Published online Mar 27, 2021. doi: 10.4254/wjh.v13.i3.362
Peer-review started: August 14, 2020
First decision: December 11, 2020
Revised: December 27, 2020
Accepted: March 12, 2021
Article in press: March 12, 2021
Published online: March 27, 2021
Processing time: 217 Days and 9.5 Hours
Tacrolimus trough levels (TTL) during the first weeks after liver transplantation (LT) have been related with long-term renal function and hepatocellular carcinoma recurrence. Nevertheless, the significance of trough levels of tacrolimus during the early post-transplant period for the long-term outcome is under debate
To evaluate the effect of TTL during the first month on the long-term outcomes after LT.
One hundred fifty-five LT recipients treated de novo with once-daily tacrolimus were retrospectively studied. Patients with repeated LT or combined transplantation were excluded as well as those who presented renal dysfunction prior to transplantation and/or those who needed induction therapy. Patients were classified into 2 groups according to their mean TTL within the first month after transplantation: ≤ 10 (n = 98) and > 10 ng/mL (n = 57). Multivariate analyses were performed to assess risk factors for patient mortality.
Mean levels within the first month post-transplant were 7.4 ± 1.7 and 12.6 ± 2.2 ng/mL in the ≤ 10 and > 10 groups, respectively. Donor age was higher in the high TTL group 62.9 ± 16.8 years vs 45.7 ± 17.5 years (P = 0.002) whilst mycophenolate-mofetil was more frequently used in the low TTL group 32.7% vs 15.8% (P = 0.02). Recipient features were generally similar across groups. After a median follow-up of 52.8 mo (range 2.8-81.1), no significant differences were observed in: Mean estimated glomerular filtration rate (P = 0.69), hepatocellular carcinoma recurrence (P = 0.44), de novo tumors (P = 0.77), new-onset diabetes (P = 0.13), or biopsy-proven acute rejection rate (12.2% and 8.8%, respectively; P = 0.50). Eighteen patients died during the follow-up and were evenly distributed across groups (P = 0.83). Five-year patient survival was 90.5% and 84.9%, respectively (P = 0.44), while 5-year graft survival was 88.2% and 80.8%, respectively (P = 0.42). Early TTL was not an independent factor for patient mortality in multivariate analyses.
Differences in tacrolimus levels restricted to the first month after transplant did not result in significant differences in long-term outcomes of LT recipients.
Core Tip: This is a retrospective study to evaluate the effect of early tacrolimus trough levels (TTL) on the long-term outcomes after liver transplantation. Patients were classified into 2 groups according to mean TTL within the first month: ≤ 10 (n = 98) and > 10 ng/mL (n = 57). After a median follow-up of 52.8 mo (range 2.8-81.1), no significant differences were observed in: Mean estimated glomerular filtration rate, hepatocellular carcinoma recurrence, de novo tumors, biopsy-proven acute rejection rate and five-year patient and graft survival. Differences in tacrolimus levels within the first month after liver transplant did not result in significant differences in long-term outcomes.
- Citation: Gastaca M, Ruiz P, Bustamante J, Martinez-Indart L, Ventoso A, Fernandez JR, Palomares I, Prieto M, Testillano M, Salvador P, Senosiain M, Suárez MJ, Valdivieso A. Early tacrolimus exposure does not impact long-term outcomes after liver transplantation. World J Hepatol 2021; 13(3): 362-374
- URL: https://www.wjgnet.com/1948-5182/full/v13/i3/362.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i3.362
Tacrolimus represents the keystone of current immunosuppressive regimens after liver transplantation (LT)[1]. Monitoring of trough drug levels is required to maintain them within the therapeutic range[2]. In the case of LT, there is some debate regarding the significance of trough levels of tacrolimus in the early post-transplant period for the long-term outcome. Initial recommendations were extrapolated from kidney transplantation, but LT does not require the high doses needed to prevent acute cellular rejection (ACR) in other allografts[3]. In this regard, various studies have explored the idea of minimizing initial tacrolimus trough levels (TTL)[4-6].
Mean TTL < 10 ng/mL within the first month after LT was associated with less renal impairment within 1 year in a recent meta-analysis[7]. In this study, tacrolimus concentration between 6 and 10 ng/mL were recommended as more appropriate after LT. Mean TTL > 10 ng/mL within the first month after LT but not thereafter has been also associated with increased risk of hepatocellular carcinoma (HCC) recurrence[8]. High exposure to calcineurin inhibitors was an independent predictor of HCC recurrence by multivariate analysis in this study (RR: 2.82; P = 0.005). Moreover, Rodríguez-Perálvarez et al[3] reported that mean TTL of 7-10 ng/mL during the first two weeks after LT was effective in preventing ACR, and was related with significantly superior results in graft survival than TTL above or below this range. More recently, the survival time of patients with mean TTL < 5 ng/mL during the first four weeks after LT was observed to be significantly shorter than that of patients with higher mean TTL[9]. Despite these studies, the actual role of initial TTL on long-term outcomes after LT is difficult to assess. Retrospective studies did not report TTL during the follow-up period[3,9], and therefore the influence of potential differences among groups in tacrolimus exposure throughout the follow-up cannot be ruled out. In addition, in some reports TTL were maintained different in the study groups not only during the first month but throughout the whole follow-up, though not significantly, with the consequent difference of long-term tacrolimus exposure and the potential influence on the outcomes[4,6,8].
Our experience with the use of once-daily tacrolimus (Tac-QD) de novo after LT has been published[10]. Outstanding long-term patient and graft survival was achieved with the use of de novo Tac-QD in a minimizing immunosuppression protocol in LT recipients. With the aim of assessing the significance of the early post-transplant period in the outcomes of LT, we conducted this study to determine the real role of early TTL within the first month on long-term outcomes after LT.
We conducted a retrospective analysis of a prospectively collected database of patients transplanted between April 2008 and May 2013. A total of 237 consecutive LTs were performed during the study period. Patients in the database with repeated LT (n = 13) or combined transplantation (n = 8) were excluded from this analysis, as were those who died within the first week after LT (n = 5) and those who did not receive Tac-QD for various reasons (n = 11). Patients who presented renal dysfunction prior to transplantation, defined as estimated glomerular filtration rate (eGFR) < 60 mL/ min/1.73 m2, and/or those who needed induction therapy (n = 45 overall) were also excluded to avoid bias in the early TTL measurements due to their particular immunosuppressive protocol with induction therapy and delayed initiation of tacrolimus. Finally, 155 adult LT recipients, whose immunosuppressive therapy was based on Tac-QD de novo, were eligible for this study and were followed up until December 31, 2015. Patients with HCC met the preoperative Milan criteria. To determine the effect of early exposure to tacrolimus on long-term outcomes and renal function, patients were classified into two groups according to their mean TTL during the first month after LT: ≤ 10 ng/mL or > 10 ng/mL. All TTL obtained during the first month were used to define the mean values.
The study was performed in accordance with relevant guidelines and regulation. No organs were procured from prisoners. The prospective database received the approval of the Research Ethics Committee of the Hospital Universitario Cruces, No. CEIC E13/08. All patients gave informed consent to be included in the prospective database; the requirement for specific informed consent was waived because of the retrospective nature of the study.
Initial immunosuppression included Tac-QD and steroids 20 mg/day, except in those patients with diabetes mellitus who were treated with Tac-QD and mycophenolate-mofetil (MMF), avoiding the use of steroids. Tac-QD was administered within the first 24 h after LT, either orally or via a nasogastric tube. Patients considered at risk of renal dysfunction received MMF at a daily dose of 1000-2000 mg. Initial Tac-QD dose was 0.15 mg/kg per day (or 0.1 mg/kg per day if combined with MMF). Subsequent doses were adjusted according to trough levels. Serum tacrolimus levels were monitored regularly every 48 h until discharge. Target TTL were 5-10 ng/mL during the first 3 mo; however, if trough levels were lower but liver function tests were normal, the TacQD dose was not preventively increased. Azathioprine was not used in our patients.
Biliary reconstruction in our patients is performed with end-to-end choledocho-choledochostomy with T-tube. When the patient progresses well, T-tube is closed on postoperative day 3 to avoid the potential effect that biliary diversion might have on TTL. Cholangiography is performed on day 7 and in the third postoperative month before T-tube removal. During these three months, patients are monitored weekly at home after hospital discharge, and also seen every two weeks at the outpatient clinic. Patients are monitored with liver function tests and TTL monthly afterwards until completion of the first year, and every 2-3 mo for a further two years. Stable patients with no relevant comorbidities are seen every 4 to 6 mo from the third year on.
The treating physicians adjusted immunosuppressive treatment according to their clinical judgment. Target TTL were progressively reduced: 4-9 ng/mL from month 3 to 6, 3-8 ng/mL from month 6 to 12, < 7 ng/mL after the first year and < 5 ng/mL after the second year onwards. Immunosuppressive protocol included steroids withdrawal 3-4 mo after transplantation, except in case of autoimmune disease (in which low-dose prednisone 5 mg/day was maintained), and in patients with hepatitis C virus (HCV), in whom withdrawal was delayed until months 12-18. Duration of treatment with MMF depended on side effects and/or clinical requirements. Adherence to treatment was assessed at each visit by asking the patients regarding any deviations from the prescribed regimen.
Outcome variables were: (1) Long-term renal function; (2) Immunosuppression-related morbidity; (3) Patient survival; and (4) Graft survival.
Long-term renal function was assessed by eGFR based on the modification of diet in renal disease formula. K/DIGO guidelines were used to define and classify chronic kidney disease[11]. Metabolic syndrome was defined according to already stablished definitions[12]. Fasting plasma glucose repeatedly > 126 mg/dL was used to define de novo diabetes whilst dyslipidemia was considered when treatment was prescribed for elevated blood cholesterol or triglycerides, and arterial hypertension when antihypertensive treatment was initiated. Patients with HCC met the Milan criteria. ACR was biopsy-proven acute rejection (BPAR) in all cases. BPAR were graded according to the Banff International Consensus Document[13]. Liver biopsy was not performed per protocol but indicated according to clinical evolution. In case of BPAR, tacrolimus exposure was further increased as the initial step. In case of severe rejection or if the graft dysfunction persisted after Tac-QD adjustments, three consecutive daily 500 mg corticosteroid boluses were used. Early graft dysfunction was defined according to previous specifications[14].
Qualitative variables are summarized as percentages and quantitative variables using means and standard deviations or median and interquartile range. Comparisons between frequencies of characteristics among trough-level groups were performed using the Chi-squared test or Fisher test, and continuous variables were compared using the Kruskall-Wallis test. Patient and graft survival were analyzed using the Kaplan-Meier method, in which patients lost to follow-up were censored at their last recorded visit. Graft loss was defined as retransplantation or death with non-functioning graft. Death with functioning graft was censored for the analysis of graft survival. The log-rank test was used to compare survival among the three groups. A univariate Cox regression analysis was performed to identify clinical and treatment factors related with patient survival including all patients in the cohort. Those variables with a P < 0.200 were included in a multivariate Cox regression model. Variables with the higher P value were excluded one by one until all variables had a P < 0.05. The proportional hazard assumption was tested. The statistical methods of this study were reviewed by Lorea Martinez-Indart from Bioinformatics and Statistics Platform, Biocruces Bizkaia Health Research Institute. Statistical analysis was performed using SPSS version 23.0.
All patients were Caucasian and received whole grafts from donation after brain-death. Ninety-eight were included in the ≤ 10 ng/mL group and 57 in the > 10 ng/mL group. A median of 7 samples of TTL (range 5-12) were used to obtain the mean TTL during the first month after transplant. Donor and recipient characteristics of the two groups are summarized in Table 1. Recipient features were generally similar across groups, including age, cause of liver disease, model for end-stage liver disease (MELD) score, baseline kidney function and pre-transplant comorbidities. The only significant difference between groups was the age of the graft donor (older for recipients whose early TTL were > 10 ng/mL); consequently, stroke as the cause of death was more frequent among those donors. Corticosteroids were similarly used in all groups; however, MMF use was significantly more common in the group with TTL ≤ 10 ng/mL.
| ≤ 10 ng/mL, n = 98 | > 10 ng/mL, n = 57 | P value | |
| Donors | |||
| Age, year (mean ± SD) | 54.7 ± 17.5 | 54.7 ± 17.5 | 0.002 |
| Male | 58 (59.2%) | 35 (61.4%) | 0.786 |
| Cause of death | 0.004 | ||
| Stroke | 57 (58.2%) | 48 (84.2%) | |
| Trauma | 27 (27.6%) | 6 (10.5%) | |
| Other | 14 (14.3%) | 3 (5.3%) | |
| Graft steatosis | 19 (19.6%) | 12 (21.1%) | 0.827 |
| Recipients | |||
| Age, years (mean ± SD) | 55.3 ± 8.4 | 53.2 ± 9.8 | 0.227 |
| Male | 81 (82.7%) | 48 (84.2%) | 0.802 |
| MELD (mean ± SD) | 13.1 ± 5.6 | 12.7 ± 5.3 | 0.618 |
| Hepatocellular carcinoma | 45 (45.9%) | 29 (50.9%) | 0.551 |
| Cause of liver disease | 0.283 | ||
| Alcohol abuse | 45 (45.9%) | 24 (42.1%) | |
| HCV | 40 (40.8%) | 18 (31.6%) | |
| HBV | 3 (3.1%) | 5 (8.8%) | |
| Cho/estatic liver disease | 3 (3.1%) | 4 (7%) | |
| Other | 7 (7.1%) | 6 (10.5%) | |
| Medical history (pre LT) | |||
| MDRD-4 (mean ± SD) | 107.8 ± 35.7 | 16.7 ± 33.7 | 0.223 |
| Diabetes mellitus | 18 (18.4%) | 9 (15.8%) | 0.683 |
| Arterial hypertension | 12 (12.2%) | 10 (17.5%) | 0.362 |
| Mean tacrolimus trough levels 1 mo (ng/mL) | 7.38 ± 1.68 | 12.62 ± 2.25 | NA |
| Corticosteroids | 80 (82.5%) | 49 (86.0%) | 0.571 |
| Mycophenolate mofetil | 32 (32.7%) | 9 (15.8%) | 0.024 |
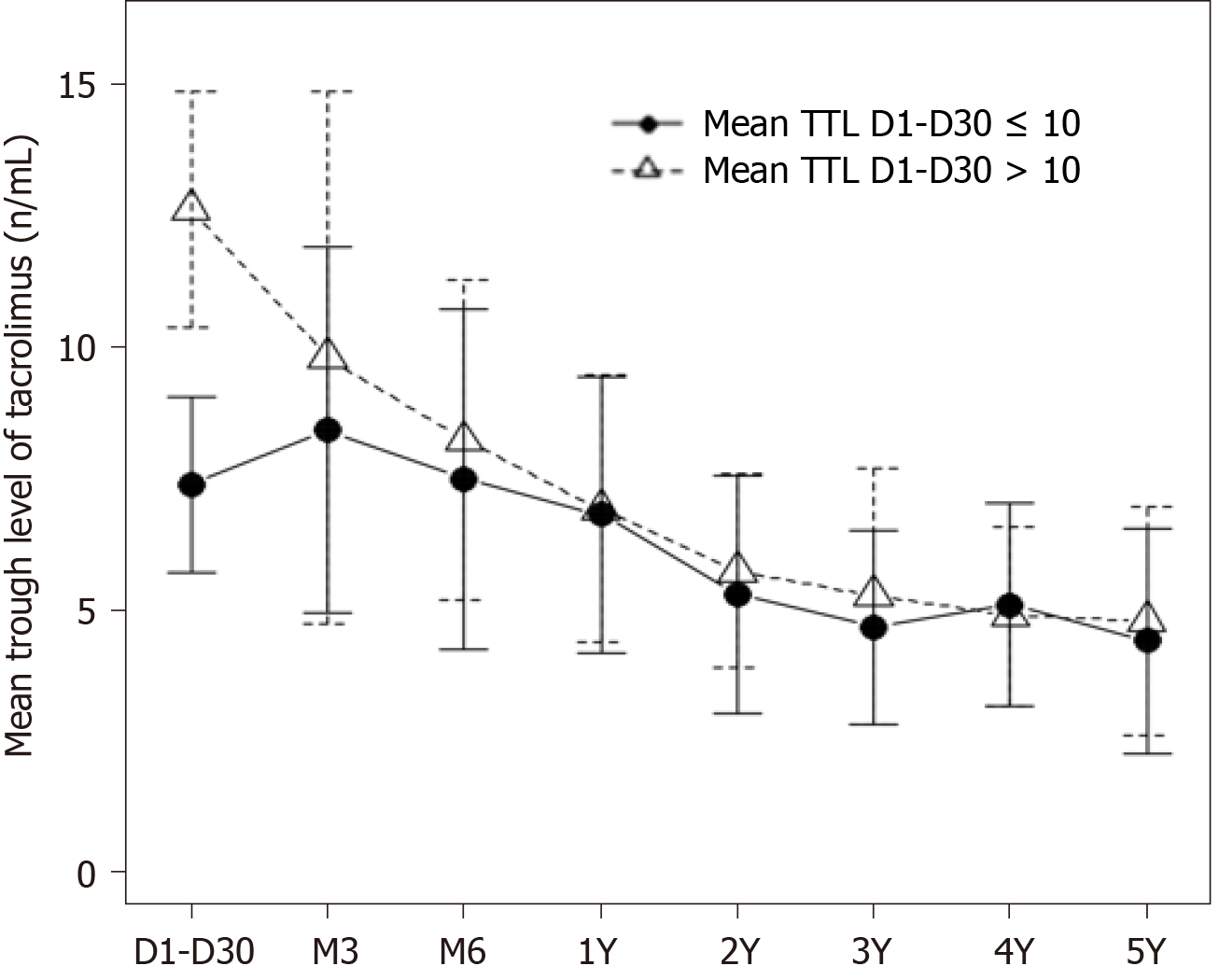
Evolution of mean TTL during the follow-up of the two groups is shown in Figure 1. Mean levels within the first month post-transplant were 7.4 ± 1.7 and 12.6 ± 2.2 ng/mL in the ≤ 10 and > 10 groups, respectively (Table 1). Levels decreased in the > 10 mg/mL group within the first three months and were similar in both groups by the third month. From the third month on, a steady decrease in TTL was observed in both groups. Of note, for the purpose of this study, TTL were significantly different among groups only during the first month after LT, but not during the rest of the follow-up (P = 0.65).
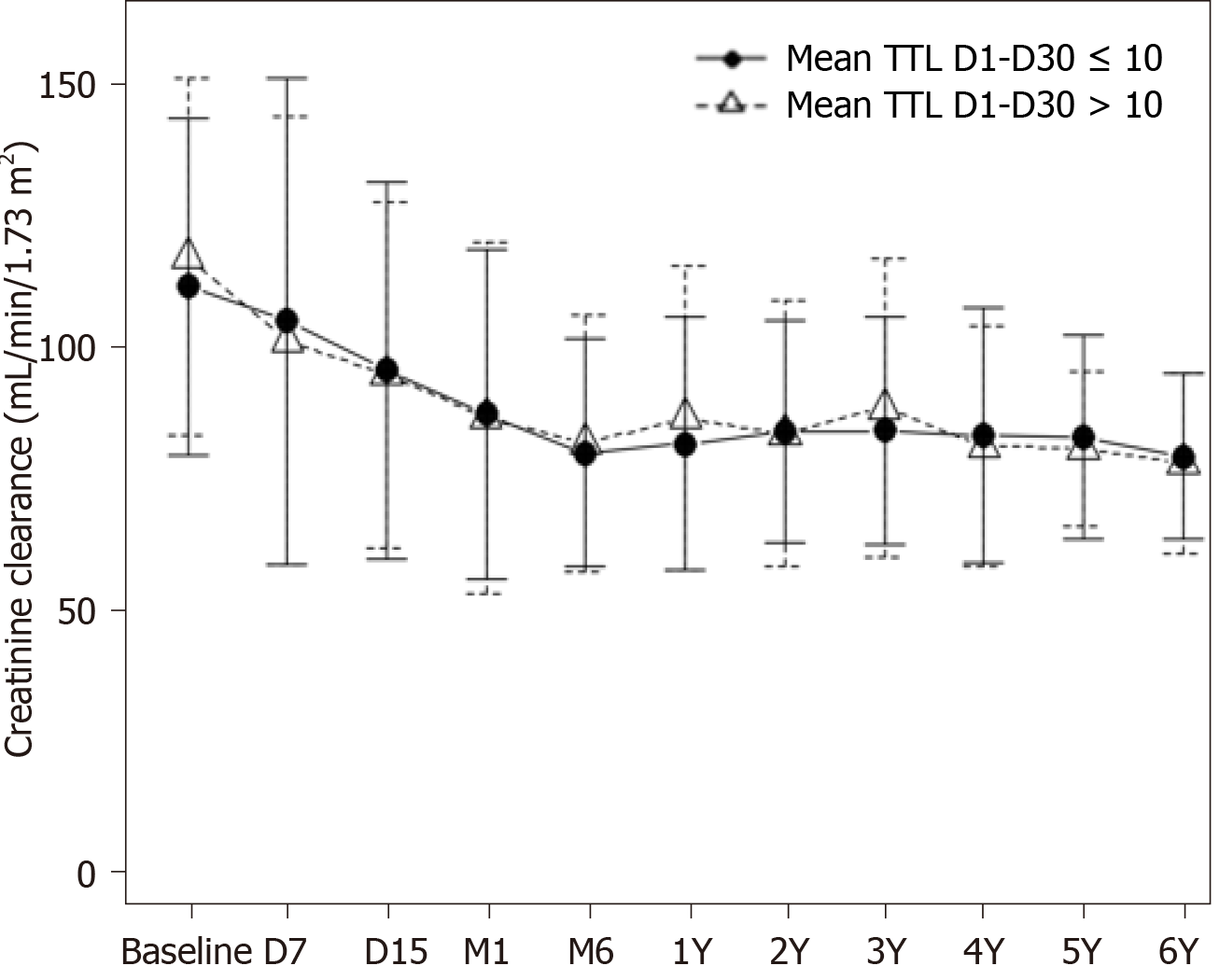
Median follow-up was 52.8 mo (range 2.8-81.1) for those patients with early levels ≤ 10 ng/mL and 52.6 mo (10.8-79.1) for patients with tacrolimus mean levels > 10 ng/mL. Patient outcomes after transplantation are summarized in Table 2. There were no statistically or clinically relevant differences among groups. Mean TTL during the early post-transplant period did not affect renal function. Creatinine clearance fell in parallel in both groups (P = 0.67), decreasing similarly during the first 6 mo to remain steady thereafter until the end of follow-up, at mean levels of approximately 80 mL/min/1.73 m2 in all groups (Figure 2). Patients with higher levels within the first month after LT did not present more immunosuppression-related toxicity including new-onset diabetes, hypertension, HCC recurrence or de novo tumors. BPAR occurred with low and similar frequency in all groups (12.2%, and 8.8% in ≤ 10 and > 10 mg/mL, respectively; P = 0.50). Only 10 patients were treated with corticosteroid boluses (8 (66.7%) and 2 (40.0%), respectively; P = 0.99), and the rest responded to tacrolimus dose escalation. There was no relationship between the decision to withdraw tacrolimus during follow-up and the initial trough level.
| ≤ 10 ng/mL, n = 98 | > 10 ng/mL, n = 57 | P value | |
| Biopsy-proven acute rejection | 12 (12.2%) | 5(8.8%) | 0.505 |
| Arterial complications | 12 (12.2%) | 7(12.3%) | 0.995 |
| Biliary complications | 13 (13.3%) | 8 (14%) | 0.893 |
| Infection (any) | 49(50.0%) | 26 (45.6%) | 0.598 |
| Cytomegalovirus infection | 26 (26.5%) | 12 (21.1%) | 0.445 |
| Retransplantation | 5 (5.1%) | 5 (8.8%) | 0.500 |
| HCC recurrence1 | 1 (2.3%) | 0 | 0.999 |
| HCV recurrence2 | 35 (87.5%) | 14 (77.8%) | 0.438 |
| De novo tumor | 10 (10.2%) | 5 (8.8%) | 0.771 |
| New-onset arterial hypertension | 35(36.1%) | 19 (36.5%) | 0.827 |
| New-onset diabetes | 21 (21.6%) | 6 (12.7%) | 0.127 |
| Tacrolimus withdrawal. Causes: | 18 (18.4%) | 8 (14.0%) | 0.486 |
| Kidney failure | 7 | 1 | |
| Neurotoxicity | 1 | 2 | |
| Metabolic syndrome | 6 | 4 | |
| Metabolic synd + kidney failure | 1 | - | |
| Other | 3 | 1 | |
| MDRD-4 at 5 yr (mean ± SD) | 82.5 ± 19.4 | 80.32 ± 14.7 | 0.686 |
| Deaths. Causes: | 10 (10.2%) | 8 (14.0%) | 0.827 |
| HCV recurrence | 5 | 3 | |
| De novo tumor | 1 | 2 | |
| Sepsis | 2 | 1 | |
| Stroke | 0 | 1 | |
| Other | 2 | 1 |
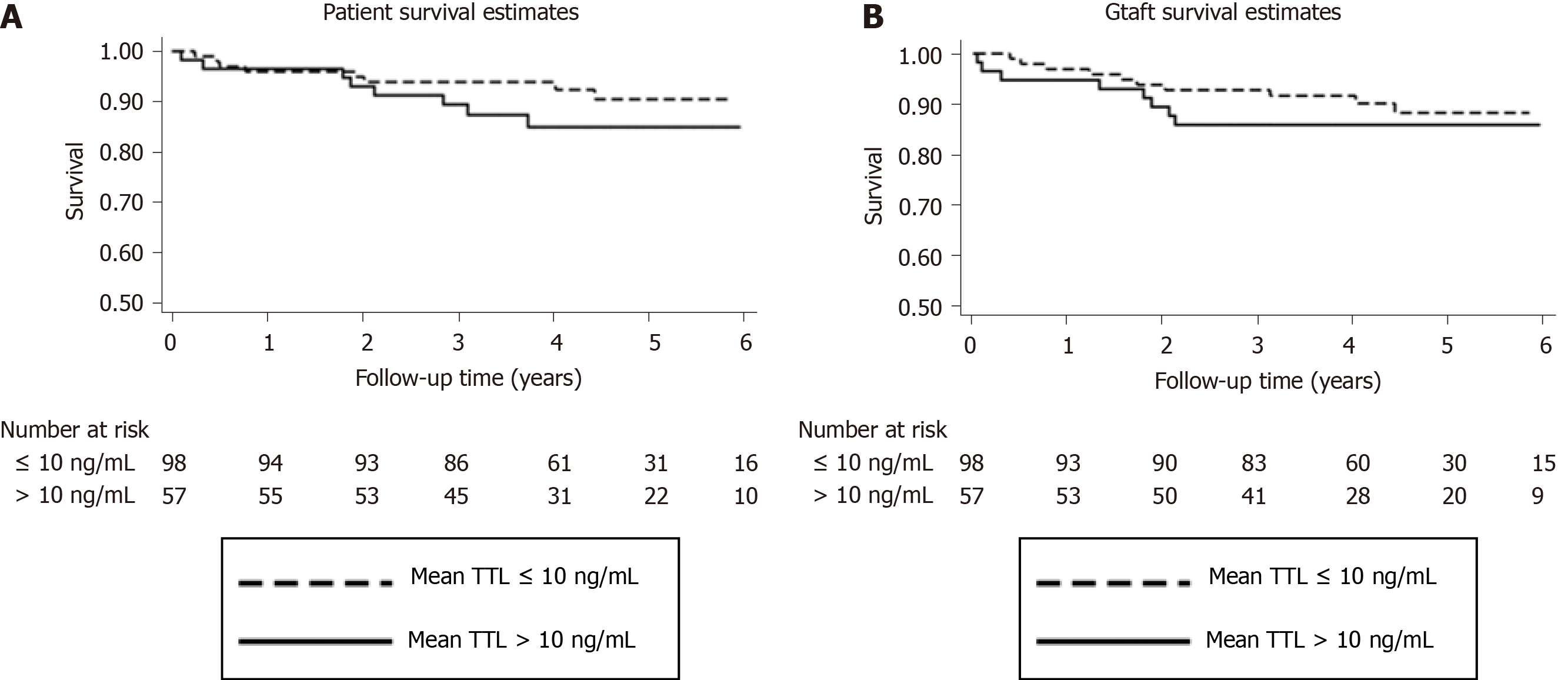
Eighteen patients died during the follow-up and were evenly distributed across groups (P = 0.83) (Table 2). The most common cause of death was HCV recurrence. Five-year patient survival in the study groups was 90.5% and 84.9%, respectively (P = 0.44) (Figure 3A), while 5-year graft survival was 88.2% and 85.8%, respectively (P = 0.42) (Figure 3B).
All patients were included in a univariate and multivariate Cox regression analysis to study factors associated with patient mortality. Multiple variables from donor and recipients were considered in the univariate analysis, as well as various outcomes and adverse events. This analysis was performed considering the two mean TTL groups described in methods, and also dividing the sample into two groups using the cut-off level 8 ng/mL or three groups using cut-off levels of < 7 ng/mL, 7-10 ng/mL and > 10 ng/mL. Multivariate analysis revealed that factors independently related with patient mortality were de novo tumor (HR = 13.8; 95%CI: 4.1-46.9; P < 0.001), MELD score ≥ 20 (HR = 6.1; 95%CI: 1.9-19.6; P = 0.003), HCV infection as the cause of liver disease (HR = 4.9; 95%CI: 1.7-14.1; P = 0.003) and arterial complications (HR = 3.7; 95%CI: 1.1-12.6; P = 0.03) (Table 3). Early TTL was not an independent factor for patient mortality.
| Univariate analysis | Multivariate analysis1 | |||
| P value | HR (95%CI) | P value | HR (95%CI) | |
| Age of donor ≥ 70 years | 0.55 | 0.73 (0.26-2.05) | ||
| Recipient | ||||
| Liver steatosis | 0.1 | 0.408 (0.09-1.79) | ||
| Age ≥ 60 years | 0.94 | 1.04 (0.39-2.78) | ||
| HCV infection as cause of liver disease | 0.02 | 3.02 (1.17-7.81) | 0.003 | 4.94 (1.72-14.17) |
| Presence of hepatocellular carcinoma | 0.57 | 1.31 (0.52-3.34) | ||
| MELD score ≥ 20 | 0.02 | 3.16 (1.12-8.91) | 0.003 | 6.06 (1.88-19.56) |
| Diabetes before transplantation | 0.63 | 1.32(0.43-4.01) | ||
| Hypertension before transplantation | 0.05 | 2.78 (0.98-7.90) | - | - |
| MDRD-4 at baseline | 0.35 | 0.99 (0.97-1.01) | ||
| Mycophenolate mofetil at initial therapy | 0.89 | 1.07 (0.38-3.02) | ||
| Outcomes and complications | ||||
| BPAR | 0.20 | 2.08 (0.67-6.43) | ||
| Arterial complications | 0.06 | 2.91 (0.94-9.06) | 0.03 | 3.76 (1.12-12.62) |
| Biliary complications | 0.59 | 1.41 (0.40-4.92) | ||
| Renal dysfunction early after transplant2 | 0.08 | 2.40 (0.90-6.38) | - | - |
| Renal hypertension | 0.82 | 1.12 (0.42-3.02) | ||
| De novo diabetes | 0.02 | 3.25 (1.24-8.55) | - | - |
| Cardiovascular | 0.14 | |||
| Arterial hypertension | 0.08 | 0.32 (0.09-1.15) | - | - |
| Heart failure | 0.26 | 0.31 (0.04-2.37) | ||
| De novo tumor | 0.005 | 4.20 (1.56-11.32) | < 0.001 | 13.82 (4.06-46.98) |
| HCV recurrence | 0.22 | 1.79 (0.70-4.53) | ||
| HCC recurrence | 0.008 | 16.61 (2.10-131.07) | - | - |
| Any infection | 0.71 | 1.12 (0.47-3.03) | ||
| Bacterial infection | 0.04 | 2.71 (1.04-7.07) | - | - |
| Viral infection | 0.39 | 0.61 (0.20-1.87) | ||
| Fungal infection | 0.87 | 1.19 (0.16-9.03) | ||
| Cytomegalovirus infection | 0.79 | 0.86 (0.28-2.62) | ||
| Normal renal function at last visit (MDRD-4 ≥ 60 mL/min/1.73 m2) | 0.92 | 1.08 (0.23-5.08) | ||
| Mean tacrolimus levels at days 1-30 after LT | ||||
| > 10 ng/mL vs ≤ 10 ng/mL | 0.44 | 1.44 (0.57-3.65) | ||
| < 7 ng/mL (reference)3 | 0.32 | |||
| 7-10 ng/mL | 0.31 | 0.49 (0.12-1.96) | ||
| > 10 ng/mL | 0.59 | 1.33 (0.47-3.73) | ||
| > 8 ng/mL vs < 8 ng/mL3 | 0.78 | 1.14 (0.44-2.95) | ||
| Early graft dysfunction | 0.08 | 2.44 (0.890-6.63) | < 0.001 | 6.02 (2.34-15.49) |
This analysis aimed to further explore factors related to long-term clinical outcomes in our LT patients treated de novo with Tac-QD, with particular interest in the effect of mean TTL during the early post-transplant period. In order to have an adequate follow-up time to study long-term outcomes, patients transplanted between 2008 and 2013 were included in the study. Considering the time when LTs were performed, we followed a policy of immunosuppression minimization with target TTL of 5-10 ng/mL during the first 3 mo; however, a significant number of patients in this cohort were outside our target levels during the first month after LT, although this was corrected afterwards, as shown in Figure 1. We divided our cohort into two groups of early TTL (within 1 mo) as previously done by Rodríguez-Perálvarez et al[7,8] who found a significant improvement of outcomes when mean TTL within the first month post-LT were ≤ 10 ng /mL, compared with patients with > 10 ng/mL[7,8]. Of note, patients treated with induction therapy and delayed introduction of low-dose tacrolimus, namely those with pretransplant renal dysfunction, were excluded in our study to avoid bias as most of these patients would have probably ended in the low mean TTL group. In contrast to the published studies, we did not find significant differences in long-term renal function, HCC recurrence, immunosuppression-related toxicity or patient and graft survival in both groups of early TTL. In addition, multivariate analysis in our study, performed three times with different cut-off values for early TTL, demonstrated the lack of influence of early TTL on long-term patient survival.
In our study, donor age was significantly higher in the group with high TTL. Aging is characterized by a decline of liver cellular function that could determine alterations in immunosuppressants liver metabolism and pharmacokinetic. In this sense, it has been suggested that aged donor livers might exhibit lower drug clearance with consequently higher TTL[15]. Nevertheless, this circumstance was not detrimental in our experience as both TTL groups achieved comparable long-term outcomes.
According to the literature, the relative risk of death more than 1 year after LT suffers a 4-fold to 5-fold increase when renal dysfunction is present[16,17]. In our study, renal function evolved similarly in the two groups, with an expected 20% decrease in eGFR during the initial period after LT-as already described by other authors[18]-and maintenance of renal function from month 6 onwards. This contrasts with the progressive decline in renal function in the Mid/long-term repeatedly reported in literature[19-21]. Although, some authors have found no relationship between TTL within 15 d after LT and chronic renal impairment[3,9], high TTL within the first month after LT has been associated with worse renal function in different studies[7,20]. Karie-Guigues et al[20] found that the introduction of MMF significantly reduced the TTL at the end of the first month after LT, and this was associated with a significantly less marked reduction of the eGFR at 12 and 60 mo. Rodríguez-Perálvarez et al[7] also observed in a meta-analysis that reduced TTL (< 10 ng/mL) within the first month after LT were associated with less renal impairment at 1 year[7]. Nevertheless, both studies can be discussed. In the former study, TTL were shown at months 1, 12 and 60 after LT; however, no data were shown on the evolution of TTL between those time points and so, results could be biased due to different exposition to tacrolimus in both groups[20]. In the latter study, only two clinical trials were used in the meta-analysis and TTL were maintained higher in both study groups along the whole follow-up although differences did not achieve significance[7].
We can hypothesize that TTL early after LT have little effect on the evolution of long-term renal function when a tacrolimus minimization policy is implemented during long-term follow-up, as in our case. A longer period of high TTL in the post-transplant period might be needed to negatively affect the mid/long-term renal function. In accordance with this idea, the role of cumulative exposure to tacrolimus in eGFR decline after LT has been recently addressed[22]. In this study, conventional/high exposure to tacrolimus within the first 3 mo resulted in a more pronounced eGRF decline as compared with minimization (23.3 mL/min vs 9.5 mL/min; P ≤ 0.001).
The role of tacrolimus exposure in HCC recurrence has been also addressed in different studies. High TTL (> 10 ng/mL) within the first month after LT but not thereafter was associated with increased risk of HCC recurrence at 5 years by Rodríguez-Perálvarez et al[8] (RR = 2.8; P = 0.005). Of note, in this study, tacrolimus levels were consistently lower during the 3-year follow-up in the non-recurrence group, although differences did not achieve significance. In another study, high exposure to tacrolimus was followed by a 50% recurrence rate vs 9.1% in patients with low exposure (P = 0.001)[23]. In this study, high exposure was described as > 10 ng/mL during the first year and not only during the first month reflecting a significant higher exposure to tacrolimus along the follow-up. In our study, overall HCC recurrence rate was extremely low and no differences were found between groups. Low exposure to tacrolimus not only during the early post-transplant period but in the long term, and our strict selection policy, all patients fulfilled Milan criteria prior to transplantation, might have positively influenced these remarkable results in our study. Recently, other authors have also reported the lack of effect of the first fifteen days of calcineurin inhibitor exposure in the development of HCC recurrence or de novo tumors after LT[24]. Again, it seems that longer periods of high exposure to tacrolimus-and not only during the first month after transplant-are needed to influence the development of de novo tumors or HCC recurrence.
Early TTL were not related with an increase in BPAR rates in our study. Reduction in early TTL was associated with the use of MMF and this could explain why the BPAR rate was not higher in patients with lower early TTL. Immunosuppression therapy with tacrolimus, MMF and steroids is currently the most common combination following LT[1], and has been demonstrated to be effective in reducing TTL while maintaining or even reducing the acute rejection rate[4,6].
We observed a relatively low rate of immunosuppression-related toxicity in terms of de novo diabetes or arterial hypertension and no differences were seen according to early TTL. In addition, development of de novo tumors was not influenced by TTL during the first month in our study.
In our study, factors associated with patient survival in multivariate analysis were de novo tumor, higher severity of liver disease (MELD score > 20), baseline HCV infection and arterial complications after LT. These factors have been repeatedly reported to be related to patient and graft survival after LT in the pre-direct-acting antivirals era[16,25,26]. Of note, early TTL were not an independent risk factors for patient survival in our study.
We recognize some limitations in our study. It is retrospective, although the data were retrieved from a prospective database. Indeed, the number of patients included in the different groups are limited and hence the number of patients who experienced adverse events of interest such as impairment of renal function or HCC recurrence are also limited. In addition, MMF was more frequently used in the lower TTL group although immunosuppression-related morbidity is more likely related with tacrolimus exposure rather than to the use of MMF. Nevertheless, our study has several strengths: (1) Median follow-up was more than 4 years in both groups, which seems sufficient to assess the long-term outcomes and draw meaningful conclusions; and (2) Regarding TTL, our study groups were significantly different only within the first month after LT, which was the target period of time in the study, but not during the rest of the follow-up, what reinforces the adequacy of the study for our purpose and avoids the significant potential bias of having not only different early TTL but different TTL during the study period.
In summary, TTL within the first month after LT had no significant effect on long-term renal function, immunosuppression-related morbidity and 5-year patient or graft survival in our study. Early post-transplant tacrolimus level was not an independent factor for long-term patient in multivariate analysis. We conclude that relatively small differences in mean tacrolimus levels restricted to the first month after LT do not determine differences in long-term immunosuppression-related morbidity and patient survival and therefore, larger exposure to tacrolimus seems to be needed to influence long-term outcomes. Larger studies should be advisable to confirm our results; however, these studies should be done on the basis of different TTL only during the early post-transplant period and not along the follow-up to avoid potential biases.
Immunosuppression is a cornerstone in liver transplantation (LT) and current immunosuppressive regimens are mostly based on tacrolimus. At present, side effects relating anticalcineurin inhibitors are one of the main concerns for long-term outcomes after LT. Side effects are commonly related with drug dose and trough levels.
Tacrolimus trough levels (TTL) above 10 ng/mL during the first weeks after liver transplant have been related with mid and long-term outcomes including impairment of renal function and an increased rate of hepatocellular recurrence, de novo tumors and new-onset diabetes.
The aim of this study was to assess the influence of the TTL during the early post-transplant period in the long-term outcomes of LT.
This was a retrospective study of 155 consecutive liver transplants treated with an immunosuppressive regimen based on de novo once-daily tacrolimus. Patients were classified into 2 groups according to their mean TTL within the first month after transplantation: ≤ 10 ng/mL (n = 98) and > 10 ng/mL (n = 57). All TTL obtained during the first month were used to define the mean values. Multivariate analyses were performed to assess risk factors for patient mortality.
TTL were significantly different among groups only during the first month after transplantation, but not during the rest of the follow-up After a median follow-up of 52.8 mo (range 2.8-81.1), no significant differences were observed in the evolution of the mean estimated glomerular filtration rate, hepatocellular carcinoma recurrence, development of de novo tumors, new-onset diabetes, new-onset arterial hypertension or biopsy-proven acute rejection rate. Five-year patient and graft survival were comparable. Early tacrolimus trough level was not an independent factor for patient mortality in multivariate analyses.
Differences in tacrolimus levels restricted to the first month after transplantation did not result in significant differences in long-term outcomes of liver transplant recipients.
Mid and long-term calcineurin inhibitors-related side effects after LT should be studied considering the cumulative exposure to tacrolimus along the follow-up and not only the trough levels observed during the early post-transplant period.
Dr. Blanca Piedrafita at Medical Statistics Consulting S.L. (Valencia) is thanked for writing assistance and Dr. José Ignacio Pijuan from the Department of Epidemiology and Biostatistics for assistance in survival analysis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Verran DJ S-Editor: Fan JR L-Editor: A P-Editor: Wang LL
| 1. | Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, Harper AM, Wainright JL, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2015 Annual Data Report: Liver. Am J Transplant. 2017;17 Suppl 1:174-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 234] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 2. | Kahan BD, Keown P, Levy GA, Johnston A. Therapeutic drug monitoring of immunosuppressant drugs in clinical practice. Clin Ther. 2002;24:330-50; discussion 329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 205] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 3. | Rodríguez-Perálvarez M, Germani G, Papastergiou V, Tsochatzis E, Thalassinos E, Luong TV, Rolando N, Dhillon AP, Patch D, O'Beirne J, Thorburn D, Burroughs AK. Early tacrolimus exposure after liver transplantation: relationship with moderate/severe acute rejection and long-term outcome. J Hepatol. 2013;58:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Neuberger JM, Mamelok RD, Neuhaus P, Pirenne J, Samuel D, Isoniemi H, Rostaing L, Rimola A, Marshall S, Mayer AD; ReSpECT Study Group. Delayed introduction of reduced-dose tacrolimus, and renal function in liver transplantation: the 'ReSpECT' study. Am J Transplant. 2009;9:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 209] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 5. | Benítez CE, Puig-Pey I, López M, Martínez-Llordella M, Lozano JJ, Bohne F, Londoño MC, García-Valdecasas JC, Bruguera M, Navasa M, Rimola A, Sánchez-Fueyo A. ATG-Fresenius treatment and low-dose tacrolimus: results of a randomized controlled trial in liver transplantation. Am J Transplant. 2010;10:2296-2304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Boudjema K, Camus C, Saliba F, Calmus Y, Salamé E, Pageaux G, Ducerf C, Duvoux C, Mouchel C, Renault A, Compagnon P, Lorho R, Bellissant E. Reduced-dose tacrolimus with mycophenolate mofetil vs. standard-dose tacrolimus in liver transplantation: a randomized study. Am J Transplant. 2011;11:965-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Rodríguez-Perálvarez M, Germani G, Darius T, Lerut J, Tsochatzis E, Burroughs AK. Tacrolimus trough levels, rejection and renal impairment in liver transplantation: a systematic review and meta-analysis. Am J Transplant. 2012;12:2797-2814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 8. | Rodríguez-Perálvarez M, Tsochatzis E, Naveas MC, Pieri G, García-Caparrós C, O'Beirne J, Poyato-González A, Ferrín-Sánchez G, Montero-Álvarez JL, Patch D, Thorburn D, Briceño J, De la Mata M, Burroughs AK. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J Hepatol. 2013;59:1193-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 188] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 9. | Jia JJ, Lin BY, He JJ, Geng L, Kadel D, Wang L, Yu DD, Shen T, Yang Z, Ye YF, Zhou L, Zheng SS. ''Minimizing tacrolimus'' strategy and long-term survival after liver transplantation. World J Gastroenterol. 2014;20:11363-11369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Gastaca M, Valdivieso A, Bustamante J, Fernández JR, Ruiz P, Ventoso A, Testillano M, Palomares I, Salvador P, Prieto M, Montejo M, Suárez MJ, de Urbina JO. Favorable longterm outcomes of liver transplant recipients treated de novo with once-daily tacrolimus: Results of a single-center cohort. Liver Transpl. 2016;22:1391-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67:2089-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2145] [Cited by in RCA: 2516] [Article Influence: 125.8] [Reference Citation Analysis (0)] |
| 12. | Charlton M. Obesity, hyperlipidemia, and metabolic syndrome. Liver Transpl. 2009;15 Suppl 2:S83-S89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 1001] [Article Influence: 35.8] [Reference Citation Analysis (1)] |
| 14. | Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, Shaked A, Christie JD. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 874] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 15. | Teperman LW, Morgan GR, Diflo T, John DG, Gopalan V, Negron CE, Tobias H. Tacrolimus dose is donor age dependent. Transplantation. 1998;66:S44. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Charlton MR. Evolution of causes and risk factors for mortality post-liver transplant: results of the NIDDK long-term follow-up study. Am J Transplant. 2010;10:1420-1427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 617] [Cited by in RCA: 586] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 17. | Lucey MR, Terrault N, Ojo L, Hay JE, Neuberger J, Blumberg E, Teperman LW. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 2013;19:3-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 362] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 18. | Bahirwani R, Reddy KR. Outcomes after liver transplantation: chronic kidney disease. Liver Transpl. 2009;15 Suppl 2:S70-S74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1703] [Cited by in RCA: 1637] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 20. | Karie-Guigues S, Janus N, Saliba F, Dumortier J, Duvoux C, Calmus Y, Lorho R, Deray G, Launay-Vacher V, Pageaux GP. Long-term renal function in liver transplant recipients and impact of immunosuppressive regimens (calcineurin inhibitors alone or in combination with mycophenolate mofetil): the TRY study. Liver Transpl. 2009;15:1083-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Allen AM, Kim WR, Therneau TM, Larson JJ, Heimbach JK, Rule AD. Chronic kidney disease and associated mortality after liver transplantation--a time-dependent analysis using measured glomerular filtration rate. J Hepatol. 2014;61:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 22. | Rodríguez-Perálvarez M, Guerrero M, De Luca L, Gros B, Thorburn D, Patch D, Aumente MD, Westbrook R, Fernández R, Amado V, Aguilar P, Montero JL, O'Beirne J, Briceño J, Tsochatzis E, De la Mata M. Area Under Trough Concentrations of Tacrolimus as a Predictor of Progressive Renal Impairment After Liver Transplantation. Transplantation. 2019;103:2539-2548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Vivarelli M, Cucchetti A, La Barba G, Ravaioli M, Del Gaudio M, Lauro A, Grazi GL, Pinna AD. Liver transplantation for hepatocellular carcinoma under calcineurin inhibitors: reassessment of risk factors for tumor recurrence. Ann Surg. 2008;248:857-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 204] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 24. | Di Maria T, Sapisochin G, Rajakumar R, Lilly L, Prieto M, Lopez-Andujar R, Berenguer M. The first fifteen days of calcineurin inhibitors exposure do not predict post-transplant malignant outcomes. Transplantation. 2018;102 Suppl 5:S167-S168. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Bruns H, Lozanovski VJ, Schultze D, Hillebrand N, Hinz U, Büchler MW, Schemmer P. Prediction of postoperative mortality in liver transplantation in the era of MELD-based liver allocation: a multivariate analysis. PLoS One. 2014;9:e98782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Jain A, Singhal A, Fontes P, Mazariegos G, DeVera ME, Cacciarelli T, Lopez RC, Sindhi R, Humar A, Marsh JW. One thousand consecutive primary liver transplants under tacrolimus immunosuppression: a 17- to 20-year longitudinal follow-up. Transplantation. 2011;91:1025-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |