Published online Dec 27, 2021. doi: 10.4254/wjh.v13.i12.1956
Peer-review started: February 25, 2021
First decision: May 13, 2021
Revised: May 27, 2021
Accepted: November 12, 2021
Article in press: November 12, 2021
Published online: December 27, 2021
Processing time: 304 Days and 7.1 Hours
Hepatobiliary manifestations are common in inflammatory bowel disease (IBD), with 30% of patients presenting abnormal liver tests and 5% developing chronic liver disease. They range from asymptomatic elevated liver tests to life-threatening disease and usually follow an independent course from IBD. The pathogenesis of liver manifestations or complications and IBD can be closely related by sharing a common auto-immune background (in primary sclerosing cholangitis, IgG4-related cholangitis, and autoimmune hepatitis), intestinal inflammation (in portal vein thrombosis and granulomatous hepatitis), metabolic impairment (in non-alcoholic fatty liver disease or cholelithiasis), or drug toxicity (in drug induced liver injury or hepatitis B virus infection reactivation). Their evaluation should prompt a full diagnostic workup to identify and readily treat all complications, improving management and outcome.
Core Tip: Hepatobiliary manifestations are common in inflammatory bowel disease (IBD), ranging from incidental findings in asymptomatic patients to life-threatening liver failure. Their pathogenesis can be intrinsically linked to IBD (auto-immune background or metabolic abnormalities) or to its medication. Early recognition of these manifestations as well as a full diagnostic workup are mandatory to improve management and prognosis. In this review, we describe all hepatobiliary manifestations in IBD.
- Citation: Gaspar R, Branco CC, Macedo G. Liver manifestations and complications in inflammatory bowel disease: A review. World J Hepatol 2021; 13(12): 1956-1967
- URL: https://www.wjgnet.com/1948-5182/full/v13/i12/1956.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i12.1956
Inflammatory bowel disease (IBD) is a group of chronic and recurrent gastrointestinal inflammatory conditions that result from the interaction of genetic, environmental, and immune factors. IBD is mainly divided into Crohn's disease (CD) and ulcerative colitis (UC), affecting equally men and women, with peak incidence between 20 and 30 and also from 50 to 60 years of age[1].
Extra-intestinal manifestations are described in up to 50% of patients, including arthropathy, metabolic bone disease, ocular, dermatological, hepatobiliary, neurologic, cardiovascular, pulmonary, and urological complications[2].
Hepatobiliary alterations are one of the most common extra-intestinal manifestations of IBD; up to 30% of patients have abnormal liver tests and 5% will develop chronic liver disease[3,4]. A wide diversity of hepatobiliary complications has been reported, ranging from incidental findings in asymptomatic patients to severe and life-threatening liver failure[5].
The pathogenesis of liver disease in IBD is not totally understood but multiple pathways may link them (Table 1)[2,5,6].
| IBD related diseases | IBD medication related diseases | ||
| Ulcerative colitis | Crohn's disease | Ulcerative colitis | Crohn's disease |
| Primary sclerosing cholangitis | Granulomatous hepatitis | Drug-induced liver injury | Drug-induced liver injury |
| Auto-immune hepatitis | Liver abscesses | HBV reactivation | HBV reactivation |
| Overlap syndromes | Cholelithiasis | ||
| Primary biliary cholangitis | Hepatic amyloidosis | ||
| Portal vein thrombosis | |||
| NAFLD | NAFLD | ||
Diseases that share a common auto-immune background include primary sclerosing cholangitis (PSC), IgG4-related cholangitis, primary biliary cholangitis (PBC), auto-immune hepatitis, and overlap syndromes.
Diseases associated with intestinal inflammation include portal vein thrombosis, Budd-Chiari syndrome, granulomatous hepatitis, and liver abscesses.
Diseases associated with malabsorption or metabolic impairment are cholelithiasis, amyloidosis, and non-alcoholic fatty liver disease (NAFLD).
Disorders associated with IBD treatment include direct hepatotoxicity with medications such as 5-aminosalicylic acid (5-ASA) compounds, methotrexate, azathioprine, or anti-TNF agents or hepatitis B reactivation due to immunosuppressants.
They can occur at any time during the natural history of disease and typically follow an independent course from the underlying intestinal disease activity. Granulomatous hepatitis, hepatic abscesses, cholelithiasis, and amyloidosis are more commonly observed in CD and PSC and auto-immune hepatitis in UC[6,7].
Moreover, these patients may present unrelated liver disease, making abnormal liver tests in IBD a challenging differential diagnosis.
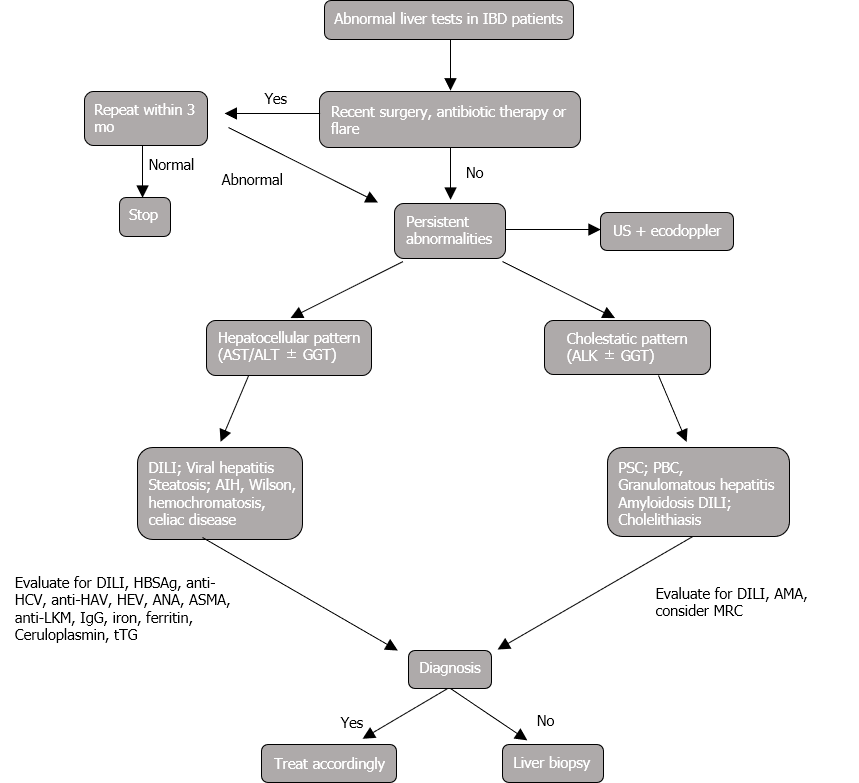
Early recognition of these manifestations is of paramount importance to avoid liver injury and improve management of both diseases (Figure 1).
The aim of this paper is to review the hepatobiliary manifestations and complications found in IBD patients.
PSC is a chronic and progressive bile duct disorder, characterized by multifocal intrahepatic and/or extrahepatic strictures and dilatations, that may result in cirrhosis and end-stage liver disease. The diagnosis is usually made by combination of clinical (jaundice, abdominal pain, and itching but it may also be asymptomatic), biochemical (elevated cholestatic liver enzymes - alkaline phosphatase and/or GGT) and imagiological [magnetic resonance cholangiography (MRCP)] findings. The mean age at diagnosis is 30 to 40 years old and it has a male predominance[8,9].
PSC is closely linked to IBD, which occurs in 70% of patients, with a UC predominance (75%). On the other hand, only up to 3% of CD and 2%-8% of UC patients develop PSC[10]. Therefore, the presence of unexplained cholestasis should prompt an immediate investigation by MRCP in those with IBD and patients with PSC should routinely undergo colonoscopy with biopsies, even in the absence of symptoms. If the index colonoscopy is negative, it should be repeated every 3 to 5 years[10,11]. The two disorders can occur at different times, but IBD diagnosis usually precedes that of PSC[12].
IBD in the setting of PSC is associated with a different clinical course, typically presenting extensive disease, rectal sparing (6% to 66% vs 2% to 25% in IBD without PSC), backwash ileitis (5% to 46% vs 3% to 24% in UC without PSC), and mild intestinal activity, as well as more frequent right colonic involvement[10,13]. Marelli et al[14] showed an inverse relationship between PSC severity and IBD activity. On the other hand, the effect of IBD in PSC prognosis is less established - higher rates of combined intrahepatic and extrahepatic involvement have been reported, although long-term outcomes of PSC do not seem to be changed[10,15,16].
PSC-IBD patients also present a greater risk of colorectal dysplasia and cancer, which supports the current recommendation of annual surveillance colonoscopy in this subset of patients. Although there are no specific recommendations, colectomy is suggested in case of indefinite or low-grade dysplasia, due to a high risk of colorectal cancer[10,17,18]. Similarly, prolonged duration of IBD was associated with an increased risk of cholangiocarcinoma, with a 33% higher risk per 10 years[19].
Small-duct PSC is very similar to large-duct PSC (close biochemical and histopathological findings) but presents a normal cholangiogram. The diagnosis requires liver biopsy and some patients may later develop the classic PSC (12%-23%)[6,20]. Almost all patients have IBD, mainly UC, and it affects females at greater rates than males. Small-duct PSC has a better prognosis and a negligible risk of cholangiocarcinoma[9,21].
IgG4-associated cholangitis, considered a secondary sclerosing cholangitis, is characterized by elevated serum levels of IgG4, dense infiltration of IgG4-positive plasma cells and lymphocytes, and fibrosis and obliterative phlebitis in the bile duct wall, being frequently associated with autoimmune pancreatitis[22]. The link between IgG4-associated cholangitis and IBD has been reported, but it is far less common than in PSC. Differential diagnosis is vital due to its responsiveness to corticosteroids[9].
PBC is an autoimmune liver disease that presents with chronic cholestasis and histological findings of nonsuppurative destructive cholangitis. The diagnosis is usually made by detection of anti-mitochondrial antibodies[23]. There are only few reports of PBC in patients with IBD, affecting mainly UC males and those at younger age[24,25].
Autoimmune hepatitis (AIH) is a rare and heterogeneous disease, affecting mostly middle-aged women. It is characterized by abnormal liver tests, hypergammaglobulinemia, circulating autoantibodies [mainly antinuclear antibody (ANA), smooth muscle antibody, and anti-liver-kidney muscle antibody], and interface hepatitis on liver histology[26].
A relationship between AIH and IBD has already been established in a study that demonstrated the presence of UC in 16% of patients with AIH[3,27].
More relevant is the fact that coexistent AIH and IBD can have a different course from either process alone - patients with UC and concurrent AIH are more likely to relapse, need proctocolectomy, have more extensive disease, and present right colon lesions[3,28]. Likewise, liver disease may also have distinct progression, developing at younger age, being more likely to be refractory to treatment, and determining higher risk of death and liver transplantation[3].
Patients with AIH may also present features of other immune-mediated liver diseases. In patients with UC, AIH-PSC is the most common overlap syndrome, described in up to 10% of PSC patients with UC[3,29]. However, cases of overlap syndrome in CD have also been described[30]. AIH-PSC is more common in children and young adults, PSC features usually develop later, and it has a better prognosis than PSC alone[31,32].
IBD is associated with a pro-inflammatory hypercoagulable state that increases the risk of portal and mesenteric vein thrombosis, with an estimated incidence of 1% to 2%[33]. Several risk factors have been identified: elevated platelet count, high fibrinogen, high factors V and VIII levels, and acquired prothrombotic factors - surgery, extent of colon disease, immobilization, inflammation, corticosteroids, and smoking[6,31]. Portal vein thrombosis has been more frequently described in UC patients after proctocolectomy and Budd-Chiari syndrome has an eight-fold risk during acute flares[31,34,35]. Anticoagulation is the mainstay of treatment, even in cases with previous gastrointestinal bleeding. Pharmacological thromboprophylaxis is recommended during hospitalizations and suggested in cases of active disease after hospital discharge and after surgery[2].
Granulomatous hepatitis is a rare complication of IBD, with a prevalence lower than 1%, mainly affecting CD patients[31]. Clinical suspicion is raised by elevated alkaline phosphatase and it is diagnosed by identification of granulomas in liver biopsy. It is mainly asymptomatic and follows a benign course, rarely requiring treatment (corticosteroids and immunosuppressants)[6,31]. It has also been associated with sulfasalazine use but differential diagnosis includes infections (tuberculosis) and malignancies[6,36].
Liver abscesses are a rare complication of IBD, but can also be its first manifestation (mainly in CD)[31]. They can result either from direct extension of an intra-abdominal abscess or from portal pyemia secondary to increased intestinal permeability[6]. They are often multiple and more frequently located in the right lobe, presenting with fever, abdominal pain, jaundice, diarrhea, and hepatosplenomegaly, as well as elevated inflammatory markers and alkaline phosphatase[31,37].
In contrast with liver abscesses in the general population, isolated Streptococcus species are the most common isolated pathogens[9,37].
The treatment of choice is prolonged intravenous antibiotics, with percutaneous drainage in case of a large abscess or refractory disease[31,38].
Cholelithiasis is a known complication of IBD, with CD patients presenting a two-fold risk of developing gallstones. On the contrary, UC is not associated with an increased risk of cholelithiasis[39]. The incidence of cholelithiasis in patients with ileal involvement or resection ranges from 13% to 34%. It is associated with malabsorption of bile salts, resulting in disruption and increased entero-hepatic circulation, which predisposes to formation of gallstones[40]. Many risk factors have been described, such as ileo-colonic localization, disease duration (> 15 years), extent of ileal resection (> 30 cm), longer hospital stay, higher number of hospitalizations (> 3), multiple total parenteral nutrition treatments, lifetime surgeries, and number of clinical recurrences (> 3)[39,40]. Complications of cholelithiasis may be an indication for cholecystectomy but systematic cholecystectomy following ileal resection is not recommended[31,40,41].
Hepatic amyloidosis is a rare complication of IBD, more frequent in CD (0.9%) than in UC (0.07%)[42]. There is a male predominance and prominent colonic involvement. It results from amyloid deposition due to chronic inflammation, presenting as asymptomatic disease or hepatomegaly. Treatment is focused on lowering systemic inflammation by controlling it in the gut[6,31,43].
NAFLD is one of the most common liver diseases with a prevalence of 25% worldwide[44]. IBD patients seem to have a higher susceptibility to NAFLD and its prevalence reaches almost 40%[45,46].
The main risk factor for NAFLD in the general population is metabolic syndrome but IBD patients develop NAFLD with fewer metabolic risk factors. In turn, IBD-associated factors that increase the risk of NAFLD include small bowel surgery, disease activity and duration, parenteral nutrition, and use of high doses of corticosteroids[47]. The influence of anti-TNF therapy on NAFLD risk is controversial: Some studies reported the development of biopsy-proven NAFLD in patients under anti-TNF therapy while others suggested a protective effect of these treatments[48,49].
There are no current guidelines for screening or assessing for NAFLD in patients with IBD.
Most drugs used for IBD treatment have been reported to cause acute and/or chronic liver injury, although the incidence of serious complications is low. The mechanism of hepatotoxicity is complex and multifactorial; thus, causality may be difficult to establish[31,50,51].
Sulfasalazine and 5-ASA compounds are used in mild-to-moderate UC. Sulfasalazine was the first aminosalicylate used for the treatment of IBD and can induce liver injury by several mechanisms[31]: (1) Hypersensitivity reaction that usually occurs within 2 mo of therapy initiation. A study revealed an incidence of 0.4% and symptoms include fever, rash, hepatomegaly, lymphadenophaty, atypical lymphocytosis, and eosinophilia. In most cases, stopping the medication is sufficient. In more severe cases, antipyretics, antihistamines, or corticosteroids may be considered[51-53]; (2) Sulfasalazine-induced granulomatous hepatitis, with elevated alkaline phosphatase and bilirubin and noncaseating granulomas on histology[51]; and (3) Cholestatic liver injury and, in rare cases, development of vanishing bile duct syndrome[54]. Mesalamine (5-ASA) is also associated with liver enzyme abnormalities in up to 2% of patients but, in most cases, it is not clinical significant[55].
Azathioprine and its principal metabolite, 6-mercaptopurine, are immunomodulators used for maintenance or achievement of remission in patients with IBD.
Azathioprine is metabolized in mercaptopurine and then thiopurine methyltransferase (TPMT) will be responsible for its conversion to 6-methylmercaptopurine. Genetic polymorphisms of TPMT determine the level of enzyme activity and should be routinely tested before initiation of these medications. In cases of absent or low activity, thiopurines should be avoided due to high risk of toxicity, whereas in intermediate activity, a dose reduction should be applied[51,56].
The annual incidence of hepatotoxicity can reach 13% in prospective studies, although most resolve spontaneously or with dose adjustment, and need for discontinuation is rare (< 4%)[31,50,57].
Most cases of liver injury result in transient elevations of AST and ALT, but there are different types of hepatotoxicity[31,51,58-61]: (1) Allergic reaction, usually within the first month of treatment, which is not dose-dependent and should prompt immediate halt; (2) Non-allergic reactions, mainly associated with TPMT activity and dose-dependent, that can cause infections, bone marrow suppression, or hepatitis. Allopurinol has been suggested to alter metabolite levels and reduce hepatotoxicity; (3) Cholestatic liver injury, usually within the first 3 mo of therapy, requiring discontinuation; and (4) Hepatic endothelial injury that may present within 3 mo up to more than 4 years after therapy initiation. It can include sinusoidal dilatation, sinusoidal obstruction syndrome, peliosis, or nodular regenerative hyperplasia (NRH). NRH occurs due to endothelial injury and/or obliterative portal venopathy, with an estimated incidence of 0.8%, and can cause non-cirrhotic portal hypertension. It is dose-dependent and should prompt drug discontinuation.
Liver tests should be checked before starting thiopurines and repeated at weeks 2, 4 and 8, and every 3 mo thereafter. In the absence of previous liver disease, the prognosis of thiopurines-induced liver injury is good[51,56].
Methotrexate is an immunosupressive and anti-proliferative agent used in the event of adverse effects or lack of efficacy of thiopurines for maintenance of clinical remission in CD[6].
Myelossupression and liver toxicity are the most common side effects, with presence of abnormal aminotransferases levels in 24% of cases[62]. This liver injury is mainly associated with alcohol consumption, while folic acid supplementation seems to be protective[6].
There are also some reports of liver fibrosis and cirrhosis development, despite being more common in rheumatologic conditions, due to higher weekly dose use[6].
Most patients with liver injury due to methotrexate will have their liver function tests back to normal while on therapy and dose adjustment or discontinuation is rarely needed[62]. Regular liver function tests are recommended but liver biopsy is not routinely performed. Transient elastography is emerging as an interesting non-invasive tool to follow these patients[31,63].
Infliximab and adalimumab are anti-TNF agents used for induction and maintenance of remission in moderate to severe CD and UC.
The main adverse effects are myelosuppression, opportunistic infections (namely tuberculosis), neurological diseases, and liver injury. There are reports of ALT increase in 39% of patients, although most (76%) of them were self-limited[64].
An auto-immune pattern of liver injury induced by anti-TNF agents with serological evidence (ANAs) has also been reported, which generally has a good prognosis as soon as the drug is stopped[51,65]. Cases of cholestatic liver injury and acute liver failure requiring liver transplant are very rare[66].
Liver functions tests should be checked in all patients before treatment institution[51].
Vedolizumab is an α4β7 integrin inhibitor used in moderate to severe CD and UC.
In the premarketing trials, significant (≥ 3 ULN) elevations occurred in less than 2% of patients, similarly to those in the placebo arm[31]. Cholestatic and hepatocellular liver injuries have already been described in the post marketing analysis, which improved after drug discontinuation[67].
Although less studied, there are several natural compounds that are tested for the treatment of IBD.
Curcumin, the main active compound of the plant Curcuma longa, has been shown to have anti-inflammatory, anti-oxidant, and antibacterial activities[68]. Kesharwani et al[69] showed that curcumin might have an important role in inhibiting IBD severity and colitis associated cancer. In addition, it has a good safety profile and is extremely well tolerated, besides some reports of its hepatoprotective effect[68,70-72].
Previous studies have suggested a higher prevalence of hepatitis B virus (HBV) and hepatitis C virus (HCV) infections in patients with IBD, due to blood transfusions and/or endoscopic procedures, which has not been demonstrated in more recent data[40,73,74].
HBV reactivation is one of the main concerns during IBD treatment, given the risk of fulminant hepatic failure and death[75]. Reactivation of HBV has already been described with high dose corticosteroids, thiopurines, and infliximab, though almost exclusively with concomitant use of other immunosuppressants[76-80]. Therefore, it is generally accepted that all patients with IBD should be screened for HBV exposure, preferably at diagnosis, which includes HBsAg and anti-HBs and anti-HBc antibodies[76,81]. According to the European Crohn's and Colitis Organisation (ECCO), IBD patients should follow these preventive measures[81]: Seronegative patients (HBsAg and anti-HBc negative) should be vaccinated and assessed for subsequent serological immune status; seropositive patients (HBsAg positive) should receive prophylactic treatment with nucleotide/nucleoside analogues for the time of treatment and at least 12 mo after stopping immunosuppressants; and HBsAg negative and anti-HBc positive patients should be monitored by HBV DNA quantification every 2-3 mo, since risk of HBV occult infection reactivation is low.
Regarding HCV infection, immunosuppressive therapy does not seem to have a detrimental effect on its course. Nevertheless, there are some reports of worsening liver function in the setting of concomitant HBV or HIV infection. Thus, the latest ECCO guidelines recommend systematic screening for HCV infection[81].
Hepatobiliary disease is one of the most common extra-intestinal manifestations in IBD patients, ranging from asymptomatic mild elevations of liver chemistries to life-threatening conditions.
Monitoring liver tests at regular intervals is crucial and must be routinely part of IBD management.
Abnormal liver tests in IBD patients may appear in the context of drug induced liver injury, common and easy to manage diseases such as NAFLD or cholelithiasis, as well as chronic and more complex diseases such as PSC or auto-immune hepatitis. As so, it should always prompt a structured and complete work-up and even benefit from a multidisciplinary approach, in order to improve patient management and outcomes.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Portugal
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Maharshi S, Valiveti CK S-Editor: Liu M L-Editor: Wang TQ P-Editor: Liu M
| 1. | Seyedian SS, Nokhostin F, Malamir MD. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J Med Life. 2019;12:113-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 417] [Article Influence: 69.5] [Reference Citation Analysis (113)] |
| 2. | Harbord M, Annese V, Vavricka SR, Allez M, Barreiro-de Acosta M, Boberg KM, Burisch J, De Vos M, De Vries AM, Dick AD, Juillerat P, Karlsen TH, Koutroubakis I, Lakatos PL, Orchard T, Papay P, Raine T, Reinshagen M, Thaci D, Tilg H, Carbonnel F; European Crohn’s and Colitis Organisation. The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease. J Crohns Colitis. 2016;10:239-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 546] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 3. | DeFilippis EM, Kumar S. Clinical Presentation and Outcomes of Autoimmune Hepatitis in Inflammatory Bowel Disease. Dig Dis Sci. 2015;60:2873-2880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (3)] |
| 4. | Yarur AJ, Czul F, Levy C. Hepatobiliary manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:1655-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Yaccob A, Mari A. Practical clinical approach to the evaluation of hepatobiliary disorders in inflammatory bowel disease. Frontline Gastroenterol. 2019;10:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Rojas-Feria M, Castro M, Suárez E, Ampuero J, Romero-Gómez M. Hepatobiliary manifestations in inflammatory bowel disease: the gut, the drugs and the liver. World J Gastroenterol. 2013;19:7327-7340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 84] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 7. | Silva J, Brito BS, Silva INN, Nóbrega VG, da Silva MCSM, Gomes HDN, Fortes FM, Pimentel AM, Mota J, Almeida N, Surlo VC, Lyra A, Rocha R, Santana GO. Frequency of Hepatobiliary Manifestations and Concomitant Liver Disease in Inflammatory Bowel Disease Patients. Biomed Res Int. 2019;2019:7604939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | European Society of Gastrointestinal Endoscopy; European Association for the Study of the Liver. Role of endoscopy in primary sclerosing cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the Liver (EASL) Clinical Guideline. J Hepatol. 2017;66:1265-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 9. | Kummen M, Schrumpf E, Boberg KM. Liver abnormalities in bowel diseases. Best Pract Res Clin Gastroenterol. 2013;27:531-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Palmela C, Peerani F, Castaneda D, Torres J, Itzkowitz SH. Inflammatory Bowel Disease and Primary Sclerosing Cholangitis: A Review of the Phenotype and Associated Specific Features. Gut Liver. 2018;12:17-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 11. | Loftus EV Jr, Harewood GC, Loftus CG, Tremaine WJ, Harmsen WS, Zinsmeister AR, Jewell DA, Sandborn WJ. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 518] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 12. | Nakazawa T, Naitoh I, Hayashi K, Sano H, Miyabe K, Shimizu S, Joh T. Inflammatory bowel disease of primary sclerosing cholangitis: a distinct entity? World J Gastroenterol. 2014;20:3245-3254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | de Vries AB, Janse M, Blokzijl H, Weersma RK. Distinctive inflammatory bowel disease phenotype in primary sclerosing cholangitis. World J Gastroenterol. 2015;21:1956-1971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 120] [Cited by in RCA: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 14. | Marelli L, Xirouchakis E, Kalambokis G, Cholongitas E, Hamilton MI, Burroughs AK. Does the severity of primary sclerosing cholangitis influence the clinical course of associated ulcerative colitis? Gut. 2011;60:1224-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Rabinovitz M, Gavaler JS, Schade RR, Dindzans VJ, Chien MC, Van Thiel DH. Does primary sclerosing cholangitis occurring in association with inflammatory bowel disease differ from that occurring in the absence of inflammatory bowel disease? Hepatology. 1990;11:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 71] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Yanai H, Matalon S, Rosenblatt A, Awadie H, Berdichevski T, Snir Y, Kopylov U, Katz L, Stein A, Mlynarsky L, Tulchinsky H, Konikoff FM, Horin SB, Braun M, Ben-Ari Z, Chowers Y, Baruch Y, Shibolet O, Dotan I. Prognosis of primary sclerosing cholangitis in israel is independent of coexisting inflammatory bowel Disease. J Crohns Colitis. 2015;9:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Zheng HH, Jiang XL. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: a meta-analysis of 16 observational studies. Eur J Gastroenterol Hepatol. 2016;28:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 18. | Farraye FA, Odze RD, Eaden J, Itzkowitz SH, McCabe RP, Dassopoulos T, Lewis JD, Ullman TA, James T 3rd, McLeod R, Burgart LJ, Allen J, Brill JV; AGA Institute Medical Position Panel on Diagnosis and Management of Colorectal Neoplasia in Inflammatory Bowel Disease. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:738-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 380] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 19. | Gulamhusein AF, Eaton JE, Tabibian JH, Atkinson EJ, Juran BD, Lazaridis KN. Duration of Inflammatory Bowel Disease Is Associated With Increased Risk of Cholangiocarcinoma in Patients With Primary Sclerosing Cholangitis and IBD. Am J Gastroenterol. 2016;111:705-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 20. | Lindor KD, Kowdley KV, Harrison ME; American College of Gastroenterology. ACG Clinical Guideline: Primary Sclerosing Cholangitis. Am J Gastroenterol. 2015;110:646-59; quiz 660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 338] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 21. | Karlsen TH, Boberg KM. Update on primary sclerosing cholangitis. J Hepatol. 2013;59:571-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Nakazawa T, Naitoh I, Hayashi K, Okumura F, Miyabe K, Yoshida M, Yamashita H, Ohara H, Joh T. Diagnostic criteria for IgG4-related sclerosing cholangitis based on cholangiographic classification. J Gastroenterol. 2012;47:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet. 2015;386:1565-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 398] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 24. | Xiao WB, Liu YL. Primary biliary cirrhosis and ulcerative colitis: a case report and review of literature. World J Gastroenterol. 2003;9:878-880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 25. | Liberal R, Gaspar R, Lopes S, Macedo G. Primary biliary cholangitis in patients with inflammatory bowel disease. Clin Res Hepatol Gastroenterol. 2020;44:e5-e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol. 2015;63:971-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 848] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 27. | Saich R, Chapman R. Primary sclerosing cholangitis, autoimmune hepatitis and overlap syndromes in inflammatory bowel disease. World J Gastroenterol. 2008;14:331-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 28. | Ordonez F, Lacaille F, Canioni D, Talbotec C, Fournet JC, Cerf-Bensussan N, Goulet O, Schmitz J, Ruemmele FM. Pediatric ulcerative colitis associated with autoimmune diseases: a distinct form of inflammatory bowel disease? Inflamm Bowel Dis. 2012;18:1809-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 29. | Agrawal M, Kim ES, Colombel JF. JAK Inhibitors Safety in Ulcerative Colitis: Practical Implications. J Crohns Colitis. 2020;14:S755-S760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 30. | Malik TA, Gutierrez AM, McGuire B, Zarzour JG, Mukhtar F, Bloomer J. Autoimmune hepatitis-primary sclerosing cholangitis overlap syndrome complicated by Crohn's disease. Digestion. 2010;82:24-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Restellini S, Chazouillères O, Frossard JL. Hepatic manifestations of inflammatory bowel diseases. Liver Int. 2017;37:475-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 32. | Czaja AJ. Diagnosis and management of the overlap syndromes of autoimmune hepatitis. Can J Gastroenterol. 2013;27:417-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Sinagra E, Aragona E, Romano C, Maisano S, Orlando A, Virdone R, Tesè L, Modesto I, Criscuoli V, Cottone M. The role of portal vein thrombosis in the clinical course of inflammatory bowel diseases: report on three cases and review of the literature. Gastroenterol Res Pract. 2012;2012:916428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Remzi FH, Fazio VW, Oncel M, Baker ME, Church JM, Ooi BS, Connor JT, Preen M, Einstein D. Portal vein thrombi after restorative proctocolectomy. Surgery. 2002;132:655-61; discussion 661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Spina L, Saibeni S, Battaglioli T, Peyvandi F, de Franchis R, Vecchi M. Thrombosis in inflammatory bowel diseases: role of inherited thrombophilia. Am J Gastroenterol. 2005;100:2036-2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Braun M, Fraser GM, Kunin M, Salamon F, Tur-Kaspa R. Mesalamine-induced granulomatous hepatitis. Am J Gastroenterol. 1999;94:1973-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Margalit M, Elinav H, Ilan Y, Shalit M. Liver abscess in inflammatory bowel disease: report of two cases and review of the literature. J Gastroenterol Hepatol. 2004;19:1338-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Albuquerque A, Magro F, Rodrigues S, Lopes S, Pereira P, Melo RB, Madureira M, Macedo G. Liver abscess of the caudate lobe due to Staphylococcus aureus in an ulcerative colitis patient: First case report. J Crohns Colitis. 2011;5:360-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Parente F, Pastore L, Bargiggia S, Cucino C, Greco S, Molteni M, Ardizzone S, Porro GB, Sampietro GM, Giorgi R, Moretti R, Gallus S. Incidence and risk factors for gallstones in patients with inflammatory bowel disease: a large case-control study. Hepatology. 2007;45:1267-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 40. | Fousekis FS, Theopistos VI, Katsanos KH, Tsianos EV, Christodoulou DK. Hepatobiliary Manifestations and Complications in Inflammatory Bowel Disease: A Review. Gastroenterology Res. 2018;11:83-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 41. | Chew SS, Ngo TQ, Douglas PR, Newstead GL, Selby W, Solomon MJ. Cholecystectomy in patients with Crohn's ileitis. Dis Colon Rectum. 2003;46:1484-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Greenstein AJ, Sachar DB, Panday AK, Dikman SH, Meyers S, Heimann T, Gumaste V, Werther JL, Janowitz HD. Amyloidosis and inflammatory bowel disease. A 50-year experience with 25 patients. Medicine (Baltimore). 1992;71:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 91] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Kato T, Komori A, Bae SK, Migita K, Ito M, Motoyoshi Y, Abiru S, Ishibashi H. Concurrent systemic AA amyloidosis can discriminate primary sclerosing cholangitis from IgG4-associated cholangitis. World J Gastroenterol. 2012;18:192-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Hoffmann P, Jung V, Behnisch R, Gauss A. Prevalence and risk factors of nonalcoholic fatty liver disease in patients with inflammatory bowel diseases: A cross-sectional and longitudinal analysis. World J Gastroenterol. 2020;26:7367-7381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 45. | Likhitsup A, Dundulis J, Ansari S, El-Halawany H, Michelson R, Hutton C, Kennedy K, Helzberg JH, Chhabra R. Prevalence of non-alcoholic fatty liver disease on computed tomography in patients with inflammatory bowel disease visiting an emergency department. Ann Gastroenterol. 2019;32:283-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Gaidos JKJ, Fuchs M. Increased Prevalence of NAFLD in IBD Patients. Dig Dis Sci. 2017;62:1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Bessissow T, Le NH, Rollet K, Afif W, Bitton A, Sebastiani G. Incidence and Predictors of Nonalcoholic Fatty Liver Disease by Serum Biomarkers in Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22:1937-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (1)] |
| 48. | Chao CY, Battat R, Al Khoury A, Restellini S, Sebastiani G, Bessissow T. Co-existence of non-alcoholic fatty liver disease and inflammatory bowel disease: A review article. World J Gastroenterol. 2016;22:7727-7734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (34)] |
| 49. | Barbuio R, Milanski M, Bertolo MB, Saad MJ, Velloso LA. Infliximab reverses steatosis and improves insulin signal transduction in liver of rats fed a high-fat diet. J Endocrinol. 2007;194:539-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 50. | Gisbert JP, Luna M, González-Lama Y, Pousa ID, Velasco M, Moreno-Otero R, Maté J. Liver injury in inflammatory bowel disease: long-term follow-up study of 786 patients. Inflamm Bowel Dis. 2007;13:1106-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 51. | Shamberg L, Vaziri H. Hepatotoxicity of Inflammatory Bowel Disease Medications. J Clin Gastroenterol. 2018;52:674-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Bashir RM, Lewis JH. Hepatotoxicity of drugs used in the treatment of gastrointestinal disorders. Gastroenterol Clin North Am. 1995;24:937-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Jobanputra P, Amarasena R, Maggs F, Homer D, Bowman S, Rankin E, Filer A, Raza K, Jubb R. Hepatotoxicity associated with sulfasalazine in inflammatory arthritis: A case series from a local surveillance of serious adverse events. BMC Musculoskelet Disord. 2008;9:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 54. | Khokhar OS, Lewis JH. Hepatotoxicity of agents used in the management of inflammatory bowel disease. Dig Dis. 2010;28:508-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 55. | Loftus EV Jr, Kane SV, Bjorkman D. Systematic review: short-term adverse effects of 5-aminosalicylic acid agents in the treatment of ulcerative colitis. Aliment Pharmacol Ther. 2004;19:179-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 56. | Kopylov U, Ben-Horin S, Seidman E. Therapeutic drug monitoring in inflammatory bowel disease. Ann Gastroenterol. 2014;27:304-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (36)] |
| 57. | Gisbert JP, González-Lama Y, Maté J. Thiopurine-induced liver injury in patients with inflammatory bowel disease: a systematic review. Am J Gastroenterol. 2007;102:1518-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 134] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 58. | Björnsson ES, Gu J, Kleiner DE, Chalasani N, Hayashi PH, Hoofnagle JH; DILIN Investigators. Azathioprine and 6-Mercaptopurine-induced Liver Injury: Clinical Features and Outcomes. J Clin Gastroenterol. 2017;51:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 59. | Morris JM, Oien KA, McMahon M, Forrest EH, Morris J, Stanley AJ, Campbell S. Nodular regenerative hyperplasia of the liver: survival and associated features in a UK case series. Eur J Gastroenterol Hepatol. 2010;22:1001-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 60. | Vernier-Massouille G, Cosnes J, Lemann M, Marteau P, Reinisch W, Laharie D, Cadiot G, Bouhnik Y, De Vos M, Boureille A, Duclos B, Seksik P, Mary JY, Colombel JF. Nodular regenerative hyperplasia in patients with inflammatory bowel disease treated with azathioprine. Gut. 2007;56:1404-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 61. | Calabrese E, Hanauer SB. Assessment of non-cirrhotic portal hypertension associated with thiopurine therapy in inflammatory bowel disease. J Crohns Colitis. 2011;5:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 62. | Fournier MR, Klein J, Minuk GY, Bernstein CN. Changes in liver biochemistry during methotrexate use for inflammatory bowel disease. Am J Gastroenterol. 2010;105:1620-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | Laharie D, Zerbib F, Adhoute X, Boué-Lahorgue X, Foucher J, Castéra L, Rullier A, Bertet J, Couzigou P, Amouretti M, de Lédinghen V. Diagnosis of liver fibrosis by transient elastography (FibroScan) and non-invasive methods in Crohn's disease patients treated with methotrexate. Aliment Pharmacol Ther. 2006;23:1621-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 64. | Rossi RE, Parisi I, Despott EJ, Burroughs AK, O'Beirne J, Conte D, Hamilton MI, Murray CD. Anti-tumour necrosis factor agent and liver injury: literature review, recommendations for management. World J Gastroenterol. 2014;20:17352-17359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 65. | Ghabril M, Bonkovsky HL, Kum C, Davern T, Hayashi PH, Kleiner DE, Serrano J, Rochon J, Fontana RJ, Bonacini M; US Drug-Induced Liver Injury Network. Liver injury from tumor necrosis factor-α antagonists: analysis of thirty-four cases. Clin Gastroenterol Hepatol 2013; 11: 558-564. e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 66. | Tobon GJ, Cañas C, Jaller JJ, Restrepo JC, Anaya JM. Serious liver disease induced by infliximab. Clin Rheumatol. 2007;26:578-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 67. | Stine JG, Wang J, Behm BW. Chronic Cholestatic Liver Injury Attributable to Vedolizumab. J Clin Transl Hepatol. 2016;4:277-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 68. | Rivera-Espinoza Y, Muriel P. Pharmacological actions of curcumin in liver diseases or damage. Liver Int. 2009;29:1457-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 69. | Kesharwani SS, Ahmad R, Bakkari MA, Rajput MKS, Dachineni R, Valiveti CK, Kapur S, Jayarama Bhat G, Singh AB, Tummala H. Site-directed non-covalent polymer-drug complexes for inflammatory bowel disease (IBD): Formulation development, characterization and pharmacological evaluation. J Control Release. 2018;290:165-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 70. | Kyung EJ, Kim HB, Hwang ES, Lee S, Choi BK, Kim JW, Kim HJ, Lim SM, Kwon OI, Woo EJ. Evaluation of Hepatoprotective Effect of Curcumin on Liver Cirrhosis Using a Combination of Biochemical Analysis and Magnetic Resonance-Based Electrical Conductivity Imaging. Mediators Inflamm. 2018;2018:5491797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 71. | Granados-Castro LF, Rodríguez-Rangel DS, Fernández-Rojas B, León-Contreras JC, Hernández-Pando R, Medina-Campos ON, Eugenio-Pérez D, Pinzón E, Pedraza-Chaverri J. Curcumin prevents paracetamol-induced liver mitochondrial alterations. J Pharm Pharmacol. 2016;68:245-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 72. | Wang X, Chang X, Zhan H, Zhang Q, Li C, Gao Q, Yang M, Luo Z, Li S, Sun Y. Curcumin and Baicalin ameliorate ethanol-induced liver oxidative damage via the Nrf2/HO-1 pathway. J Food Biochem. 2020;e13425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 73. | Biancone L, Pavia M, Del Vecchio Blanco G, D'Incà R, Castiglione F, De Nigris F, Doldo P, Cosco F, Vavassori P, Bresci GP, Arrigoni A, Cadau G, Monteleone I, Rispo A, Fries W, Mallardi B, Sturniolo GC, Pallone F; Italian Group for the Study of the Colon and Rectum (GISC). Hepatitis B and C virus infection in Crohn's disease. Inflamm Bowel Dis. 2001;7:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 74. | Mahfouz M, Martin P, Carrion AF. Hepatic Complications of Inflammatory Bowel Disease. Clin Liver Dis. 2019;23:191-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | Hou JK, Velayos F, Terrault N, Mahadevan U. Viral hepatitis and inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:925-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 76. | Gisbert JP, Chaparro M, Esteve M. Review article: prevention and management of hepatitis B and C infection in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:619-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 77. | Cheng AL, Hsiung CA, Su IJ, Chen PJ, Chang MC, Tsao CJ, Kao WY, Uen WC, Hsu CH, Tien HF, Chao TY, Chen LT, Whang-Peng J; Lymphoma Committee of Taiwan Cooperative Oncology Group. Steroid-free chemotherapy decreases risk of hepatitis B virus (HBV) reactivation in HBV-carriers with lymphoma. Hepatology. 2003;37:1320-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 229] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 78. | Sacco R, Bertini M, Bresci G, Romano A, Altomare E, Capria A. Entecavir for hepatitis B virus flare treatment in patients with Crohn's disease. Hepatogastroenterology. 2010;57:242-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 79. | Ostuni P, Botsios C, Punzi L, Sfriso P, Todesco S. Hepatitis B reactivation in a chronic hepatitis B surface antigen carrier with rheumatoid arthritis treated with infliximab and low dose methotrexate. Ann Rheum Dis. 2003;62:686-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 157] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 80. | Michel M, Duvoux C, Hezode C, Cherqui D. Fulminant hepatitis after infliximab in a patient with hepatitis B virus treated for an adult onset still's disease. J Rheumatol. 2003;30:1624-1625. [PubMed] |
| 81. | Rahier JF, Magro F, Abreu C, Armuzzi A, Ben-Horin S, Chowers Y, Cottone M, de Ridder L, Doherty G, Ehehalt R, Esteve M, Katsanos K, Lees CW, Macmahon E, Moreels T, Reinisch W, Tilg H, Tremblay L, Veereman-Wauters G, Viget N, Yazdanpanah Y, Eliakim R, Colombel JF; European Crohn's and Colitis Organisation (ECCO). Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 746] [Article Influence: 67.8] [Reference Citation Analysis (0)] |