Published online Mar 27, 2021. doi: 10.4254/wjh.v13.i3.384
Peer-review started: January 6, 2021
First decision: January 25, 2021
Revised: January 31, 2021
Accepted: March 10, 2021
Article in press: March 10, 2021
Published online: March 27, 2021
Processing time: 72 Days and 23.5 Hours
Hepatocellular carcinoma (HCC) accompanied by portal vein tumour thrombus (PVTT) presents an aggressive disease course, worsening liver function reserve, and a high recurrence rate. Clinical practice guidelines recommend systemic therapy as the first-line option for HCC with portal invasion. However, to achieve longer survival in these patients, the treatment strategy should be concluded with removal of the tumour by locoregional therapy. We experienced a case of initially unresectable HCC with main PVTT converted to radical hepatectomy after lenvatinib treatment.
A 59-year-old male with chronic hepatitis C infection visited our clinic as a regular post-surgery follow-up. Contrast-enhanced abdominal computed tomography revealed a liver mass diffusely located at the lateral segment with a massive PVTT extending from the umbilical portion to the main and contralateral third-order portal branches. With the diagnosis of unresectable HCC with Vp4 (main trunk/contralateral branch) PVTT, lenvatinib was started at 12 mg/d. The computed tomography taken 3 mo after starting lenvatinib showed regression of the PVTT, which had retreated to the contralateral first-order portal branch. He tolerated the full dose without major adverse effects. With cessation of lenvatinib for 7 d, radical left lobectomy and PVTT thrombectomy were conducted. The patient’s postoperative course was uneventful. Microscopically, the primary lesion showed fibrotic changes, with moderately to poorly differentiated tumour cells surrounded by granulation tissues in some areas. The majority of the PVTT showed necrosis. He was alive without recurrence for 8 mo.
This is the first case of HCC with Vp4 PVTT in which radical conversion hepatectomy was succeeded after lenvatinib treatment.
Core Tip: Patients with hepatocellular carcinoma (HCC) with portal vein tumour thrombus demonstrate an aggressive disease course, decreased liver function reserve, and higher recurrence rates after treatment. Clinical practice guidelines recommend systemic therapy as the first-line option for HCC with portal invasion. However, to achieve longer survival in these patients, the treatment strategy should be concluded with removal of the tumour. We report the first case of HCC with main portal vein tumour thrombus, in which radical conversion hepatectomy was successfully performed after lenvatinib treatment. Lenvatinib has several strengths that validate its use for targeting conversion hepatectomy for unresectable HCC.
- Citation: Takahashi K, Kim J, Takahashi A, Hashimoto S, Doi M, Furuya K, Hashimoto R, Owada Y, Ogawa K, Ohara Y, Akashi Y, Hisakura K, Enomoto T, Shimomura O, Noguchi M, Oda T. Conversion hepatectomy for hepatocellular carcinoma with main portal vein tumour thrombus after lenvatinib treatment: A case report. World J Hepatol 2021; 13(3): 384-392
- URL: https://www.wjgnet.com/1948-5182/full/v13/i3/384.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i3.384
Portal vein tumour thrombus (PVTT) is a condition of hepatocellular carcinoma (HCC) that leads to the wide dissemination of tumours throughout the liver and causes a deterioration of liver function, leading to poor prognosis. PVTT is classified as Vp1 (segmentary), Vp2 (secondary order branch), Vp3 (first order branch), and Vp4 (main trunk/contralateral branch)[1], and clinical practice guidelines recommend systemic therapy as the first-line option for HCC with portal invasion[2,3]. Current systemic therapy for HCC consists of receptor tyrosine kinase inhibitors (TKIs) and checkpoint inhibitors[4]. As a newly introduced TKI, lenvatinib is a multitargeted TKI that inhibits vascular endothelial growth factor receptor 1-3, platelet-derived growth factor receptor-alpha, rearranged during transfection, and stem cell factor receptor. Lenvatinib is characterized by high tumour regression and tumour necrosis effects[4,5]. However, post progression survival is recognized as being short[6], and the post hoc exploratory analysis disclosed severe morbidities related to lenvatinib treatment in patients with HCC with Vp4 PVTT (data not shown). To achieve longer survival in patients with advanced HCC, the treatment strategy should be concluded with removal of the tumour by locoregional therapy (LRT) because of the limitation of systemic therapy alone[7-9]. Here, we present a case of initially unresectable HCC with Vp4 PVTT converted to radical hepatectomy after lenvatinib treatment.
A 59-year-old male presented to our clinic as a regular post-surgery follow-up for HCC.
The patient received segmentectomy 5 and cholecystectomy for a single HCC 2 years prior.
He had hepatitis C virus infection with genotypes 1a which was treated with 24 wk of ledipasvir/sofosbuvir 5 years prior, and sustained virologic response rate was achieved. He received the radiofrequency ablation a year before the first hepatectomy.
The patient had a history of alcohol use with 200 mL daily intake for 35 years. Since HCC was diagnosed, the patient had quitted alcohol drinking. He had no family history of cancer.
The patient’s temperature was 36.5 °C, heart rate was 74 bpm, respiratory rate was 14 breath/min, blood pressure was 128/81 mmHg and oxygen saturation in room air was 98%. There was an operative scar for a J-shaped incision on the abdomen from the previous liver resection. No ascites and encephalopathy were detected.
Laboratory exams were normal except for a slight increase in aspartate aminotransferase levels of 52 U/L and protein induced by des-γ-carboxy prothrombin of 107 mAU/mL. Electrocardiogram, chest X-ray and arterial blood gas were also normal.
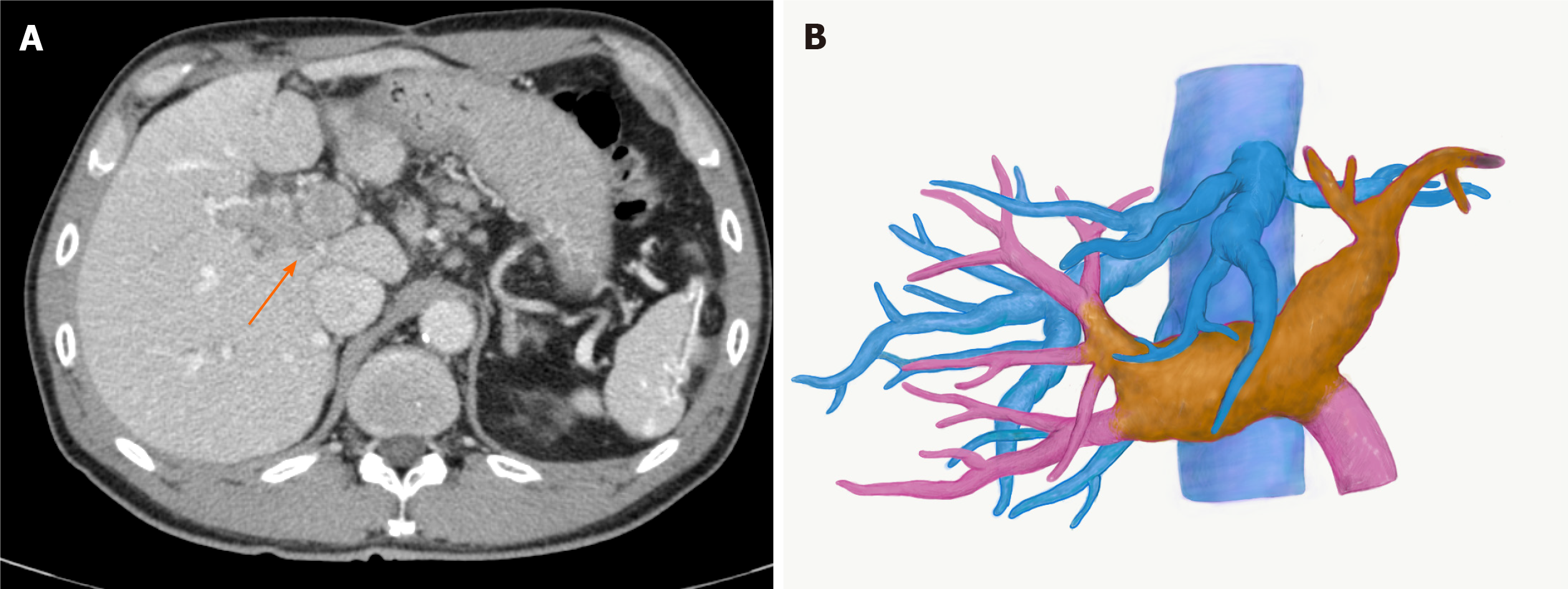
Contrast-enhanced (CE) abdominal computed tomography (CT) revealed a liver mass diffusely located at the lateral segment with a massive PVTT extending from the umbilical portion to the main portal and the contralateral third portal branches (Vp4) (Figure 1A and B).
As a treatment strategy, we should administer lenvatinib at a dose of 12 mg, following the clinical guidelines. The reason for choosing lenvatinib, not sorafenib was that lenvatinib demonstrated higher response rate compared with sorafenib in an open-label, phase III, multicentre, non-inferiority trial involving patients with advanced HCC (the REFLECT trial). If the PVTT exhibited shrinkage to the contralateral first portal branch, we would be able to remove the tumour surgically. We should be careful to follow the liver function during lenvatinib treatment, since the post hoc exploratory analysis revealed severe morbidities including liver failure in cases with Vp4 PVTT.
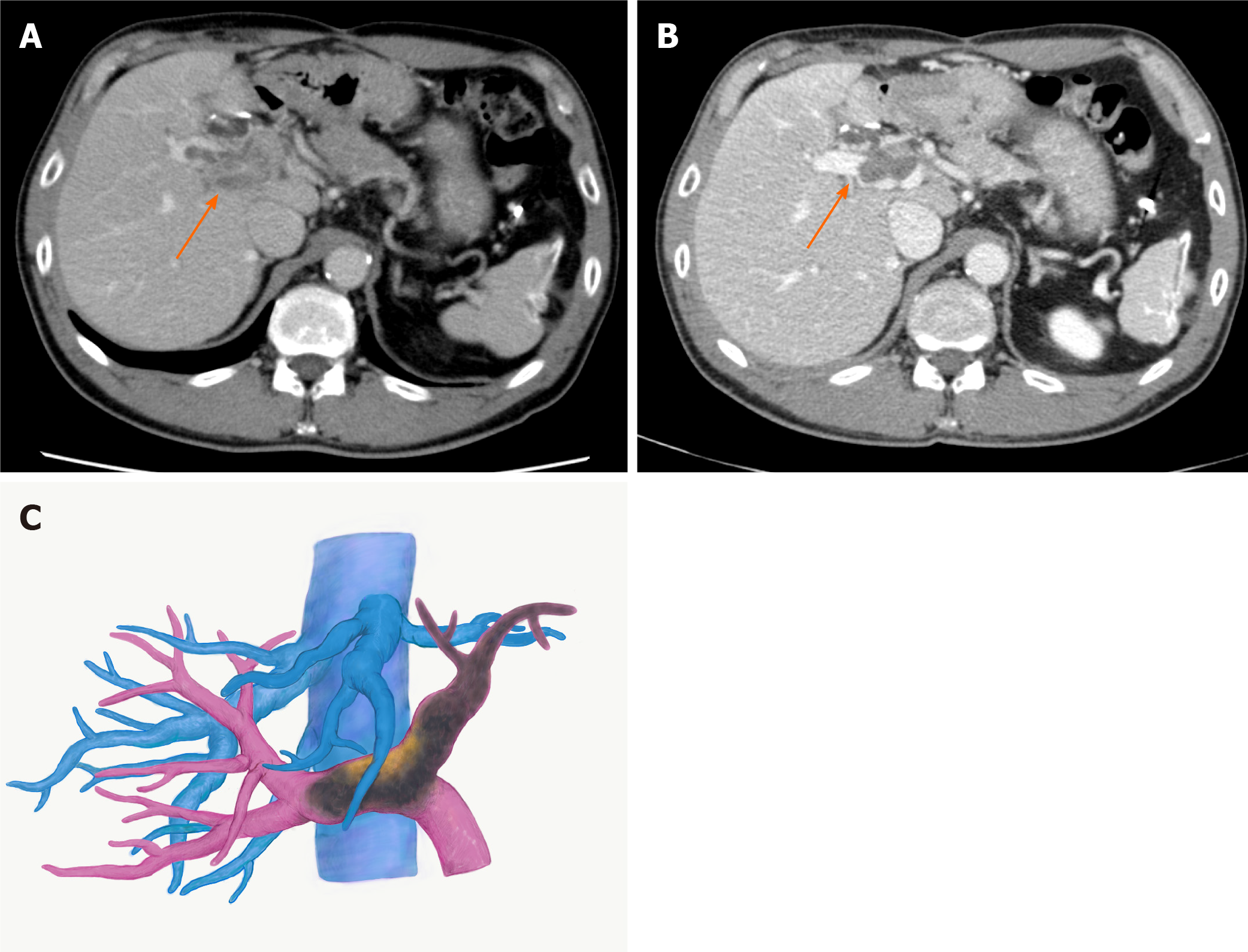
With the diagnosis of unresectable HCC with Vp4 PVTT, lenvatinib was started at 12 mg/d. CT taken two weeks after starting lenvatinib showed regression of PVTT (by 11%) with partial disappearance of contrast enhancement, retreating to the contralateral second-order PV (Figure 2A). At 3 mo, the PVTT regressed further to the contralateral first-order branch with more loss of contrast enhancement (by 58%), meeting the definition of partial response according to the modified Response Evaluation Criteria in Solid Tumours criteria (Figure 2B and C). During lenvatinib treatment, liver function was maintained within Child-Pugh A (5 points), and the albumin-bilirubin (ALBI) score was -3.45 to -2.93 (Grade 1). He tolerated the full dose without treatment-related adverse effects (TRAEs).
After cessation of lenvatinib for 7 d, left lobectomy with PVTT thrombectomy was performed. Intraoperatively, no intrahepatic satellite lesions, ascites or disseminated nodules were identified. The left hepatic artery, left PV and left hepatic duct were isolated at the hilum. After ligating and disconnecting the left hepatic artery, the right, left and main PVs were exposed. After checking the PVTT by ultrasound, the PVs were clamped by Satinsky forceps (Figure 3A). Venotomy was placed at the bifurcation of the left PV, and the PVTT was thrombectomized (Figure 3B). After flushing the PV with normal saline and confirming that no PVTT remained, the left PV stump was closed by 6-0 proline (Figure 3C). Liver dissection was completed along the middle hepatic vein. The left hepatic duct and hepatic vein were cut and closed with 6-0 proline. The specimen was finally removed.
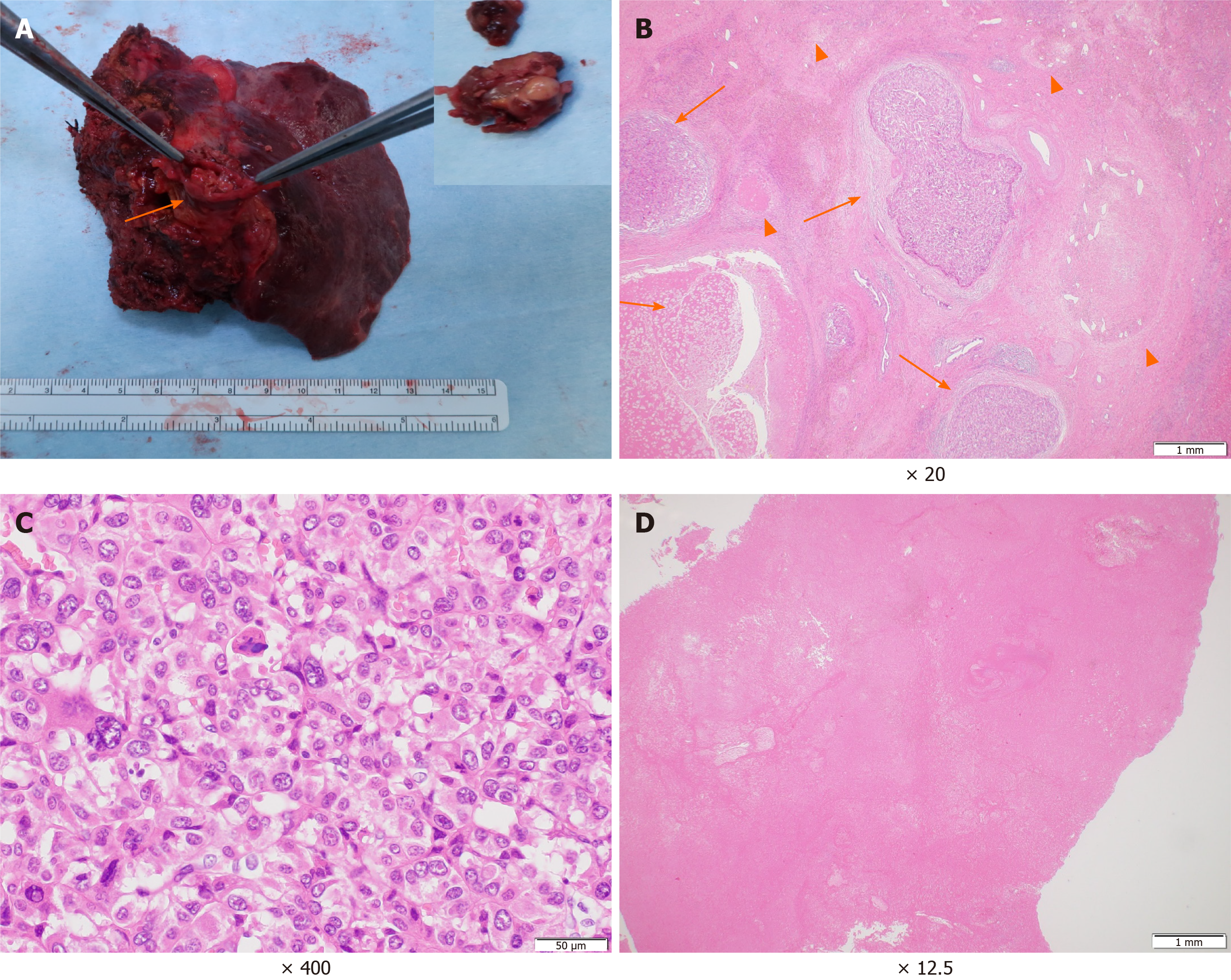
The patient’s postoperative course was uneventful. Macroscopically, the primary tumour at the parenchyma was obscure. The PVTT demonstrated a white to brownish nodule with a size of 60 mm × 30 mm × 25 mm (Figure 4A). Microscopically, the primary lesion demonstrated fibrotic changes with haemosiderin deposition. In some areas, moderately to poorly differentiated tumour cells and tumour cells with necrotic changes were surrounded by granulation tissues and fibrosis (Figure 4B and C). The majority of the PVTT showed necrosis (Figure 4D). According to the Union for International Cancer Control classification, the tumour was finally staged as T3 N0 M0 Stage IIIB. He is alive with no evidence of recurrence 8 mo post-surgery.
Patients with HCC with PVTT usually have an aggressive disease course, decreased liver function reserve, limited treatment options, higher recurrence rates after treatment and poor overall survival (OS). The median OS is reported to be as poor as 2-4 mo with best supporting care[10]. The Barcelona Clinic Liver Cancer (BCLC) staging classifies patients with PVTT with Child-Pugh A or B liver function reserve as advanced HCC with BCLC stage C. The recommended treatment option for this group is systemic therapy with sorafenib or lenvatinib as the first-line treatment[4]. LRT including hepatectomy was not recommended over systemic therapy since there was inadequate evidence to inform the balance of benefit vs harm[2]. LRT could only be considered for HCC with Vp1/2, only as an option within research settings[2]. In a Japanese nationwide surveillance study consisting of more than 6000 patients with HCC with PVTT, propensity score matching analysis demonstrated a longer median OS in the surgical group than in the non-surgery group (2.87 years vs 1.10 years, P < 0.001)[11]. However, surgical benefit was acknowledged when PVTT was limited to the first-order branch (Vp3), and no surgical benefit was observed among patients with Vp4 PVTT. The problem in this study was that more than half of the patients with Vp3/4 underwent non-curative resection, and the impact of curative resection was not clarified in this cohort. Several retrospective studies have demonstrated survival benefits of curative hepatectomies with aggressive PV thrombectomy or en block resection for Vp4 PVTT[12-14]. Hatano et al[14] conducted a retrospective multi-institutional study regarding the outcome of macroscopically curative hepatic resection in 400 patients with HCC with Vp3/4 PVTT. The results demonstrated a median survival time and 5-year OS rate of 21.5 mo and 25.7%, respectively. OS time showed no statistically significant difference between Vp3 and Vp4.
Lenvatinib was initially approved as the first-line therapy for advanced HCC in Japan in 2018. The REFLECT trial met its primary endpoint of non-inferiority to sorafenib in OS[6]. Lenvatinib was superior to sorafenib in progression-free survival (PFS) and time to tumour progression (TTP). Although the complete response rate was low, the objective response rate (ORR) in the lenvatinib group was significantly higher than that in the sorafenib group (40.6% vs 12.4%, P < 0.001). TRAEs, including hand-foot syndrome, hypertension, proteinuria, and anorexia, were comparable between lenvatinib and sorafenib. These side effects are not life-threatening, and they can usually be controlled by supportive medical treatments. Subsequent studies have reported a relatively high ORR with lenvatinib of 29.4%-45.0%[15-17]. On the other hand, the REFLECT trial excluded HCC cases that had main PV invasion, and the outcomes of this cohort were unclear. The efficacy of lenvatinib treatment for unresectable HCC with major PVTT has been reported in some case reports and retrospective studies[18-20]. Kuzuya et al[20] compared the outcomes of advanced HCC with Vp3/4 PVTT between sorafenib and lenvatinib as the first-line systemic therapy. The ORR was significantly higher using lenvatinib (53.8% vs 14.3% P = 0.0193), and the median OS and TTP were significantly longer in the lenvatinib group than in the sorafenib group. No patient discontinued lenvatinib treatment secondary to TRAEs. These reports may characterize lenvatinib as having a relatively strong antitumour effect against HCC including PVTT, with less emergence of serious side effects.
Other characteristics of lenvatinib treatment are the rapid antitumour effects and preservation and fast recovery of liver function[20]. The antitumour effects of lenvatinib have been described as quick, which could be confirmed in 2 wk, and these early radiologic changes could be biomarkers to predict clinical outcomes, including OS[16]. Another group similarly stated that the changes in arterial tumour perfusion on CE-ultrasound at 1 wk were associated with the radiological antitumour response on CE-CT at 8 wk[21]. Regarding the preservation of liver function, patients treated with lenvatinib maintained liver functional reserves better than those treated with sorafenib[22]. Furthermore, ALBI scores in the lenvatinib group improved faster than those in the sorafenib group[20]. In our case, the patient tolerated the full dose while maintaining liver function without major side effects. The tumour including the PVTT showed early necrotic changes 2 wk after lenvatinib treatment.
Based on these reports, lenvatinib is characterized by the following strengths: (1) Relatively strong antitumour effect not only on the main tumour but also on PVTT; (2) Quick antitumour effects that could be noted in 1-2 wk; and (3) Preservation and early recovery of liver function with less incidence of life-threatening TRAEs. Because of these strengths, lenvatinib can be considered an optimal chemotherapeutic agent targeting radical conversion hepatectomy for unresectable HCC. The good indication might be unresectable HCC with a large size or with PV invasion. Multiple intra-extra hepatic HCC can be considered as long as curative resection is feasible, since pathological complete response is usually difficult to attain by lenvatinib alone, and the tumours can quickly regrow during the drug cessation period[19,23]. Lenvatinib demonstrates quick antitumour effects, and it deteriorates liver function temporally[17]. The treatment effects on the tumour and liver function reserve should be evaluated in a short period to avoid missing the best timing for conversion. Since severe morbidities related to lenvatinib treatment were reported in advanced HCC with PVTT (data not shown), physicians should be reminded to perform careful observation during the treatment period, especially in cases with Vp3/4 PVTT, since liver function could deteriorate quickly.
Identification of serum biomarkers for the prediction of lenvatinib response would be of significant benefit for the proper selection of patients for treatment. The post hoc exploratory analysis of the REFLECT trial revealed that the occurrence of hypertension, diarrhoea, proteinuria, or hypothyroidism was generally associated with longer OS in patients with unresectable HCC treated with lenvatinib[24]. Another group stated that maintaining a higher relative dose intensity (RDI) in the early period after starting lenvatinib was associated with a higher ORR and longer PFS[25]. In our case, the patient did not complain of any TRAEs that deteriorated his quality of life, and he could continue lenvatinib with RDI of 100% without decreasing the lenvatinib dose. It is reasonable to think that the high RDI might be the main reason for this significant antitumour effect, leading to PR and conversion hepatectomy.
Four conversion cases with lenvatinib treatment, including ours, were reported in the previous literature (Table 1)[23,25,26]. Three cases were treated with lenvatinib monotherapy, and one case was treated with a combination of lenvatinib and nivolmab. Unresectable factors in these cases were large tumour size with inadequate residual liver volume, lung metastasis, and Vp4 PVTT. The duration of lenvatinib treatment in cases of large tumours and PVTT cases was short, 3-6 mo, and RDIs before conversion were all high (over 70%) in these cases. All cases demonstrated good postoperative courses with no evidence of tumour recurrence. Since the length of its market use is still short, it is necessary to gain experience and cases to clarify which cohort is suitable for targeting conversion.
| Ref. | Age/sex | Background disease | Regimen | Reason for unresectivity | Former treatment | Child-Pugh classification | Duration | RDI (%) | Type of hepatectomy | Prognosis |
| Sato et al[23] (2019) | 66/F | HCV | Lenvatinib | Large size | TACE | 8 (B) | 6 mo | 70 | Extended right hepatectomy | 3 mo alive with no recurrence |
| Chen et al[26] (2019) | 69/F | HBV | Lenvatinib, nivolmab | Large size | Sorafenib TACE | 8 (B) | 3.5 mo | 100 | Extended right hepatectomy | 3 mo alive with no recurrence |
| Takahashi et al[25] (2019) | 82/F | Non B/C | Lenvatinib | Lung metastasis | None | 5 (A) | 13 mo | 38 | Extended posterior segmentectomy | 5 mo alive with no recurrence |
| Present study | 59/M | HCV | Lenvatinib | PVTT (Vp4) | None | 5 (A) | 3 mo | 100 | Left hepatectomy | 8 mo alive with no recurrence |
Recently, there have been reports regarding the efficacy of proton beam therapy for advanced HCC with PVTT[27,28]. Proton beam therapy has advantages in that it is less invasive to patients; however, it requires high medical expenses and a large-scale facility that is not widely available worldwide. Because of its strong and quick antitumour effects with fewer TRAEs, conversion hepatectomy using lenvatinib could be an ideal strategy. A clinical trial is currently underway in Japan regarding conversion surgery during lenvatinib administration for unresectable HCC. Several molecular targeting agents and checkpoint inhibitors are being developed and will be coming to the market soon. These sequential flows could explore a new strategy against unresectable HCC.
In conclusion, we experienced the first case of HCC with Vp4 PVTT in which radical conversion hepatectomy was successfully performed after lenvatinib treatment. Lenvatinib has several strengths that validate its use for targeting radical conversion hepatectomy for unresectable HCC. A multicentre prospective trial is needed to clarify its clinical utility.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kang KJ, Kumar R S-Editor: Gao CC L-Editor: A P-Editor: Li JH
| 1. | Shi J, Lai EC, Li N, Guo WX, Xue J, Lau WY, Wu MC, Cheng SQ. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2010;17:2073-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 2. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6063] [Article Influence: 866.1] [Reference Citation Analysis (3)] |
| 3. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3242] [Article Influence: 463.1] [Reference Citation Analysis (1)] |
| 4. | Doycheva I, Thuluvath PJ. Systemic Therapy for Advanced Hepatocellular Carcinoma: An Update of a Rapidly Evolving Field. J Clin Exp Hepatol. 2019;9:588-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 5. | Liu PH, Huo TI, Miksad RA. Hepatocellular Carcinoma with Portal Vein Tumor Involvement: Best Management Strategies. Semin Liver Dis. 2018;38:242-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 6. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib vs sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3830] [Article Influence: 547.1] [Reference Citation Analysis (1)] |
| 7. | Ye JZ, Wang YY, Bai T, Chen J, Xiang BD, Wu FX, Li LQ. Surgical resection for hepatocellular carcinoma with portal vein tumor thrombus in the Asia-Pacific region beyond the Barcelona Clinic Liver Cancer treatment algorithms: a review and update. Oncotarget. 2017;8:93258-93278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Zhang ZY, Dong KS, Zhang EL, Zhang LW, Chen XP, Dong HH. Resection might be a meaningful choice for hepatocellular carcinoma with portal vein thrombosis: A systematic review and meta-analysis. Medicine (Baltimore). 2019;98:e18362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Peng SY, Wang XA, Huang CY, Li JT, Hong DF, Wang YF, Xu B. Better surgical treatment method for hepatocellular carcinoma with portal vein tumor thrombus. World J Gastroenterol. 2018;24:4527-4535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (5)] |
| 10. | Chan SL, Chong CC, Chan AW, Poon DM, Chok KS. Management of hepatocellular carcinoma with portal vein tumor thrombosis: Review and update at 2016. World J Gastroenterol. 2016;22:7289-7300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 133] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 11. | Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, Kudo M, Ku Y, Sakamoto M, Nakashima O, Kaneko S, Kokudo N; Liver Cancer Study Group of Japan. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol. 2016;65:938-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 365] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 12. | Fukumoto T, Kido M, Takebe A, Tanaka M, Kinoshita H, Kuramitsu K, Komatsu S, Tsugawa D, Goto T, Asari S, Toyama H, Ajiki T, Ku Y. New macroscopic classification and back-flow thrombectomy for advanced hepatocellular carcinoma with portal vein tumor thrombus invading the contralateral second portal branch. Surg Today. 2017;47:1094-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Chok KS, Cheung TT, Chan SC, Poon RT, Fan ST, Lo CM. Surgical outcomes in hepatocellular carcinoma patients with portal vein tumor thrombosis. World J Surg. 2014;38:490-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Hatano E, Uemoto S, Yamaue H, Yamamoto M; Japanese Society of Hepato-Biliary-Pancreatic Surgery. Significance of hepatic resection and adjuvant hepatic arterial infusion chemotherapy for hepatocellular carcinoma with portal vein tumor thrombus in the first branch of portal vein and the main portal trunk: a project study for hepatic surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2018;25:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Kodama K, Kawaoka T, Namba M, Uchikawa S, Ohya K, Morio K, Nakahara T, Murakami E, Yamauchi M, Hiramatsu A, Imamura M, Chayama K, Aikata H. Correlation between Early Tumor Marker Response and Imaging Response in Patients with Advanced Hepatocellular Carcinoma Treated with Lenvatinib. Oncology. 2019;97:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Kuzuya T, Ishigami M, Ito T, Ishizu Y, Honda T, Ishikawa T, Fujishiro M. Favorable radiological antitumor response at 2 weeks after starting lenvatinib for patients with advanced hepatocellular carcinoma. Hepatol Res. 2020;50:374-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Hiraoka A, Kumada T, Kariyama K, Takaguchi K, Atsukawa M, Itobayashi E, Tsuji K, Tajiri K, Hirooka M, Shimada N, Shibata H, Ishikawa T, Ochi H, Tada T, Toyoda H, Nouso K, Tsutsui A, Itokawa N, Imai M, Joko K, Hiasa Y, Michitaka K; Real-life Practice Experts for HCC (RELPEC) Study Group; HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan). Clinical features of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions: Multicenter analysis. Cancer Med. 2019;8:137-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 18. | Kosaka Y, Kawaoka T, Aikata H, Suehiro Y, Yamaoka K, Ando Y, Namba M, Takeuchi Y, Fujii Y, Uchikawa S, Kodama K, Oya K, Morio K, Fujino H, Nakahara T, Murakami E, Yamauchi M, Tsuge M, Hiramatsu A, Imamura M, Baba Y, Awai K, Kimura T, Nagata Y, Chayama K. A case of advanced HCC treated with lenvatinib after hepatic arterial infusion chemotherapy combined with radiation therapy treatment for portal vein tumor thrombosis in the main trunk. Clin J Gastroenterol. 2020;13:839-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Takeda H, Nishijima N, Nasu A, Komekado H, Kita R, Kimura T, Kudo M, Osaki Y. Long-term antitumor effect of lenvatinib on unresectable hepatocellular carcinoma with portal vein invasion. Hepatol Res. 2019;49:594-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Kuzuya T, Ishigami M, Ito T, Ishizu Y, Honda T, Ishikawa T, Fujishiro M. Sorafenib vs. Lenvatinib as First-line Therapy for Advanced Hepatocellular Carcinoma With Portal Vein Tumor Thrombosis. Anticancer Res. 2020;40:2283-2290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 21. | Kuorda H, Abe T, Fujiwara Y, Okamoto T, Yonezawa M, Sato H, Endo K, Oikawa T, Sawara K, Takikawa Y. Change in arterial tumor perfusion is an early biomarker of lenvatinib efficacy in patients with unresectable hepatocellular carcinoma. World J Gastroenterol. 2019;25:2365-2372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Terashima T, Yamashita T, Takata N, Toyama T, Shimakami T, Takatori H, Arai K, Kawaguchi K, Kitamura K, Sakai Y, Mizukoshi E, Honda M, Kaneko S. Comparative analysis of liver functional reserve during lenvatinib and sorafenib for advanced hepatocellular carcinoma. Hepatol Res. 2020;50:871-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Sato N, Beppu T, Kinoshita K, Yuki H, Suyama K, Chiyonaga S, Motohara T, Komohara Y, Hara A, Akahoshi S. Conversion Hepatectomy for Huge Hepatocellular Carcinoma With Arterioportal Shunt After Chemoembolization and Lenvatinib Therapy. Anticancer Res. 2019;39:5695-5701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Sung M, Finn RS, Qin S, Han G, Ikeda K, Cheng A, Kudo M, Tateishi R, Ikeda M, Breder V, Rau K, Ma Y, Alisina A, Ryoo B, Ren Z, Mody K, Ductcus C, Tamai T, Saito K, Piscagilia F. Association between overall survival and adverse events with lenvatinib treatment in patients with hepatocellular carcinoma (REFLECT). J Clin Oncol. 2019;37:317. |
| 25. | Takahashi A, Moriguchi M, Seko Y, Ishikawa H, Yo T, Kimura H, Fujii H, Shima T, Mitsumoto Y, Ishiba H, Takashima H, Nagao Y, Jo M, Arai M, Hara T, Okajima A, Muramatsu A, Morita A, Yoshinami N, Nakajima T, Mitsuyoshi H, Umemura A, Nishikawa T, Yamaguchi K, Itoh Y. Impact of Relative Dose Intensity of Early-phase Lenvatinib Treatment on Therapeutic Response in Hepatocellular Carcinoma. Anticancer Res. 2019;39:5149-5156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 26. | Chen X, Zhang Y, Zhang N, Ge Y, Jia W. Lenvatinib combined nivolumab injection followed by extended right hepatectomy is a feasible treatment for patients with massive hepatocellular carcinoma: a case report. Onco Targets Ther. 2019;12:7355-7359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Sugahara S, Nakayama H, Fukuda K, Mizumoto M, Tokita M, Abei M, Shoda J, Matsuzaki Y, Thono E, Tsuboi K, Tokuuye K. Proton-beam therapy for hepatocellular carcinoma associated with portal vein tumor thrombosis. Strahlenther Onkol. 2009;185:782-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 28. | Lee SU, Park JW, Kim TH, Kim YJ, Woo SM, Koh YH, Lee WJ, Park SJ, Kim DY, Kim CM. Effectiveness and safety of proton beam therapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Strahlenther Onkol. 2014;190:806-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |