Published online May 27, 2021. doi: 10.4254/wjh.v13.i5.522
Peer-review started: March 7, 2021
First decision: March 29, 2021
Revised: March 30, 2021
Accepted: April 29, 2021
Article in press: April 29, 2021
Published online: May 27, 2021
Processing time: 73 Days and 20 Hours
The coronavirus disease 2019 (COVID-19) pandemic has caused unprecedented pressure on public health and healthcare. The pandemic surge and resultant lockdown have affected the standard-of-care of many medical conditions and diseases. The initial uncertainty and fear of cross transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have changed the routine management of patients with pre-existing liver diseases, hepatocellular carcinoma, and patients either listed for or received a liver transplant. COVID-19 is best described as a multisystem disease caused by SARS-CoV-2, and it can cause acute liver injury or decompensation of the pre-existing liver disease. There has been considerable research on the pathophysiology, infection transmission, and treatment of COVID-19 in the last few months. The pathogenesis of liver involvement in COVID-19 includes viral cytotoxicity, the secondary effect of immune dysregulation, hypoxia resulting from respiratory failure, ischemic damage caused by vascular endotheliitis, congestion because of right heart failure, or drug-induced liver injury. Patients with chronic liver diseases, cirrhosis, and hepatocellular carcinoma are at high risk for severe COVID-19 and mortality. The phase III trials of recently approved vaccines for SARS-CoV-2 did not include enough patients with pre-existing liver diseases and excluded immunocompromised patients or those on immunomodulators. This article reviews the currently published research on the effect of COVID-19 on the liver and the management of patients with pre-existing liver disease, including SARS-CoV-2 vaccines.
Core Tip: Liver involvement in coronavirus disease 2019 (COVID-19) is caused by either viral cytotoxicity or secondary to systemic immune dysregulation. Patients with pre-existing liver disease are at high risk of disease progression, morbidity, and mortality. Chronic liver disease with COVID-19 should be managed as per the standard guidelines, with education on hand hygiene, social distancing, and face masks to reduce hospital admissions. There is no evidence that currently available vaccines for severe acute respiratory syndrome coronavirus 2 will have any complications different from other inactivated vaccines and are recommended for patients with pre-existing liver disease, hepatocellular carcinoma, or liver transplant recipients.
- Citation: Nasa P, Alexander G. COVID-19 and the liver: What do we know so far? World J Hepatol 2021; 13(5): 522-532
- URL: https://www.wjgnet.com/1948-5182/full/v13/i5/522.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i5.522
Coronavirus is an enveloped single-stranded RNA virus belonging to the Coronaviridae family and Orthocoronavirinae subfamily. Severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) respectively caused epidemics in 2003 and 2012. The pandemic of coronavirus disease 2019 (COVID-19) caused by the SARS-CoV-2 was first reported from Wuhan, China on December 31, 2019 in patients with atypical pneumonia[1]. While symptoms are mild in most patients, severe and critical symptoms (in 10%-15% of patients) like hypoxemia (SpO2 < 94%), acute respiratory distress syndrome, multiorgan failure, or shock; may need hospitalization and respiratory support[2,3]. Older patients, especially those with comorbidities like hypertension, diabetes, chronic liver disease (CLD), and heart disease, are at risk of severe disease and mortality[2,3]. With the rapid spread of COVID-19, there has been significant concern regarding the safe management of patients with pre-existing liver disease (CLD), hepatocellular carcinoma (HCC), and candidates for a liver transplant. This review discusses the current evidence on liver involvement in COVID-19 and its impact on managing patients with CLD, including current recommendations for SARS-CoV-2 vaccines.
Based on the published literature, 14%-53% of patients with COVID-19 developed hepatic dysfunction, and 2%-11% of the patients were reported to have underlying CLD[4-9]. Hepatic dysfunction characterized by elevated liver enzymes was significantly higher in severe and critical COVID-19 and was associated with poor outcomes[4]. In a meta-analysis of 45 studies, the most common biochemical abnormality of the liver in COVID-19 was hypoalbuminemia (39.8%), followed by elevation of gamma-glutamyl transferase (GGT 35.8%), or aminotransferases [aspartate aminotransferase (AST 21.8%) and alanine aminotransferase (ALT 20.4%)][10]. The incidence of elevated hepatic enzymes was also higher in COVID-19 patients requiring intensive care unit (ICU) admission as compared to non-ICU patients (62% vs 23%)[4]. In another meta-analysis of 128 studies, the most common hepatic abnormality was hypoalbuminemia (61.3%), followed by elevation of GGT (27.9%), ALT (23.3%), and AST (23.4%) in the patients with COVID-19. The degree of the hepatic abnormalities was directly proportional to the severity of the disease[11].
The pathogenic properties of SARS-CoV-2 depend on the binding of viral spike proteins to the host angiotensin-converting enzyme 2 (ACE-2) receptors, which allows the virus to enter the target cells along with priming by the host transmembrane serine protease 2 (TMPRSS2)[12-14]. The ACE-2-TMPRSS2 is expressed in the ileum, liver, lung, nasal mucosa, bladder, testis, prostate, and kidney (in that order)[14-17]. SARS-CoV-2 binding to ACE-2 receptors in the upper respiratory tract is the primary site of replication and entry to the body[14]. ACE-2-TMPRSS2-positive cells in the gastrointestinal tract include enterocytes in the biliary duct or pancreatic duct epithelium and hepatocytes[14,17].
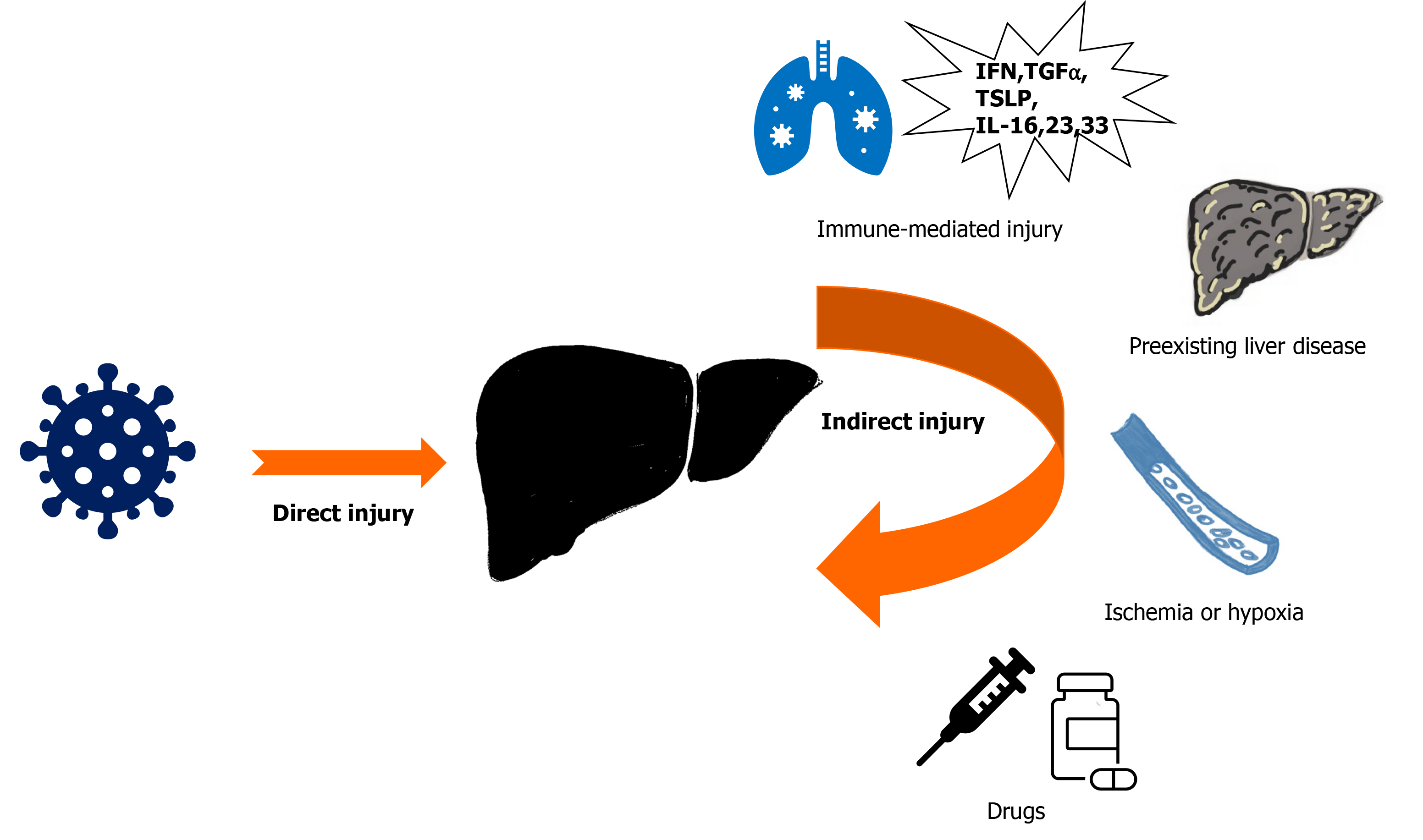
The mechanism of liver injury in COVID-19 is possibly multifactorial. SARS-CoV-2 might induce direct hepatoxicity (SARS-CoV-2 enters into the liver via cholangiocytes or translocation from gut to the liver) or indirect hepatic injury (from systemic inflammation with immune dysregulation, hypoxia from respiratory failure, ischemic damage due to coagulopathy or endotheliitis, right heart failure due to myocarditis, deterioration of pre-existing liver diseases, or drug-induced liver injury)[15] (Figure 1). The liver function abnormalities like increased GGT are consistent with a direct cytotoxic effect of SARS-CoV-2 on cholangiocytes[15,18]. However, the expression of ACE-2 receptors is minimal on hepatocytes suggesting a significant contribution of indirect causes of liver damage rather than direct hepatoxicity[16,18]. The treatment of severe COVID-19 with antiviral agents, immunomodulators, antibiotics, or supportive agents, may also cause hepatotoxicity. Among those agents, remdesivir, favipiravir, lopinavir/ritonavir combination, corticosteroids, and tocilizumab could increase liver enzyme levels[18-20]. Adjuvant drugs like acetaminophen and antibiotics may also cause hepatoxicity[20] (Table 1).
| Drug | Mechanism of action | Impact on CLD management |
| Remdesivir | Viral RNA-dependent RNA polymerase inhibitor | Liver toxicity possible; No liver relevant drug-drug interactions |
| Lopinavir/ritonavir | Protease inhibitors | mTOR inhibitors (sirolimus, everolimus) should not be co-administered; Close monitoring of drug level is required for calcineurin inhibitors (cyclosporine, tacrolimus); The risk of lopinavir-associated hepatotoxicity in patients with very advanced liver disease is low; Patients with decompensated cirrhosis should not be treated |
| Tocilizumab | Humanized monoclonal antibody targeting interleukin-6 receptor | Patients with decompensated cirrhosis should not be treatedConsider risk of HBV reactivation |
| Methylprednisolone (steroids) | Bind nuclear receptors todampen proinflammatory cytokines | The risk of other infections (e.g., spontaneous bacterial peritonitis) and viral shedding may increase in patents with decompensated liver cirrhosis; Consider antimicrobial prophylaxis; Consider risk of HBV reactivation |
| Favipiravir | Guanine analogue, RNA-dependent RNA polymerase | Elevation of ALT and AST possible; No data in cirrhosis available |
In a systematic review and meta-analysis of 73 studies, the prevalence of CLD was 3% in 24299 COVID-19 patients[21]. Other studies reported a 3%-11% prevalence of underlying CLD with COVID-19[4-9,22]. The patients with CLD may also be more susceptible to contract SARS-CoV-2 infection[23]. Besides, the presence of CLD increased the risk of severe COVID-19 [pooled odds ratio (OR) 1.48] and overall mortality (pooled OR 1.78)[21,24]. Two other meta-analyses found that pre-existing liver diseases increase the risk of severe COVID-19, decompensation, and mortality[24,25]. From an extensive registry of over 17 million patients from the United Kingdom, COVID-19 was associated with a 2.34 (95% confidence interval: 1.94-2.83) times increased risk of mortality with liver disease[26]. The evidence is conflicting on the increased risk of severe COVID-19 in patients with chronic viral hepatitis[4,27]. However, SARS-CoV-2 infection in patients with chronic hepatitis B could have an increased risk of reactivation. A study of 21 patients with known chronic hepatitis B, SARS-CoV-2 infection was associated with hepatitis B reactivation in three patients[28].
In a multicenter retrospective study from the United States, CLD and nonalcoholic fatty liver disease (NAFLD) were independent risk factors for ICU admission and invasive mechanical ventilation[29]. NAFLD was also associated with the progression of COVID-19 to severe disease in other studies[30-32]. The Asian Pacific Association for the Study of the Liver COVID-19 Liver Injury Spectrum Study (APCOLIS) study included 228 confirmed COVID-19 patients from 13 Asian countries with pre-existing liver disease. Metabolism associated fatty liver disease (MAFLD) was the commonest (61%) etiology[33]. In a retrospective study, a history of NAFLD/MAFLD was associated with increased odds of admission for COVID-19[34]. Obesity is common in patients with NAFLD and is an independent risk factor for severe COVID-19, invasive mechanical ventilation, and increased mortality[31,35]. However, in a study by Hashemi et al[29], the clinical severity of COVID-19 in patients of NAFLD was observed to be independent of obesity. The deleterious interplay of chronic inflammation observed in NAFLD with an acute inflammatory response to SARS-CoV-2 could explain the higher hepatic injury and a worse outcome in metabolically compromised NAFLD patients[36]. In another study, the extent of liver fat was correlated with serum markers of inflammation and oxidative stress[37]. It explains the multifaceted impact of NAFLD on the pathophysiology and clinical course of COVID-19. However, effective treatment for metabolic disease can mitigate the increased risk from NAFLD[29,36].
Patients with cirrhosis are also at increased risk of decompensation with SARS-CoV-2 infection[38]. The presence of cirrhosis was also found to be an independent predictor of mortality in COVID-19[29,31]. In a study from the United States, the risk factors related to higher mortality in COVID-19 and CLD were alcoholic liver disease, decompensated cirrhosis, and HCC[39]. The worse outcomes in patients with cirrhosis can be multifactorial and likely due to cirrhosis-associated immune and inflammation modulation, limited physiological reserves, and increased risk of severe COVID-19[39]. Other large registries of patients with cirrhosis and COVID-19, like SECURE-cirrhosis and COVID-Hep.net, reported a case fatality rate of 38%, which may be as high as 70% in the Child-Pugh C category[40].
Patients with malignancy are vulnerable during the COVID-19 pandemic, with an increased risk of SARS-CoV-2 infection[41,42]. The overall prognosis of COVID-19 in cancer patients is poor, with high ICU admissions and mortality[41-43]. A small retrospective study of 28 cancer patients with COVID-19, including 2 HCC patients, had worse outcomes than the general population[43]. The increased risk may be attributed to age, multiple comorbidities, and the presence of cirrhosis. In patients with HCC, COVID-19 may exacerbate existing CLD and complicate the management of cancer.
The SARS-CoV-2 infection in patients with pre-existing liver pathology may increase the risk of decompensation, acute liver injury, or a combination of both. Acute liver injury was the most observed presentation (43%) in CLD patients without cirrhosis, while acute-on-chronic liver failure (11.6%) and decompensation (9%) were more common in patients with cirrhosis[34]. The risk factors for decompensation include comorbid illnesses like diabetes or obesity. The AST/ALT ratio, total bilirubin, and R-value (ALT/ALP ratio) can predict survival in cirrhotic patients[34]. The residual hepatic synthetic function in CLD patients is inversely proportional to liver-related complications with COVID-19. Liver injury has been seen in the third week in CLD patients without cirrhosis and in the first week in cirrhotic patients[34].
Being an immunocompromised host, liver transplant recipients have an increased risk of acquiring SARS-CoV-2 infection and progression to severe disease. The outcome of COVID-19 in liver transplant patients was evaluated in a prospective study of 111 patients in Spain. Of 96 patients (86.5%) who were diagnosed with COVID-19 requiring hospital admission, 22 patients (19.8%) needed respiratory support, 12 (10.8%) required ICU admission, and the case fatality rate was 18% which was relatively lower than the matched general population despite higher severity of disease[44]. Similar results were found in another multicenter study of 112 patients from the United States. The hospital and ICU mortality rates were 22.3% and 26.8%, respectively, which was lower than the rates in matched patients of CLD without liver transplant[45]. The postulated hypothesis for better outcomes was ongoing immunomodulatory therapies that may ameliorate a harmful immune response (i.e. cytokine storm), reducing mortality[45,46]. However, immunosuppressants may delay viral clearance, explaining the severe disease[44]. The factors associated with mortality in liver transplant recipients were new liver injury, younger age, hispanic ethnicity, metabolic syndrome, vasopressor requirement, and antibiotic usage. Moderate liver injury [ALT 2-5 times the upper limit of normal (ULN)] and severe liver injury (ALT more than five times the ULN) was significantly associated (P = 0.007) with mortality and ICU admission[45].
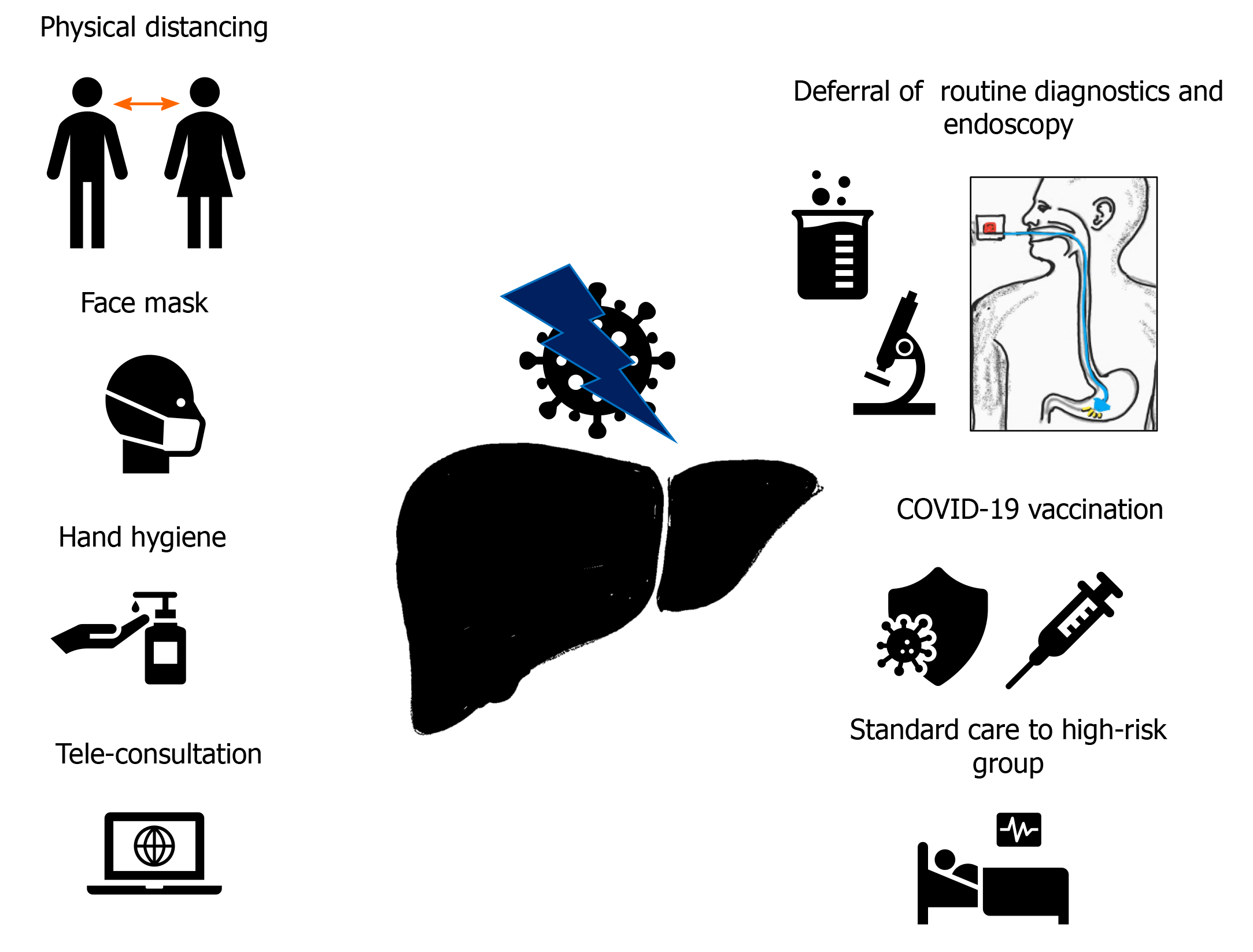
The COVID-19 pandemic had a considerable impact on the management of CLD. Various factors must be considered and monitored while managing this group of patients. There is a potential threat of cross transmission of SARS-CoV-2 among patients and health care workers (HCWs) during physical assessment and treatment. However, it is imperative to maintain the continuity of care of patients with CLD to reduce the risk of decompensation and hospital admission. The measures recommended for safe and effective management of CLD patients can be divided into general and specific (Figure 2).
Physical distancing, avoiding closed spaces without a face mask, and hand hygiene are vital pillars of SARS-CoV-2 infection prevention. Education on infection prevention measures should be included with other social measures like abstinence from alcohol and medication compliance. The screening of fever or respiratory symptoms should be performed on all patients and HCWs at the entrance of the hospital premises. Telemedicine, postponing routine outpatient visits, or periodic laboratory testing are other strategies that can be considered, depending on the available resources and patient condition[1]. The patient education must include prophylactic vaccination for streptococcus pneumonia or influenza.
Compensated liver disease: There is no evidence that initial clinical symptoms of SARS-CoV-2 are different in patients with CLD. Patients with NAFLD/MAFLD may suffer from other metabolic comorbidities like diabetes mellitus, hypertension, hyperlipidemia, and obesity, which need optimization and regular monitoring. Experts recommend against the alteration of immunosuppression in autoimmune hepatitis and liver transplant patients to reduce the risk of severe COVID-19[47]. The risk of aerosolization of SARS-CoV-2 during endoscopy must be considered during routine management of esophageal varices. Experts recommended non-endoscopic pathways to assess esophageal varices, especially during periods of high community transmission[47]. Any acute decompensation in patients with known CLD needs exclusion of SARS-CoV-2 coinfection. The potential reactivation of hepatitis B in patients with COVID-19 and chronic hepatitis B mandates monitoring of liver function tests and hepatitis B virus -DNA levels[28].
Decompensated liver disease: The care of the patients should follow standard guidelines while reducing direct visits to the healthcare facility (e.g., using telemedicine or telephone consultation) wherever feasible. The standard management of these patients, like prophylaxis for variceal bleeding, spontaneous bacterial peritonitis, or hepatic encephalopathy, should be continued unaltered to prevent further worsening and reduce admissions[47].
Liver transplantation: The liver transplant recipients are at increased risk of contracting COVID-19, like patients with CLD. The general measures can include teleconsultation to shorten in-hospital stay and interactions with other HCWs. There were attempts to generate international consensus on treatment protocols of liver transplant recipients during this pandemic to reduce the risk of cross-transmission of SARS-CoV-2 and optimize healthcare resources[47]. The immunosuppression in liver transplant recipients may interfere with the immune response against the virus, while any alteration in the treatment may cause acute graft rejection. Also, the use of various therapeutics to treat COVID-19 and drug-drug interactions with immunomodulators raises concerns of hepatotoxicity. In a prospective cohort study by Colmenero et al[44], mycophenolate at doses higher than 1000 mg/d was an independent predictor of severe COVID-19 in 111 liver transplant patients diagnosed with COVID-19. The synergistic effect of mycophenolate and SARS-CoV-2 may deplete peripheral lymphocytes responsible for an aberrant immune reconstitution to SARS-CoV-2[11,48]. In a multicenter study from the United States of COVID-19 in 112 liver transplant patients, new liver injury was associated increased mortality and ICU admission[45].
The close monitoring of liver enzymes in liver transplant patients and COVID-19 is suggested to watch for new liver injury or graft rejection. The immunosuppression regimen preferably should not be altered, except in the case of a mycophenolate-based regimen. Hypothermia is associated with worsening liver functions in severe COVID-19 and should be corrected with appropriate interventions[45].
Candidates for liver transplant: SARS-CoV-2 routine testing should be performed for both the recipient and donor before transplantation. However, a single negative RT-PCR test cannot exclude an asymptomatic infection[47]. During high community transmission or inundated healthcare resources, the transplantation should be offered only to select patients with poor short-term prognosis. It includes acute or acute-on-chronic liver failure, a high Model for End-stage Liver Disease score, or HCC with upper limits of the Milan criteria[45,49]. The diagnostic workup and procedure for the transplant program must be performed rapidly, with a short hospital stay[49].
Hepatocellular Carcinoma: Although the number of patients with HCC in the published COVID-19 studies is minimal, similar infection risk mitigation should be implemented in patients with CLD. The clinical services of cancer patients have been significantly affected by the current coronavirus pandemic, with decreased referral of the patients to the multidisciplinary tumor board (MTB), and treatment delays[50]. The evaluation, treatment and monitoring of HCC should be personalized based on the availability of medical resources and level of infection risk of SARS-CoV-2. Guidelines on the management of liver disease and HCC have been published by various academic societies[47,51]. The recommendations include virtual MTB meetings, prioritizing surgery on a case-to-case basis with preference to patients with low disease burdens and alternative therapies like radiofrequency and microwave ablation in selected patients. Treatment deferral or modification should be based on the best available evidence and availability of resources[52].
Scientists developed vaccines against SARS-CoV-2 with unprecedented speed. The vaccines have been found effective in reducing the incidence of severe disease, hospitalization, and mortality. Vaccines based on various platforms, like mRNA, nonhuman viral vectors, and inactivated whole SARS-CoV-2 were developed. Despite more than 200000 participants in phase III trials, there is minimal data on efficacy in patients with pre-existing liver diseases. In the BNT162b2 (Pfizer/BioNTech) vaccination study, 217 participants (0.6%) had CLD and only 3 (< 0.1%) had moderate to severe liver disease[53]. Similarly, only 196 liver disease patients (0.6%) were included in the mRNA-1273 (Moderna) trial[54]. Data on patients with pre-existing liver disease is not available from the ChAdOx1-nCoV-19 (Oxford–AstraZeneca) vaccine trial[55]. Patients on systemic immunosuppression were excluded in all phase III trials, undermining the role of vaccines in the liver transplant recipients or patients with autoimmune liver disease on immunosuppressants. However, in the real world, millions are already vaccinated, including patients with liver disease; thus, data on safety and effectiveness are expected to be available soon. The deficiencies of innate or adaptive immune responses and an attenuated response to others vaccines are well recognized in CLD patients. A similar altered response to SARS-CoV-2 vaccines is also suspected. Nevertheless, based on an increased risk of severe disease, and in the absence of any data suggesting harm, the European Association for the Study of the Liver (EASL), the American Association for the Study of Liver Diseases (AASLD), and British Association for the Study of Liver currently recommend the available SARS-CoV-2 vaccines for patients with CLD, and liver transplant recipients[56-58]. Although the vaccines may be less effective in patients with CLD and liver transplant recipients, they still provide protection[58].
Emerging research suggests that liver injury is common in COVID-19 patients and associated with worse outcomes. Patients with CLD and post liver transplant patients are at risk of SARS-CoV-2 infection, with an increased risk of complications and mortality. The management of this vulnerable group of patients should be prioritized based on their clinical condition, strategies to reduce cross transmission, and optimizing limited resources. Liver transplant and HCC management programs should be modified depending on the prevalence of community transmission of SARS-CoV-2. Specific management issues should be considered during the treatment of COVID-19 in patients with pre-existing liver diseases.
Manuscript source: Invited manuscript
Specialty type: Medicine, general and internal
Country/Territory of origin: United Arab Emirates
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Fan Y, Gallo G, Liu D S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Li JH
| 1. | World Health Organization. COVID-19 Clinical management: living guidance. Updated 25 January 2021. [cited 5 March 2021]. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1. |
| 2. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18175] [Article Influence: 3635.0] [Reference Citation Analysis (0)] |
| 3. | Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, Ji R, Wang H, Wang Y, Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2672] [Cited by in RCA: 2506] [Article Influence: 501.2] [Reference Citation Analysis (2)] |
| 4. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1293] [Article Influence: 258.6] [Reference Citation Analysis (4)] |
| 5. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 554] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 6. | Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 661] [Article Influence: 132.2] [Reference Citation Analysis (0)] |
| 7. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14749] [Article Influence: 2949.8] [Reference Citation Analysis (0)] |
| 8. | Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2493] [Cited by in RCA: 2308] [Article Influence: 461.6] [Reference Citation Analysis (0)] |
| 9. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18839] [Article Influence: 3767.8] [Reference Citation Analysis (7)] |
| 10. | Wu Y, Li H, Guo X, Yoshida EM, Mendez-Sanchez N, Levi Sandri GB, Teschke R, Romeiro FG, Shukla A, Qi X. Incidence, risk factors, and prognosis of abnormal liver biochemical tests in COVID-19 patients: a systematic review and meta-analysis. Hepatol Int. 2020;14:621-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 11. | Kumar-M P, Mishra S, Jha DK, Shukla J, Choudhury A, Mohindra R, Mandavdhare HS, Dutta U, Sharma V. Coronavirus disease (COVID-19) and the liver: a comprehensive systematic review and meta-analysis. Hepatol Int. 2020;14:711-722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 12. | Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1286] [Cited by in RCA: 1526] [Article Influence: 305.2] [Reference Citation Analysis (0)] |
| 13. | Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 739] [Article Influence: 147.8] [Reference Citation Analysis (0)] |
| 14. | Jia HP, Look DC, Shi L, Hickey M, Pewe L, Netland J, Farzan M, Wohlford-Lenane C, Perlman S, McCray PB Jr. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol. 2005;79:14614-14621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 643] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 15. | Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41:20-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 271] [Article Influence: 67.8] [Reference Citation Analysis (2)] |
| 16. | Shojaee A, Vahedian-Azimi A, Faizi F, Rahimi-Bashar F, Shahriary A, Galeh HEG, Nehrir B, Guest PC, Sahebkar A. Relationship Between COVID-19 and Angiotensin-Converting Enzyme 2: A Scoping Review. Adv Exp Med Biol. 2021;1321:53-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Muus C, Luecken MD, Eraslan G, Sikkema L, Waghray A, Heimberg G, Kobayashi Y, Vaishnav ED, Subramanian A, Smillie C, Jagadeesh KA, Duong ET, Fiskin E, Triglia ET, Ansari M, Cai P, Lin B, Buchanan J, Chen S, Shu J, Haber AL, Chung H, Montoro DT, Adams T, Aliee H, Allon SJ, Andrusivova Z, Angelidis I, Ashenberg O, Bassler K, Bécavin C, Benhar I, Bergenstråhle J, Bergenstråhle L, Bolt L, Braun E, Bui LT, Callori S, Chaffin M, Chichelnitskiy E, Chiou J, Conlon TM, Cuoco MS, Cuomo ASE, Deprez M, Duclos G, Fine D, Fischer DS, Ghazanfar S, Gillich A, Giotti B, Gould J, Guo M, Gutierrez AJ, Habermann AC, Harvey T, He P, Hou X, Hu L, Hu Y, Jaiswal A, Ji L, Jiang P, Kapellos TS, Kuo CS, Larsson L, Leney-Greene MA, Lim K, Litviňuková M, Ludwig LS, Lukassen S, Luo W, Maatz H, Madissoon E, Mamanova L, Manakongtreecheep K, Leroy S, Mayr CH, Mbano IM, McAdams AM, Nabhan AN, Nyquist SK, Penland L, Poirion OB, Poli S, Qi C, Queen R, Reichart D, Rosas I, Schupp JC, Shea CV, Shi X, Sinha R, Sit RV, Slowikowski K, Slyper M, Smith NP, Sountoulidis A, Strunz M, Sullivan TB, Sun D, Talavera-López C, Tan P, Tantivit J, Travaglini KJ, Tucker NR, Vernon KA, Wadsworth MH, Waldman J, Wang X, Xu K, Yan W, Zhao W, Ziegler CGK; NHLBI LungMap Consortium; Human Cell Atlas Lung Biological Network. Single-cell meta-analysis of SARS-CoV-2 entry genes across tissues and demographics. Nat Med. 2021;27:546-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 237] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 18. | Bertolini A, van de Peppel IP, Bodewes FAJA, Moshage H, Fantin A, Farinati F, Fiorotto R, Jonker JW, Strazzabosco M, Verkade HJ, Peserico G. Abnormal Liver Function Tests in Patients With COVID-19: Relevance and Potential Pathogenesis. Hepatology. 2020;72:1864-1872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 193] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 19. | Genovese MC, Kremer JM, van Vollenhoven RF, Alten R, Scali JJ, Kelman A, Dimonaco S, Brockwell L. Transaminase Levels and Hepatic Events During Tocilizumab Treatment: Pooled Analysis of Long-Term Clinical Trial Safety Data in Rheumatoid Arthritis. Arthritis Rheumatol. 2017;69:1751-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 20. | Metawea MI, Yousif WI, Moheb I. COVID 19 and liver: An A-Z literature review. Dig Liver Dis. 2021;53:146-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (2)] |
| 21. | Kovalic AJ, Satapathy SK, Thuluvath PJ. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: a systematic review and meta-analysis. Hepatol Int. 2020;14:612-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 22. | Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium; Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 6502] [Article Influence: 1300.4] [Reference Citation Analysis (0)] |
| 23. | Mantovani A, Beatrice G, Dalbeni A. Coronavirus disease 2019 and prevalence of chronic liver disease: A meta-analysis. Liver Int. 2020;40:1316-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 24. | Oyelade T, Alqahtani J, Canciani G. Prognosis of COVID-19 in Patients with Liver and Kidney Diseases: An Early Systematic Review and Meta-Analysis. Trop Med Infect Dis. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 25. | Del Zompo F, De Siena M, Ianiro G, Gasbarrini A, Pompili M, Ponziani FR. Prevalence of liver injury and correlation with clinical outcomes in patients with COVID-19: systematic review with meta-analysis. Eur Rev Med Pharmacol Sci. 2020;24:13072-13088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 26. | The OpenSAFELY Collaborative, Williamson E, Walker AJ, Bhaskaran KJ, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, Mcdonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong A, Grieve R, Harrison D, Forbes H, Schultze A, Croker RT, Parry J, Hester F, Harper S, Perera R, Evans S, Smeeth L, Goldacre B. OpenSAFELY: factors associated with COVID‐19‐related hospital death in the linked electronic health records of 17 million adult NHS patients. 2020 Preprint. Available from: medRxiv: 2020.05.06.20092999. [DOI] [Full Text] |
| 27. | Chen X, Jiang Q, Ma Z, Ling J, Hu W, Cao Q, Mo P, Yao L, Yang R, Gao S, Gui X, Hou W, Xiong Y, Li J, Zhang Y. Clinical Characteristics of Hospitalized Patients with SARS-CoV-2 and Hepatitis B Virus Co-infection. Virol Sin. 2020;35:842-845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 28. | Liu J, Wang T, Cai Q, Sun L, Huang D, Zhou G, He Q, Wang FS, Liu L, Chen J. Longitudinal changes of liver function and hepatitis B reactivation in COVID-19 patients with pre-existing chronic hepatitis B virus infection. Hepatol Res. 2020;50:1211-1221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 29. | Hashemi N, Viveiros K, Redd WD, Zhou JC, McCarty TR, Bazarbashi AN, Hathorn KE, Wong D, Njie C, Shen L, Chan WW. Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: A multicentre United States experience. Liver Int. 2020;40:2515-2521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (2)] |
| 30. | Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (2)] |
| 31. | Cabibbo G, Rizzo GEM, Stornello C, Craxì A. SARS-CoV-2 infection in patients with a normal or abnormal liver. J Viral Hepat. 2021;28:4-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Kushner T, Cafardi J. Chronic Liver Disease and COVID-19: Alcohol Use Disorder/Alcohol-Associated Liver Disease, Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis, Autoimmune Liver Disease, and Compensated Cirrhosis. Clin Liver Dis (Hoboken). 2020;15:195-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 33. | Sarin SK, Choudhury A, Lau GK, Zheng MH, Ji D, Abd-Elsalam S, Hwang J, Qi X, Cua IH, Suh JI, Park JG, Putcharoen O, Kaewdech A, Piratvisuth T, Treeprasertsuk S, Park S, Wejnaruemarn S, Payawal DA, Baatarkhuu O, Ahn SH, Yeo CD, Alonzo UR, Chinbayar T, Loho IM, Yokosuka O, Jafri W, Tan S, Soo LI, Tanwandee T, Gani R, Anand L, Esmail ES, Khalaf M, Alam S, Lin CY, Chuang WL, Soin AS, Garg HK, Kalista K, Batsukh B, Purnomo HD, Dara VP, Rathi P, Al Mahtab M, Shukla A, Sharma MK, Omata M; APASL COVID Task Force; APASL COVID Liver Injury Spectrum Study (APCOLIS Study-NCT 04345640). Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study). Hepatol Int. 2020;14:690-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (1)] |
| 34. | Bramante C, Tignanelli CJ, Dutta N, Jones E, Tamariz L, Clark JM, Usher M, Metlon-Meaux G, Ikramuddin S. Non-alcoholic fatty liver disease (NAFLD) and risk of hospitalization for Covid-19. medRxiv. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 35. | Abiri B, Guest PC, Vafa M. Obesity and Risk of COVID-19 Infection and Severity: Available Evidence and Mechanisms. Adv Exp Med Biol. 2021;1321:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Portincasa P, Krawczyk M, Smyk W, Lammert F, Di Ciaula A. COVID-19 and non-alcoholic fatty liver disease: Two intersecting pandemics. Eur J Clin Invest. 2020;50:e13338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 37. | Fricker ZP, Pedley A, Massaro JM, Vasan RS, Hoffmann U, Benjamin EJ, Long MT. Liver Fat Is Associated With Markers of Inflammation and Oxidative Stress in Analysis of Data From the Framingham Heart Study. Clin Gastroenterol Hepatol 2019; 17: 1157-1164. e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 38. | Ji D, Zhang D, Yang T, Mu J, Zhao P, Xu J, Li C, Cheng G, Wang Y, Chen Z, Qin E, Lau G. Effect of COVID-19 on patients with compensated chronic liver diseases. Hepatol Int. 2020;14:701-710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 39. | Kim D, Adeniji N, Latt N, Kumar S, Bloom PP, Aby ES, Perumalswami P, Roytman M, Li M, Vogel AS, Catana AM, Wegermann K, Carr RM, Aloman C, Chen V, Rabiee A, Sadowski B, Nguyen V, Dunn W, Chavin K, Zhou K, Lizaola-Mayo B, Moghe A, Debes J, Lee TH, Branch A, Viveiros K, Chan W, Chascsa D, Kwo P, Dhanasekaran R. Predictors of Outcomes of COVID-19 in Patients with Chronic Liver Disease: US Multi-center Study. Clin Gastroenterol Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 183] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 40. | Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74:567-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 383] [Article Influence: 95.8] [Reference Citation Analysis (0)] |
| 41. | Tsamakis K, Gavriatopoulou M, Schizas D, Stravodimou A, Mougkou A, Tsiptsios D, Sioulas V, Spartalis E, Sioulas AD, Tsamakis C, Charalampakis N, Mueller C, Arya D, Zarogoulidis P, Spandidos DA, Dimopoulos MA, Papageorgiou C, Rizos E. Oncology during the COVID-19 pandemic: challenges, dilemmas and the psychosocial impact on cancer patients. Oncol Lett. 2020;20:441-447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 42. | Xia Y, Jin R, Zhao J, Li W, Shen H. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21:e180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 307] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 43. | Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, Jia P, Guan HQ, Peng L, Chen Y, Peng P, Zhang P, Chu Q, Shen Q, Wang Y, Xu SY, Zhao JP, Zhou M. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31:894-901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 819] [Cited by in RCA: 1014] [Article Influence: 202.8] [Reference Citation Analysis (0)] |
| 44. | Colmenero J, Rodríguez-Perálvarez M, Salcedo M, Arias-Milla A, Muñoz-Serrano A, Graus J, Nuño J, Gastaca M, Bustamante-Schneider J, Cachero A, Lladó L, Caballero A, Fernández-Yunquera A, Loinaz C, Fernández I, Fondevila C, Navasa M, Iñarrairaegui M, Castells L, Pascual S, Ramírez P, Vinaixa C, González-Dieguez ML, González-Grande R, Hierro L, Nogueras F, Otero A, Álamo JM, Blanco-Fernández G, Fábrega E, García-Pajares F, Montero JL, Tomé S, De la Rosa G, Pons JA. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74:148-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 270] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 45. | Rabiee A, Sadowski B, Adeniji N, Perumalswami PV, Nguyen V, Moghe A, Latt NL, Kumar S, Aloman C, Catana AM, Bloom PP, Chavin KD, Carr RM, Dunn W, Chen VL, Aby ES, Debes JD, Dhanasekaran R; COLD Consortium. Liver Injury in Liver Transplant Recipients With Coronavirus Disease 2019 (COVID-19): U.S. Multicenter Experience. Hepatology. 2020;72:1900-1911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 46. | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration; UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6366] [Cited by in RCA: 6735] [Article Influence: 1347.0] [Reference Citation Analysis (0)] |
| 47. | Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, Berg T. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2:100113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 334] [Article Influence: 66.8] [Reference Citation Analysis (1)] |
| 48. | Brennan DC, Legendre C, Patel D, Mange K, Wiland A, McCague K, Shihab FS. Cytomegalovirus incidence between everolimus versus mycophenolate in de novo renal transplants: pooled analysis of three clinical trials. Am J Transplant. 2011;11:2453-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 49. | Chew CA, Iyer SG, Kow AWC, Madhavan K, Wong AST, Halazun KJ, Battula N, Scalera I, Angelico R, Farid S, Buchholz BM, Rotellar F, Chan AC, Kim JM, Wang CC, Pitchaimuthu M, Reddy MS, Soin AS, Derosas C, Imventarza O, Isaac J, Muiesan P, Mirza DF, Bonney GK. An international multicenter study of protocols for liver transplantation during a pandemic: A case for quadripartite equipoise. J Hepatol. 2020;73:873-881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 50. | Amaddeo G, Brustia R, Allaire M, Lequoy M, Hollande C, Regnault H, Blaise L, Ganne-Carrié N, Séror O, Larrey E, Lim C, Scatton O, El Mouhadi S, Ozenne V, Paye F, Balladur P, Dohan A, Massault PP, Pol S, Dioguardi Burgio M, Vilgrain V, Sepulveda A, Cauchy F, Luciani A, Sommacale D, Leroy V, Roudot-Thoraval F, Bouattour M, Nault JC; Paris Liver Cancer Group. Impact of COVID-19 on the management of hepatocellular carcinoma in a high-prevalence area. JHEP Rep. 2021;3:100199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 51. | Shiina S, Gani RA, Yokosuka O, Maruyama H, Nagamatsu H, Payawal DA, Dokmeci AK, Lesmana LA, Tanwandee T, Lau G, Sarin SK, Omata M. APASL practical recommendations for the management of hepatocellular carcinoma in the era of COVID-19. Hepatol Int. 2020;14:920-929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 52. | Chan SL, Kudo M. Impacts of COVID-19 on Liver Cancers: During and after the Pandemic. Liver Cancer. 2020;9:491-502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 53. | Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603-2615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10556] [Cited by in RCA: 10644] [Article Influence: 2128.8] [Reference Citation Analysis (1)] |
| 54. | Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T; COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7073] [Cited by in RCA: 7513] [Article Influence: 1878.3] [Reference Citation Analysis (1)] |
| 55. | Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, Bibi S, Briner C, Cicconi P, Collins AM, Colin-Jones R, Cutland CL, Darton TC, Dheda K, Duncan CJA, Emary KRW, Ewer KJ, Fairlie L, Faust SN, Feng S, Ferreira DM, Finn A, Goodman AL, Green CM, Green CA, Heath PT, Hill C, Hill H, Hirsch I, Hodgson SHC, Izu A, Jackson S, Jenkin D, Joe CCD, Kerridge S, Koen A, Kwatra G, Lazarus R, Lawrie AM, Lelliott A, Libri V, Lillie PJ, Mallory R, Mendes AVA, Milan EP, Minassian AM, McGregor A, Morrison H, Mujadidi YF, Nana A, O'Reilly PJ, Padayachee SD, Pittella A, Plested E, Pollock KM, Ramasamy MN, Rhead S, Schwarzbold AV, Singh N, Smith A, Song R, Snape MD, Sprinz E, Sutherland RK, Tarrant R, Thomson EC, Török ME, Toshner M, Turner DPJ, Vekemans J, Villafana TL, Watson MEE, Williams CJ, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ; Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3551] [Cited by in RCA: 3426] [Article Influence: 856.5] [Reference Citation Analysis (0)] |
| 56. | Cornberg M, Buti M, Eberhardt CS, Grossi PA, Shouval D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. 2021;74:944-951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 174] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 57. | Fix OK, Blumberg EA, Chang KM, Chu J, Chung RT, Goacher EK, Hameed B, Kaul DR, Kulik LM, Kwok RM, McGuire BM, Mulligan DC, Price JC, Reau NS, Reddy KR, Reynolds A, Rosen HR, Russo MW, Schilsky ML, Verna EC, Ward JW, Fontana RJ; AASLD COVID-19 Vaccine Working Group. AASLD Expert Panel Consensus Statement: Vaccines to Prevent COVID-19 Infection in Patients with Liver Disease. Hepatology. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 137] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 58. | British Society of Gastroenterology. A joint statement on vaccination for SARS-CoV-2 in patients with Liver diseases. Updated 03 March 2021. [cited 5 March 2021]. Available from: https://www.bsg.org.uk/covid-19-advice/a-joint-statement-on-vaccination-for-sars-cov2-in-patients-with-liver-disease/. |