Published online Dec 27, 2021. doi: 10.4254/wjh.v13.i12.2113
Peer-review started: May 27, 2021
First decision: July 6, 2021
Revised: July 7, 2021
Accepted: November 25, 2021
Article in press: November 25, 2021
Published online: December 27, 2021
Processing time: 213 Days and 16.9 Hours
Previous reports showed some beneficial effect of oral vancomycin treatment (OVT) in children with primary sclerosing cholangitis; conversely, the experience in patients with other autoimmune liver diseases (AILD), including autoimmune hepatitis (AIH) and autoimmune sclerosing cholangitis (ASC), is scant.
To assess the response to immunosuppressive treatment (IS) and to OVT in children diagnosed with AILD.
Retrospective study of children diagnosed with AIH (normal biliary tree at cholangiography) and ASC (abnormal biliary tree at cholangiography) in the last 10 years. All underwent standard immunosuppressive therapy (IS), but non-responders received also OVT. Biochemical remission [normal aspartate aminotransferase (AST)] and immunological remission (normal IgG and negative autoantibodies) rates and Sclerosing Cholangitis Outcomes in Pediatrics (SCOPE) index were assessed and compared during the follow up.
75 children were included [69% female, median age 10.5 years (5.6-13.4 years), AIH = 54, ASC= 21]. Sixty-three patients (84%, AIH = 52, ASC = 11) were treated with standard IS and 61 achieved biochemical remission, whereas 12 not responding to IS [16%, F = 75%, median age 13.5 years, (12.2-15.7), 10 with ASC] required OVT and 8 achieved biochemical remission. Overall OVT increased the biochemical remission rate of the whole group of AILD patients from 81% (61/75) to 92% (69/75). Median values of AST, alanine aminotransferase (ALT) and gamma-glutamyl transferase (GGT) decreased significantly after OVT start (P < 0.05). Complete normalization of livers enzymes (AST, ALT and GGT) was observed in 6/12 patients (50%). Decrease in SCOPE index score was reported in 5/12 patients (42%). At last follow up (median of 4.4 years, range 0.6-13.8 years) all 75 patients are alive, 6 (8%, 1 with ASC) successfully discontinued medications, 1 (with ASC) required liver transplantation.
Children with AIH and ASC respond well to IS treatment. OVT may represent a valuable treatment option to achieve biochemical remission in patients not responding to standard IS. These promising preliminary results suggest that a prospective study is indicated to define the efficacy of OVT in AILD.
Core Tip: Experience with oral vancomycin in children with autoimmune liver disease (AILD) is limited. We enrolled 75 children [median age 10.5 years (5.6-13.4)], 54 with autoimmune hepatitis and 21 with autoimmune sclerosing cholangitis; 63/75 achieved remission by standard immunosuppressive therapy (IS), whereas 12/75 (16%) required oral vancomycin treatment (OVT). In 6/12 patients (50%) the response was complete, whereas it was partial in 2/12 (17%), and absent in 4/12 (33%). Overall OVT increased the remission rate of the whole group of AILD patients from 81% to 92%. OVT may represent a valuable treatment option in children with AILD who do not respond to standard IS.
- Citation: Di Giorgio A, Tulone A, Nicastro E, Norsa L, Sonzogni A, D'Antiga L. Use of oral vancomycin in children with autoimmune liver disease: A single centre experience. World J Hepatol 2021; 13(12): 2113-2127
- URL: https://www.wjgnet.com/1948-5182/full/v13/i12/2113.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i12.2113
Pediatric autoimmune liver disease (AILD) is a progressive inflammatory condition including autoimmune hepatitis (AIH), (diagnosed with the standard criteria) and autoimmune sclerosing cholangitis (ASC), (defined as patients fulfilling the criteria for AIH but with an abnormal biliary tree at cholangiography)[1-3].
Children with AILD respond well to immunosuppressive (IS) treatment, although some patients progress to cirrhosis despite normal liver enzymes; a low proportion (30%-40%) achieve immunological remission (normal IgG and negative autoanti
Empirical use of candidate therapies for AILD has significantly increased in the last decades, in the attempt of finding effective medications to normalize liver enzymes and improve outcomes; oral vancomycin is one of the most common drugs empirically used in patients with SC[5-7]. Oral vancomycin is supposed to have an immunomodulatory effect by inducing an increase of T-regs lymphocytes and TGF-β (both with anti-inflammatory activity) without alterations in Th1 or Th2 cytokine production patterns[6-9]. Cox et al[12] reported benefits from oral vancomycin treatment (OVT) in children with primary SC (PSC) and inflammatory bowel disease (IBD). Interestingly, OVT seems to be able to modify the gut microbiota and bile acid metabolism, that may have a protective effect on PSC recurrence after LT[10-12].
Previous studies have offered information on the use of OVT in adults and children with PSC; conversely the experience with OVT in children with AIH or ASC not responding to standard IS is very limited[5-7].
In our center we empirically used oral vancomycin in a small series of children with AIH and ASC not responding to standard IS to gather insights that could guide us to the design of a prospective clinical trial.
In this study, we aimed to review our cohort of pediatric patients with AILD to assess: (1) The response to standard IS treatment; and (2) The efficacy of OVT to achieve biochemical and immunological remission in patients not responding to standard IS.
We reviewed retrospectively the medical records of children diagnosed with AILD (AIH or ASC) at Hospital Papa Giovanni XXIII, Bergamo, Italy, between 2010 and 2021. During this period of time all patients were diagnosed by the standard diagnostic criteria including magnetic resonance cholangiopancreatography (MRCP) performed at diagnosis; OVT was regularly adopted in patients not responding to standard treatment. Biochemical and clinical features, histology, and data on outcomes were collected in all patients and compared between the two groups divided according to the diagnosis (AIH vs ASC) and OVT.
The diagnosis of AILD was based on elevated transaminases and IgG levels, positive autoantibodies, compatible liver histology, and exclusion of other liver diseases[13]. A lower threshold for autoantibody positivity was applied to children compared to adults, i.e., titre ≥ 1:20 for antinuclear antibodies (ANA) and smooth muscle antibodies (SMA) and ≥ 1:10 for anti-liver kidney microsomal type 1 (anti-LKM-1) were used, as indicated by the International Autoimmune Hepatitis Group (IAIHG) consensus statement on liver autoimmune serology[14] and more recently by the European Society of Paediatric Gastroenterology, Hepatology and Nutrition[3]. Patients without cholangiopathy on MRCP were diagnosed as AIH type 1 (AIH-1, positivity for SMA and/or ANA) or type 2 (AIH-2, positivity for LKM-1 and/or LC1)[1,3]. Patients with cholangiopathy were diagnosed as ASC[1,3]. Patients with histological diagnosis of ASC but normal cholangiogram were classified as small duct ASC[3].
Clinical presentation was classified as: (1) Acute (malaise, nausea/vomiting, abdominal pain, jaundice, dark urine, pale stools); (2) Insidious (fatigue, headache, amenorrhoea, joint pain); and (3) Asymptomatic (incidental finding of abnormal liver function tests during investigation of non-hepatic conditions, including IBD). Protocol and description of autoantibodies detection and histological features suggestive for biliopathy are reported in our previous studies[4].
IS treatment consisted of first line use of oral prednisone at a dose of 2 mg/kg/d (up to a maximum of 60 mg/d) for 10-14 d followed by 4-6 wk tapering schedule to reach a total maintenance dose of 5 or 2.5 mg/d (depending on age). Blood tests during induction of remission were checked weekly to monitor the response to treatment and side effects. If the response was not satisfactory, azathioprine was added [starting dose 0.5 mg/kg/d, increased weekly to 1.5 mg/kg/d (maximum dose 2-2.5 mg/kg/d) in the absence of side effects or leukopenia] until normal transaminase levels were achieved. Mycophenolate mofetil (MMF, as second line treatment) and calcineurin inhibitors (cyclosporine or tacrolimus, as third line treatment) were used when standard treatment failed or azathioprine was contraindicated. Patients with ASC were also administered ursodehoxycholic acid (UDCA) at the dose of 15-20 mg/kg/d[3,15].
Patients not responding to standard immunosuppression underwent liver biopsy to assess the degree of inflammation and the stage of biliopathy as per criteria defined in our previous study[16].
OVT was given to patients who did not respond to first/second line treatment and who had on histology features of biliopathy without (or mild) portal-periportal inflammation. OVT was started at the dose of 50 mg/kg/d (divided in 3 doses, maximum dose 1500 mg/d), for 6 mo. In patients who did not respond, OVT was discontinued after 6 mo, whereas it was continued in responders.
Conversely, in children having on histology moderate/severe inflammatory infiltrate, a temporary increase of oral prednisone and conversion from azathioprine to MMF or from MMF to tacrolimus were prescribed[3], and OVT was not commenced.
We considered OVT-related side-effects the following symptoms: Fever, chills, rash, fatigue, gastroenterological symptoms (abdominal pain, persistent diarrhea), nephrotoxicity, neutropenia, ototoxicity, thrombocytopenia, antibiotic-resistant infections and neurological symptoms[5].
Biochemical remission was defined as normal transaminase levels; immunological remission was normal transaminase and IgG levels with negative/Low titer (ANA/SMA < 1:20) of autoantibodies; histological remission was the absence of inflammation on liver histology. Relapse was defined as transaminase levels ≥ 2-fold the upper limit of normal (ULN)[3].
In patients receiving OVT, the values of aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), serum IgG and autoantibodies were reported before and after treatment.
Biochemical response to OVT was classified as follows:
Complete response: AST, ALT and GGT returning within normal values (NV);
Partial response: AST, ALT or GGT levels decreasing to < 1.5 × ULN, but not reaching NV;
No response: No significant changes in liver enzymes.
Discontinuation of IS treatment was attempted in patients with normal transaminases and IgG, negative or low positive titer of autoantibodies at least 3 years after starting IS treatment, and no inflammation on follow up histology[3].
The Sclerosing Cholangitis Outcomes in Pediatrics (SCOPE) index includes 5 parameters which correlate with long-term outcomes in children with SC. The model stratifies patients as low, medium, or high risk based on progression to transplant or death (rates of < 1%, 3%, or 9% annually) and to hepatobiliary complications, including portal hypertension or biliary strictures (rates of 2%, 6%, and 13% annually)[17]. In this study, we assessed whether the SCOPE index score was improved, stable or worsened after OVT.
Data are reported as medians and interquartile range unless specified differently. Baseline measures and data on outcome were compared between AIH and ASC to see whether they differed. Paired t test/Mann-Whitney U test were used for continuous variables and chi-square/Fisher exact test for categorical variables. A P value of 0.05 or less was assigned significance. The analysis was performed with IBM-SPSS 13.0 for Windows. The statistical methods of this study were reviewed by one of the authors (EN) who is an expert statistician.
Seventy-five patients were diagnosed with AILD [AIH = 54 (type 1 n = 42, type 2 n = 12), ASC = 21] during the study period. Median age at diagnosis was 10.5 years (5.6-13.4) without differences between the two groups (P > 0.05). Female predominance was 69% (AIH = 72 %, ASC = 62%). The most common type of presentation was acute (35%, 43% in AIH vs 14% in ASC, P = 0.011), followed by asymptomatic (33%) and insidious (32%), the latter more common in ASC group (57% in ASC vs 22% in AIH, P = 0.005). IBD was reported in 18 patients [24%, ulcerative colitis (UC) in 12, Crohn’s disease (CD) in 2 and IBD-unclassified (IBD-U) in 4 patients], mainly in ASC group (57% vs 11% in AIH group, P < 0.001). Associated autoimmune disorders were reported in 13/75 patients (17%, AIH = 17% and ASC = 14%) including coeliac disease in 4 (with AIH), autoimmune thyroiditis in 3 (1 with AIH), diabetes mellitus type 1 in 2 (both with AIH), psoriasis in 2 (both with AIH), idiopathic arthritis in 1 (with ASC), nephrotic syndrome in 1 (with ASC).
At diagnosis, all but one patient (F, with ASC, already on treatment for IBD) had raised transaminases; GGT was increased in 63 patients (84%) and normal in 12 (16%, all with AIH). Median values of AST, ALT, GGT, total bilirubin, ALT/AST ratio, international normalized ratio and platelets were significantly different between AIH and ASC (Table 1). Autoantibodies were positive in all (100%). No patient with ASC was positive for anti-LKM-1 and/or LC-1. Positivity for anti-neutrophil cytoplasmatic antibodies was more common in ASC patients (71% vs 41% in AIH, P < 0.05). Raised IgG was reported in 68% of patients (51/75) without differences between the two groups (P > 0.05). Liver biopsy was performed in all patients with similar prevalence of interface hepatitis, cirrhosis and biliary features in the two groups (P > 0.05) (Table 1).
| All patients, n = 75 | AIH, n = 54 | ASC, n = 21 | P value | |
| Biochemical profile | ||||
| AST U/L (NV ≤ 45) | 438 (129-982) | 678 (204-1200) | 150 (94-333) | < 0.001 |
| GGT U/L (NV ≤ 50) | 116 (60-296) | 107 (54-196) | 360 (68-607) | < 0.001 |
| Total bilirubin (NV ≤ 1 mg/dL) | 1.7 (0.6-4.5) | 2.7 (0.6-5.3) | 1.2 (0.7-2.5) | 0.05 |
| ALP (NV ≤ 350 U/L) | 296 (204-469) | 283 (199-462) | 301 (242-494) | 0.328 |
| ALP/AST ratio | 0.7 (0.3-2.2) | 0.4 (0.2-1.6) | 2.3 (0.7-3.5) | 0.002 |
| Albumin (NV: 30-50 g/dL) | 42 (38-44) | 42 (37-44) | 42 (40-46) | 0.082 |
| INR (NV: 0.9-1.2) | 1.2 (1.1-1.5) | 1.2 (1.1-1.6) | 1.1 (1.0-1.2) | < 0.05 |
| Platelet (109/L) | 252 (180-350) | 234 (167-314) | 319 (251-393) | < 0.05 |
| Autoimmune profile | ||||
| ANA (≥ 1:20): n (%) | 55 (73) | 38 (70) | 17 (81) | 0.777 |
| SMA (≥ 1:20): n (%) | 53 (71) | 38 (70) | 15 (71) | 1 |
| Anti-LKM-1 (≥ 1:10): n (%) | 12 (16) | 12 (22) | 0 (0) | < 0.05 |
| Anti-LC1: n (%) | 9 (12) | 9 (17) | 0 (0) | < 0.05 |
| ANCA: n (%) | 37 (49) | 22 (41) | 15 (71) | < 0.05 |
| IgG g/dL (NV: 0.5-1.8 g/dL) | 2.0 (1.4-3.2) | 2.3 (1.4-3.3) | 1.7 (1.5-2.2) | 0.325 |
| IgG > ULN: n (%) | 51 (68) | 37 (69) | 14 (67) | 1 |
| Histology, n (%) | ||||
| Interface hepatitis | 51 (68) | 42 (78) | 12 (57) | 0.09 |
| Fibrosis | 61 (81) | 42 (78) | 19 (90) | 0.324 |
| Cirrhosis | 17 (23) | 15 (28) | 2 (10) | 0.127 |
| Features of biliopathy1 | 62 (83) | 37 (68) | 17 (81) | 0.764 |
Medications used in our cohort of patients are reported in Table 2. The association between prednisone/azathioprine was more common in AIH patients (52% vs 10% in ASC, P < 0.001); conversely the association between prednisone/MMF/OVT was commonly used in ASC patients (23% vs 2% in AIH, P = 0.005) (Table 2).
| Variables | All patients, n = 75 | AIH, n = 54 | ASC, n = 21 | P value |
| Treatment, n | ||||
| Prednisone alone | 26 (35%) | 19 (35%) | 7 (33%) | 1 |
| Prednisone + Azathioprine | 30 (40%) | 28 (52%) | 2 (10%) | < 0.001 |
| Prednisone + MMF | 5 (7%) | 3 (5%) | 2 (10%) | 0.615 |
| Prednisone + Vancomycin | 4 (5%) | 1 (2%) | 3 (14%) | 0.064 |
| Prednisone + Azathioprine + Vancomycin | 2 (3%) | 0 (0%) | 2 (10%) | 0.075 |
| Prednisone + MMF + Vancomycin | 6 (8%) | 1 (2%) | 5 (23%) | 0.005 |
| Prednisone + Tacrolimus | 1 (1%) | 1 (2%) | 0 | NA |
| Cyclosporine | 1 (1%) | 1 (2%) | 0 | NA |
| Response to treatment | ||||
| Normal AST (NV ≤ 45 U/L): n | 69 (92%) | 52 (96%) | 17 (81%) | 0.048 |
| Time to normalize AST (yr) | 0.1 (0.1-0.5) | 0.2 (0.1-0.6) | 0.1 (0.1-0.2) | 0.19 |
| GGT (< 50 UI/L), n | 68 (91%) | 51 (94%) | 17 (81%) | 0.811 |
| Time to normalize GGT (yr) | 0.3 (0.2-0.6) | 0.3 (0.2-0.5) | 0.3 (0.2-1.1) | 0.062 |
| Immunological remission1: n | 25 (33%) | 22 (40%) | 3 (14%) | 0.032 |
| Time to immunological remission | 3.1 (2.2-4.2) | 3.8 (2.9-4.3) | 3.4 (3.2-3.7) | 0.86 |
| Relapse of AILD during treatment, n | ||||
| At least one relapse | 36 (48%) | 22 (41%) | 14 (67%) | < 0.070 |
| 1 relapse alone | 26 (35%) | 17 (31%) | 9 (43%) | 0.421 |
| ≥ 2 relapses | 10 (13%) | 5 (9%) | 5 (24%) | 0.131 |
| Outcome at last follow up | ||||
| Median follow up, yr (range) | 4.4 (0.6-13.8) | 4.1 (1.2-11.7) | 4.5 (0.6-13.8) | 0.079 |
| Alive | 75 (100%) | 54 (100%) | 21 (100%) | NA |
| OFF-IS therapy | 6 (8%) | 5 (9%) | 1 (5%) | 0.666 |
| Medical treatment | 68 (91%) | 49 (91%) | 19 (90%) | 1 |
| Liver transplant | 1 (1%) | 0 (0%) | 1 (5%) | 0.28 |
Sixty-nine patients (92%, AIH = 96% vs ASC = 81%, P = 0.048) normalized transaminase levels and achieved biochemical remission at a median of 0.1 years (0.1-0.5) after starting standard medical treatment; 74 patients (98%, AIH = 100% and ASC = 95%) reduced AST levels to < 2 × ULN (AST NV 45 IU/L).
Sixty-eight patients (91%) normalized GGT levels at a median of 0.3 years (0.2-0.9) after starting standard medical treatment. Median time to GGT normalization tended to be significantly higher in ASC patients (P = 0.06); 71 patients (95%, AIH 98% and ASC = 85%) reduced GGT levels to < 2 × ULN (GGT < 100 U/L).
One patient with ASC (F, age at diagnosis 13.1 years, with CD) did not respond to first and second line treatment and required LT (details below). Immunological remission was achieved in 25 patients (33%, AIH 40% and ASC = 14%) at a median of 3.1 years (2.2-4.2) after starting standard IS treatment.
Thirty-six patients experienced at least 1 episode of relapse (1 episode n = 16 patients; ≥ 2 episodes n = 10) managed with a temporary increase of prednisolone dose in 10 patients, with the addition of azathioprine in 15, and conversion from azathioprine to MMF in 11. Suboptimal adherence to treatment was detected in 8% (n = 3, AIH = 2, ASC = 1) of those who relapsed.
Of 75 patients, 12 [16%, F = 75%, median age 13.5 years, (12.2-15.7)] required OVT after a median time from the diagnosis of 2.2 years (0.8-4.3) (Table 3). Ten patients were diagnosed with ASC and 2 with AIH; 10/12 had IBD (83%) (Table 3). Liver biopsy performed before starting OVT showed absent (or mild) inflammatory infiltrate in all, and biliary features including inflammatory injury of the bile duct in 8 (67%) patients, ductular reaction in 11 (92%), biliary metaplasia in 7 (58%), and periductular fibrosis in 6 (50%). Need for OVT was significantly higher in ASC group compared to AIH [10/12 (83%) in ASC vs 2/54 (4%) in AIH, P < 0.001]. Immunological profile, histology and medications are reported in Table 3.
| Patients/ diagnosis | Gender | Age at diagnosis (yr) | Type of presentation | IBD | Splenomegaly1 | IgG > ULN | Auto-antibodies | Histology | Medications | |||
| Interface hepatitis | Fibrosis | Cirrhosis | Biliopathy3 | |||||||||
| AIH | F | 4.2 | Asymptomatic | IC | Not | Yes | SMA 1:40; p-ANCA | No | Yes | No | Yes | Pred/MMF/UDCA/Mesa |
| AIH | F | 10.9 | Asymptomatic | UC | Not | Yes | ANA 1:320; SMA 1:160; p-ANCA positive | Yes | Yes | No | Yes | Pred/UDCA/Mesa |
| ASC | F | 16 | Insidious | None | Yes | Not | ANA 1:160; p-ANCA positive | Yes | Yes | Yes | No | Pred/MMF/UDCA |
| ASC | M | 4.3 | Asymptomatic | CD | Not | Yes | ANA 1:160; SMA 1:160; p-ANCA +++ | Yes | Yes | No | Yes | Pred/UDCA/Mesa |
| ASC | F | 8.6 | Insidious | UC | Not | Not | SMA 1:40; p-ANCA positive | No | No | No | Yes | Pred/AZA/UDCA/Mesa |
| ASC | F | 12.1 | Insidious | UC2 | Not | Not | SMA 1:40; p-ANCA positive | No | No | No | Yes | Pred/AZA/UDCA |
| ASC | M | 14.1 | Insidious | None | Not | Not | SMA 1:40; p-ANCA positive | Yes | Yes | No | Yes | Pred/AZA/UDCA |
| ASC | M | 14.3 | Acute | UC | Not | Yes | ANA 1:320; SMA 1:320 | Yes | No | No | Yes | Pred/UDCA/Mesa |
| ASC | F | 13.8 | Asymptomatic | UC | Yes | Yes | ANA 1:640; p-ANCA positive | No | Yes | Yes | Yes | Pred/MMF/UDCA/Mesa |
| ASC | F | 5.1 | Acute | IC | Not | Not | ANA 1:160; p-ANCA positive | No | Yes | No | Yes | Pred/MMF/UDCA/Mesa |
| ASC | F | 13.1 | Acute | CD | Yes | Yes | ANA 1:80; p-ANCA positive | No | Yes | No | Yes | Pred/MMF/UDCA/Mesa |
| ASC | F | 3.6 | Asymptomatic | UC | Yes | Not | ANA 1:160; SMA 1:80; p-ANCA positive | Yes | Yes | No | Yes | Pred/MMF/UDCA/Mesa |
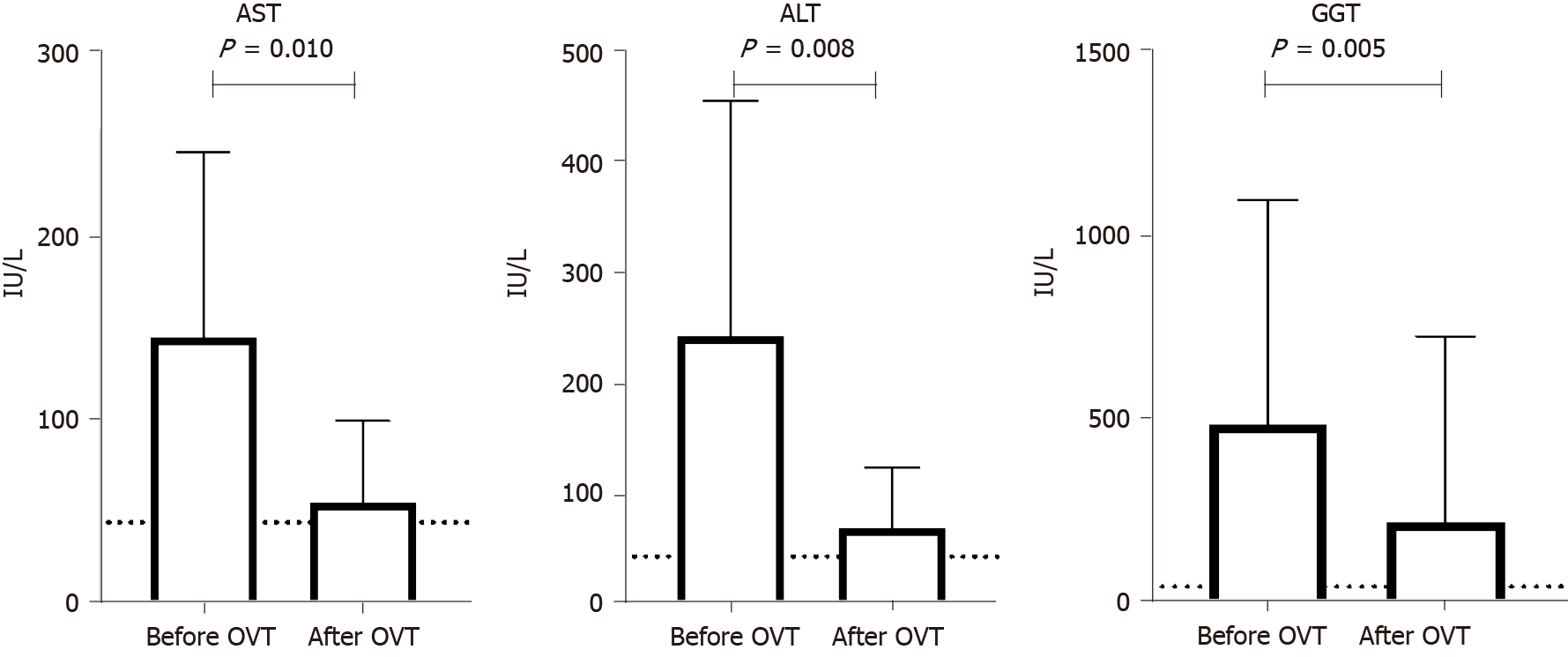
Median values of AST, ALT and GGT significantly decreased during OVT [AST levels from 107 UI/L (83-158) to 38 UI/L (31-65), P = 0.010; ALT from 160 UI/L (140-335) to 40 UI/L (37-87), P = 0.008; GGT from 279 (150-498) to 63 (32-143), P = 0.005] (Figure 1).
AST levels decreased in 10/12 patients (83%, within normal range in 8 patients and < 1.5 × ULN in 2), ALT levels in 9/12 patients (75%, within normal range in 7 patients and < 1.5 × ULN in 2), and GGT levels in 8/12 patients (67%, within normal range in 6 patients and < 1.5 × ULN in 2) (Table 2). Median time to normalization of AST, ALT and GGT levels were 2 mo (1.7-3.2), 5 mo (2.7-6.2), and 5 mo (3.2-6.0) respectively.
A complete response to OVT (normalization of AST, ALT and GGT) was observed in 6/12 patients (50%, cases n. 1, 2, 4, 5, 8, 10), a partial response in 2/12 (17%, cases n. 3 and 9) (Table 4).
| Patients/diagno | Age at OVT (yr) | AST (NV ≤ 45 U/L) | ALT (NV ≤ 45 U/L) | GGT (NV ≤ 50 U/L) | Response to OVT 1 | SCOPE index score2 | Time on OVT (mo) | OVT side-effect | Overall FU3 (mo) | ||||||||||
| Before OVT | After OVT | TTN | Result | Before OVT | After OVT | TTN | Result | Before OVT | After OVT | TTN | Result | Before OVT | After OVT | ||||||
| AIH | 5.4 | 212 | 39 | 4 mo | NV | 147 | 17 | 6 mo | NV | 73 | 22 | 6 mo | NV | Complete | 3 low risk | 0 low risk | 99 | None | 113 |
| AIH | 11.8 | 251 | 31 | 2 mo | NV | 359 | 39 | 9 mo | NV | 26 | 34 | 8 mo | NV | Complete | 3 low risk | 0 low risk | 72 | None | 73 |
| ASC | 16.8 | 98 | 47 | 3 mo | < 1.5 NV | 140 | 70 | 4 | < 1.5 NV | 39 | 83 | 4 | < 1.5 NV | Partial | 8 high risk | 8 high risk | 16 | None | 26 |
| ASC | 4.8 | 86 | 28 | 7 d | NV | 156 | 38 | 14 d | NV | 84 | 44 | 14 d | NV | Complete | 4 medium risk | 1 low risk | 37 | None | 39 |
| ASC | 13.1 | 60 | 14 | 1 mo | NV | 365 | 38 | 3 mo | NV | 68 | 27 | 4 mo | NV | Complete | 5 medium risk | 2 low risk | 31 | None | 84 |
| ASC | 15.2 | 71 | 40 | 14 mo | NV | 140 | 56 | 14 mo | < 1.5 NV | 52 | 164 | 12 mo | - | None | 6 high risk | 6 high risk | 6 | None | 68 |
| ASC | 17.4 | 113 | 65 | 1 mo | < 1.5 NV | 205 | 141 | 1 mo | - | 49 | 226 | 1 mo | - | None | 6 high risk | 4 medium risk | 3 | None | 52 |
| ASC | 15 | 407 | 30 | 6 mo | NV | 856 | 35 | 6 mo | NV | 61 | 28 | 1 mo | NV | Complete | 5 medium risk | 2 low risk | 40 | None | 49 |
| ASC | 17.3 | 102 | 37 | 2 mo | NV | 111 | 36 | 2 mo | NV | 135 | 82 | 5 mo | < 1.5 NV | Partial | 6 high risk | 4 medium risk | 18 | None | 61 |
| ASC | 12.5 | 76 | 31 | 2 mo | NV | 124 | 40 | 7 mo | NV | 86; TX | 42 | 6 mo | NV | Complete | 5 medium risk | 4 medium risk | 47 | None | 135 |
| ASC | 13.9 | 123 | 155 | - | - | 165 | 154 | - | - | 165 | 1800 | - | - | None | 8 high risk | 8 high risk | 6 | None | 86; TX |
| ASC | 13.2 | 141 | 135 | - | - | 156 | 180 | - | - | 71 mo (range 26-165) | 136 | - | - | None | 7 high risk | 7 high risk | 4 | None | 165 |
| Response to OVT | 10/12 (83%) | 9/12 (75%) | 8/12 (67%) | Median: 34 (range 1-99) | 71 (range 26-165) | ||||||||||||||
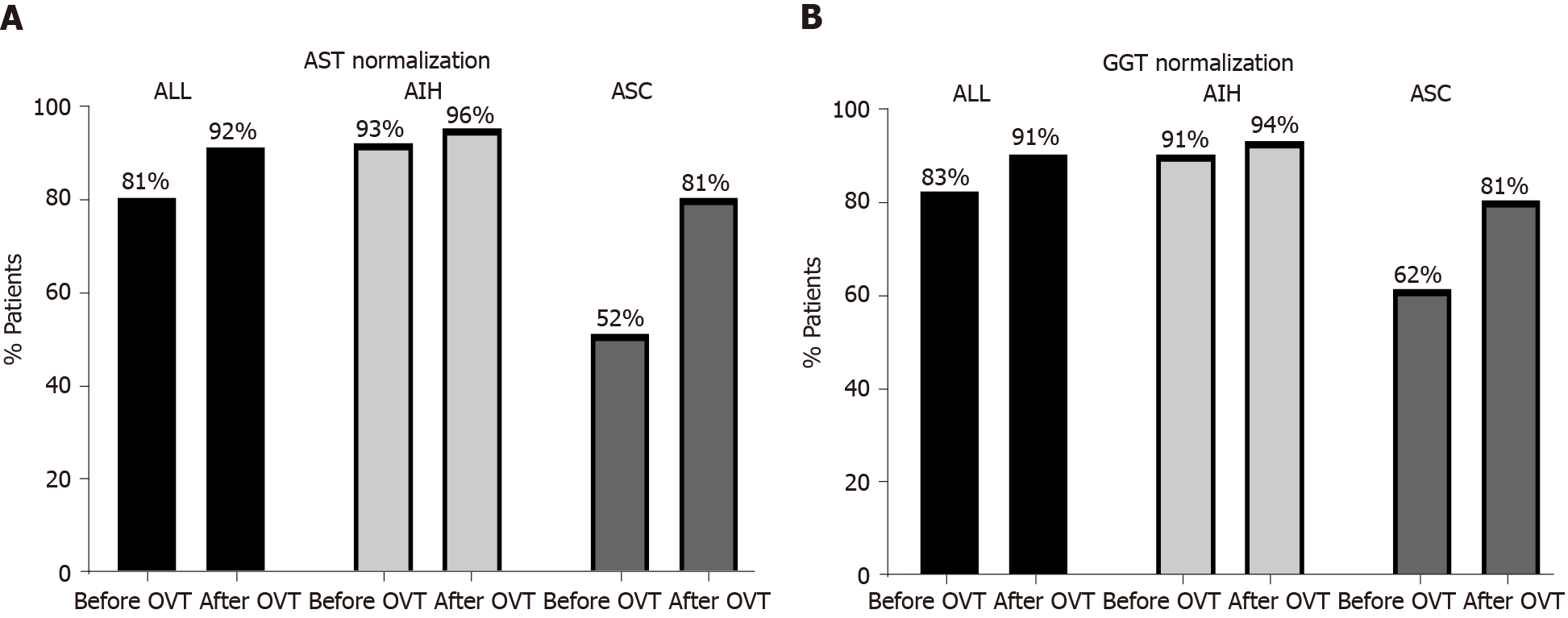
After OVT, the percentage of patients who achieved biochemical remission increased overall from 81% (61/75 patients) to 92% (69/75), [from 93% (50/54) to 96% (52/54) in AIH, and from 52% (11/21) to 81% (17/21) in ASC] (Figure 2). Similarly, the percentage of patients who normalized GGT levels increased after OVT, mainly in ASC patients (from 62% to 81%) (Figure 2). No significant changes were observed in the other biochemical parameters including total bilirubin, serum albumin, and platelet count, nor in the prevalence of high IgG and positive autoantibodies (P > 0.05).
Based on SCOPE index score, all 6 patients who showed a complete response to OVT were classified as low risk (cases 1, 2) or medium risk (cases 4, 5, 8, 10); the other 6 patients (cases 3, 6, 7, 9, 11, 12) were classified as high risk. Decrease in SCOPE index score was reported in 5/12 patients (42%), from high to medium risk in 2 patients (cases 7, 9) and from medium to low risk in 3 (cases 4, 5, 8) (Table 4). After a median time of 24 mo (range 1-99), none of 12 patients complained of side effects related to OVT.
Four of 12 patients (33%, cases 6, 7, 11, 12, all with ASC) did not respond to OVT. One patient (n. 6) underwent colectomy at the age of 14 years due to a severe form of IBD. She never normalized her liver enzymes. A course of OVT was commenced at the age of 15.2 years, was not successful and was therefore discontinued 6 mo later. At the age of 16 years she was diagnosed also with juvenile arthritis, and was treated with adalimumab. Another patient (n. 7) achieved histological remission 3 years after the diagnosis, and IS treatment was gradually discontinued. Six months later he developed a relapse of ASC not responding to prednisone and azathioprine. A follow up liver biopsy showed fibro-obliterative lesions around the bile ducts and OVT was commenced, though without success. One patient (n. 11) developed progressive cholestasis and complications of portal hypertension requiring LT at age 17 years. One year later she developed ASC disease recurrence requiring re-transplantation at age 21 years. A second ASC recurrence occurred 10 mo later leading to multiple episodes of cholangitis. A new course of OVT was commenced unsuccessfully. The patient was re-listed for the third LT.
The last patient (n. 12) did not respond to first and second line treatment nor to OVT and developed features of portal hypertension (splenomegaly and hypersplenism) and incomplete cirrhosis on histology.
At last follow up (median of 4.4 years, range 0.6-13.8 years) all patients are alive. Only 1 patient (F, with ASC) underwent LT at the age of 17 years and re-LT at the age of 21 years, due to recurrence of ASC (details above). Of 74 patients not requiring LT, 68 (92%) at last follow-up were still on medical treatment. In one patient (n. 5) who responded to OVT, we tried to reduce the dose of vancomycin from 1500 mg/d (divided in 3 doses) to 1000 mg/d (in 2 doses). However, few weeks later, AST and GGT increased 3 × ULN and normalized again when OVT went back to full dose (1500 mg/times for day).
Based on histological remission, IS withdrawal was attempted in 8 patients [7 females, median age 10.4 years (8.1-15.1), 7 AIH-1, 1 ASC] after a median of 4.0 years (3.9-5.3) from the diagnosis; 2/8 (n. 1,2) received OVT at the age of 5.4 and 11.8 years respectively. Two of these 8 patients (F, both with AIH-1) relapsed 1 and 4 mo after stopping treatment and responded successfully to IS treatment re-introduction. The other 6 (8%), including 1 patient with ASC, remained off treatment. One patient (n.1), discontinued prednisone and MMF 7.6 years after the diagnosis remaining on OVT alone, and her AST and GGT levels remained normal. Sixteen months later (at age of 13.8 years) on routine blood tests she had an increase of AST and GGT > 3 × ULN. The patient confessed a low adherence to treatment; once she re-started OVT regularly, AST and GGT returned normal.
In pediatrics, there are few published studies focusing on the differences between AIH and ASC. Furthermore, experience on empirical use of oral vancomycin in children with AILD not responding to standard immunosuppression is limited.
In this study, MRCP performed at diagnosis allowed us to differentiate children with AIH from those with ASC, and see whether they differ in terms of characteristics at presentation, response to medical treatment and outcome.
Our results show that characteristics at presentation were different between AIH and ASC, similarly to other studies[4,18]. All patients with ASC were positive for ANA and/or SMA, none for anti-LKM-1 confirming the rare association between LKM-1 positivity and ASC[18-20]. IBD was more common in ASC patients compared those with AIH, UC being more common[4,18-21]. On histology, cirrhosis was reported in 23% of patients, similar to previous studies (from 11% to 68%), suggesting a late diagnosis in a proportion of cases[4,18,19]. Features of biliopathy were equally reported in AIH and ASC confirming that both conditions are not easily distinguishable on histological ground making the cholangiogram the only effective tool to differentiate patients with AIH from those with ASC[16,18].
Pediatric patients with AILD respond well to IS treatment although the efficacy of second and third line treatment remains to be demonstrated, particularly in patients ASC[3].
The first study reporting benefits from OVT in children with ASC and IBD (n = 3 patients) was reported by Cox et al[12] in 1998. In that study OVT was administered to 3 patients (1 aged 15 years and 2 aged 14 years) diagnosed with PSC and IBD who showed improvements in gastrointestinal symptoms and liver enzymes after OVT[12].
However, this is the first study that aims to assess consistently the efficacy of OVT in a cohort of children and adolescents with AIH and ASC who did not respond to standard treatment and were treated according to a single protocol.
At our center OVT was given to children with AILD who failed to respond to first/second line IS treatment and had, on histology, features of biliopathy without (or mild) inflammation. To our opinion, in these patients an escalation of IS therapy (third line treatment) was not indicated due to the absence of significant lymphoplasmacytic infiltrate.
In this cohort a high proportion of patients normalized transaminases and GGT levels on standard IS; the majority of patients (40%) required an association between prednisone plus azathioprine, mainly in AIH group. Of interest, 10/12 patients who required OVT had ASC and 2/12 with AIH; on histology all had strong features of biliopathy, with mild or no inflammation.
Similarly to our study, improvements in liver enzymes after OVT were reported in Davies et al[7]’s study (n = 14 children with PSC and IBD), and in two randomized clinical trials on a total of 64 adult patients with PSC[5,6]. In Abarbanel et al[8]’s study the authors showed that all children with PSC and IBD experienced a reduction in GGT and ALT levels and improvement of biliary imaging, biopsies of the liver and intestine, and IBD symptoms while on OVT. In our study, median time to normalize liver enzymes ranged from 2 to 5 mo suggesting that a course of OVT should last at least 6 mo before assessing a biochemical response to treatment. Of note, no improvements were observed in the other biochemical parameters similar to Davies et al[7].
In a recent prospective study including pediatric patients (42% with small and 48% with large duct PSC), 49% (22/45), 20% (9/40), and 62.2% (28/45) of children experienced normalization of GGT, ALP, and ALT, respectively. Of note, the biochemical response to OVT was more favorable in the pediatric compared to the adult group. Besides, a significant proportion of patients showed improvements on histologic features and cholangiopathy[22]. Conversely, in a recent retrospective study on a large cohort of children with PSC the authors did not show improvement in outcomes of children treated with OVT or UDCA compared to those with “no treatment”[23], although several limitations were recorded in the study design[24]. The median OVT dose in Deneau et al[23]’s study was 21 mg/kg/d, which was substantially lower than the 50 mg/kg/d typically used in our and others’ studies[5,6].
In Tabibian et al[6]’s work (n = 35 adult patients with PSC) the authors experienced a significant improvement in pruritus only in the high-dose vancomycin group. In our study we observed a temporary increase in AST and GGT levels after OVT dose reduction. In Cox et al[12]’s study, 3 children with SC and IBD had a normalization of liver tests while on OVT and return to abnormal values upon OVT discontinuation. These results confirm the efficacy of OVT and the importance of maintaining full doses regularly during the treatment.
The mechanisms by which OVT leads to biochemical improvement are still undefined. Previous studies suggested that OVT may have an immunomodulatory effect on regulatory T cells (Treg)[5,6-8]. Some authors suggest that the response to OVT is likely due to its antimicrobial effects on unknown pathogens or normal flora that cause abnormal immunological reactions following migration to the liver[7]. Several lines of experimental evidence from animal models demonstrate that enteric dysbiosis and/or administration of bacterial antigens can lead to hepatobiliary inflammation with various features of PSC[6]. In this study we found that the prevalence of IBD was similar in patients responding to OVT compared to those not responding, suggesting no role of IBD in the pathogenic mechanism of OVT.
Overall, the need for OVT emerged mainly in ASC group, and the percentage of patients who achieved the biochemical remission increased mainly in ASC group (from 52% to 81%) rather than in AIH (from 93% to 96%) (Figure 2)[4,25].
Of 75 patients, only 33% achieved immunological remission and no significant changes in IgG levels and autoantibody positivity were observed after OVT. This may imply an ongoing disease activity despite normal transaminase levels, possibly explaining the low proportion of children able to stop treatment successfully (8% in this study).
Interestingly, all 6 patients who showed a complete response to OVT were classified as low or medium SCOPE index strata, none as high risk, suggesting that probably the patients achieving a biochemical response to OVT are those with a milder disease activity. Similar results were reported in Deneau et al[17]’s study showing that a low SCOPE index at treatment initiation was an independent predictor of response. Moreover, the authors showed that the rate of response to OVT was similar in the group that started it as primary treatment and another that had it as second line[17]. Remarkably, in this study, OVT was associated with prednisone alone in 3 cases (100% responded to treatment) and with a second IS drug in the other 9 (55% responded to treatment, P > 0.05).
The decrease in the SCOPE index score (42% in this study) may suggest a potential benefit of OVT on long-term outcomes. Similar results were reported in a triple blinded, randomized, placebo-controlled clinical trial on adult patients with PSC where the analysis showed a significant decrease in the Mayo PSC score in the vancomycin group at the third month comparing to the baseline evaluation[5].
Similarly to previous studies, we did not observe side effects or infectious complications from long-term OVT during the study period[6,7,22]. However, whether the use of this antibiotic may lead to vancomycin-resistant organisms is still an open issue. All 4 patients not responding to OVT (all with ASC) showed a progression of liver disease. One patient developed recurrence of ASC after the LT (twice) and did not respond to OVT confirming the high recurrence rate post-LT[3]. Differently from our experience, OVT has been reported to be effective in the treatment of a pediatric patient with recurrent PSC after LT, suggesting a disease mechanism with some causes external to the liver—potentially from the gut bacteria[26].
Overall, the outcome in our cohort was excellent, with 100% of patients alive at last follow up and 8% off IS treatment. Only 1 patient required LT, although the median follow up of our cohort of patients is relatively short.
This is the first study reporting data on the consistent use of OVT in children with AILD not responding to standard treatment. Our results show that AIH and ASC have different characteristics at presentation although both respond well to medical therapy. For children not responding to standard IS, OVT may represent a valuable option to achieve biochemical remission, particularly in ASC patients. This study adds timely insights into the highly engaged discussion about the use of OVT for children with AILD, confirming the need of further structured studies assessing the efficacy and safety of OVT as well as its potential benefits on long-term outcomes.
Pediatric autoimmune liver disease (AILD) includes autoimmune hepatitis (AIH) and autoimmune sclerosing cholangitis (ASC). Children with AILD not responding to standard immunosuppression (IS) may progress to end-stage liver disease and require liver transplantation.
Despite the absence of strong evidences the empirical use of candidate therapies has significantly increased in the last decades. Oral vancomycin has an immunomodulatory effect and it has been used in patients with primary sclerosing cholangitis. In pediatrics, the experience with oral vancomycin treatment (OVT) in patients with AIH or ASC is very limited.
In this study we evaluated: (1) The response to standard IS in a large cohort of pediatric patients with AILD; and (2) The efficacy of OVT to normalize transaminases (biochemical remission) and to achieve immunological remission in patients not responding to standard IS.
Retrospective study of children diagnosed with AILD (AIH or ASC) at Hospital Papa Giovanni XXIII, Bergamo, Italy, in the last decade. Response to IS treatment and need for OVT was reported in all patients and compared between the two groups (AIH vs ASC).
Seventy-five patients diagnosed with AILD were included in this study (median age 10.5 years, range 5.6-13.4; F = 69%); 12 patients (16%, 10 with ASC) required OVT. Response to OVT was observed in 75% of patients and the percentage of those who achieved biochemical remission increased overall from 81% to 92%. Decrease in Sclerosing Cholangitis Outcomes in Pediatrics (SCOPE) index was reported in 42% of patients.
This study shows that OVT may be considered as a valuable treatment option to achieve biochemical remission in children with AILD not responding to standard IS. Decrease in SCOPE index after OVT may suggest improvements in the long-term outcome.
These promising preliminary results suggest that further prospective studies are needed to better define the efficacy of OVT in AILD.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gadour E S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Liberal R, Vergani D, Mieli-Vergani G. Paediatric Autoimmune Liver Disease. Dig Dis. 2015;33 Suppl 2:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol. 2015;63:971-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 848] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 3. | Mieli-Vergani G, Vergani D, Baumann U, Czubkowski P, Debray D, Dezsofi A, Fischler B, Gupte G, Hierro L, Indolfi G, Jahnel J, Smets F, Verkade HJ, Hadžić N. Diagnosis and Management of Pediatric Autoimmune Liver Disease: ESPGHAN Hepatology Committee Position Statement. J Pediatr Gastroenterol Nutr. 2018;66:345-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 212] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 4. | Di Giorgio A, Hadzic N, Dhawan A, Deheragoda M, Heneghan MA, Vergani D, Mieli-Vergani G, Samyn M. Seamless Management of Juvenile Autoimmune Liver Disease: Long-Term Medical and Social Outcome. J Pediatr. 2020;218:121-129.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Rahimpour S, Nasiri-Toosi M, Khalili H, Ebrahimi-Daryani N, Nouri-Taromlou MK, Azizi Z. A Triple Blinded, Randomized, Placebo-Controlled Clinical Trial to Evaluate the Efficacy and Safety of Oral Vancomycin in Primary Sclerosing Cholangitis: a Pilot Study. J Gastrointestin Liver Dis. 2016;25:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 6. | Tabibian JH, Weeding E, Jorgensen RA, Petz JL, Keach JC, Talwalkar JA, Lindor KD. Randomised clinical trial: vancomycin or metronidazole in patients with primary sclerosing cholangitis - a pilot study. Aliment Pharmacol Ther. 2013;37:604-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 199] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 7. | Davies YK, Cox KM, Abdullah BA, Safta A, Terry AB, Cox KL. Long-term treatment of primary sclerosing cholangitis in children with oral vancomycin: an immunomodulating antibiotic. J Pediatr Gastroenterol Nutr. 2008;47:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Abarbanel DN, Seki SM, Davies Y, Marlen N, Benavides JA, Cox K, Nadeau KC, Cox KL. Immunomodulatory effect of vancomycin on Treg in pediatric inflammatory bowel disease and primary sclerosing cholangitis. J Clin Immunol. 2013;33:397-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Buness C, Lindor KD, Miloh T. Oral Vancomycin Therapy in a Child with Primary Sclerosing Cholangitis and Severe Ulcerative Colitis. Pediatr Gastroenterol Hepatol Nutr. 2016;19:210-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Vrieze A, Out C, Fuentes S, Jonker L, Reuling I, Kootte RS, van Nood E, Holleman F, Knaapen M, Romijn JA, Soeters MR, Blaak EE, Dallinga-Thie GM, Reijnders D, Ackermans MT, Serlie MJ, Knop FK, Holst JJ, van der Ley C, Kema IP, Zoetendal EG, de Vos WM, Hoekstra JB, Stroes ES, Groen AK, Nieuwdorp M. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J Hepatol. 2014;60:824-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 428] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 11. | Davies YK, Tsay CJ, Caccamo DV, Cox KM, Castillo RO, Cox KL. Successful treatment of recurrent primary sclerosing cholangitis after orthotopic liver transplantation with oral vancomycin. Case Rep Transplant. 2013;2013:314292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Cox KL, Cox KM. Oral vancomycin: treatment of primary sclerosing cholangitis in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1998;27:580-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Sebode M, Hartl J, Vergani D, Lohse AW; International Autoimmune Hepatitis Group (IAIHG). Autoimmune hepatitis: From current knowledge and clinical practice to future research agenda. Liver Int. 2018;38:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | Vergani D, Alvarez F, Bianchi FB, Cançado EL, Mackay IR, Manns MP, Nishioka M, Penner E; International Autoimmune Hepatitis Group. Liver autoimmune serology: a consensus statement from the committee for autoimmune serology of the International Autoimmune Hepatitis Group. J Hepatol. 2004;41:677-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 288] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 15. | Kerkar N, Chan A. Autoimmune Hepatitis, Sclerosing Cholangitis, and Autoimmune Sclerosing Cholangitis or Overlap Syndrome. Clin Liver Dis. 2018;22:689-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Di Giorgio A, D'Adda A, Marseglia A, Sonzogni A, Licini L, Nicastro E, D'Antiga L. Biliary features in liver histology of children with autoimmune liver disease. Hepatol Int. 2019;13:510-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Deneau MR, Mack C, Perito ER, Ricciuto A, Valentino PL, Amin M, Amir AZ, Aumar M, Auth M, Broderick A, DiGuglielmo M, Draijer LG, Tavares Fagundes ED, El-Matary W, Ferrari F, Furuya KN, Gupta N, Hochberg JT, Homan M, Horslen S, Iorio R, Jensen MK, Jonas MM, Kamath BM, Kerkar N, Kim KM, Kolho KL, Koot BGP, Laborda TJ, Lee CK, Loomes KM, Martinez M, Miethke A, Miloh T, Mogul D, Mohammad S, Mohan P, Moroz S, Ovchinsky N, Palle S, Papadopoulou A, Rao G, Rodrigues Ferreira A, Sathya P, Schwarz KB, Shah U, Shteyer E, Singh R, Smolka V, Soufi N, Tanaka A, Varier R, Vitola B, Woynarowski M, Zerofsky M, Zizzo A, Guthery SL. The Sclerosing Cholangitis Outcomes in Pediatrics (SCOPE) Index: A Prognostic Tool for Children. Hepatology. 2021;73:1074-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Sokollik C, McLin VA, Vergani D, Terziroli Beretta-Piccoli B, Mieli-Vergani G. Juvenile autoimmune hepatitis: A comprehensive review. J Autoimmun. 2018;95:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Terziroli Beretta-Piccoli B, Mieli-Vergani G, Vergani D. Serology in autoimmune hepatitis: A clinical-practice approach. Eur J Intern Med. 2018;48:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Giorgio AD, Vergani D, Mieli-Vergani G. Cutting edge issues in juvenile sclerosing cholangitis. Dig Liver Dis. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Smolka V, Karaskova E, Tkachyk O, Aiglova K, Ehrmann J, Michalkova K, Konecny M, Volejnikova J. Long-term follow-up of children and adolescents with primary sclerosing cholangitis and autoimmune sclerosing cholangitis. Hepatobiliary Pancreat Dis Int. 2016;15:412-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Ali AH, Damman J, Shah SB, Davies Y, Hurwitz M, Stephen M, Lemos LM, Carey EJ, Lindor KD, Buness CW, Alrabadi L, Berquist WE, Cox KL. Open-label prospective therapeutic clinical trials: oral vancomycin in children and adults with primary sclerosing cholangitis. Scand J Gastroenterol. 2020;55:941-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 23. | Deneau MR, Mack C, Mogul D, Perito ER, Valentino PL, Amir AZ, DiGuglielmo M, Draijer LG, El-Matary W, Furuya KN, Gupta N, Hochberg JT, Horslen S, Jensen MK, Jonas MM, Kerkar N, Koot BGP, Laborda TJ, Lee CK, Loomes KM, Martinez M, Miethke A, Miloh T, Mohammad S, Ovchinsky N, Rao G, Ricciuto A, Sathya P, Schwarz KB, Shah U, Singh R, Vitola B, Zizzo A, Guthery SL. Oral Vancomycin, Ursodeoxycholic Acid, or No Therapy for Pediatric Primary Sclerosing Cholangitis: A Matched Analysis. Hepatology. 2021;73:1061-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 24. | Assis DN, Levy C. Oral Vancomycin or Ursodeoxycholic Acid for Pediatric Primary Sclerosing Cholangitis? Hepatology. 2021;73:887-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Rojas CP, Bodicharla R, Campuzano-Zuluaga G, Hernandez L, Rodriguez MM. Autoimmune hepatitis and primary sclerosing cholangitis in children and adolescents. Fetal Pediatr Pathol. 2014;33:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Tabibian JH, Talwalkar JA, Lindor KD. Role of the microbiota and antibiotics in primary sclerosing cholangitis. Biomed Res Int. 2013;2013:389537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |