Published online Dec 27, 2021. doi: 10.4254/wjh.v13.i12.2081
Peer-review started: March 9, 2021
First decision: May 2, 2021
Revised: May 2, 2021
Accepted: October 24, 2021
Article in press: October 24, 2021
Published online: December 27, 2021
Processing time: 292 Days and 10 Hours
Biliary complications (BCs) after liver transplantation (LT) remain a considerable cause of morbidity, mortality, increased cost, and graft loss.
To investigate the impact of BCs on chronic graft rejection, graft failure and mortality.
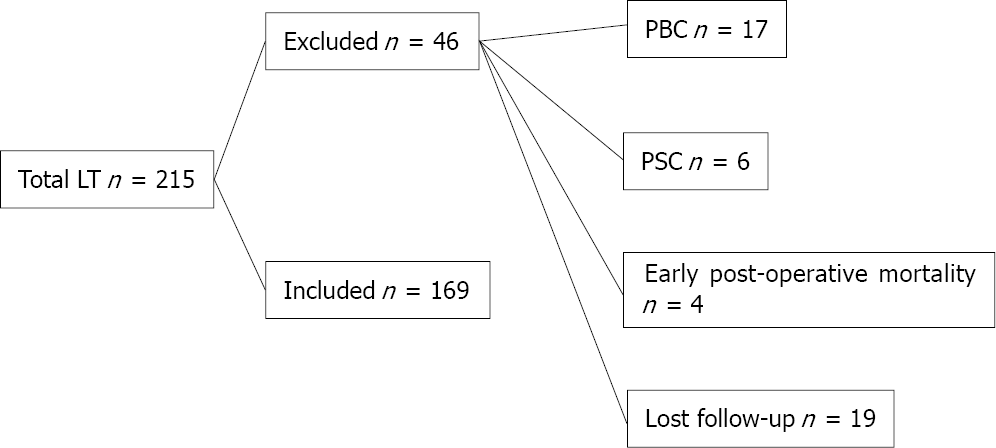
From 2011 to 2016, 215 adult recipients underwent right-lobe living-donor liver transplantation (RT-LDLT) at our centre. We excluded 46 recipients who met the exclusion criteria, and 169 recipients were included in the final analysis. Donors’ and recipients’ demographic data, clinical data, operative details and postoperative course information were collected. We also reviewed the management and outcomes of BCs. Recipients were followed for at least 12 mo post-LT until December 2017 or graft or patient loss.
The overall incidence rate of BCs including biliary leakage, biliary infection and biliary stricture was 57.4%. Twenty-seven (16%) patients experienced chronic graft rejection. Graft failure developed in 20 (11.8%) patients. A total of 28 (16.6%) deaths occurred during follow-up. BCs were a risk factor for the occurrence of chronic graft rejection and failure; however, mortality was determined by recurrent hepatitis C virus infection.
Biliary complications after RT-LDLT represent an independent risk factor for chronic graft rejection and graft failure; nonetheless, effective management of these complications can improve patient and graft survival.
Core Tip: We included 169 right lobe living-donor liver transplantation recipients in this retrospective study. The overall incidence rate of biliary complications including biliary leakage, biliary infection and biliary stricture was 57.4%. Twenty-seven (16%) patients experienced chronic graft rejection. Graft failure developed in 20 (11.8%) patients. A total of 28 (16.6%) deaths occurred during follow-up. Biliary complications were an independent risk factor for the occurrence of chronic graft rejection and failure; however, mortality was determined by unresolved recurrent hepatitis C virus infection. In conclusion, biliary complications represent an independent risk factor for chronic graft rejection and graft failure; nonetheless, effective management of these complications can improve patient and graft survival.
- Citation: Guirguis RN, Nashaat EH, Yassin AE, Ibrahim WA, Saleh SA, Bahaa M, El-Meteini M, Fathy M, Dabbous HM, Montasser IF, Salah M, Mohamed GA. Biliary complications in recipients of living donor liver transplantation: A single-centre study . World J Hepatol 2021; 13(12): 2081-2103
- URL: https://www.wjgnet.com/1948-5182/full/v13/i12/2081.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i12.2081
Liver transplantation (LT) is a life-saving therapeutic modality for patients with end-stage hepatic disease[1]. Despite considerable progress in LT surgical performance and peri-operative management, post-LT biliary complications (BCs) remain a considerable cause of morbidity, mortality, increased cost, and graft loss[2,3].
Living-donor liver transplantation (LDLT) is a well-established substitute to deceased-donor LT (DDLT)[4,5]. LDLT has potential advantages over DDLT, such as lower cost, superior graft vitality, shorter cold ischemia time, and lower prevalence of steroid-resistant graft rejection[6]. However, it has been reported that LDLT is related to higher post-LT morbidity, hospitalization rates and duration of stay. This is mainly referred to the higher incidence rate of BCs in LDLT ranging from 10% to 67% compared to DDLT[7-9], which could be attributed to the technically challenging biliary reconstruction during LDLT[9]. Technical skilfulness is mandatory to reduce the incidence of BCs[10], and the most critical key step is to maintain the blood supply to the biliary ducts in donor surgery[11].
Post-LT BCs include biliary strictures (BSs), biliary leaks (BLs), and biliary infection. There are two types of BLs post-LDLT: Anastomotic and cut surface BLs[12,13]. BLs occur commonly at the T-tube insertion site and less frequently at the anastomosis site[14]. Most BLs occur within the first post-transplant month and are mostly related to inadequate surgical skills or biliary duct ischemia[15].
BSs are the most common BC, accounting for 40% of BCs following LT. Like BLs, BSs are more prevalent post-LDLT when compared to DDLT, mostly due to the more technically challenging biliary anastomosis in LDLT due to the small-sized ducts requiring multiple biliary anastomoses[7,16]. BSs typically present after one month post-LT; in addition, they can be anastomotic or non-anastomotic[12]. Anastomotic strictures account for approximately 80% of post-LT BSs and commonly occur in LDLT and at the anastomotic site[7,17]. Non-anastomotic strictures account for approximately 10%-25% of post-LT BSs[18]. BSs are mainly linked to surgical skills, patients with small-sized ducts, donor-recipient bile duct size mismatch, longer operative time, total ischemia time, local ischemia, chronic rejection, older donor age, donor and recipient gender matching and initial disease recurrence like primary sclerosing cholangitis (PSC)[2,3,19,20].
Duct-to-duct anastomosis (DDA) has developed into the preferred biliary reconstruction method due to its benefits of a shorter total operative time, less incidence of post-operative infections, more physiological enteric functions and the enablement of access to the biliary tree in case of complications. Roux-en-Y hepaticojejunostomy (RYHJ) is performed in the case of re-transplantation or short or diseased bile ducts[21]. However, diversity in the results regarding the superiority of both of the two biliary reconstruction and suturing techniques is still present[3,8,15,22].
Similarly, the use of biliary drainage remains controversial[10]. The post-LT stent represents a method for biliary tract decompression, as well as the facilitation of postoperative cholangiography[22]. However, this technique is predisposed to BL at the entry site and thus has become less commonly used[14]. Also, temporary internal biliary stents may be applied to cross the anastomosis site[19]; however, it has been reported that the incidence of BCs may increase with this technique[23].
There is considerable overlap in the diagnostic and therapeutic modalities in patients with post-LT BCs. Frequently used diagnostic modalities include abdominal ultrasonography, computed tomography scan, magnetic retrograde cholangiopancreatography (MRCP), magnetic resonance imaging, percutaneous transhepatic cholangiography (PTC) and endoscopic retrograde cholangiopancreatography (ERCP). Currently, the preferred imaging method for the biliary tract is MRCP; it provides a guide for further interventional approaches[14].
In the case of isolated deranged liver functions post-LT, it is crucial to make an accurate diagnosis of other parenchymal hepatic diseases such as acute or chronic rejection, drug-induced hepatotoxicity, recurrence of primary cholestatic disease or viral hepatitis to further apply the appropriate management plan. Liver biopsy is a conclusive diagnostic procedure for these patients[4,7].
The management of BCs depends on a multidisciplinary approach including endoscopic, percutaneous and surgical interventions. Currently, ERCP is the preferable first-line therapeutic modality, especially in cases of DDA[4,17]. The success rate of this technique is variable, ranging from 51% to 100%[24]. If ERCP fails, PTC can be tried; also, it is the preferred therapeutic modality in cases of RYHJ. Surgical intervention is a last option for BCs management[2,20]. However, the optimal strategy for managing post-LT BCs remains undefined.
Based on the published literature, BC causes significant morbidity following LDLT. If not managed properly, it leads to cholestasis, progressive bridging fibrosis, secondary biliary cirrhosis and eventually graft failure. Hence, we aimed to investigate its impact on chronic graft rejection, graft failure and mortality.
This retrospective cohort study was conducted at Ain Shams Centre for Organ Transplantation, Ain Shams Specialized Hospital, Cairo, Egypt, from January 2011 to December 2016. This study was performed according to the ethical guidelines of the Declaration of Helsinki and was approved by the ethical review board of the Faculty of Medicine, Ain Shams University (No. FMASU MD 187/2016), which waived the requisite of informed consent owing to the retrospective nature of the study.
During the study period, 215 adult recipients underwent right lobe-LDLT (RL-LDLT) at our centre. We excluded 46 patients who met the exclusion criteria, and 169 recipients were enrolled in the final analysis. We included cirrhotic patients who met the transplantation criteria of our institution [a Child-Pugh score of ≥ 7 and model for end-stage liver disease (MELD) score of ≥ 15]. Patients with hepatocellular carcinoma (HCC) were enrolled if they met the Milan criteria, defined as a single lesion ≤ 5 cm or up to three lesions of ≤ 3 cm each with the absence of vascular invasion and extra-hepatic metastases[25]. We excluded patients with cholestatic hepatic diseases [primary biliary cirrhosis (PBC) or PSC] and early postoperative mortality and patients lost on follow-up (Figure 1).
Donors’ and recipients’ demographic data, clinical data, operative details and postoperative course information were collected. We also reviewed the management and outcomes of BCs. Recipients were followed for at least 12 mo post-LT until December 2017 or graft or patient loss.
The following BCs and their management were recorded from data files:
BL: Clinically suspected due to the existence of bile in the surgical drains or the presence of an intra-abdominal biloma and confirmed by imaging studies.
Biliary infection: Clinically suspected due to fever, abdominal pain, rigours, biochemical cultures and elevated inflammatory markers, including levels of C-reactive protein.
BS: Clinically suspected due to jaundice, pruritus, and elevated levels of serum bilirubin and/or alkaline phosphatase and confirmed by imaging studies as a narrowing at any site of the biliary tree whether at an anastomotic or non-anastomotic site with proximal dilatation.
Graft failure: Confirmed by histological evidence as graft cirrhosis, the need for re-transplantation because of graft failure and/or allograft-associated mortality.
Chronic ductopenic graft rejection: Proven by liver biopsy.
Recurrent hepatitis C virus (HCV) infection: Proven by high viral load, elevated transaminases and liver biopsy.
A right-lobe graft was used without the middle hepatic vein by the piggyback technique. Biliary anastomosis was done by DDA with an end-to-end interrupted style using absorbable polydioxanone (PDS-II; Ethicon) 6-0 sutures[26]. A ductoplasty was conducted if one duct was approximately twice the size of the other. A routine external biliary stent was inserted for three months post-operation. Three drains were placed postoperatively: In the right subphrenic space, the right Morrison’s pouch and at the cut surface of the graft. Internal biliary stents were used selectively if indicated. Arterial reconstruction was described previously[27]. The ratio of graft weight to recipient body weight was used to assess the relation of the graft size for recipients[27]. The accepted ratio was 1.2 ± 0.2%. All recipients had the same ABO blood group as the donors.
Data were analysed using IBM© SPSS© Statistics version 23 (IBM© Corp., Armonk, NY) and MedCalc© version 18.2.1 (MedCalc© Software bv, Ostend, Belgium). Non-parametric numerical variables were presented as medians and interquartile ranges, whereas between-group differences were analysed using the Mann-Whitney test and, in the case of paired data, the Wilcoxon signed-rank test. Parametric numerical data were shown as mean ± standard deviation, and between-group differences were analysed using a t-test and, in the case of paired data, a paired t-test. Nominal variables were shown as number and percentage, and differences were analysed using Pearson’s chi-squared test or Fisher’s exact test. Ordinal data were analysed using the chi-squared test for trend. Multivariable binary logistic regression analysis was used to define the independent risk factors. Univariable time-to-event analysis was done using the Kaplan-Meier method. Cox proportional hazard regression analysis was used for multivariable time-to-event analysis. Two-sided P values of < 0.05 were considered statistically significant.
This study included 169 adult RL-LDLT recipients. At the time of operation, the mean age of the recipient was 50 ± 8 years, and 150 (88.8%) were male. The indications for LT were HCC [60 (35.5%)] and liver cirrhosis because of HCV [148 (87.6%)], hepatitis B virus (HBV) [5 (3%)], HCV and HBV coinfection [4 (2.4%)], and other aetiologies including vascular, autoimmune, and cryptogenic cirrhosis [12 (7.1%); Tables 1 and 2].
| Variable | n (%) | |
| Etiology of cirrhosis | HCV | 148 (87.6) |
| HBV | 5 (3) | |
| Combined HCV & HBV | 4 (2.4) | |
| Others | 12 (7.1) | |
| Hepatocellular carcinoma | - | 109 (64.5) |
| + | 60 (35.5) | |
| Donors’ gender | Male | 141 (83.4) |
| Female | 28 (16.6) | |
| Recipients’ gender | Male | 150 (88.8) |
| Female | 19 (11.2) | |
| HCV PCR viremia prior to transplantation | Negative | 33 (19.52) |
| Below 200 000 IU | 59 (34.91) | |
| 200000 to 2 million | 69 (40.82) | |
| More than 2 million | 8 (4.73) | |
| Antiviral treatment for HCV prior to transplantation | - | 138 (81.7) |
| + | 31 (18.3) | |
| Arterial complications | - | 155 (91.7) |
| + | 14 (8.3) | |
| Number of anastomosis | 1 Anastomosis | 109 (64.5) |
| 2 Anastomosis | 57 (33.7) | |
| 3 Anastomosis | 3 (1.8) | |
| Number of ducts | 1 Duct | 78 (46.2) |
| 2 Ducts | 78 (46.2) | |
| 3 Ducts | 12 (7.1) | |
| 4 Ducts | 1 (0.6) | |
| Number of stents introduced at surgery | Nil | 7 (4.1) |
| 1 Stent | 71 (42) | |
| 2 Stents | 79 (46.7) | |
| 3 Stents | 11 (6.5) | |
| 4 Stents | 1 (0.6) | |
| Immunosuppressant | Tacrolimus | 118 (69.8) |
| Cyclosporine | 51 (30.2) | |
| Biliary leakage | - | 114 (67.5) |
| + | 55 (32.5) | |
| Need of pigtail catheter for biloma | - | 46 (83.6) |
| + | 9 (16.4) | |
| Biliary infection | - | 72 (42.6) |
| + | 97 (57.4) | |
| Frequency of biliary infection | 1-2 Episodes | 84 (49.7) |
| ≥ 3 Episodes | 13 (7.7) | |
| Biliary stricture | - | 109 (64.5) |
| + | 60 (35.5) | |
| Frequency of biliary stricture | 1-2 Episodes | 43 (25.4) |
| ≥ 3 Episodes | 17 (10.1) | |
| Need for ERCP | - | 109 (64.5) |
| + | 60 (35.5) | |
| Frequency of ERCP | 1-2 ERCP | 42 (24.9) |
| ≥ 3 ERCP | 18 (10.7) | |
| Need for PTC | - | 161 (95.3) |
| + | 8 (4.7) | |
| Frequency of PTC | 1 PTC | 7 (4.1) |
| 2 PTC | 1 (0.6) | |
| Surgical intervention for stricture | - | 168 (99.4) |
| + | 1 (0.6) | |
| HCV PCR during occurrence of stricture | Negative | 15 (25) |
| Below 200 000 IU | 15 (25) | |
| 200000 to 2 million | 19 (31.7) | |
| More than 2 million | 11 (18.3) | |
| HCV antiviral treatment in relation to stricture diagnosis | No treatment | 27 (45) |
| Before stricture | 14 (23.3) | |
| During occurrence of stricture | 13 (21.7) | |
| After stricture | 6 (10) | |
| Admission related to BC | - | 95 (56.2) |
| + | 74 (43.8) | |
| Mortality | - | 141 (83.4) |
| + | 28 (16.6) | |
| Cause of mortality (total number: 28) | Biliary sepsis | 5 (17.9) |
| Graft rejection | 4 (14.3) | |
| Recurrent HCV | 3 (10.7) | |
| Other causes | 16 (57.1) | |
| Chronic rejection | - | 142 (84) |
| + | 27 (16) | |
| Recurrent HCV infection | - | 128 (75.7) |
| + | 41 (24.3) | |
| Resolution of recurrent HCV | - | 4 (9.8) |
| + | 37 (90.2) | |
| Graft failure | - | 149 (88.2) |
| + | 20 (11.8) | |
| Causes of graft failure (total number: 20) | Biliary sepsis | 5 (25) |
| Graft rejection | 6 (30) | |
| Recurrent HCV | 3 (15) | |
| Other causes | 6 (30) | |
| Early biliary infection (total = 97) | - | 6 (6.18) |
| + | 91 (93.81) | |
| Early biliary stricture (total = 60) | - | 45 (75) |
| + | 15 (25) |
| Variable | Data |
| MELD score | 16 ± 4 |
| Child score | 10 ± 2 |
| Donors’ age (yr) | 27 ± 6 |
| Donors’ BMI (kg/m2) | 24 ± 3 |
| Recipient's age (yr) | 50 ± 8 |
| Recipient's BMI (kg/m2) | 28 ± 4 |
| Total bilirubin (mg/dL) | 2.6 (1.9-3.8) |
| Direct bilirubin (mg/dL) | 1.3 (0.7-2.1) |
| Alkaline phosphatase (IU/L) | 104 ± 48 |
| Gamma-glutamyl transferase (IU/L) | 36 (19-61) |
| Platelets (109/L) | 79 ± 35 |
| Cold ischemia time (min) | 49 ± 24 |
| Warm ischemia time (min) | 48 ± 20 |
| Graft arterialization time (min) | 141 ± 51 |
| Time to biliary infection (d) | 16 (11-30) |
| Time to biliary stricture (d) | 150 (120-218) |
| Time to mortality (d) | 285 (55-808) |
| Time to chronic graft rejection (d) | 490 (230-920) |
| Time to recurrent HCV (d) | 391 (180-714) |
| Time to graft failure (d) | 556 (135-1267) |
Prior to LT, 33 (19.52%) patients were HCV RNA negative, and 136 (80.46%) were HCV RNA positive. Thirty-one (18.3%) patients received antiviral treatment prior to LT. Forty-one (24.3%) patients experienced recurrent HCV infection, which was resolved in 37 (90.2%) patients (Table 1). Before the direct-acting antivirals (DAA) era, a Peg-interferon alfa-2a/Ribavirin (Peg-IFN/RBV) regimen was used for eligible patients, whereas after the availability of DAA therapy, sofosbuvir/daclatasvir ± RBV, sofosbuvir/simeprevir and ledipasvir/sofosbuvir regimens were used.
The majority of grafts had one or two ducts [both n = 78 (46.2%)], and the majority of patients needed one anastomosis [109 (64.5%)]. One to two stents were used in the majority of grafts [71 (42%) and 79 (46.7%), respectively; Table 1].
Fourteen (8.3%) patients experienced arterial complications; 12 patients had hepatic artery thrombosis (HAT), and two patients had hepatic artery stenosis (HAS; Table 1). In case HAT was detected not beyond two weeks post-LT, re-exploration was done, and after implementing inflow from the hepatic artery as well as backflow from the graft artery by embolectomy, re-anastomosis was conducted. In case of late presented HAT, interventional radiology and anticoagulation were done. In the case of HAS, a stent was inserted.
Among the 169 RT-LDLT recipients included in this study, minor BLs occurred in 55 patients (32.5%) and stopped spontaneously without further management. Only in nine (16.4%) patients were pigtail insertion and further interventional management needed. Ninety-seven (57.4%) patients suffered from biliary infection; it mostly occurred early [91 (93.81%)], and 13 (7.7%) patients had three or more episodes (Table 1).
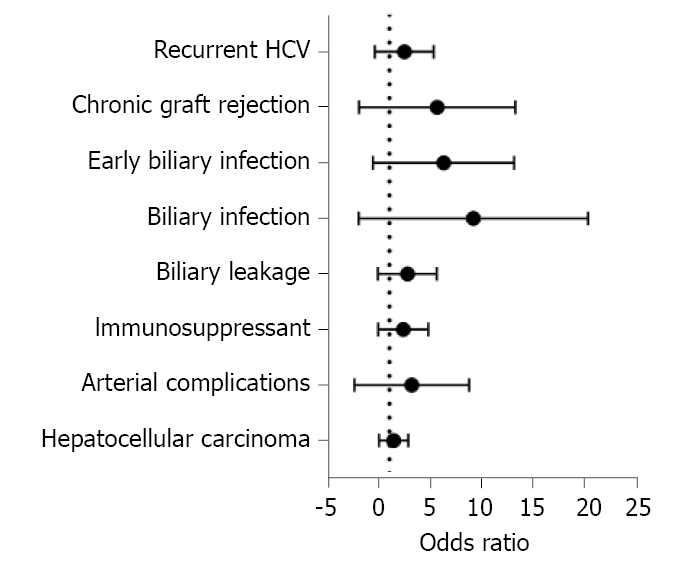
Sixty (35.5%) patients developed BS, most of which were anastomotic [59 (98.33%)], presented late [45 (75%)] and in one to two episodes [43 (25.4%)]. Most patients [45/60 (75%)] were HCV PCR positive during the occurrence of BS. Twenty-seven (45%) patients were not eligible for HCV antiviral treatment, while 14 (23.3%), 13 (21.7%) and 6 (10%) patients were treated before, during, and after the occurrence of BS, respectively (Table 1). Risk factors for BS were BL, biliary infection (especially if early or frequent), chronic graft rejection and longer graft arterialization time (Tables 3, 4 and Figure 2). In the multivariate analysis, graft arterialisation time > 130 min and biliary infection were the two determinants of BS (Table 5).
| Variable | Biliary strictures | OR | CI | P value1 | |||
| No stricture (n = 109) | Stricture (n = 60) | 95% LCL | 95% UCL | ||||
| n, Row % | n, Row % | ||||||
| Etiology of cirrhosis | HCV | 95 (64.2) | 53 (35.8) | 0.1422 | |||
| Isolated HBV | 5 (100) | 0 (0) | |||||
| Combined HCV & HBV | 1 (25) | 3 (75) | |||||
| Causes other than viral hepatitis | 8 (66.7) | 4 (33.3) | |||||
| Donors’ gender | Male | 90 (63.8) | 51 (36.2) | 0.8 | 0.4 | 2.0 | 0.684 |
| Female | 19 (67.9) | 9 (32.1) | |||||
| Recipients’ gender | Male | 96 (64) | 54 (36) | 0.8 | 0.3 | 2.3 | 0.704 |
| Female | 13 (68.4) | 6 (31.6) | |||||
| HCV PCR viremia prior to transplantation | Negative | 20 (60.6) | 13 (39.4) | 0.7683 | |||
| Below 200000 IU | 41 (69.5) | 18 (30.5) | |||||
| 200000 to 2 million | 44 (63.8) | 25 (36.2) | |||||
| More than 2 million | 4 (50) | 4 (50) | |||||
| Antiviral treatment prior to transplantation | - | 92 (66.7) | 46 (33.3) | 1.6 | 0.7 | 3.6 | 0.214 |
| + | 17 (54.8) | 14 (45.2) | |||||
| Hepatocellular carcinoma | - | 71 (65.1) | 38 (34.9) | 1.1 | 0.6 | 2.1 | 0.815 |
| + | 38 (63.3) | 22 (36.7) | |||||
| Arterial complications | - | 102 (65.8) | 53 (34.2) | 1.9 | 0.6 | 5.8 | 0.2552 |
| + | 7 (50) | 7 (50) | |||||
| Number of anastomoses | One | 70 (64.2) | 39 (35.8) | 0.9103 | |||
| Two | 37 (64.9) | 20 (35.1) | |||||
| Three | 2 (66.7) | 1 (33.3) | |||||
| Number of ducts | 1 Duct | 50 (64.1) | 28 (35.9) | 0.8573 | |||
| 2 Ducts | 52 (66.7) | 26 (33.3) | |||||
| 3 Ducts | 6 (50) | 6 (50) | |||||
| 4 Ducts | 1 (100) | 0 (0) | |||||
| Number of stents | Nil | 5 (71.4) | 2 (28.6) | 0.5783 | |||
| 1 Stent | 43 (60.6) | 28 (39.4) | |||||
| 2 Stents | 53 (67.1) | 26 (32.9) | |||||
| 3 Stents | 7 (63.6) | 4 (36.4) | |||||
| 4 Stents | 1 (100) | 0 (0) | |||||
| Immunosuppressant | Tacrolimus | 81 (68.6) | 37 (31.4) | 1.8 | 0.9 | 3.5 | 0.087 |
| Cyclosporine | 28 (54.9) | 23 (45.1) | |||||
| Biliary leakage | - | 80 (70.2) | 34 (29.8) | 2.1 | 1.1 | 4.1 | 0.026 |
| + | 29 (52.7) | 26 (47.3) | |||||
| Biliary infection | - | 62 (86.1) | 10 (13.9) | 6.6 | 3.0 | 14.4 | < 0.001 |
| + | 47 (48.5) | 50 (51.5) | |||||
| Frequency of biliary infection | Nil | 62 (86.1) | 10 (13.9) | < 0.0013 | |||
| 1-2 Episodes | 45 (53.6) | 39 (46.4) | |||||
| ≥ 3 Episodes | 2 (15.4) | 11 (84.6) | |||||
| Early biliary infection | - | 64 (82.1) | 14 (17.9) | 4.7 | 2.3 | 9.5 | < 0.001 |
| + | 45 (49.5) | 46 (50.5) | |||||
| Chronic graft rejection | - | 99 (69.7) | 43 (30.3) | 3.9 | 1.7 | 9.2 | 0.001 |
| + | 10 (37) | 17 (63) | |||||
| Recurrent HCV | - | 87 (68) | 41 (32) | 1.8 | 0.9 | 3.8 | 0.096 |
| + | 22 (53.7) | 19 (46.3) | |||||
| Variable | No biliary stricture (n = 109) | Biliary stricture (n = 60) | P value1 |
| MELD score | 15 (13-18) | 15 (13-19) | 0.588 |
| CHILD score | 10 (9-11) | 9 (8-11) | 0.198 |
| Donors’ age (yr) | 27 (23-30) | 25 (24-30) | 0.727 |
| Donors’ BMI (kg/m2) | 25 (23-26) | 24 (22-26) | 0.155 |
| Recipient's age (yr) | 51 (46-56) | 52 (48-55) | 0.961 |
| Recipient's BMI (kg/m2) | 27 (25-30) | 27 (26-30) | 0.219 |
| Total bilirubin (mg/dL) | 2.6 (1.9-3.7) | 2.5 (1.9-4.1) | 0.911 |
| Direct bilirubin (mg/dL) | 1.3 (0.8-2.1) | 1.3 (0.7-1.9) | 0.405 |
| Alkaline phosphatase (IU/L) | 99 (70-118) | 84 (68-143) | 0.982 |
| GGT (IU/L) | 36 (19-63) | 34 (22-60) | 0.992 |
| Platelets (109/L) | 70 (51-104) | 68 (51-102) | 0.830 |
| Cold ischemia time (min) | 45 (30-60) | 45 (30-60) | 0.929 |
| Warm ischemia time (min) | 45 (35-60) | 45 (35-60) | 0.860 |
| Graft arterialization time (min) | 120 (90-150) | 155 (120-205) | < 0.001 |
| Variable | P value | Odds ratio | 95%CI |
| Graft arterializations time > 130 min | 0.001 | 3.705 | 1.669-8.224 |
| Biliary leakage | 0.649 | 1.208 | 0.536-2.726 |
| > 1 Episode of biliary infection | < 0.0001 | 9.892 | 4.086-23.952 |
| Chronic graft rejection | 0.173 | 2.088 | 0.725-6.014 |
With respect to the management of BCs, ERCP with stenting ± dilatation was done for 60 (35.5%) patients, with 18 (10.7%) patients needing ≥ 3 ERCP sessions. PTC was attempted only in 8 (4.7%) patients, with one patient needing another session. These methods only failed in one patient who needed surgical reconstruction of BSs (Table 1).
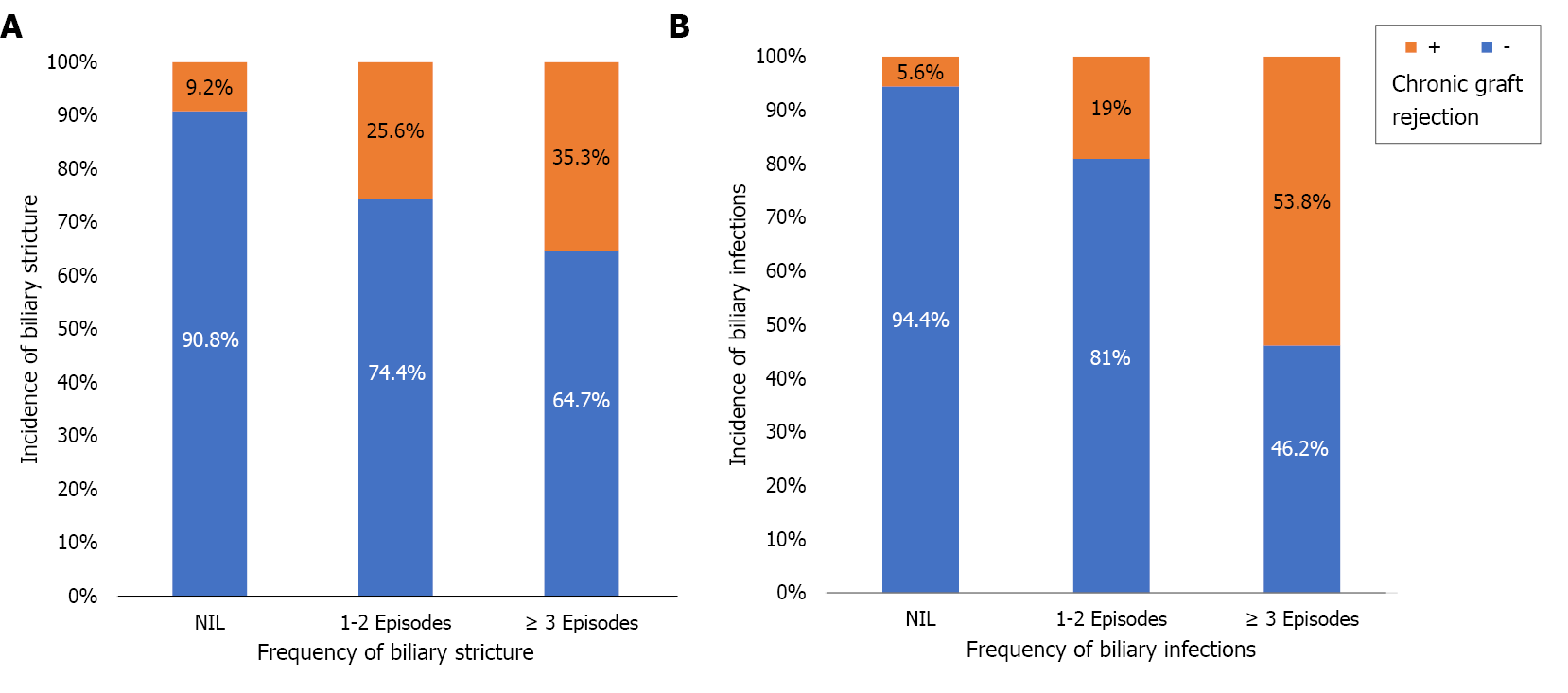
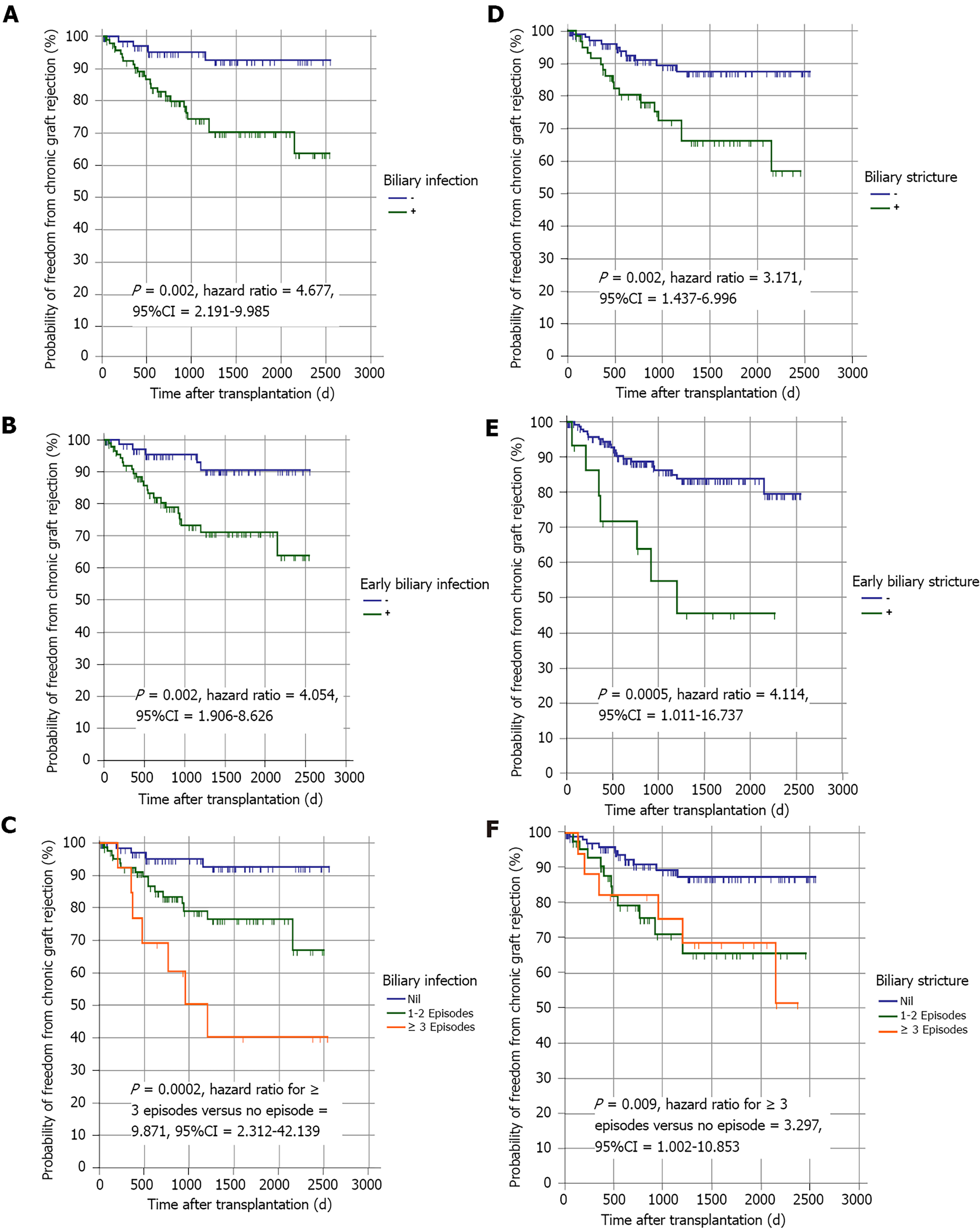
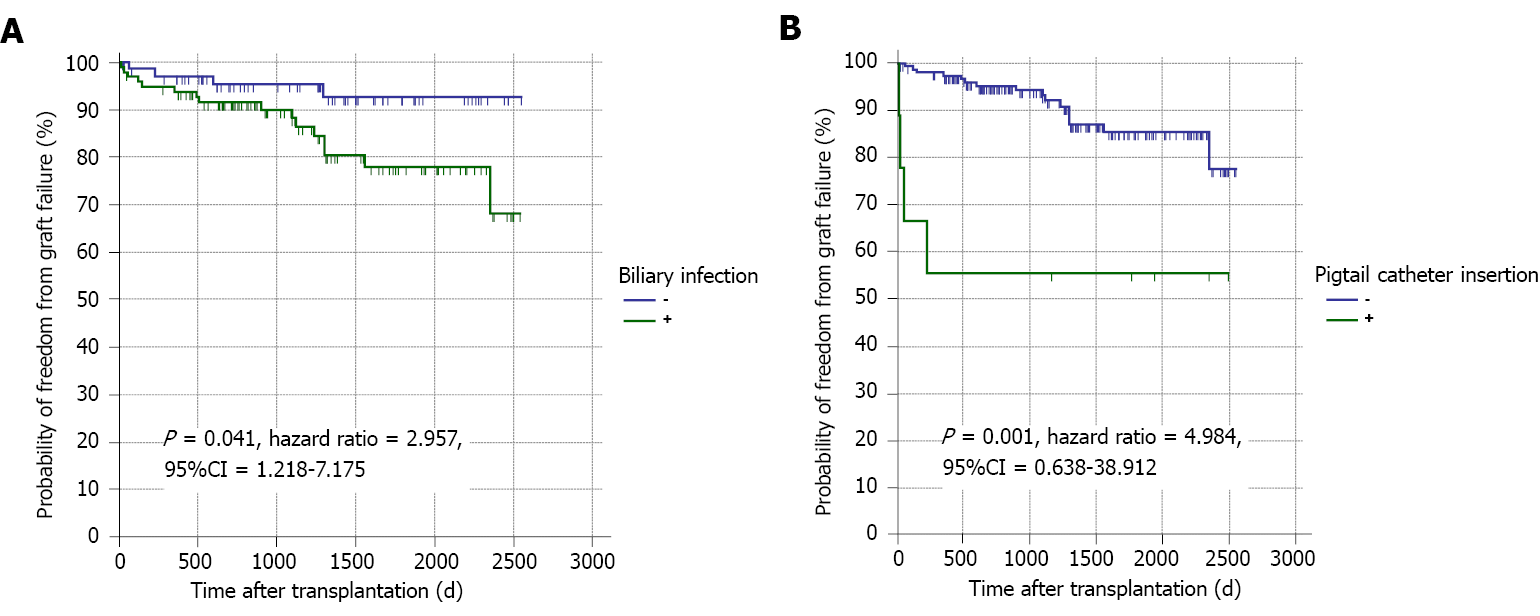
Twenty-seven (16%) patients experienced chronic graft rejection. It was determined by biliary infection (especially if early or frequent), BS (especially if early or frequent), the need of ERCP (especially if multiple sessions), the number of stents used for BS treatment, hospital admission (especially if frequent) and recurrent HCV infection (Tables 1, 6 and Figure 3). The impact of these parameters on graft rejection was further demonstrated by multivariate analysis and Kaplan-Meier analysis (Table 7, Figure 4, and Supplementary material).
| Variable | No Chronic graft rejection (n = 142), n (%) | Chronic graft rejection (n = 27), n (%) | OR | CI | P value1 | ||
| 95% LCL | 95% UCL | ||||||
| Biliary leakage | - | 100 (87.7) | 14 (12.3) | 2.2 | 1.0 | 5.1 | 0.059 |
| + | 42 (76.4) | 13 (23.6) | |||||
| Insertion of pigtail catheter for biliary leakage | - | 135 (84.4) | 25 (15.6) | 1.5 | 0.3 | 7.9 | 0.6372 |
| + | 7 (77.8) | 2 (22.2) | |||||
| Biliary infection | - | 68 (94.4) | 4 (5.6) | 5.3 | 1.7 | 16.1 | 0.001 |
| + | 74 (76.3) | 23 (23.7) | |||||
| Frequency of biliary infection | Nil | 68 (94.4) | 4 (5.6) | < 0.0013 | |||
| 1-2 Episodes | 68 (81) | 16 (19) | |||||
| ≥ 3 Episodes | 6 (46.2) | 7 (53.8) | |||||
| Early biliary infection | - | 73 (93.6) | 5 (6.4) | 4.7 | 1.7 | 13.0 | 0.002 |
| + | 69 (75.8) | 22 (24.2) | |||||
| Biliary stricture | - | 99 (90.8) | 10 (9.2) | 3.9 | 1.7 | 9.2 | 0.001 |
| + | 43 (71.7) | 17 (28.3) | |||||
| Frequency of biliary strictures | Nil | 99 (90.8) | 10 (9.2) | 0.0013 | |||
| 1-2 Episodes | 32 (74.4) | 11 (25.6) | |||||
| ≥ 3 Episodes | 11 (64.7) | 6 (35.3) | |||||
| Early biliary stricture | - | 134 (87) | 20 (13) | 5.9 | 1.9 | 17.9 | 0.0032 |
| + | 8 (53.3) | 7 (46.7) | |||||
| Need for ERCP | - | 99 (90.8) | 10 (9.2) | 3.9 | 1.7 | 9.2 | 0.001 |
| + | 43 (71.7) | 17 (28.3) | |||||
| Frequency of ERCP | Nil | 99 (90.8) | 10 (9.2) | 0.0013 | |||
| 1-2 ERCP | 31 (73.8) | 11 (26.2) | |||||
| ≥ 3 ERCP | 12 (66.7) | 6 (33.3) | |||||
| Number of stents introduced for stricture | Nil | 102 (91.1) | 10 (8.9) | 0.0023 | |||
| 1-2 stents | 25 (73.5) | 9 (26.5) | |||||
| ≥ 3 stents | 15 (65.2) | 8 (34.8) | |||||
| Need for PTC | - | 136 (84.5) | 25 (15.5) | 1.8 | 0.3 | 9.5 | 0.6152 |
| + | 6 (75) | 2 (25) | |||||
| Frequency of PTC | Nil | 136 (84.5) | 25 (15.5) | 0.1903 | |||
| 1 PTC | 6 (85.7) | 1 (14.3) | |||||
| 2 PTC | 0 (0) | 1 (100) | |||||
| Surgical intervention for stricture | - | 141 (83.9) | 27 (16.1) | 0.8 | 0.8 | 0.9 | 1.0002 |
| + | 1 (100) | 0 (0) | |||||
| HCV PCR at occurrence of stricture | Negative | 10 (66.7) | 5 (33.3) | 0.6603 | |||
| Below 200000 IU | 12 (80) | 3 (20) | |||||
| 200000 to 2 million | 15 (78.9) | 4 (21.1) | |||||
| More than 2 million | 6 (54.5) | 5 (45.5) | |||||
| Antiviral treatment in relation to stricture | Not given | 21 (77.8) | 6 (22.2) | 0.5362 | |||
| Before stricture | 9 (64.3) | 5 (35.7) | |||||
| After stricture | 4 (66.7) | 2 (33.3) | |||||
| During occurrence of stricture | 9 (69.2) | 4 (30.8) | |||||
| Admission related to BC | - | 85 (89.5) | 10 (10.5) | 2.5 | 1.1 | 5.9 | 0.028 |
| + | 57 (77) | 17 (23) | |||||
| Frequency of admissions related to biliary complications | Nil | 85 (89.5) | 10 (10.5) | 0.0023 | |||
| 1-2 | 35 (87.5) | 5 (12.5) | |||||
| ≥ 3 | 22 (64.7) | 12 (35.3) | |||||
| Recurrent HCV | - | 116 (90.6) | 12 (9.4) | 5.6 | 2.3 | 13.3 | < 0.001 |
| + | 26 (63.4) | 15 (36.6) | |||||
| Resolution of recurrent HCV | - | 1 (25) | 3 (75) | 0.2 | 0.0 | 1.7 | 0.1302 |
| + | 25 (67.6) | 12 (32.4) | |||||
| Variable | P value | Odds ratio | 95%CI |
| Biliary infection | 0.001 | 4.301 | 1.97-8.224 |
| Early biliary infection | 0.061 | 1.105 | 0.89-1.20 |
| Frequency of biliary infection | 0.025 | 1.208 | 0.536-2.726 |
| Biliary stricture | < 0.0001 | 3.882 | 4.056-9.952 |
| Need for ERCP | 0.02 | 2.91 | 1.85-7.97 |
| Frequency of ERCP | 0.074 | 1.098 | 0.99-1.114 |
| Number of stents | 0.62 | 1.22 | 0.57-2.42 |
| Admission related to BCs | 0.082 | 1.102 | 0.99-1.40 |
| Frequency of admission | 0.51 | 1.73 | 0.56-7.5 |
| Recurrent HCV | 0.032 | 3.11 | 1.97-8.07 |
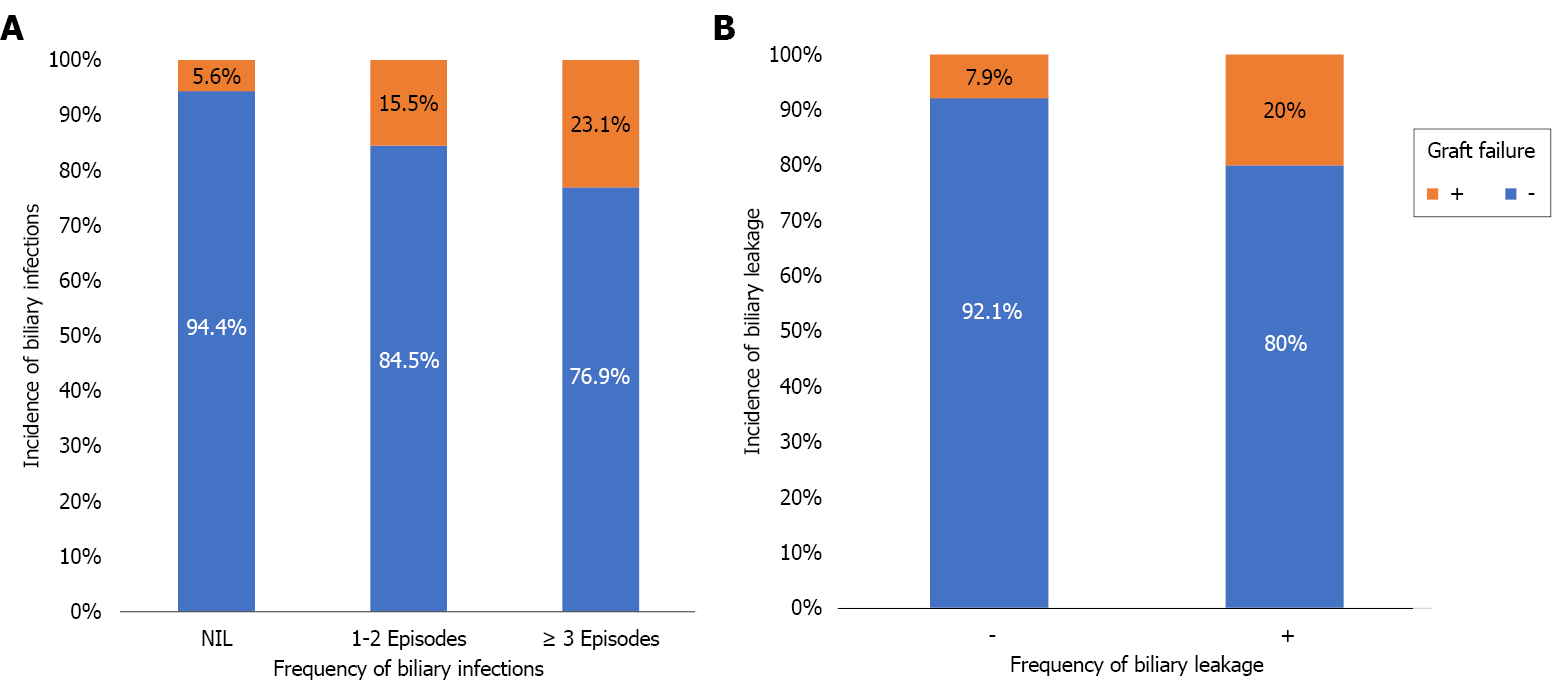
Graft failure developed in 20 (11.8%) patients; the causes were chronic graft rejection [6 (30%)], biliary infection [5 (25%)], recurrent HCV infection [3 (15%)], and other causes [6 (30%); Table 1]. BL, the need for pigtail catheter insertion, biliary infection (especially if frequent), recurrent HCV infection and non-response to HCV therapy were the risk factors of graft failure (Tables 8, 9 and Figure 5). Kaplan-Meier survival analysis further proved the impact of major BL and biliary infection on graft survival (Figure 6).
| Variable | No graft failure (n = 149), n (%) | Graft failure (n = 20), n (%) | OR | CI | P value1 | ||
| 95% LCL | 95% UCL | ||||||
| Biliary leakage | - | 105 (92.1) | 9 (7.9) | 2.9 | 1.1 | 7.5 | 0.022 |
| + | 44 (80) | 11 (20) | |||||
| Insertion of pigtail catheter | - | 144 (90) | 16 (10) | 7.2 | 1.8 | 29.6 | 0.0122 |
| + | 5 (55.6) | 4 (44.4) | |||||
| Biliary infection | - | 68 (94.4) | 4 (5.6) | 3.4 | 1.1 | 10.5 | 0.029 |
| + | 81 (83.5) | 16 (16.5) | |||||
| Frequency of biliary infection | Nil | 68 (94.4) | 4 (5.6) | 0.0213 | |||
| 1-2 Episodes | 71 (84.5) | 13 (15.5) | |||||
| ≥ 3 Episodes | 10 (76.9) | 3 (23.1) | |||||
| Early biliary infection | - | 73 (93.6) | 5 (6.4) | 2.9 | 1.0 | 8.3 | 0.043 |
| + | 76 (83.5) | 15 (16.5) | |||||
| Biliary stricture | - | 98 (89.9) | 11 (10.1) | 1.6 | 0.6 | 4.0 | 0.345 |
| + | 51 (85) | 9 (15) | |||||
| Frequency of biliary stricture | Nil | 98 (89.9) | 11 (10.1) | 0.1683 | |||
| 1-2 Episodes | 38 (88.4) | 5 (11.6) | |||||
| ≥ 3 Episodes | 13 (76.5) | 4 (23.5) | |||||
| Early biliary stricture | - | 137 (89) | 17 (11) | 2.0 | 0.5 | 7.9 | 0.3922 |
| + | 12 (80) | 3 (20.0) | |||||
| Need for ERCP | - | 98 (89.9) | 11 (10.1) | 1.6 | 0.6 | 4.0 | 0.345 |
| + | 51 (85) | 9 (15.0) | |||||
| Frequency of ERCP | Nil | 98 (89.9) | 11 (10.1) | 0.1883 | |||
| 1-2 ERCP | 37 (88.1) | 5 (11.9) | |||||
| ≥ 3 ERCP | 14 (77.8) | 4 (22.2) | |||||
| Number of stents introduced for stricture | Nil | 101 (90.2) | 11 (9.8) | 0.1363 | |||
| 1-2 Stents | 30 (88.2) | 4 (11.8) | |||||
| ≥ 3 Stents | 18 (78.3) | 5 (21.7) | |||||
| Need for PTC | - | 142 (88.2) | 19 (11.8) | 1.1 | 0.1 | 9.2 | 1.0002 |
| + | 7 (87.5) | 1 (12.5) | |||||
| Frequency of PTC | Nil | 142 (88.2) | 19 (11.8) | 0.3743 | |||
| 1 PTC | 7 (100) | 0 (0) | |||||
| 2 PTC | 0 (0) | 1 (100) | |||||
| Surgical intervention for stricture | - | 148 (88.1) | 20 (11.9) | 0.9 | 0.8 | 0.9 | 1.0002 |
| + | 1 (100) | 0 (0) | |||||
| HCV PCR at occurrence of stricture | Negative | 13 (86.7) | 2 (13.3) | 0.2923 | |||
| Below 200000 IU | 13 (86.7) | 2 (13.3) | |||||
| 200000 to 2 million | 18 (94.7) | 1 (5.3) | |||||
| More than 2 million | 7 (63.6) | 4 (36.4) | |||||
| Antiviral treatment in relation to stricture | Not given | 24 (88.9) | 3 (11.1) | 0.8362 | |||
| Before stricture | 11 (78.6) | 3 (21.4) | |||||
| After stricture | 5 (83.3) | 1 (16.7) | |||||
| During occurrence of stricture | 11 (84.6) | 2 (15.4) | |||||
| Admission related to BC | - | 85 (89.5) | 10 (10.5) | 1.3 | 0.5 | 3.4 | 0.551 |
| + | 64 (86.5) | 10 (13.5) | |||||
| Frequency of admissions related to BC | Nil | 85 (89.5) | 10 (10.5) | 0.119 | |||
| 1-2 ERCP | 38 (95) | 2 (5) | |||||
| ≥3 ERCP | 26 (76.5) | 8 (23.5) | |||||
| Recurrent HCV infection | - | 118 (92.2) | 10 (7.8) | 3.8 | 1.5 | 10.0 | 0.0102 |
| + | 31 (75.6) | 10 (24.4) | |||||
| Resolution of recurrent HCV | - | 0 (0) | 4 (100) | 6.2 | 3.0 | 12.8 | 0.0022 |
| + | 31 (83.8) | 6 (16.2) | |||||
| Variable | P value | Odds ratio | 95%CI |
| Biliary leakage | 0.021 | 1.82 | 1.34-5.57 |
| Insertion of pigtail catheter | 0.010 | 3.76 | 1.45-11.83 |
| Biliary infection | 0.032 | 3.11 | 1.03-9.06 |
| Early biliary infection | 0.05 | 1.34 | 0.65-2.86 |
| Frequency of biliary infection | 0.001 | 2.52 | 1.28-4.91 |
| Nonresponse to HCV anti-viral therapy | 0.001 | 3.6 | 1.8-9.34 |
| Recurrent HCV | 0.001 | 3.56 | 1.86-10.71 |
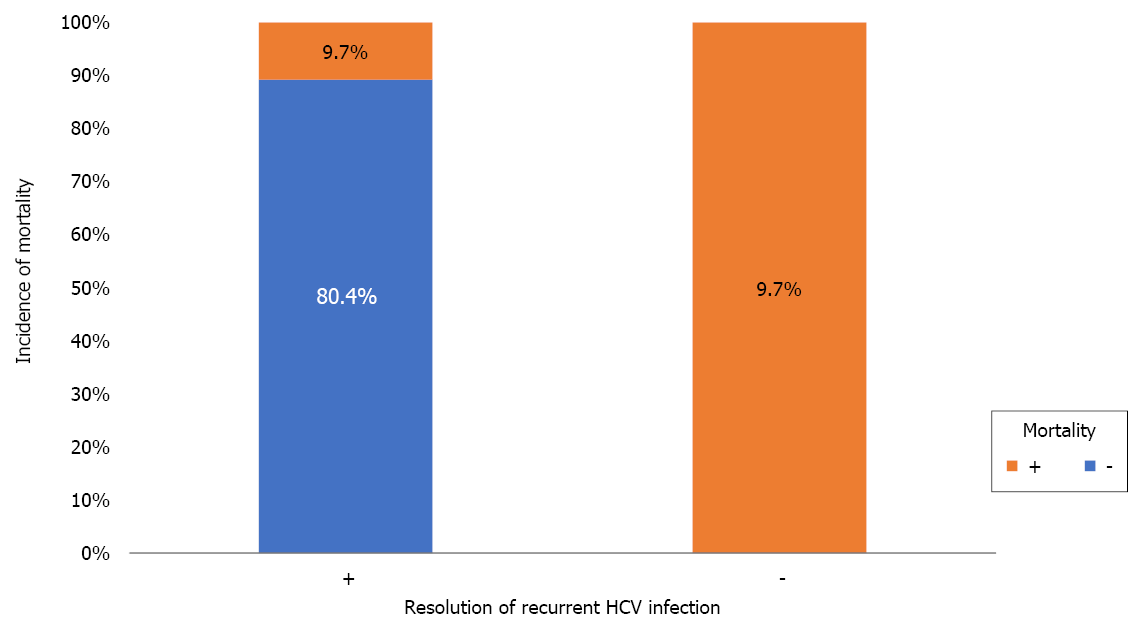
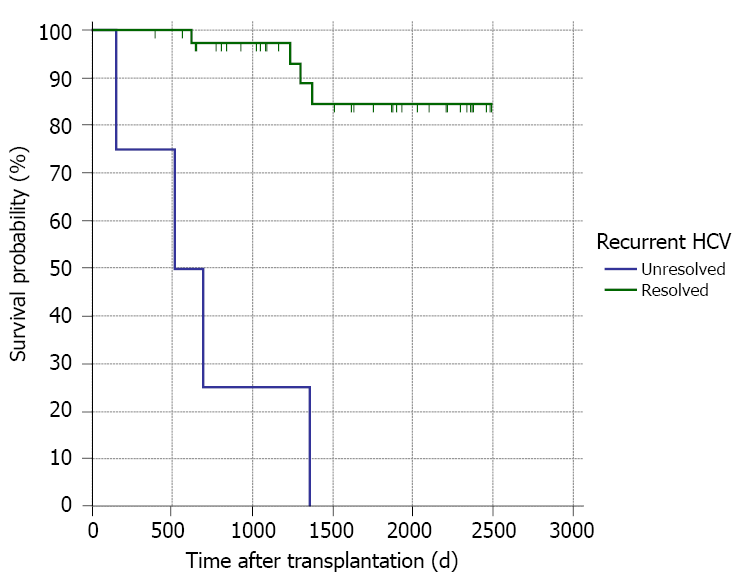
A total of 28 (16.6%) deaths occurred during follow-up. The aetiologies of mortality were biliary infection [5 (17.9%)], chronic graft rejection [4 (14.3%)], recurrent HCV infection [3 (10.7%)], and other causes [16 (57.1%); Table 1]. Unresolved recurrent HCV infection was the only risk factor for mortality (Table 10 and Figure 7). This was further proved by Kaplan-Meier survival analysis (Figure 8).
| Variable | Survivors (n = 141), n (%) | Non-survivors (n = 28), n (%) | OR | CI | P value1 | ||
| 95% LCL | 95% UCL | ||||||
| Biliary leakage | - | 97 (85.1) | 17 (14.9) | 1.4 | 0.6 | 3.3 | 0.405 |
| + | 44 (80) | 11 (20) | |||||
| Biliary infection | - | 60 (83.3) | 12 (16.7) | 1.0 | 0.4 | 2.2 | 1.0002 |
| + | 81 (83.5) | 16 (16.5) | |||||
| Frequency of biliary infection | Nil | 60 (83.3) | 12 (16.7) | 0.9403 | |||
| 1-2 Episodes | 70 (83.3) | 14 (16.7) | |||||
| ≥ 3 Episodes | 11 (84.6) | 2 (15.4) | |||||
| Early biliary infection | - | 64 (82.1) | 14 (17.9) | 0.8 | 0.4 | 1.9 | 0.6552 |
| + | 77 (84.6) | 14 (15.4) | |||||
| Biliary stricture | - | 89 (81.7) | 20 (18.3) | 0.7 | 0.3 | 1.7 | 0.4012 |
| + | 52 (86.7) | 8 (13.3) | |||||
| Frequency of biliary strictures | Nil | 89 (81.7) | 20 (18.3) | 0.3963 | |||
| 1-2 Episodes | 37 (86) | 6 (14) | |||||
| ≥ 3 Episodes | 15 (88.2) | 2 (11.8) | |||||
| Early biliary stricture | - | 128 (83.1) | 26 (16.9) | 0.8 | 0.2 | 3.6 | 1.0002 |
| + | 13 (86.7) | 2 (13.3) | |||||
| Need for ERCP | - | 89 (81.7) | 20 (18.3) | 0.7 | 0.3 | 1.7 | 0.4012 |
| + | 52 (86.7) | 8 (13.3) | |||||
| Frequency of ERCP | Nil | 89 (81.7) | 20 (18.3) | 0.3753 | |||
| 1-2 ERCP | 36 (85.7) | 6 (14.3) | |||||
| ≥ 3 ERCP | 16 (88.9) | 2 (11.1) | |||||
| Number of stents introduced for stricture | Nil | 92 (82.1) | 20 (17.9) | 0.5203 | |||
| 1-2 Stents | 29 (85.3) | 5 (14.7) | |||||
| ≥ 3 Stents | 20 (87) | 3 (13) | |||||
| Need for PTC | - | 134 (83.2) | 27 (16.8) | 0.7 | 0.1 | 6.0 | 1.0002 |
| + | 7 (87.5) | 1 (12.5) | |||||
| Frequency of PTC | Nil | 134 (83.2) | 27 (16.8) | 0.6743 | |||
| 1 PTC | 7 (100) | 0 (0) | |||||
| 2 PTC | 0 (0) | 1 (100) | |||||
| Surgical intervention for stricture | - | 140 (83.3) | 28 (16.7) | 1.0002 | |||
| + | 1 (100) | 0 (0) | |||||
| HCV PCR at occurrence of stricture | Negative | 12 (80) | 3 (20) | 0.8493 | |||
| Below 200 000 IU | 14 (93.3) | 1 (6.7) | |||||
| 200000 to 2 million | 18 (94.7) | 1 (5.3) | |||||
| More than 2 million | 8 (72.7) | 3 (27.3) | |||||
| Antiviral treatment in relation to stricture | Not given | 23 (85.2) | 4 (14.8) | 1.0002 | |||
| Before stricture | 12 (85.7) | 2 (14.3) | |||||
| After stricture | 5 (83.3) | 1 (16.7) | |||||
| During occurrence of stricture | 12 (92.3) | 1 (7.7) | |||||
| Admission related to BC | - | 75 (78.9) | 20 (21.1) | 0.5 | 0.2 | 1.1 | 0.0762 |
| + | 66 (89.2) | 8 (10.8) | |||||
| Recurrent HCV | - | 108 (84.4) | 20 (15.6) | 1.3 | 0.5 | 3.2 | 0.5602 |
| + | 33 (80.5) | 8 (19.5) | |||||
| Resolution of recurrent HCV (n = 41) | - | 0 (0) | 4 (9.7) | 9.3 | 3.7 | 23.3 | 0.0012 |
| + | 33 (80.4) | 4 (9.7) | |||||
LT is considered the only curative therapeutic option for patients with end-stage hepatic disease. Several complications, especially BC, still endanger its short and long-term outcomes[21,28,29]. Many studies have focused on BC to improve care for transplanted recipients; however, data on long-term outcomes remains scarce[28].
The BC incidence rate is extremely diverse between centres. The overall incidence of BC, including BL, biliary infection and BS, in our study was 57.4%. This rate is comparable to previous reports[17,30-33]; however, it is higher than other published data[8,15,21,34,35]. This difference can be attributed to the heterogeneous structure between the different studies regarding the type of graft, surgical techniques and the inconsistent inclusion of biliary infection and bile stones as a part of BC.
In addition to surgical techniques, several risk factors for BC have been defined in the published literature[3,7,14,21,36], such as older recipients and donors, female recipients and recipients of female donors, ABO mismatch, a prolonged anhepatic phase and prolonged ischemia times. However, the current study and other investigators[15,22,34] were unable to establish any of these conditions as risk factors for BC. This may be attributed to the inclusion of only ABO-matched living grafts, the younger age of our donors and recipients and the male predominance in our cohort.
Additionally, cholestatic liver diseases and the use of RYHJ technique were independent risk factors for BS in previous reports[15,37]. However, this is not the case in our study because DDA was used in all the grafts; besides, we excluded patients with PBC and PSC from the final analysis to avoid the bias of primary disease recurrence as a confounding factor during analysis of BC.
In accordance with published data[15,17], no association between BC and MELD score was observed. This result differs from studies recognizing a higher MELD score as a risk factor for BC[3,28,34]. This can be explained by the lower MELD scores in our patients. Also, these conflicting results may reflect the well-established limits of the MELD score in predicting post-LT outcomes[38].
The ideal material and style of sutures in biliary reconstruction has been argued since the early development of LT. Kaldas et al[17] reported that the use of non-absorbable sutures for biliary reconstruction was an independent risk factor for BC. However, this was not the case in the present study due to the different suture material.
In accordance with previous results[22], we observed that the occurrence of BS was not related to the number of bile ducts or stent insertion. In contrast, Miyagi et al[8] and Ogiso et al[34] identified the number of bile ducts as a risk factor for BC. Furthermore, Senter-Zapata et al[15] reported that internal biliary stents and T-tube insertion were risk factors for BC post-LT. However, in our centre, we prefer external drainage for easy accessibility of biliary ducts for postoperative cholangiography to manage any strictures[22]; on the contrary, other centres do not prefer this due to the higher incidence of postoperative BL and biliary infections[14].
BCs are mostly identified in the first three to 12 mo post-LT[8,17]. Similarly, in consistence with other reports[7,15,17,30,31,33], we detected BL early in 55/169 (32.5%) patients, and BS in 60/169 (35.5%) patients. The majority of BSs were anastomotic and presented late.
In a similar management plan as other centres[22,24,29,30,34], minor BLs were treated conservatively; nonetheless, major BL required percutaneous drainage and/or stenting. ERCP was the treatment of choice for all patients. PTC was the treatment option if ERCP failed, and surgical intervention was performed as a last option.
In consistence with our results, other investigators[7,8,21,39] observed that BL and cholangitis were risk factors for the development of BS. This can be explained by the inflammatory process with the resultant progression of fibrosis and stricture formation[40].
In agreement with Rammohan et al[39], we identified longer arterialization time as a risk factor for BS. This finding is predictable because biliary tract vascularization is supplied exclusively by the hepatic artery[41-43], and a longer arterialization time of the graft may cause biliary ischemia and subsequently BS[28].
In contrast to the present and Ogiso et al[34] studies, other investigators[15,17,28,29,41] reported that hepatic artery complications were linked to the incidence of BC. This conflicting result can be attributed to the low incidence of arterial complications in our cohort as well as the early effective intervention for such complications.
It was previously reported that graft rejection and BC are interrelated conditions[15,35]; however, there are limited data concerning the impact of BC on chronic graft rejection. The incidence rate of chronic ductopenic rejection in our study was 27 (16%) patients; 23 (85.18%),17 (63%) and 13 (48.1%) of them had biliary infection, BS and BL, respectively. Additionally, chronic graft rejection was a risk factor for BS. Similar findings were reported by other investigators[44]. This is consistent with the histopathological findings of chronic ductopenic rejection where ductal inflammation and proliferation are seen in early stages and biliary duct fibrosis with progressive ductopenia is seen in late stages, which is manifested as intrahepatic BS by MRCP[45].
Biliary infection was a risk factor for chronic graft rejection and graft failure, which is explained by interrupted immunosuppressive therapy during times of sepsis[46,47].
In agreement with previous results[15,17,34,48], we found that the main reasons for graft failure were chronic ductopenic rejection, biliary infection, BL, and recurrent HCV infection, while Egeli et al[49] reported that HCC recurrence was the main cause of graft failure. This is justified by the inclusion of many patients beyond Milan criteria in their study.
In contrast to Mathur et al[50] and in consistence with other investigators[8,17,34,41], there was no association between BS and graft failure. This proves that early detection and efficient management of BS can prevent graft loss.
In the current study, recurrent HCV infection was a risk factor for chronic graft rejection, graft failure and mortality. This is predictable due to the aggressive course of HCV recurrence in LT recipients through direct cytotoxic effects on the graft, resulting in graft failure[48,49,51-53]. It is noteworthy that DAA were not FDA approved during the first three years of the study duration; thus, many patients were ineligible for the Peg-IFN/RBV regimen at that time.
Similar to Takagi et al[54] study, the overall mortality rate for recipients was 28 (16.56%). Unresolved HCV recurrence was the only significant risk factor for mortality, while BC had no impact on recipients’ survival in the present study. This is similar to previous results[17,21,39,41,49]. In contrast, other investigators[15,33] observed a worse survival rate in recipients with BC. This indicates that early detection and effective management of BC can improve recipients’ survival[2,17].
This study has the strength of being large volume with a long duration of follow-up, as well as the exclusion of LDLT recipients because of cholestatic hepatic diseases; however, it is limited by being a single-centre retrospective study. Multi-centre large-scale studies are required to comprehensively investigate the risk factors for the occurrence and impacts of BC.
In conclusion, biliary complications after RT-LDLT represent an independent risk factor for chronic graft rejection and graft failure; nonetheless, effective management of these complications can improve patient and graft survival.
Despite considerable progress in liver transplantation (LT) surgical performance and peri-operative management, post-LT biliary complications (BCs) remain a considerable cause of morbidity, mortality, increased cost, and graft loss.
Many studies have focused on biliary complications to improve care for transplanted recipients; however, data on long-term outcomes remain scarce.
We aimed to investigate the impact of BCs after right lobe-LDLT (RL-LDLT) on chronic graft rejection, graft failure and mortality.
From 2011 to 2016, 215 adult recipients underwent RL-LDLT at our centre. We excluded 46 recipients who met the exclusion criteria, and 169 recipients were included in the final analysis. Donors’ and recipients’ demographic data, clinical data, operative details and postoperative course information were collected. We also reviewed the management and outcomes of BCs. Recipients were followed for at least 12 mo post-LT until December 2017 or graft or patient loss.
The overall incidence rate of BCs including biliary leakage, biliary infection and biliary stricture was 57.4%. Twenty-seven (16%) patients experienced chronic graft rejection. Graft failure developed in 20 (11.8%) patients. A total of 28 (16.6%) deaths occurred during follow-up. BCs were a risk factor for the occurrence of chronic graft rejection and failure; however, mortality was determined by recurrent hepatitis C virus infection.
Biliary complications after RT-LDLT represent an independent risk factor for chronic graft rejection and graft failure; nonetheless, effective management of these complications can improve patient and graft survival.
Multi-centre large-scale studies are required to comprehensively investigate the risk factors for the occurrence and impacts of BC.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lee Y, Ou HY, Zhang W S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Jadlowiec CC, Taner T. Liver transplantation: Current status and challenges. World J Gastroenterol. 2016;22:4438-4445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 238] [Cited by in RCA: 211] [Article Influence: 23.4] [Reference Citation Analysis (2)] |
| 2. | Chang JH, Lee I, Choi MG, Han SW. Current diagnosis and treatment of benign biliary strictures after living donor liver transplantation. World J Gastroenterol. 2016;22:1593-1606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 3. | Nemes B, Gámán G, Doros A. Biliary complications after liver transplantation. Expert Rev Gastroenterol Hepatol. 2015;9:447-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | Tsujino T, Isayama H, Kogure H, Sato T, Nakai Y, Koike K. Endoscopic management of biliary strictures after living donor liver transplantation. Clin J Gastroenterol. 2017;10:297-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Rela M, Reddy MS. Living donor liver transplant (LDLT) is the way forward in Asia. Hepatol Int. 2017;11:148-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Doyle MB, Maynard E, Lin Y, Vachharajani N, Shenoy S, Anderson C, Earl M, Lowell JA, Chapman WC. Outcomes with split liver transplantation are equivalent to those with whole organ transplantation. J Am Coll Surg. 2013;217:102-12; discussion 113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Rao HB, Prakash A, Sudhindran S, Venu RP. Biliary strictures complicating living donor liver transplantation: Problems, novel insights and solutions. World J Gastroenterol. 2018;24:2061-2072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 8. | Miyagi S, Kakizaki Y, Shimizu K, Miyazawa K, Nakanishi W, Hara Y, Tokodai K, Nakanishi C, Kamei T, Ohuchi N, Satomi S. Arterial and biliary complications after living donor liver transplantation: a single-center retrospective study and literature review. Surg Today. 2018;48:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Baker TB, Zimmerman MA, Goodrich NP, Samstein B, Pomfret EA, Pomposelli JJ, Gillespie BW, Berg CL, Emond JC, Merion RM. Biliary reconstructive techniques and associated anatomic variants in adult living donor liver transplantations: The adult-to-adult living donor liver transplantation cohort study experience. Liver Transpl. 2017;23:1519-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Lee SG. A complete treatment of adult living donor liver transplantation: a review of surgical technique and current challenges to expand indication of patients. Am J Transplant. 2015;15:17-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 270] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 11. | Chok KS, Lo CM. Prevention and management of biliary anastomotic stricture in right-lobe living-donor liver transplantation. J Gastroenterol Hepatol. 2014;29:1756-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Samstein B, Smith AR, Freise CE, Zimmerman MA, Baker T, Olthoff KM, Fisher RA, Merion RM. Complications and Their Resolution in Recipients of Deceased and Living Donor Liver Transplants: Findings From the A2ALL Cohort Study. Am J Transplant. 2016;16:594-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Simoes P, Kesar V, Ahmad J. Spectrum of biliary complications following live donor liver transplantation. World J Hepatol. 2015;7:1856-1865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Girometti R, Pancot M, Como G, Zuiani C. Imaging of liver transplantation. Eur J Radiol. 2017;93:295-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Senter-Zapata M, Khan AS, Subramanian T, Vachharajani N, Dageforde LA, Wellen JR, Shenoy S, Majella Doyle MB, Chapman WC. Patient and Graft Survival: Biliary Complications after Liver Transplantation. J Am Coll Surg. 2018;226:484-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Oh HC. Percutaneous Transhepatic Cholangioscopy in Bilioenteric Anastomosis Stricture. Clin Endosc. 2016;49:530-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Kaldas FM, Korayem IM, Russell TA, Agopian VG, Aziz A, DiNorcia J, Farmer DG, Yersiz H, Hiatt JR, Busuttil RW. Assessment of Anastomotic Biliary Complications in Adult Patients Undergoing High-Acuity Liver Transplant. JAMA Surg. 2019;154:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Lee DW, Jo HH, Abdullah J, Kahaleh M. Endoscopic Management of Anastomotic Strictures after Liver Transplantation. Clin Endosc. 2016;49:457-461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Koo PT, Medici V, Tabibian JH. Anastomotic Biliary Stricture Development after Liver Transplantation in the Setting of Retained Prophylactic Intraductal Pediatric Feeding Tube: Case and Review. Case Reports Hepatol. 2018;2018:4707389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Crismale JF, Ahmad J. Endoscopic Management of Biliary Issues in the Liver Transplant Patient. Gastrointest Endosc Clin N Am. 2019;29:237-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (37)] |
| 21. | Hong SY, Hu XG, Lee HY, Won JH, Kim JW, Shen XY, Wang HJ, Kim BW. Longterm Analysis of Biliary Complications After Duct-to-Duct Biliary Reconstruction in Living Donor Liver Transplantations. Liver Transpl. 2018;24:1050-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Azzam AZ, Tanaka K. Biliary complications after living donor liver transplantation: A retrospective analysis of the Kyoto experience 1999-2004. Indian J Gastroenterol. 2017;36:296-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Santosh Kumar KY, Mathew JS, Balakrishnan D, Bharathan VK, Thankamony Amma BSP, Gopalakrishnan U, Narayana Menon R, Dhar P, Vayoth SO, Sudhindran S. Intraductal Transanastomotic Stenting in Duct-to-Duct Biliary Reconstruction after Living-Donor Liver Transplantation: A Randomized Trial. J Am Coll Surg. 2017;225:747-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Elwir S, Thompson J, Amateau SK, Trikudanathan G, Attam R, Hassan M, Kandaswamy R, Pruett T, Lake J, Chinnakotla S, Freeman ML, Arain MA. Endoscopic Management of Biliary Leaks and Strictures After Living Donor Liver Transplantation: Optimizing Techniques for Successful Management. Dig Dis Sci. 2017;62:244-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5313] [Article Influence: 183.2] [Reference Citation Analysis (0)] |
| 26. | Ishiko T, Egawa H, Kasahara M, Nakamura T, Oike F, Kaihara S, Kiuchi T, Uemoto S, Inomata Y, Tanaka K. Duct-to-duct biliary reconstruction in living donor liver transplantation utilizing right lobe graft. Ann Surg. 2002;236:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Tanaka K, Uemoto S, Tokunaga Y, Fujita S, Sano K, Nishizawa T, Sawada H, Shirahase I, Kim HJ, Yamaoka Y. Surgical techniques and innovations in living related liver transplantation. Ann Surg. 1993;217:82-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 436] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 28. | Kaltenborn A, Gutcke A, Gwiasda J, Klempnauer J, Schrem H. Biliary complications following liver transplantation: Single-center experience over three decades and recent risk factors. World J Hepatol. 2017;9:147-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Coelho JCU, Leite LO, Molena A, Freitas ACT, Matias JEF. BILIARY COMPLICATIONS AFTER LIVER TRANSPLANTATION. Arq Bras Cir Dig. 2017;30:127-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Rao HB, Koshy AK, Sudhindran S, Prabhu NK, Venu RP. Paradigm shift in the management of bile duct strictures complicating living donor liver transplantation. Indian J Gastroenterol. 2019;38:488-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Iesari S, Inostroza Núñez ME, Rico Juri JM, Ciccarelli O, Bonaccorsi-Riani E, Coubeau L, Laterre PF, Goffette P, De Reyck C, Lengelé B, Gianello P, Lerut J. Adult-to-adult living-donor liver transplantation: The experience of the Université catholique de Louvain. Hepatobiliary Pancreat Dis Int. 2019;18:132-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Boraschi P, Donati F, Pacciardi F, Ghinolfi D, Falaschi F. Biliary complications after liver transplantation: Assessment with MR cholangiopancreatography and MR imaging at 3T device. Eur J Radiol. 2018;106:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Hafeez Bhatti AB, Dar FS, Qureshi AI, Khan NY, Zia HH, Khan EUD, Khan NA, Salih M, Shah NH. Failure to rescue in living donor liver transplantation: Patterns and predictors. Int J Surg. 2017;44:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Ogiso S, Kamei H, Onishi Y, Kurata N, Jobara K, Kawashima H, Ogura Y. Decreased long-term graft survival in persistent biliary complications after right-lobe living-donor liver transplantation. Clin Transplant. 2020;34:e13771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Dogan N, Hüsing-Kabar A, Schmidt HH, Cicinnati VR, Beckebaum S, Kabar I. Acute allograft rejection in liver transplant recipients: Incidence, risk factors, treatment success, and impact on graft failure. J Int Med Res. 2018;46:3979-3990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 36. | Memeo R, Piardi T, Sangiuolo F, Sommacale D, Pessaux P. Management of biliary complications after liver transplantation. World J Hepatol. 2015;7:2890-2895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Pena Polanco NA, Levy C, Martin EF. Cholestatic Liver Diseases After Liver Transplant. Clin Liver Dis. 2017;21:403-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Rana A, Hardy MA, Halazun KJ, Woodland DC, Ratner LE, Samstein B, Guarrera JV, Brown RS Jr, Emond JC. Survival outcomes following liver transplantation (SOFT) score: a novel method to predict patient survival following liver transplantation. Am J Transplant. 2008;8:2537-2546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 350] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 39. | Rammohan A, Govil S, Vargese J, Kota V, Reddy MS, Rela M. Changing pattern of biliary complications in an evolving liver transplant unit. Liver Transpl. 2017;23:478-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Daniel K, Said A. Early Biliary complications after liver transplantation. Clin Liver Dis (Hoboken). 2017;10:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Jeong S, Wang X, Wan P, Sha M, Zhang J, Xia L, Tong Y, Luo Y, Xia Q. Risk factors and survival outcomes of biliary complications after adult-to-adult living donor liver transplantation. United European Gastroenterol J. 2017;5:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Kim PT, Fernandez H, Gupta A, Saracino G, Ramsay M, McKenna GJ, Testa G, Anthony T, Onaca N, Ruiz RM, Klintmalm GB. Low Measured Hepatic Artery Flow Increases Rate of Biliary Strictures in Deceased Donor Liver Transplantation: An Age-Dependent Phenomenon. Transplantation. 2017;101:332-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Nacif LS, Ducatti L, Andraus W, D’Albuquerque LC. Hepatic Artery Thrombosis after Orthotopic Liver Transplantation. Adv Res Gastroentero Hepatol. 2015;1:555560. [DOI] [Full Text] |
| 44. | Mocchegiani F, Vincenzi P, Lanari J, Montalti R, Nicolini D, Svegliati Baroni G, Risaliti A, Vivarelli M. Immunological risk factors in biliary strictures after liver transplantation. Ann Transplant. 2015;20:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Koukoulis GK, Shen J, Karademir S, Jensen D, Williams J. Cholangiocytic apoptosis in chronic ductopenic rejection. Hum Pathol. 2001;32:823-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 46. | Tannuri AC, Lima F, Mello ES, Tanigawa RY, Tannuri U. Prognostic factors for the evolution and reversibility of chronic rejection in pediatric liver transplantation. Clinics (Sao Paulo). 2016;71:216-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Horster S, Bäuerlein FJ, Mandel P, Raziorrouh B, Hopf C, Stemmler HJ, Guba M, Angele M, Stangl M, Rentsch M, Frey L, Kaspar M, Kaczmarek I, Eberle J, Nickel T, Gruener N, Zachoval R, Diepolder H. Influence of hepatitis C virus infection and high virus serum load on biliary complications in liver transplantation. Transpl Infect Dis. 2013;15:306-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Huesing-Kabar A, Dohna CZ, Heinzow H, Cicinnati VR, Beckebaum S, Schmidt M, Gerth HU, Pohlen M, Wilms C, Palmes D, Schmidt HH, Kabar I. Risk factors for allograft failure in liver transplant recipients. Z Gastroenterol. 2018;56:745-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Egeli T, Unek T, Ağalar C, Derici S, Ozbilgin M, Akarasu M, Bacakoglu A, Ellidokuz H, Astarcıoglu I. Analysis of Causes and Risk Factors for Late Mortality After Liver Transplant: How Can We Obtain Better Long-Term Survival? Exp Clin Transplant. 2020;18:182-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 50. | Mathur AK, Nadig SN, Kingman S, Lee D, Kinkade K, Sonnenday CJ, Welling TH. Internal biliary stenting during orthotopic liver transplantation: anastomotic complications, post-transplant biliary interventions, and survival. Clin Transplant. 2015;29:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 51. | Berge E, Otón E, Reina Z, Díaz L, Márquez A, Cejas L, Acosta S, Pérez F. Predictors of Poor Prognosis in Recurrent Hepatitis C After Liver Transplantation. Transplant Proc. 2016;48:2997-2999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 52. | Shiba H, Hashimoto K, Kelly D, Fujiki M, Quintini C, Aucejo F, Uso TD, Yerian L, Yanaga K, Matsushima M, Eghtesad B, Fung J, Miller C. Risk stratification of allograft failure secondary to hepatitis C recurrence after liver transplantation. Hepatol Res. 2016;46:1099-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 53. | Llovet LP, Sciarrone S, Rodríguez-Tajes S, Montironi C, Mescoli C, Rugge M, Crespo G, Burra P, Forns X, Diaz A, Londoño MC. Ductular reaction and hepatocyte ballooning identify patients with fibrosing cholestatic hepatitits after liver transplantation. Gastroenterol Hepatol. 2020;43:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 54. | Takagi K, Domagala P, Polak WG, Ijzermans JNM, Boehnert MU. Right posterior segment graft for living donor liver transplantation: A systematic review. Transplant Rev (Orlando). 2020;34:100510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |