Published online May 27, 2021. doi: 10.4254/wjh.v13.i5.584
Peer-review started: January 14, 2021
First decision: February 13, 2021
Revised: February 24, 2021
Accepted: April 25, 2021
Article in press: April 25, 2021
Published online: May 27, 2021
Processing time: 126 Days and 14.2 Hours
Primary hepatic sarcoma is a rare tumor originated from mesenchymal tissue. There are various pathologic types of primary hepatic sarcoma and the treatment outcome of this tumor was usually disappointing. Unlike hepatocellular carcinoma, outcome of primary hepatic sarcoma is not well-known due to it’s rarity. However, with development of medical technology, surgical treatment may lead to better survival.
To investigate the surgical outcomes of primary hepatic sarcoma, we gathered and analyzed the cases of a single institute.
From August 2001 to September 2016, a total of nine patients were surgically treated for primary hepatic sarcoma after exclusion of cases with open and closure, early loss to follow-up and sarcomatoid hepatocellular carcinoma and sarcomatoid cholangiocellular carcinoma. Baseline characteristics, tumor characteristics such as tumor pathology, size and number, surgical and adjuvant treatments were reviewed. Tumor recurrence, and patient survival were analyzed with retrospective approach.
The enrolled participants included five patients with angiosarcoma and four patients with undifferentiated sarcoma. All patients experienced tumor recurrence at a median of 52 post-operative days. Only two patients survived and the 5-year survival rate was 29.6%. One patient with angiosarcoma who received central hepatectomy for primary tumor and received radiofrequency ablation for recurrent tumor still lives for 11 years. One patient with undifferentiated sarcoma received Rt. lobectomy for primary tumor followed by chemotherapy and radiation therapy still lives around 30 mo even though she got additional operation for recurrent tumor. Two patients who received living donor liver transplantation due to angiosarcoma died. Only adjuvant therapy was associated with survival gain (P = 0.002).
Patients with primary hepatic sarcoma may gain survival benefit with surgical resection followed by adjuvant therapy, even though the outcome remains relatively poor.
Core Tip: This is a retrospective study to analyze the outcomes of primary hepatic sarcoma. A total of nine patients were included, five of them are with angiosarcoma and four are with undifferentiated sarcoma. While all patients experienced tumor recurrence, one patient with angiosarcoma and another patient with undifferentiated sarcoma still survive for 11 years and 30 mo respectively, after receiving effective local treatment for recurrent tumors. Although the outcome of primary hepatic sarcoma is known to be poor, surgical treatment with appropriate adjuvant therapy may support the long-term survival.
- Citation: Kim SJ, Rhu J, Kim JM, Choi GS, Joh JW. Surgical treatment outcomes of primary hepatic sarcomas: A single-center experience. World J Hepatol 2021; 13(5): 584-594
- URL: https://www.wjgnet.com/1948-5182/full/v13/i5/584.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i5.584
Sarcoma is a malignant tumor that usually arises from mesenchymal tissue. Most sarcomas originate in the extremities, and the prognosis of sarcoma in these sites is well known due to its prevalence[1]. Primary hepatic sarcoma is a rare and aggressive tumor with poor outcomes. Most malignant tumors in the liver are hepatocellular carcinomas (HCC); hepatic sarcomas represent less than 1%[2]. Hepatic sarcoma has various pathologic types, including angiosarcoma, undifferentiated (embryonal) sarcoma, leiomyosarcoma, and epithelioid hemangioendothelioma, among others. For most of these tumors, the cause is still unknown, and there are usually no specific symptoms until abdominal pain presents due to the effect of the mass increasing in size[3,4]. The various types, the rarity, and the difficulty in diagnosis of primary hepatic sarcoma results in various prognoses, thereby making it difficult to set a treatment plan. The aim of our study is to analyze the outcomes of primary hepatic sarcoma following surgical resection in a single institute.
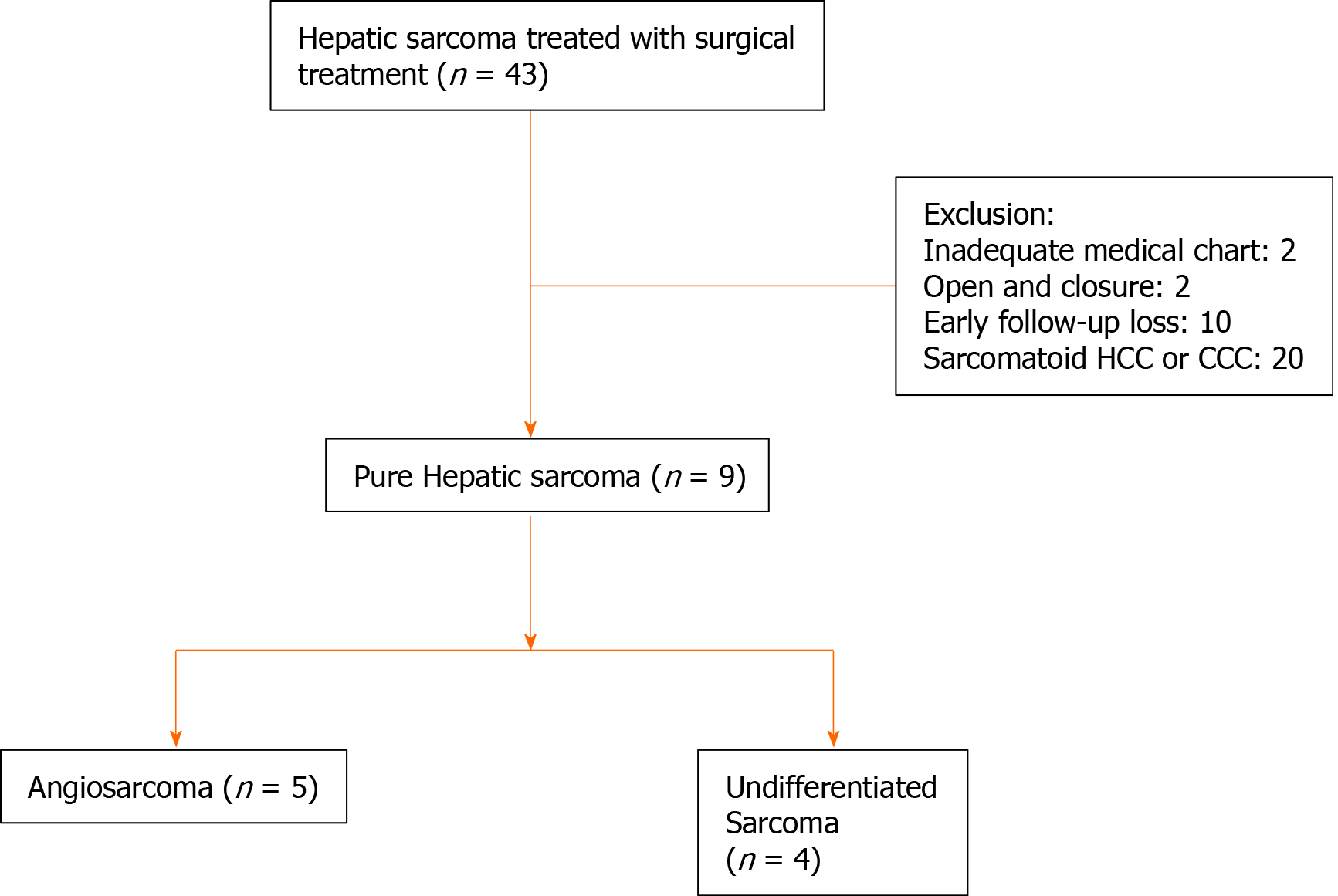
From August 2001 to September 2016, a total of 43 patients underwent surgical treatment for primary hepatic sarcoma at Samsung Medical Center, South Korea. These patients were selected by searching the word “sarcoma” in liver pathological report through all time of our institute. Open and closure, inadequate medical chart, and sarcomatoid HCC or cholangiocellular carcinoma cases were excluded. Six early-follow up loss patients who were treated with resection in our center and then transferred to other hospitals were also excluded. Four patients who did not want additional therapy and did not come to outpatient clinic were also excluded. Data was collected by retrospective approach.
Patient age, sex, and pre-operative blood tests such as total bilirubin, aspartate transaminase, alanine aminotransferase, alpha fetoprotein, and CA 19-9 were evaluated. Operation types were reviewed, and pathological diagnosis, tumor size and number were evaluated. Disease recurrence was evaluated using computed tomography or positron emission tomography scan. Patient death was the primary end point.
Operation type was decided by tumor size, location and liver function and the target of operation was R0 (microscopically margin-negative) resection. However, patients with R1 (macroscopically no remnant tumor, but margin-positive microscopically) and R2 (seen remnant tumor) resection were also included in the study population. Patients usually received chemotherapy when the pathologic report was diagnosed as primary hepatic sarcoma, unless the patient was in poor general condition and could not endure chemotherapy. For cases of recurrence or metastasis of cancer at the vertebrae, radiation therapy (RT) was performed. Resectable recurrent tumors were excised surgically. For small recurrences in the liver, radiofrequency ablation (RFA) could also be performed.
Baseline characteristics and tumor markers were compared using the Mann-Whitney test. Cox proportional hazards regression analysis and the Kaplan-Meier method were used to analyze disease-free survival, overall survival and corresponding risk factor. The independent variables were age, sex, tumor size and number, tumor markers and adjuvant therapy. Disease free-survival and overall survival was compared according to pathologic type of sarcoma and adjuvant therapy due to known different prognosis of each sarcoma type. Statistical analysis was executed using IBM SPSS-24 statistical program (IBM Institute, NY, United States).
After exclusion of open and closure, early loss to follow-up, inadequate medical chart, and sarcomatoid HCC or cholangiocellular carcinoma among 43 patients, total nine patients with pure hepatic sarcomas were involved in this study (Figure 1). According to pathological diagnoses, we divided the patients into an angiosarcoma group (n = 5) and an undifferentiated sarcoma group (n = 4, Table 1). Baseline characteristics of each group showed no statistical differences in any variables including tumor size, number of tumors, and tumor markers (Table 2). Median size of the largest tumor was 13 cm. Median age of the patients was 57, and only one patient was pediatric (a 2-year-old female).
| Case | Sex | Age | Pathology | Size (cm) | Operation, R Status | Adjuvant therapy | Recurrence (mo) | Follow-up (mo) | Outcomes |
| 1 | M | 57 | HAS | 13.5 | Lt. lobectomy, R0 | 1.7 | 2.2 | Dead | |
| 2 | F | 2 | HAS | 21 | Living donor LT, R0 | CTx1 | 1.8 | 31.2 | Dead |
| 3 | M | 52 | HAS | 7.3 | Rt. Trisectionectomy, R0 | 1.2 | 4.2 | Dead | |
| 4 | F | 48 | UDS | 13 | Rt. lobectomy, R0 | CTx2 | 1.6 | 68.2 | Dead |
| 5 | M | 60 | HAS | 2.4 | Living donor LT, R0 | RT | 11.0 | 13.4 | Dead |
| 6 | F | 53 | UDS | 25 | Rt. Trisectionectomy, R2 | 0 | 1.3 | Dead | |
| 7 | M | 62 | HAS | 2 | Central hepatectomy, R0 | RFA | 59.9 | 135.1 | Alive |
| 8 | F | 60 | UDS | 11.5 | Rt. lobectomy, R0 | CTx, RT, Exc3 | 14.6 | 29.9 | Alive |
| 9 | M | 60 | UDS | 24 | Rt. lobectomy and Rt. Npx, R0 | CTx4 | 1.2 | 9.9 | Dead |
| Total (n = 9) | Angiosarcoma (n = 5) | Undifferentiated sarcoma (n = 4) | P value | |
| Age (range)1 | 57 (2-62) | 57 (2-62) | 56 (48-60) | 0.999 |
| Sex, male (%) | 5 (55.6) | 4 (80) | 1 (25) | 0.120 |
| Largest tumor size (cm)1 | 13.0 | 7.3 | 18.5 | 0.142 |
| Tumor number1 | 1.0 | 2 | 1 | 0.371 |
| AFP1 | 2.8 | 2.6 | 2.8 | 0.999 |
| CA19-91 | 13.2 | 3.2 | 1970 | 0.180 |
| Recurrence (%) | 9 (100) | 5 (100) | 4 (100) | 0.999 |
| Disease-free survival days1 | 52 (0-1798) | 53 (36-1798) | 35 (0-439) | 0.221 |
| Death (%) | 7 (77.8) | 4 (80) | 3 (75) | 0.866 |
| Survival days1 | 402 | 402 | 596 | 0.806 |
Among the five patients with angiosarcoma, only one patient survived. Median survival duration was 13 mo, and median disease-free survival was 53 d. Two patients (a 57-year-old male and a 52-year-old male, case 1 and 3, respectively) could not receive adjuvant chemotherapy due to poor general condition and expired relatively early (66 d and 127 d, respectively). The pediatric patient (case 2) underwent living donor liver transplantation (LDLT) for angiosarcoma and was diagnosed with multiple bone metastasis at the extremities on post-operative day 53. She received six cycles of ifosfamide/carboplatin/etoposide chemotherapy and expired at post-operative 31 mo. Case 5, a 60-year-old male, received LDLT and experienced recurrence at the liver and metastasis at the vertebra and rib at post-operative 11 mo. He received palliative RT on the bone metastases and expired at post-operative 13 mo.
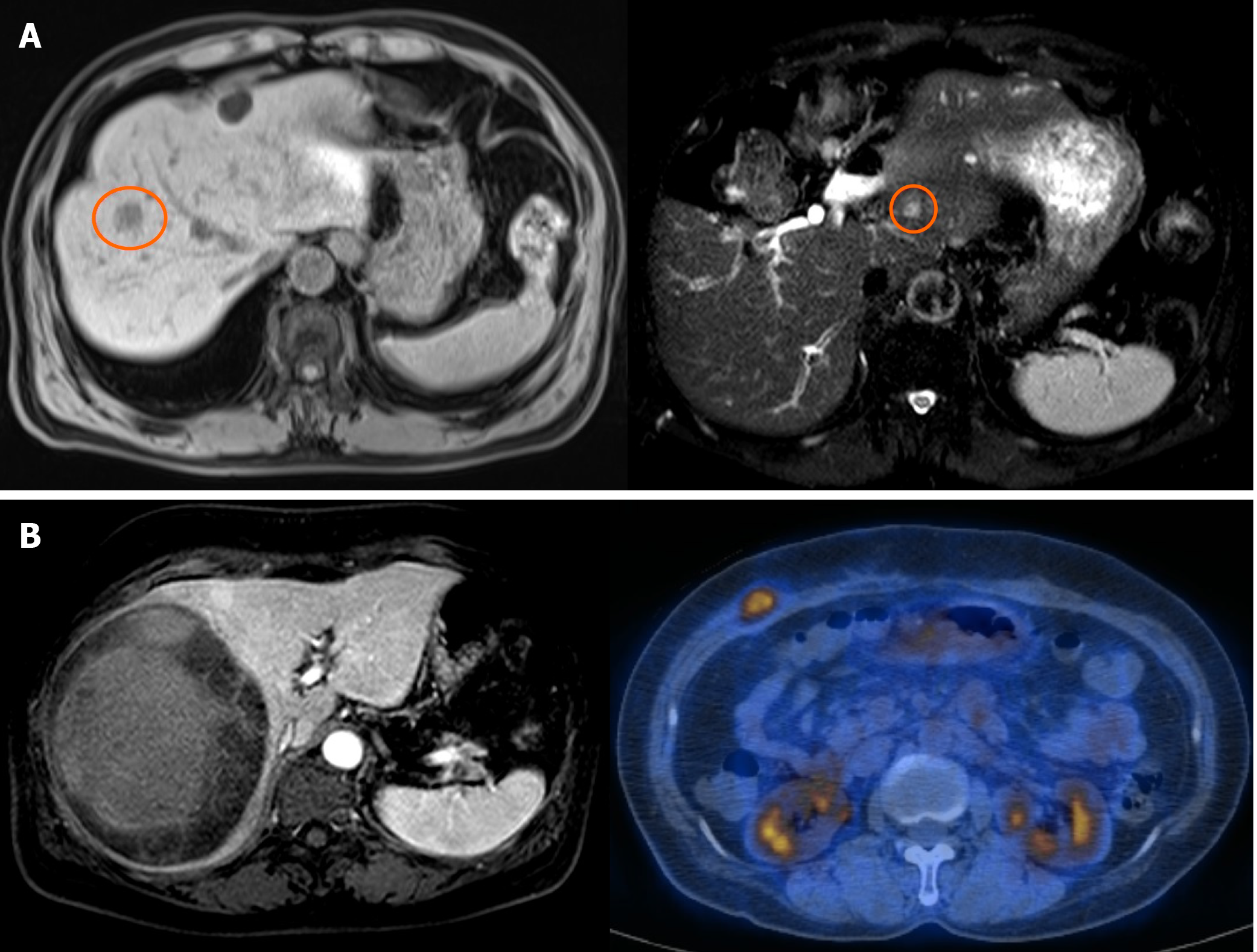
Case 7, a 62-year-old male who received central hepatectomy for a 2 cm angiosarcoma on segment 8, is still alive after 11 years (Figure 2A). The pathologic resection margin had no cancer cells, and the tumor was the infiltrative type without invasion to any other organs. After operation, the patient experienced recurrence on segment 2 with a 0.9 cm tumor. Successful RFA was done, and he has been cancer-free for six years.
Among the four patients with undifferentiated sarcoma, only one patient survived. Median survival duration was 20 mo, and median disease-free survival was 53 d. Case 6, a 53-year-old female, received Rt. trisectionectomy for a 25 cm undifferentiated sarcoma associated with partial cholangiocellular carcinoma. The tumor was partially ruptured, and a cytology test of ascites was positive for malignant cells with two regional lymph node metastases. The patient expired at post-operative 40 d before receiving chemotherapy. Case 9, a 60-year-old male, received Rt. lobectomy, Rt. nephrectomy, and diaphragm resection due to a 24 cm undifferentiated sarcoma, followed by a diagnosis of lung metastasis at post-operative 35 d. He received four cycles of AI regimen (doxorubicin/ifosfamide) and one cycle of docetaxel/gemcitabine chemotherapy until he expired at post-operative 10 mo.
Case 4, a 48-year-old female, received Rt. lobectomy due to a 13 cm undifferentiated sarcoma. The tumor had already penetrated to the liver capsule and had a high mitotic count (10/10 HPFs), and tumor emboli were in the Rt. portal vein. Multiple tumors recurred on the liver resection margin and the remnant liver at post-operative 49 d. The patient received multiple series of chemotherapy, including five cycles of VIP regimen (etoposide/ifosfamide/cisplatin) followed by five cycles of AI regimen (doxorubicin/ifosfamide) and two cycles of docetaxel/gemcitabine. The patient survived 68 mo until she died due to tumor progression, lung metastasis, and liver abscess.
Case 8, a 60-year-old female who received Rt. lobectomy for an 11.5 cm undifferentiated sarcoma, is still alive after 30 mo (Figure 2B). The tumor had high cellularity, moderate pleomorphism, and tumor necrosis of more than 50% with a negative resection margin. After operation, the patient received six cycles of chemotherapy with the VIP regimen (etoposide/ifosfamide/cisplatin), followed by RT to the pre-operatively diagnosed vertebral metastasis (1.5 cm tumor at T9). She had no cancer recurrence until abdominal metastasis was detected at post-operative 14 mo. The metastatic tumor was 2 cm, located between the abdominal investing fascia and the external oblique muscle. After a wide excision, the patient has been cancer-free for 30 mo.
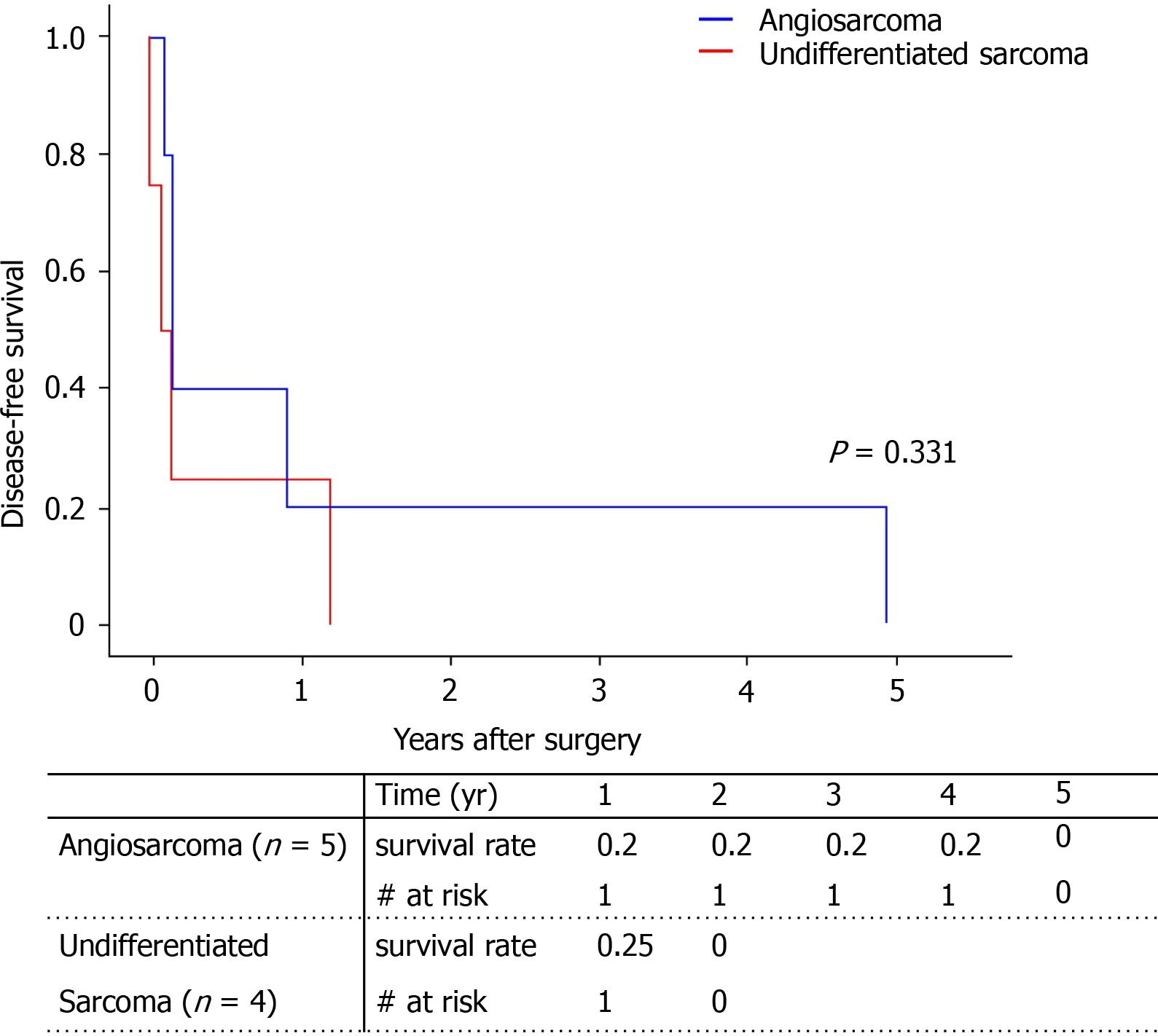
All patients experienced recurrence of the primary cancer. Median disease-free survival was 52 d. Because one patient with angiosarcoma had tumor recurrence in the Left lobe at post-operative 4 years and 11 mo, the disease-free survival of angiosarcoma looks higher than that of undifferentiated sarcoma; however, this was not statistically significant (Figure 3). The age, sex, pathology type, and tumor markers showed no statistical influence on cancer recurrence (Table 3). Only the largest tumor size was associated with higher cancer recurrence, but only in univariate analysis (HR = 1.13, P = 0.49) and not in multivariate analysis.
| Univariate HR (95%CI) | P value | Multivariate HR (95%CI) | P value | |
| Sex (male) | 0.82 (0.20-3.31) | 0.779 | ||
| Age | 0.99 (0.96-1.03) | 0.694 | ||
| Pathology (HAS/UDS) | 0.50 (0.12-2.07) | 0.339 | 0.63 (0.11-3.55) | 0.602 |
| Largest tumor size | 1.13 (1.00-1.27) | 0.049 | 1.12 (0.97-1.28) | 0.115 |
| Tumor number | 1.28 (0.79-2.07) | 0.311 | ||
| AFP | 1.02 (0.77-1.33) | 0.917 | ||
| CA 19-9 | 1.00 (1.00-1.00) | 0.527 | ||
| Adjuvant therapy | 0.24 (0.04-1.47) | 0.121 | 0.25 (0.04-1.71) | 0.157 |
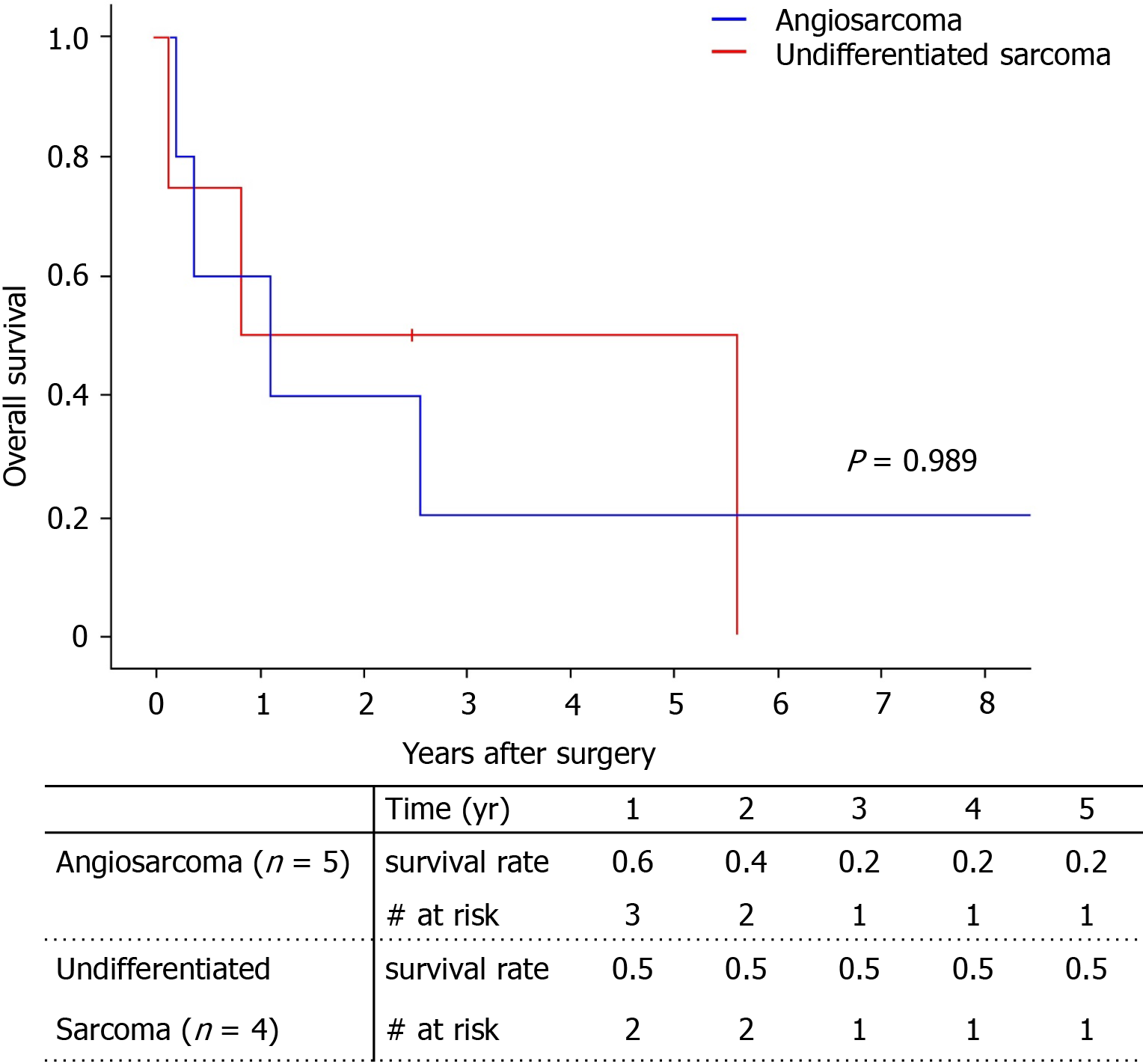
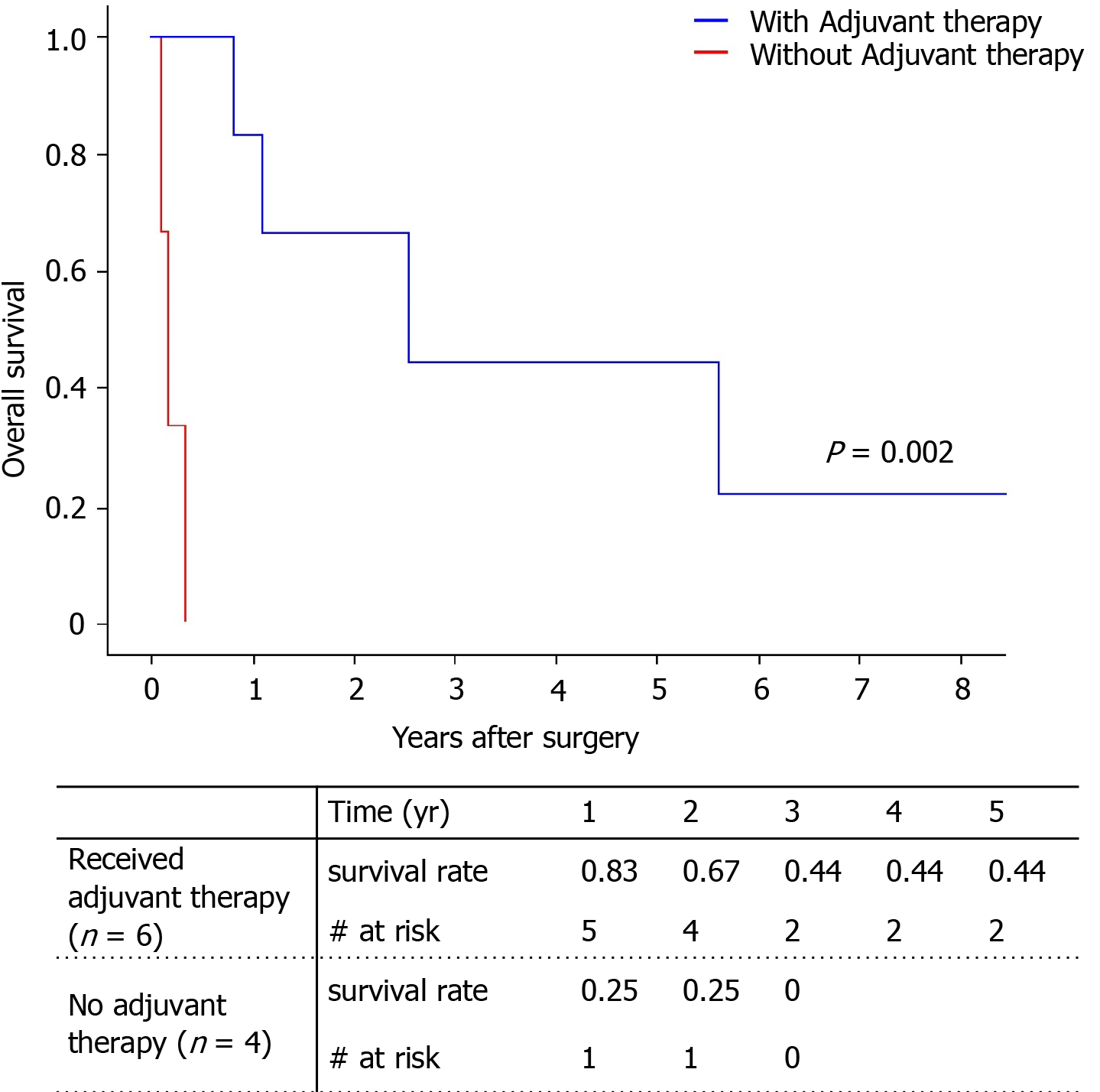
Among the total of nine patients, only two patients survived (one with angiosarcoma, one with undifferentiated sarcoma). The 5-year survival rate was 29.6% (Figure 4). There was no significant difference between survival of the angiosarcoma and undifferentiated sarcoma groups. Pathologic type, largest tumor size, number of tumors, and tumor markers did not influence patient death with univariate analysis (Table 4). Only adjuvant therapy had an effect on patient survival. The 5-year survival of patients who received adjuvant therapy was 44.4%, while all patients without adjuvant therapy expired within 1 year (Figure 5).
| Univariate HR (95%CI) | P value | |
| Sex (male) | 1.36 (0.30-6.24) | 0.693 |
| Age | 1.00 (0.96-1.04) | 0.922 |
| Pathology (HAS/UDS) | 0.99 (0.22-4.47) | 0.989 |
| Largest tumor size | 1.08 (0.97-1.20) | 0.179 |
| Tumor number | 1.28 (0.79-2.07) | 0.311 |
| AFP | 0.99 (0.74-1.31) | 0.922 |
| CA 19-9 | 1.00 (0.99-1.01) | 0.677 |
| Adjuvant therapy | 0.00 (0.03-2779) | 0.002 |
In our study, all patients who received surgical treatment for primary hepatic sarcoma had tumor recurrence, and the 5-year survival rate was relatively low (29.6%). However, one patient with angiosarcoma is still alive after 11 years, and one with undifferentiated sarcoma is still alive after 30 mo.
With recent medical advances, survival outcomes after surgical resection of primary hepatic sarcoma are slightly increasing. One study with 22 patients who received primary surgical treatment showed a 5-year survival rate of 65.2%[5]. However, that study population included various sarcoma types, including rhabdomyosarcoma, leiomyosarcoma, and hemangiopericytoma, while the five cases of angiosarcoma had much poorer outcomes with only one patient surviving for six months. Another review article including 64 cases of hepatic angiosarcoma showed a median survival time of five months[6]. In this article, median survival of patients who received local excision alone or local excision combined with chemotherapy was 17 mo. In our study, the angiosarcoma group had a median survival of 13 mo, with a 5-year survival rate of 20%. One patient who received central hepatectomy and RFA for recurrence at the Lt. lateral section still lives after 11 years.
Hepatic undifferentiated sarcoma is also known to have poor outcomes[7]. Sometimes, it is misdiagnosed as other cystic tumor on pre-operative images and revealed as undifferentiated sarcoma on pathologic review after surgical resection[8,9]. However, it may have better outcomes compared to angiosarcoma when surgically resected. A recent review article including 271 patients who received partial hepatectomy showed a 5-year survival rate of 70%[10]. The 5-year survival rate for the undifferentiated sarcoma group in our study was 50%. One patient who still lives, received Right lobectomy for an 11.5 cm tumor, followed by chemotherapy and RT for vertebral metastasis. About 14 mo later, she underwent a local excision at the abdominal wall metastasis and has maintained a disease-free state for 15 mo. A 48-year-old female patient who received Rt. lobectomy followed by chemotherapy lived more than five years but expired at 5 years and 7 mo due to tumor cachexia.
Even though the outcome of surgical resection for primary hepatic sarcoma is not ideal, surgical resection is still considered to be a better treatment than chemotherapy alone. In a study with 30 primary hepatic sarcoma patients, those who received R0-surgical resection experienced much better outcomes than those who did not, except for patients with the specific pathologic type of epithelioid hemangioendothelioma[2]. Another study in which 6 patients received transcatheter arterial chemoembolization or transcatheter arterial embolization alone for hepatic angiosarcoma resulted in all patients expiring within 1 year[11]. A recent study of 8 patients with R0-resected hepatic angiosarcoma showed median survival and disease-free survival of 59 and 11 mo which emphasizes the radical surgical resection is best approach for long-term survival[12].
Still, adjuvant chemotherapy after resection is recommended for hepatic sarcoma. In cases of angiosarcoma, one study showed that a combination of surgical treatment and adjuvant chemotherapy may be beneficial[13]. A review article with 64 cases of angiosarcoma suggested that surgery with chemotherapy is the optimal choice for survival[6]. May et al[14] studied five pediatric patients with hepatic undifferentiated sarcoma who underwent a uniform approach of local resection and vincristine, actinomycin-D, cyclophosphamide. All patients survived with median survival of 53 mo. Lenze et al[15] described 14 patients with undifferentiated sarcoma who remained alive for a median of 28.5 mo after receiving both surgical resection and adjuvant chemotherapy, which was a significantly better outcome compared to patients without adjuvant chemotherapy. However, the optimal regimen of chemotherapy for hepatic sarcoma has not yet been established. Kim et al[16] showed that palliative chemotherapy may be beneficial to survival even if the hepatic angiosarcoma is unresectable. Transarterial chemoembolization showed some effectiveness in acute intra-abdominal hemorrhage of hepatic angiosarcoma cases[17]. In our study, three patients who did not receive adjuvant therapy had poorer survival than patients who received adjuvant therapy. However, two of them could not receive chemotherapy due to poor general condition (angiosarcoma patients), and one patient expired before scheduled chemotherapy (undifferentiated sarcoma). There is a case report of immunotherapy about a patient with primary hepatic angiosarcoma with multiple liver metastasis treated by pazopanib plus procedural death factors-1 inhibitor and RAK cells showing stable disease after treatment[18]. Although this is only one case report, this study showed a hope of new era of treatment which may aid surgical resection of hepatic angiosarcoma.
In our study, two patients with hepatic angiosarcoma received living donor liver transplantation (LT). The 2-year-old girl had recurrence at 53 d and expired at 31 mo after LT, while the 60-year-old male had recurrence at 11 mo and expired at 14 mo. Selection of treatment between surgical resection and LT is an issue of concern. A study of 237 patients registered in the National Cancer Database of North America concluded that both hepatic resection and LT may lead to similar long-term survival with selected pathologic cases such as epithelioid hemangioendotheliomas[19]. However, this study also found that the prognosis of angiosarcoma was worse with both resection and transplantation. A review article of 64 angiosarcoma cases did not recommend LT for angiosarcoma due to the higher recurrence rate[6]. This result accords with the poor outcomes of LT seen in the hepatic angiosarcoma patients in our study. For hepatic undifferentiated sarcoma, liver transplantation cases are rare. Walther et al[20] studied 3 patients who received LT and remained in clinical remission for a mean of 35 mo. Wu et al[10] showed 14 patients who received LT for hepatic undifferentiated sarcoma with a 5-year survival rate of 78.9%. When weighing the benefits of LT against the risks for the liver donor or the shortage of deceased donor, LT in hepatic undifferentiated sarcoma is still controversial and needs further research.
Our study has limitations in that it is a retrospective study and has a small number of patients due to the rarity of the tumor. Furthermore, three patients did not receive adjuvant therapy, not due to clinical decision, but due to either poor patient condition or the patient expiring prior to receiving the adjuvant therapy.
Primary hepatic sarcoma has poor outcomes even after surgical resection. However, surgical resection may have some benefit for extending long term life expectancy in some cases. Adjuvant therapy may support the outcomes. Liver transplantation for primary hepatic angiosarcoma also continues to have poor survival outcomes.
Primary hepatic sarcoma is a malignant tumor which arises from hepatic mesenchymal tissue. It consists of angiosarcoma, undifferentiated (embryonal) sarcoma, leiomyosarcoma, and epithelioid hemangioendothelioma.
Due to it’s rarity and various prognosis, the treatment plan of primary hepatic sarcoma is not established yet.
We aim to analyze the tumor characteristics, treatment and prognosis of the primary hepatic sarcoma cases which was surgically resected in a single center.
After exclusion of cases with open and closure, early loss to follow-up and sarcomatoid tumors, total nine cases of primary hepatic sarcoma were surgically resected from August 2001 to September 2016. The research data collection and analysis were achieved with retrospective approach. Baseline patient’s characteristics, tumor characteristics and treatment modality with tumor recurrence and patient’s survival were analyzed. The analysis was done separately according to tumor pathologic type.
Among five angiosarcoma and four undifferentiated sarcoma patients, only two patients survived and all patients experienced tumor recurrences (5-year survival rate: 29.6%). Follow-up post-operative durations of survived angiosarcoma patient and undifferentiated sarcoma patient were 11 years and 30 mo, respectively. Adjuvant therapy had a positive role on survival gain (P = 0.002). However, this study has a limitation of a retrospective approach and a small case number.
In spite of known poor prognosis, surgical resection of primary hepatic sarcoma may help extending the life expectancy of patient. Aggressive adjuvant treatment after resection may aid the better outcome.
Accumulation of primary hepatic sarcoma data followed by finding of specific prognostic factor should be researched. New era of adjuvant therapies, such as immunotherapy for primary hepatic sarcoma is also needed to be developed.
Manuscript source: Invited manuscript
Specialty type: Surgery
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jiménez Pérez M, Rodrigues AT, Takemura N S-Editor: Zhang L L-Editor: A P-Editor: Wang LL
| 1. | Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 941] [Cited by in RCA: 920] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 2. | Weitz J, Klimstra DS, Cymes K, Jarnagin WR, D'Angelica M, La Quaglia MP, Fong Y, Brennan MF, Blumgart LH, Dematteo RP. Management of primary liver sarcomas. Cancer. 2007;109:1391-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Falk H, Thomas LB, Popper H, Ishak KG. Hepatic angiosarcoma associated with androgenic-anabolic steroids. Lancet. 1979;2:1120-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 104] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Forbes A, Portmann B, Johnson P, Williams R. Hepatic sarcomas in adults: a review of 25 cases. Gut. 1987;28:668-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Matthaei H, Krieg A, Schmelzle M, Boelke E, Poremba C, Rogiers X, Knoefel WT, Peiper M. Long-term survival after surgery for primary hepatic sarcoma in adults. Arch Surg. 2009;144:339-44; discussion 344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Zheng YW, Zhang XW, Zhang JL, Hui ZZ, Du WJ, Li RM, Ren XB. Primary hepatic angiosarcoma and potential treatment options. J Gastroenterol Hepatol. 2014;29:906-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Stocker JT, Ishak KG. Undifferentiated (embryonal) sarcoma of the liver: report of 31 cases. Cancer. 1978;42:336-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 8. | Manabe Y, Uojima H, Hidaka H, Shao X, Iwasaki S, Wada N, Kubota K, Tanaka Y, Nakazawa T, Shibuya A, Ichinoe M, Kumamoto Y, Kaizu T, Koizumi W. Undifferentiated Embryonal Sarcoma of the Liver Identified after the Initial Diagnosis of a Hepatic Cyst. Intern Med. 2020;59:2375-2382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Mori A, Fukase K, Masuda K, Sakata N, Mizuma M, Ohtsuka H, Morikawa T, Nakagawa K, Hayashi H, Motoi F, Naitoh T, Murakami K, Unno M. A case of adult undifferentiated embryonal sarcoma of the liver successfully treated with right trisectionectomy: a case report. Surg Case Rep. 2017;3:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Wu Z, Wei Y, Cai Z, Zhou Y. Long-term survival outcomes of undifferentiated embryonal sarcoma of the liver: a pooled analysis of 308 patients. ANZ J Surg. 2020;90:1615-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Park YS, Kim JH, Kim KW, Lee IS, Yoon HK, Ko GY, Sung KB. Primary hepatic angiosarcoma: imaging findings and palliative treatment with transcatheter arterial chemoembolization or embolization. Clin Radiol. 2009;64:779-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Tripke V, Heinrich S, Huber T, Mittler J, Hoppe-Lotichius M, Straub BK, Lang H. Surgical therapy of primary hepatic angiosarcoma. BMC Surg. 2019;19:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Huang NC, Kuo YC, Chiang JC, Hung SY, Wang HM, Hung YM, Chang YT, Wann SR, Chang HT, Wang JS, Ho SY, Guo HR. Hepatic angiosarcoma may have fair survival nowadays. Medicine (Baltimore). 2015;94:e816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | May LT, Wang M, Albano E, Garrington T, Dishop M, Macy ME. Undifferentiated sarcoma of the liver: a single institution experience using a uniform treatment approach. J Pediatr Hematol Oncol. 2012;34:e114-e116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Lenze F, Birkfellner T, Lenz P, Hussein K, Länger F, Kreipe H, Domschke W. Undifferentiated embryonal sarcoma of the liver in adults. Cancer. 2008;112:2274-2282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Kim HR, Rha SY, Cheon SH, Roh JK, Park YN, Yoo NC. Clinical features and treatment outcomes of advanced stage primary hepatic angiosarcoma. Ann Oncol. 2009;20:780-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 17. | Stambo GW, Guiney MJ. Hepatic angiosarcoma presenting as an acute intraabdominal hemorrhage treated with transarterial chemoembolization. Sarcoma. 2007;2007:90169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Qiao Y, Yang J, Liu L, Zeng Y, Ma J, Jia J, Zhang L, Li X, Wu P, Wang W, Liu D, Chen H, Zhao Y, Xi H, Wang Y. Successful treatment with pazopanib plus PD-1 inhibitor and RAK cells for advanced primary hepatic angiosarcoma: a case report. BMC Cancer. 2018;18:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Konstantinidis IT, Nota C, Jutric Z, Ituarte P, Chow W, Chu P, Singh G, Warner SG, Melstrom LG, Fong Y. Primary liver sarcomas in the modern era: Resection or transplantation? J Surg Oncol. 2018;117:886-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Walther A, Geller J, Coots A, Towbin A, Nathan J, Alonso M, Sheridan R, Tiao G. Multimodal therapy including liver transplantation for hepatic undifferentiated embryonal sarcoma. Liver Transpl. 2014;20:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |