Published online Dec 27, 2021. doi: 10.4254/wjh.v13.i12.2024
Peer-review started: May 22, 2021
First decision: July 27, 2021
Revised: August 19, 2021
Accepted: November 4, 0202
Article in press: November 4, 2021
Published online: December 27, 2021
Processing time: 218 Days and 19.6 Hours
Progressive familial intrahepatic cholestasis (PFIC) is a heterogeneous group of disorders characterized by defects in bile secretion and presentation with intrahepatic cholestasis in infancy or childhood. The most common types include PFIC 1 (deficiency of FIC1 protein, ATP8B1 gene mutation), PFIC 2 (bile salt export pump deficiency, ABCB11 gene mutation), and PFIC 3 (multidrug resistance protein-3 deficiency, ABCB4 gene mutation). Mutational analysis of subjects with normal gamma-glutamyl transferase cholestasis of unknown etiology has led to the identification of newer variants of PFIC, known as PFIC 4, 5, and MYO5B related (sometimes known as PFIC 6). PFIC 4 is caused by the loss of function of tight junction protein 2 (TJP2) and PFIC 5 is due to NR1H4 mutation causing Farnesoid X receptor deficiency. MYO5B gene mutation causes microvillous inclusion disease (MVID) and is also associated with isolated cholestasis. Children with TJP2 related cholestasis (PFIC-4) have a variable spectrum of presentation. Some have a self-limiting disease, while others have progressive liver disease with an increased risk of hepatocellular carcinoma. Hence, frequent surveillance for hepatocellular carcinoma is recommended from infancy. PFIC-5 patients usually have rapidly progressive liver disease with early onset coagulopathy, high alpha-fetoprotein and ultimately require a liver transplant. Subjects with MYO5 B-related disease can present with isolated cholestasis or cholestasis with intractable diarrhea (MVID). These children are at risk of worsening cholestasis post intestinal transplant (IT) for MVID, hence combined intestinal and liver transplant or IT with biliary diversion is preferred. Immunohistochemistry can differentiate most of the variants of PFIC but confirmation requires genetic analysis.
Core Tip: Progressive familial intrahepatic cholestasis (PFIC) manifests with a varying spectrum of clinical features, with some variants progressing rapidly into end stage liver disease. Recently, newer variants of PFIC have been described including PFIC 4 due to tight junction protein 2 (TJP2) mutation, PFIC 5 due to NR1H4 mutation and MYO5B related cholestasis also sometimes known as PFIC 6. TJP2 related PFIC also has a risk of hepatocellular carcinoma. This article describes the pathogenesis and clinical features of the newer variants of PFIC.
- Citation: Vinayagamoorthy V, Srivastava A, Sarma MS. Newer variants of progressive familial intrahepatic cholestasis. World J Hepatol 2021; 13(12): 2024-2038
- URL: https://www.wjgnet.com/1948-5182/full/v13/i12/2024.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i12.2024
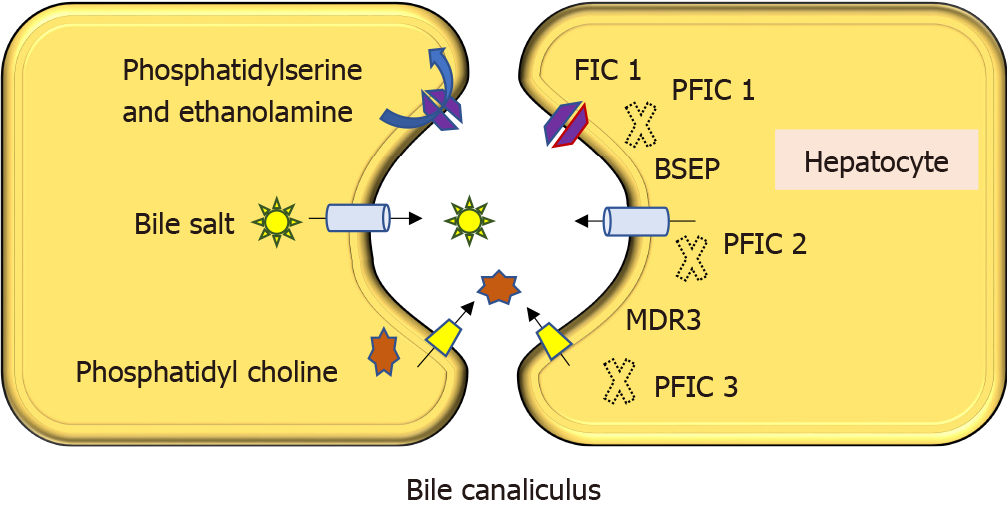
Progressive familial intrahepatic cholestasis (PFIC) is a heterogeneous group of intrahepatic cholestatic disorders caused by a defect in bile transport and secretion. It manifests in infancy or childhood and can progress to end-stage liver disease[1-3]. Genetically confirmed PFIC accounts for 12%-13% of cholestatic disorders in infants and children[4]. Disease variants are classified based on the specific bile transporter defects and all of them have an autosomal recessive inheritance. The three most prominent varieties are familial intrahepatic cholestasis-1, 2 and 3, which are caused by mutations in ATP8B1 gene encoding FIC1, ABCB11 gene encoding bile salt export pump, and ABCB4 gene encoding multidrug resistance protein-3 respectively (Figure 1). Nearly two-thirds of subjects with normal gamma-glutamyl transpeptidase (GGT) cholestasis (normally associated with PFIC except PFIC 3) do not have any mutations identified in ATP8B1 or ABCB11 genes[3]. Detailed mutational analysis in patients with this phenotype has led to the identification of 3 more conditions, often known as PFIC 4, 5, and 6. PFIC 4 is caused by the loss of function of tight junction protein 2 (TJP2)[5], and PFIC 5 is due to NR1H4 mutation causing farnesoid X receptor (FXR) deficiency[6,7]. MYO5B mutation, known to cause microvillous inclusion disease (MVID), is also reported to cause isolated cholestasis and is sometimes known as PFIC 6 though it is not yet recognized by the Online Mendelian Inheritance in Man[8]. The exact incidence of newer variants of PFIC is not known due to the limited number of studies, which are mostly case reports or small case series. Based on the available literature, this review attempts to sensitize physicians to the disease.
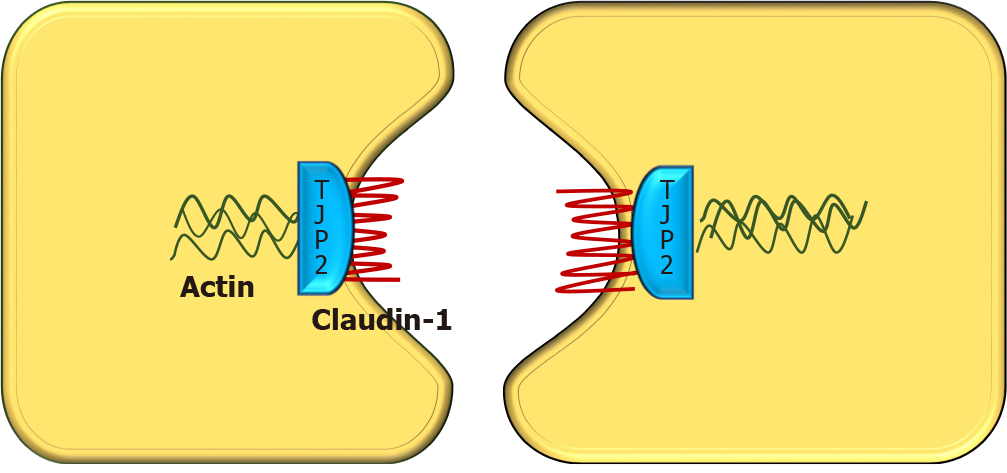
TJP2 gene, located in chromosome 9q21 was first discovered in 1991 by Gumbiner et al[9]. It encodes a protein called tight junction protein 2 or zona occludens-2. Though named as tight junction protein, it is not present in the tight junction. Instead, TJP2 is a cytosolic protein, involved in maintaining cell-to-cell adhesion by linking the transmembrane tight junction proteins like claudin with the actin cytoskeleton. There are two types of claudin i.e., claudin-1 (CLDN1) and claudin-2 (CLDN2), both of which are localized to the bile canalicular membrane[10]. In TJP2 mutation, CLDN1 fails to localize to the bile canalicular membrane (Figure 2). This results in reduced integrity of the canalicular membrane and reflux of toxic bile acids through the paracellular spaces into hepatocytes, causing hepatocyte damage and cholestasis[11]. TJP2 has a widespread expression, including the respiratory and central nervous systems. This may explain the systemic features reported in a few cases[11]. The detergent action of the bile potentiates damage in the liver, which explains the predominant hepatic manifestations in this condition.
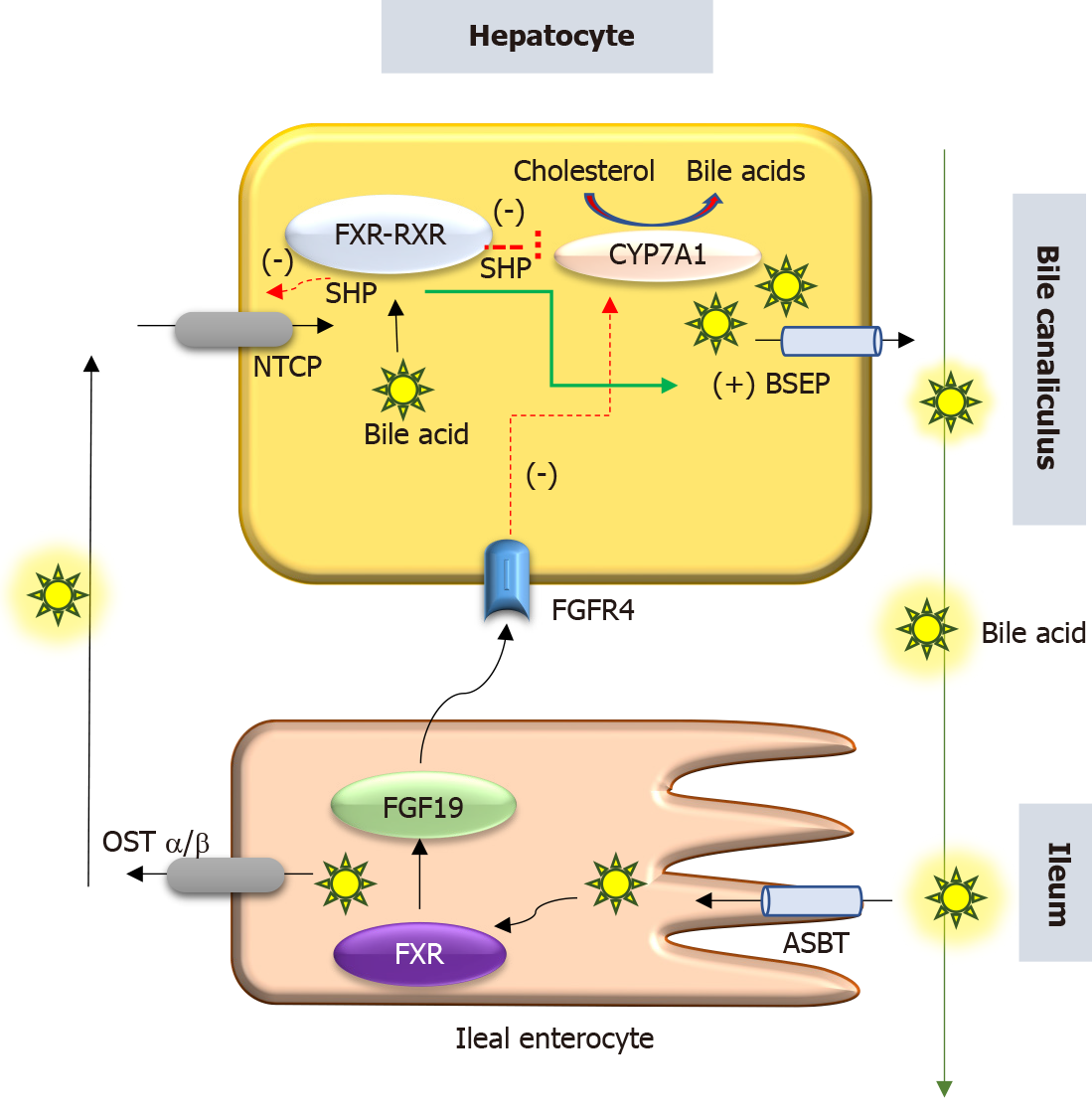
PFIC 5 is related to a deficiency of the FXR due to loss of function mutation in the NR1H4 gene located in chromosome 12q23. NR1H4 related PFIC 5 is a less commonly reported variant, with < 10 cases reported by 2020. FXR, a protein translated from the NR1H4 gene was first described in 1995 by Forman et al[12]. It belongs to a nuclear receptor group activated by farnesyl, an intermediate metabolite of the mevalonic acid synthesis pathway. FXR is the master regulator of cholesterol, bile acid, triglyceride and various sterol ring-containing compounds (Vitamin D, carotenoids, retinoids, etc.)[13]. In the liver, the FXR acts as a nuclear bile acid-sensing receptor involved in the expression of bile salt export protein (BSEP) and sometimes MDR3[6,14]. Apart from the liver, FXR is also expressed in the small intestine. Whenever bile acid levels are elevated in the ileal enterocytes, FXR is activated to induce the synthesis of fibroblast growth factor 19 (FGF19). FGF 19 is then transported via enterohepatic recirculation to the liver, where it binds to the fibroblast growth factor receptor 4/β-Klotho complex, and causes inhibition of bile acid synthesis by repressing CYP7A1. Elevated bile acid inside hepatocytes also activates FXR which induces ABCB11gene transcription, BSEP synthesis, and bile acid export from the liver. Hence, the NR1H4 mutation causes loss of BSEP expression, leading to the accumulation of toxic bile and hepatocellular damage (Figure 3). FXR is also involved in the regulation of coagulation factor synthesis by transactivating fibrinogen and kininogen genes. Thus, the FXR mutation leads to the development of vitamin K independent, early-onset coagulopathy, well before liver failure sets in[6].
Homozygous or compound heterozygous loss of function mutations (c.526C>T and c.419 420insAAA/intragenic 31.7-kb deletion, respectively) have been described[7]. In one woman with intrahepatic cholestasis of pregnancy, NR1H4 heterozygous variant (c.-1G>T) was found to be associated with cholestasis[15].
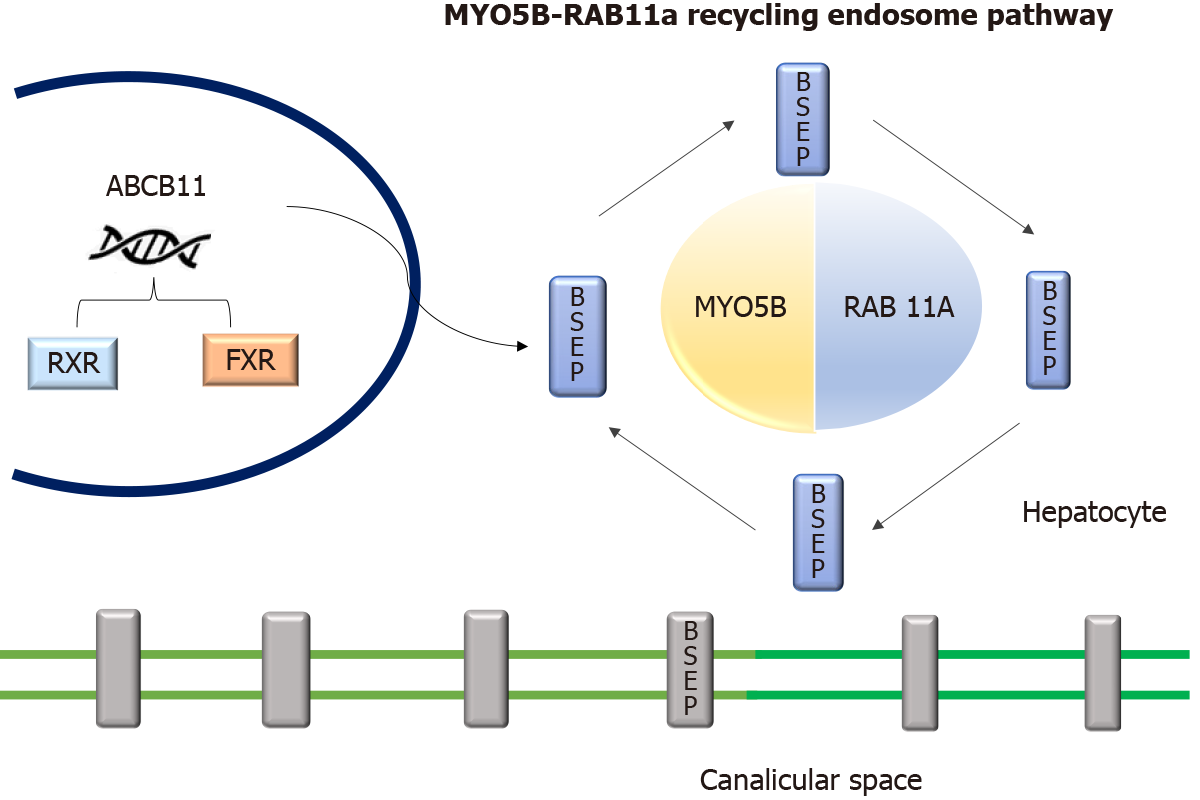
The MYO5B gene located in chromosome 18q21.1 encodes an actin-associated molecular motor protein called MYO5B. MYO5B and RAS-related GTP-binding protein 11A (RAB11A) is essential for the epithelial cell polarization in multiple tissues (Figure 4). In hepatocytes, it is important for the localization of ATP-dependent bile canalicular transporters like BSEP to the canalicular membrane, and in the intestine, it is important for maintaining enterocyte polarity[16]. MYO5B mutations disrupt the MYO5B/RAB11A recycling endosome pathway leading to defective targeting of BSEP[17]. MYO5B gene mutations can result in cholestatic liver disease with or without associated MVID, which presents as intractable diarrhea in infancy[8,18]. Staining of BSEP and MDR3 by immunohistochemistry in these patients is sub-canalicular in the location instead of the regular localization in the canalicular membrane[8].
There is a suggestion that the type of MYO5B mutation affects the clinical presentation[18,19]. Less severe mutations have a loss of canalicular transporter function in hepatocytes without any loss of enterocytes functionality. These patients present with isolated cholestasis. In severe variants of mutations, there is a dys
Intrahepatic cholestasis is the hallmark of how these 3 genetic conditions present. Most often, patients present with variable combinations of pruritus, jaundice, pale stools, and failure to thrive. The published literature on each of these three entities (TJP2, FXR, and MYO5B) is limited and has been summarized in Tables 1-3 respectively.
| Ref. | n | Age at onset of symptoms | Symptoms | Other symptoms | Treatment | Liver transplant | Outcome |
| Sambrotta et al[5] | 12 | 1 wk-3 mo | NC-12/12 | Chronic respiratory disease-1, recurrent unexplained hematoma-1 | UDCA, PEBD-2 | 9/12 cases at the age of 1.5-10 yr | Post-transplant-9 (doing well, no disease recurrence); Stable liver disease with PHT-2; Mortality-1 at 13 mo age |
| Zhang et al[22] | 7 (M = 6, F = 1) | 3 d-2 mo | NC-6/7, pruritus at 7 mo-1/7 | Gallstones 2/7 | Response to UDCA, cholestyramine | None | Resolved cholestasis (n = 6) over 7-26 mo; Persisting icterus-1 |
| Ge et al[46] | 1 (F) | 6mo | Jaundice, pruritus, FTT | - | Responded to medical treatment | None | Resolved cholestasis |
| Mirza et al[47] | 1 (M) | 4 yr | Jaundice, pruritus | - | Medical treatment | None | Cirrhosis, PHT with variceal bleed at 15 yr |
| Wei et al[24] | Index case (M) with multiple affected family members1 | 19 yr | Cirrhosis, PHT with variceal bleed, HCC at 22 yr | - | Medical treatment including EVL | 23 yr | Well in post-transplant period |
| Ref. | Sex | Age at onset of symptoms | Age at initial evaluation | Symptoms | Lab parameters | Histology/IHC | Age at LTx | Outcome | |||
| GGT | INR (at onset) | AFP ng/mL | |||||||||
| Gomez-Ospina et al[6], 2016 | All cases had homozygous mutations | ||||||||||
| 1Patient 1 | F | 2 wk | 20 mo | J, FTT | 53 | 2 | 716 | Cirrhosis | 22 mo | 10 yr4 | |
| 1Patient 2 | M | 2 wk | 7 wk | J, FTT | 45 | 2 | 146000 | Fibrosis | 4.4 mo | 15 mo4 | |
| 2Patient 3 | F | 6 wk | 6 wk | J | 59 | 1.4 | 13900 | Fibrosis | ND | Died 8 mo | |
| 2Patient 4 | M | Birth | Birth | J, ascites, pleural effusion, ICB | - | - | Fibrosis | ND | Died at 4 wk | ||
| Himes et al[7], 2020 | Patient 5 and 7 had homozygous mutations | ||||||||||
| Patient 5 | M | 16 mo | 17 mo | J, ascites | 81 | 1.9 | 9610 | Cirrhosis | 20 mo | Alive at 8 yr of age, no graft steatosis | |
| 3Patient 6 | M | 3 wk | 1 mo | J, FTT, hydrothorax | - | - | - | - | ND | Died at 8 mo, liver failure | |
| 3Patient 7 | F | 1 wk | 4 mo | J, FTT, hydrothorax | - | - | > 100000 | - | ND | Died at 7 mo, liver failure | |
| Chen et al[27], 2019 | Patient had compound heterozygote mutation | ||||||||||
| Patient 8 | N/A | 3 mo | J, splenomegaly | 3.0 | > 80000 | - | ND | Died at 5 mo | |||
| Ref. | Age at onset of symptom | Age at initial evaluation | Symptoms | Treatment | Lab parameters | Outcome | |||
| GGT (IU/L) | AST (IU/L) | ALT (IU/L) | |||||||
| Qiu et al[20], 2017 | n = 10, M-8, F-2, 4 had affected siblings | 2 d-19 mo | 1 mo-10 yr | Jaundice and pruritus; No diarrhea | UDCA, cholestyramine | 9-99 | 24-255 | 41-432 | Recurrent-3, persistent-2, transient cholestasis-2, lost to follow-3, listed for LT -1 (died) |
| Cockar et al[19], 2020 | n = 6, M-3, F-3 | - | 6 mo-15 yr | Pruritus with pale stools-6, Jaundice-3; FTT-3; Diarrhea-2, (intractable and settled at 3 yr and 7 yr), gallstone-1 | Antipruritic medications-6; PIBD-1; PIBD followed by PEBD-1; ENBD followed by PEBD-1 | 10-22 | - | 15-177 | 1-LT for poor QOL and pruritus; 5-Partial response with mild pruritus while on medications |
| Gonzales et al[8], 2017 | n = 5, M-4, F-1 | - | 7-15 mo | Pruritus-5; Jaundice-5; Pale stools-5 hepatomegaly-5; Language delay-1 episodes of severe diarrhea before 3 yr of age-1 | UDCA and rifampicin-5; PEBD-1 | 7-11 | 31-170 | 57-207 | Followed till 3.5-13.5 yr of age; Fluctuating cholestasis-4; Cholestasis resolved after 1 mo of PEBD, well till 7 yr of age |
| Girard et al[17], 2014 | n = 8/28 MVID, patients with cholestasis M-5, F-3 | 3-60 mo | Jaundice, pruritus, hepatomegaly-8; Pre Int Tx-5, post Int Tx-3 | Antipruritic medications-8; PIBD followed by PEBD-1; PIBD-1; PEBD-1; Combined liver and Int Tx-1 | 8-42 | 51-124 | 52-121 | Follow up till 2.8-14 yr of age, remission-6, partial remission-2; Removal of small bowel graft due to acute rejection in 2 cases improved cholestasis | |
A varying spectrum of clinical presentation, ranging from mild anicteric illness, recurrent jaundice to severe progressive liver disease has been described[5,11]. Incomplete penetrant, homozygous, missense mutations affecting both isoforms of TJP2 have been shown to cause familial hypercholanemia in the Amish population which manifests as a mild anicteric disease with pruritus and steatorrhea. In this condition, the binding of TJP2 to claudins is impaired[21]. Milder mutations of TJP2 are also known to be associated with intrahepatic cholestasis of pregnancy[15].
In the 12 cases reported by Sambrotta et al[5], 9 (75%) required liver transplantation (LT) while 2 had portal hypertension. In contrast, none of the 7 cases reported by Zhang et al[22] required LT, and cholestasis responded to medical therapy in a majority. Zhang et al[22] also showed that truncating or canonical splice-site biallelic TJP2 mutations caused a more severe presentation due to a complete loss of protein expression. In their study, 3 children with severe mutations had growth failure. While the other 3 cases with missense variants had normal growth and sustained response in pruritus with ursodeoxycholic acid (UDCA) and cholestyramine.
All homozygous mutations are predicted to abolish protein translation and a complete loss of function[5]. Mutations involving missense and frame deletion lead to less severe clinical disease due to residual TJP2 protein expression[22]. This suggests the presence of a genotype-phenotype correlation based on the amount of remnant functional TJP2 activity.
There is a higher risk of developing hepatocellular carcinoma (HCC) in these cases, similar to that seen in PFIC 2 patients. Subjects can either present with a space-occupying lesion (SOL) in the liver or are detected to have HCC after LT on histology of the explanted liver[23,24]. This predisposition to HCC highlights the importance of close follow-up and regular monitoring.
FXR is the master player of bile acid regulation and plays an important role in reducing bile acid-induced hepatotoxicity. Rapidly progressive liver disease and early onset vitamin K independent coagulopathy are the main features of this condition. The details of the 8 published cases are given in Table 2. A majority of patients presented early in the first 3 mo of life and progressed rapidly to liver failure. Patients have markedly increased alpha-fetoprotein and deranged international normalized ratio. Without a liver transplant, 5/8 died in infancy itself. Three cases survived post-liver transplant, of which 2 were found to have liver function abnormality with graft steatosis in the follow-up[6]. This post-transplant hepatic damage may be attributed to the altered enterohepatic circulation and FXR signalling in these cases. The absence of FXR in the intestine leads to low FGF 19 levels and this allows for continued and increased synthesis of bile acids by the liver[25]. Intrahepatic cholestasis of pregnancy has been reported and attributed to the downregulation of BSEP in this condition[26].
Patients with MYO5B mutations can present with isolated cholestasis, isolated MVID, or both MVID and cholestasis. Typically, the child presents with jaundice, pruritus, and hepatomegaly. In patients with MVID and cholestasis, the onset of cholestasis may be pre or post-small bowel transplant. The exact explanation as to why some MVID cases develop cholestasis while others do not is unclear but it may be related to the severity of mutation (vide supra). The summary of the clinical presentation of 29 cases with MYO5B mutation, as reported in 4 papers, is shown in Table 3. Even in siblings with the same mutation and presentation with cholestasis, the disease severity may vary[20]. This suggests the possible role of modifier genes or environmental factors. Among Han Chinese children, defects in MYO5B accounted for approximately 20% of cases of idiopathic low-normal GGT intrahepatic cholestasis[20].
The main steps for making a diagnosis of PFIC and determining the specific type in any given child with cholestasis are as follows: Step 1: Detailed history and physical examination including family history, consanguinity, extraintestinal symptoms, growth, nutritional deficiencies, and features of advanced liver disease; Step 2: Complete liver function test with GGT. Low-normal GGT is seen in ATP8B1, ABCB11, TJP2, NR1H4, and MYO5B disease. Early-onset of vitamin K unresponsive coagulopathy is a feature of NR1H4 disease; Step 3: Radiologic imaging. Ultrasonography (USG) of the abdomen is useful to exclude structural causes of neonatal cholestasis, like biliary atresia or choledochal cyst. The presence of biliary radicle dilatation may suggest sclerosing cholangitis, which needs to be confirmed by MRCP. USG is also useful to document features of advanced liver disease like ascites, splenomegaly, dilated portal vein, and collaterals. Gall stones have been reported in TJP2 disease, as also in PFIC 2 and 3. The presence of hepatic SOL raises suspicion of HCC and needs evaluation by triple-phase CT and alpha-fetoprotein. Early HCC is a feature of TJP2 disease; Step 4: Liver histology including immunohistochemistry and next-generation sequencing (NGS). Liver biopsy shows canalicular cholestasis in all three types (TJP2, NR1H4, and MYO5B defects) along with a variable degree of fibrosis and giant cell transformation[6]. On electron microscopy, the tight junctions appear elongated and lack the densest part of the zona occludens in PFIC 4[6]. In subjects with MYO5B and liver disease, electron microscopy will show dilatation of the bile canalicular lumen, canalicular thickening, and disappearance of the microvilli apart from cholestasis[17]. Inclusion bodies are not seen in the hepatocytes on transmission electron microscopy, in contrast to the findings in intestines in MVID[17]. The comparative features at histology in these three types are given in Table 4.
| PFIC 4 | PFIC 5 | PFIC 6 | |
| Gene mutation | TJP2/Zona occludens-2 located in 9q21.11 | NR1H4/FXR-located in 12q23.1 | MYO5B located in 18q21.1 |
| Clinical features | |||
| Clinical features | Cholestatic jaundice with pruritus | Rapidly progressive neonatal-onset cholestasis with uncorrectable coagulopathy | Cholestasis with pruritus, with/without transient, recurrent or progressive diarrhea (association with MVID) |
| Extrahepatic features | Neurological and respiratory symptoms | - | - |
| ICP | Yes | Yes (uncommon) | No |
| Laboratory parameters | |||
| AST/ALT | Elevated | Moderate elevation | Mild to moderate elevation |
| GGT | Normal or mild elevation | Normal | Normal |
| Coagulopathy | Late-onset | Early-onset | Late-onset |
| Alpha fetoprotein | Normal, elevated in cases with HCC | Elevated | Normal |
| S. Bile acids | Elevated | Elevated | Elevated |
| Histopathology | |||
| Canalicular cholestasis | Yes | Yes | Yes |
| Portal/lobular fibrosis | Yes | Yes | Yes |
| Giant-cell transformation | Yes | Diffuse | Sparse |
| Ductular reaction | No | Yes | Yes |
| Hepatocyte necrosis | Yes | - | - |
| Cirrhosis | Yes | Yes | Less common |
| Immunohistochemistry | |||
| BSEP | Present | Absent BSEP staining on bile canaliculus | Abnormally thick, irregular and granular positivity that overflows into subcanalicular area |
| MDR3 | Present | Present | Thickened canalicular staining granular and patchy pattern overflows into subcanalicular area |
| TJP2 | Absent expression in canalicular membrane | Present | Present |
| Claudin1 | Absent or reduced staining on bile canaliculi | Present | Present |
| FXR | Normal | Absent staining on bile canaliculus | Normal |
| MYO5B/RAB11 | Normal | Normal | Intense, granular staining pattern in hepatocyte cytoplasm, and weak/loss of canalicular expression |
| Progression | Rapid | Very rapid | Slow |
| Complications | Hepatocellular carcinoma | Post-transplant graft steatosis similar to PFIC1 | Worsening of cholestasis post intestinal transplant |
| Treatment | |||
| Medical management | UDCA, Rifampicin | Minimal role | UDCA, rifampin, cholestyramine |
| Biliary diversion | PEBD some role | Not tried | Cholestasis subsides after BD in MVID patients with cholestasis |
| Liver transplant | Yes | Yes | Yes. Combined liver intestinal transplant in children with MVID and ongoing cholestasis |
A complete panel of immunohistochemistry including BSEP, MDR3, TJP2, FXR, MYO5B, and Claudin1 can help in identifying the subtype of PFIC as shown in Table 4. However, simultaneous NGS for multiple genes (cholestasis panel) is a rapid and affordable way of confirming the molecular diagnosis[27]. A recent study has shown that a molecular genetic diagnosis can be made in a quarter of cases with neonatal cholestasis using NGS[28]. A study with a 66-gene cholestasis panel in 2171 cholestatic children and young adults, had a diagnostic yield of 12% and turnaround time of only 21 d[29]. The simultaneous testing for multiple genes helps in not only confirming the diagnosis but also in excluding other conditions. NGS is becoming the test of choice in the primary evaluation of patients with PFIC phenotype as it is non-invasive in comparison to liver biopsy and immunohistochemistry. For cases in which the panel yields a negative result and the index of suspicion is high, further testing by the whole exome (WES) or whole-genome (WGS) sequencing may be done. The presence of variables of unknown significance and monoallelic pathogenic/likely pathogenic variants in a significant proportion of cases highlights the complexity of analysis and the need for expertise for proper interpretation. Also, the ongoing discovery of new genes requires expansion of the genetic testing panel from time to time.
The main differentials to be considered in a patient with intrahepatic cholestasis with low-normal GGT (< 100 U/L) include bile acid synthetic defect (BASD), arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome, and USP53 related cholestasis, apart from the different types of PFIC (1, 2, 4, 5 and MYO5B associated). Type 3 PFIC (ABCB4) has raised GGT[1,30]. The serum bile acids are raised in PFIC and ARC syndrome, while they are low in the BASD. These entities can be differentiated by their distinct clinical presentation and liver histopathology with immunohistochemistry. However, the confirmation of diagnoses is best done by genetic analysis.
In bile acid synthetic defects (BASD) there is an accumulation of toxic bile acid intermediates in the hepatocytes due to deficiency of various enzymes involved in bile acid synthesis. Patients present with cholestatic jaundice, overt steatorrhea and florid manifestations of fat-soluble vitamin deficiencies like rickets. Pruritus is distinctly uncommon. Sometimes they may also present with neonatal liver failure, and cholestatic liver disease along with neurological manifestations like hypotonia, seizures[31]. BASD is diagnosed with fast-atom bombardment mass spectrometry of urine, which shows the accumulation of the distinct bile acid intermediaries due to a block in the bile acid synthesis pathway. Genetic analysis is confirmatory. Supplementation of cholic acid (CA) and chenodeoxycholic acid (CDCA) along with fat-soluble vitamins is the mainstay of therapy[32].
ARC syndrome (MIM 208085) is a rare multisystem disorder with autosomal recessive inheritance. It includes a triad of arthrogryposis, renal tubular acidosis, and neonatal cholestatic jaundice. Some patients may have accompanying features like ichthyosis (approximately 50%), platelet anomalies (approximately 25%), agenesis of the corpus callosum (> 20%), congenital cardiovascular anomalies (approximately 10%), and deafness. The clinical features are very useful to suspect the diagnosis, which is confirmed by a demonstration of mutations in the VPS33B or VIPAR gene. Histopathology shows bile duct paucity, giant cell transformation, bile plugs, and portal fibrosis. Caution is required before proceeding with a renal or liver biopsy due to the increased risk of a life-threatening bleed. Treatment is supportive and includes management of joint contractures, renal tubular acidosis, and cholestasis (UDCA, fat-soluble vitamins)[33].
USP53 encodes an enzyme known as ubiquitin carboxyl-terminal hydrolase 53, which belongs to the de-ubiquitinating enzyme family and helps in maintaining cell integrity by interacting with TJP2 in hepatocytes. Whole-exome sequencing in 69 Han Chinese infants, with low GGT cholestasis without any pathological variants in ATP8B1, ABCB11, NR1H4, TJP2, and MYO5B genes, showed the presence of biallelic USP53 mutations (homozygous or compound heterozygous) in 7 patients[34]. All these children had cholestatic jaundice in infancy and responded to medications (UDCA, cholestyramine). Liver biopsy showed varying levels of lobular disarray and hepatocellular and canalicular cholestasis, rosetting, portal tract fibrosis, ductular proliferation, and giant-cell transformation. Ultrastructural examination in 2 cases revealed abnormality of tight junction complexes and expression of TJP2 and CLDN1 were reduced. Two children also had sensorineural hearing loss. In another report on the novel USP53 mutation, three members from the same family (2 sisters and a cousin) had low-GGT cholestasis, pruritus, elevated transaminases, very high alkaline phosphatase, and sensorineural hearing loss (n = 2). One of them required LT because of intractable pruritus[35].
Alagille syndrome is also known as arteriohepatic dysplasia or syndromic paucity of interlobular bile ducts. This disorder is autosomal dominant with variable phenotypic penetrance. Alagille syndrome is one of the commonest causes of genetic cholestasis[36]. The defining feature is cholestasis with multisystemic involvement. Features include neonatal cholestasis in 95%, extrahepatic biliary hypoplasia, pruritus, xanthoma and associated facial dysmorphism. Structural cardiac defects such as peripheral pulmonary stenosis and septal defects are seen in 88%. Vertebral anomalies, ocular abnormalities most commonly posterior embryotoxon, renal dysplasia, vascular anomalies like Moyamoya disease, carotid and subclavian artery aneurysm are the other systemic features. Genetic analysis reveals JAG1 mutation in the majority (approximately 90%) and NOTCH2 mutation in minority[35].
It is caused due to SLC25A13 (Solute Carrier family 25) gene mutation located in chromosome 7q21.3. The disease spectrum includes neonatal intrahepatic cholestasis, failure to thrive and dyslipidemia, and adult-onset type II citrullinemia. Chubby cheeks in infancy are a hallmark finding. These children also have a characteristic history of aversion to carbohydrates and a dietary preference towards a protein and lipid-rich diet[37].
Neonatal Ichthyosis Sclerosing cholangitis is a rare cause of neonatal cholestasis with an autosomal recessive inheritance pattern. It is caused due to a mutation in the CLDN1 gene which encodes the CLDN1 protein located at the tight junction. This condition presents with neonatal cholestasis, cicatricial alopecia, ichthyosis and pruritus. Magnetic resonance cholangiopancreatography will show features of sclerosing cholangitis[38].
Amongst the different PFIC subtypes, PFIC 1, 2, 4, 5 and 6 have low-normal GGT cholestasis. The presence of diarrhea is a feature of PFIC 1 and MYO5B disease. While neurological symptoms may be seen in ARC syndrome and sometimes in patients with MVID, a higher risk of HCC is a feature of TJP2 and BSEP deficiency. Table 4 gives the comparison of the clinical features and investigations in TJP2, FXR, and MYO5B defects. A detailed description and comparison of PFIC 1 and 2 are given elsewhere[39].
The main components include counselling of parents in detail, providing adequate nutrition, correcting vitamin deficiencies, controlling pruritus, managing complications like ascites, variceal bleeding etc., growth monitoring, and vaccination[40].
Nutritional therapy: A diet that provides adequate calories (125%-140% of RDA) and protein (2-3 g/kg) with supplementation of medium-chain triglyceride and fat-soluble vitamins is recommended[41]. The doses of vitamin supplementation may need modification based on clinical signs and symptoms of vitamin deficiency and serum level monitoring (if available). Anemia, if present, needs to be corrected. Age-appropriate immunization including vaccination against hepatotropic viruses (hepatitis A and hepatitis B) is essential.
Management of pruritus: Pruritus is one of the most disabling symptoms in these children. Apart from skincare, medications such as UDCA, cholestyramine, rifampicin, naltrexone, and sertraline are used for controlling pruritus. These aspects have been addressed in detail elsewhere[42]. There are no published reports on the use of FXR agonists like obeticholic acid, or apical sodium–bile acid transporter inhibitors like maralixibat in PFIC 4, 5 and MYO5B related diseases. Long-term follow-up includes growth monitoring, monitoring for nutritional deficiencies, and HCC surveillance, especially in TJP2 related cholestasis.
Biliary diversion (BD) takes away bile from the intestine, thereby reducing the reabsorption of bile acids through the enterohepatic circulation[43]. It has an important role in the alleviation of pruritus that is refractory to medical management in PFIC 1 and 2[44]. The role of BD is not well known in the newer variants of PFIC. BD has been tried in MVID patients who developed worsened cholestasis post intestinal transplant and was found to be helpful[17]. In MYO5B mutation, the ongoing cholestatic liver disease worsens after the intestinal transplant, leading to progressive liver fibrosis. Hence combined liver and intestinal transplantation are preferred. But in cases of isolated intestinal transplants, gallbladders should be preserved so that in case the cholestasis worsens, partial external biliary drainage can be attempted. The ileal bypass should be avoided as it removes a part of the transplanted bowel and doesn’t result in long-term remission of cholestasis[16].
LT is to be considered in children with decompensated chronic liver disease, growth failure (not amenable to dietary modification), refractory pruritus, or associated complications like hepato-pulmonary syndrome. In NR1H4 related PFIC, an early transplant may be required due to progressive liver disease with decompensation. Post liver transplant graft steatosis may develop in patients with NR1H4 mutation-associated cholestasis[6].
Once a child is confirmed to have PFIC, parents need to be counselled about the nature of the disease and the autosomal recessive pattern of inheritance. A geneticist should be involved in counselling about future pregnancies and testing during pregnancy[45].
TJP2, FXR, and MYO5B are recent additions to the three well-known types of PFIC (1, 2, and 3). This review has described the genetics, clinical profile, investigative findings, and treatments of these newer entities. There are gaps in our understanding of these conditions due to the limited literature at present. Advances in bioinformatics and techniques of next-generation gene-sequencing will help us study the genotype-phenotype correlation and synergistic effect of multiple mutations. Despite the recognition of these entities, not all cases with the PFIC phenotype have a confirmed genetic diagnosis, which indicates the presence of other causative genes that are waiting to be discovered.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Andreone P S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
| 1. | Davit-Spraul A, Gonzales E, Baussan C, Jacquemin E. Progressive familial intrahepatic cholestasis. Orphanet J Rare Dis. 2009;4:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 244] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 2. | Hori T, Nguyen JH, Uemoto S. Progressive familial intrahepatic cholestasis. Hepatobiliary Pancreat Dis Int. 2010;9:570-578. [PubMed] |
| 3. | Jacquemin E. Progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol. 2012;36 Suppl 1:S26-S35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 4. | Baker A, Kerkar N, Todorova L, Kamath BM, Houwen RHJ. Systematic review of progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol. 2019;43:20-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 5. | Sambrotta M, Strautnieks S, Papouli E, Rushton P, Clark BE, Parry DA, Logan CV, Newbury LJ, Kamath BM, Ling S, Grammatikopoulos T, Wagner BE, Magee JC, Sokol RJ, Mieli-Vergani G; University of Washington Center for Mendelian Genomics, Smith JD, Johnson CA, McClean P, Simpson MA, Knisely AS, Bull LN, Thompson RJ. Mutations in TJP2 cause progressive cholestatic liver disease. Nat Genet. 2014;46:326-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 203] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 6. | Gomez-Ospina N, Potter CJ, Xiao R, Manickam K, Kim MS, Kim KH, Shneider BL, Picarsic JL, Jacobson TA, Zhang J, He W, Liu P, Knisely AS, Finegold MJ, Muzny DM, Boerwinkle E, Lupski JR, Plon SE, Gibbs RA, Eng CM, Yang Y, Washington GC, Porteus MH, Berquist WE, Kambham N, Singh RJ, Xia F, Enns GM, Moore DD. Mutations in the nuclear bile acid receptor FXR cause progressive familial intrahepatic cholestasis. Nat Commun. 2016;7:10713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 200] [Article Influence: 22.2] [Reference Citation Analysis (2)] |
| 7. | Himes RW, Mojarrad M, Eslahi A, Finegold MJ, Maroofian R, Moore DD. NR1H4-related Progressive Familial Intrahepatic Cholestasis 5: Further Evidence for Rapidly Progressive Liver Failure. J Pediatr Gastroenterol Nutr. 2020;70:e111-e113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Gonzales E, Taylor SA, Davit-Spraul A, Thébaut A, Thomassin N, Guettier C, Whitington PF, Jacquemin E. MYO5B mutations cause cholestasis with normal serum gamma-glutamyl transferase activity in children without microvillous inclusion disease. Hepatology. 2017;65:164-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 9. | Gumbiner B, Lowenkopf T, Apatira D. Identification of a 160-kDa polypeptide that binds to the tight junction protein ZO-1. Proc Natl Acad Sci U S A. 1991;88:3460-3464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 366] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 10. | Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539-1550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1524] [Cited by in RCA: 1568] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 11. | Sambrotta M, Thompson RJ. Mutations in TJP2, encoding zona occludens 2, and liver disease. Tissue Barriers. 2015;3:e1026537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (4)] |
| 12. | Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW, Evans RM, Weinberger C. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 309] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 13. | Trauner M, Claudel T, Fickert P, Moustafa T, Wagner M. Bile acids as regulators of hepatic lipid and glucose metabolism. Dig Dis. 2010;28:220-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 237] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 14. | Huang L, Zhao A, Lew JL, Zhang T, Hrywna Y, Thompson JR, de Pedro N, Royo I, Blevins RA, Peláez F, Wright SD, Cui J. Farnesoid X receptor activates transcription of the phospholipid pump MDR3. J Biol Chem. 2003;278:51085-51090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 164] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Dixon PH, Sambrotta M, Chambers J, Taylor-Harris P, Syngelaki A, Nicolaides K, Knisely AS, Thompson RJ, Williamson C. An expanded role for heterozygous mutations of ABCB4, ABCB11, ATP8B1, ABCC2 and TJP2 in intrahepatic cholestasis of pregnancy. Sci Rep. 2017;7:11823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 16. | Lapierre LA, Kumar R, Hales CM, Navarre J, Bhartur SG, Burnette JO, Provance DW Jr, Mercer JA, Bähler M, Goldenring JR. Myosin vb is associated with plasma membrane recycling systems. Mol Biol Cell. 2001;12:1843-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 335] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 17. | Girard M, Lacaille F, Verkarre V, Mategot R, Feldmann G, Grodet A, Sauvat F, Irtan S, Davit-Spraul A, Jacquemin E, Ruemmele F, Rainteau D, Goulet O, Colomb V, Chardot C, Henrion-Caude A, Debray D. MYO5B and bile salt export pump contribute to cholestatic liver disorder in microvillous inclusion disease. Hepatology. 2014;60:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 18. | Aldrian D, Vogel GF, Frey TK, Ayyıldız Civan H, Aksu AÜ, Avitzur Y, Ramos Boluda E, Çakır M, Demir AM, Deppisch C, Duba HC, Düker G, Gerner P, Hertecant J, Hornová J, Kathemann S, Koeglmeier J, Koutroumpa A, Lanzersdorfer R, Lev-Tzion R, Lima R, Mansour S, Meissl M, Melek J, Miqdady M, Montoya JH, Posovszky C, Rachman Y, Siahanidou T, Tabbers M, Uhlig HH, Ünal S, Wirth S, Ruemmele FM, Hess MW, Huber LA, Müller T, Sturm E, Janecke AR. Congenital Diarrhea and Cholestatic Liver Disease: Phenotypic Spectrum Associated with MYO5B Mutations. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Cockar I, Foskett P, Strautnieks S, Clinch Y, Fustok J, Rahman O, Sutton H, Mtegha M, Fessatou S, Kontaki E, Papaevangelou V, Deheragoda M, Thompson RJ, Grammatikopoulos T. Mutations in Myosin 5B in Children With Early-onset Cholestasis. J Pediatr Gastroenterol Nutr. 2020;71:184-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Qiu YL, Gong JY, Feng JY, Wang RX, Han J, Liu T, Lu Y, Li LT, Zhang MH, Sheps JA, Wang NL, Yan YY, Li JQ, Chen L, Borchers CH, Sipos B, Knisely AS, Ling V, Xing QH, Wang JS. Defects in myosin VB are associated with a spectrum of previously undiagnosed low γ-glutamyltransferase cholestasis. Hepatology. 2017;65:1655-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 21. | Carlton VE, Harris BZ, Puffenberger EG, Batta AK, Knisely AS, Robinson DL, Strauss KA, Shneider BL, Lim WA, Salen G, Morton DH, Bull LN. Complex inheritance of familial hypercholanemia with associated mutations in TJP2 and BAAT. Nat Genet. 2003;34:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 205] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 22. | Zhang J, Liu LL, Gong JY, Hao CZ, Qiu YL, Lu Y, Feng JY, Li JQ, Li ZD, Wang MX, Xing QH, Knisely AS, Wang JS. TJP2 hepatobiliary disorders: Novel variants and clinical diversity. Hum Mutat. 2020;41:502-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Vij M, Shanmugam NP, Reddy MS, Sankaranarayanan S, Rela M. Paediatric hepatocellular carcinoma in tight junction protein 2 (TJP2) deficiency. Virchows Arch. 2017;471:679-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Wei CS, Becher N, Friis JB, Ott P, Vogel I, Grønbæk H. New tight junction protein 2 variant causing progressive familial intrahepatic cholestasis type 4 in adults: A case report. World J Gastroenterol. 2020;26:550-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Kliewer SA, Mangelsdorf DJ. Bile Acids as Hormones: The FXR-FGF15/19 Pathway. Dig Dis. 2015;33:327-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 318] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 26. | Van Mil SW, Milona A, Dixon PH, Mullenbach R, Geenes VL, Chambers J, Shevchuk V, Moore GE, Lammert F, Glantz AG, Mattsson LA, Whittaker J, Parker MG, White R, Williamson C. Functional variants of the central bile acid sensor FXR identified in intrahepatic cholestasis of pregnancy. Gastroenterology. 2007;133:507-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 179] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 27. | Chen HL, Li HY, Wu JF, Wu SH, Chen HL, Yang YH, Hsu YH, Liou BY, Chang MH, Ni YH. Panel-Based Next-Generation Sequencing for the Diagnosis of Cholestatic Genetic Liver Diseases: Clinical Utility and Challenges. J Pediatr. 2019;205:153-159.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 28. | Togawa T, Sugiura T, Ito K, Endo T, Aoyama K, Ohashi K, Negishi Y, Kudo T, Ito R, Kikuchi A, Arai-Ichinoi N, Kure S, Saitoh S. Molecular Genetic Dissection and Neonatal/Infantile Intrahepatic Cholestasis Using Targeted Next-Generation Sequencing. J Pediatr. 2016;171:171-7.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 29. | Feldman AG, Sokol RJ. Neonatal cholestasis: emerging molecular diagnostics and potential novel therapeutics. Nat Rev Gastroenterol Hepatol. 2019;16:346-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 30. | de Vree JM, Jacquemin E, Sturm E, Cresteil D, Bosma PJ, Aten J, Deleuze JF, Desrochers M, Burdelski M, Bernard O, Oude Elferink RP, Hadchouel M. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc Natl Acad Sci U S A. 1998;95:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 456] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 31. | Ravindranath A, Sen Sarma M, Yachha SK. Bile acid synthetic defects: Simplified approach in a nutshell. Hepatobiliary Pancreat Dis Int. 2020;19:80-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Gonzales E, Matarazzo L, Franchi-Abella S, Dabadie A, Cohen J, Habes D, Hillaire S, Guettier C, Taburet AM, Myara A, Jacquemin E. Cholic acid for primary bile acid synthesis defects: a life-saving therapy allowing a favorable outcome in adulthood. Orphanet J Rare Dis. 2018;13:190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Zhou Y, Zhang J. Arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome: from molecular genetics to clinical features. Ital J Pediatr. 2014;40:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Zhang J, Yang Y, Gong JY, Li LT, Li JQ, Zhang MH, Lu Y, Xie XB, Hong YR, Yu Z, Knisely AS, Wang JS. Low-GGT intrahepatic cholestasis associated with biallelic USP53 variants: Clinical, histological and ultrastructural characterization. Liver Int. 2020;40:1142-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 35. | Maddirevula S, Alhebbi H, Alqahtani A, Algoufi T, Alsaif HS, Ibrahim N, Abdulwahab F, Barr M, Alzaidan H, Almehaideb A, AlSasi O, Alhashem A, Hussaini HA, Wali S, Alkuraya FS. Identification of novel loci for pediatric cholestatic liver disease defined by KIF12, PPM1F, USP53, LSR, and WDR83OS pathogenic variants. Genet Med. 2019;21:1164-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 36. | Mitchell E, Gilbert M, Loomes KM. Alagille Syndrome. Clin Liver Dis. 2018;22:625-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 37. | Hayasaka K. Metabolic basis and treatment of citrin deficiency. J Inherit Metab Dis. 2021;44:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 38. | Nagtzaam IF, van Geel M, Driessen A, Steijlen PM, van Steensel MA. Bile duct paucity is part of the neonatal ichthyosis-sclerosing cholangitis phenotype. Br J Dermatol. 2010;163:205-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Srivastava A. Progressive familial intrahepatic cholestasis. J Clin Exp Hepatol. 2014;4:25-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 40. | Jagadisan B, Srivastava A. Child with Jaundice and Pruritus: How to Evaluate? Indian J Pediatr. 2016;83:1311-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Lane E, Murray KF. Neonatal Cholestasis. Pediatr Clin North Am. 2017;64:621-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Thébaut A, Debray D, Gonzales E. An update on the physiopathology and therapeutic management of cholestatic pruritus in children. Clin Res Hepatol Gastroenterol. 2018;42:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Lemoine C, Superina R. Surgical diversion of enterohepatic circulation in pediatric cholestasis. Semin Pediatr Surg. 2020;29:150946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Verkade HJ, Thompson RJ, Arnell H, Fischler B, Gillberg PG, Mattsson JP, Torfgård K, Lindström E. Systematic Review and Meta-analysis: Partial External Biliary Diversion in Progressive Familial Intrahepatic Cholestasis. J Pediatr Gastroenterol Nutr. 2020;71:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Fonda Allen J, Stoll K, Bernhardt BA. Pre- and post-test genetic counseling for chromosomal and Mendelian disorders. Semin Perinatol. 2016;40:44-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 46. | Ge T, Zhang X, Xiao Y, Wang Y, Zhang T. Novel compound heterozygote mutations of TJP2 in a Chinese child with progressive cholestatic liver disease. BMC Med Genet. 2019;20:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 47. | Mirza N, Bharadwaj R, Malhotra S, Sibal A. Progressive familial intrahepatic cholestasis type 4 in an Indian child: presentation, initial course and novel compound heterozygous mutation. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |