Published online Dec 27, 2021. doi: 10.4254/wjh.v13.i12.2179
Peer-review started: July 1, 2021
First decision: July 13, 2021
Revised: July 25, 2021
Accepted: November 14, 2021
Article in press: November 14, 2021
Published online: December 27, 2021
Processing time: 178 Days and 16 Hours
Nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) seem common after liver transplantation.
To investigate incidence and predictors of NAFLD and NASH by employing noninvasive testing in liver transplant recipients, namely controlled attenuation parameter (CAP) and the serum biomarker cytokeratin 18 (CK-18). We also evaluated the diagnostic accuracy of CK-18 and CAP compared to liver histology.
We prospectively recruited consecutive adult patients who received liver transplant at the McGill University Health Centre between 2015-2018. Serial measurements of CK-18 and CAP were recorded. NAFLD and NASH were diagnosed by CAP ≥ 270 dB/m, and a combination of CAP ≥ 270 dB/m with CK-18 > 130.5 U/L, respectively. Incidences and predictors of NAFLD and NASH were investigated using survival analysis and Cox proportional hazards.
Overall, 40 liver transplant recipients (mean age 57 years; 70% males) were included. During a median follow-up of 16.8 mo (interquartile range 15.6-18.0), 63.0% and 48.5% of patients developed NAFLD and NASH, respectively. On multivariable analysis, after adjusting for sex and alanine aminotransferase, body mass index was an independent predictor of development of NAFLD [adjusted hazard ratio (aHR): 1.21, 95% confidence interval (CI): 1.04-1.41; P = 0.01] and NASH (aHR: 1.26, 95%CI: 1.06-1.49; P < 0.01). Compared to liver histology, CAP had a 76% accuracy to diagnose NAFLD, while the accuracy of CAP plus CK-18 to diagnose NASH was 82%.
NAFLD and NASH diagnosed non-invasively are frequent in liver transplant recipients within the first 18 mo. Close follow-up and nutritional counselling should be planned in overweight patients.
Core Tip: This is the first prospective study using cytokeratin 18 in association with transient elastography with controlled association parameter to investigate nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) in liver transplant recipients. NAFLD and NASH diagnosed by non-invasive tests occur frequently in the first 18 mo from liver transplant. Overweight is the main risk factor. Non-invasive liver fibrosis markers have suboptimal accuracy.
- Citation: Alhinai A, Qayyum-Khan A, Zhang X, Samaha P, Metrakos P, Deschenes M, Wong P, Ghali P, Chen TY, Sebastiani G. Non-alcoholic steatohepatitis in liver transplant recipients diagnosed by serum cytokeratin 18 and transient elastography: A prospective study. World J Hepatol 2021; 13(12): 2179-2191
- URL: https://www.wjgnet.com/1948-5182/full/v13/i12/2179.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i12.2179
In recent years, there has been a shift in the etiologies of liver diseases leading to liver transplantation (LT): Chronic hepatitis C is declining, while nonalcoholic fatty liver disease (NAFLD) is on the rise. NAFLD affects 25.24% of the general population globally, driven by the epidemic of metabolic conditions such as obesity and type 2 diabetes mellitus[1-3]. NAFLD is an umbrella term encompassing a spectrum of clinical and pathologic features characterized by a fatty overload involving over 5% of the liver weight in the absence of other causes of liver disease. It ranges from simple steatosis or nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH). Without treatment, NAFL can evolve to NASH, liver fibrosis and cirrhosis, eventually resulting in liver failure and hepatocellular carcinoma (HCC)[2,4]. NASH is now the second leading indication for liver transplant in North America and is projected to become the main indication in the next 10 years[5,6].
In contrast to alcoholic liver disease, the mitigation of NASH risk factors is not a requirement for transplant eligibility. Hence, risk factors for NASH may persist or worsen after LT, placing these recipients at risk for recurrence. De novo NASH in patients transplanted for other etiologies of liver disease can also occur due to excess of metabolic risk factors following LT, including type 2 diabetes mellitus, rapid weight gain, hypertension, hyperlipidemia. Immunosuppressive medications may also play a role, as both corticosteroids and calcineurin inhibitors promote diabetes, hypertension and hypercholesterolemia[7,8]. About 20% and 10% of LT recipients develop de novo NAFLD and NASH, respectively[8]. Recurrent NAFLD and NASH can be as frequent as 62% and 33%, respectively. NAFLD is a common occurrence within 6 mo, whereas the onset of NASH occurs in a period of 6 mo to 1 year in several studies[9]. Due to these reasons, LT recipients may require monitoring to detect changes to the liver graft and prevent hepatic failure and mortality. The majority of studies evaluating recurrent NAFLD and NASH in LT recipients have been of retrospective nature, with no serial monitoring. Hence, longitudinal, prospective data on the frequency of NAFLD and NASH are lacking in the first months following LT. Protocol biopsies have long been used to identify liver disease recurrence and guide management. However, liver biopsy is invasive, costly and prone to sampling error[10]. Recent non-invasive tools for the diagnosis of hepatic steatosis and fibrosis include the measurement of liver stiffness by transient elastography (TE) and the associated controlled attenuation parameter (CAP)[2,11-13]. The accuracy of TE for the diagnosis of liver graft fibrosis seems similar to the non-transplant population[14]. Few studies have investigated the accuracy of CAP in the post-transplant setting[15,16]. Serum cytokeratin 18 (CK-18) has been proposed for the non-invasive diagnosis of NASH. CK-18 is the major intermediate filament protein in the liver and one of the most prominent substrates of caspases during hepatocyte apoptosis. Apoptotic cell death of hepatocytes is associated with the release of caspase-cleaved CK-18 fragments into the bloodstream[17]. Apoptotic activity occurs in NASH but not in NAFL, as such the presence of CK-18 fragments in the blood may differentiate the two conditions[17-19]. In a meta-analysis of over 1600 patients, CK-18 predicted the presence of NASH with a pooled area under the curve (AUC) of 0.82[20]. One report suggests that CK-18 could also have a prognostic value in predicting one-year survival post-LT[21]. No study has employed CK-18 to diagnose NASH in LT recipients.
We prospectively investigated incidence and predictors of NAFLD and NASH diagnosed by TE with CAP and CK-18 in LT recipients within the first 18 mo post-transplantation. We also studied the diagnostic accuracy of non-invasive tests compared to paired liver biopsies performed as a part of clinical care.
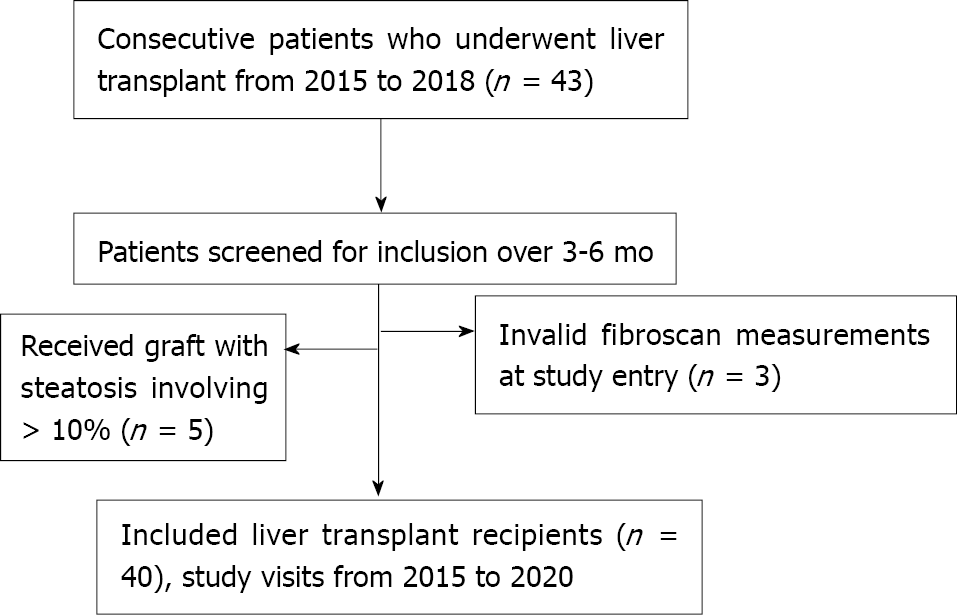
This was a prospective, longitudinal study conducted at a single site, the McGill University Health Center (MUHC) Solid Organ Transplant Unit, and it included all eligible and consecutive patients who underwent LT between March 2015 and June 2018. Since 1990, a computerized database on all LT recipients has been maintained into which demographic data, clinical diagnosis, laboratory results, and prescription information had been prospectively entered. In order to be included, patients had to fulfill the following criteria: Age > 18 years; patient and graft survival > 6 mo; a minimum follow-up of 1 year. Exclusion criteria were any of the following: LT due to chronic hepatitis C, genotype 3; patients who received liver grafts involving more than 10% steatosis; failure of TE with CAP examination or unreliable measurement at study entry. The immunosuppressive regimen used as a standard by the LT program is induction with anti-thymocyte globulin, tacrolimus and mycophenolate mofetil as maintenance immunosuppression and rapid prednisone taper. Overweight and obesity were defined as body mass index (BMI) > 25 and > 30 kg/m2, respectively.
The study was approved by the Research Ethics Board of the Research Institute of MUHC (code 15-002-MUHC) and was registered at ClinicalTrials.gov (NCT03128918). The study was conducted according to the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided their informed written consent prior to participation.
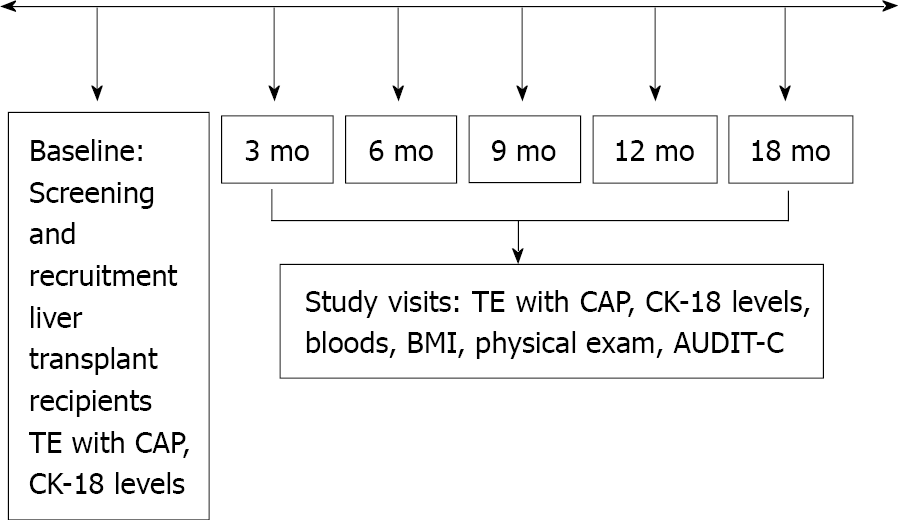
Study visits were scheduled at baseline, month 3, 6, 9, 12 and 18, for a total of 5 visits (Figure 1). The following parameters were collected at each study visit: BMI, laboratory tests for hematology, blood chemistry. The questionnaire Alcohol Use Disorders Identification Test (AUDIT-C) was administered[22]. TE with CAP measurement and plasma to measure CK-18 were also acquired at each study visit. TE examination was performed in patients fasting for at least 3 h using FibroScan 502 Touch (Echosens, Paris, France). The same two experienced operators performed all elastographic measurements. The standard M probe was used in all patients. The XL probe was used in cases of failure of TE with the M probe or if BMI > 30 kg/m2. The following criteria were applied to define the result of TE as reliable: At least 10 validated measurements and an interquartile range (IQR) < 30% of the median liver stiffness measurement (LSM)[23]. Available liver biopsies were used for the diagnostic accuracy study. Liver biopsy was performed at the discretion of the treating transplant hepatologist, as part of standard of care. All biopsies were obtained with a 16G Tru-Cut type needle and interpreted by two experienced liver pathologists. The stage of fibrosis was reported according to the Kleiner classification[24]. The NAFLD activity score (NAS) was calculated as the unweighted sum of the scores for steatosis (0-3), lobular inflammation (0-3) and hepatocellular ballooning (0-2). A diagnosis of NASH was made if NAS ≥ 5[24]. The CAP cut-off used for diagnosis of NAFLD was 270 dB/m, as recently reported in LT recipients[16]. Plasma stored at -80 °C was used for quantitative measurement of CK-18 levels by the Human cytokeratin ELISA kit (MJS Biolynx inc, Brockville Ontario, Canada). A cut-off of CK-18 > 130.5 U/L was used to indicate significant hepatocyte apoptosis, diagnostic for NASH when combined with CAP > 270 dB/m[25,26]. Liver fibrosis (stage ≥ 1 out of 4) was diagnosed as LSM ≥ 7.4 kPa[16]. The following simple serum fibrosis biomarkers were also computed: Hepatic steatosis index (HSI), defined as 8 × aspartate aminotransferase (AST)/alanine aminotransferase (ALT) + BMI (+ 2, if female; +2, if diabetes mellitus present)[27], fibrosis-4 (FIB-4), calculated as [age (years) × AST]/[platelet count (109/L) × ALT][28], and AST to platelet ratio (APRI), calculated as {[AST level/AST (upper limit of normal)]/platelet count (109/L) × 100}[29]. Liver fibrosis was defined as FIB-4 > 3.64 and APRI > 1, as previously described in the liver transplant setting[30].
The performance of the non-invasive tests to diagnose NAFLD, NASH and liver fibrosis was measured with the following: Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy, positive and negative likelihood ratios (LR+ and LR−, respectively). Correlation coefficients of TE with CAP with serum biomarkers were calculated using the Pearson correlation analysis. For the longitudinal analysis, baseline (study entry) corresponded to the day of LT. Patients were followed until March 2020 or were censored either when they developed the outcome or at their last study visit (18 mo post-LT). At each visit, complete medical history and physical examination were performed along with routine laboratory work-up. Standard diagnostic and therapeutic management following LT was offered during the follow-up. Continuous variables were expressed as mean (standard deviation), and categorical variables were presented as numbers (%). We estimated incidence rates of NAFLD and NASH by dividing the number of participants developing the outcome by the number of person-years (PY) of follow-up. Poisson count models were used to calculate CI for incidence rates. Multivariable time-dependent Cox regression models were constructed to assess predictors of the development of NAFLD and NASH and included covariates that were determined a priori to be clinically important and with a P-value < 0.1 on univariable analysis. The final model was adjusted for sex, BMI and ALT. Robust variance estimation was used in all Cox regression analyses to account for the correlation of data contributed by the same participant at multiple visits. We considered an association with the outcome significant when the 95%CI excluded one. We generated Kaplan-Meier curves to illustrate and compare the cumulative incidence of NAFLD and NASH in overweight vs normal weight patients. The log-rank test was used to evaluate differences among incidences. All tests were two-tailed and with a significance level of α = 0.05. Statistical analysis was performed using STATA 15 (StataCorp LP, TX, United States).
After applying exclusion criteria, 40 LT recipients were included in this prospective study (Figure 2). The main demographic, clinical and biochemical characteristics of the study population at baseline are summarized in Table 1. Univariable analysis by outcome category of NAFLD and NASH is also reported. Overall, mean age was 57.3 years and 70% of patients were male. The most frequent indications for LT were NASH and HCC. Metabolic comorbidities were frequent, with overweight, type 2 diabetes mellitus and hypertension affecting 40%, 35% and 37.5% of the patients, respectively. Patients who developed NAFLD and NASH during the follow-up period were more frequently transplanted for NASH and on tacrolimus as immunosuppressant.
| Whole cohort | Patients who developed NAFLD | Patients who developed NASH | |
| n = 40 | n = 22 | n = 17 | |
| Age (yr) | 57.3 ± 8.5 | 55.5 ± 9.2 | 56.3 ± 7.9 |
| Male (%) | 28 (70) | 18 (82) | 14 (82) |
| Ethnicity (%) | |||
| Caucasian | 32 (80) | 19 (86) | 15 (88) |
| Other (Asian, Black, Arab) | 8 (20) | 3 (14) | 2 (11) |
| Etiology of liver disease (%) | |||
| NASH | 21 (52.5) | 13 (52) | 12 (70) |
| HCC | 9 (22.5) | 2 (9) | 2 (12) |
| HCV (excluding genotype 3) | 8 (20) | 6 (27) | 3 (18) |
| Alcoholic liver disease | 1 (2.5) | 1 (4.5) | 0 |
| Other | 1 (2.5) | 0 | 0 |
| BMI (kg/m2) | 24.8 ± 4.6 | 26.2 ± 5.1 | 26.6 ± 4.5 |
| BMI >25 (%) | 18 (40) | 14 (64) | 12 (70) |
| Comorbidities (%) | |||
| Diabetes | 14 (35) | 9 (41) | 8 (47) |
| Hypertension | 15 (37.5) | 7 (32) | 8 (47) |
| Dyslipidemia | 6 (15) | 6 (27) | 5 (29) |
| MELD-Na Score | < 9 | < 9 | < 9 |
| Laboratory | |||
| AST (U/L) | 27.6 ± 33 | 31.8 ± 41.2 | 34.5 ± 45.1 |
| ALT (U/L) | 32.8 ± 42.8 | 37.6 ± 52.6 | 40.6 ± 57.7 |
| GGT (U/L) | 177.5 ± 256.6 | 177.7 ± 271.4 | 188.1 ± 297.6 |
| Bilirubin (µmol/L) | 17 ± 15.9 | 18.2 ± 17.3 | 18 ± 18.2 |
| INR | 1.25 ± 1.39 | 1.05 ± 0.12 | 1.04 ± 1.3 |
| Albumin (g/L) | 39.6 ± 3.69 | 38.7 ± 4.3 | 39.4 ± 3.9 |
| Platelets (109/L) | 172.3 ± 86.9 | 185 ± 92.5 | 170.5 ± 93.6 |
During the study period, 35 liver biopsies (mean length ± SD: 1.7 ± 0.4 cm) from 24 patients were available. The median time between liver biopsies and non-invasive diagnostic testing was 38.6 ± 30 d. Table 2 shows the performance of non-invasive tests compared to liver histology. The diagnostic accuracy of CAP and HSI for NAFLD was 76% and 45.7%, respectively. The diagnostic accuracy of a combination of CAP ≥ 270 dB/m and CK-18 > 130.5 to diagnose NASH was 82%. The diagnostic accuracy of LSM, FIB-4 and APRI for liver fibrosis was low at 57.8%, 48.7% and 54.1%, respectively. There was a medium positive correlation between CAP and HSI of 0.4. There was a medium positive correlation between LSM and FIB-4 of 0.4, and a weak positive correlation between LSM and APRI of 0.1.
| NAFLD | NASH | Liver fibrosis | ||||
| CAP | HSI | CAP + CK-18 | LSM | FIB-4 | APRI | |
| Sensitivity (%) | 58 | 64.3 | 75 | 61.9 | 7.1 | 14.3 |
| Specificity (%) | 86 | 33 | 83 | 54.2 | 73.9 | 78.3 |
| PPV (%) | 70 | 39 | 37 | 54.2 | 14.3 | 28.6 |
| NPV (%) | 79 | 58 | 96 | 61.9 | 56.7 | 60 |
| LR+ | 4.28 | 0.96 | 4.5 | 1.35 | 0.27 | 0.66 |
| LR- | 0.48 | 1.07 | 0.3 | 0.7 | 1.26 | 1.1 |
| Accuracy (%) | 76 | 45.7 | 82 | 57.8 | 48.7 | 54.1 |
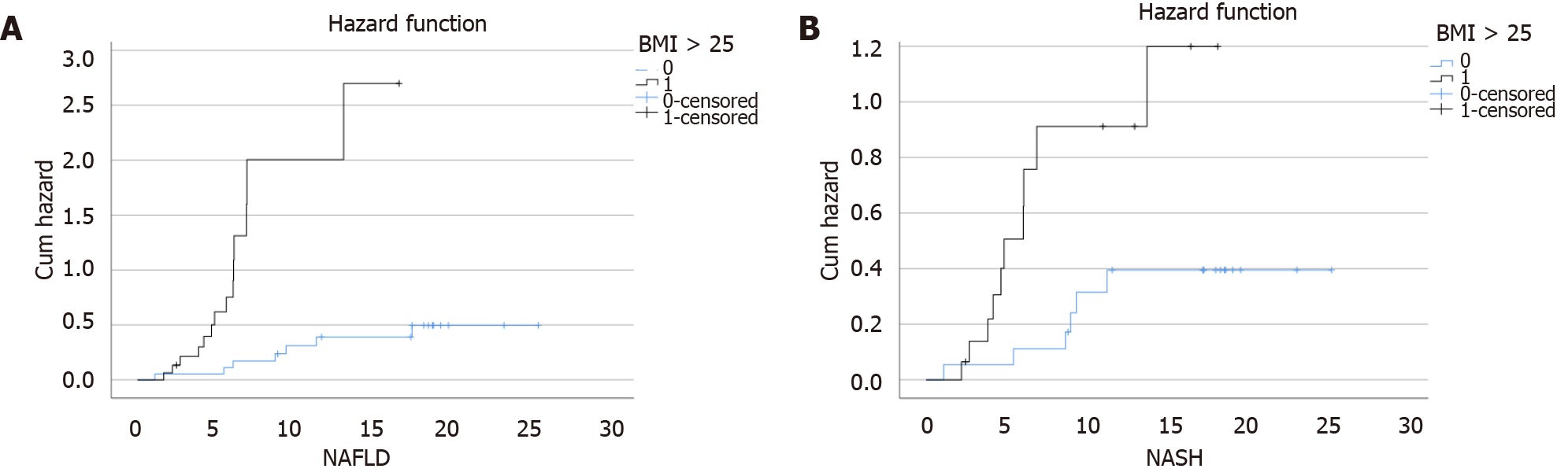
During a median follow-up of 16.8 mo (IQR: 15.6-18.0), 22 patients (63.0%) developed NAFLD (incidence rate: 71.0 per 100 PY, 95%CI: 45.0-78.0), and 17 patients (48.5%) developed NASH (incidence rate: 48.6 per 100 PY, 95%CI: 31.4-66.0). On multivariate Cox regression analysis, BMI was an independent predictor of both NAFLD (adjusted HR: 1.1, 95%: 1.0-1.2) and NASH (adjusted HR: 1.1, 95%CI: 1.0-1.3) (Table 3). To further elaborate on the effect of high BMI on the incidence of NAFLD and NASH, a hazard plot was performed and showed that overweight was a significant risk factor for both NAFLD and NASH (log-rank, P < 0.01, respectively) (Figure 3).
| NAFLD | NASH | |||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| HR (95%CI) | P value | aHR (95%CI) | P value | HR (95%CI) | P value | aHR (95%CI) | P value | |
| Female sex (yes vs no) | 0.6 (0.4-1.2) | 0.1 | 0.9 (0.3-1.7) | 0.5 | 0.6 (0.3-1.1) | 0.1 | 0.9 (0.4-2.1) | 0.8 |
| Age (per year) | 1.0 (0.9-1.0) | 0.6 | 1.0 (0.9-1.0) | 0.9 | ||||
| BMI (per kg/m2) | 1.1 (1.0-1.2) | < 0.01 | 1.1 (1.0-1.2) | < 0.01 | 1.1 (1.0-1.2) | 0.01 | 1.1 (1.0-1.3) | < 0.01 |
| Diabetes (yes vs no) | 1.7 (1.0-2.7) | 0.02 | 1.3 (0.7-2.1) | 0.3 | ||||
| Dyslipidemia (yes vs no) | 4.6 (1.7-12.8) | < 0.01 | 4.4 (1.5-13) | 0.007 | ||||
| ALT (per U/L) | 1.0 (0.9-1.0) | 0.09 | 1 (0.9-1.0) | 0.3 | 1.0 (1.0-1.0) | 0.03 | 1 (0.9-1.0) | 0.1 |
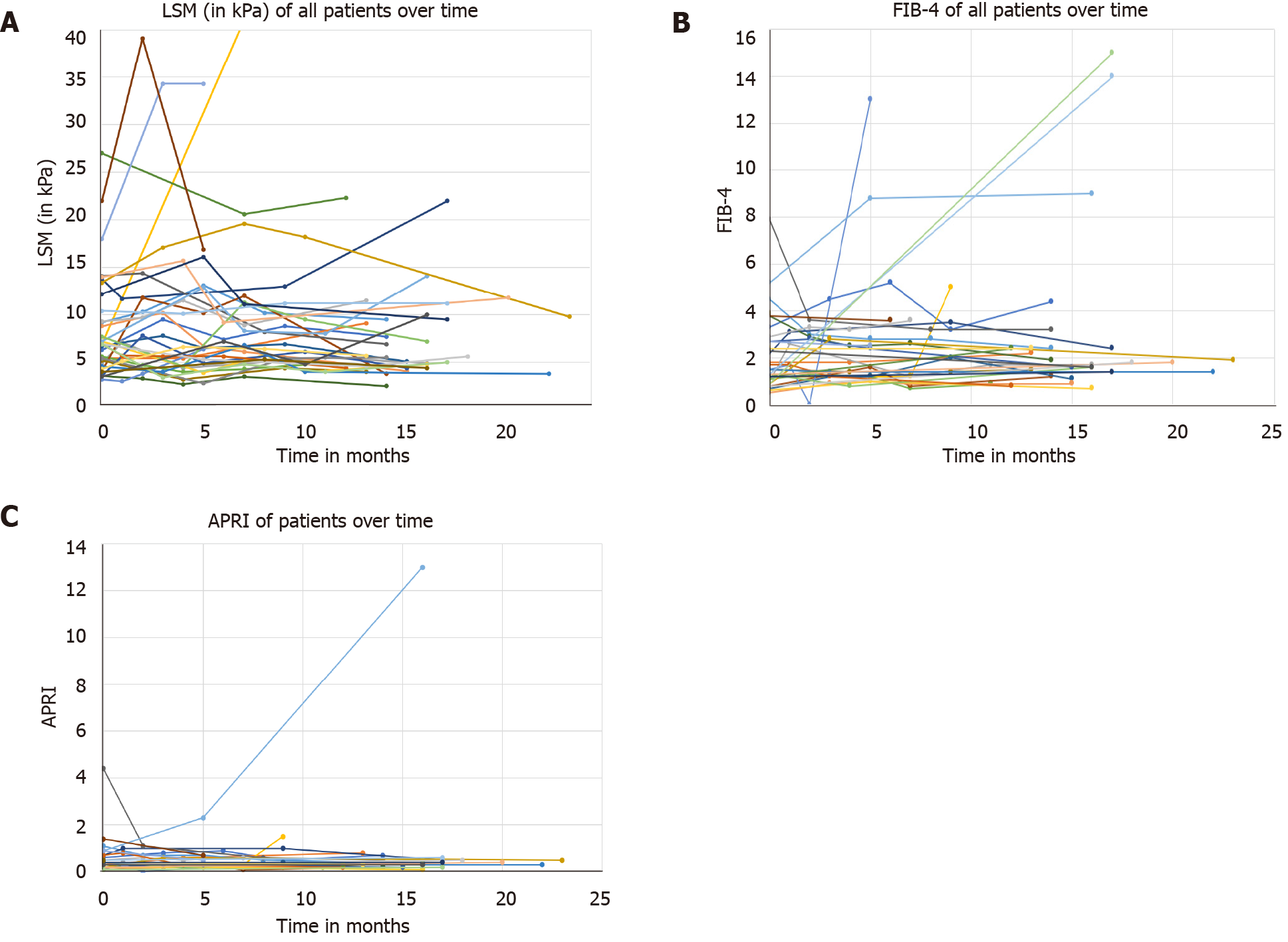
Given the low accuracy for the non-invasive fibrosis tests, we studied changes in LSM, FIB-4 and APRI during the follow-up. While the majority of patients had an LSM ranging from 2.5 to 15 kPa, there were patients who developed marked increases, and these were observed in the first six months of follow-up (Figure 4A). Similarly, while most of the patients had FIB-4 and APRI ranging from 1 to 2.5 and from 0.5 to 1.5, respectively, there were patients who developed marked increases during the first six months of follow-up (Figures 4B and 4C).
In this prospective study, we have shown that NAFLD and NASH diagnosed non-invasively are frequent occurrences in the first 18 mo from LT. Similar to results reported in previous retrospective studies, the majority of incident NAFLD and NASH in our population occurred within the first year of LT[31-33]. The main predictor of these events was high BMI, thus underlying the importance of controlling the weight beginning from the first 3 mo post-LT. We also showed that the diagnostic accuracy of non-invasive tests for NAFLD is good and similar to previously reported, while non-invasive fibrosis tests have low accuracy in the first months following LT. Finally, we first report the accuracy of the apoptotic biomarker CK-18 combined with CAP for the diagnosis of NASH.
We compared the performance of non-invasive tests to liver biopsy. We used a CAP cut-off ≥ 270 dB/m, as referenced by Siddiqui et al[16], and compared it to the presence of steatosis grade 0 vs 1-3 on liver biopsy. Our results showed a lower sensitivity (58% vs 74%), however the specificity (86% vs 87%), PPV (70% vs 78%) and NPV (79% vs 84%) were similar. The variations can be explained by the different population sizes, number of available liver biopsies and the timing of the study conducted within the first 18 mo from LT. When HSI was compared to histology, it showed less accuracy than CAP as demonstrated before in other studies on non-LT populations[34,35]. Secondly, we used a combination of CK-18 > 130.5 with CAP ≥ 270 dB/m and compared it to the presence of NASH (NAS ≥ 5 or proven NASH) on liver histology. To our knowledge, this is the first study to use CK-18 to detect NASH in LT patients. Compared to one meta-analysis of over 1600 patients that assessed the accuracy of CK-18 (cut-off range: 121.6-380.2 U/L) in non-transplanted patients with NASH, our results are similar for both sensitivity (75% vs 78%) and specificity (83% vs 87%)[20]. Compared to another more recent meta-analysis of over 1400 patients that evaluated the diagnostic value of CK-18 for the diagnosis of NASH, our results also reported similar sensitivity (75% vs 75%), specificity (83% vs 77%), LR+ (4.5 vs 3.3), and LR- (0.3 vs 0.3)[36].
There are two interesting points. Firstly, our cut-off values of all the non-invasive biomarkers reported a higher NPV than PPV which could indicate that these tests are more efficient at ruling-out NAFLD, NASH and liver fibrosis rather than ruling-in these diseases, as previously described[16,37]. However, their ability to minimize the need for liver biopsy in this clinical setting still requires further validation. Secondly, while we combined CK-18 with CAP to diagnose NASH, our results are very closely related to those the two meta-analyses which used CK-18 alone to diagnose NASH. This makes us question the role of combining CAP with CK-18 to diagnose NASH. Two studies investigated the combined use of CK-18 with TE to detect fibrosis and found either no significant improvement or only some improvement in AUC by combining CK-18 and TE compared to using a single test[38,39]. Yet, other studies have shown that combining CK-18 with other biomarkers improves the accuracy to diagnose NASH[40,41]. Our analysis must be replicated in a larger sample using different combinations of biomarkers to better understand this.
Our results are comparable to a recent cross-sectional study by Mikolasevic et al[15] which reported a prevalence of liver steatosis of 68.6% and severe liver steatosis of 46.8% in LT recipients using CAP and LSM. Our incidence rates are also comparable to previously published meta-analyses and retrospective studies, while minor variations are most likely due to the difference in populations, the cut-off values to define steatosis/NAFLD and NASH, and the absence of the use of CK-18 as a diagnostic tool in those studies[15,31-33]. On multivariate Cox regression analysis, high BMI was the main risk factor for the development of NAFLD and NASH in patients post LT, conceding with results from previous studies[15,31]. Obesity is an independent risk factor for the development of NAFLD and NASH and can occur or continue to be present even during the first months post-LT. Indeed, other studies have shown that the maximum weight gain occurs in the first year post LT mainly because of the use of immunosuppressive medications[42,43]. Type 2 diabetes mellitus and dyslipidemia were significant risk factors on univariate analysis, also in line with previous results[15]. The presence of these risk factors poses a risk for the development of fatty deposits in the graft and progression to NAFLD and NASH. Therefore, strategies must be implemented both before and after LT to control and prevent the progression of liver disease. These strategies include weight reduction with a low carbohydrate diet and performing regular exercise, avoiding alcohol and smoking, controlling of comorbid metabolic diseases, and controlling immunosuppression medications post-LT.
We also reported a low performance of non-invasive fibrosis tests during the first 18 mo following LT. Similar findings have been reported previously in post-LT patients with HCV recurrence. El-Meteini et al[44] concluded that TE and APRI were not correlated with the degree of fibrosis in liver biopsy done at 3 mo post-LT in 31 patients. Other studies reported a poor diagnostic accuracy of APRI and FIB-4 compared to liver biopsy for the presence of advanced fibrosis post-LT[45,46]. Indeed, some of our patients experienced an important variation in LSM, FIB-4 and APRI particularly during the first 6 mo post-LT. This could be due to several reasons. Inflammation due to congestion or cholestasis is common post-LT and could be one reason for the inaccuracy of fibrosis tests. Fluctuations in liver enzymes and platelets during the first 6 mo may also account for these findings as LT recipients have started receiving and adjusting their immunosuppressive medications. Since a majority of our liver recipients were overweight, this could have interfered with the LSM results[47]. Since our study and the previous studies were performed on small cohorts, a conclusion regarding the accuracy of non-invasive fibrosis tests cannot be made.
There are limitations to our study. The sample size was small which could have interfered with the interpretation of the results. Nevertheless, our incidence rates and predictors are similar to previous retrospective studies[15,31-33]. Additionally, not all patients had available liver biopsy to compare with non-invasive tests. Only 24 out of 40 patients required liver biopsy during follow up therefore the comparison was only possible in these patients, for a total of 35 liver biopsies. Regardless of this, the results obtained from our study provide a rationale for the use of non-invasive tests to frequently monitor this patient population, which could not be feasible with liver biopsy, and can be viewed as an opportunity for larger studies to be done on this topic. Another limitation of our study is that CK-18 is not currently a routine test, as such its application to clinical practice should be further explored. The median study length was 16.8 mo, so in the future we plan to continue following these patients for a longer duration by monitoring CAP scores and re-occurrence of steatosis.
In conclusion, our study showed that LT recipients have a high risk of developing NAFLD and NASH during the first 18 mo following LT, mainly driven by high BMI. While CAP and CK-18 are promising non-invasive tools for diagnosing NAFLD and NASH, LSM and other fibrosis biomarkers are not reliable tests in detecting liver fibrosis in the first month post-transplant. Larger scale, long-term data on the use of non-invasive tests is needed to determine their accuracy to diagnose and monitor disease progression, as well as their prognostic value. These data may result in the implementation of non-invasive tests and optimization of surveillance.
Nonalcoholic fatty liver disease (NAFLD) is a major indication for liver transplant (LT) globally. NAFLD and nonalcoholic steatohepatitis (NASH) may occur after LT.
Studies on the incidence of NASH and NAFLD in the first months following LT are limited.
This work aimed to determine the incidence of NASH and NAFLD in the first 18 mo following LT by means of non-invasive diagnostic tests. It also aimed to investigate the diagnostic accuracy of these non-invasive tests compared to liver histology.
Consecutive adult patients who received LT at a single center were recruited between 2015-2018. Serial measurements of the biomarker cytokeratin 18 (CK-18) and controlled attenuation parameter (CAP) were recorded. NAFLD and NASH were diagnosed by CAP ≥ 270 dB/m, and a combination of CAP ≥ 270 dB/m with CK-18 > 130.5 U/L, respectively. Incidence and predictors of NAFLD and NASH were investigated using survival analysis.
During a median follow-up of 16.8 mo, 63% and 48.5% of 40 LT recipients developed NAFLD and NASH, respectively. The diagnostic accuracy for NAFLD and NASH was 76% and 82%, respectively.
NAFLD and NASH diagnosed by CAP and CK-18 are frequent in LT recipients within the first 18 mo.
To improve post-transplant outcomes, close follow-up with non-invasive tests and metabolic counselling could be considered.
Part of this work has been presented at the Liver Meeting of the American Association for the study of Liver Diseases 2020.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Association for the Study of Liver Diseases; European Association for the Study of the Liver; Canadian Association for the Study of the Liver.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Sporea I, Xu R, Tomoki Sempokuya S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1725] [Cited by in RCA: 1745] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 2. | Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 791] [Article Influence: 52.7] [Reference Citation Analysis (1)] |
| 3. | Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1522] [Cited by in RCA: 1620] [Article Influence: 115.7] [Reference Citation Analysis (1)] |
| 4. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4954] [Article Influence: 707.7] [Reference Citation Analysis (9)] |
| 5. | Mikolasevic I, Filipec-Kanizaj T, Mijic M, Jakopcic I, Milic S, Hrstic I, Sobocan N, Stimac D, Burra P. Nonalcoholic fatty liver disease and liver transplantation - Where do we stand? World J Gastroenterol. 2018;24:1491-1506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 94] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 6. | Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1211] [Cited by in RCA: 1384] [Article Influence: 138.4] [Reference Citation Analysis (1)] |
| 7. | Marchetti P, Navalesi R. The metabolic effects of cyclosporin and tacrolimus. J Endocrinol Invest. 2000;23:482-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Seo S, Maganti K, Khehra M, Ramsamooj R, Tsodikov A, Bowlus C, McVicar J, Zern M, Torok N. De novo nonalcoholic fatty liver disease after liver transplantation. Liver Transpl. 2007;13:844-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Patil DT, Yerian LM. Evolution of nonalcoholic fatty liver disease recurrence after liver transplantation. Liver Transpl. 2012;18:1147-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Sebastiani G, Gkouvatsos K, Plebani M. Non-invasive assessment of liver fibrosis: it is time for laboratory medicine. Clin Chem Lab Med. 2011;49:13-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | European Association for the Study of the Liver. . EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 919] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 12. | Sherman M, Shafran S, Burak K, Doucette K, Wong W, Girgrah N, Yoshida E, Renner E, Wong P, Deschênes M. Management of chronic hepatitis B: consensus guidelines. Can J Gastroenterol. 2007;21 Suppl C:5C-24C. [PubMed] |
| 13. | Shiha G, Sarin SK, Ibrahim AE, Omata M, Kumar A, Lesmana LA, Leung N, Tozun N, Hamid S, Jafri W, Maruyama H, Bedossa P, Pinzani M, Chawla Y, Esmat G, Doss W, Elzanaty T, Sakhuja P, Nasr AM, Omar A, Wai CT, Abdallah A, Salama M, Hamed A, Yousry A, Waked I, Elsahar M, Fateen A, Mogawer S, Hamdy H, Elwakil R; Jury of the APASL Consensus Development Meeting 29 January 2008 on Liver Fibrosis With Without Hepatitis B or C. Liver fibrosis: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL). Hepatol Int. 2009;3:323-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Barrault C, Roudot-Thoraval F, Tran Van Nhieu J, Atanasiu C, Kluger MD, Medkour F, Douvin C, Mallat A, Zafrani ES, Cherqui D, Duvoux C. Non-invasive assessment of liver graft fibrosis by transient elastography after liver transplantation. Clin Res Hepatol Gastroenterol. 2013;37:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Mikolasevic I, Hauser G, Mijic M, Domislovic V, Radic-Kristo D, Krznaric Z, Razov-Radas M, Pavic T, Matasin M, Filipec Kanizaj T. Assessment of Steatosis and Fibrosis in Liver Transplant Recipients Using Controlled Attenuation Parameter and Liver Stiffness Measurements. Can J Gastroenterol Hepatol. 2021;2021:6657047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Siddiqui MS, Idowu MO, Stromberg K, Sima A, Lee E, Patel S, Ghaus S, Driscoll C, Sterling RK, John B, Bhati CS. Diagnostic Performance of Vibration-Controlled Transient Elastography in Liver Transplant Recipients. Clin Gastroenterol Hepatol. 2021;19:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50:1072-1078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 550] [Cited by in RCA: 516] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 18. | Diab DL, Yerian L, Schauer P, Kashyap SR, Lopez R, Hazen SL, Feldstein AE. Cytokeratin 18 fragment levels as a noninvasive biomarker for nonalcoholic steatohepatitis in bariatric surgery patients. Clin Gastroenterol Hepatol. 2008;6:1249-1254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 19. | Feldstein AE, Alkhouri N, De Vito R, Alisi A, Lopez R, Nobili V. Serum cytokeratin-18 fragment levels are useful biomarkers for nonalcoholic steatohepatitis in children. Am J Gastroenterol. 2013;108:1526-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 919] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 21. | Lorente L, Rodriguez ST, Sanz P, Pérez-Cejas A, Padilla J, Díaz D, González A, Martín MM, Jiménez A, Barrera MA. Prognostic Value of Serum Caspase-Cleaved Cytokeratin-18 Levels before Liver Transplantation for One-Year Survival of Patients with Hepatocellular Carcinoma. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Higgins-Biddle JC, Babor TF. A review of the Alcohol Use Disorders Identification Test (AUDIT), AUDIT-C, and USAUDIT for screening in the United States: Past issues and future directions. Am J Drug Alcohol Abuse. 2018;44:578-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 199] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 23. | Tapper EB, Loomba R. Noninvasive imaging biomarker assessment of liver fibrosis by elastography in NAFLD. Nat Rev Gastroenterol Hepatol. 2018;15:274-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 24. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8248] [Article Influence: 412.4] [Reference Citation Analysis (5)] |
| 25. | Arab JP, Hernández-Rocha C, Morales C, Vargas JI, Solís N, Pizarro M, Robles C, Sandoval D, Ponthus S, Benítez C, Barrera F, Soza A, Riquelme A, Arrese M. Serum cytokeratin-18 fragment levels as noninvasive marker of nonalcoholic steatohepatitis in the chilean population. Gastroenterol Hepatol. 2017;40:388-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Benmassaoud A, Ghali P, Cox J, Wong P, Szabo J, Deschenes M, Osikowicz M, Lebouche B, Klein MB, Sebastiani G. Screening for nonalcoholic steatohepatitis by using cytokeratin 18 and transient elastography in HIV mono-infection. PLoS One. 2018;13:e0191985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, Kim YJ, Yoon JH, Cho SH, Sung MW, Lee HS. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1102] [Cited by in RCA: 1070] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 28. | Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1205] [Cited by in RCA: 1168] [Article Influence: 73.0] [Reference Citation Analysis (1)] |
| 29. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3246] [Article Influence: 147.5] [Reference Citation Analysis (0)] |
| 30. | Imai H, Kamei H, Onishi Y, Ishizu Y, Ishigami M, Goto H, Ogura Y. Diagnostic Usefulness of APRI and FIB-4 for the Prediction of Liver Fibrosis After Liver Transplantation in Patients Infected with Hepatitis C Virus. Transplant Proc. 2018;50:1431-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Chayanupatkul M, Dasani DB, Sogaard K, Schiano TD. The Utility of Assessing Liver Allograft Fibrosis and Steatosis Post-Liver Transplantation Using Transient Elastography With Controlled Attenuation Parameter. Transplant Proc. 2021;53:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Karlas T, Kollmeier J, Böhm S, Müller J, Kovacs P, Tröltzsch M, Weimann A, Bartels M, Rosendahl J, Mössner J, Berg T, Keim V, Wiegand J. Noninvasive characterization of graft steatosis after liver transplantation. Scand J Gastroenterol. 2015;50:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Saeed N, Glass L, Sharma P, Shannon C, Sonnenday CJ, Tincopa MA. Incidence and Risks for Nonalcoholic Fatty Liver Disease and Steatohepatitis Post-liver Transplant: Systematic Review and Meta-analysis. Transplantation. 2019;103:e345-e354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 34. | Chon YE, Jung KS, Kim SU, Park JY, Park YN, Kim DY, Ahn SH, Chon CY, Lee HW, Park Y, Han KH. Controlled attenuation parameter (CAP) for detection of hepatic steatosis in patients with chronic liver diseases: a prospective study of a native Korean population. Liver Int. 2014;34:102-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 35. | Xu L, Lu W, Li P, Shen F, Mi YQ, Fan JG. A comparison of hepatic steatosis index, controlled attenuation parameter and ultrasound as noninvasive diagnostic tools for steatosis in chronic hepatitis B. Dig Liver Dis. 2017;49:910-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | He L, Deng L, Zhang Q, Guo J, Zhou J, Song W, Yuan F. Diagnostic Value of CK-18, FGF-21, and Related Biomarker Panel in Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Biomed Res Int. 2017;2017:9729107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 37. | Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology. 2017;66:1486-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 641] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 38. | Ergelen R, Akyuz U, Aydin Y, Eren F, Yilmaz Y. Measurements of serum procollagen-III peptide and M30 do not improve the diagnostic accuracy of transient elastography for the detection of hepatic fibrosis in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2015;27:667-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Rosso C, Caviglia GP, Abate ML, Vanni E, Mezzabotta L, Touscoz GA, Olivero A, Marengo A, Rizzetto M, Bugianesi E, Smedile A. Cytokeratin 18-Aspartate396 apoptotic fragment for fibrosis detection in patients with non-alcoholic fatty liver disease and chronic viral hepatitis. Dig Liver Dis. 2016;48:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Cao W, Zhao C, Shen C, Wang Y. Cytokeratin 18, alanine aminotransferase, platelets and triglycerides predict the presence of nonalcoholic steatohepatitis. PLoS One. 2013;8:e82092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 41. | Pirvulescu I, Gheorghe L, Csiki I, Becheanu G, Dumbravă M, Fica S, Martin S, Sarbu A, Gheorghe C, Diculescu M, Copăescu C. Noninvasive clinical model for the diagnosis of nonalcoholic steatohepatitis in overweight and morbidly obese patients undergoing bariatric surgery. Chirurgia (Bucur). 2012;107:772-779. [PubMed] |
| 42. | Krasnoff JB, Vintro AQ, Ascher NL, Bass NM, Paul SM, Dodd MJ, Painter PL. A randomized trial of exercise and dietary counseling after liver transplantation. Am J Transplant. 2006;6:1896-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 43. | Richards J, Gunson B, Johnson J, Neuberger J. Weight gain and obesity after liver transplantation. Transpl Int. 2005;18:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 224] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 44. | El-Meteini M, Sakr M, Eldorry A, Mohran Z, Abdelkader NA, Dabbous H, Montasser I, Refaie R, Salah M, Aly M. Non-Invasive Assessment of Graft Fibrosis After Living Donor Liver Transplantation: Is There Still a Role for Liver Biopsy? Transplant Proc. 2019;51:2451-2456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Kabbany MN, Conjeevaram Selvakumar PK, Guirguis J, Rivas J, Akras Z, Lopez R, Hanouneh I, Eghtesad B, Alkhouri N. Accuracy of Noninvasive Fibrosis Scores in Predicting the Presence of Fibrosis in Patients after Liver Transplantation. Exp Clin Transplant. 2018;16:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 46. | Kamphues C, Lotz K, Röcken C, Berg T, Eurich D, Pratschke J, Neuhaus P, Neumann UP. Chances and limitations of non-invasive tests in the assessment of liver fibrosis in liver transplant patients. Clin Transplant. 2010;24:652-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Wong GL, Wong VW, Chim AM, Yiu KK, Chu SH, Li MK, Chan HL. Factors associated with unreliable liver stiffness measurement and its failure with transient elastography in the Chinese population. J Gastroenterol Hepatol. 2011;26:300-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |