepatocellular carcinoma (HCC) tumors are growing up to more than 2 cm in diameter, they are often associated with microvascular invasion and/or satellites, which are major predictors of recurrence after initial effective treatments[1]. The same tendency was observed by Stravitz et al[2], who reported that the early detection of HCC improves the prognosis. Therefore, we must identify very small HCC nodules (≤ 2 cm in maximum diameter) in the surveillance of HCC.
Recently, we demonstrated that more than one third of patients with very small HCC nodules were dropped from surveillance using the tumor markers alpha-fetoprotein (AFP) and PIVKA-Ⅱ[3]. Therefore, we must survey patients with liver diseases using imaging modalities.
Surveillance of HCC in liver diseases, especially in liver cirrhosis, has been conducted by ultrasound (US) or magnetic resonance imaging (MRI) throughout the world.
Although US was performed more popularly than MRI in the surveillance of HCC, the superiority of MRI over US has been demonstrated in many studies since 2001-2003[4,5]. Although enhanced MRI is now performed for the accurate diagnosis of HCC[5-9], in conventional clinical practice for HCC surveillance in liver diseases, unenhanced MRI is widely performed throughout the world. On the other hand, MRI has made much progress in recent years.
In this study, a comparison of unenhanced MRI and US in surveying very small HCC was made. In order to conduct precise evaluation, we selected patients in whom MRI and US were performed at about the same time.
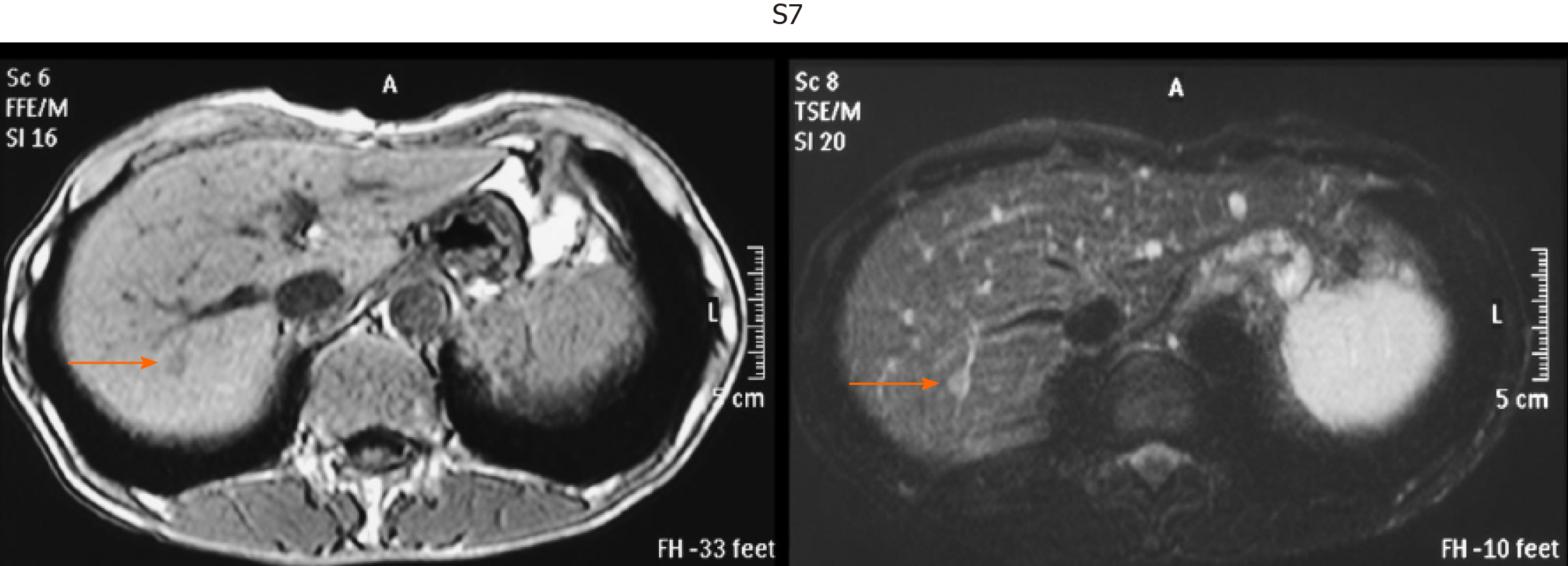
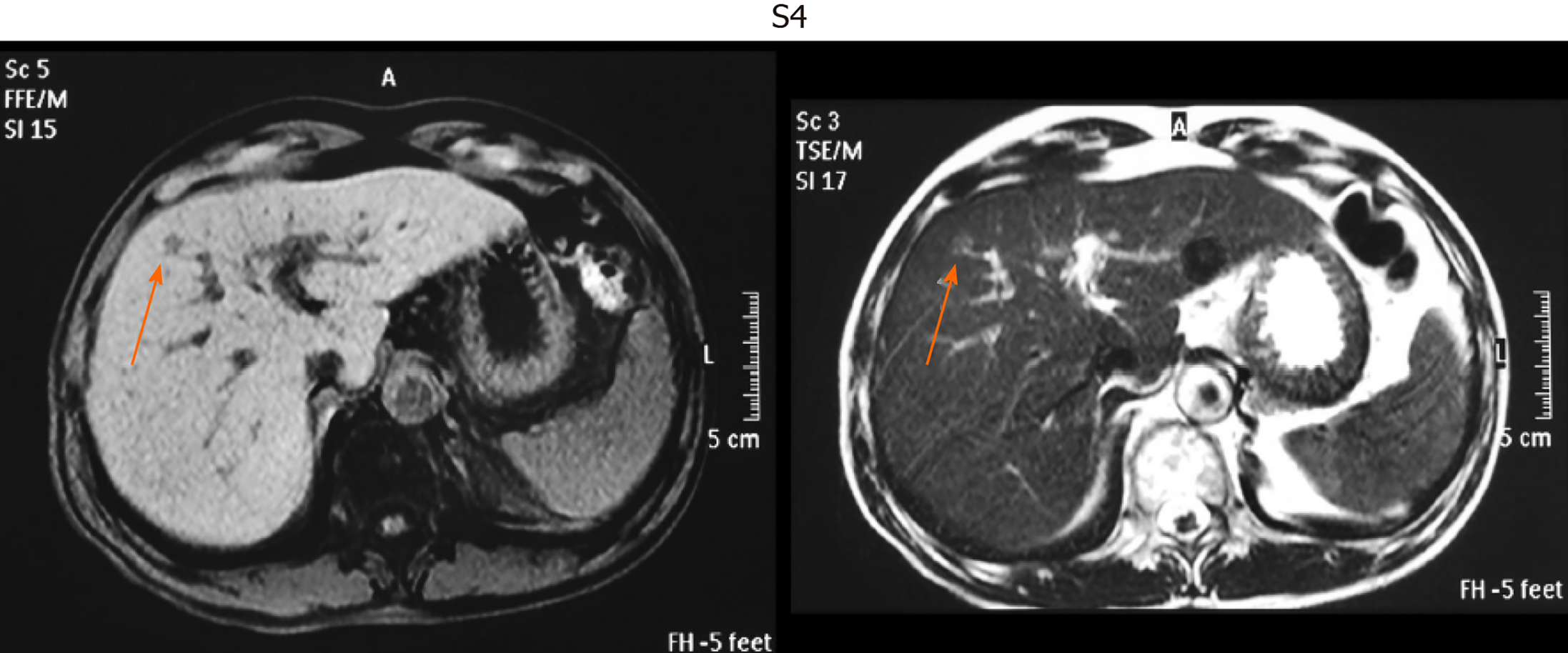
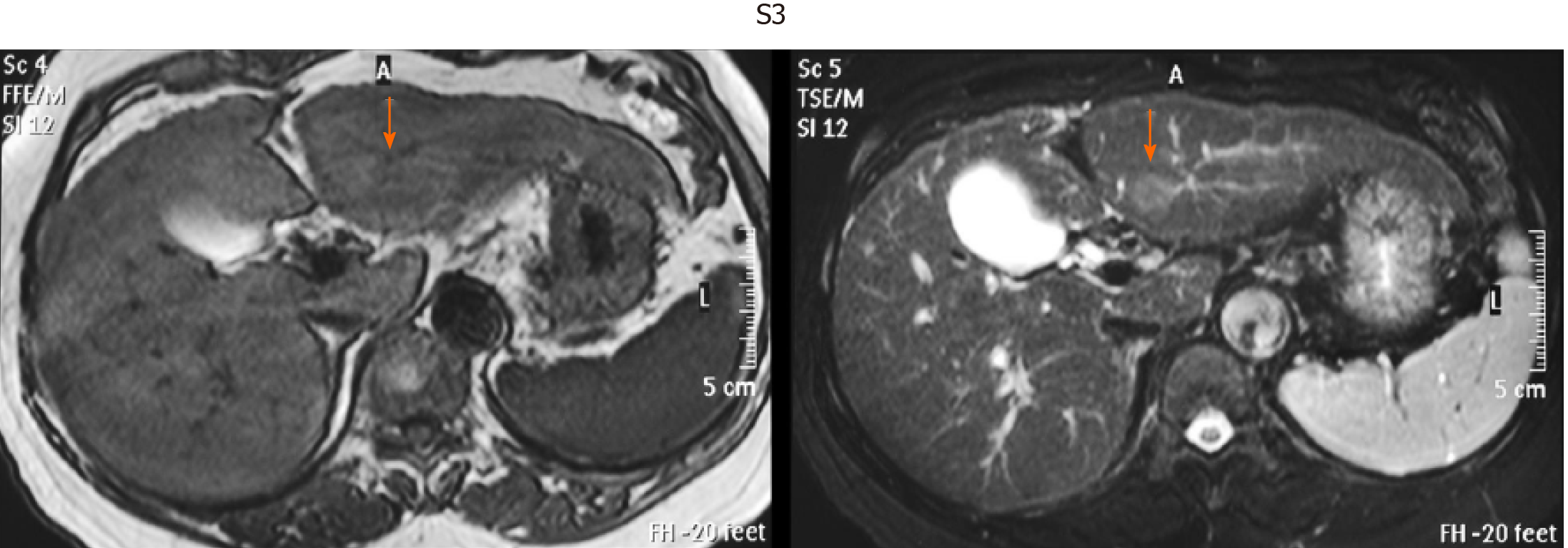
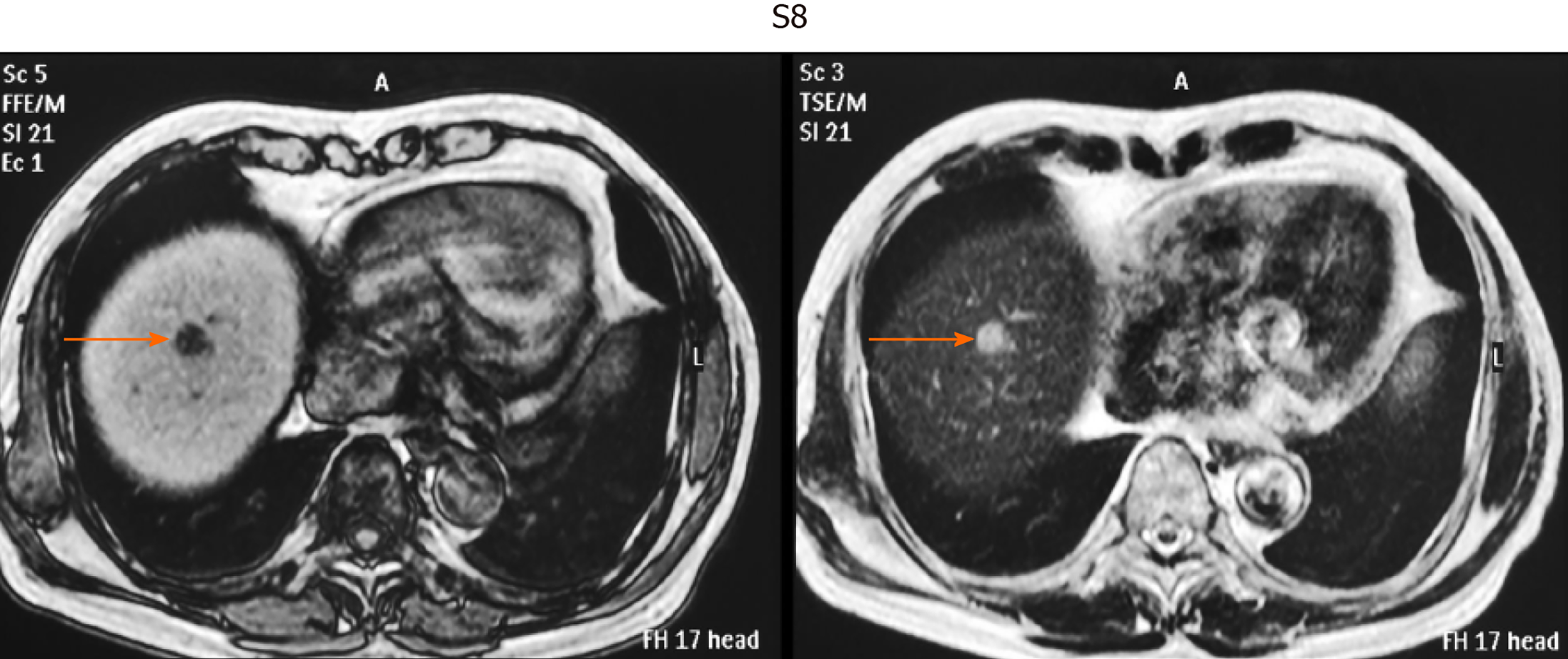
This was a retrospective observational study that included 403 patients with small single HCC nodules (≤ 2 cm in maximum diameter, Barcelona clinical stage 0) who visited the following three hospitals and one clinic in Yokohama City for the first time between January 2008 and September 2020: Gastroenterological Center, Medical Center, Yokohama City University; Department of Gastroenterology, Yokohama Municipal Citizen's Hospital; Department of Gastroenterology, National Hospital Organization, Yokohama Medical Center; and Tarao’s Gastroenterological Clinic. Of the 403 patients with very small HCC, 102 were selected in whom MRI and US were conducted simultaneously (on the same day or at least within 14 days of one another) (Figure 1). In this series of the study, MRI and US were performed in unenhanced states because we wanted to study the usefulness to survey HCC in routine follow-up study. In the unenhanced MRI, a very small HCC usually appears as a dark spot in T1 image and light white spot in T2 image (see Figures 2-5). It is important that characteristics of both T1 and T2 images were present at the same time. In the US images, it usually appears as a dark round spot.
HCCs were diagnosed chiefly by dynamic computed tomography (CT) and abdominal angiography, which showed early enhancement and early washout. This work was performed in accordance with the Declaration of Helsinki.
Previously diagnosed HCC was excluded from the protocol. This study was performed after approval by the respective institutional review boards.
The patients were classified according to the etiologies of liver diseases (Table 1).
| Background of patients | |
| Number of patients | 102 |
| Age (yr) | 72.4 ± 9.6 |
| Sex (%) | |
| Male | 52 (51.0) |
| Female | 50 (49.0) |
| Etiology (%) | |
| HBV | 13 (12.9) |
| HCV | 61 (60.3) |
| Alcohol | 14 (13.9) |
| NBNC | 7 (6.9) |
| Autoimmune | 2 (2.0) |
| NASH | 2 (2.0) |
| PBC | 1 (1.0) |
| Others | 2 (2.0) |
The diagnosis of HCC was confirmed by US, MRI, CT, enhanced dynamic CT, and abdominal angiography. All patients underwent abdominal angiography to confirm the single nodules. The maximum diameter of the HCC nodules was scaled by US or MRI.
Helical dynamic CT and abdominal angiography were performed in almost all patients except those with hypersensitivity to iodine and advanced kidney disease. In the helical dynamic CT, an intravenous bolus injection of contrast material and sequential scanning were performed, and an intense homogenous arterial phase (early enhancement) and early washout in the venous phase were considered to be characteristic of HCC[10-12]. Abdominal angiography was also performed to exclude the benign nodular lesions and exclude HCC patients with macrovascular invasion. Of course, the characteristic features of very small HCC in unenhanced MRI as mentioned above were taken into account.
Patients with macrovascular invasion or extrahepatic metastasis were excluded. In patients undergoing hepatectomy, the final decision on HCC was made by pathological diagnosis, and cases of benign nodules were excluded.
We calculated the detection rate and its 95% confidence interval (CI) for each method. We then compared the detection rates between MRI and US using McNemar's test.
The sensitivity of unenhanced MRI for detecting very small HCC (≤ 2 cm in diameter) was 95.1% [97/102, 95%CI: 90.9-99.3] and that of unenhanced US was 69.6% (71/102, 95%CI: 60.7-78.5) (P < 0.001).
Table 2 shows the location of the HCC in the liver of patients in whom detection by US was unsuccessful. S7-8 was the site in 51.7% of these patients. Thus, HCC lesions in S7-8 may be difficult to identify by US. Representative images of four cases of very small HCC (A, B, C, and D) by unenhanced MRI are shown in Figures 2-5. In all the four cases, HCC was confirmed using hepatectomized specimens.
| Location in the liver | Number of patients (%) |
| S1-4 | 6 (20.7) |
| S5-6 | 8 (27.6) |
| S7-8 | 15 (51.7) |
Moreover, the treatment methods for 102 HCC patients are shown in Table 3.
| Therapy | Number of treated patients |
| Hepatectomy | 19 |
| RFA | 58 |
| TACE | 14 |
| TACE + RFA | 2 |
| TAI | 1 |
| Chemotherapy | 2 |
| BSC | 3 |
| Others | 3 |
For the surveillance of very small HCC, US was hitherto performed worldwide. However, in recent years, the superiority of MRI over US to detect very small HCC has been reported in many articles.
Colli et al[4] conducted a systemic review on this issue, and found that the pooled estimate of 14 US studies was 60.5% (95%CI: 44-76) for sensitivity[13-25], and that of 9 MRI studies was 80.6% (95%CI: 70-91) for sensitivity[9,23,24,26-31]. The difference in sensitivity between US and MRI may be due to the fact that MRI is less influenced by the operator's technique, patient's body type, and location of HCC lesions.
More recently, in 2017, Kim et al[5] compared MRI and US in a cohort of 407 patients with cirrhosis who underwent 1100 surveillance examinations, and found that MRI had a sensitivity of 83.7% (95%CI: 69.7-92.2) for early HCC detection, which was significantly higher than that of US (25.6%, 95%CI: 14.8-49.4).
We demonstrated in this study that 95% of cases with very small HCC can be detected by unenhanced MRI. This figure is very high compared with previous reports published between 2001 and 2003 concerning the sensitivity of unenhanced MRI for detecting very small HCC. Table 4 shows the reported sensitivity of unenhanced MRI for detecting very small HCC between 2001 and 2003 when MRI used 1.5-tesla (T) imaging. The average sensitivity in that period was 60.3% (95%CI: 52.2-68.4)[25,27,28,30,31].
The reasons why this marked improvement appeared in the sensitivity of unenhanced MRI with regard to detecting very small HCC must be considered.
First of all, MRI has made marked progress in its ability in recent years. Recent technological development of MRI scanners has allowed high-quality multiphasic imaging of the entire liver. Since 2003-2005, the 3.0-T magnetic resonance (MR) scanner with a higher field strength has been increasingly used because improved lesion detection can be expected as a result of the increased signal-to-noise ratio (SNR), which is theoretically twice the SNR at 1.5-T[32,33]. Indeed, it was demonstrated that 3.0-T images were superior to 1.5 T images for detecting hepatic metastases[34]. Previous misdiagnoses of HCC on MRI maybe have been due to poor patient compliance, especially the inability to suspend respiration. These problems can be resolved by the new advancements mentioned above to develop faster and motion-robust sequences.
Another important improvement of MRI is the practical use of diffusion-weighted imaging. Indeed, it was demonstrated that the sensitivity of detecting pancreatic cancer rose with the use of diffusion-weighted imaging[35].
On the other hand, the sensitivity of unenhanced US in our study for detecting very small HCC was 69.6%, which was nearly the same as those in previous reports[9,22,24,26-29]. One of the reasons for the inferiority of US may be the location of HCC in the liver. A lesion located at S7-8 (the most frequent HCC lesion in the liver) may be difficult to identify by US.
Our present study indicates the importance of unenhanced MRI in detecting very small HCC, because more than one third of these patients were dropped from surveillance by tumor markers AFP and PVKA-II. However, there are two limitations of unenhanced MRI. First, it is more expensive than US. Second, in case of very tiny HCC (3-5 mm), it is difficult to find HCC by unenhanced MRI.
Considering the above-mentioned facts, unenhanced MRI is a very useful tool for detecting very small HCC in the conventional follow-up of patients with liver diseases, especially liver cirrhosis.
Nowadays advancement of magnetic resonance imaging (MRI) has markedly improved the quality of liver imaging. We believe that a high-speed scan and diffusion-weighted imaging are two major factors that have contributed to the improved detection of hepatocellular carcinomas (HCCs). In early MRI, a respiration artifact was the most troublesome factor deteriorating the quality of images of the liver. A high-speed scan brought by the conversion from 1.5-tesla (T) to 3.0-T facilitates whole-liver MRI while patients hold their breath. Breath-holding scans reduce motion and misregistration artifacts, and create high-quality liver images. In addition, the practical use of diffusion-weighted imaging has contributed to the detection of cell-rich lesions. Tumors are proper objects of these sequences. There is a report (or several reports) that the sensitivity of detecting pancreatic cancer rose with the use of diffusion-weighted imaging. We believe that the same can be applied to detect HCC. Currently, dynamic MRI with contrast media is considered the standard procedure to diagnose HCC. However, with improved images, non-contrasted liver MRI is still a useful modality to detect HCCs.
Previous reports in 2001-2003 stated that the sensitivity of unenhanced MRI to detect very small HCC (≤ 2 cm in diameter) was about 60%. Since then, there have been few reports on the sensitivity to detect very small HCC, especially in recent years.
Surveillance of HCC in liver diseases, especially in liver cirrhosis, has been conducted by ultrasound (US) or MRI throughout the world. Although US was performed more popularly than MRI in the surveillance of HCC, the superiority of MRI over US has been demonstrated in many studies since 2001-2003. Although enhanced MRI is now performed for the accurate diagnosis of HCC, in conventional clinical practice for HCC surveillance in liver diseases, unenhanced MRI is widely performed throughout the world. On the other hand, MRI has made marked improvements in recent years. In this study, a comparison of unenhanced MRI and US in detecting very small HCC was made. In order to conduct precise evaluation, we selected patients in whom MRI and US were performed at about the same time (on the same day whenever possible or at least within 14 d of one another).
Out of the 403 patients with very small HCC nodules (≤ 2 cm in maximal diameter), 102 who underwent unenhanced MRI and US at nearly the same time (on the same day whenever possible or at least within 14 d of one another) at the first diagnosis of HCC were selected. The detection rate of HCC by unenhanced MRI was studied in comparison with unenhanced US.
We found that the sensitivity of unenhanced MRI for detecting very small HCC was as high as 95.1%, as compared with 69.6% by unenhanced US (P < 0.001).
Currently, unenhanced MRI is a very important imaging modality for picking up very small HCC in usual clinical practice.
As in this study, the marked superiority of unenhanced MRI to detect very small HCC as compared with unenhanced US was confirmed, and it may be desirable to perform routine surveillance of HCC in liver diseases by unenhanced MRI.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: The Japanese Society of Gastroenterology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elsayed M, Yang L S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3587] [Article Influence: 275.9] [Reference Citation Analysis (4)] |
| 2. | Stravitz RT, Heuman DM, Chand N, Sterling RK, Shiffman ML, Luketic VA, Sanyal AJ, Habib A, Mihas AA, Giles HC, Maluf DG, Cotterell AH, Posner MP, Fisher RA. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008;121:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Tarao K, Nozaki A, Komatsu H, Komatsu T, Taguri M, Tanaka K, Chuma M, Numata K, Maeda S. Real impact of tumor marker AFP and PIVKA-II in detecting very small hepatocellular carcinoma (≤ 2 cm, Barcelona stage 0) - assessment with large number of cases. World J Hepatol. 2020;12:1046-1054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Colli A, Fraquelli M, Casazza G, Massironi S, Colucci A, Conte D, Duca P. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol. 2006;101:513-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 386] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 5. | Kim SY, An J, Lim YS, Han S, Lee JY, Byun JH, Won HJ, Lee SJ, Lee HC, Lee YS. MRI With Liver-Specific Contrast for Surveillance of Patients With Cirrhosis at High Risk of Hepatocellular Carcinoma. JAMA Oncol. 2017;3:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 259] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 6. | Motosugi U, Ichikawa T, Morisaka H, Sou H, Muhi A, Kimura K, Sano K, Araki T. Detection of pancreatic carcinoma and liver metastases with gadoxetic acid-enhanced MR imaging: comparison with contrast-enhanced multi-detector row CT. Radiology. 2011;260:446-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 7. | Di Martino M, Marin D, Guerrisi A, Baski M, Galati F, Rossi M, Brozzetti S, Masciangelo R, Passariello R, Catalano C. Intraindividual comparison of gadoxetate disodium-enhanced MR imaging and 64-section multidetector CT in the Detection of hepatocellular carcinoma in patients with cirrhosis. Radiology. 2010;256:806-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 8. | Park MJ, Kim YK, Lee MH, Lee JH. Validation of diagnostic criteria using gadoxetic acid-enhanced and diffusion-weighted MR imaging for small hepatocellular carcinoma (<= 2.0 cm) in patients with hepatitis-induced liver cirrhosis. Acta Radiol. 2013;54:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Rode A, Bancel B, Douek P, Chevallier M, Vilgrain V, Picaud G, Henry L, Berger F, Bizollon T, Gaudin JL, Ducerf C. Small nodule detection in cirrhotic livers: evaluation with US, spiral CT, and MRI and correlation with pathologic examination of explanted liver. J Comput Assist Tomogr. 2001;25:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 203] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 10. | Hayashi M, Matsui O, Ueda K, Kawamori Y, Kadoya M, Yoshikawa J, Gabata T, Takashima T, Nonomura A, Nakanuma Y. Correlation between the blood supply and grade of malignancy of hepatocellular nodules associated with liver cirrhosis: evaluation by CT during intraarterial injection of contrast medium. AJR Am J Roentgenol. 1999;172:969-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 249] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 11. | Ueda K, Terada T, Nakanuma Y, Matsui O. Vascular supply in adenomatous hyperplasia of the liver and hepatocellular carcinoma: a morphometric study. Hum Pathol. 1992;23:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 109] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Roncalli M, Roz E, Coggi G, Di Rocco MG, Bossi P, Minola E, Gambacorta M, Borzio M. The vascular profile of regenerative and dysplastic nodules of the cirrhotic liver: implications for diagnosis and classification. Hepatology. 1999;30:1174-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 133] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Okazaki N, Yoshida T, Yoshino M, Matue H. Screening of patients with chronic liver disease for hepatocellular carcinoma by ultrasonography. Clin Oncol. 1984;10:241-246. [PubMed] |
| 14. | Maringhini A, Cottone M, Sciarrino E, Marcenò MP, La Seta F, Rinaldi F, Pagliaro L. Ultrasonographic and radionuclide detection of hepatocellular carcinoma in cirrhotics with low alpha-fetoprotein levels. Cancer. 1984;54:2924-2926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Kobayashi K, Sugimoto T, Makino H, Kumagai M, Unoura M, Tanaka N, Kato Y, Hattori N. Screening methods for early detection of hepatocellular carcinoma. Hepatology. 1985;5:1100-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Tanaka S, Kitamura T, Ohshima A, Umeda K, Okuda S, Ohtani T, Tatsuta M, Yamamoto K. Diagnostic accuracy of ultrasonography for hepatocellular carcinoma. Cancer. 1986;58:344-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Dodd GD 3rd, Miller WJ, Baron RL, Skolnick ML, Campbell WL. Detection of malignant tumors in end-stage cirrhotic livers: efficacy of sonography as a screening technique. AJR Am J Roentgenol. 1992;159:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 106] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Saada J, Bhattacharya S, Dhillon AP, Dick R, Burroughs AK, Rolles K, Davidson BR. Detection of small hepatocellular carcinomas in cirrhotic livers using iodised oil computed tomography. Gut. 1997;41:404-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Chalasani N, Horlander JC Sr, Said A, Hoen H, Kopecky KK, Stockberger SM Jr, Manam R, Kwo PY, Lumeng L. Screening for hepatocellular carcinoma in patients with advanced cirrhosis. Am J Gastroenterol. 1999;94:2988-2993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Gambarin-Gelwan M, Wolf DC, Shapiro R, Schwartz ME, Min AD. Sensitivity of commonly available screening tests in detecting hepatocellular carcinoma in cirrhotic patients undergoing liver transplantation. Am J Gastroenterol. 2000;95:1535-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 116] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Kim CK, Lim JH, Lee WJ. Detection of hepatocellular carcinomas and dysplastic nodules in cirrhotic liver: accuracy of ultrasonography in transplant patients. J Ultrasound Med. 2001;20:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 99] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Bennett GL, Krinsky GA, Abitbol RJ, Kim SY, Theise ND, Teperman LW. Sonographic detection of hepatocellular carcinoma and dysplastic nodules in cirrhosis: correlation of pretransplantation sonography and liver explant pathology in 200 patients. AJR Am J Roentgenol. 2002;179:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Teefey SA, Hildeboldt CC, Dehdashti F, Siegel BA, Peters MG, Heiken JP, Brown JJ, McFarland EG, Middleton WD, Balfe DM, Ritter JH. Detection of primary hepatic malignancy in liver transplant candidates: prospective comparison of CT, MR imaging, US, and PET. Radiology. 2003;226:533-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Tong MJ, Blatt LM, Kao VW. Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United States of America. J Gastroenterol Hepatol. 2001;16:553-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Libbrecht L, Bielen D, Verslype C, Vanbeckevoort D, Pirenne J, Nevens F, Desmet V, Roskams T. Focal lesions in cirrhotic explant livers: pathological evaluation and accuracy of pretransplantation imaging examinations. Liver Transpl. 2002;8:749-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Born M, Layer G, Kreft B, Schwarz N, Schild H. [MRI, CT and CT arterial portography in the diagnosis of malignant liver tumors in liver cirrhosis]. Rofo. 1998;168:567-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Krinsky GA, Lee VS, Theise ND, Weinreb JC, Rofsky NM, Diflo T, Teperman LW. Hepatocellular carcinoma and dysplastic nodules in patients with cirrhosis: prospective diagnosis with MR imaging and explantation correlation. Radiology. 2001;219:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 230] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 28. | de Lédinghen V, Laharie D, Lecesne R, Le Bail B, Winnock M, Bernard PH, Saric J, Couzigou P, Balabaud C, Bioulac-Sage P, Drouillard J. Detection of nodules in liver cirrhosis: spiral computed tomography or magnetic resonance imaging? Eur J Gastroenterol Hepatol. 2002;14:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Mori K, Scheidler J, Helmberger T, Holzknecht N, Schauer R, Schirren CA, Bittmann I, Dugas M, Reiser M. Detection of malignant hepatic lesions before orthotopic liver transplantation: accuracy of ferumoxides-enhanced MR imaging. AJR Am J Roentgenol. 2002;179:1045-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Bhartia B, Ward J, Guthrie JA, Robinson PJ. Hepatocellular carcinoma in cirrhotic livers: double-contrast thin-section MR imaging with pathologic correlation of explanted tissue. AJR Am J Roentgenol. 2003;180:577-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Burrel M, Llovet JM, Ayuso C, Iglesias C, Sala M, Miquel R, Caralt T, Ayuso JR, Solé M, Sanchez M, Brú C, Bruix J; Barcelona Clínic Liver Cancer Group. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology. 2003;38:1034-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Norris DG. High field human imaging. J Magn Reson Imaging. 2003;18:519-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 126] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Schick F. Whole-body MRI at high field: technical limits and clinical potential. Eur Radiol. 2005;15:946-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 237] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 34. | Sofue K, Tsurusaki M, Miyake M, Sakurada A, Arai Y, Sugimura K. Detection of hepatic metastases by superparamagnetic iron oxide-enhanced MR imaging: prospective comparison between 1.5-T and 3.0-T images in the same patients. Eur Radiol. 2010;20:2265-2273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Matsuki M, Inada Y, Nakai G, Tatsugami F, Tanikake M, Narabayashi I, Masuda D, Arisaka Y, Takaori K, Tanigawa N. Diffusion-weighed MR imaging of pancreatic carcinoma. Abdom Imaging. 2007;32:481-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |