Published online Dec 27, 2021. doi: 10.4254/wjh.v13.i12.2071
Peer-review started: January 4, 2021
First decision: July 8, 2021
Revised: July 22, 2021
Accepted: November 24, 2021
Article in press: November 24, 2021
Published online: December 27, 2021
Processing time: 356 Days and 10.2 Hours
The importance of early diagnosis of alcoholic liver disease underscores the need to seek better and especially non-invasive diagnostic procedures. Leukocyte cell-derived chemotaxin-2 (LECT2) has been widely studied to determine its usefulness in monitoring the course of non-alcoholic fatty liver disease but not for alcoholic liver cirrhosis (ALC).
To determine the concentration of LECT2 in the blood serum of patients in relation to progressive stages of ALC, its relation to fibroblast growth factor 1 (FGF-1) and FGF-21, and to examine the possible wider use of LECT2 in diagnosing ALC.
A retrospective case-control study was conducted with 69 ALC cases and 17 controls with no ALC. Subjects were recruited from the region of Lublin (eastern Poland). Liver cirrhosis was diagnosed based on clinical features, history of heavy alcohol consumption, laboratory tests, and abdominal ultrasonography. The degree of ALC was evaluated according to Pugh-Child criteria (the Pugh-Child score). Blood was drawn and, after centrifugation, serum was collected for analysis. LECT2, FGF-1, and FGF-21 were determined using enzyme-linked immunosorbent assay kits.
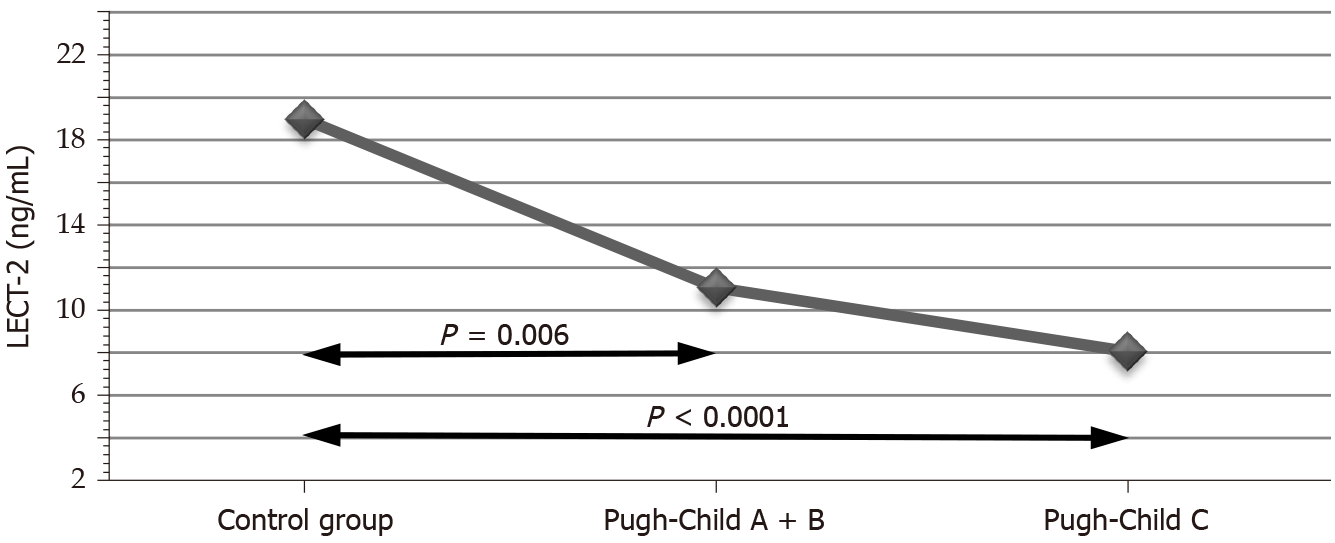
The LECT2 Levels in the control group were 18.99 ± 5.36 ng/mL. In the study groups, they declined with the progression of cirrhosis to 11.06 ± 6.47 ng/mL in one group and to 8.06 ± 5.74 ng/mL in the other (P < 0.0001). Multiple comparison tests confirmed the statistically significant differences in LECT2 Levels between the control group and both test groups (P = 0.006 and P < 0.0001). FGF-21 Levels were 44.27 ± 64.19 pg/mL in the first test group, 45.4 ± 51.69 pg/mL in the second (P = 0.008), and 13.52 ± 7.51 pg/mL in the control group. The difference between the control group and the second test group was statistically significant (P = 0.007).
We suggest that LECT2 may be a non-invasive diagnostic factor for alcohol-induced liver cirrhosis. The usefulness of LECT2 for non-invasive monitoring of alcohol-induced liver cirrhosis was indirectly confirmed by the multiple regression model developed on the basis of our statistical analysis.
Core Tip: Leukocyte cell-derived chemotaxin-2 (LECT2) was first described in 1996 as a novel chemotactic factor for neutrophils. It has been widely studied to determine its usefulness for monitoring the course of non-alcoholic fatty liver disease but not for alcoholic liver cirrhosis (ALC). We suggest that LECT2 may be used for the non-invasive diagnosis of ALC.
- Citation: Sak JJ, Prystupa A, Kiciński P, Luchowska-Kocot D, Kurys-Denis E, Bis-Wencel H. Leukocyte cell-derived chemotaxin-2 and fibroblast growth factor 21 in alcohol-induced liver cirrhosis. World J Hepatol 2021; 13(12): 2071-2080
- URL: https://www.wjgnet.com/1948-5182/full/v13/i12/2071.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i12.2071
Alcoholic liver disease (ALD) occurs in three stages: fatty liver, alcoholic hepatitis, and liver cirrhosis. In the present study, the role of leukocyte cell-derived chemotaxin-2 (LECT2) in the development of alcohol-induced liver cirrhosis was investigated.
In recent decades, there have been significant developments in research on the biochemical possibilities for the early diagnosis and monitoring of non-alcoholic fatty liver disease (NAFLD)[1]. Hepatokines were found to be extremely useful for NAFLD monitoring[2]. Moreover, relationships between the stages of NAFLD and fetuin-A[3,4], selenoprotein-P[5,6], and fibroblast growth factor 21 (FGF-21)[7] have been demonstrated. Fibroblast growth factor mimicking has been developed as a novel therapeutic option[8]. The analogues of hepatokines, such as a pegylated FGF-21 analogue[9], have been used in NAFLD therapies. However, finding similar diagnostic options for ALD remains valid[10]. ALD is among the most prevalent diseases in Western countries. It has recently been recognized as an increasingly serious epidemiological and therapeutic problem in developing countries[11,12].
Therefore, finding new possibilities for the early diagnosis of ALD, especially novel and precise non-invasive diagnostic procedures, is a real challenge for modern hepatological practice.
LECT2 has been widely studied to determine its usefulness in monitoring the course of NAFLD. According to the available study findings, serum LECT2 concentrations increase with the advancement of NAFLD[13,14]. LECT2 was first described by Yamagoe et al[15] in 1996 as a novel chemotactic factor for neutrophils. Subsequent studies identified its expression in human hepatocytes and classified it as a hepatokine[16-18]. Clinical observations have demonstrated that LECT2-associated amyloidosis is a frequent cause of hepatic amyloidosis in the United States[19]. Studies in animal models have reported that LECT2 overexpression increases fibrosis, promotes sinusoid capillarization, and inhibits portal angiogenesis. LECT2 is a functional ligand of Tie1. Xu et al[20] suggested that serum LECT2 Levels may be a potential biomarker for the diagnosis or screening of liver fibrosis, and LECT2/Tie1 signaling may be used for the development of new drugs.
It seems that LECT2 could be of great importance in the diagnosis of fatty liver. In a cross-sectional study, Okumura et al[13] showed statistically significant higher levels of LECT2 in fatty liver and obesity. However, the possibility of diagnosing and monitoring the course of alcohol-induced liver cirrhosis using LECT2 has not yet been assessed.
The aim of our study was to determine the concentration of LECT2 in the blood serum of patients at progressive stages of alcoholic liver cirrhosis to determine the relation to FGF-1 and FGF-21, and to discuss the possible wider use of LECT2 in the diagnosis of ALC.
The study protocol was approved by the Bioethics Committee. All patients gave their written informed consent prior to participating in the study.
The study was conducted at the Department of Internal Medicine, Medical University of Lublin, Poland, and included 69 patients from the region of Lublin (eastern Poland) with alcoholic cirrhosis. Liver cirrhosis was diagnosed based on clinical features, history of heavy alcohol consumption, laboratory tests, and abdominal ultrasonography. Heavy alcohol consumption was defined according to the guidelines of the National Institute on Alcohol Abuse and Alcoholism (NIAAA) as consuming more than four drinks on any day or more than 14 drinks per week for men and three drinks on any day or more than seven drinks per week for women[21]. Patients with alcoholic hepatitis, hepatocellular carcinoma, or viral and autoimmune diseases were excluded from the study. Other exclusion criteria were type 2 diabetes, obesity, acute infections (e.g., pneumonia, spontaneous bacterial peritonitis), acute and chronic heart failure (> NYHA I—i.e. slight or marked limitation of physical activity, ordinary physical activity results in fatigue, palpitation, dyspnea), acute and chronic respiratory disorders resulting in respiratory insufficiency, acute kidney injury, and chronic kidney disease (> stage G2—i.e. an estimated glomerular filtration rate < 60 mL/min). Both clinical assessments and laboratory tests were used to exclude underlying liver diseases in the control group. The degree of liver cirrhosis was evaluated according to Pugh-Child criteria (the Pugh-Child score), and on that basis, patients were assigned to one of three groups: Pugh-Child (P-Ch) A (n = 21) with stage A, P-Ch B (n = 23) with stage B, and P-Ch C (n = 28) with stage C liver cirrhosis (Table 1). The control group consisted of 17 healthy individuals without liver disease who did not abuse alcohol. Detailed demographic, clinical, and biochemical characteristics of the patients are presented in Tables 1 and 2.
| Control group (n = 17) | Liver cirrhosis | P value | ||
| Pugh-Child A + B (n = 37) | Pugh-Child C (n = 32) | |||
| Age (yr) | 43.7 ± 14.6 | 55.7 ± 12.1 | 55.9 ± 10.2 | 0.021 |
| Percentage of males (%) | 64.3% | 73% | 72.7% | 0.52 |
| Body weight (kg) | 67.6 ± 8.9 | 73 ± 11.4 | 75.5 ± 12.8 | 0.17 |
| Height (cm) | 173 ± 5.9 | 174 ± 8 | 173 ± 7.6 | 0.64 |
| Duration of alcohol abuse (yr) | - | 15.7 ± 8.2 | 18.7 ± 8.3 | 0.98 |
| Oesophageal varices (%) | - | 32.4% | 81.8% | < 0.0001 |
| Encephalopathy (%) | - | 32.4% | 83.9% | < 0.0001 |
| Ascites (%) | - | 40.5% | 90.9% | < 0.0001 |
| Total bilirubin (mg/dL) | 0.6 ± 0.3 | 4.6 ± 6.9 | 10.5 ± 9.2 | < 0.0001 |
| INR | - | 1.36 ± 0.35 | 1.95 ± 0.56 | < 0.0001 |
| Albumin (g/dL) | - | 3.1±0.8 | 2.4±0.4 | 0.0002 |
| Total protein (g/dL) | 6.3 ± 0.3 | 6.4 ± 1 | 5.9 ± 0.9 | 0.16 |
| Alanine aminotransferase (U/L) | 17.9 ± 6 | 65.3 ± 139.9 | 50.6 ± 87.3 | 0.018 |
| Aspartate aminotransferase (U/l) | 18.3 ± 7 | 128.1 ± 173.5 | 120 ± 164.7 | < 0.0001 |
| Platelets (G/L) | 231.4 ± 29.8 | 173 ± 105.4 | 127.8 ± 72.3 | 0.0004 |
| Mean corpuscular volume (fL) | 84.8 ± 3.5 | 91.2 ± 9.1 | 95.5 ± 9 | 0.0002 |
| Urea (mg/dL) | - | 27.5 ± 16.1 | 58.2 ± 43.7 | 0.065 |
| Sodium (mmol/l) | 140 ± 3.3 | 133.8 ± 5 | 131.9 ± 6.7 | < 0.0001 |
| Potassium (mmol/L) | 4.4 ± 0.4 | 3.8 ± 0.7 | 3.9 ± 0.8 | 0.019 |
| C-reactive protein (mg/L) | 2.5 ± 2.3 | 19.8 ± 21 | 32.7 ± 27.8 | < 0.0001 |
| Angiotensinogen (ng/mL) | 1006.91 ± 610.49 | 1117.04 ± 873.69 | 1468.7 ± 817.33 | 0.22 |
| Control group | Liver cirrhosis | P value | ||
| Pugh-Child A + B | Pugh-Child C | |||
| LECT2 (ng/mL) | 18.99 ± 5.36 | 11.06 ± 6.47 | 8.06 ± 5.74 | < 0.0001 |
| FGF-1 (pg/mL) | 37.94 ± 40.4 | 144.77 ± 14.42 | 164.52 ± 169.46 | 0.01 |
| FGF-21 (pg/mL) | 13.52 ± 7.51 | 44.27 ± 64.19 | 45.4 ± 51.69 | 0.008 |
Blood was drawn, and after centrifugation, serum was collected for analysis. Human LECT2, FGF-1, and FGF-21 were determined using enzyme-linked immunosorbent assay (ELISA) kits. All absorbance readings were conducted using an Epoch Microplate Spectrophotometer (BioTek Instrumentals, Inc., Winooski, VT, United States). LECT2 concentrations were determined using a BioVendor Human LECT2 ELISA kit (BioVendor, Laboratorni medicina a.s., Brno, Czech Republic). FGF-1 and FGF-21 concentrations were quantified using sandwich enzyme immunoassay kits produced by Cloud-Clone Corp. (Katy, TX, United States). Serum samples had been suitably diluted (20-fold dilution for LECT2) or used without dilution (FGF-1 and FGF 21) prior to testing, in accordance with the manufacturers’ recommendations. Testing was carried out in accordance with the typical standard applicable for enzyme-linked immunoassays: samples, standards, and blanks were applied to a plate pre-coated with a factor-specific antibody. Subsequently, horseradish peroxidase conjugated avidin was added to each well, and the plate was incubated for one hour at room temperature (LECT2) or at 37°C (FGF-1 and FGF-21). Next, TMB substrate was added; the wells containing biotin-conjugated antibody and enzyme-conjugated avidin exhibited a change in color. The enzyme-substrate reaction was terminated by adding acidic solution, and the absorbance of the complex formed was measured at a wavelength of 450 nm. The concentrations of the study parameters were determined using a standard curve. Results were multiplied by the dilution factor, when necessary.
Statistica 13.3 (TIBCO Software, Inc.) was used for data analysis. Continuous variables were expressed as mean ± SD. Before calculations, variables were checked for normality using the Shapiro-Wilk test. To compare the results between more than two groups, one-way ANOVA and the Kruskal-Wallis test were used, depending on distribution. Correlations among variables were tested using Pearson’s and Spearman’s correlation tests, depending on distribution. Qualitative variables were shown as indicators of structure (percentage). For intergroup comparisons, the χ2 test was used. For all tests, P < 0.05 was considered statistically significant.
The study group consisted of 69 patients (50 men), including 37 with P-Ch A or P-Ch B cirrhosis and 32 with P-Ch C. The control group included 17 gender-matched individuals (P = 0.52). The age of patients in the control group was lower than that of patients with cirrhosis (P = 0.021). The duration of alcohol abuse in the study group was, on average, 15.7 ± 8.2 years in the P-Ch A + B subgroup and 18.7 ± 8.3 years in the P-Ch C subgroup.
As expected, patients with liver cirrhosis were characterized by significantly lower albumin levels and higher total bilirubin (TB), alanine aminotransferase, aspartate aminotransferase (AST), international normalized ratio, and C-reactive protein levels (Table 1).
Angiotensinogen levels increased with the progression of cirrhosis, reaching the highest in the P-Ch C group of 1468.7 ± 817.33 ng/mL. However, the differences observed were not statistically significant (P = 0.22).
The LECT2 Levels in the control group were 18.99 ± 5.36 ng/mL. With the progression of cirrhosis in the P-Ch A + B group, this value dropped to 11.06 ± 6.47 ng/mL and to 8.06 ± 5.74 ng/mL in the P-Ch C group (P < 0.0001) (Table 2). Multiple comparisons confirmed the statistically significant differences in LECT2 Levels between the control group and the P-Ch A + B (P = 0.006) and between the control group and P-Ch C (P < 0.0001) (Figure 1).
Otherwise, the lowest FGF-1 Level was found in the control group—37.94 ± 40.4 pg/mL—and was higher in patients with cirrhosis, increasing to 144.77 ± 1 in the P-Ch A + B group and to 164.52 ± 169.46 pg/mL in the P-Ch C group (P < 0.01). The difference between the control group and P-Ch C was statistically significant (P = 0.002) (Table 2).
A similar trend was observed for FGF-21. Its concentration in the control group was 13.52 ± 7.51 pg/mL, 44.27 ± 64.19 pg/mL in the P-Ch A + B group, and 45.4 ± 51.69 pg/mL in the P-Ch C group (P = 0.008). The difference between the control group and the P-Ch C group was statistically significant (P = 0.007) (Table 2).
The strongest correlations were observed between LECT2 and TB (r = –0.59; P < 0.0001) and angiotensinogen (r = –0.51; P < 0.0001) (Table 3).
| Pair of variables | Correlation coefficient | ||
| R | P value | ||
| LECT2 | Age | -0.29 | 0.048 |
| Total bilirubin | -0.59 | < 0.0001 | |
| Platelets | 0.34 | 0.02 | |
| Alanine transaminase | -0.43 | 0.003 | |
| C-reactive protein | -0.4 | 0.008 | |
| Angiotensinogen | -0.51 | < 0.0001 | |
| FGF-1 | -0.38 | 0.004 | |
| FGF-21 | -0.39 | 0.004 | |
In the multiple regression model, angiotensinogen, AST, TB, and age were observed to be independent LECT2-related variables (Table 4). This model was statistically significant (P < 0.0001) and explained less than two-thirds of variability (adjusted R2 = 0.59).
| Effect | B* | SE with B* | B | SE with B | P value |
| Constant | 30.64 | 3.68 | < 0.0001 | ||
| Angiotensinogen | -0.423 | 0.114 | -0.004 | 0.001 | 0.001 |
| Alanine aminotransferase | -0.341 | 0.115 | -0.02 | 0.005 | 0.005 |
| Total bilirubin | -0.279 | 0.108 | -0.25 | 0.099 | 0.014 |
| Age | -0.275 | 0.109 | -0.16 | 0.064 | 0.016 |
ALD is a serious health consequence of excessive alcohol consumption. The spectrum of clinical-histologic ALD changes includes fatty liver, alcoholic hepatitis, and cirrhosis[22]. It is estimated that over 90% of all heavy drinkers have fatty liver; about 25% of them have alcoholic hepatitis, and 15% have cirrhosis. According to a meta-analysis conducted by Askgaard et al[23], the probability of alcoholic liver cirrhosis reaches 16% after 8–12 years of alcoholization; 45% of patients with cirrhosis had been consuming more than 110 g of alcohol daily. The above results correspond to our observations based on a relatively small sample. Alcohol-induced liver cirrhosis accounts for half of all cirrhosis cases in the United States. In recent years, the importance of finding new non-invasive methods to diagnose more severe forms of ALD and predict prognosis has been strongly emphasized[24,25].
In our study, the serum levels of FGF-1 and FGF-21 in the study groups and control group were determined to obtain biochemical reference points for levels of LECT2. FGF-1 is an angiogenic factor that modifies the migration and proliferation of endothelial cells and regulates the metabolism of lipids and carbohydrates. FGF-1 is involved in response to injury and fibrosis. The highest expression of FGF has been observed in the late stages of hepatic morphogenesis in animal models, as well as during hepatic differentiation in the adult liver. FGF-1 is present in perisinusoidal hepatic stellate cells (HSCs) during liver regeneration. The chronic activation of nonparenchymal HSCs (also called Ito cells and fat-storing cells) is the major contributor to liver fibrogenesis resulting from chronic toxic insult primarily through its production of extracellular matrix components.
FGF-1 reduces hepatic lipid accumulation independently of insulin and is important in the pathogenesis of NAFLD. Moreover, it has therapeutic potential for the treatment of ischemic disease[26]. Previous studies have demonstrated an inverse relationship between this factor and portal pressure in patients after liver transplantation[27]. In animal model studies, the protective effect of FGF-1 on liver cells was confirmed, as it prevented acute inflammation and apoptosis induced by acetaminophen[28]. The main source of FGF-1 in the human body is liver cells. However, this protein is also expressed in the pancreas, testes, duodenum, and adipose tissue. For this reason, its use as an indicator of liver function is clearly limited, and in recent years this problem has not been studied. Among fibroblast growth factors, FGF-21 has been tested as a marker of liver function[29,30]. According to a Chinese prospective study, this protein is an independent predictor of NAFLD[31]. The possible use of FGF-21 as an NAFLD marker has also been described in an American study conducted in children[32]. However, the above study demonstrated significant relationships between the level of this marker and the prevalence of obesity, with or without insulin resistance. In a study on ALD, Yang et al[33] suggested that FGF-21 may indicate a progression from heavy drinking to alcoholic cirrhosis. In their latest study, Willis et al[34] indicated that acute high-fat overfeeding augments circulating concentrations of FGF-21, LECT2, and fetuin-A in healthy men. Perhaps a slightly opposite effect than in this subgroup occurs in patients with cirrhosis with regard to correlation of LECT2 and FGF-21. The results of our study showed that LECT2 Levels correlated inversely with FGF-1 and FGF-21 in ALD. However, based on our results, it is not possible to state whether this is specific to ALD. Previous studies have shown that LECT2 could be of great importance in the diagnosis of NAFLD[13,14]. We suggest the need for further, more extensive, including prospective, studies.
Our study is the first attempt to assess the usefulness of LECT2 in the non-invasive diagnosis of alcohol-induced liver cirrhosis. Therefore, the points of reference are scarce. However, considering the above-mentioned studies on the marker function of FGF-21, it is worth noting that our results are compatible with those reported by Yang et al[33] In our study, the concentration of FGF-21 in the control group, that is, patients without cirrhosis, was significantly lower compared to both subgroups of the study group. However, the differences in FGF-21 concentrations between the two subgroups (P-Ch A + B and P-Ch C) were not statistically significant. FGF-21 may play an important role in supporting non-invasive diagnostics of alcohol-induced liver cirrhosis and in monitoring the course of NAFLD. We did not find it useful in non-invasive monitoring of alcohol-induced liver cirrhosis, contrary to the level of serum taurine/glycine-conjugated bile acids as a non-invasive marker to predict the severity of alcohol-induced liver cirrhosis, as tested by Yang et al[33]. Our results suggest that LECT2 might be used as a diagnostic and monitoring marker to determine the severity of alcohol-induced liver cirrhosis. Its highest statistically significant concentration was observed in the control group. In the study groups, as cirrhosis progressed, the plasma levels of LECT2 dropped. The lowest values of LECT2 were observed in P-Ch C stage patients, that is, in the most advanced stage of the disease.
LECT2 Levels correlated inversely with TB, AST, and angiotensinogen (AGT). Although strong correlations were identified between LECT2 and cirrhosis progression, and between AGT and LECT2, we did not observe an analogous relationship between AGT and cirrhosis progression. We suggest that this may be caused by low sample size and decreased power. The liver’s renin-angiotensin system plays an important role in the development of liver cirrhosis. The levels of total bilirubin, AST, and AGT increase as alcohol-induced liver cirrhosis progresses. Higher serum concentration of AGT indicates unfavorable histological remodeling of the liver parenchyma closely related to liver dysfunction. Previous studies on animal models have indicated that AGT plays an important role in NAFLD[35-37]. AGT is an important precursor of hepatic fibrogenesis, which has been confirmed in animal studies[38]. According to the reported data, AGT inhibition could be an effective anti-liver fibrosis strategy.
Our research suggests that LECT2 may be used for the non-invasive diagnosis of alcohol-induced liver cirrhosis. The usefulness of LECT2 for non-invasive monitoring of alcohol-induced liver cirrhosis was indirectly confirmed by the multiple regression model developed on the basis of our statistical analysis.
Leukocyte cell-derived chemotaxin-2 (LECT2) has been widely studied to determine its usefulness for monitoring the course of non-alcoholic fatty liver disease but not for alcoholic liver cirrhosis (ALC).
The aim of our study was to assess and discuss LECT2’s possible wider use in the diagnosis of ALC.
The purpose of this study was to determine the concentration of LECT2 in the blood serum of patients in accordance with progressive stages of ALC and its relation to fibroblast growth factor 1 (FGF-1) and FGF-21.
A study was conducted with an ALC group and a control group with no ALC. The extent of ALC was evaluated according to Pugh-Child criteria (the Pugh-Child score). LECT2, FGF-1, and FGF-21 were determined using enzyme-linked immunosorbent assay kits.
Our study showed strong correlations between LECT2 and cirrhosis progression. LECT2 levels correlated inversely with FGF-1 and FGF-21.
LECT2 may be used for the non-invasive diagnosis of alcohol-induced liver cirrhosis.
Further prospective studies should be conducted to explore whether the inverse correlation of LECT2 and FGF-21 is specific to ALD.
This study was performed at the Medical University of Lublin, Poland. The authors thank Anna Misiuna, who provided medical writing services on behalf of the Medical University of Lublin, Poland.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yeoh SW S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Benedict M, Zhang X. Non-alcoholic fatty liver disease: An expanded review. World J Hepatol. 2017;9:715-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 547] [Cited by in RCA: 511] [Article Influence: 63.9] [Reference Citation Analysis (18)] |
| 2. | Ke Y, Xu C, Lin J, Li Y. Role of Hepatokines in Non-alcoholic Fatty Liver Disease. J Transl Int Med. 2019;7:143-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Sato M, Kamada Y, Takeda Y, Kida S, Ohara Y, Fujii H, Akita M, Mizutani K, Yoshida Y, Yamada M, Hougaku H, Takehara T, Miyoshi E. Fetuin-A negatively correlates with liver and vascular fibrosis in nonalcoholic fatty liver disease subjects. Liver Int. 2015;35:925-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Celebi G, Genc H, Gurel H, Sertoglu E, Kara M, Tapan S, Acikel C, Karslioglu Y, Ercin CN, Dogru T. The relationship of circulating fetuin-a with liver histology and biomarkers of systemic inflammation in nondiabetic subjects with nonalcoholic fatty liver disease. Saudi J Gastroenterol. 2015;21:139-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Choi HY, Hwang SY, Lee CH, Hong HC, Yang SJ, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Increased selenoprotein p levels in subjects with visceral obesity and nonalcoholic Fatty liver disease. Diabetes Metab J. 2013;37:63-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Polyzos SA, Kountouras J, Mavrouli M, Katsinelos P, Doulberis M, Gavana E, Duntas L. Selenoprotein P in Patients with Nonalcoholic Fatty Liver Disease. Exp Clin Endocrinol Diabetes. 2019;127:598-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Praktiknjo M, Djayadi N, Mohr R, Schierwagen R, Bischoff J, Dold L, Pohlmann A, Schwarze-Zander C, Wasmuth JC, Boesecke C, Rockstroh JK, Trebicka J. Fibroblast growth factor 21 is independently associated with severe hepatic steatosis in non-obese HIV-infected patients. Liver Int. 2019;39:1514-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Ocker M. Fibroblast growth factor signaling in non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: Paving the way to hepatocellular carcinoma. World J Gastroenterol. 2020;26:279-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 9. | Sanyal A, Charles ED, Neuschwander-Tetri BA, Loomba R, Harrison SA, Abdelmalek MF, Lawitz EJ, Halegoua-DeMarzio D, Kundu S, Noviello S, Luo Y, Christian R. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet. 2019;392:2705-2717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 422] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 10. | Mueller S, Seitz HK, Rausch V. Non-invasive diagnosis of alcoholic liver disease. World J Gastroenterol. 2014;20:14626-14641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 99] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (2)] |
| 11. | Addolorato G, Mirijello A, Barrio P, Gual A. Treatment of alcohol use disorders in patients with alcoholic liver disease. J Hepatol. 2016;65:618-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 12. | Arab JP, Roblero JP, Altamirano J, Bessone F, Chaves Araujo R, Higuera-De la Tijera F, Restrepo JC, Torre A, Urzua A, Simonetto DA, Abraldes JG, Méndez-Sánchez N, Contreras F, Lucey MR, Shah VH, Cortez-Pinto H, Bataller R. Alcohol-related liver disease: Clinical practice guidelines by the Latin American Association for the Study of the Liver (ALEH). Ann Hepatol. 2019;18:518-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 13. | Okumura A, Unoki-Kubota H, Matsushita Y, Shiga T, Moriyoshi Y, Yamagoe S, Kaburagi Y. Increased serum leukocyte cell-derived chemotaxin 2 (LECT2) levels in obesity and fatty liver. Biosci Trends. 2013;7:276-283. [PubMed] |
| 14. | Yoo HJ, Hwang SY, Choi JH, Lee HJ, Chung HS, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Association of leukocyte cell-derived chemotaxin 2 (LECT2) with NAFLD, metabolic syndrome, and atherosclerosis. PLoS One. 2017;12:e0174717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Yamagoe S, Yamakawa Y, Matsuo Y, Minowada J, Mizuno S, Suzuki K. Purification and primary amino acid sequence of a novel neutrophil chemotactic factor LECT2. Immunol Lett. 1996;52:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 148] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Lebensztejn DM, Flisiak-Jackiewicz M, Białokoz-Kalinowska I, Bobrus-Chociej A, Kowalska I. Hepatokines and non-alcoholic fatty liver disease. Acta Biochim Pol. 2016;63:459-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Yamagoe S, Mizuno S, Suzuki K. Molecular cloning of human and bovine LECT2 having a neutrophil chemotactic activity and its specific expression in the liver. Biochim Biophys Acta. 1998;1396:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Lan F, Misu H, Chikamoto K, Takayama H, Kikuchi A, Mohri K, Takata N, Hayashi H, Matsuzawa-Nagata N, Takeshita Y, Noda H, Matsumoto Y, Ota T, Nagano T, Nakagen M, Miyamoto K, Takatsuki K, Seo T, Iwayama K, Tokuyama K, Matsugo S, Tang H, Saito Y, Yamagoe S, Kaneko S, Takamura T. LECT2 functions as a hepatokine that links obesity to skeletal muscle insulin resistance. Diabetes. 2014;63:1649-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 19. | Mereuta OM, Theis JD, Vrana JA, Law ME, Grogg KL, Dasari S, Chandan VS, Wu TT, Jimenez-Zepeda VH, Fonseca R, Dispenzieri A, Kurtin PJ, Dogan A. Leukocyte cell-derived chemotaxin 2 (LECT2)-associated amyloidosis is a frequent cause of hepatic amyloidosis in the United States. Blood. 2014;123:1479-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Xu M, Xu HH, Lin Y, Sun X, Wang LJ, Fang ZP, Su XH, Liang XJ, Hu Y, Liu ZM, Cheng Y, Wei Y, Li J, Li L, Liu HJ, Cheng Z, Tang N, Peng C, Li T, Liu T, Qiao L, Wu D, Ding YQ, Zhou WJ. LECT2, a Ligand for Tie1, Plays a Crucial Role in Liver Fibrogenesis. Cell 2019; 178: 1478-1492. e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 156] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 21. | National Institute on Alcohol Abuse and Alcoholism (NIAAA). Drinking Levels Defined. [cited 4 Jan 2021]. Available from: https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking. |
| 22. | Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG Clinical Guideline: Alcoholic Liver Disease. Am J Gastroenterol. 2018;113:175-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 561] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 23. | Askgaard G, Kjær MS, Tolstrup JS. Opportunities to Prevent Alcoholic Liver Cirrhosis in High-Risk Populations: A Systematic Review With Meta-Analysis. Am J Gastroenterol. 2019;114:221-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Moreno C, Mueller S, Szabo G. Non-invasive diagnosis and biomarkers in alcohol-related liver disease. J Hepatol. 2019;70:273-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 25. | Lucey MR. Alcohol-Associated Cirrhosis. Clin Liver Dis. 2019;23:115-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Xia X, Babcock JP, Blaber SI, Harper KM, Blaber M. Pharmacokinetic properties of 2nd-generation fibroblast growth factor-1 mutants for therapeutic application. PLoS One. 2012;7:e48210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Serrano CA, Ling SC, Verdaguer S, León M, Jarufe N, Guerra JF, Pattillo JC, Benítez C, Villagrán A, Torres J, Concha M, Villarroel L, Dellepiane P, Domínguez P, Martínez J, Gana JC. Portal Angiogenesis in Chronic Liver Disease Patients Correlates with Portal Pressure and Collateral Formation. Dig Dis. 2019;37:498-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Wang X, Zhang X, Wang F, Pang L, Xu Z, Li X, Wu J, Song Y, Xiao J, Lin H, Liu Y. FGF1 protects against APAP-induced hepatotoxicity via suppression of oxidative and endoplasmic reticulum stress. Clin Res Hepatol Gastroenterol. 2019;43:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Yang M, Xu D, Liu Y, Guo X, Li W, Guo C, Zhang H, Gao Y, Mao Y, Zhao J. Combined Serum Biomarkers in Non-Invasive Diagnosis of Non-Alcoholic Steatohepatitis. PLoS One. 2015;10:e0131664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 30. | Gong Z, Tas E, Yakar S, Muzumdar R. Hepatic lipid metabolism and non-alcoholic fatty liver disease in aging. Mol Cell Endocrinol. 2017;455:115-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 31. | Li H, Dong K, Fang Q, Hou X, Zhou M, Bao Y, Xiang K, Xu A, Jia W. High serum level of fibroblast growth factor 21 is an independent predictor of non-alcoholic fatty liver disease: a 3-year prospective study in China. J Hepatol. 2013;58:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 32. | Sodhi K, Bracero L, Feyh A, Nichols A, Srikanthan K, Latif T, Preston D, Shapiro JI, Elitsur Y. Role of Serum Biomarkers in Early Detection of Non-Alcoholic Steatohepatitis and Fibrosis in West Virginian Children. J Clin Cell Immunol. 2016;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Yang Z, Kusumanchi P, Ross RA, Heathers L, Chandler K, Oshodi A, Thoudam T, Li F, Wang L, Liangpunsakul S. Serum Metabolomic Profiling Identifies Key Metabolic Signatures Associated With Pathogenesis of Alcoholic Liver Disease in Humans. Hepatol Commun. 2019;3:542-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 34. | Willis SA, Sargeant JA, Yates T, Takamura T, Takayama H, Gupta V, Brittain E, Crawford J, Parry SA, Thackray AE, Varela-Mato V, Stensel DJ, Woods RM, Hulston CJ, Aithal GP, King JA. Acute Hyperenergetic, High-Fat Feeding Increases Circulating FGF21, LECT2, and Fetuin-A in Healthy Men. J Nutr. 2020;150:1076-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Takahashi N, Li F, Hua K, Deng J, Wang CH, Bowers RR, Bartness TJ, Kim HS, Harp JB. Increased energy expenditure, dietary fat wasting, and resistance to diet-induced obesity in mice lacking renin. Cell Metab. 2007;6:506-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 36. | Frantz ED, Penna-de-Carvalho A, Batista Tde M, Aguila MB, Mandarim-de-Lacerda CA. Comparative effects of the renin-angiotensin system blockers on nonalcoholic fatty liver disease and insulin resistance in C57BL/6 mice. Metab Syndr Relat Disord. 2014;12:191-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Tao XR, Rong JB, Lu HS, Daugherty A, Shi P, Ke CL, Zhang ZC, Xu YC, Wang JA. Angiotensinogen in hepatocytes contributes to Western diet-induced liver steatosis. J Lipid Res. 2019;60:1983-1995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Lu P, Liu H, Yin H, Yang L. Expression of angiotensinogen during hepatic fibrogenesis and its effect on hepatic stellate cells. Med Sci Monit. 2011;17:BR248-BR256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |