Published online May 27, 2021. doi: 10.4254/wjh.v13.i5.595
Peer-review started: January 13, 2021
First decision: February 24, 2021
Revised: March 11, 2021
Accepted: March 18, 2021
Article in press: March 18, 2021
Published online: May 27, 2021
Processing time: 127 Days and 12 Hours
Biliary drainage, either by the stent-in-stent (SIS) or side-by-side (SBS) technique, is often required when treating a malignant hilar biliary obstruction (MHBO). Both methods differ from each other and have distinct advantages.
To compare both techniques regarding their efficacy and safety in achieving drainage of MHBO.
A comprehensive search of multiple electronic databases (MEDLINE, Embase, LILACS, BIREME, Cochrane) was conducted and grey literature from their inception until December 2020 with no restrictions regarding the year of publication or language, since there was at least an abstract in English. The included studies compared SIS and SBS techniques through endoscopic retrograde cholangiopancreatography. Outcomes analyzed included technical and clinical success, early and late adverse events (AEs), stent patency, reintervention, and procedure-related mortality.
Four cohort studies and one randomized controlled trial evaluating a total of 250 patients (127 in the SIS group and 123 in the SBS group) were included in this study. There were no statistically significant differences between the two groups concerning the evaluated outcomes, except for stent patency, which was higher in the SIS compared with the SBS technique [mean difference (d) = 33.31; 95% confidence interval: 9.73 to 56.90, I2 = 45%, P = 0.006].
The SIS method showed superior stent patency when compared to SBS for achieving bilateral drainage in MHBO. Both techniques are equivalent in terms of technical success, clinical success, rates of both early and late AEs, reintervention, and procedure-related mortality.
Core Tip: Biliary drainage is often required when treating a malignant hilar biliary obstruction. There are two types of drainage: Stent-in-stent (SIS) and side-by-side (SBS) techniques. Both of them differ from each other and have distinct advantages. This study aimed to compare both techniques regarding their efficacy and safety. Our systematic review and meta-analysis demonstrated no statistically significant differences between the SIS and SBS techniques; except for stent patency which was superior in the SIS technique. The choice of palliation for drainage must be guided by both local expertise and resource availability.
- Citation: de Souza GMV, Ribeiro IB, Funari MP, de Moura DTH, Scatimburgo MVCV, de Freitas Júnior JR, Sánchez-Luna SA, Baracat R, de Moura ETH, Bernardo WM, de Moura EGH. Endoscopic retrograde cholangiopancreatography drainage for palliation of malignant hilar biliary obstruction — stent-in-stent or side-by-side? A systematic review and meta-analysis. World J Hepatol 2021; 13(5): 595-610
- URL: https://www.wjgnet.com/1948-5182/full/v13/i5/595.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i5.595
Malignant hilar biliary obstruction (MHBO) is a late manifestation of certain types of cancer. This is diagnosed as unresectable in up to 80% of cases, and capable of causing potentially fatal complications, such as cholangitis and sepsis[1-6]. Thus, aimed at improving the quality of life and survival rate of patients, a discussion on the optimal method for palliation of drainage is very valuable[7-10].
The endoscopic biliary stent, introduced at the beginning of the 1980s, was a significant advance in the treatment of extrahepatic obstruction[11-13]. In biliary obstruction, self-expandable metal stents (SEMS) seem to provide prolonged patency of drainage when compared to plastic stents[3,4,14-17]. The endoscopic approach is preferred for drainage over the percutaneous and surgical approaches due to its more physiological nature, minimal invasiveness[3,4,6,18-20], low rate of adverse events (AEs), and shorter hospital stays[21]. One predictor of the effectiveness of biliary drainage is when the drained hepatic volume is above 50%. This often requires a bilateral decompression[15,22], which is associated with a lower chance of reintervention when compared to unilateral drainage in the palliation of drainage of MHBOs[23].
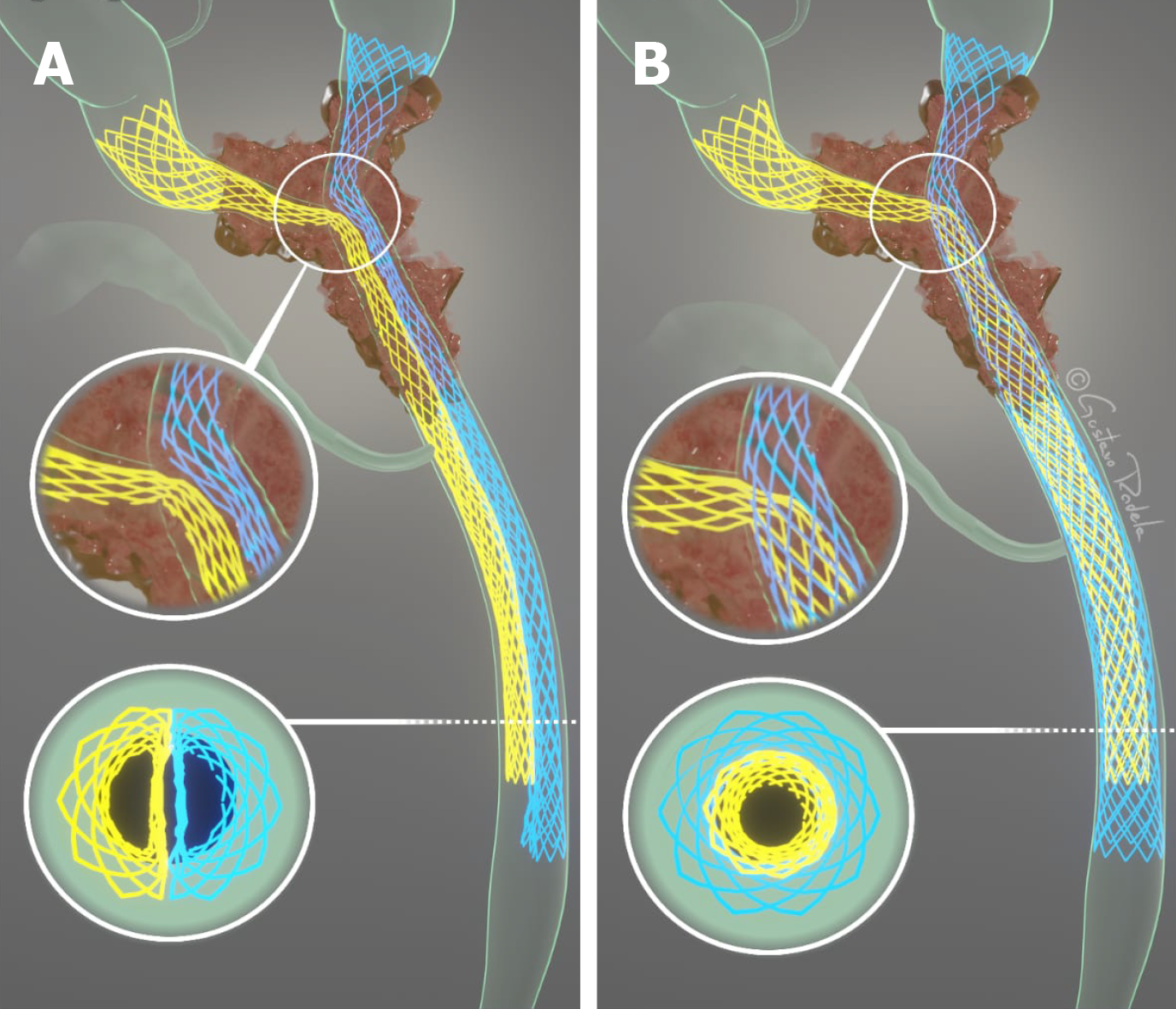
Bilateral drainage of the bile ducts can be performed via two methods: Stent-in-stent (SIS) or side-by-side (SBS)[15] placement of metal stents (Figure 1). In the SIS technique, one of the stents is positioned through the wire mesh of the other, configuring into a Y-shaped aspect. On the other hand, in the SBS method, both stents are placed side by side[22]. The SIS technique, in contrast to the SBS technique, does not require a dilated common bile duct, and thus allows the placement of higher caliber biliary stents[17], and presents a more physiological nature of drainage[3]. The SBS technique provides an easier procedural execution[3,15], and in the case of stent occlusion, reintervention is often more feasible[17].
In theory, there are advantages to both techniques, which casts doubt whether there is enough evidence to favor one method to the detriment of the other. Furthermore, few comparative studies have addressed the subject, making it still unclear which of the two methods is the optimal approach. To gather the best available data in the literature, we have designed this systematic review and meta-analysis on the subject. We aimed to compare the feasibility, safety, and efficacy of both the SIS and SBS techniques for palliative drainage in MHBO.
This study was performed in conformity with the PRISMA[24] and it was registered in the International Prospective Register of Systematic Reviews under the file number CRD42020191262. The study was approved by the Ethics Committee of Hospital das Clínicas, Faculty of Medicine at The University of São Paulo.
The data search was made without limitations of publication date or language, since there was at least an abstract in English. We considered clinical trials or observational studies published either as full text or as an abstract with the necessary data, comparing SIS and SBS metal stent placement in patients with malignant hilar biliary strictures. The following outcomes were observed: Technical and clinical success, early AEs (occurring within the first month after the procedure), late AEs (occurring after 30 d), stent patency, reintervention, and procedural-related mortality.
The exclusion criteria were studies using non-human subjects and trials that evaluated percutaneous biliary access drainage.
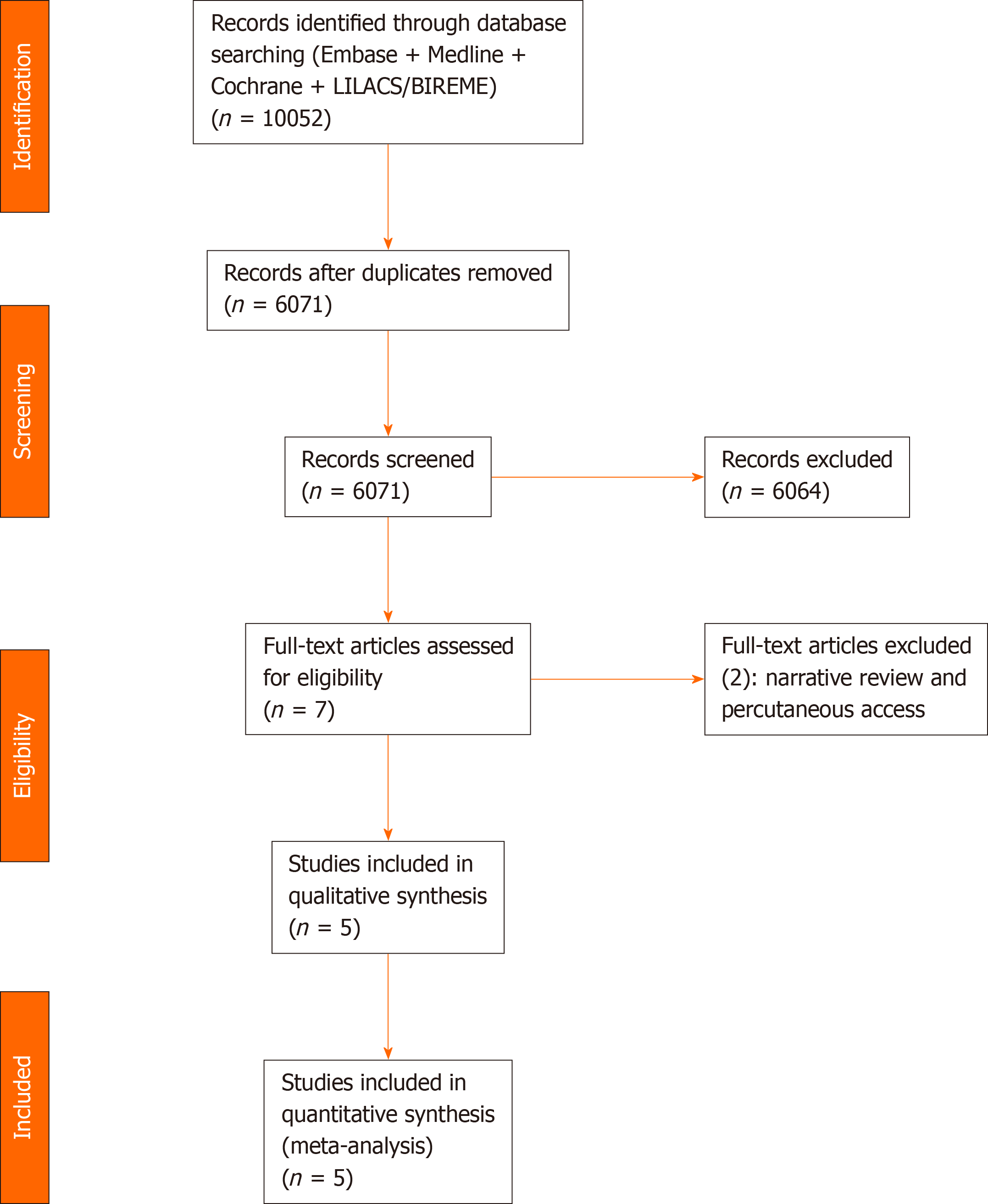
We identified the studies by searching electronic databases and scanning reference lists of the selected articles. This search strategy was applied in electronic databases [MEDLINE, Embase, Central Cochrane, LILACS (via BVS), BIREME, and Google Scholar] and grey literature from their inception until December 2020 (Figure 2).
The following search strategy was used in all databases: [(Neoplasia OR Neoplasias OR Neoplasm OR Neoplasms OR Tumors OR Tumor OR Cancer OR Cancers OR Malignancy OR Malignancies) AND (Biliary Tract OR Biliary Tree OR Biliary System OR Bile Duct OR Bile Ducts)] OR [(Bile Duct Neoplasms OR Bile Duct Neoplasm OR Bile Duct Cancer OR Bile Duct Cancers OR Biliary Tract Neoplasm OR Biliary Tract Neoplasms OR Biliary Tract Cancer OR Biliary Tract Cancers) AND (Prostheses and Implants)] OR Prosthetic OR Implants OR Implant OR Prostheses OR Prosthesis OR Endoprosthesis OR Endoprostheses OR Stent OR Stents OR Stent-in-stent OR Side-by-Side.
Two researchers reviewed the title and abstract of each article after the removal of duplicated articles. Articles that were found to be relevant were selected for full-text review. The final decision on the selection of the studies was based on predetermined inclusion and exclusion criteria. Any disagreement on the selection of studies was resolved by consensus with a third experienced researcher. The target data of the selected studies were entered and organized in a Microsoft Excel spreadsheet by the same two reviewers who conducted the selection. The reviewers extracted from the articles the outcomes of interest and information concerning the population and study characteristics. When the data of the published articles were insufficient, the corresponding authors were consulted by e-mail for further elucidation.
The risk of bias in the cohort studies was assessed by the Risk of Bias in Non-randomized Studies-of Interventions (ROBINS I) Cochrane tool[25]. For randomized clinical trials, the risk of bias was defined by version 2 of the Cochrane Risk-of-Bias tool for Randomized Trials (RoB2)[26].
The quality of evidence, expressed as high, moderate, low, and very low, was assessed utilizing the objective criteria from GRADE (Grading Recommendations Assessment, Development, and Evaluation) for each of the pre-specified results and outcomes using GRADEpro-Guideline Development Tool software (McMaster University, 2015; Evidence Prime, Inc., Ontario, Canada)[27].
For continuous variables, we used mean or median values[28] along with the standard deviation and the total number of patients. Regarding the outcomes expressed by categorical variables, the absolute number of events and the total number of patients was employed, with calculation of the regular and absolute risk differences for each group utilizing the Mantel-Haenszel test. The mean values of each continuous outcome were calculated, as well as the 95% confidence interval (CI). P values < 0.05 were considered statistically significant and the results were exposed through forest plots.
Heterogeneity was calculated using the Higgins method (I2). When heterogeneity < 50% was found, the fixed-effect model was used. In outcomes with high heterogeneity among studies (I2> 50%), sensitivity analysis employing funnel plots were conducted to identify publication bias (outliers). If the heterogeneity levels were still high even after outlier exclusion, we maintained the outlier and applied the random-effects model to express the results (true heterogeneity). If the heterogeneity levels were low after outlier exclusion, we applied the fixed-effects model.
The data of interest extracted from the selected studies were meta-analyzed using RevMan software (Review Manager Software version 5.4—Cochrane Collaboration Copyright© 2020).
A total of 10052 articles were identified through our searches in the MEDLINE, Embase, LILACS, BIREME, and Central Cochrane databases. After the removal of duplicates, evaluation of the titles and abstracts, and text analysis, four retrospective cohort studies[29-32] and one randomized controlled trial (RCT)[33] were included in the meta-analysis (Figure 2). The characteristics of the included studies are summarized in Table 1.
| Technical success | Clinical success | Rate of early adverse events | Rate of late adverse events | Stent patency | Reintervention | Procedure-related mortality | ||||||||||
| Ref. | Design | Year | SIS | SBS | SIS | SBS | SIS | SBS | SIS | SBS | SIS | SBS | SIS | SBS | SIS | SBS |
| Lee et al[33] | RCT | 2019 | 34/34 | 32/35 | 32/34 | 29/35 | 4/34 | 4/35 | 6/34 | 8/35 | Median 253 d (28-420); SD 98; mean 253 | Median 262 d (9-455); SD 111.5; mean 262 | 15/34 | 12/35 | 0/34 | 0/35 |
| Naitoh et al[30] | Cohort | 2012 | 24/24 | 25/28 | 24/24 | 24/28 | 1/24 | 3/28 | 2/24 | 8/28 | Median 104 d (20-600); SD 145; mean 207 | Median 155 d (15–881); SD 216.5; mean 155 | NA | NA | 0/24 | 0/28 |
| Kim et al[31] | Cohort | 2012 | 18/22 | 15/19 | NA | NA | 5/22 | 6/19 | 11/22 | 7/19 | NA | NA | NA | NA | NA | NA |
| Law et al[29] | Cohort | 2013 | 7/7 | 17/17 | NA | NA | NA | NA | 0/7 | 0/17 | NA | NA | 3/7 | 9/17 | 0/7 | 0/17 |
| Ishigaki et al[32] | Cohort | 2020 | 40/40 | 23/24 | 37/40 | 23/24 | 9/40 | 11/24 | 4/40 | 3/24 | Median 169 d (108-445); SD 84.25; mean 169 | Median 205 d (85-NA); SD 24.39; mean 123.75 | NA | NA | NA | NA |
Three[29,30,32] of the four retrospective studies presented a moderate overall risk of bias, assessed by the ROBINS-I tool, mainly due to confounding, the bias in the selection of participants, and bias in the selection of the reported results. The other included study[31] presented a serious risk of bias. The RCT study[33] presented a low risk of bias in our analysis (RoB2) (Tables 2 and 3). Detailed information concerning the risk of bias for each outcome is described in Table 4.
| Ref. | D1 | D2 | D3 | D4 | D5 | D6 | D7 | Overall |
| Naitoh et al[30] 2012 | Moderate | Moderate | Low | Low | Low | Moderate | Moderate | Moderate |
| Kim et al[31] 2012 | Serious | Serious | Low | Serious | Serious | Serious | Serious | Serious |
| Law et al[29] 2013 | Moderate | Moderate | Low | Low | Moderate | Moderate | Serious | Moderate |
| Ishigaki et al[32] 2020 | Moderate | Moderate | Low | Low | Low | Moderate | Moderate | Moderate |
| Certainty assessment | Summary of findings | ||||||||||
| Participants (studies) follow up | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall certainty of evidence | Study event rates (%) | Relative effect (95%CI) | Anticipated absolute effects | ||
| With SBS | With SIS | Risk with SBS | Risk difference with SIS | ||||||||
| Early adverse events: Cohorts | |||||||||||
| 157 (3 observational studies) | Serious1 | Not serious | Not serious | Not serious | None | Moderate | 20/71 (28.2) | 15/86 (17.4) | RR 0.54 (0.31 to 0.96) | 282 per 1.000 | 130 fewer per 1.000 (from 194 fewer to 11 fewer) |
| Early adverse events: RCT | |||||||||||
| 69 (1 RCT) | Not serious | Not serious | Not serious | Serious2 | None | Moderate | 4/35 (11.4) | 4/34 (11.8) | RR 1.03 (0.28 to 3.79) | 114 per 1.000 | 3 more per 1.000 (from 82 fewer to 319 more) |
| Late adverse events: Cohorts | |||||||||||
| 181 (4 observational studies) | Serious1 | Not serious | Not serious | Not serious | None | Moderate | 18/88 (20.5) | 17/93 (18.3) | RR 0.82 (0.46 to 1.47) | 205 per 1.000 | 37 fewer per 1.000 (from 110 fewer to 96 more) |
| Late adverse events: RCT | |||||||||||
| 69 (1 RCT) | Not serious | Not serious | Not serious | Serious2 | None | Moderate | 8/35 (22.9) | 6/34 (17.6) | RR 0.77 (0.30 to 1.99) | 229 per 1.000 | 53 fewer per 1.000 (from 160 fewer to 226 more) |
| Procedural-related mortality: Cohorts | |||||||||||
| 76 (2 observational studies) | Serious1 | Not serious | Not serious | Not serious | None | Moderate | 0/45 (0.0) | 0/31 (0.0) | Not pooled | Not pooled | Not pooled |
| Procedural-related mortality: RCT | |||||||||||
| 69 (1 RCT) | Not serious | Not serious | Not serious | Not serious | None | High | 0/35 (0.0) | 0/34 (0.0) | RR 0.00 (-0.05 to 0.05) | 0 per 1.000 | - per 1.000 (from 0 fewer to 0 fewer) |
| Technical success: Cohorts | |||||||||||
| 181 (4 observational studies) | Serious1 | Not serious | Not serious | Not serious | None | Moderate | 80/88 (90.9) | 89/93 (95.7) | RR 1.06 (0.97 to 1.16) | 909 per 1.000 | 55 more per 1.000 (from 27 fewer to 145 more) |
| Technical success: RCT | |||||||||||
| 69 (1 RCT) | Not serious | Not serious | Not serious | Not serious | None | High | 32/35 (91.4) | 34/34 (100.0) | RR 1.09 (0.97 to 1.22) | 914 per 1.000 | 82 more per 1.000 (from 27 fewer to 201 more) |
| Clinical success: Cohort | |||||||||||
| 116 (2 observational studies) | Serious1 | Serious3 | Not serious | Not serious | None | Low | 47/52 (90.4) | 61/64 (95.3) | RR 1.05 (0.87 to 1.26) | 904 per 1.000 | 45 more per 1.000 (from 118 fewer to 235 more) |
| Clinical success: RCT | |||||||||||
| 69 (1 RCT) | Not serious | Not serious | Not serious | Not serious | None | High | 29/35 (82.9) | 32/34 (94.1) | RR 1.14 (0.96 to 1.35) | 829 per 1.000 | 116 more per 1.000 (from 33 fewer to 290 more) |
| Reintervention: Cohort | |||||||||||
| 24 (1 observational study) | Serious1 | Not serious | Not serious | Serious2 | None | Low | 9/17 (52.9) | 3/7 (42.9) | RR 0.81 (0.31 to 2.13) | 529 per 1.000 | 101 fewer per 1.000 (from 365 fewer to 598 more) |
| Reintervention: RCT | |||||||||||
| 69 (1 RCT) | Not serious | Not serious | Not serious | Not serious | None | High | 12/35 (34.3) | 15/34 (44.1) | RR 1.29 (0.71 to 2.33) | 343 per 1.000 | 99 more per 1.000 (from 99 fewer to 456 more) |
| Stent patency: Cohort | |||||||||||
| 116 (2 observational studies) | Serious1 | Not serious | Not serious | Not serious | None | Moderate | 52 | 64 | - | The mean stent patency: Cohort was 0 | MD 45.75 higher (18.92 higher to 72.58 higher) |
| Stent patency: RCT | |||||||||||
| 69 (1 RCT) | Not serious | Not serious | Not serious | Not serious | None | High | 35 | 34 | - | The mean stent patency: RCT was 0 | MD 9 lower (58.49 lower to 40.49 higher) |
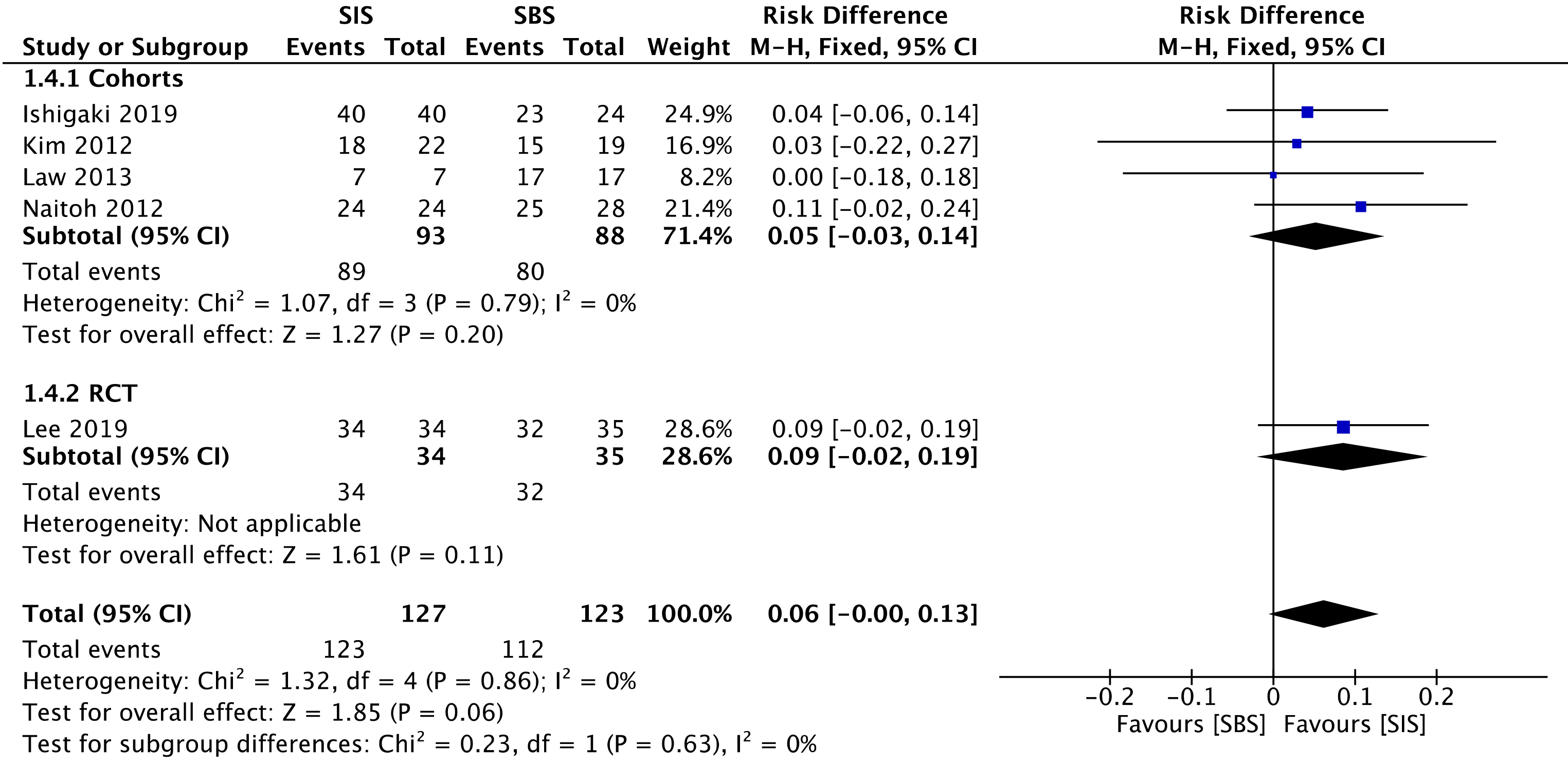
All four cohorts[29-32] (181 patients) and the RCT study[33] (69 patients) assessed technical success. The overall analysis showed no difference between both SIS and SBS [risk difference (RD) = 0.06; 95%CI: -0.00 to 0.13, I2 = 0%, P = 0.06] (Figure 3).
The overall certainty of the evidence was moderate for the cohorts and high for the RCT study, according to GRADE.
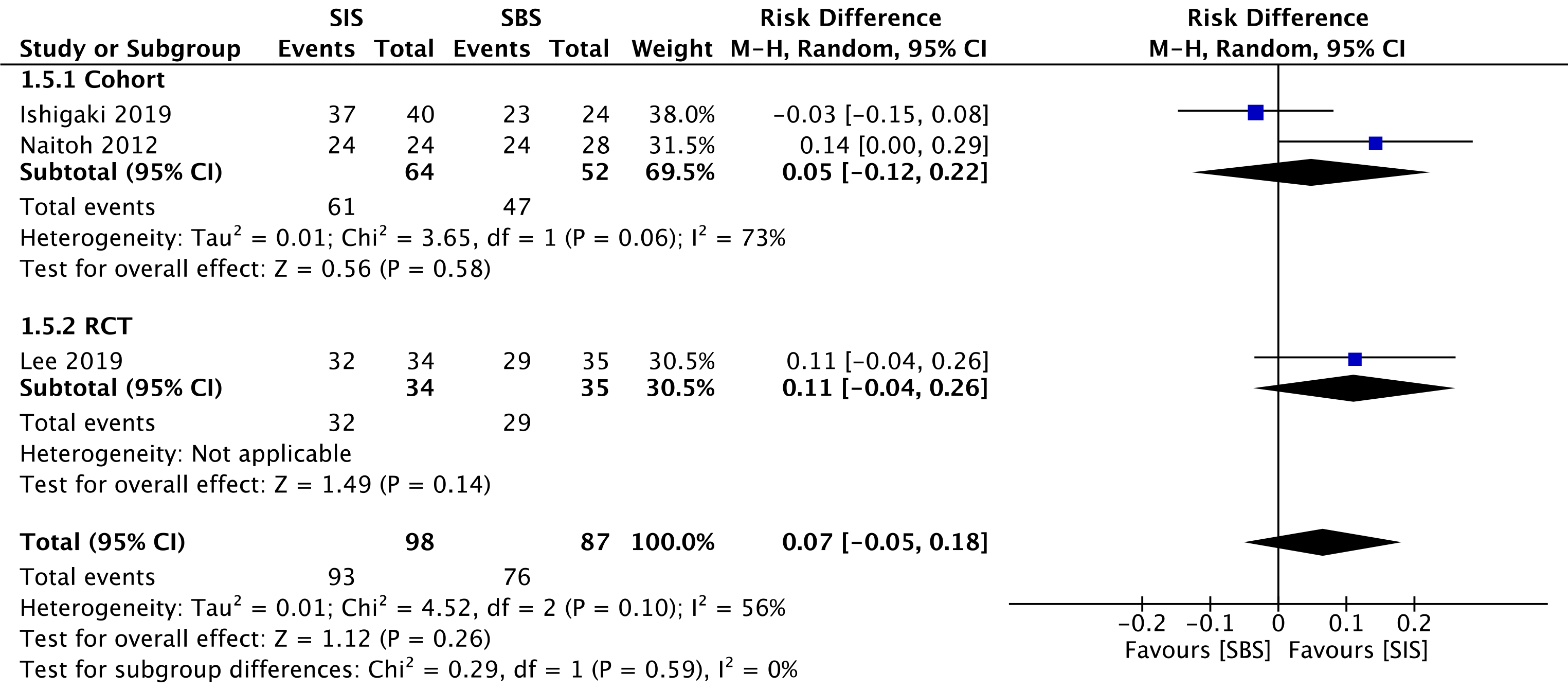
Three studies evaluated clinical success, namely two cohorts[30,32] (116 patients) and the RCT study[33] (69 patients). This outcome was similar for both SIS and SBS techniques in the overall analysis (RD = 0.07; 95%CI: -0.05 to 0.18, I2 = 56%, P =0.26) (Figure 4).
The overall certainty of the evidence was low for the cohort and high for the RCT study, according to GRADE.
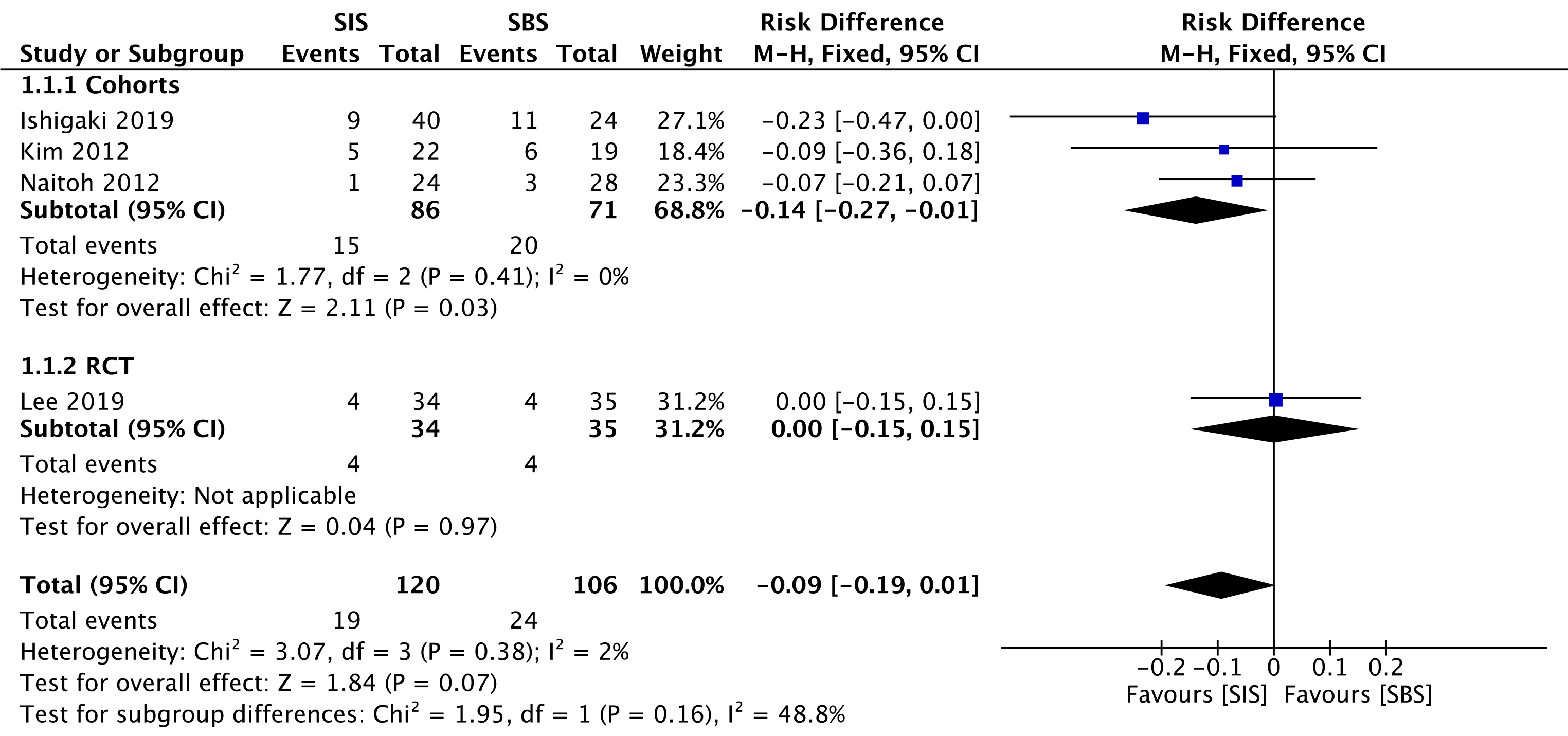
Three cohorts[30-32] (157 patients) and the RCT study[33] (69 patients) evaluated early complications. In the overall analysis, both SIS and SBS techniques performed similarly regarding this outcome (RD = -0.09; 95%CI: -0.19 to 0.01, I2 = 2%, P =0.07) (Figure 5).
The overall certainty of the evidence was moderate for both cohorts and the RCT study, according to GRADE.
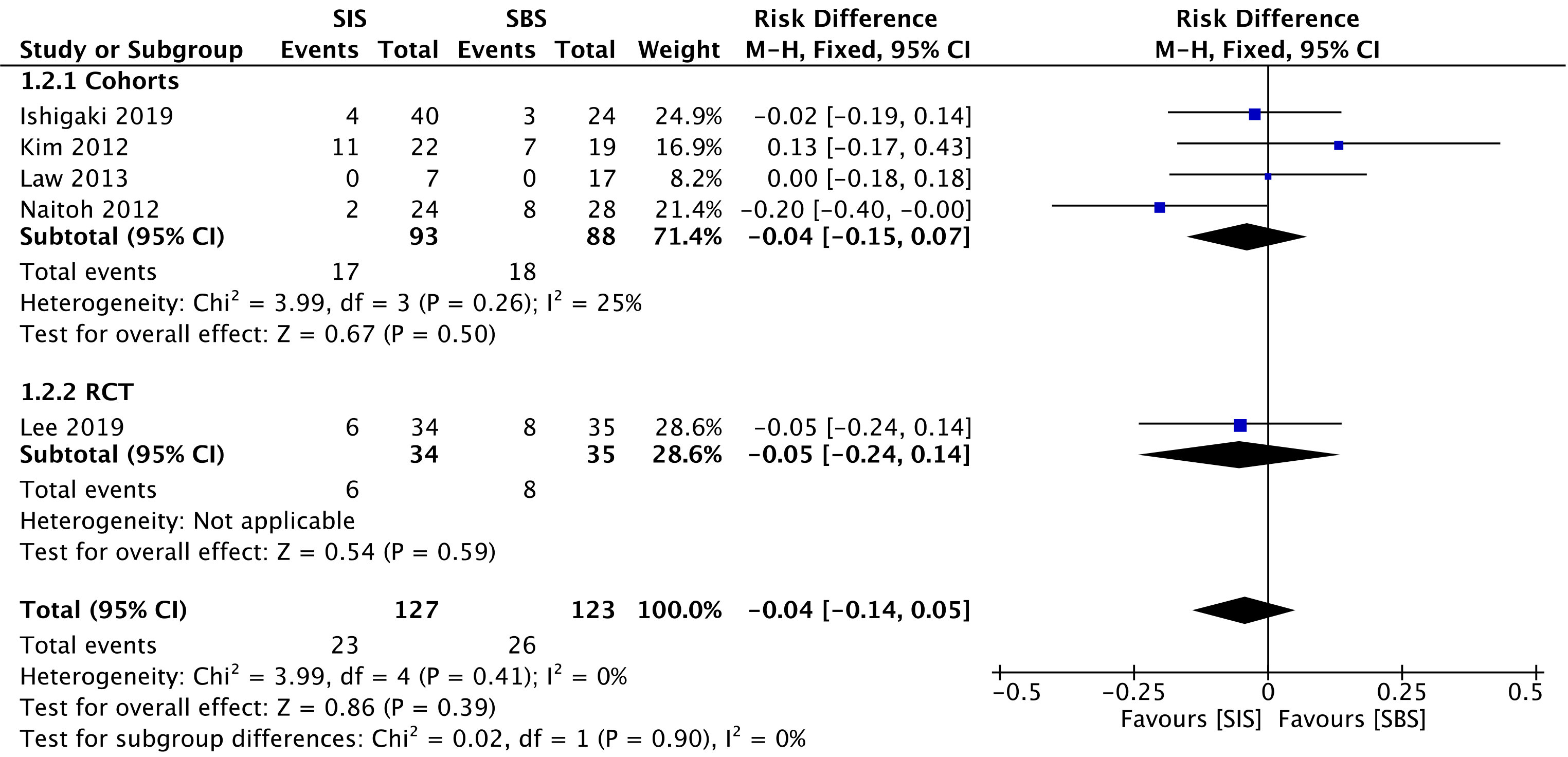
Five studies[29-33] compared late complication rates, evaluating a total of 181 patients in the cohorts and 69 patients in the RCT. In the overall analysis, there was no significant difference between the two groups (RD = -0.04; 95%CI: -0.14 to 0.05, I2 = 0%, P = 0.39) (Figure 6).
The overall certainty of the evidence was moderate for both cohorts and the RCT study, according to GRADE.
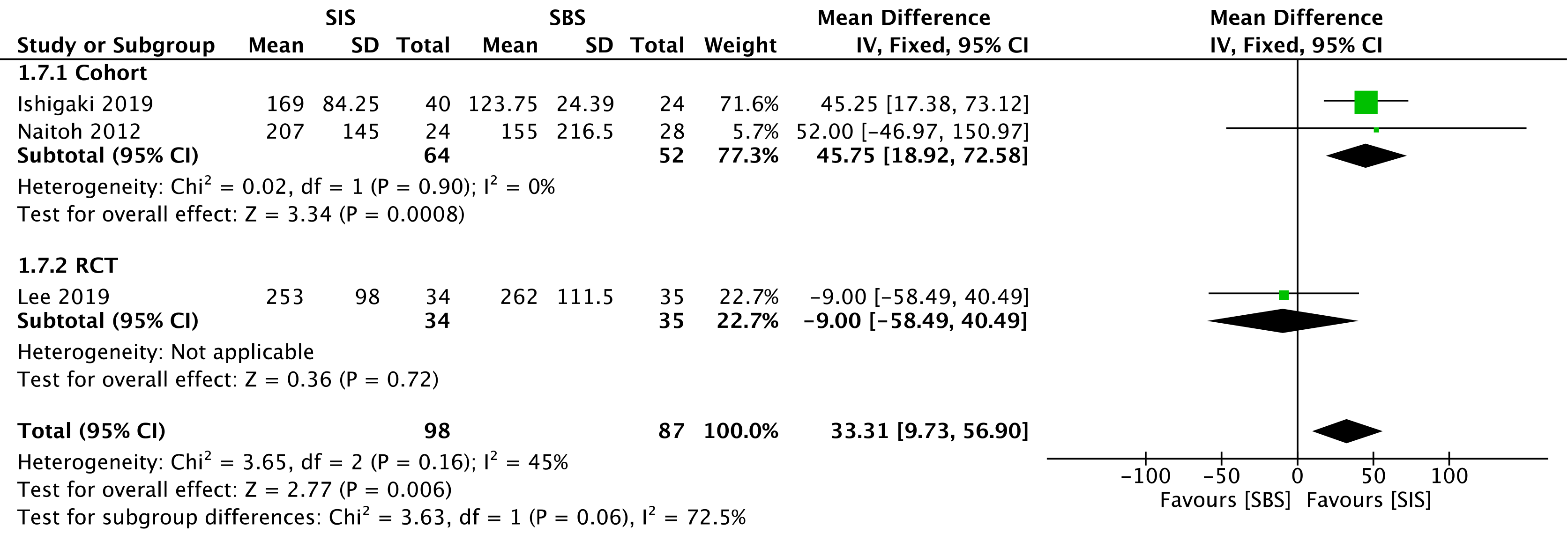
Three studies assessed stent patency: two cohorts[30,32] (116 patients) and the RCT[33] (69 patients). The overall analysis revealed increased stent patency when SIS was performed [mean deviation (MD) = 33.31; 95%CI: 9.73 to 56.90, I2 = 45%, P = 0.006] (Figure 7).
The overall certainty of the evidence was moderate for the cohort and high for the RCT study, according to GRADE.
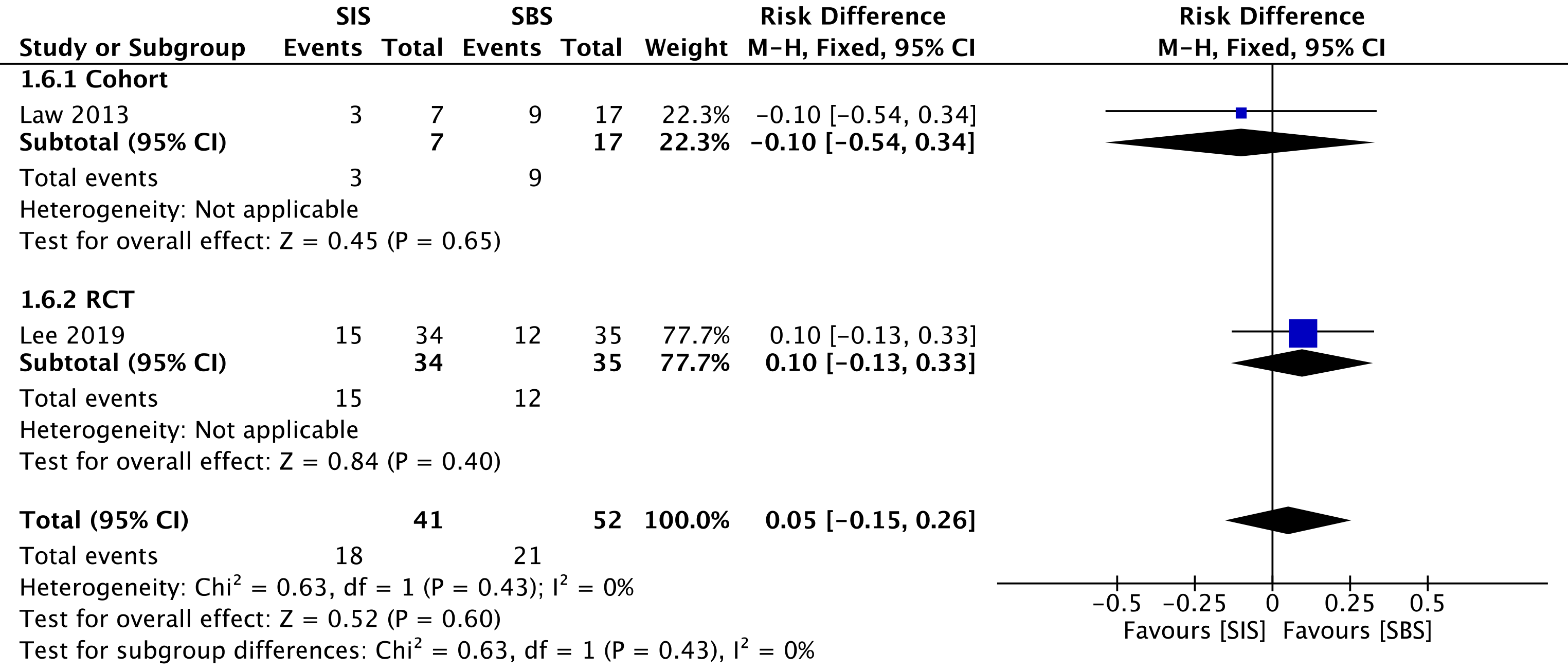
One cohort[29] compared reintervention rates, evaluating a total of 24 procedures—7 in the SIS group and 17 in the SBS group. We found no difference between the two groups in the overall analysis (RD = 0.05; 95%CI: -0.15 to 0.26, I2 = 0%, P = 0.60) (Figure 8).
The overall certainty of the evidence was low for the cohort and high for the RCT study, according to GRADE.
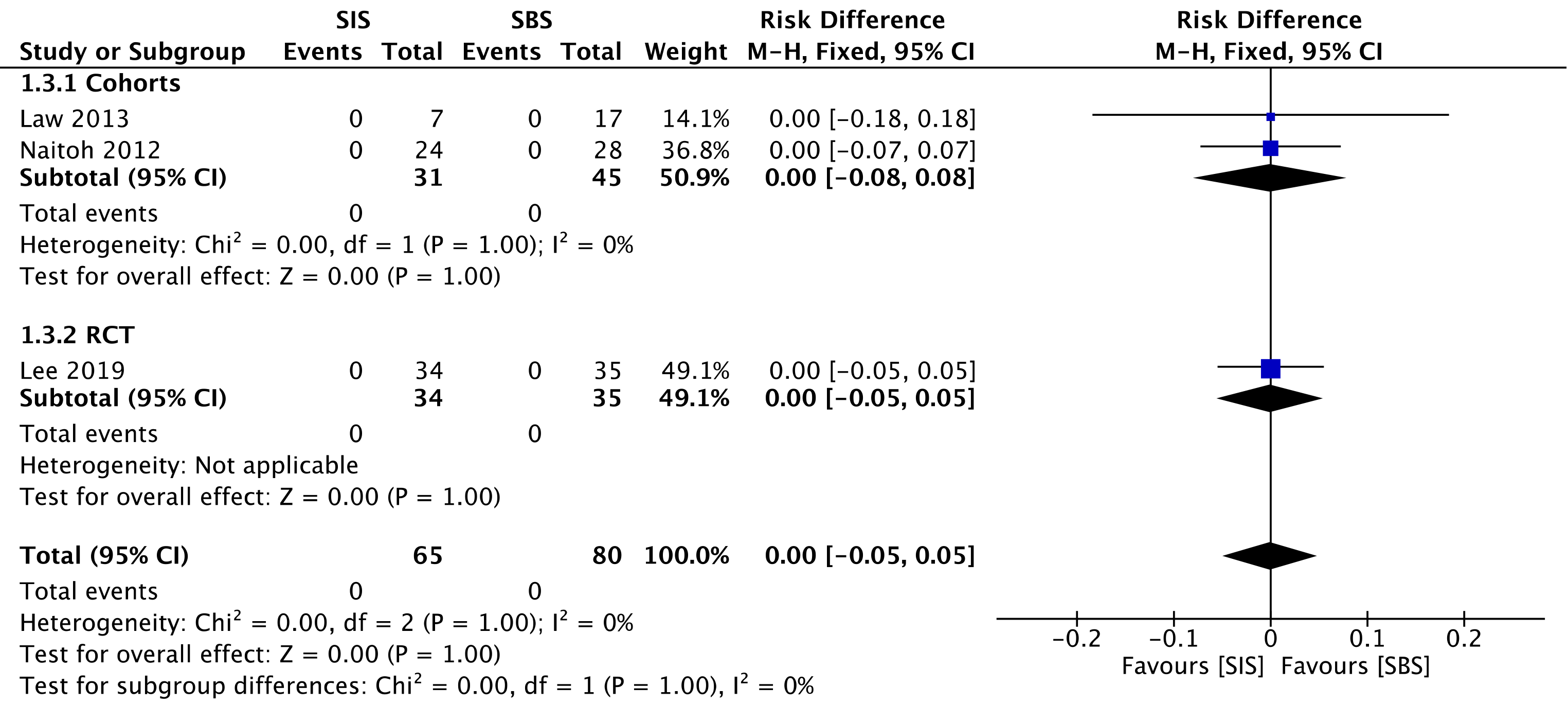
Two cohorts[29,30] compared procedure-related mortality, evaluating a total of 76 procedures—31 in the SIS group and 45 in the SBS group. We found no difference between the two groups (RD = 0.00; 95%CI: -0.05 to 0.05, I2 = 0%, P = 1.00) (Figure 9).
The overall certainty of the evidence was moderate for the cohorts and high for the RCT study, according to GRADE.
Despite being targeted by promising therapies in several clinical trials[34,35], bile duct tumors are often diagnosed as unresectable when they present with biliary obstruction. Therefore, internal drainage via the endoscopic deployment of stents has a pivotal role in this condition.
To the best of our knowledge, this is the first systematic review and meta-analysis comparing both the SIS and SBS techniques for the palliation of biliary drainage in MHBOs. This is a relevant topic for clinical practice, and many studies have non-comparatively evaluated these biliary drainage methods in the past. Despite presenting higher stent patency with the SIS method, we have found through our meta-analysis that there were no statistically significant differences concerning technical success, clinical success, early AEs, late AEs, reintervention, and procedure-related mortality.
For both groups, technical success was achieved in most cases, and we consider that the included studies were conducted at high-volume centers. The main challenge in the SBS method consists of the deployment of the second stent along with the first one. This is especially important since the distal end of both stents should ideally remain at the same level to facilitate an eventual reintervention. New devices have been developed, including systems with a thinner delivery system, which allows the simultaneous deployment of both prostheses. This system prevents the risk of a failed second placement and is associated with a shorter procedural time, as reported by Inoue et al[36]. Traditionally, the dilation on the wire mesh of the first stent before inserting the second one is necessary for the SIS technique. This prerequisite increases the difficulty and cost of the procedure. However, stents with larger cells have been developed, specifically for this usage, with high rates of technical success for the SIS method[37]. We consider that despite the fact that achieving bilateral biliary drainage in the MHBOs is technically challenging, technical success rates were increased and equivalent between both SIS and SBS, probably due to the endoscopist’s vast expertise and the availability of suitable material.
Clinical success was defined in the studies as a total bilirubin decrease in the first month to at least 50% or 75% of the pre-treatment value. Although there was no statistical difference between the groups, we have reservations regarding this outcome definition and we think this outcome should be evaluated very carefully. One reason for this could be that the studies that evaluated this outcome opted for a conservative definition, based on a little significant drop in bilirubin levels, and not on laboratory level standards. Also, they failed to assess other laboratory or clinical parameters.
The use of uncovered SEMS is preferred over fully covered SEMS (FCSEMS) for palliative drainage of malignant biliary obstructions[21], just as it was done in the assessed studies. This is due to the risk of obstruction in intrahepatic lateral branches and cystic and pancreatic ducts, abscess-related factors, cholecystitis, and acute pancreatitis (AP). Inoue et al[38] and Yoshida et al[39] reported the occurrence of hepatic abscesses (11.8% and 6.3% of cases, respectively) when using 6 mm FCSEMS. Although these results cannot be attributed to the stents, they allow us to consider such a hypothesis. In our study, the SIS and SBS techniques presented similar results regarding late complications, such as cholangitis, cholecystitis, and biloma formation. In the cohort meta-analysis, SBS resulted in higher early complication rates (RD = -0.14; 95%CI: -0.27 to -0.01, I2 = 0%, P = 0.03), such as AP. Tarnasky et al[40] had already reported a higher risk of AP in patients referred to biliary stenting for hilar biliary stricture. Furthermore, stent deployment in SBS with the distal end of the stent across the papilla, instead of above the papilla, seems to raise the risk of AP[41]. Nevertheless, in the cohorts meta-analyzed in the present study both techniques were utilized, thus impeding the attribution of the aforementioned complication exclusively to that reason. However, a RCT and general analysis showed no statistically significant differences. These data suggest that both techniques are safe as part of a minimally invasive treatment, with no differences regarding the occlusion of intrahepatic, cystic, or pancreatic ducts. Even if it is not possible to arrive at this conclusion from only this meta-analysis, it seems to us that the stent type has more influence on the complication rates than the drainage technique itself. The safety of endoscopic treatment and each specific technique, is reinforced by the absence of procedural-related deaths in all the casuistry of this study.
The outcome of stent patency, evaluated as moderate and high levels of evidence for the cohort and the RCT, respectively, showed a MD = 33.31, favoring SIS, with a 95%CI: 9.73 to 56.90. Although the reason behind such a difference is unclear, we believe that the SIS technique may allow greater stent expandability, and consequently larger internal caliber, in comparison with the SBS technique. Nevertheless, this result should be analyzed very carefully since some studies do not specify the exact caliber of the employed stent, and one of them disclosed the use of calibers slightly larger in the SIS technique. The use of SEMS in the studies is a positive factor regarding stent patency, corroborating the findings of the specific study that showed higher patency with these types of stents when compared with the plastic stents (131 d vs 47 d)[42]. Our study found no difference regarding the reintervention rate. The main cause of post-procedural obstruction was tumor progression (ingrowth or overgrowth) provoking cholestasis and cholangitis, and thus requiring reintervention. The reintervention approach usually adopted in these cases is the placement of an inner metallic stent, after the cleansing of ductal debris with a balloon extraction and/or cholangioscopy. Radiofrequency ablation can also be considered, but related studies are still scarce[21].
Our study has some limitations. There is only one RCT in the literature comparing both analyzed techniques. Besides the RCT, only 4 comparative retrospective observational studies are available in the literature. Furthermore, the number of patients in the included studies is small, perhaps because this disease does not have a high prevalence. Although the study by Kim et al[31] is available only as an abstract, it possessed all the necessary data for this analysis. Moreover, the prostheses used in different studies were from different manufacturers, with no information on diameter measurements for comparison. Given such limitations, new RCTs may have a valuable role in new systematic reviews, thus improving the quality of evidence.
Despite the aforementioned limitations and to the best of our knowledge, our study is the first systematic review with a meta-analysis on this topic. We firmly believe this has significant clinical applicability given the increasing demand for bile duct drainage in the palliation of malignant hilar tumors.
There is no significant difference between the SIS or SBS techniques in terms of early and late complication rates, technical success, clinical success, reintervention, and procedural-related mortality. The SIS technique was superior in terms of stent patency when compared to the SBS technique, which may guide decision-making regarding the best therapeutic modality for each patient.
Patients with malignant hilar biliary obstruction (MHBO) benefit from bilateral palliative endoscopic drainage. However, there is no consensus on which is the optimal technique for placing a metal stent: Stent-in-stent (SIS) or side-by-side (SBS).
Many patients undergo palliative endoscopic retrograde cholangiopancreatography (ERCP) drainage, due to the advanced stage of the disease at the time of diagnosis, unresectable in most cases. However, choosing the best management for drainage can be a real technical challenge. Therefore, we aimed to compare both drainage techniques in an attempt to identify the optimal approach.
To perform a systematic review and meta-analysis of available studies that compare SIS and SBS deployment in patients with MHBO undergoing ERCP drainage.
The systematic review and meta-analysis followed the PRISMA Guidelines. Electronic searches were performed in MEDLINE, Embase, Cochrane, LILACS, and BIREME databases, and the grey literature. Comparative cohorts and randomized controlled trials (RCTs) were included. Studied outcomes were technical and clinical success, early and late adverse events (AEs), stent patency, reintervention, and procedure-related mortality.
Four comparative cohorts and one RCT were included in the final analysis with a total of 250 patients, of whom 127 belonged to the SIS group and 123 to the SBS group. Stent patency was significantly higher in the SIS group. Procedure-related mortality was similar in both groups, and no significant differences were found in the rates of technical success, clinical success, early AEs, late AEs, and reintervention.
There was no difference between the groups concerning technical and clinical success, early and late AEs, reintervention, and procedure-related mortality. However, there was longer stent patency in patients undergoing the SIS technique. This result suggests that SIS may be the preferred technique for bilateral palliative metal stent deployment in patients with inoperable MHBO.
Palliative biliary drainage is an increasingly performed procedure, but without consensus on the optimal technique, SIS or SBS. There is a small number of comparative studies in the literature. Future RCTs will have an important role in elucidating the most optimal drainage technique.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang WH, Zorron Cheng Tao Pu L S-Editor: Gao CC L-Editor: Webster JR P-Editor: Li JH
| 1. | Chen G, Zhang M, Sheng YG, Yang F, Li ZQ, Liu TG, Fu YF. Stent with radioactive seeds strand insertion for malignant hilar biliary obstruction. Minim Invasive Ther Allied Technol. 2020;1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Lamarca A, Edeline J, McNamara MG, Hubner RA, Nagino M, Bridgewater J, Primrose J, Valle JW. Current standards and future perspectives in adjuvant treatment for biliary tract cancers. Cancer Treat Rev. 2020;84:101936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 3. | Heo JY, Lee HS, Son JH, Lee SH, Bang S. Clinical Outcomes of Bilateral Stent-in-Stent Placement Using Self-Expandable Metallic Stent for High-Grade Malignant Hilar Biliary Obstruction. Yonsei Med J. 2018;59:827-833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Okuno M, Mukai T, Iwashita T, Ichikawa H, Iwasa Y, Mita N, Yoshida K, Iwata K, Tomita E, Shimizu M. Evaluation of endoscopic reintervention for self-expandable metallic stent obstruction after stent-in-stent placement for malignant hilar biliary obstruction. J Hepatobiliary Pancreat Sci. 2019;26:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Fu YF, Xu YS, Shi YB, Zong RL, Cao C. Percutaneous metal stenting for malignant hilar biliary obstruction: a systematic review and meta-analysis of unilateral vs bilateral stenting. Abdom Radiol (NY). 2021;46:749-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Drapek LC, Kerlan RK Jr, Acquisto S. Guidelines for biliary stents and drains. Chin Clin Oncol. 2020;9:9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Ribeiro IB, Bernardo WM, Martins BDC, de Moura DTH, Baba ER, Josino IR, Miyahima NT, Coronel Cordero MA, Visconti TAC, Ide E, Sakai P, de Moura EGH. Colonic stent vs emergency surgery as treatment of malignant colonic obstruction in the palliative setting: a systematic review and meta-analysis. Endosc Int Open. 2018;6:E558-E567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 8. | Ferraz Gonçalves JA, Rosendo E, Sousa L, Lopes AR, Leão I, Queirós R, Marote S, Sousa MJ. Complications of Biliary Drainage in Patients with Malignant Biliary Obstruction. J Gastrointest Cancer. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | García-Cano J, Viñuelas-Chicano M, Valiente GonzÁlez L. Straight plastic stents in tumors at the hepatic hilum and related duodenal perforations. Rev Esp Enferm Dig. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Ribeiro IB, de Moura DTH, Sachdev AH, Guimarães Hourneaux de Moura E. Stent as a bridge to surgery for colonic obstruction: Do we really need more systematic reviews with meta-analysis of the same articles? Gastrointest Endosc. 2019;90:704-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Shepherd HA, Royle G, Ross AP, Diba A, Arthur M, Colin-Jones D. Endoscopic biliary endoprosthesis in the palliation of malignant obstruction of the distal common bile duct: a randomized trial. Br J Surg. 1988;75:1166-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 337] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 13. | Andersen JR, Sørensen SM, Kruse A, Rokkjaer M, Matzen P. Randomised trial of endoscopic endoprosthesis vs operative bypass in malignant obstructive jaundice. Gut. 1989;30:1132-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 393] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 14. | Zorrón Pu L, de Moura EG, Bernardo WM, Baracat FI, Mendonça EQ, Kondo A, Luz GO, Furuya Júnior CK, Artifon EL. Endoscopic stenting for inoperable malignant biliary obstruction: A systematic review and meta-analysis. World J Gastroenterol. 2015;21:13374-13385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 15. | Ogura T, Takenaka M, Shiomi H, Nishioka N, Ueno S, Miyano A, Kamiyama R, Higuchi K. Single-session multiple stent deployment using moving cell stent without dilating initial stent mesh to treat malignant hilar biliary obstruction (with videos). J Hepatobiliary Pancreat Sci. 2020;27:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Xia MX, Cai XB, Pan YL, Wu J, Gao DJ, Ye X, Wang TT, Hu B. Optimal stent placement strategy for malignant hilar biliary obstruction: a large multicenter parallel study. Gastrointest Endosc 2020; 91: 1117-1128. e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Ahmed O, Lee JH. Modern gastrointestinal endoscopic techniques for biliary tract cancers. Chin Clin Oncol. 2020;9:3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Sugiura R, Kuwatani M, Kato S, Kawakubo K, Kamachi H, Taketomi A, Noji T, Okamura K, Hirano S, Sakamoto N. Risk factors for dysfunction of preoperative endoscopic biliary drainage for malignant hilar biliary obstruction. J Hepatobiliary Pancreat Sci. 2020;27:851-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Ribeiro IB, de Moura DTH, Thompson CC, de Moura EGH. Acute abdominal obstruction: Colon stent or emergency surgery? World J Gastrointest Endosc. 2019;11:193-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 20. | Liu N, Yang D, Draganov PV. Endoscopic Stenting for Malignant Hilar Biliary Obstruction: After You Double Down, Are You In or Out? Dig Dis Sci. 2020;65:3428-3430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Dumonceau JM, Tringali A, Papanikolaou IS, Blero D, Mangiavillano B, Schmidt A, Vanbiervliet G, Costamagna G, Devière J, García-Cano J, Gyökeres T, Hassan C, Prat F, Siersema PD, van Hooft JE. Endoscopic biliary stenting: indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated October 2017. Endoscopy. 2018;50:910-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 476] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 22. | Naitoh I, Inoue T, Hayashi K. Which is better for unresectable malignant hilar biliary obstruction: Side-by-side vs stent-in-stent? Gastrointest Interv. 2018;7:78-84. [DOI] [Full Text] |
| 23. | Ashat M, Arora S, Klair JS, Childs CA, Murali AR, Johlin FC. Bilateral vs unilateral placement of metal stents for inoperable high-grade hilar biliary strictures: A systemic review and meta-analysis. World J Gastroenterol. 2019;25:5210-5219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 46975] [Article Influence: 2935.9] [Reference Citation Analysis (0)] |
| 25. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7683] [Cited by in RCA: 10691] [Article Influence: 1187.9] [Reference Citation Analysis (2)] |
| 26. | Chandler J, McKenzie J, Boutron I, Welch V. Cochrane Methods 2016. Cochrane Database Syst Rev. 2016;. [DOI] [Full Text] |
| 27. | Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11058] [Cited by in RCA: 14767] [Article Influence: 868.6] [Reference Citation Analysis (0)] |
| 28. | Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4895] [Cited by in RCA: 6846] [Article Influence: 342.3] [Reference Citation Analysis (0)] |
| 29. | Law R, Baron TH. Bilateral metal stents for hilar biliary obstruction using a 6Fr delivery system: outcomes following bilateral and side-by-side stent deployment. Dig Dis Sci. 2013;58:2667-2672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Naitoh I, Hayashi K, Nakazawa T, Okumura F, Miyabe K, Shimizu S, Yoshida M, Yamashita H, Ohara H, Joh T. Side-by-side vs stent-in-stent deployment in bilateral endoscopic metal stenting for malignant hilar biliary obstruction. Dig Dis Sci. 2012;57:3279-3285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 31. | Kim KM, Lee KH, Chung YH, Shin JU, Lee JK, Lee KT, Shim SG. A comparison of bilateral stenting methods for malignant hilar biliary obstruction. Hepatogastroenterology. 2012;59:341-346. [PubMed] |
| 32. | Ishigaki K, Hamada T, Nakai Y, Isayama H, Sato T, Hakuta R, Saito K, Saito T, Takahara N, Mizuno S, Kogure H, Ito Y, Yagioka H, Matsubara S, Akiyama D, Mohri D, Tada M, Koike K. Retrospective Comparative Study of Side-by-Side and Stent-in-Stent Metal Stent Placement for Hilar Malignant Biliary Obstruction. Dig Dis Sci. 2020;65:3710-3718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 33. | Lee TH, Moon JH, Choi JH, Lee SH, Lee YN, Paik WH, Jang DK, Cho BW, Yang JK, Hwangbo Y, Park SH. Prospective comparison of endoscopic bilateral stent-in-stent vs stent-by-stent deployment for inoperable advanced malignant hilar biliary stricture. Gastrointest Endosc. 2019;90:222-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 34. | Zayac A, Almhanna K. Hepatobiliary cancers and immunotherapy: where are we now and where are we heading? Transl Gastroenterol Hepatol. 2020;5:8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | He X, Zhu Y, Wang Y, Hao Y, Hong J. Advances in stent therapy for malignant biliary obstruction. Abdom Radiol (NY). 2021;46:351-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Inoue T, Ishii N, Kobayashi Y, Kitano R, Sakamoto K, Ohashi T, Nakade Y, Sumida Y, Ito K, Nakao H, Yoneda M. Simultaneous Versus Sequential Side-by-Side Bilateral Metal Stent Placement for Malignant Hilar Biliary Obstructions. Dig Dis Sci. 2017;62:2542-2549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Kogure H, Isayama H, Nakai Y, Tsujino T, Ito Y, Yamamoto K, Mizuno S, Yagioka H, Kawakubo K, Sasaki T, Hirano K, Sasahira N, Tada M, Omata M, Koike K. Newly designed large cell Niti-S stent for malignant hilar biliary obstruction: a pilot study. Surg Endosc. 2011;25:463-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Inoue T, Okumura F, Naitoh I, Fukusada S, Kachi K, Ozeki T, Anbe K, Iwasaki H, Mizushima T, Kobayashi Y, Ishii N, Ito K, Kondo H, Hayashi K, Yoneda M, Sano H. Feasibility of the placement of a novel 6-mm diameter threaded fully covered self-expandable metal stent for malignant hilar biliary obstructions (with videos). Gastrointest Endosc. 2016;84:352-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Yoshida T, Hara K, Imaoka H, Hijioka S, Mizuno N, Ishihara M, Tanaka T, Tajika M, Niwa Y, Yamao K. Benefits of side-by-side deployment of 6-mm covered self-expandable metal stents for hilar malignant biliary obstructions. J Hepatobiliary Pancreat Sci. 2016;23:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Tarnasky PR, Cunningham JT, Hawes RH, Hoffman BJ, Uflacker R, Vujic I, Cotton PB. Transpapillary stenting of proximal biliary strictures: does biliary sphincterotomy reduce the risk of postprocedure pancreatitis? Gastrointest Endosc. 1997;45:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Cosgrove N, Siddiqui AA, Adler DG, Shahid H, Sarkar A, Sharma A, Kowalski TE, Loren D, Warndorf M, Chennat J, Munigala S, Papachristou GI. A Comparison of Bilateral Side-by-Side Metal Stents Deployed Above and Across the Sphincter of Oddi in the Management of Malignant Hilar Biliary Obstruction. J Clin Gastroenterol. 2017;51:528-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Inoue T, Naitoh I, Okumura F, Ozeki T, Anbe K, Iwasaki H, Nishie H, Mizushima T, Sano H, Nakazawa T, Yoneda M, Joh T. Reintervention for stent occlusion after bilateral self-expandable metallic stent placement for malignant hilar biliary obstruction. Dig Endosc. 2016;28:731-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |