Published online Dec 27, 2021. doi: 10.4254/wjh.v13.i12.2104
Peer-review started: April 21, 2021
First decision: June 23, 2021
Revised: July 2, 2021
Accepted: November 15, 2021
Article in press: November 15, 2021
Published online: December 27, 2021
Processing time: 249 Days and 20 Hours
Metabolic-associated fatty liver disease (MAFLD) is the commonest cause of abnormal liver function tests (LFTs). Current upper normal of limit (UNL) of LFTs was derived from a “healthy” population, where undiagnosed MAFLD and viral hepatitis might be suspected.
To evaluated potential implications of changes in UNL of alanine aminotransferase (ALT) in MAFLD.
We retrospectively assessed consecutive first referrals with a diagnosis of MAFLD from 2010 to 2017. The conventional UNL of ALT was 45 IU/L for men and 34 IU/L for women, while a low UNL of ALT was 30 IU/L for men and 19 IU/L for women. The UNL of aspartate aminotransferase (AST) was 40 IU/L.
Total 436 patients were enrolled; of these, 288 underwent liver biopsy. Setting a lower UNL reduced the percentage of those with significant disease despite normal ALT; specifically, patients with advanced fibrosis (F ≥ F3) or definite “metabolic-associated steato-hepatitis (MASH)” (NAS ≥ 5) within normal ALT decreased from 10% to 1% and from 28% to 4% respectively. However, the proportion of those with elevated ALT and no evidence of advanced fibrosis or “definite MASH” increased from 39% to 47% and from 3% to 19%. Overall, LFTs performed poorly in distinguishing “definite MASH” from simple steatosis (receiver operating characteristic areas under the curves 0.59 for ALT and 0.55 for AST).
Liver function tests might both under- and overestimate MASH-related liver disease. Reducing the UNL might not be beneficial and imply an increase in healthcare burden. Risk stratification in MAFLD should rely on a combination of risk factors, not on LFTs alone.
Core Tip: In the United Kingdom, the hepatologists receive increasing demand for secondary care services to investigate liver function tests (LFTs), especially with the suspicion of metabolic-associated fatty liver disease (MAFLD). With current upper normal limit (UNL), patients without liver diseases but elevated LFTs is high (27%), as well as those with significant fibrosis or metabolic-associated steato-hepatitis and normal LFTs (10%). Here, we aimed to evaluate the potential implications of changes in UNL of LFTs. Our data show that reducing the UNL would lead to an increase in overall healthcare burden. In MAFLD, the risk-stratification should rely on a combination of risk factors, rather than on LFTs alone.
- Citation: Forlano R, Mullish BH, Dhar A, Goldin RD, Thursz M, Manousou P. Liver function tests and metabolic-associated fatty liver disease: Changes in upper normal limits, does it really matter? World J Hepatol 2021; 13(12): 2104-2112
- URL: https://www.wjgnet.com/1948-5182/full/v13/i12/2104.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i12.2104
Metabolic-associated fatty liver disease (MAFLD) is emerging as the most prevalent chronic liver disease worldwide secondary to the epidemic of obesity and metabolic syndrome. MAFLD also represents the commonest cause of abnormal liver function tests (LFTs) in Western countries[1]. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are enzymes which transfer amino groups to different substrates, with ALT being more liver-specific[2]. Notably, the patient’s metabolic status (such as the presence of obesity and/or insulin resistance) may directly influence LFTs values[3,4]. Moreover, current upper normal limits (UNL) were derived in a population with highly-prevalent MAFLD but unrecognised as a disease entity at the time. As such, several studies have questioned whether current UNL of ALT should be revised although giving contrasting results[5,6].
LFTs are often the first-line investigation for any suspected liver disease with or without imaging[2]. However, the role of LFTs in diagnosing metabolic-associated steato-hepatitis (MASH)-related liver disease, such as the presence of advance fibrosis and/or steatohepatitis, is currently limited. In particular, the full spectrum of MAFLD has been reported in patients with normal LFTs[7,8]. Although histology represents the “gold standard” for diagnosing and staging MASH, the costs and invasive nature of the procedure limit its widespread applicability. Therefore, non-invasive markers are an established part of the investigation of MAFLD. In particular, transient elastography has been validated as marker of fibrosis and represents the typical second-line investigation for MAFLD[2,9].
The aim of this study was to evaluate potential implications of lowering the UNL of ALT in patients with a clinical or histological diagnosis of MAFLD.
We retrospectively assessed all consecutive referrals with a clinical or histological diagnosis of MAFLD followed-up at the Liver Unit of St. Mary’s Hospital, Imperial College Healthcare NHS Trust, from January 2010 to May 2017.
At the time of liver biopsy or Liver Stiffness Measurement, clinical parameters were recorded, including demographic, anthropometric and biochemical data. The use of steatogenic drugs, chronic alcohol consumption, as well as other liver disease were considered as exclusion criteria[9]. Fibrosis-4 index and non-alcoholic fatty liver disease (NAFLD) fibrosis score were calculated based on published formulas[10,11].
The conventional upper normal limit (UNL) of ALT from the Imperial College NHS Trust laboratory was 45 IU/L for men and 34 IU/L for women. The effect of the application of a lower value of ALT was then investigated. This UNL was set at 30 IU/L for men and 19 IU/L for women, in keeping with previous studies aiming to increase the sensitivity in diagnosing active chronic hepatitis C in the general population[5]. Similarly, this lower ALT UNL helped with differentiating active from inactive chronic hepatitis B carriers[12].
The whole study population was then stratified into three subgroups: the group with ALT higher than the conventional UNL (ALT ≥ 45 IU/L for men and ≥ 34 IU/L for women), the group with ALT within the conventional and the low UNL (ALT 31-45 IU/L for men and 20-34 UI/L for women), and the group with ALT lower than the low UNL (ALT ≤ 30 IU/L for men and ≤ 19 IU/L for women). The UNL for AST was set as 40 IU/L, as per laboratory range.
Liver stiffness measurement (LSM) was obtained using FibroScan™. Scans were performed after 4 h fasting. LSM was interpreted according to interquartile range/median ratio: “poorly reliable” LSM values were not considered[13]. Advanced fibrosis was defined as LSM ≥ 8.1 kPa[14].
Liver biopsies were performed using a 16-Gauge Trucut needle (Argon, Athens Tx, USA). Specimens were formalin-fixed and paraffin-embedded; thick sections were stained with Hematoxylin and Eosin and Sirius Red. All biopsies were scored using the NASH CRN scoring system. Advanced fibrosis was defined as fibrosis stage ≥ F3. “Definite MASH”, “possible MASH” and “non-MASH” were defined as per NAFLD activity score (NAS)[15].
The distribution of variables was explored using the Shapiro-Wilk test. Since the variables were normally distributed, continuous variables were expressed as medians and SD, and categorical variables were expressed as relative frequencies. Differences between the groups were tested using one-way ANOVA for categorical and Mann-Whitney or Kruskal Wallis for categorical variables. Correlation was measured using Pearson’s Rho coefficient. Receiver operating characteristic (ROC) areas under the curves (AUROC) were used to assess the diagnostic performance of ALT and AST. Statistical analysis was performed using SPSS© (version 24.0; SPSS Inc. Chicago, IL).
This study was considered a service evaluation project, using routinely collected patient data, therefore no ethical approval was required under the United Kingdom (UK) policy framework for health and social care.
Four hundred thirty-six patients underwent LSM. Overall, 330 (76%) patients had ALT higher than the conventional UNL, 73 (17%) had ALT within the conventional and the low UNL and 33 (7%) had ALT lower than the low UNL. AST and γ-GT levels only were significantly different between the three groups (P < 0.0001 and P = 0.008 respectively). There was no difference in terms of use of statin therapy between the groups (Table 1).
| Variable | ALT lower than the low cut-off (n = 33) | ALT within the conventional and the low cut-off (n = 73) | ALT higher than the conventional cut-off (n = 330) | P value |
| Age (yr) | 52 ± 13.3 | 52.1 ± 12.1 | 52.5 ± 13.1 | 0.52 |
| BMI (kg/m2) | 29.9 ± 4.2 | 30 ± 5.5 | 29.3 ± 4.5 | 0.23 |
| T-Cholesterol (mmol/L) | 4.2 ± 1.4 | 4.4 ± 1 | 4.7 ± 2 | 0.3 |
| HDL (mmol/L) | 1 ± 0.3 | 1.1 ± 0.3 | 1 ± 0.8 | 0.81 |
| LDL (mmol/L) | 2.4 ± 1.1 | 2.5 ± 0.9 | 2.6 ± 1 | 0.27 |
| Triglycerides (mmol/L) | 1.9 ± 1 | 1.6 ± 0.9 | 1.7 ± 1.4 | 0.28 |
| HbA1c (mmol/L) | 41 ± 21 | 42 ± 16 | 45 ± 15.8 | 0.75 |
| AST (IU/L) | 25 ± 8 | 31 ± 7.7 | 51 ± 37 | < 0.00011 |
| γGT (IU/L) | 32 ± 41 | 38 ± 62 | 81 ± 76 | 0.0081 |
| Platelet (109 /L) | 208 ± 70 | 225 ± 72 | 229 ± 72 | 0.39 |
| Albumin (g/L) | 40 ± 6.1 | 41 ± 3.4 | 41 ± 3.2 | 0.62 |
| Ferritin (µg/L) | 58 ± 145 | 104 ± 150 | 163 ± 120 | 0.13 |
| Male gender | 21 (65) | 52 (62) | 207 (63) | 0.13 |
| Diabetes Mellitus | 19 (58) | 46 (55) | 161 (49) | 0.12 |
| Ethnicity | ||||
| Caucasian | 17 (5) | 35 (48) | 163 (49) | 0.79 |
| Arab | 8 (24) | 11 (15) | 66 (20) | 0.31 |
| Hispanic and Latinos | 2 (6) | 5 (6) | 20 (7) | 0.99 |
| South Asian | 4 (12) | 11 (15) | 41 (12) | 0.95 |
| East Asian | 1 (3) | 6 (6) | 25 (7) | 0.26 |
| African/Afrocaribbean | 1 (3) | 5 (6) | 15 (4) | 0.73 |
| Hypertension | 15 (45) | 33 (39) | 112 (34) | 0.2 |
| Dyslipidemia | 13 (39) | 37 (44) | 141 (43) | 0.93 |
| Statin treatment | 14 (42) | 34 (46) | 152 (46) | 0.54 |
Using the conventional UNL as reference, 10% of the patients had evidence of advanced fibrosis (LSM ≥ 8.1 kPa) despite normal ALT. When the low UNL for ALT was applied, this percentage reduced to 3%. However, applying the low UNL determined also the increase in the proportion of those with elevated ALT but not showing evidence of advanced fibrosis (LSM ≥ 8.1 kPa) from 42% to 52% (Supple
In the whole population, there was no linear association between ALT and age, as Pearson’s correlation was not significant (Rho = -0.86, P = 0.07). Moreover, the distribution of ALT across age groups was similar when patients were further stratified per gender (Kruskal Wallis).
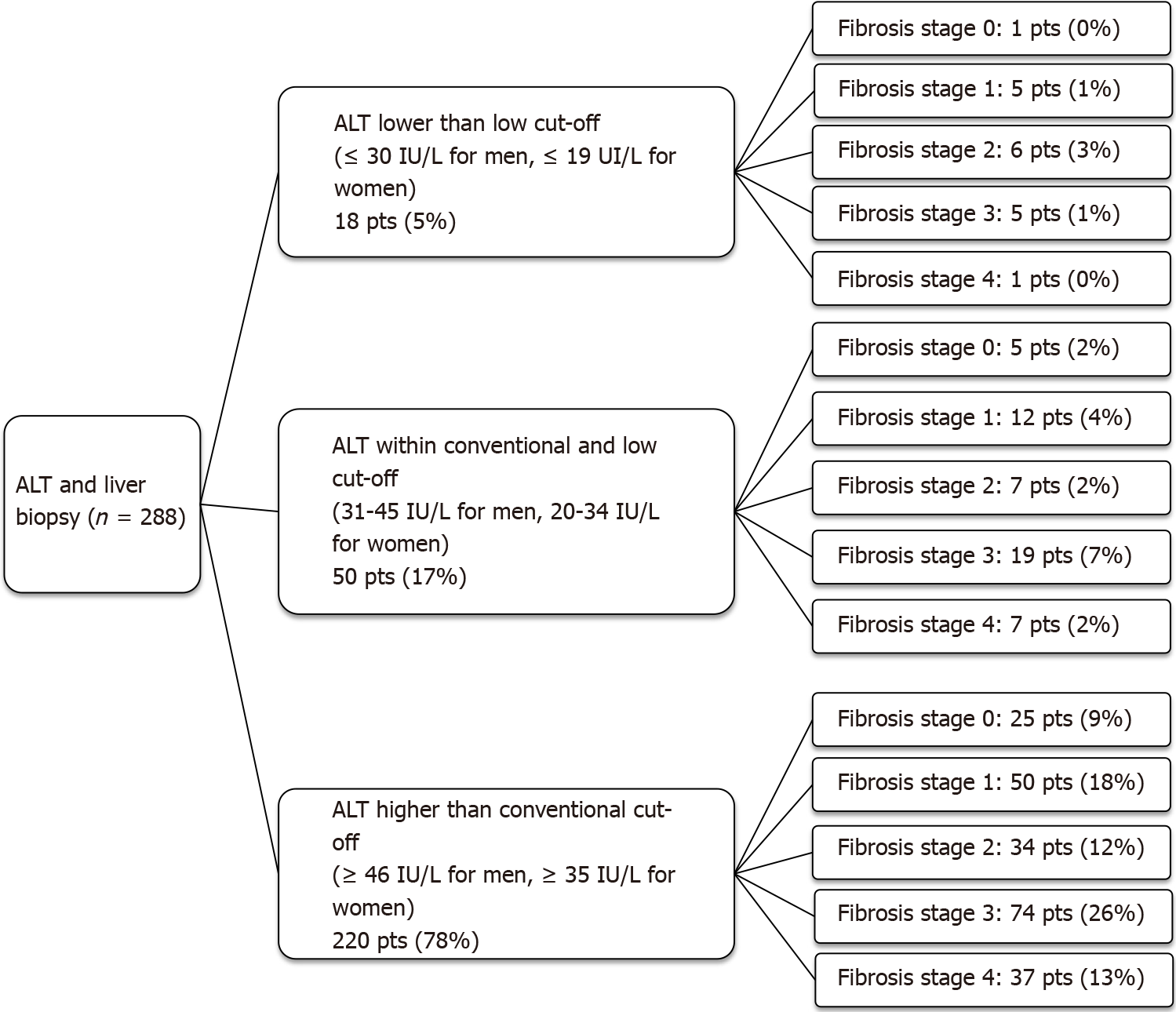
A subgroup of 288 patients underwent a liver biopsy. Overall, 220 (78%) patients had ALT higher than the conventional UNL, 50 (17%) had ALT within the conventional and the low UNL and 18 (5%) had ALT lower than the low UNL.
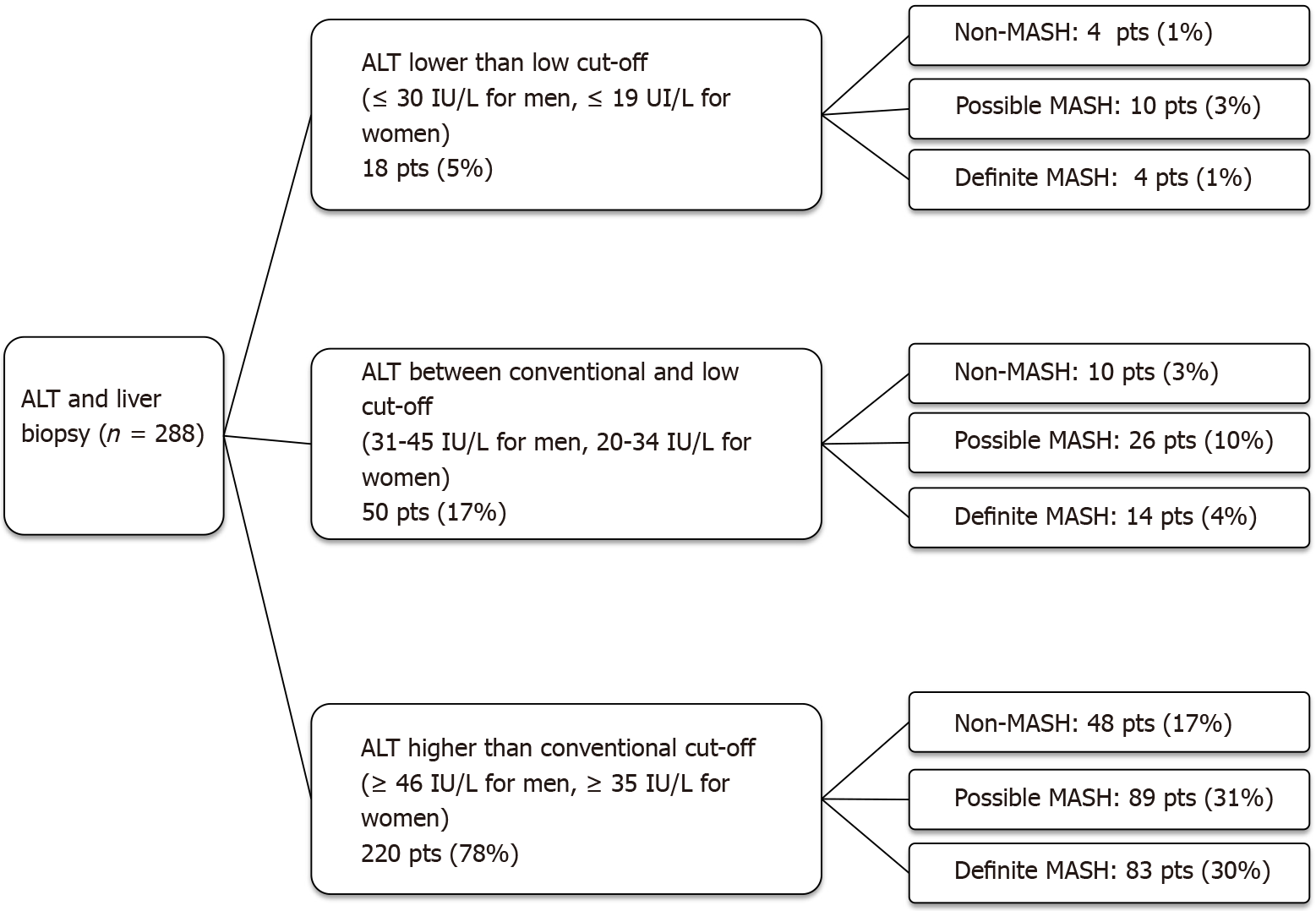
Using the conventional UNL as reference, 10% of patients had advanced fibrosis (F ≥ F3) on histology despite normal ALT. When the low UNL for ALT was applied, this percentage reduced to 1%. However, applying the low UNL determined also the increase in the proportion of those with elevated ALT but not showing advanced fibrosis from 39% to 47% (Figure 1). Similarly, lowering the UNL of ALT, the percentage of those with “definite MASH” (NAS ≥ 5) despite normal ALT decreased from 28% to 4%, whilst the percentage of patients without “definite MASH” but showing elevated ALT increased from 3% to 19% (Figure 2).
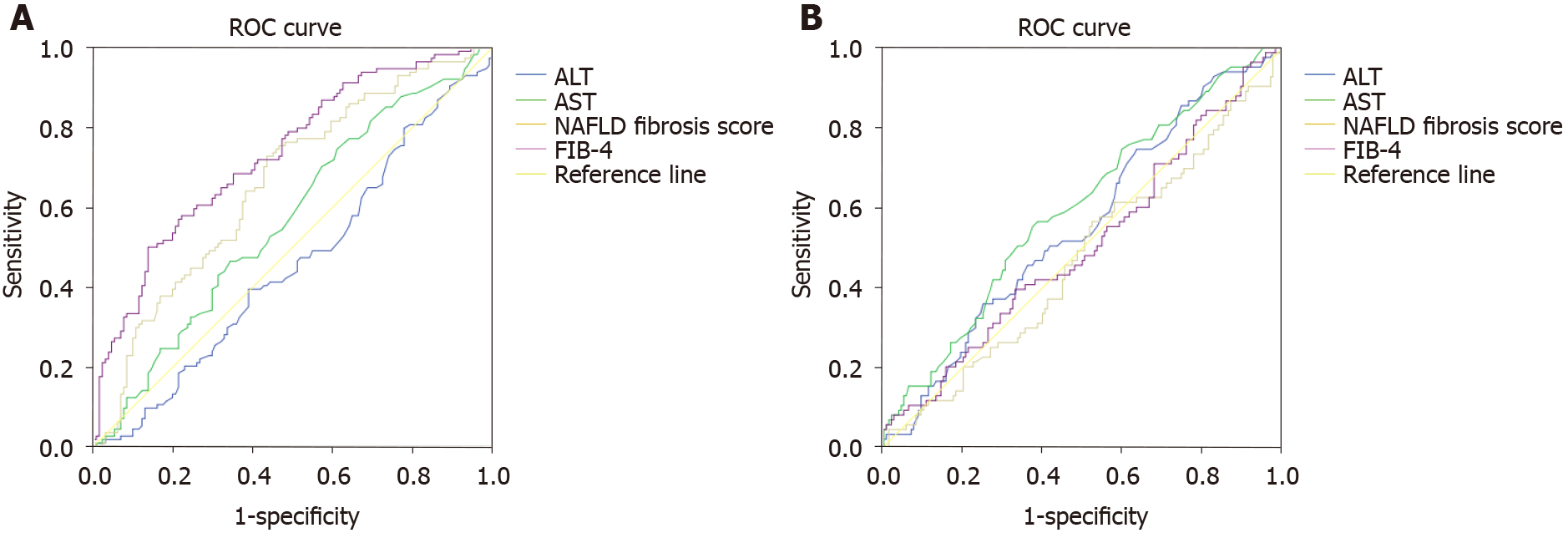
Overall, FIB-4 and NAFLD fibrosis scored performed better than ALT in diagnosing F > F3. Specifically, the AUROC of ALT for diagnosing F ≥ F3 was 0.45 (95%CI: 0.38-0.53, P = 0.05) compared to 0.71 (95%CI: 0.63-0.79, P = 0.0001) for FIB-4 and 0.65 (95%CI: 0.59-0.72, P = 0.0001) for NAFLD fibrosis score. However, ALT, FIB-4 and NAFLD fibrosis score performed similarly in diagnosing “definite MASH”. In particular, the AUROC of ALT was 0.55 (95%CI: 0.47-0.62, P = 0.049), compared to 0.47 (95%CI: 0.39-0.54, P = 0.01) for FIB-4 and 0.5 (95%CI: 0.42-0.58, P = 0.05) for NAFLD fibrosis score (Figure 3A and B).
Overall, 235 (54%) patients had elevated AST and 201 (46%) had normal AST. ALT, γ-GT and ferritin only were significantly different between the groups (P < 0.0001, P < 0.0001 and P = 0.008 respectively). There was no difference in terms of statin therapy (Supplementary Table 1).
Advanced fibrosis (LSM ≥ 8.1 kPa) was diagnosed despite normal AST in 16% of the cases, while the proportion of those with elevated AST but LSM <8.1 kPa was 27%.
In the whole population, there was no linear association between AST and age, as Pearson’s correlation was not significant (Rho = 0.01, P = 0.99). Moreover, the distribution of AST across age groups was similar when patients were further stratified per gender (Kruskal Wallis).
In the subgroup of patients who underwent a liver biopsy, 155 (54%) patients had elevated AST and 133 (46%) had normal AST. Advanced fibrosis (F ≥ F3) was diagnosed despite normal AST in 21% of the cases, while the proportion of those with elevated AST and no advanced fibrosis (F ≥ F3) was 26%. “Definite MASH” was diagnosed in presence of normal AST in 37% cases.
Overall, FIB-4 and NAFLD fibrosis scored performed better than AST in diagnosing F > F3, while the three performed similarly in diagnosing “definite MASH”. Specifically, the AUROC of AST for diagnosing F ≥ F3 was 0.56 (95%CI: 0.49-0.64, P = 0.05) and 0.59 (95%CI: 0.52-0.67, P = 0.049) for diagnosing “definite MASH” (Figure 3A and B).
Metabolic-associated Fatty Liver Disease is a major cause of chronic liver disease and the commonest cause of elevated liver enzymes[16,17]. In the UK, referrals for abnormal LFTs are increasing (> 300 referrals/year), and this often represents the first step in diagnosing MAFLD[18].
Several factors may influence ALT, such as age, gender, BMI, insulin resistance and triglycerides[3,4,19]. Overall, ALT is more commonly elevated than AST in chronic liver disease, with the notable exception of alcohol-induced liver injury[20]. Since transaminases are released following hepatocellular injury, AST and ALT are markers of cytolysis and not necessarily associated with inflammation or steatosis[21]. Nevertheless, LFTs are often used as a surrogate markers to assess the anti-inflammatory effect in clinical trials in MAFLD[22].
While the diagnosis and management of MAFLD has been streamlined in secondary and tertiary care centres, there is still a high variability in how the disease is assessed within the community. In particular, general practitioners (GPs) in primary care rely heavily on LFTs measurement, consistent with pragmatic guidelines which have been developed only recently in the UK[2]. It is also evident from a recent survey study that diagnosing MAFLD is perceived as challenging even to experienced GPs, with the overall perception of overlooking the disease especially in high-risk groups[23].
In this retrospective cohort of patients diagnosed with MAFLD, LFTs were frequently normal despite the presence of advanced liver disease. Moreover, transaminases could not distinguish simple steatosis from “definite MASH” (AUROC 0.59 for ALT and 0.55 for AST) at first referral, giving false reassurance in 10%-15% of patients. Conversely, decision-making based on LFTs alone might have implied unnecessary second-line investigations in approximately 27%-42% of cases. Our results confirm that non-invasive markers based on blood tests (i.e., FIB-4 and NAFLD fibrosis score) perform better than LFTs alone in assessing the severity of liver disease from NAFLD.
The actual normal ALT value is an area of ongoing controversy. Differences in the UNL used between studies are consistent, resulting from laboratory setting and populations tested[24]. Interestingly, the ALT normal range has been derived from “healthy” subjects in the general population[1], where MAFLD and obesity were highly prevalent[24]. Moreover, the UNL was first described in the 1980s, when LFTs were used to rule out ‘non-A and non-B hepatitis’ positivity amongst blood donors, in a time when anti-HCV antibodies were not available[25]. As such, both undiagnosed cases of MAFLD and chronic viral hepatitis may have contributed to the current definition of the UNL.
In this cohort, when a lower UNL was applied, the proportion of patients with advanced fibrosis or definite MASH on biopsy and normal biochemistry fell substantially, providing a rationale for revising current UNL. However, reducing the ALT normal range might lead to an increase in unnecessary second-line investigations (from 27% to 33% in based on histology this population) for a disease which is already highly prevalent in the general population. As a result, health costs would overwhelm the healthcare system with no clear clinical benefit[5].
Liver function tests might both underestimate and overestimate MASH-associated liver disease. Changing the UNL of ALT is not beneficial, as it might increase healthcare burden. Referral/management pathways and risk-stratification strategies are most needed for primary and they should rely on a combination of risk factors and non-invasive markers, not on LFTs alone.
Elevated liver function tests (LFTs) often represent the main reason for referring patients with metabolic-associated fatty liver disease (MAFLD) to secondary and tertiary care.
In MAFLD, liver function tests may both under and over-estimate liver disease. Moreover, difference in upper normal limit (UNL) of LFTs is consistent across the literature.
As such, we investigated the potential use of different UNLs of LFTs in MAFLD.
We evaluated the use of a lower UNL of ALT vs histology and liver stiffness measurement in a cohort of 436 patients with non-alcoholic fatty liver disease in a tertiary care centre.
Modifying the upper normal limit of LFTs does not improve the diagnostic performance of the test in MAFLD.
In MAFLD, the risk-stratification should rely on a combination of risk factors and non-invasive markers, rather than on LFTs alone.
Future research should focus on identifying biomarkers for diagnosing metabolic-associated steato-hepatitis and advanced fibrosis.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: European Association for the Study of the Liver.
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Shahini E, Tsou MT S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Liangpunsakul S, Chalasani N. Unexplained elevations in alanine aminotransferase in individuals with the metabolic syndrome: results from the third National Health and Nutrition Survey (NHANES III). Am J Med Sci. 2005;329:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 2. | Newsome PN, Cramb R, Davison SM, Dillon JF, Foulerton M, Godfrey EM, Hall R, Harrower U, Hudson M, Langford A, Mackie A, Mitchell-Thain R, Sennett K, Sheron NC, Verne J, Walmsley M, Yeoman A. Guidelines on the management of abnormal liver blood tests. Gut. 2018;67:6-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 332] [Article Influence: 47.4] [Reference Citation Analysis (1)] |
| 3. | Maximos M, Bril F, Portillo Sanchez P, Lomonaco R, Orsak B, Biernacki D, Suman A, Weber M, Cusi K. The role of liver fat and insulin resistance as determinants of plasma aminotransferase elevation in nonalcoholic fatty liver disease. Hepatology. 2015;61:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 143] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 4. | Fracanzani AL, Valenti L, Bugianesi E, Andreoletti M, Colli A, Vanni E, Bertelli C, Fatta E, Bignamini D, Marchesini G, Fargion S. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 2008;48:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 467] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 5. | Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, Vianello L, Zanuso F, Mozzi F, Milani S, Conte D, Colombo M, Sirchia G. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1025] [Cited by in RCA: 1048] [Article Influence: 45.6] [Reference Citation Analysis (3)] |
| 6. | Wejstål R, Hansson G, Lindholm A, Norkrans G. Persistent alanine aminotransferase elevation in healthy Swedish blood donors--mainly caused by obesity. Vox Sang. 1988;55:152-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Kunde SS, Lazenby AJ, Clements RH, Abrams GA. Spectrum of NAFLD and diagnostic implications of the proposed new normal range for serum ALT in obese women. Hepatology. 2005;42:650-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 143] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, Sterling RK, Shiffman ML, Stravitz RT, Sanyal AJ. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 812] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 9. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3156] [Article Influence: 350.7] [Reference Citation Analysis (4)] |
| 10. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3499] [Article Influence: 184.2] [Reference Citation Analysis (0)] |
| 11. | Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, Lindor K, Sanderson SO, Lenzi M, Adams LA, Kench J, Therneau TM, Day CP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1917] [Cited by in RCA: 2261] [Article Influence: 125.6] [Reference Citation Analysis (1)] |
| 12. | Assy N, Beniashvili Z, Djibre A, Nasser G, Grosovski M, Nseir W. Lower baseline ALT cut-off values and HBV DNA levels better differentiate HBeAg- chronic hepatitis B patients from inactive chronic carriers. World J Gastroenterol. 2009;15:3025-3031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Boursier J, Zarski JP, de Ledinghen V, Rousselet MC, Sturm N, Lebail B, Fouchard-Hubert I, Gallois Y, Oberti F, Bertrais S, Calès P; Multicentric Group from ANRS/HC/EP23 FIBROSTAR Studies. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology. 2013;57:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 487] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 14. | Cassinotto C, Boursier J, de Lédinghen V, Lebigot J, Lapuyade B, Cales P, Hiriart JB, Michalak S, Bail BL, Cartier V, Mouries A, Oberti F, Fouchard-Hubert I, Vergniol J, Aubé C. Liver stiffness in nonalcoholic fatty liver disease: A comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63:1817-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 382] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 15. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8160] [Article Influence: 408.0] [Reference Citation Analysis (5)] |
| 16. | Armstrong MJ, Houlihan DD, Bentham L, Shaw JC, Cramb R, Olliff S, Gill PS, Neuberger JM, Lilford RJ, Newsome PN. Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J Hepatol. 2012;56:234-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 17. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 3739] [Article Influence: 534.1] [Reference Citation Analysis (2)] |
| 18. | Sheridan DA, Aithal G, Alazawi W, Allison M, Anstee Q, Cobbold J, Khan S, Fowell A, McPherson S, Newsome PN, Oben J, Tomlinson J, Tsochatzis E. Care standards for non-alcoholic fatty liver disease in the United Kingdom 2016: a cross-sectional survey. Frontline Gastroenterol. 2017;8:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Piton A, Poynard T, Imbert-Bismut F, Khalil L, Delattre J, Pelissier E, Sansonetti N, Opolon P. Factors associated with serum alanine transaminase activity in healthy subjects: consequences for the definition of normal values, for selection of blood donors, and for patients with chronic hepatitis C. MULTIVIRC Group. Hepatology. 1998;27:1213-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 205] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 20. | Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC; Public Policy Committee of the American Association for the Study of Liver Disease. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 582] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 21. | Verma S, Jensen D, Hart J, Mohanty SR. Predictive value of ALT levels for non-alcoholic steatohepatitis (NASH) and advanced fibrosis in non-alcoholic fatty liver disease (NAFLD). Liver Int. 2013;33:1398-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 235] [Article Influence: 19.6] [Reference Citation Analysis (1)] |
| 22. | Ratziu V. A critical review of endpoints for non-cirrhotic NASH therapeutic trials. J Hepatol. 2018;68:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 23. | Standing HC, Jarvis H, Orr J, Exley C, Hudson M, Kaner E, Hanratty B. GPs' experiences and perceptions of early detection of liver disease: a qualitative study in primary care. Br J Gen Pract. 2018;68:e743-e749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Neuschwander-Tetri BA, Unalp A, Creer MH; Nonalcoholic Steatohepatitis Clinical Research Network. Influence of local reference populations on upper limits of normal for serum alanine aminotransferase levels. Arch Intern Med. 2008;168:663-666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med. 2000;342:1266-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 692] [Article Influence: 27.7] [Reference Citation Analysis (4)] |