Published online Dec 27, 2021. doi: 10.4254/wjh.v13.i12.2161
Peer-review started: March 26, 2021
First decision: June 15, 2021
Revised: June 29, 2021
Accepted: October 17, 2021
Article in press: October 17, 2021
Published online: December 27, 2021
Processing time: 275 Days and 16.2 Hours
The coronavirus disease-2019 (COVID-19) pandemic has had a profound worldwide impact. Indeed, it has led to a vast decrease in organ transplantation, including liver transplants (LT). There is little data regarding adjustments made by LT centers as a response to the COVID-19 pandemic.
To assess the experience of LT centers in the United States during the pandemic.
We performed an observational survey study from May 11, 2020 to June 5, 2020. We sent out a 13 question survey to 15 LT centers across the southeastern United States.
Eleven LT centers responded to the survey. We found that (11/11) 100% of transplant centers made adjustments because of the COVID-19 pandemic. At least 50% of transplant centers had at least one transplant recipient infected with COVID-19. To adjust, greater than 50% of centers performed fewer LT, 100% of patients were tested for COVID-19, and most centers implemented a virtual platform.
The COVID-19 pandemic greatly affected liver transplantation in the southeastern United States. It was evident that a concerted effort was made by LT centers to protect their patients and employees from COVID-19 but also to continue the life-saving procedure of LT in this sick patient population. Further studies are needed to assess how LT centers around the world managed the pandemic in order to learn strategies to continue life-saving procedures in this patient population.
Core Tip: The coronavirus disease-2019 (COVID-19) pandemic tremendously affected solid organ transplantation around the world, but little information has been published regarding adaptation from transplant centers. We performed a survey study of 11 Liver transplant (LT) centers in the southeastern United States. 100% of transplant centers made adjustments. COVID-19 testing of transplant candidates, virtual clinic visits, and use of remote allocation of staff were among the most commonly utilized strategies. These strategies can be advantageously used in LT centers in the future. We recommend contingency plans be in place in case of future unprecedented states of emergency.
- Citation: Gonzalez AJ, Kapila N, Thomas E, Pinna A, Tzakis A, Zervos XB. Managing liver transplantation during the COVID-19 pandemic: A survey among transplant centers in the Southeast United States. World J Hepatol 2021; 13(12): 2161-2167
- URL: https://www.wjgnet.com/1948-5182/full/v13/i12/2161.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i12.2161
The coronavirus disease-2019 (COVID-19) pandemic brought forth new challenges for transplant centers in countries all around the world. Concern for the safety of transplant donors, recipients and hospital staff, in addition to a scarcity of hospital resources allocated to organ transplantation, led to a steep decline in the number of transplanted organs worldwide[1].
In the early stages of the pandemic, limited guidance was offered to liver transplant (LT) centers in regards to the appropriate policies and practices of proceeding with transplantation. To date, there is little data regarding adjustments made by LT centers in response to the COVID-19 pandemic. In this study, we assess the impact of COVID-19 on LT centers early in the pandemic and the adjustments that these centers made in the setting of an unprecedented crisis.
We performed an observational, survey-based study using a 13-question survey (Figure 1). The questionnaire (Table 1) was created and distributed using an emailed link to Qualtrics (Provo, UT). The questionnaire included both automatic and fill in responses. The technical functionality and ease of use of the electronic questionnaire had been tested before sending out the questionnaire. We identified transplant hepatologists from 15 LT centers in the Southeast United States. Contact information of transplant hepatologists was obtained from a database maintained by the Southeastern division of the American Liver Foundation. Participants were not compensated. Survey participants were informed of the survey details via electronic mail. On May 11, 2020, the questionnaire was sent via electronic mail. The deadline to respond to the questionnaire was June 5, 2020. Only questionnaires that were entirely completed were analyzed. The CHERRIES guidelines were used to further describe the methodology and results of our survey.
| Questionnaire |
| 1 What percentage of your office staff is working remotely? |
| 2 What percentage of your visits is now virtual? |
| 3 How many transplants have been performed in the last 2 mo? |
| 4 What percentage of your donors is screened for COVID-19? |
| 5 What percentage of your candidates is screened for COVID-19? |
| 6 Do you have a dedicated COVID-free ICU space? |
| 7 Is there a current MELD cut-off for new evaluations to occur? |
| 8 Are you currently rotating providers in teams to minimize exposure? |
| 9 Are you flying out for donors? |
| 10 Is there direct communication with UNOS regarding operations of your program? |
| 11 What is the comparison of liver transplants in the past 2 mo to the same time frame in 2019? |
| 12 How many of your transplanted patients contracted the COVID-19 virus? |
| 13 What were the outcomes of those infected? |
Results of the questionnaire were analyzed using statistics of central tendency. All data analyses were conducted using SAS version 9.4 (Cary, NC). As this was a survey study without the review of specific patient data, IRB approval was not obtained.
Of the 15 transplant centers, 11 (73.3%) responded to the questionnaire. All of the centers are academic-based institutions. Nine different cities in 6 different states across the southeastern United States were represented. Ten (91%) of the transplant centers had a dedicated COVID-free space in the intensive care unit (ICU).
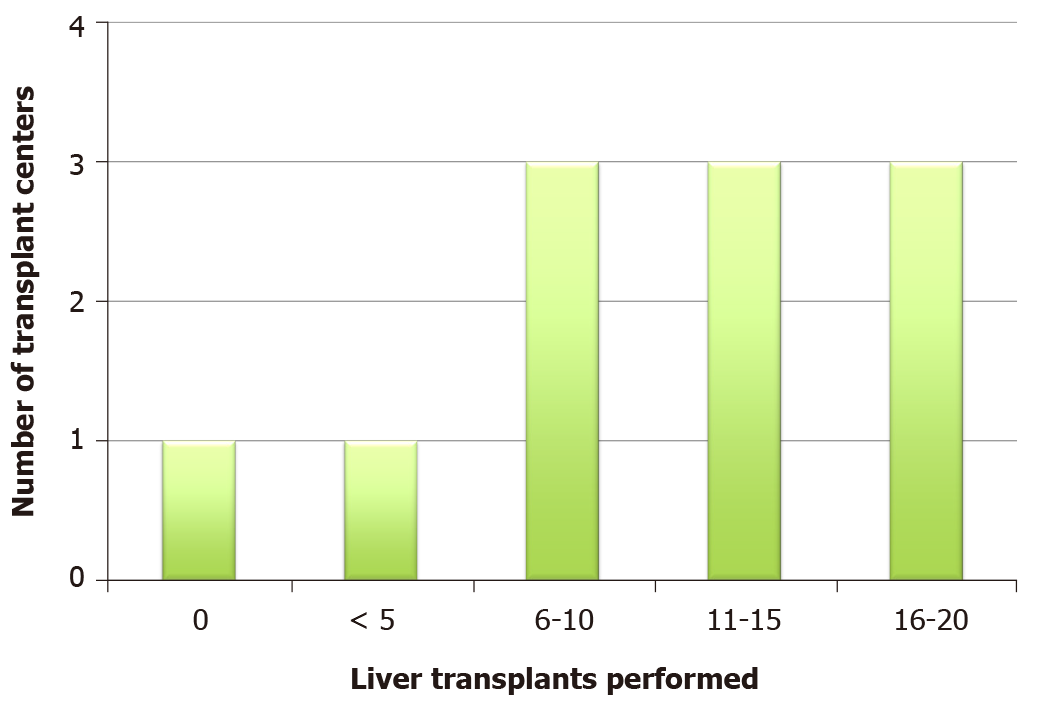
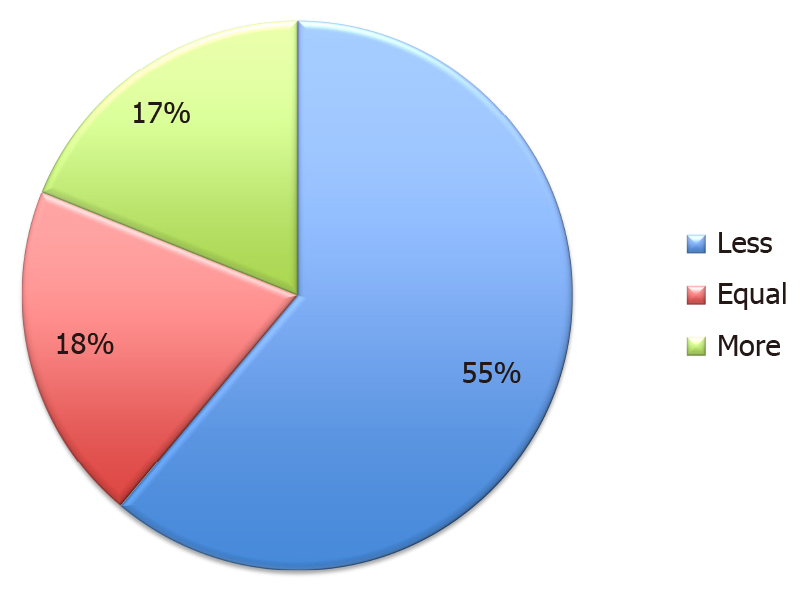
Most participating centers performed at least 11 transplants during the preceding 8 wk (Figure 1), ranging from 0 to 20 transplants. Five of 11 centers performed less than 10 transplants. Compared to the previous year, 6 (55%) centers performed less LTs (Figure 2). This included a single center where LT services were stopped altogether. Six (55%) centers had at least 1 recipient infected with COVID-19. During the study period, the mean number of infected transplant recipients per center was 1.8.
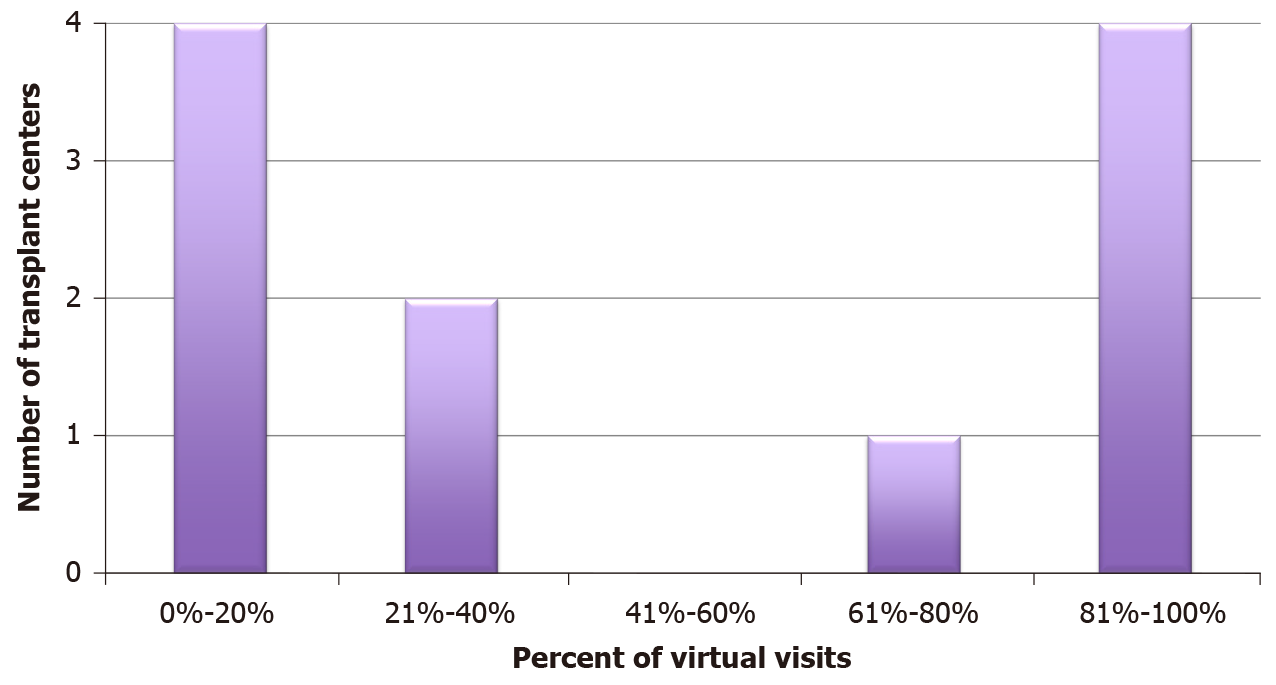
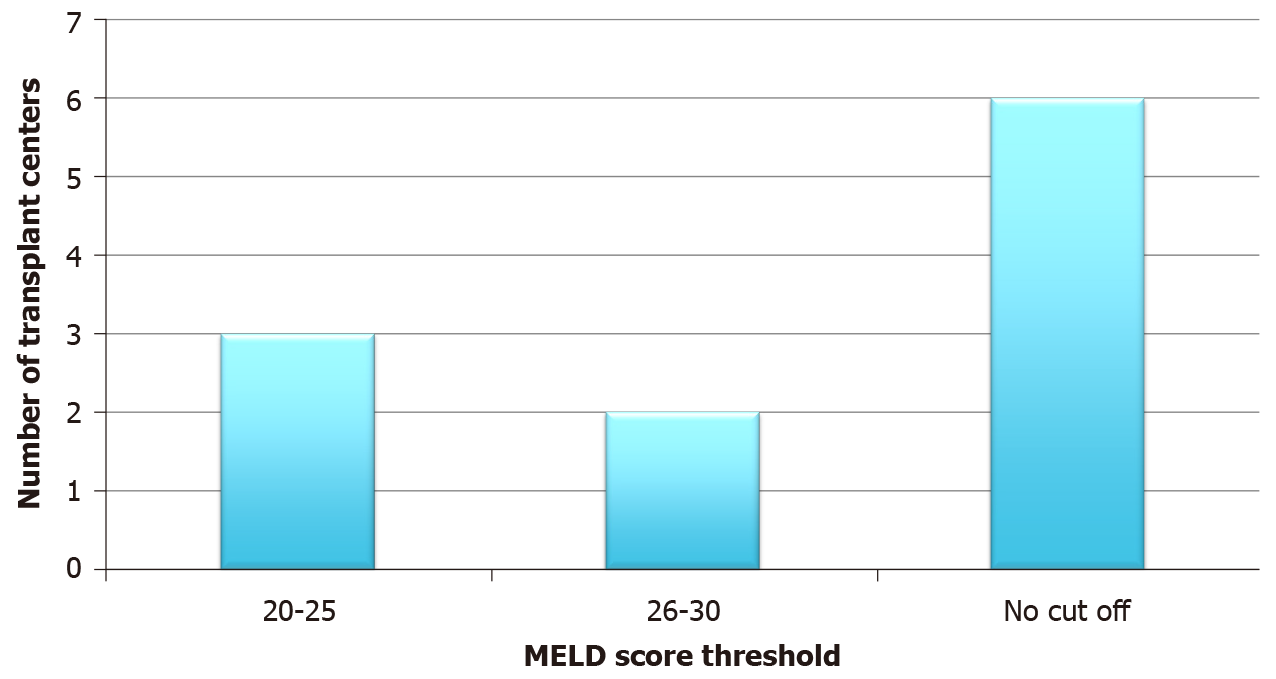
All centers routinely tested donors and recipients for COVID-19. During the study period, 58% of clinic visits were conducted virtually, and all centers reported at least some degree of telehealth medicine (Figure 3). On average, 73% of each transplant center’s staff was assigned to work remotely. Transplant centers attempted to minimize exposure and institutions rotated 72.7% of their providers to minimize exposure. Less than half (45%) of transplant centers had a model for end stage liver disease (MELD) cut-off. For those centers that implemented a cut-off, 25 was the median MELD (Figure 4). All 5 centers that used a MELD cut-off performed less transplants than the year prior. More than half (55%) of the centers continued to fly to procure organs. Centers that continued to fly out for donors performed an average of 15 transplants compared to 9 transplants in centers that stopped flying out for donors. Fifty-five percent of centers had direct communication with United Network for Organ Sharing (UNOS). The centers that did not communicate with UNOS also did not fly out for organs and performed fewer transplants on average (8 vs 12).
The COVID-19 pandemic presented transplant centers with the unique challenge of providing potential life-saving therapy in the midst of an unprecedented public health crisis. Although several studies have investigated the effects of COVID-19 on rates of transplantation and outcomes in LT recipients[2-5], few have assessed the policy adjustments that centers were forced to implement[6]. To our knowledge, our study is the first to study the early effects of the COVID-19 pandemic, specifically on liver transplant centers, and the steps taken by these centers to provide care to their patients.
The response rate to our survey was at 73%. A recent study that surveyed clinicians on practices and policies at abdominal transplant programs in the United States found a similarly high response rate of 79.3%[6]. This suggests that transplant physicians have a keen interest to improve their understanding and adjust their practice in the midst of the COVID-19 pandemic. At the time of our study, there was limited guidance on appropriate practices and policies for LT programs during the pandemic. In fact, it was not until the third week of April 2020 that the American Association for Study of Liver Disease released a consensus statement from a panel of experts that offered guidance on management during the pandemic[7]. Nearly half of the surveyed centers maintained direct communication with UNOS for guidance[8]. Considering the magnitude of the pandemic and the many challenges that LT programs were therefore forced to manage, we expected more programs to have been in communication with UNOS for guidance during this unprecedented period.
Over the past year, several studies[1] have shown decreases in all types of solid organ transplantation due to the COVID-19 pandemic similar to our findings. The decrease in transplantation is due to many reasons including a paucity of supplies, limited ICU space[6], decreased nursing and medical staff, and the uncertainty of post-transplant care and immunosuppression during the pandemic[9,10]. The majority (90.9%) of centers in our study continued performing LT, albeit often at a limited capacity, thus highlighting the importance of continuing these life-saving procedures. A single center ceased performing all LT. It was also the only center without a dedicated, COVID-free space in the ICU, thus underscoring the tremendous impact that limited resources had on transplant centers during the pandemic. Due to concerns for safety and limited resources, nearly half of centers stopped flying for organ procurement and made use of locally available donors. This may serve as a future impetus for an increased focus on local organ donations.
The safety of liver transplant recipients and hospital staff has been an area of concern since the onset of the COVID-19 pandemic. Nearly 3% of people that have been infected with COVID-19 are healthcare workers[11]. Additionally, several studies have shown that COVID-19 infection rate may be higher in LT recipients, although outcomes are similar when compared to the general population[3,5]. During the study period, a majority (55%) of centers reported at least one transplant recipient with COVID-19 infection. No center reported a COVID-19 related mortality; however, since the survey was conducted the number of patients infected and the mortality is likely to have changed.
At the onset of the pandemic, transplant centers took steps to ensure the safety of liver transplant staff and recipients. Some of the interventions put in place included testing all LT candidates and donors for COVID-19, utilizing a virtual visit platform, and rotating staff to work remotely. Similar to what was reported in other studies[12,13], all centers used telemedicine to some capacity. Transplant centers may have been better equipped to adapt to telemedicine due the basic infrastructure that is required for normal operations. Our survey shows that the pandemic changed centers’ approach to telemedicine. Though imperfect in many ways, telemedicine has broadened the reach of transplant programs and has given patients increased access to transplant providers[13].
Our study adds to the growing data[6,14,15] regarding the management and policies of LT during the COVID-19 pandemic. Our study provides a unique perspective to the practice of transplant centers in the Southeast United States, which was a “hotspot” for COVID-19, albeit after the initial wave that affected the New York City region. Also, we had a high response rate to our survey, allowing us to better understand the practices in the majority of centers in the region.
We had several limitations to our study. The primary limitation was the sample size with the inclusion of 11 transplant centers. Though the number of centers was limited, our goal was to highlight the practices of a unique region in the United States. Our survey was only distributed to transplant hepatologists and did not include surgeons and other transplant staff that may have offered more perspective on their centers’ practices. Although the peak of the pandemic has passed, this study is a learning opportunity and an encouragement to develop contingency plans for possible future public health emergencies. Finally, due to the nature of the study, there is the possibility of recall bias.
COVID-19 changed the practice of medicine across the world, and in our study, we highlight how COVID-19 affected LT practices in the Southeast United States. Our study offers a unique perspective to how individual transplant centers adapted their practice and created their own strategies in response to the COVID-19 public health emergency, despite the lack of clear guidelines. Moving forward, the transplant community should use this experience as an important learning opportunity and as a chance to develop contingency plans for future public health emergencies, natural disasters, and other emergency situations. This may be in the form of specific preemptive guidelines, emergency committees, and resources for communication. These strategies are imperative to continue efficiently performing these life-saving procedures, even during unprecedented situations.
The coronavirus disease-2019 (COVID-19) pandemic greatly affected liver transplant (LT) centers. This is the first study to investigate the effects of COVID-19 specifically on LT centers and the adjustments made by them to provide care to their patients.
There is limited data on policy adjustments made by LT centers during the pandemic. Our findings can help guide transplant centers during future health care emergencies but also to encourage the development of contingency plans for possible future public health emergencies.
Our main aim was to assess the experience of southeastern United States LT centers during the COVID-19 pandemic. Specifically, we wanted to see how the pandemic affected LT centers and the adjustments made by the centers. We were able to realize these objectives.
We performed an observation, survey-based study using a 13-question survey. The survey was sent via electronic mail to 15 LT centers across the Southeastern United States.
Eleven of fifteen LT centers responded. 100% of centers made adjustments during the COVID-19 pandemic. Greater than 50% of centers performed fewer LTs. 100% of patients were tested for COVID-19, and most centers implemented a virtual platform.
LT centers varied in their policy adjustments during the COVID-19 pandemic. This was likely due to the lack of clear guidelines. Going forward, the transplant community should use this experience as an important learning opportunity and galvanize contingency plans for possible future public health emergencies.
Future studies should assess the most effective way to establish and implement clear guidelines to continue liver transplantation during emergency situations. Future studies should also assess which policy adjustments made during the COVID-19 pandemic were safest and most effective in continuing liver transplantation.
Provenance and peer review: Invited article; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gallo G S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Loupy A, Aubert O, Reese PP, Bastien O, Bayer F, Jacquelinet C. Organ procurement and transplantation during the COVID-19 pandemic. Lancet. 2020;395:e95-e96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 209] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 2. | Sahin TT, Akbulut S, Yilmaz S. COVID-19 pandemic: Its impact on liver disease and liver transplantation. World J Gastroenterol. 2020;26:2987-2999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 3. | Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5:532-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 205] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 4. | Becchetti C, Zambelli MF, Pasulo L, Donato MF, Invernizzi F, Detry O, Dahlqvist G, Ciccarelli O, Morelli MC, Fraga M, Svegliati-Baroni G, van Vlierberghe H, Coenraad MJ, Romero MC, de Gottardi A, Toniutto P, Del Prete L, Abbati C, Samuel D, Pirenne J, Nevens F, Dufour JF; COVID-LT group. COVID-19 in an international European liver transplant recipient cohort. Gut. 2020;69:1832-1840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 5. | Colmenero J, Rodríguez-Perálvarez M, Salcedo M, Arias-Milla A, Muñoz-Serrano A, Graus J, Nuño J, Gastaca M, Bustamante-Schneider J, Cachero A, Lladó L, Caballero A, Fernández-Yunquera A, Loinaz C, Fernández I, Fondevila C, Navasa M, Iñarrairaegui M, Castells L, Pascual S, Ramírez P, Vinaixa C, González-Dieguez ML, González-Grande R, Hierro L, Nogueras F, Otero A, Álamo JM, Blanco-Fernández G, Fábrega E, García-Pajares F, Montero JL, Tomé S, De la Rosa G, Pons JA. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74:148-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 270] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 6. | Boyarsky BJ, Po-Yu Chiang T, Werbel WA, Durand CM, Avery RK, Getsin SN, Jackson KR, Kernodle AB, Van Pilsum Rasmussen SE, Massie AB, Segev DL, Garonzik-Wang JM. Early impact of COVID-19 on transplant center practices and policies in the United States. Am J Transplant. 2020;20:1809-1818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 192] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 7. | Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, Pratt DS, Russo MW, Schilsky ML, Verna EC, Loomba R, Cohen DE, Bezerra JA, Reddy KR, Chung RT. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72:287-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 422] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 8. | COVID-19 Resources for Organ Transplants and Donations. [cited 25 March 2021]. In: UNOS. Available from: https://unos.org/covid/. |
| 9. | Forns X, Navasa M. Liver transplant immunosuppression during the covid-19 pandemic. Gastroenterol Hepatol. 2020;43:457-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Ridruejo E, Soza A. The liver in times of COVID-19: What hepatologists should know. Ann Hepatol. 2020;19:353-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 11. | CDC COVID-19 Response Team. Characteristics of Health Care Personnel with COVID-19 - United States, February 12-April 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:477-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 522] [Cited by in RCA: 581] [Article Influence: 116.2] [Reference Citation Analysis (0)] |
| 12. | Bokolo AJ. Exploring the adoption of telemedicine and virtual software for care of outpatients during and after COVID-19 pandemic. Ir J Med Sci. 2021;190:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 194] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 13. | Smith AC, Thomas E, Snoswell CL, Haydon H, Mehrotra A, Clemensen J, Caffery LJ. Telehealth for global emergencies: Implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare. 2020;26:309-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1194] [Cited by in RCA: 1045] [Article Influence: 209.0] [Reference Citation Analysis (0)] |
| 14. | Strauss AT, Boyarsky BJ, Garonzik-Wang JM, Werbel W, Durand CM, Avery RK, Jackson KR, Kernodle AB, Baker T, Snyder J, Segev DL, Massie AB. Liver transplantation in the United States during the COVID-19 pandemic: National and center-level responses. Am J Transplant. 2021;21:1838-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 15. | Raveh Y, Simkins J, Vianna R, Tekin A, Nicolau-Raducu R. A Less Restrictive Policy for Liver Transplantation in Coronavirus Disease 2019 Positive Patients, Based Upon Cycle Threshold Values. Transplant Proc. 2021;53:1126-1131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |