Published online Dec 27, 2021. doi: 10.4254/wjh.v13.i12.1850
Peer-review started: May 6, 2021
First decision: June 15, 2021
Revised: July 20, 2021
Accepted: November 15, 2021
Article in press: November 15, 2021
Published online: December 27, 2021
Processing time: 234 Days and 11.2 Hours
The outbreak of coronavirus disease 2019 (COVID-19) is a global pandemic. Many clinical trials have been performed to investigate potential treatments or vaccines for this disease to reduce the high morbidity and mortality. The drugs of higher interest include umifenovir, bromhexine, remdesivir, lopinavir/ritonavir, steroid, tocilizumab, interferon alpha or beta, ribavirin, fivapiravir, nitazoxanide, ivermectin, molnupiravir, hydroxychloroquine/chloroquine alone or in com
Core Tip: Gastrointestinal symptoms such as anorexia, dyspepsia, nausea, vomiting, diarrhea and abdominal pain are common among patients with coronavirus disease 2019 (COVID-19). Liver injury can be a result of systemic inflammation or cytokine storm, or due to the adverse drug reactions of different treatments. Regular monitoring of liver function is recommended. Patients with inflammatory bowel disease, chronic liver diseases or liver transplant recipients are encouraged to receive the COVID-19 vaccine, and the benefits will outweigh the risks in the vast majority of patients.
- Citation: Law MF, Ho R, Law KWT, Cheung CKM. Gastrointestinal and hepatic side effects of potential treatment for COVID-19 and vaccination in patients with chronic liver diseases. World J Hepatol 2021; 13(12): 1850-1874
- URL: https://www.wjgnet.com/1948-5182/full/v13/i12/1850.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i12.1850
The outbreak of coronavirus disease 2019 (COVID-19) is a global pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It is a very contagious virus and has infected millions of people worldwide causing numerous deaths. There are many clinical trials investigating potential treatments or vaccines for this disease to reduce the high morbidity and mortality.
Drugs with potential utility include remdesivir, lopinavir/ritonavir (LPV/r), steroids, tocilizumab, interferon alpha or beta, ribavirin, hydroxychloroquine/chloro
The common GI symptoms in patients with COVID-19 include anorexia, dyspepsia, nausea, vomiting, diarrhea and abdominal pain[3-11]. The pooled prevalence of GI symptoms is 17.6% according to a recent meta-analysis[12]. The hepatic manifestations of COVID-19 include elevated liver enzymes and less commonly elevated bilirubin levels. The incidence of liver injury ranges from 14.8% to 53% as indicated by abnormal alanine transaminase (ALT)/aspartate aminotransferase (AST) levels with slight elevation of bilirubin levels[2,7]. Patients with liver dysfunction also tend to have severe COVID-19, and the liver injury in these patients can be a result of systemic inflammation or cytokine storm, or due to the adverse drug reactions in severe COVID-19 patients who have been receiving different treatments. While cholangiocytes may contribute to hepatic regeneration and immune response, it has been suggested that bile duct epithelial cells play a greater role in hepatic injury due to SARS-CoV-2 infection than cholangiocytes do[13]. The aim of the current article is to review the GI and hepatic side effects associated with the potential agents for the treatment of COVID-19, focusing particularly on redemsivir, LPV/r and steroids which have shown beneficial effects in the treatment of COVID-19. COVID-19 vaccines are now available in many countries and an increasing number of people are getting vaccinated. We will discuss their side effects and the current views on whether patients with chronic liver diseases (CLD), liver transplantation or inflammatory bowel disease (IBD) should receive the vaccine.
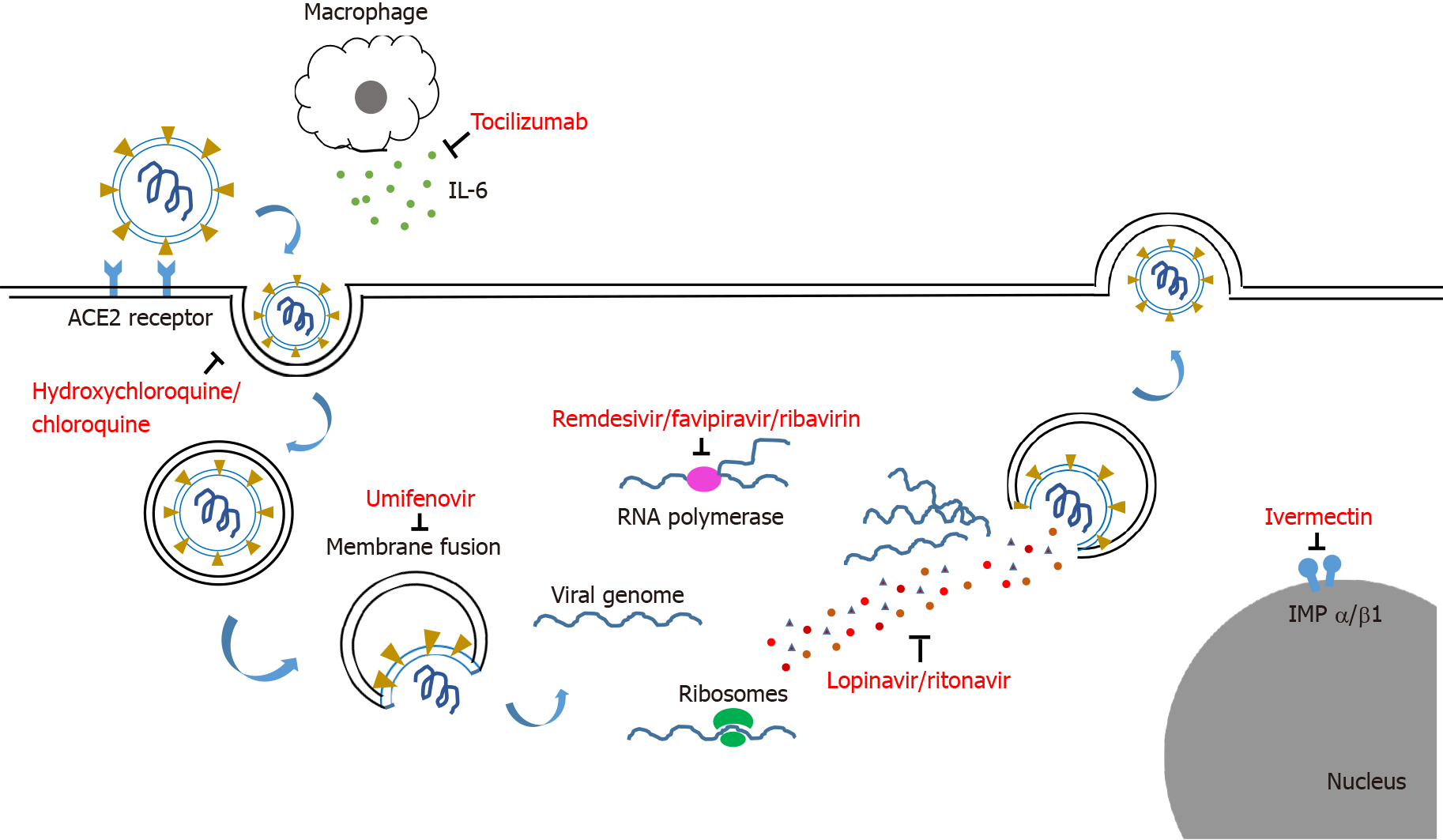
The agents used for COVID-19 treatment can be classified according to the type of agents, such as antiviral, antiparasitic, antibacterial and immunomodulatory agents, or according to the site of action on the SARS-CoV-2 virus such as blocking the entry of virus, inhibition of viral replication and anti-inflammatory effect.
Viral entry can be blocked by proteins, peptides, or small molecule compounds that bind to the viral S protein, thereby preventing the virus from interacting with the host membrane. Examples are umifenovir and bromhexine[14].
Inhibitors of viral nucleic acid synthesis are the best represented class of antiviral drugs that suppress viral replication in host cells[15]. Examples include lopinavir-ritonavir, remdesivir, ribavirin, chloroquine or hydroxychloroquine, favipiravir, nitazoxanide, ivermectin and molnupiravir.
The RNA-dependent RNA polymerase (RdRp) is found in the core of the coronavirus replication machinery, nsp12 protein, and has an important role in the viral life cycle[16]. Inhibition of RdRp is a possible target for therapeutic interventions. Examples of RdRP inhibitors include favipiravir and ribavirin.
Excessive inflammatory responses and cytokine release are found in patients with severe cases of COVID-19. This mechanism contributes to the worsening of the disease and stimulates lung and other systemic injuries. The early modulation of these responses can reduce the risk of acute respiratory distress[17]. Examples of agents that target the inflammatory response include steroids, tocilizumab [an anti-interleukin (IL)-6 monoclonal antibody] and baricitinib. The mechanisms of agents used for the treatment of COVID-19 are shown in Figure 1.
Umifenovir is used for the treatment of some enveloped and non-enveloped viral infection. It can also effectively block SARS-CoV-2 entry into cells and inhibits post-entry stages of infection[18]. The efficacy of the drug was assessed in an open-label randomized controlled trial (RCT). One hundred patients were randomly assigned to two treatment groups receiving either hydroxychloroquine followed by LPV/r or hydroxychloroquine followed by umifenovir[19]. The primary outcome was hospitalization duration and clinical improvement 7 d after admission.
Umifenovir significantly improved clinical and laboratory parameters including peripheral oxygen saturation, intensive care unit (ICU) admission rate, duration of hospitalization, white blood cell (WBC), and erythrocyte sedimentation rate when compared with LPV/r. The duration of hospitalization in the umifenovir group was significantly shorter than in the LPV/r arm (7.2 d vs 9.6 d; P = 0.02)[19].
Nausea, vomiting and liver function test (LFT) derangements are the major GI and hepatic abnormalities that can occur in patients receiving umifenovir. Clinicians should use the drug with caution in those patients with hepatic impairment.
SARS-CoV-2 invades the human body through the angiotensin-converting enzyme 2 (ACE-2)/transmembrane protease serine 2 (TMPRSS2). In addition to host cell entry, TMPRSS2 is involved in the maturation and release of the virus, which ultimately increase the viral infectivity[20]. Therefore, a possible useful therapeutic approach for COVID-19 is the inhibition of TMPRSS2[21].
Bromhexine has strong inhibitory effect on TMPRSS2 and can be used to block pulmonary virus infection[22]. Therefore, it may exert a protective effect against COVID-19-induced acute lung injury. The effect and safety of bromhexine was assessed in patients with mild or moderate COVID-19 who were randomly assigned to a bromhexine group or a control group at a 2:1 ratio[22]. The primary end points were the time to clinical recovery and the rate of deterioration after initiation of medi
There were no significant differences in the outcomes between the two treatment groups. The side effects include LFT derangement (38.9%), gingivitis (11.1%), insomnia (11.1%), headache (5.6%), and elevated WBCs in urine (5.6%). However, all side effects were mild and no patient stopped the treatment because of the adverse effects[22].
Another randomized, open-label clinical trial study involving 78 patients was performed to assess the efficacy of bromhexine. Patients were randomized to the bromhexine group or the control group. The primary outcomes were the rate of ICU admissions, intubation and then mechanical ventilation, and 28-d mortality[23]. When compared with the standard treatment group, the bromhexine-treated group showed a significant reduction in ICU admissions (5.1% vs 28.2%, P = 0.006), intubation (2.6% vs 23.1%, P = 0.007) and death (0 vs 5, P = 0.027)[23].
LPV/r is a co-formulation of two structurally related protease inhibitor (PI) antiretroviral agents widely used to treat HIV infections[24]. Ritonavir substantially increases the half-life of lopinavir by inhibiting cytochrome P450 (CYP) isoenzyme 3A4[25]. PIs prevent cleavage of gag and gag-pol protein precursors in infected cells, arresting maturation and inhibiting the formation of infectious virions, thereby preventing subsequent waves of infection[26].
Lopinavir demonstrated in vitro inhibitory activity against SARS-CoV and Middle East respiratory syndrome coronavirus[27-29]. Addition of LPV/r to ribavirin in treating SARS patients showed a reduction of adverse outcomes [death or development of acute respiratory distress syndrome (ARDS) requiring intensive care] compared to ribavirin alone[30]. Conflicting results of published data have stirred controversy concerning the use of LPV/r in COVID-19 patients. Cao et al[31] con
GI adverse events were common in patients receiving LPV/r. The most common GI adverse event in patients receiving LPV/r was diarrhea (occurring in 20% of patients); others included nausea, vomiting abdominal pain and gastroenteritis[35]. In the study by Cao et al[31], 14% of patients were unable to complete the full 14-d course of LPV/r because of GI adverse events (Table 1). In the study by Li et al[32], one patient with
| Ref. | Dosage | n | Age, yr | Gender, male (%) | Incidence of adverse events in treatment vs control arm, n (%) | ||||||
| Diarrhea | Vomiting | Abdominal pain | Constipation | Increased AST | Increased ALT | Drug termination due to AE | |||||
| Lopinavir/ritonavir | |||||||||||
| Cao et al[31] | 400/100 mg twice a day for 14 d | Tx 99; control 100 | Median 58 (IQR 49-68) | 120 (60.3) | 4 (4.2) vs 0 | 6 (6.3) vs 0 | 4 (4.2) vs 2 (2.1) | NA | 2 (2.1) vs 5 (5.1) | 1 (1.1) vs 4 (4.0) | 14% |
| Li et al[32] | 200/50 mg, twice a day for 7-14 d | Tx 34; control 17 | mean ± SD, 49.4 ± 14.7 | 40 (46.5) | 9/34 (26.5) vs 0 | NA | NA | NA | NA | 1/21 (4.8) vs 0 | 1/34 (2.94) |
| Remdesivir | |||||||||||
| Beigel et al[50] | 200 mg daily on day 1, followed by 100 mg daily on day 2-10 | Tx 538; control 521 | mean ± SD, 58.9 ± 15.0 | 684 (64.3) | NA | NA | NA | NA | 15 (2.8) vs 20 (3.8) | 8 (1.5) vs 9 (1.7) | 49 (9.1) |
| Wang et al[51] | 200 mg daily on day 1, followed by 100 mg daily on day 2-10 | Tx 158; control 79 | Median (IQR) 65 (56-71) | 89 (56) | 5 (3) vs 2 (3) | 4 (3) vs 2 (3%) | NA | 21 (14) vs 12 (15) | 7 (5) vs 9 (12) | NA | 18 (12) |
| Spinner et al[53] | 200 mg daily on day 1, followed by 100 mg daily on day 2-5 or day 2-10 | 193; 193; 200 | Median (IQR) 56 (45-66) | 118 (61), 114 (60) | 5% vs 6% vs 7% | NA | NA | NA | 32 vs 32 vs 33 | 32 vs 34 vs 39 | 31 (7.8) |
| Hydroxychloroquine | |||||||||||
| Cavalcanti et al[70] | 400 mg daily | Tx 221; control 227 | mean ± SD, 50.3 ± 14.6 | 388 (55.3) | NA | 0 vs 1 (0.6) | NA | NA | 17 (8.5) vs 6 (3.4) | NA | NA |
| Boulware et al[71] | 800 mg once, followed by 600 mg | Tx 414; control 407 | Median (IQR) 41 (33-51) | 196 (47.3) | 81 (23.2) vs 15 (4.3) for diarrhoea or abdominal pain or vomiting | 81 (23.2) vs 15 (4.3) for diarrhoea or abdominal pain or vomiting | 81 (23.2) vs 15 (4.3) for diarrhoea or abdominal pain or vomiting | NA | NA | NA | 17 (4.1) |
| Favipiravir | |||||||||||
| Chen et al[80] | 1600 mg twice a day on day 1, followed by 600 mg twice daily on day 2-10 | Tx 116; control 120 | NA | 59 (50.86) | NA | NA | NA | NA | 10 (8.62) | NA | Nil |
| Nitazoxanide | |||||||||||
| Rocco et al[82] | 500 mg 3 times per day | Tx 194; control 198 | 18-77 | 101 (52) | 57 (29.4) vs 49 (24.7) | 9 (4.6) vs 3 (1.5) | 10 (5.2) vs 5 (2.5) | NA | NA | NA | Nil |
| Tocilizumab | |||||||||||
| Stone et al[120] | Tocilizumab 8 mg/kg IV inf not to exceed 800 mg | Tx 161; control 82 | Median (IQR) 61.6 (46.4-69.7) | 96 (60) | NA | NA | NA | NA | 6 (3.7) vs 3 (3.7) for grade 3 or 4 | 8 (5.0) vs 4 (4.9) for grade 3 or 4 | NA |
Ritonavir use is associated with a 5-fold higher incidence of severe hepatotoxicity compared with other PIs[39]. Hepatitis including elevation of AST, ALT, and gamma-glutamyl transferase levels has been reported in 3.5% of patients taking LPV/r, according to the package insert[35]. This drug is principally metabolized by the hepatic CYP3A4 isoenzyme[40] and therefore, caution should be exercised when administering this drug to patients with hepatic impairment. Safety data on LPV/r use in patients with cirrhosis do exist[41]. Coinfection with hepatitis B virus (HBV) and/or hepatitis C virus (HCV) increases the risk hepatotoxicity and patients with such infections should be monitored closely[42]. Patients with severe liver disease such as cirrhosis or those with significant elevation of liver enzyme were excluded from RCTs[31,32]. Concomitant use of tenofovir with LPV/r is not recommended since this will lead to elevated levels of tenofovir. Physicians may consider switching from tenofovir to entecavir during treatment with LPV/r.
Remdesivir was initially under clinical development for the treatment of Ebola virus disease[43]. It is a monophosphoramidate prodrug of an adenosine analog, which is then metabolized in cells to an active nucleoside triphosphate that inhibits viral RdRp early in the viral infectious cycle. It has demonstrated antiviral activity against coronavirus including SARS-CoV-2[44-47]. Other potential antiviral mechanisms involve lethal mutagenesis and chain termination[48,49].
Remdesivir was used to treat the first case of COVID-19 infection in the United States[3]. Thereafter, numerous clinical trials focusing on its efficacy and safety have been published. In a multicenter RCT led by Beigel et al[50] including 1059 hospitalized patients with evidence of lower respiratory tract involvement, remdesivir was administered intravenously as a 200-mg loading dose on day 1, followed by 100-mg daily on days 2 through 10 or until hospital discharge or death. Patients who received treatment had a shorter time to recovery than patients who received placebo (median 11 d vs 15 d; rate ratio for recovery, 1.32; 95%CI: 1.12 to 1.55; P < 0.001). Recovery was defined as patients not requiring supplemental oxygen or ongoing medical care except for infection-control reasons. Mortality was numerically lower in the treatment group than the placebo group, but the difference was not significant (HR for death, 0.70; 95%CI: 0.47 to 1.04)[50]. Another RCT from China enrolled 237 patients, but failed to demonstrate a significant difference in the time to clinical improvement with remdesivir in severe patients [21.0 d in remdesivir group vs 23.0 d in the control group, HR 1.23 (95%CI: 0.87 to 1.75)][51]. Nevertheless, the results should be interpreted with caution as the power of this study was limited by failure to complete full enrolment due to control of the outbreak in Wuhan.
Several studies have compared the efficacy and safety of 5 d vs 10 d of remdesivir treatment in patients with COVID-19[52,53]. Goldman et al[52] enrolled 397 COVID-19 patients with evidence of pneumonia and reduced oxygen levels but not requiring mechanical ventilation or extracorporeal membrane oxygenation. Similar clinical improvement was observed in the 5-d group and 10-d group based on assessment on day 14 (P = 0.14). The most common GI/hepatic adverse events were nausea (10% in the 5-d group vs 9% in the 10-d group), increased ALT (6% vs 8%), and constipation (7% in both groups)[52]. Spinner et al[53] randomized 596 patients with moderate COVID-19 to a 10-d course of remdesivir, a 5-d course of remdesivir, or standard care in a 1:1:1 ratio. At 11 d after starting treatment, those randomized to the 5-d course of remdesivir had a statistically significant difference in clinical status compared with standard care[53]. However, those receiving the 10-d course of remdesivir did not have a statistically significant difference in clinical outcome compared with standard care. Common side effects included nausea, hypokalemia, and headache. Elevated liver enzymes were observed in one-third of patients, and were of grade ≥ 3 severity in 2% of patients[53].
GI/hepatic adverse events were similar in the treatment and control arms of the two RCTs described above[50,51]. One patient receiving remdesivir developed a hemorrhage of the lower digestive tract and three patients discontinued treatment as a result of liver enzyme elevation in the study by Wang et al[51]. No serious grade 3 or 4 liver dysfunction was reported in either arm[51].
GI and hepatic adverse events have also been reported in case series of patients receiving remdesivir. In a remdesivir compassionate use program (n = 53), 12 patients (23%) developed elevated hepatic enzymes, and 5 (9%) had diarrhea[54]. Two patients (3.8%) discontinued remdesivir prematurely because of elevated aminotransferases[54]. In another case series in 35 patients who received compassionate remdesivir treatment in Italy, hepatotoxicity was the most frequent adverse event, with a grade 3 to 4 increase in transaminase levels observed in 42.8% of the patients[55]. In the first 12 COVID-19 patients in United States, all 3 patients who received remdesivir experienced transient transaminitis and GI symptoms including nausea, vomiting, gastroparesis or rectal bleeding[56]. Another case series of critically ill patients receiving remdesivir in Italy reported that three of these four patients had elevated ALT and AST levels, ranging from 5 times to 8 times the upper limit of normal[57].
Hepatic adverse events are not unexpected with nucleoside analogues; these agents can cause direct hepatotoxicity by inducing mitochondrial dysfunction and/or idiosyncratic hepatotoxicity via an acute hypersensitivity reaction or the production of toxic intermediates[58]. Asymptomatic grade 1 or 2 ALT elevations were observed in healthy individuals who received remdesivir in phase 1 studies[59]. Pharmacokinetic studies in patients with hepatic impairment were limited, but remdesivir should be used with caution in patients with existing liver disease, and only if the potential benefit outweighs the risk[60]. Regular monitoring of liver function should be performed if possible[61].
Hydroxychloroquine/chloroquine are drugs commonly used in the management of rheumatoid arthritis, systemic lupus erythematosus and malaria. SARS-CoV-2 enters cells by binding to the ACE-2 receptor. Chloroquine may inhibit terminal glycosylation, thus preventing the virus from binding to the ACE-2 receptor[62]. Hydroxychloroquine prevents SARS-CoV-2 from binding to gangliosides which in turn prevents the virion from engaging with the ACE-2 receptor[63].
The use of hydroxychloroquine/chloroquine in the treatment of COVID-19 is controversial[64-71]. A multicenter, RCT was conducted in 504 hospitalized patients with COVID-19 who were receiving either no supplemental oxygen or a maximum of 4 L/min of supplemental oxygen. Patients were randomly assigned in a 1:1:1 ratio to receive standard care, standard care plus hydroxychloroquine 400 mg twice daily, or standard care plus hydroxychloroquine 400 mg twice daily and azithromycin 500 mg once daily for 7 d[70]. Active treatment had no effect on patients’ clinical status at 15 d compared with standard care. The proportional odds of having a higher score on the seven-point ordinal scale at 15 d was not increased by either hydroxychloroquine alone [odds ratio (OR) 1.21; 95%CI: 0.69 to 2.11; P = 1.00] or hydroxychloroquine plus azithromycin (OR, 0.99; 95%CI: 0.57 to 1.73; P = 1.00). In addition, a higher proportion of patients receiving hydroxychloroquine alone (8.5%) or with azithromycin (10.9%) developed elevated liver enzymes compared those who did not receive either agent (3.4%)[70]. Further randomized studies are needed to clarify the efficacy of hydroxychloroquine or chloroquine in the treatment of COVID-19.
These drugs also have a number of side effects. Apart from the well-known arrhythmogenic cardiotoxicity of the drugs, the most common adverse events of hydroxychloroquine and chloroquine are GI, including GI upset, nausea, vomiting, diarrhea, abdominal cramps, and a metallic taste[72-74]. In a study evaluating the use of chloroquine, nearly 24% of patients suffered from nausea or abdominal cramps and 17% reported diarrhea as side effects[75]. Up to 50% of patients receiving hydroxychloroquine in another study reported some GI side effects; the frequency was dose-dependent with GI events occurring more commonly with loading doses of 800 mg or higher[76].
Chloroquine and hydroxychloroquine should be administered with food to reduce nausea and vomiting. At the same time, chloroquine can be crushed and mixed with flavored syrups to mask the bitter taste. It is also recommended to avoid taking antacids within 4 h of chloroquine because of a potential for chelation and reduced bioavailability, but this drug interaction does not occur with hydroxychloroquine.
Azithromycin is a semisynthetic macrolide antibiotic that is commonly prescribed to treat infections with Gram-positive, Gram-negative and atypical pathogens. It has been used for the treatment of COVID-19 in combination with hydroxychloroquine or chloroquine and has produced synergistic effects in the context of combination therapy[77]. Azithromycin may cause GI side effects such as nausea and vomiting.
Ribavirin is a guanine derivative used for the treatment of respiratory syncytial virus and HCV infections. It has been used in combination with other agents for the treatment of COVID-19[78]. In a prospective study of patients with mild to moderate COVID-19, the combination of interferon-beta, oral LPV/r and ribavirin produced a significantly shorter median time from start of study treatment to negative nasopharyngeal swab compared with LPV/r alone[78]. Patients in the combination group also had earlier relief of symptoms compared with the control group (4 d vs 8 d, P < 0.0001). This study suggests that combination therapy is more potent than single-agent antiviral therapy against COVID-19[78].
The common side effects observed in the combination therapy group included diarrhea (40%), fever (37%), nausea (35%) and elevated ALT levels (13%)[78]. Since CYP enzymes are not involved in the metabolism and elimination of ribavirin, there is minimal potential for drug-drug interactions.
Favipiravir is an RdRp inhibitor[79]. Once inside cells, favipiravir is converted into an active phosphoribosylated form, which acts as a substrate for viral RNA polymerase, and then inhibits RNA polymerase activity. It is a broad-spectrum antiviral drug approved in Japan for the treatment of influenza. It has also been used for the treatment of Ebola and Lassa virus infection.
Chen et al[80] conducted a prospective, randomized, open-label multicenter clinical trial involving 240 adult patients with COVID-19 comparing the efficacy and safety of favipiravir vs umifenovir. The clinical recovery rate on day 7 was better in the favipiravir arm than in the umifenovir arm (71.43% vs 55.86%, P = 0.01). Favipiravir significantly shortened the latency to relief for pyrexia and cough compared with umifenovir, and dyspnea was significantly (P = 0.017) less common in the favipiravir group than in the umifenovir group. Deranged LFT is a common side effect of favipiravir and was found in 8.6% of patients.
Cai et al[81] conducted an open-label study in 80 patients with mild to moderate COVID-19 and assessed the effects of favipiravir in comparison with LPV/r for the treatment of COVID-19. Favipiravir was shown to have shorter viral clearance time (median 4 d vs 11 d). In addition, a higher proportion of patients in the favipiravir than the LPV/r groups showed improvement in chest imaging (91.43% vs 62.22%; P = 0.004), particularly in the group with viral clearance within 7 d of starting treatment. Multivariable Cox regression showed that favipiravir was significantly (P = 0.026) associated with faster viral clearance[81].
The most common side effects of favipiravir were liver enzyme abnormalities, GI symptoms like diarrhea, and serum uric acid elevations. We would be cautious about prescribing favipiravir in patients with abnormal LFT results.
Nitazoxanide is an antiparasitic prodrug with antiviral properties that is approved by the U.S. Food and Drug Administration (FDA). The effects of nitazoxanide against COVID-19 were examined in a multicenter, randomized, double-blind, placebo-controlled trial recruiting 392 patients presenting up to 3 d after onset of symptoms including fever, dry cough, and/or fatigue. The patients were randomized in a 1:1 ratio to receive either nitazoxanide 500 mg 3 times/d or matching placebo for 5 d after the diagnosis of SARS-CoV2 infection was made by reverse transcription polymerase chain reaction (RT-PCR) on a nasopharyngeal sample[82].
Although there was no difference between the nitazoxanide and placebo groups in the resolution of symptoms at the 5-d study visit, a significantly higher proportion of patients in the nitazoxanide group (29.9%) returned a negative PCR result for SARS-CoV-2 compared with the placebo group (18.2%; P = 0.009). There was also significantly greater reduction in viral load between the start and end of therapy in patients receiving nitazoxanide (55%) compared with placebo (45%; P = 0.013). GI side effects included nausea (14.4%), vomiting (4.6%), diarrhea (29.4%), and abdominal pain (5.2%) were reported in patients receiving nitazoxanide in the study[82].
Ivermectin is an antiparasitic drug and was found to have a broad range of antiviral activity against many RNA and DNA viruses in vitro. It was also shown to be highly effective in vitro against SARS-CoV-2[83].
It was shown that the combined use of ivermectin, nitazoxanide and ribavirin plus zinc supplement achieved better clearance of the SARS-COV2 from the nasopharynx in a shorter time than symptomatic therapy in a non-RCT[84]. The viral clearance rates on the 7th day were 0% and 58.1%, respectively, in the groups receiving supportive treatment and combined antiviral therapy, and were 13.7% and 73.1%, respectively, on the 15th day. The corresponding cumulative viral clearance rates on the 15th day were 13.7% and 88.7%, respectively. Overall, 11.3% of patients had elevation of LFTs and 22.6% of developed GI upset during the study period.
Rajter et al[85] performed a retrospective study of 280 COVID-19 patients to assess the efficacy of ivermectin, in which 173 had been treated with ivermectin and 107 had not. Most patients in both groups also received hydroxychloroquine, azithromycin, or both. Mortality was significantly lower in the ivermectin group (13.3% vs 24.5%; P < 0.05). Mortality was also lower among ivermectin-treated patients with severe pulmonary involvement (38.8% vs 80.7%; P = 0.001). Eleven percent of phas a broad range of antiviral activity against many RNA and DNA viruses in vitro has a broad range of antiviral activity against many RNA and DNA viruses in vitro. Ivermectin has a broad range of antiviral activity against many RNA and DNA viruses in vitro.
Molnupiravir is an oral, direct-acting antiviral agent which was shown to be highly effective in reducing nasopharyngeal SARS-CoV-2 infectious virus and viral RNA. It is well absorbed after oral administration. Fischer et al[86] randomized 202 patients to molnupiravir (200, 400 or 800 mg) or placebo twice-daily for 5 d. Antiviral activity was assessed as time to undetectable levels of viral RNA by RT-PCR and time to elimination of infectious virus isolation from nasopharyngeal swabs.
The results showed a significant reduction in virus isolation in participants receiving 800 mg molnupiravir (1.9%) vs placebo (16.7%) at day 3 (P = 0.02). Virus was not isolated from any patient receiving 400 mg or 800 mg molnupiravir while 11.1% of patients receiving placebo had virus isolated at day 5 (P = 0.03).
There was decrease in the time to viral RNA clearance in patients given 800 mg molnupiravir compared with placebo (14 d vs 27 d, P = 0.001). There was also a higher rate of overall clearance in patients receiving molnupiravir. The side effects of molnupiravir include headache, insomnia, and increased ALT. We would be cautious using molnupiravir in patient with hepatic dysfunction.
Cytokine storm is an important pathogenic process in COVID-19 patients[87]. SARS-CoV-2 binds to the toll-like receptor, activating the nuclear factor (NF)-κB pathway and pro-inflammatory cytokines[88]. Cytokines are signalling molecules that recruit immune cells to the site of inflammation, induce vascular leakage and exudation, and stimulate the generation of free radicals and proteases[89]. Pro-inflammatory cytokines induce alveolar injury and reduced alveolar fluid clearance resulted in ARDS[90]. Compared with mild or moderate cases, patients with severe COVID-19 have higher levels of circulating IL-2, IL-6, IL-7, IL-10, interferon gamma, granulocyte colony stimulating factor, interferon-inducible protein 10, monocyte chemoattractant peptide , macrophage inflammatory protein-1A, and tumor necrosis factor (TNF)-α[7,91-93]. This raises the possibility of using immunomodulatory agents to control the inflammatory response, and thereby improve the prognosis of COVID-19[94].
Corticosteroids inhibit NF-κB signalling and various pro-inflammatory cytokines such as IL-1β, IL-2, IL-6, TNF-α, and IL-17. It also reduces the proliferation, activation, differentiation, and survival of T cells and macrophages[95]. Steroids may play a protective role in the respiratory and digestive systems by activating ACE-2 and suppressing the cytokine storm, in particular reducing IL-6 levels, in patients with severe or critical COVID-19[96]. Corticosteroids were used in early reports from Wuhan, China, where they were used in an attempt to reduce inflammation-induced lung injury[90].
Dexamethasone is the first treatment that has been shown to reduce mortality in severely ill COVID-19 patients[97,98]. The randomized evaluation of COVID-19 therapy (RECOVERY) trial compared 2104 patients receiving oral or intravenous dexamethasone (at a dose of 6 mg once daily) for up to 10 d with 4321 patients receiving usual care alone. The 28-d mortality rate was lower in the group receiving dexamethasone compared with usual care group in patients who were receiving invasive mechanical ventilation (29.3% vs 41.4%; rate ratio, 0.64; 95%CI: 0.51 to 0.81) or receiving oxygen without invasive mechanical ventilation (23.3% vs 26.2%; rate ratio, 0.82; 95%CI: 0.72 to 0.94). No survival benefit was seen among those who were receiving no respiratory support at randomization. Dexamethasone also reduced mortality in patients with symptoms for more than 7 d but not in those with more recent symptom onset[97].
The positive impact of steroids was confirmed in a prospective meta-analysis of seven clinical trials involving 1703 critically ill patients with COVID-19 conducted in 12 countries[99]. The meta-analysis showed that the use of systemic corticosteroids reduced all-cause 28-d mortality compared with usual care or placebo. The number of deaths was 222 in those receiving corticosteroids compared to 425 deaths in the usual care or placebo group. Dexamethasone could significantly suppress the odds of all-cause mortality.
The preliminary report of the RECOVERY study did not describe side effects. Previously reported side effects of steroids include hyperglycemia, hypokalemia, delayed viral clearance, risk of secondary bacterial infection, psychosis and avascular osteonecrosis[100-104]. Corticosteroids may induce various GI adverse events such as gastritis, peptic ulcer formation and GI bleeding, with the risk of bleeding significantly increased by concomitant non-steroidal anti-inflammatory drug use[105,106]. Direct SARS-CoV-2 invasion of the GI tract, causing erosion and ulcers in severe patients, may increase the risk further[1]. Prophylactic proton pump inhibitors should be considered in patients who receive dexamethasone[107].
Steroids increase the risk of acute pancreatitis by an unknown mechanism[108]. Steroids activate triglyceride synthesis and accumulation, increase fatty acid uptake and inhibit fatty acid beta-oxidation in the liver, while they also increase lipolysis, lipogenesis and the secretion of non-esterified fatty acids and adipokines in adipose tissue, which results in hepatic steatosis[109]. Diabetes and obesity are associated with the development of non-alcoholic fatty liver disease[110]. These metabolic risk factors may result in deleterious effects on host immunity, and are closely related to disease severity and mortality in patients with COVID-19[111-115]. Regular monitoring of liver function and glucose level is recommended for this high-risk group of patients receiving dexamethasone.
COVID-19 can trigger aggressive an inflammatory response resulting in cytokine release syndrome (CRS), which is associated with an unfavorable prognosis[116]. A meta-analysis of 6 studies including 1302 patients demonstrated 2.9-fold higher levels of IL-6 in patients with complicated COVID-19 compared with patients with non-complicated disease[117]. IL-6 is an important cytokine responsible for an inflammatory storm that leads to impaired oxygen diffusion in the lungs[7]. Tocilizumab is a recombinant humanized monoclonal antibody against the IL-6 receptor and reduces the effects of CRS. This led to speculation that it could be used in the treatment of COVID-19, especially in severe patients with high IL-6 levels.
A retrospective, observational cohort study was carried out to investigate mortality in 544 patients with severe COVID-19 requiring support in the ICU; 179 patients received tocilizumab and 365 patients received standard care. There was an improvement in median overall survival from time of hospital admission in patients receiving tocilizumab when compared with the standard care cohort (20% vs 7%; P < 0.001)[118].
Another multicenter retrospective cohort study investigated outcomes in 4485 adults with COVID-19 admitted to ICU in 68 hospitals. Among critically ill patients, the risk of in-hospital mortality was lower in patients treated with tocilizumab in the first 2 d of ICU admission compared with patients whose early treatment did not include tocilizumab (HR, 0.71; 95%CI: 0.56 to 0.92)[119].
However, similar favorable results were not seen in a RCT involving 243 patients with hyperinflammatory states. Tocilizumab was not shown to be effective enough to prevent intubation or death in moderately ill, hospitalized COVID-19 patients in this trial[120]. Further research in RCTs is needed.
Reports have emerged of liver injury with an increase in transaminase levels associated with tocilizumab use in COVID-19 patients[121], and increases in liver enzyme levels were seen in 5% of patients in one of the cohort studies described above and in 1% of patients in the RCT[118,120]. In the cohort study by Gupta et al[119], 16.6% of patients receiving tocilizumab developed an AST of more than 250 U/L and 8.5% developed an ALT level of more than 500 U/L. Tocilizumab can interfere with serum concentrations of CYP3A4 substrates. It should be used with caution and liver function regularly monitored, especially when used in combination with another hepatotoxic drug or in patients receiving multiple concomitant medications.
Baricitinib is a selective inhibitor of Janus kinase (JAK) 1 and 2, and orally administered. It was originally developed for the treatment of rheumatoid arthritis. Inhibition of JAK blocks intracellular signal transmission from cytokine or growth factor receptors and leads to reduced hematopoiesis[17]. This inhibition of signal transmission prevents phosphorylation and then activation of signal transducers and activators of transcription.
Baricitinib was used in combination with remdesivir in a RCT involving 1033 patients with COVID-19. The rationale for combining these two therapies is that clinical outcomes would be improved by reducing the immune response and preventing a hyperinflammatory state[122]. The combination was found to be significantly better than remdesivir alone in reducing recovery time and accelerating clinical improvement in patients with COVID-19. This effect was more marked in patients receiving high-flow oxygen or non-invasive ventilation. The time to recovery was 10 d in patients who received combination treatment compared with 18 d in patients who received remdesivir alone. The 28-d mortality was 5.1% in the combination group and 7.8% in the control group (HR for death, 0.65; 95%CI: 0.39 to 1.09).
The combination was associated with fewer serious adverse events. Transaminases increased in 1.2% of patients receiving combination therapy and 2% of patients receiving remdesivir, and bilirubin increased in 0.4% and 1.6%, respectively. Regular monitoring of liver function is recommended, especially when used in combination with remdesivir.
A summary of the side effects of the potential treatments for COVID-19 is shown in Table 2.
| Drug name | Gastrointestinal and hepatic side effects |
| Remdesivir | Elevation of liver enzymes |
| Lopinavir-ritonavir | Nausea, vomiting, abdominal pain, gastroenteritis |
| Hydroxychloroquine/chloroquine | Nausea, vomiting, abdominal pain, diarrhea |
| Steroids | Epigastric pain, peptic ulcer, risk of HBV reactivation |
| Interferon | Diarrhea, nausea, elevated alanine aminotransferase level |
| Ribavirin | Elevated liver enzyme levels |
| Umifenovir | Nausea, vomiting and deranged liver function |
| Bromhexine | Deranged liver function |
| Favipiravir | Diarrhoea, liver enzyme abnormalities |
| Nitazoxanide | Nausea, vomiting, diarrhoea and abdominal pain |
| Imervectin | Elevation of liver enzymes |
| Molnupiravir | Elevated alanine aminotransferase |
| Tocilizumab | Liver dysfunction |
| Baricitinib | Nausea, liver dysfunction |
| Azithromycin | Nausea, vomiting |
Vaccination is an important method to protect the population from COVID-19 and is likely to be especially important in high-risk individuals, such as those with pre-existing health conditions. A minimum vaccine efficacy of 50% is necessary to get regulatory approval from the World Health Organization (WHO). Patients with chronic diseases have a higher mortality when they get infected with COVID-19. Therefore, this group of patients will benefit more from the vaccination. However, the phase 1-3 studies of the COVID-19 vaccines mainly recruited healthy individuals, so data are limited in patients with chronic diseases. The decision to be vaccinated may also depend on the stability of the patient’s chronic illness and the prevalence of COVID-19 in the relevant country or region.
Different technologies were applied to the development of the vaccines including mRNA, viral vectors, inactivated viruses, recombinant DNA, protein subunits and live attenuated viruses.
The BNT162b2 mRNA vaccine (manufactured by Pfizer BioNTec) and the mRNA-1273 mRNA vaccine (manufactured by Moderna-NIH) was developed based on mRNAs that encode variants of the SARS-CoV-2 spike glycoprotein and are encapsulated into lipid nanoparticles[123-125]. The ChAdOx1 nCoV-19 vaccine (manufactured by AstraZeneca) uses an adenoviral vector and is approved by the WHO is currently being used in Europe, the United States and many other countries[126]. Another WHO-approved COVID-19 vaccine is Ad26.COV2.S, developed by Janssen (Johnson & Johnson); this is a single-dose viral vector vaccine based on a human adenovirus that has been modified to contain the gene for making the spike protein of the SARS-CoV-2 virus[127]. However, the use of this vaccine was stopped by the WHO because of the risk of thrombotic complications.
The two mRNA vaccines described above got the earliest approval from the WHO and are now being used, but these vaccines must be stored in very low temperature freezers. Common acute side effects of the vaccines include myalgia, fatigue, low-grade fever, headache, nausea and redness or soreness at the injection site. There do not appear to be many GI and hepatic side effects.
BNT162b2 was chosen by Pfizer/BioNTec as the most promising of two potential mRNA vaccine candidates based on safety and immunogenicity data from phase I studies in younger and older adults[123]. A two-dose regimen of BNT162b2 confirmed a 95% protection rate against COVID-19 in persons 16 years of age or older. The side effect profile was characterized mainly by fatigue, mild to moderate pain at the injection site, and headache[124].
A phase III study of the mRNA-1273 vaccine was carried out in 30420 healthy individuals aged 18 or above randomly assigned in a 1:1 ratio to receive either vaccine or placebo. It showed an efficacy of 94.1% at preventing COVID-19 illness, including severe disease[125]. There were no major safety concerns apart from transient local and systemic reactions.
The third approved vaccine is ChAdOx1 nCoV-19 vaccine (AZD1222) which was developed at Oxford University. It consists of a replication-deficient chimpanzee adenoviral vector ChAdOx1 which contains the SARS-CoV-2 structural surface glycoprotein antigen (spike protein; nCoV-19) gene. After receiving two standard doses of vaccine, the efficacy of the vaccine was 62.1% vs 1.6% of 4455 participants in the control group[126].
Recently, however, safety concerns have emerged about the thrombotic risk associated with the vaccine. A pathogenic PF4-dependent syndrome, which was unrelated to the use of heparin, was identified after the administration of the vaccine[128]. Clinicians should pay particular attention to individuals with thrombotic risk factors.
The fifth vaccine is an inactivated vaccine developed by Sinovac Life Sciences and is being used in some countries. CoronaVac was well tolerated and induced humoral responses against SARS-CoV-2, and it was approved for emergency use in China and some other countries and regions. Efficacy and safety were demonstrated in two phase I/II double-blind, placebo-controlled RCTs in healthy adults aged 18-59 years and 60 years or older[129,130]. A phase III, randomized, multicenter, double-blind, placebo-controlled clinical study is being carried out to assess the efficacy and safety of the adsorbed vaccine COVID-19 (inactivated) produced by Sinovac in two age groups: 18 years to 59 years and 60 years or more[131].
Another vaccine, Sinopharm, which is an inactivated vaccine developed in China, has been approved and used in some countries and regions. It showed promising results in phase I/II trials[132]. The phase III trial data will provide more information on the safety, efficacy and immunogenicity of the vaccine. A summary of the available COVID-19 vaccines is shown in Table 3. There are ongoing studies for these and other vaccines and more choices will become available over time.
| Vaccine | Mechanism | Number of participants | Efficacy |
| mRNA-1273 (Moderna)[125] | RNA (embedded in lipid nanoparticles)encodes a variant of the SARS-CoV-2 spike protein | 30420 participants (randomized 1:1 vaccine vs placebo) | Efficacy 94.1% (11 vaccinated vs 185 controls with COVID-19) |
| BNT162b2 (BioNTech and Pfizer)[124] | RNA (embedded in lipid nanoparticles) encodes a variant of the SARS-CoV-2 spike protein | 43548 participants (randomized 1:1 vaccine vs placebo) | Efficacy 95% (9 vaccinated vs 169 controls with COVID-19) |
| ChAdOx1 nCoV-19 (AZD122; AstraZenenca and University of Oxford)[126] | Replication-deficient chimpanzee adenovirus vector, containing the full-length codon-optimized coding sequence of SARS-CoV-2 spike protein | 23848 participants (randomized 1:1 vaccine vs placebo) | Efficacy 70.4% [30 (0.5%) of 5807 vaccine recipients vs 101 (1.7%) of 5829 controls with COVID-19] |
| CoronaVac (Sinovac Life Sciences, Beijing, China)[129,131] | Inactivated vaccine candidate against COVID-19 | 600 participants | Seroconversion was seen in 114 (97%) of 117 in the 3 μg group, 118 (100%) of 118 in the 6 μg group, and none (0%) of 59 in the placebo group |
| Sinopharm vaccine[132] | Inactivated vaccine candidate against COVID-19 | 448 participants | Neutralizing antibodies were detected in 100% of recipients |
Patients with CLD, liver cirrhosis, hepatobiliary malignancies, and candidates for liver transplantation are at higher risk of COVID-19 infections. At the same time, these groups of patients have a lower immune response to vaccines.
The benefits and risks of vaccination for patients with chronic disease or immunocompromised patients should be weighed individually, taking into account the incidence of the infection in the country or community, the vaccine formulation, the type of immunosuppressive therapy (e.g., chemotherapy, transplantation) the patient is receiving, and the extent of their immunosuppression.
There is a reduction of immune memory against and immune responses to certain vaccines as patients age and their CLD progresses[133]. Moreover, patients with alcohol-associated liver disease, CLD and cirrhosis may have an impaired immune response to vaccination. At the same time, they are more susceptible to infections and infection-related complications[134].
Patients with immunosuppressive conditions or liver diseases were usually excluded from the studies of the COVID-19 vaccines. A post-marketing study in a nationwide mass vaccination setting in Israel suggests that the BNT162b2 mRNA vaccine is effective for a wide range of COVID-19-related outcomes, a finding con
There are currently limited published data on specific patient subgroups. Investigators have performed subgroup analyses, each time restricting the matching process to persons with a specific condition of interest, in order to maximize the sample size[136]. The results on the subgroup with CLD are not yet known.
Patients with CLD infected with SARS-CoV-2 infection have higher risk of adverse outcome than the general population. There are on-going trials in patients with liver diseases worldwide and the results are pending[137].
In view of the high rate of complications and decompensation caused by COVID 19 in CLD, we recommend SARS-CoV-2 vaccination in patients with CLD, and in candidates for liver transplantation, with prioritization of patients with risk factors for severe COVID-19.
In general, professional bodies like the European Association for the Study of the Liver and the American Association for the Study of Liver Disease recommend COVID-19 vaccination for patients with CLD as the benefits likely outweigh the risks[138,139].
Rituximab may be used for the treatment of CLD such as autoimmune hepatitis and its efficacy is shown in a recent retrospective study[140]. There is usually a blunted vaccine response after vaccination in patients with lymphoma[141-144] or autoimmune disorders[145-148] treated with rituximab. B cells are required for the development of humoral immune responses to neoantigens. Therefore, depletion of B cells following rituximab will likely reduce the humoral immune responses to the COVID-19 vaccine. Both T cell-dependent and -independent responses are also significantly impaired for at least 6 mo after rituximab treatment[148].
Assuming that immunological response to the COVID-19 vaccine correlates with disease protection, it is recommended that vaccination be performed at least 6 mo after rituximab infusion.
Solid organ transplant (SOT) recipients are on immunosuppression to prevent graft rejection, so they are at a higher risk of infection and infective complications. Vaccination is useful to prevent infections and the associated complications in transplant recipients.
COVID-19 vaccination is recommended for all SOT recipients including liver transplant recipients, and vaccination can be given 3-6 mo after SOT. Since the current approved vaccines do not contain live or attenuated virus, they are likely to be safe in immunosuppressed patients[139,149].
The immunogenicity of vaccines in SOT recipients is lower than in immunocompetent individuals because of the immunosuppressive therapy and the underlying chronic disease. Therefore, vaccination against COVID-19 is recommended for family members and healthcare professionals caring for these patients to reduce exposure to SARS-CoV-2[138].
IBD is an umbrella term for the immune-mediated inflammatory conditions of Crohn’s disease and ulcerative colitis.
IBD patients may receive immunosuppressive drugs such as high-dose corticosteroids, immunomodulators (thiopurines, methotrexate, and calcineurin inhibitors), anticytokine therapies (including anti-TNF and anti-IL-12p40 biologics), anti-integrin therapies (vedolizumab), and small-molecule inhibitors of signalling (tofacitinib), which could leave them susceptible to infection.
Immunosuppressive drugs may reduce the humoral response to vaccines and thus their effectiveness, which could have major implications for the safety of immunosuppressed patients in the COVID-19 era. The risks associated with current COVID-19 vaccines are low, and guidelines recommend vaccination for patients with IBD[150,151].
COVID-19 vaccination is also advocated for IBD patients younger than 16 years. Although pediatric patients may experience milder illness if they get infected by SARS-CoV-2[152,153], they can be the source of ongoing outbreaks and transmission[154]. The cessation of the COVID-19 pandemic relies on maximal community uptake of the COVID-19 vaccine in order to achieve herd immunity. On May 10, 2021, the U.S. FDA expanded the Emergency Use Authorization for the BNT162b2 mRNA vaccine to include people aged 12 years to 15 years[155]. This is based on the results of an RCT enrolling 2260 adolescents (12-15-year-old) who were randomized 1:1 to receive the BNT162b2 or placebo[156]. In 7 d after the second dose of BNT162b2, there were zero new case of COVID-19, translating into 100% vaccine efficacy, while there were 16 confirmed cases in the placebo group. Vaccinated adolescents 12- to 15-year-old had higher geometric mean titers of SARS-CoV-2 neutralizing antibodies (1239.5 vs 705.1) compared with recipients aged 16 years to 25 years. A favorable safety and side effect profile, similar to other age groups, was also demonstrated in the 12- to 15-year-old recipients of BNT162b2[156].
The use of COVID-19 vaccines is not recommended in pregnant women and there are no safety data of the vaccines in these women to date.
Another point to consider is that patients with IBD are at risk of thromboembolic complications, and COVID-19 increases the risk of thromboembolic events. Studies have shown that prophylactic anticoagulation can reduce the 30-d mortality risk in patients with COVID-19[157].
COVID-19 is a pandemic infection with a high burden of morbidity and mortality. Various drugs are under investigation for the treatment of the disease, but many are associated with GI and hepatic side effects. Caution and careful monitoring should be exercised when prescribing these therapies in patients with GI symptoms like diarrhea and vomiting. As liver impairment is a common observation among patients with COVID-19, we recommend that all patients with COVID-19 and liver impairment undergo investigations for potential causes of liver disease, including viral hepatitis serology, particularly in areas where HBV is prevalent.
Furthermore, increasing rates of liver dysfunction have been correlated with the severity of COVID-19[158]. We need to maintain a high index of suspicion as hepatotoxic drug effects may be difficult to detect in this condition.
High-dose corticosteroids and tocilizumab have been used for the treatment of patients with severe COVID-19. There is a risk of HBV reactivation, hepatitis flare, and even acute liver failure in patients with chronic HBV infection receiving this regimen. Screening for HBsAg is recommended, and antiviral prophylaxis with nucleoside analogs should be given to patients with COVID-19 who are positive for HBsAg during steroid therapy.
COVID-19 vaccines have been rapidly developed. Patients with CLD or IBD and liver transplant recipients are encouraged to receive vaccination. The benefits will outweigh the risks.
Vaccination against COVID-19 is also recommended for family members and healthcare professionals caring for these patients to reduce exposure to SARS-CoV-2. The vaccination against COVID-19 is encouraged for all individuals at risk of SARS-CoV-2 infection, including those with underlying chronic diseases. Recommendations by professional bodies, governments and health authorities will be important driver of COVID-19 vaccination[159].
Extensive research has been performed to identify potential treatments for SARS-CoV-2 infection. GI symptoms and liver dysfunction in COVID-19 patients could be due to disease manifestations or treatment side effects, which physicians should take into consideration when choosing the best therapeutic strategy. The development of effective and safe vaccines is the light at the end of the tunnel to end the pandemic and should be encouraged, including for patients with CLD, IBD, liver transplant recipients their family members, and healthcare professionals.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Bendary M, Kim JM S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
| 1. | Lin L, Jiang X, Zhang Z, Huang S, Fang Z, Gu Z, Gao L, Shi H, Mai L, Liu Y, Lin X, Lai R, Yan Z, Li X, Shan H. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997-1001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 630] [Cited by in RCA: 658] [Article Influence: 131.6] [Reference Citation Analysis (0)] |
| 2. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 556] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 3. | Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK; Washington State 2019-nCoV Case Investigation Team. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382:929-936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4155] [Cited by in RCA: 3822] [Article Influence: 764.4] [Reference Citation Analysis (1)] |
| 4. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18879] [Article Influence: 3775.8] [Reference Citation Analysis (7)] |
| 5. | Wong SH, Lui RN, Sung JJ. Covid-19 and the digestive system. J Gastroenterol Hepatol. 2020;35:744-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 445] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 6. | Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, Xing F, Liu J, Yip CC, Poon RW, Tsoi HW, Lo SK, Chan KH, Poon VK, Chan WM, Ip JD, Cai JP, Cheng VC, Chen H, Hui CK, Yuen KY. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514-523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6483] [Cited by in RCA: 5423] [Article Influence: 1084.6] [Reference Citation Analysis (0)] |
| 7. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30124] [Article Influence: 6024.8] [Reference Citation Analysis (3)] |
| 8. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12978] [Article Influence: 2595.6] [Reference Citation Analysis (1)] |
| 9. | Luo S, Zhang X, Xu H. Don't Overlook Digestive Symptoms in Patients With 2019 Novel Coronavirus Disease (COVID-19). Clin Gastroenterol Hepatol. 2020;18:1636-1637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 233] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 10. | Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, Hao SR, Jia HY, Cai H, Zhang XL, Yu GD, Xu KJ, Wang XY, Gu JQ, Zhang SY, Ye CY, Jin CL, Lu YF, Yu X, Yu XP, Huang JR, Xu KL, Ni Q, Yu CB, Zhu B, Li YT, Liu J, Zhao H, Zhang X, Yu L, Guo YZ, Su JW, Tao JJ, Lang GJ, Wu XX, Wu WR, Qv TT, Xiang DR, Yi P, Shi D, Chen Y, Ren Y, Qiu YQ, Li LJ, Sheng J, Yang Y. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002-1009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 944] [Cited by in RCA: 871] [Article Influence: 174.2] [Reference Citation Analysis (0)] |
| 11. | Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol. 2020;115:766-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1160] [Cited by in RCA: 1206] [Article Influence: 241.2] [Reference Citation Analysis (0)] |
| 12. | Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, Ng YY, Chu MY, Chung TWH, Tam AR, Yip CCY, Leung KH, Fung AY, Zhang RR, Lin Y, Cheng HM, Zhang AJX, To KKW, Chan KH, Yuen KY, Leung WK. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology. 2020;159:81-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1113] [Cited by in RCA: 1133] [Article Influence: 226.6] [Reference Citation Analysis (1)] |
| 13. | Banales JM, Huebert RC, Karlsen T, Strazzabosco M, LaRusso NF, Gores GJ. Cholangiocyte pathobiology. Nat Rev Gastroenterol Hepatol. 2019;16:269-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 347] [Article Influence: 57.8] [Reference Citation Analysis (1)] |
| 14. | Shyr ZA, Gorshkov K, Chen CZ, Zheng W. Drug Discovery Strategies for SARS-CoV-2. J Pharmacol Exp Ther. 2020;375:127-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 15. | Hoenen T, Groseth A, Feldmann H. Therapeutic strategies to target the Ebola virus life cycle. Nat Rev Microbiol. 2019;17:593-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 16. | Shannon A, Selisko B, Le N, Huchting J, Touret F, Piorkowski G, Fattorini V, Ferron F, Decroly E, Meier C, Coutard B, Peersen O, Canard B. Favipiravir strikes the SARS-CoV-2 at its Achilles heel, the RNA polymerase. bioRxiv. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Barlow A, Landolf KM, Barlow B, Yeung SYA, Heavner JJ, Claassen CW, Heavner MS. Review of Emerging Pharmacotherapy for the Treatment of Coronavirus Disease 2019. Pharmacotherapy. 2020;40:416-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 139] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 18. | Wang X, Cao R, Zhang H, Liu J, Xu M, Hu H, Li Y, Zhao L, Li W, Sun X, Yang X, Shi Z, Deng F, Hu Z, Zhong W, Wang M. The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell Discov. 2020;6:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 215] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 19. | Nojomi M, Yassin Z, Keyvani H, Makiani MJ, Roham M, Laali A, Dehghan N, Navaei M, Ranjbar M. Effect of Arbidol (Umifenovir) on COVID-19: a randomized controlled trial. BMC Infect Dis. 2020;20:954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 20. | Limburg H, Harbig A, Bestle D, Stein DA, Moulton HM, Jaeger J, Janga H, Hardes K, Koepke J, Schulte L, Koczulla AR, Schmeck B, Klenk HD, Böttcher-Friebertshäuser E. TMPRSS2 Is the Major Activating Protease of Influenza A Virus in Primary Human Airway Cells and Influenza B Virus in Human Type II Pneumocytes. J Virol. 2019;93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 21. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14274] [Article Influence: 2854.8] [Reference Citation Analysis (0)] |
| 22. | Li T, Sun L, Zhang W, Zheng C, Jiang C, Chen M, Chen D, Dai Z, Bao S, Shen X. Bromhexine Hydrochloride Tablets for the Treatment of Moderate COVID-19: An Open-Label Randomized Controlled Pilot Study. Clin Transl Sci. 2020;13:1096-1102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Ansarin K, Tolouian R, Ardalan M, Taghizadieh A, Varshochi M, Teimouri S, Vaezi T, Valizadeh H, Saleh P, Safiri S, Chapman KR. Effect of bromhexine on clinical outcomes and mortality in COVID-19 patients: A randomized clinical trial. Bioimpacts. 2020;10:209-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 24. | Cvetkovic RS, Goa KL. Lopinavir/ritonavir: a review of its use in the management of HIV infection. Drugs. 2003;63:769-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 298] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 25. | Mangum EM, Graham KK. Lopinavir-Ritonavir: a new protease inhibitor. Pharmacotherapy. 2001;21:1352-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Flexner C. HIV-protease inhibitors. N Engl J Med. 1998;338:1281-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 602] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 27. | Chen F, Chan KH, Jiang Y, Kao RY, Lu HT, Fan KW, Cheng VC, Tsui WH, Hung IF, Lee TS, Guan Y, Peiris JS, Yuen KY. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31:69-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 385] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 28. | de Wilde AH, Jochmans D, Posthuma CC, Zevenhoven-Dobbe JC, van Nieuwkoop S, Bestebroer TM, van den Hoogen BG, Neyts J, Snijder EJ. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014;58:4875-4884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 539] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 29. | Chu CM, Cheng VC, Hung IF, Wong MM, Chan KH, Chan KS, Kao RY, Poon LL, Wong CL, Guan Y, Peiris JS, Yuen KY; HKU/UCH SARS Study Group. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1117] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 30. | Chan KS, Lai ST, Chu CM, Tsui E, Tam CY, Wong MM, Tse MW, Que TL, Peiris JS, Sung J, Wong VC, Yuen KY. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med J. 2003;9:399-406. [PubMed] |
| 31. | Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382:1787-1799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3386] [Cited by in RCA: 3627] [Article Influence: 725.4] [Reference Citation Analysis (0)] |
| 32. | Li Y, Xie Z, Lin W, Cai W, Wen C, Guan Y, Mo X, Wang J, Wang Y, Peng P, Chen X, Hong W, Xiao G, Liu J, Zhang L, Hu F, Li F, Zhang F, Deng X, Li L. Efficacy and Safety of Lopinavir/Ritonavir or Arbidol in Adult Patients with Mild/Moderate COVID-19: An Exploratory Randomized Controlled Trial. Med (N Y). 2020;1:105-113.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 33. | Yan D, Liu XY, Zhu YN, Huang L, Dan BT, Zhang GJ, Gao YH. Factors associated with prolonged viral shedding and impact of lopinavir/ritonavir treatment in hospitalised non-critically ill patients with SARS-CoV-2 infection. Eur Respir J. 2020;56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 126] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 34. | Ye XT, Luo YL, Xia SC, Sun QF, Ding JG, Zhou Y, Chen W, Wang XF, Zhang WW, Du WJ, Ruan ZW, Hong L. Clinical efficacy of lopinavir/ritonavir in the treatment of Coronavirus disease 2019. Eur Rev Med Pharmacol Sci. 2020;24:3390-3396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 73] [Reference Citation Analysis (0)] |
| 35. | AbbVie Inc. KALETRA (lopinavir and ritonavir) [package insert] North Chicago, IL: AbbVie Inc., 2016. |
| 36. | Molina JM, Podsadecki TJ, Johnson MA, Wilkin A, Domingo P, Myers R, Hairrell JM, Rode RA, King MS, Hanna GJ. A lopinavir/ritonavir-based once-daily regimen results in better compliance and is non-inferior to a twice-daily regimen through 96 wk. AIDS Res Hum Retroviruses. 2007;23:1505-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Guest JL, Ruffin C, Tschampa JM, DeSilva KE, Rimland D. Differences in rates of diarrhea in patients with human immunodeficiency virus receiving lopinavir-ritonavir or nelfinavir. Pharmacotherapy. 2004;24:727-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Liu F, Xu A, Zhang Y, Xuan W, Yan T, Pan K, Yu W, Zhang J. Patients of COVID-19 may benefit from sustained Lopinavir-combined regimen and the increase of Eosinophil may predict the outcome of COVID-19 progression. Int J Infect Dis. 2020;95:183-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 229] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 39. | Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA. 2000;283:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 680] [Cited by in RCA: 644] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 40. | Rock BM, Hengel SM, Rock DA, Wienkers LC, Kunze KL. Characterization of ritonavir-mediated inactivation of cytochrome P450 3A4. Mol Pharmacol. 2014;86:665-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Casado JL, Del Palacio M, Moya J, Rodriguez JM, Moreno A, Perez-Elías MJ, Belso A, Dronda F, Moreno S. Safety and pharmacokinetics of lopinavir in HIV/HCV coinfected patients with advanced liver disease. HIV Clin Trials. 2011;12:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 42. | Sulkowski MS. Hepatotoxicity associated with antiretroviral therapy containing HIV-1 protease inhibitors. Semin Liver Dis. 2003;23:183-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Mulangu S, Dodd LE, Davey RT Jr, Tshiani Mbaya O, Proschan M, Mukadi D, Lusakibanza Manzo M, Nzolo D, Tshomba Oloma A, Ibanda A, Ali R, Coulibaly S, Levine AC, Grais R, Diaz J, Lane HC, Muyembe-Tamfum JJ; PALM Writing Group, Sivahera B, Camara M, Kojan R, Walker R, Dighero-Kemp B, Cao H, Mukumbayi P, Mbala-Kingebeni P, Ahuka S, Albert S, Bonnett T, Crozier I, Duvenhage M, Proffitt C, Teitelbaum M, Moench T, Aboulhab J, Barrett K, Cahill K, Cone K, Eckes R, Hensley L, Herpin B, Higgs E, Ledgerwood J, Pierson J, Smolskis M, Sow Y, Tierney J, Sivapalasingam S, Holman W, Gettinger N, Vallée D, Nordwall J; PALM Consortium Study Team. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N Engl J Med. 2019;381:2293-2303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1120] [Cited by in RCA: 1092] [Article Influence: 182.0] [Reference Citation Analysis (0)] |
| 44. | Brown AJ, Won JJ, Graham RL, Dinnon KH 3rd, Sims AC, Feng JY, Cihlar T, Denison MR, Baric RS, Sheahan TP. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res. 2019;169:104541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 302] [Cited by in RCA: 358] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 45. | Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, Leist SR, Pyrc K, Feng JY, Trantcheva I, Bannister R, Park Y, Babusis D, Clarke MO, Mackman RL, Spahn JE, Palmiotti CA, Siegel D, Ray AS, Cihlar T, Jordan R, Denison MR, Baric RS. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1011] [Cited by in RCA: 1126] [Article Influence: 160.9] [Reference Citation Analysis (0)] |
| 46. | Zhang L, Zhou R. Structural Basis of the Potential Binding Mechanism of Remdesivir to SARS-CoV-2 RNA-Dependent RNA Polymerase. J Phys Chem B. 2020;124:6955-6962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 47. | Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4289] [Cited by in RCA: 4569] [Article Influence: 913.8] [Reference Citation Analysis (0)] |
| 48. | Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X, Smith EC, Case JB, Feng JY, Jordan R, Ray AS, Cihlar T, Siegel D, Mackman RL, Clarke MO, Baric RS, Denison MR. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio. 2018;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 920] [Cited by in RCA: 998] [Article Influence: 142.6] [Reference Citation Analysis (0)] |
| 49. | Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, Götte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem. 2020;295:4773-4779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 507] [Cited by in RCA: 560] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 50. | Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC; ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383:1813-1826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5711] [Cited by in RCA: 5121] [Article Influence: 1024.2] [Reference Citation Analysis (0)] |
| 51. | Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569-1578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2337] [Cited by in RCA: 2488] [Article Influence: 497.6] [Reference Citation Analysis (0)] |
| 52. | Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, Spinner CD, Galli M, Ahn MY, Nahass RG, Chen YS, SenGupta D, Hyland RH, Osinusi AO, Cao H, Blair C, Wei X, Gaggar A, Brainard DM, Towner WJ, Muñoz J, Mullane KM, Marty FM, Tashima KT, Diaz G, Subramanian A; GS-US-540-5773 Investigators. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020;383:1827-1837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 987] [Cited by in RCA: 987] [Article Influence: 197.4] [Reference Citation Analysis (0)] |
| 53. | Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A, Ogbuagu O, Malhotra P, Mullane KM, Castagna A, Chai LYA, Roestenberg M, Tsang OTY, Bernasconi E, Le Turnier P, Chang SC, SenGupta D, Hyland RH, Osinusi AO, Cao H, Blair C, Wang H, Gaggar A, Brainard DM, McPhail MJ, Bhagani S, Ahn MY, Sanyal AJ, Huhn G, Marty FM; GS-US-540-5774 Investigators. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial. JAMA. 2020;324:1048-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 986] [Cited by in RCA: 922] [Article Influence: 184.4] [Reference Citation Analysis (0)] |
| 54. | Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure FX, Nicastri E, Oda R, Yo K, Quiros-Roldan E, Studemeister A, Redinski J, Ahmed S, Bernett J, Chelliah D, Chen D, Chihara S, Cohen SH, Cunningham J, D'Arminio Monforte A, Ismail S, Kato H, Lapadula G, L'Her E, Maeno T, Majumder S, Massari M, Mora-Rillo M, Mutoh Y, Nguyen D, Verweij E, Zoufaly A, Osinusi AO, DeZure A, Zhao Y, Zhong L, Chokkalingam A, Elboudwarej E, Telep L, Timbs L, Henne I, Sellers S, Cao H, Tan SK, Winterbourne L, Desai P, Mera R, Gaggar A, Myers RP, Brainard DM, Childs R, Flanigan T. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl J Med. 2020;382:2327-2336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1926] [Cited by in RCA: 1884] [Article Influence: 376.8] [Reference Citation Analysis (0)] |
| 55. | Antinori S, Cossu MV, Ridolfo AL, Rech R, Bonazzetti C, Pagani G, Gubertini G, Coen M, Magni C, Castelli A, Borghi B, Colombo R, Giorgi R, Angeli E, Mileto D, Milazzo L, Vimercati S, Pellicciotta M, Corbellino M, Torre A, Rusconi S, Oreni L, Gismondo MR, Giacomelli A, Meroni L, Rizzardini G, Galli M. Compassionate remdesivir treatment of severe Covid-19 pneumonia in intensive care unit (ICU) and Non-ICU patients: Clinical outcome and differences in post-treatment hospitalisation status. Pharmacol Res. 2020;158:104899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (1)] |
| 56. | COVID-19 Investigation Team. Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States. Nat Med. 2020;26:861-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 245] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 57. | Durante-Mangoni E, Andini R, Bertolino L, Mele F, Florio LL, Murino P, Corcione A, Zampino R. Early experience with remdesivir in SARS-CoV-2 pneumonia. Infection. 2020;48:779-782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 58. | National Institute of Diabetes and Digestive and Kidney Diseases. Nucleoside Analogues. In: Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2020. |
| 59. | Jorgensen SCJ, Kebriaei R, Dresser LD. Remdesivir: Review of Pharmacology, Pre-clinical Data, and Emerging Clinical Experience for COVID-19. Pharmacotherapy. 2020;40:659-671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 169] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 60. | U. S. Food and Drug Administration. Fact sheet for health care providers Emergency Use Authorization (EUA) of Remdesivir (GS-5734™). [cited 1 May 2020]. In: U.S. Food and Drug Administration [Internet]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment. |
| 61. | Kang JE, Rhie SJ. Practice considerations on the use of investigational anti-COVID-19 medications: Dosage, administration and monitoring. J Clin Pharm Ther. 2020;45:1199-1205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 62. | Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, Seidah NG, Nichol ST. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1198] [Cited by in RCA: 1225] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 63. | Fantini J, Di Scala C, Chahinian H, Yahi N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents. 2020;55:105960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 354] [Cited by in RCA: 400] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 64. | Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Tissot Dupont H, Honoré S, Colson P, Chabrière E, La Scola B, Rolain JM, Brouqui P, Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3279] [Cited by in RCA: 3272] [Article Influence: 654.4] [Reference Citation Analysis (0)] |
| 65. | Chen Z, Hu J, Zhang Z, Jiang S, Han S, Yan D, Zhuang R, Hu B. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. 2020 Preprint. Available from: medRxiv:2020.03.22.20040758. [DOI] [Full Text] |
| 66. | Molina JM, Delaugerre C, Le Goff J, Mela-Lima B, Ponscarme D, Goldwirt L, de Castro N. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect. 2020;50:384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 492] [Cited by in RCA: 513] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 67. | Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Sevestre J, Mailhe M, Doudier B, Aubry C, Amrane S, Seng P, Hocquart M, Eldin C, Finance J, Vieira VE, Tissot-Dupont HT, Honoré S, Stein A, Million M, Colson P, La Scola B, Veit V, Jacquier A, Deharo JC, Drancourt M, Fournier PE, Rolain JM, Brouqui P, Raoult D. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med Infect Dis. 2020;34:101663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 512] [Cited by in RCA: 477] [Article Influence: 95.4] [Reference Citation Analysis (0)] |
| 68. | Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1597] [Cited by in RCA: 1602] [Article Influence: 320.4] [Reference Citation Analysis (0)] |
| 69. | Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, Labella A, Manson DK, Kubin C, Barr RG, Sobieszczyk ME, Schluger NW. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020;382:2411-2418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1226] [Cited by in RCA: 1154] [Article Influence: 230.8] [Reference Citation Analysis (0)] |
| 70. | Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, Damiani LP, Marcadenti A, Kawano-Dourado L, Lisboa T, Junqueira DLM, de Barros E Silva PGM, Tramujas L, Abreu-Silva EO, Laranjeira LN, Soares AT, Echenique LS, Pereira AJ, Freitas FGR, Gebara OCE, Dantas VCS, Furtado RHM, Milan EP, Golin NA, Cardoso FF, Maia IS, Hoffmann Filho CR, Kormann APM, Amazonas RB, Bocchi de Oliveira MF, Serpa-Neto A, Falavigna M, Lopes RD, Machado FR, Berwanger O; Coalition Covid-19 Brazil I Investigators. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med. 2020;383:2041-2052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 751] [Cited by in RCA: 768] [Article Influence: 153.6] [Reference Citation Analysis (0)] |
| 71. | Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, Skipper CP, Nascene AA, Nicol MR, Abassi M, Engen NW, Cheng MP, LaBar D, Lother SA, MacKenzie LJ, Drobot G, Marten N, Zarychanski R, Kelly LE, Schwartz IS, McDonald EG, Rajasingham R, Lee TC, Hullsiek KH. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. N Engl J Med. 2020;383:517-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 994] [Cited by in RCA: 923] [Article Influence: 184.6] [Reference Citation Analysis (0)] |
| 72. | Drent M, Proesmans VLJ, Elfferich MDP, Jessurun NT, de Jong SMG, Ebner NM, Lewis EDO, Bast A. Ranking Self-reported Gastrointestinal Side Effects of Pharmacotherapy in Sarcoidosis. Lung. 2020;198:395-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 73. | Srinivasa A, Tosounidou S, Gordon C. Increased Incidence of Gastrointestinal Side Effects in Patients Taking Hydroxychloroquine: A Brand-related Issue? J Rheumatol. 2017;44:398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 74. | Tétu P, Hamelin A, Lebrun-Vignes B, Soria A, Barbaud A, Francès C, Chasset F. [Prevalence of hydroxychloroquine-induced side-effects in dermatology patients: A retrospective survey of 102 patients]. Ann Dermatol Venereol. 2018;145:395-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 75. | Arnaout A, Robertson SJ, Pond GR, Lee H, Jeong A, Ianni L, Kroeger L, Hilton J, Coupland S, Gottlieb C, Hurley B, McCarthy A, Clemons M. A randomized, double-blind, window of opportunity trial evaluating the effects of chloroquine in breast cancer patients. Breast Cancer Res Treat. 2019;178:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 76. | Furst DE, Lindsley H, Baethge B, Botstein GR, Caldwell J, Dietz F, Ettlinger R, Golden HE, McLaughlin GE, Moreland LW, Roberts WN, Rooney TW, Rothschild B, Sack M, Sebba AI, Weisman M, Welch KE, Yocum D. Dose-loading with hydroxychloroquine improves the rate of response in early, active rheumatoid arthritis: a randomized, double-blind six-week trial with eighteen-week extension. Arthritis Rheum. 1999;42:357-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 77. | Arshad S, Kilgore P, Chaudhry ZS, Jacobsen G, Wang DD, Huitsing K, Brar I, Alangaden GJ, Ramesh MS, McKinnon JE, O'Neill W, Zervos M; Henry Ford COVID-19 Task Force. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020;97:396-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 392] [Cited by in RCA: 371] [Article Influence: 74.2] [Reference Citation Analysis (0)] |
| 78. | Hung IF, Lung KC, Tso EY, Liu R, Chung TW, Chu MY, Ng YY, Lo J, Chan J, Tam AR, Shum HP, Chan V, Wu AK, Sin KM, Leung WS, Law WL, Lung DC, Sin S, Yeung P, Yip CC, Zhang RR, Fung AY, Yan EY, Leung KH, Ip JD, Chu AW, Chan WM, Ng AC, Lee R, Fung K, Yeung A, Wu TC, Chan JW, Yan WW, Chan JF, Lie AK, Tsang OT, Cheng VC, Que TL, Lau CS, Chan KH, To KK, Yuen KY. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695-1704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1028] [Cited by in RCA: 1061] [Article Influence: 212.2] [Reference Citation Analysis (1)] |
| 79. | Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther. 2020;14:58-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 834] [Cited by in RCA: 873] [Article Influence: 174.6] [Reference Citation Analysis (0)] |
| 80. |
Chen C, Zhang Y, Huang J, Yin P, Cheng Z, Wu J, Chen S, Chen Bo, Lu M, Luo Y, Ju L, Zhang J, Wang X.
Favipiravir |
| 81. | Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, Liao X, Gu Y, Cai Q, Yang Y, Shen C, Li X, Peng L, Huang D, Zhang J, Zhang S, Wang F, Liu J, Chen L, Chen S, Wang Z, Zhang Z, Cao R, Zhong W, Liu Y, Liu L. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering (Beijing). 2020;6:1192-1198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 702] [Cited by in RCA: 774] [Article Influence: 154.8] [Reference Citation Analysis (0)] |
| 82. | Rocco PRM, Silva PL, Cruz FF, Melo-Junior MAC, Tierno PFGMM, Moura MA, De Oliveira LFG, Lima CC, Dos Santos EA, Junior WF, Fernandes APSM, Franchini KG, Magri E, de Moraes NF, Gonçalves JMJ, Carbonieri MN, Dos Santos IS, Paes NF, Maciel PVM, Rocha RP, de Carvalho AF, Alves PA, Proença-Módena JL, Cordeiro AT, Trivella DBB, Marques RE, Luiz RR, Pelosi P, Lapa E Silva JR; SARITA-2 investigators. Early use of nitazoxanide in mild COVID-19 disease: randomised, placebo-controlled trial. Eur Respir J. 2021;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 83. | Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1171] [Cited by in RCA: 1302] [Article Influence: 260.4] [Reference Citation Analysis (0)] |
| 84. | Elalfy H, Besheer T, El-Mesery A, El-Gilany AH, Soliman MA, Alhawarey A, Alegezy M, Elhadidy T, Hewidy AA, Zaghloul H, Neamatallah MAM, Raafat D, El-Emshaty WM, Abo El Kheir NY, El-Bendary M. Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-19. J Med Virol. 2021;93:3176-3183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 85. | Rajter JC, Sherman MS, Fatteh N, Vogel F, Sacks J, Rajter JJ. Use of Ivermectin Is Associated With Lower Mortality in Hospitalized Patients With Coronavirus Disease 2019: The Ivermectin in COVID Nineteen Study. Chest. 2021;159:85-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 152] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 86. | Fischer W, Eron JJ, Holman W, Cohen MS, Fang L, Szewczyk LJ, Sheahan TP, Baric R, Mollan KR, Wolfe CR, Duke ER, Azizad MM, Borroto-Esoda K, Wohl DA, Loftis AJ, Alabanza P, Lipansky F, Painter WP. Molnupiravir, an Oral Antiviral Treatment for COVID-19. medRxiv. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 87. | Azkur AK, Akdis M, Azkur D, Sokolowska M, van de Veen W, Brüggen MC, O'Mahony L, Gao Y, Nadeau K, Akdis CA. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75:1564-1581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 820] [Cited by in RCA: 743] [Article Influence: 148.6] [Reference Citation Analysis (0)] |
| 88. | Conti P, Ronconi G, Caraffa A, Gallenga CE, Ross R, Frydas I, Kritas SK. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34:327-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 594] [Reference Citation Analysis (0)] |
| 89. | Wu J. Tackle the free radicals damage in COVID-19. Nitric Oxide. 2020;102:39-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 90. | Huppert LA, Matthay MA, Ware LB. Pathogenesis of Acute Respiratory Distress Syndrome. Semin Respir Crit Care Med. 2019;40:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 317] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 91. | Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620-2629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2835] [Cited by in RCA: 3419] [Article Influence: 683.8] [Reference Citation Analysis (0)] |
| 92. | Liu J, Li S, Liu J, Liang B, Wang X, Wang H, Li W, Tong Q, Yi J, Zhao L, Xiong L, Guo C, Tian J, Luo J, Yao J, Pang R, Shen H, Peng C, Liu T, Zhang Q, Wu J, Xu L, Lu S, Wang B, Weng Z, Han C, Zhu H, Zhou R, Zhou H, Chen X, Ye P, Zhu B, Wang L, Zhou W, He S, He Y, Jie S, Wei P, Zhang J, Lu Y, Wang W, Zhang L, Li L, Zhou F, Wang J, Dittmer U, Lu M, Hu Y, Yang D, Zheng X. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1171] [Cited by in RCA: 1208] [Article Influence: 241.6] [Reference Citation Analysis (0)] |
| 93. | Gao Y, Li T, Han M, Li X, Wu D, Xu Y, Zhu Y, Liu Y, Wang X, Wang L. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020;92:791-796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 548] [Cited by in RCA: 635] [Article Influence: 127.0] [Reference Citation Analysis (0)] |
| 94. | Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infect. 2020;80:607-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1858] [Cited by in RCA: 1960] [Article Influence: 392.0] [Reference Citation Analysis (0)] |
| 95. | Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front Immunol. 2020;11:1708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 768] [Cited by in RCA: 726] [Article Influence: 145.2] [Reference Citation Analysis (0)] |
| 96. | Xiang Z, Liu J, Shi D, Chen W, Li J, Yan R, Bi Y, Hu W, Zhu Z, Yu Y, Yang Z. Glucocorticoids improve severe or critical COVID-19 by activating ACE2 and reducing IL-6 Levels. Int J Biol Sci. 2020;16:2382-2391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 97. | RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6762] [Cited by in RCA: 7390] [Article Influence: 1847.5] [Reference Citation Analysis (1)] |
| 98. | Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. 2020;582:469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 252] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 99. | WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, Annane D, Azevedo LCP, Berwanger O, Cavalcanti AB, Dequin PF, Du B, Emberson J, Fisher D, Giraudeau B, Gordon AC, Granholm A, Green C, Haynes R, Heming N, Higgins JPT, Horby P, Jüni P, Landray MJ, Le Gouge A, Leclerc M, Lim WS, Machado FR, McArthur C, Meziani F, Møller MH, Perner A, Petersen MW, Savovic J, Tomazini B, Veiga VC, Webb S, Marshall JC. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA. 2020;324:1330-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1683] [Cited by in RCA: 1681] [Article Influence: 336.2] [Reference Citation Analysis (0)] |
| 100. | Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473-475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1415] [Cited by in RCA: 1444] [Article Influence: 288.8] [Reference Citation Analysis (0)] |
| 101. | Lee DT, Wing YK, Leung HC, Sung JJ, Ng YK, Yiu GC, Chen RY, Chiu HF. Factors associated with psychosis among patients with severe acute respiratory syndrome: a case-control study. Clin Infect Dis. 2004;39:1247-1249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 102. | Xiao JZ, Ma L, Gao J, Yang ZJ, Xing XY, Zhao HC, Jiao JS, Li GW. [Glucocorticoid-induced diabetes in severe acute respiratory syndrome: the impact of high dosage and duration of methylprednisolone therapy]. Zhonghua Nei Ke Za Zhi. 2004;43:179-182. [PubMed] |
| 103. | Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, Hussein MA, Jose J, Pinto R, Al-Omari A, Kharaba A, Almotairi A, Al Khatib K, Alraddadi B, Shalhoub S, Abdulmomen A, Qushmaq I, Mady A, Solaiman O, Al-Aithan AM, Al-Raddadi R, Ragab A, Balkhy HH, Al Harthy A, Deeb AM, Al Mutairi H, Al-Dawood A, Merson L, Hayden FG, Fowler RA; Saudi Critical Care Trial Group. Corticosteroid Therapy for Critically Ill Patients with Middle East Respiratory Syndrome. Am J Respir Crit Care Med. 2018;197:757-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 791] [Article Influence: 131.8] [Reference Citation Analysis (0)] |
| 104. | Yang Z, Liu J, Zhou Y, Zhao X, Zhao Q. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect. 2020;81:e13-e20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 239] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 105. | Piper JM, Ray WA, Daugherty JR, Griffin MR. Corticosteroid use and peptic ulcer disease: role of nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1991;114:735-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 372] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 106. | Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. A meta-analysis. Ann Intern Med. 1991;115:787-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 859] [Cited by in RCA: 800] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 107. | Caplan A, Fett N, Rosenbach M, Werth VP, Micheletti RG. Prevention and management of glucocorticoid-induced side effects: A comprehensive review: Gastrointestinal and endocrinologic side effects. J Am Acad Dermatol. 2017;76:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 108. | Sadr-Azodi O, Mattsson F, Bexlius TS, Lindblad M, Lagergren J, Ljung R. Association of oral glucocorticoid use with an increased risk of acute pancreatitis: a population-based nested case-control study. JAMA Intern Med. 2013;173:444-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 109. | Woods CP, Hazlehurst JM, Tomlinson JW. Glucocorticoids and non-alcoholic fatty liver disease. J Steroid Biochem Mol Biol. 2015;154:94-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 110. | Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2:901-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 938] [Article Influence: 85.3] [Reference Citation Analysis (0)] |
| 111. | Hu Y, Sun J, Dai Z, Deng H, Li X, Huang Q, Wu Y, Sun L, Xu Y. Prevalence and severity of corona virus disease 2019 (COVID-19): A systematic review and meta-analysis. J Clin Virol. 2020;127:104371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 471] [Cited by in RCA: 387] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 112. | Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736-E741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 503] [Article Influence: 100.6] [Reference Citation Analysis (0)] |
| 113. | Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, Labreuche J, Mathieu D, Pattou F, Jourdain M; LICORN and the Lille COVID-19 and Obesity study group. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity (Silver Spring). 2020;28:1195-1199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1263] [Cited by in RCA: 1577] [Article Influence: 315.4] [Reference Citation Analysis (0)] |
| 114. | Yang J, Hu J, Zhu C. Obesity aggravates COVID-19: A systematic review and meta-analysis. J Med Virol. 2021;93:257-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 211] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 115. | Zhou Y, Chi J, Lv W, Wang Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (Covid-19). Diabetes Metab Res Rev. 2021;37:e3377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 358] [Cited by in RCA: 316] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 116. | Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 Cytokine Storm; What We Know So Far. Front Immunol. 2020;11:1446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1044] [Cited by in RCA: 1097] [Article Influence: 219.4] [Reference Citation Analysis (0)] |
| 117. | Coomes EA, Haghbayan H. Interleukin-6 in Covid-19: A systematic review and meta-analysis. Rev Med Virol. 2020;30:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 493] [Cited by in RCA: 513] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 118. | Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, Franceschini E, Cuomo G, Orlando G, Borghi V, Santoro A, Di Gaetano M, Puzzolante C, Carli F, Bedini A, Corradi L, Fantini R, Castaniere I, Tabbì L, Girardis M, Tedeschi S, Giannella M, Bartoletti M, Pascale R, Dolci G, Brugioni L, Pietrangelo A, Cossarizza A, Pea F, Clini E, Salvarani C, Massari M, Viale PL, Mussini C. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474-e484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 604] [Cited by in RCA: 688] [Article Influence: 137.6] [Reference Citation Analysis (0)] |
| 119. | Gupta S, Wang W, Hayek SS, Chan L, Mathews KS, Melamed ML, Brenner SK, Leonberg-Yoo A, Schenck EJ, Radbel J, Reiser J, Bansal A, Srivastava A, Zhou Y, Finkel D, Green A, Mallappallil M, Faugno AJ, Zhang J, Velez JCQ, Shaefi S, Parikh CR, Charytan DM, Athavale AM, Friedman AN, Redfern RE, Short SAP, Correa S, Pokharel KK, Admon AJ, Donnelly JP, Gershengorn HB, Douin DJ, Semler MW, Hernán MA, Leaf DE; STOP-COVID Investigators. Association Between Early Treatment With Tocilizumab and Mortality Among Critically Ill Patients With COVID-19. JAMA Intern Med. 2021;181:41-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 358] [Article Influence: 89.5] [Reference Citation Analysis (0)] |
| 120. | Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, Horick NK, Healy BC, Shah R, Bensaci AM, Woolley AE, Nikiforow S, Lin N, Sagar M, Schrager H, Huckins DS, Axelrod M, Pincus MD, Fleisher J, Sacks CA, Dougan M, North CM, Halvorsen YD, Thurber TK, Dagher Z, Scherer A, Wallwork RS, Kim AY, Schoenfeld S, Sen P, Neilan TG, Perugino CA, Unizony SH, Collier DS, Matza MA, Yinh JM, Bowman KA, Meyerowitz E, Zafar A, Drobni ZD, Bolster MB, Kohler M, D'Silva KM, Dau J, Lockwood MM, Cubbison C, Weber BN, Mansour MK; BACC Bay Tocilizumab Trial Investigators. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N Engl J Med. 2020;383:2333-2344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 998] [Cited by in RCA: 1014] [Article Influence: 202.8] [Reference Citation Analysis (0)] |
| 121. | Muhović D, Bojović J, Bulatović A, Vukčević B, Ratković M, Lazović R, Smolović B. First case of drug-induced liver injury associated with the use of tocilizumab in a patient with COVID-19. Liver Int. 2020;40:1901-1905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 122. | Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, Marconi VC, Ruiz-Palacios GM, Hsieh L, Kline S, Tapson V, Iovine NM, Jain MK, Sweeney DA, El Sahly HM, Branche AR, Regalado Pineda J, Lye DC, Sandkovsky U, Luetkemeyer AF, Cohen SH, Finberg RW, Jackson PEH, Taiwo B, Paules CI, Arguinchona H, Erdmann N, Ahuja N, Frank M, Oh MD, Kim ES, Tan SY, Mularski RA, Nielsen H, Ponce PO, Taylor BS, Larson L, Rouphael NG, Saklawi Y, Cantos VD, Ko ER, Engemann JJ, Amin AN, Watanabe M, Billings J, Elie MC, Davey RT, Burgess TH, Ferreira J, Green M, Makowski M, Cardoso A, de Bono S, Bonnett T, Proschan M, Deye GA, Dempsey W, Nayak SU, Dodd LE, Beigel JH; ACTT-2 Study Group Members. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med. 2021;384:795-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1159] [Cited by in RCA: 1333] [Article Influence: 333.3] [Reference Citation Analysis (1)] |
| 123. | Walsh EE, Frenck RW Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, Swanson KA, Li P, Koury K, Kalina W, Cooper D, Fontes-Garfias C, Shi PY, Türeci Ö, Tompkins KR, Lyke KE, Raabe V, Dormitzer PR, Jansen KU, Şahin U, Gruber WC. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med. 2020;383:2439-2450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1747] [Cited by in RCA: 1902] [Article Influence: 380.4] [Reference Citation Analysis (0)] |
| 124. | Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603-2615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10556] [Cited by in RCA: 10719] [Article Influence: 2143.8] [Reference Citation Analysis (1)] |
| 125. | Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T; COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7073] [Cited by in RCA: 7580] [Article Influence: 1895.0] [Reference Citation Analysis (1)] |
| 126. | Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, Bibi S, Briner C, Cicconi P, Collins AM, Colin-Jones R, Cutland CL, Darton TC, Dheda K, Duncan CJA, Emary KRW, Ewer KJ, Fairlie L, Faust SN, Feng S, Ferreira DM, Finn A, Goodman AL, Green CM, Green CA, Heath PT, Hill C, Hill H, Hirsch I, Hodgson SHC, Izu A, Jackson S, Jenkin D, Joe CCD, Kerridge S, Koen A, Kwatra G, Lazarus R, Lawrie AM, Lelliott A, Libri V, Lillie PJ, Mallory R, Mendes AVA, Milan EP, Minassian AM, McGregor A, Morrison H, Mujadidi YF, Nana A, O'Reilly PJ, Padayachee SD, Pittella A, Plested E, Pollock KM, Ramasamy MN, Rhead S, Schwarzbold AV, Singh N, Smith A, Song R, Snape MD, Sprinz E, Sutherland RK, Tarrant R, Thomson EC, Török ME, Toshner M, Turner DPJ, Vekemans J, Villafana TL, Watson MEE, Williams CJ, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ; Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3551] [Cited by in RCA: 3443] [Article Influence: 860.8] [Reference Citation Analysis (0)] |
| 127. | Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, de Groot AM, Stoop J, Tete S, Van Damme W, Leroux-Roels I, Berghmans PJ, Kimmel M, Van Damme P, de Hoon J, Smith W, Stephenson KE, De Rosa SC, Cohen KW, McElrath MJ, Cormier E, Scheper G, Barouch DH, Hendriks J, Struyf F, Douoguih M, Van Hoof J, Schuitemaker H. Interim Results of a Phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine. N Engl J Med. 2021;384:1824-1835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 877] [Cited by in RCA: 861] [Article Influence: 215.3] [Reference Citation Analysis (0)] |
| 128. | Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, Goldblatt D, Kotoucek P, Thomas W, Lester W. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. 2021;384:2202-2211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 651] [Cited by in RCA: 754] [Article Influence: 188.5] [Reference Citation Analysis (0)] |
| 129. | Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, Han W, Chen Z, Tang R, Yin W, Chen X, Liu X, Jiang C, Li J, Yang M, Song Y, Wang X, Gao Q, Zhu F. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 743] [Cited by in RCA: 967] [Article Influence: 193.4] [Reference Citation Analysis (0)] |
| 130. | Wu Z, Hu Y, Xu M, Chen Z, Yang W, Jiang Z, Li M, Jin H, Cui G, Chen P, Wang L, Zhao G, Ding Y, Zhao Y, Yin W. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:803-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 371] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 131. | Palacios R, Patiño EG, de Oliveira Piorelli R, Conde MTRP, Batista AP, Zeng G, Xin Q, Kallas EG, Flores J, Ockenhouse CF, Gast C. Double-Blind, Randomized, Placebo-Controlled Phase III Clinical Trial to Evaluate the Efficacy and Safety of treating Healthcare Professionals with the Adsorbed COVID-19 (Inactivated) Vaccine Manufactured by Sinovac - PROFISCOV: A structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 180] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 132. | Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, Tan W, Wu G, Xu M, Lou Z, Huang W, Xu W, Huang B, Wang W, Zhang W, Li N, Xie Z, Ding L, You W, Zhao Y, Yang X, Liu Y, Wang Q, Huang L, Xu G, Luo B, Liu P, Guo W. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 899] [Cited by in RCA: 817] [Article Influence: 204.3] [Reference Citation Analysis (0)] |
| 133. | McMahon BJ, Wainwright K, Bulkow L, Parkinson AJ, Lindenbaum M, Wainwright R, Helminiak C. Response to hepatitis B vaccine in Alaska natives with chronic alcoholism compared with non-alcoholic control subjects. Am J Med. 1990;88:460-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 134. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1821] [Article Influence: 260.1] [Reference Citation Analysis (2)] |
| 135. | Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, Hernán MA, Lipsitch M, Reis B, Balicer RD. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med. 2021;384:1412-1423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1992] [Cited by in RCA: 1823] [Article Influence: 455.8] [Reference Citation Analysis (0)] |
| 136. | Barda N, Dagan N, Balicer RD. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. Reply. N Engl J Med. 2021;384:1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 137. | Sharma A, Patnaik I, Kumar A, Gupta R. COVID-19 vaccines in patients with chronic liver disease. J Clin Exp Hepatol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 138. | Cornberg M, Buti M, Eberhardt CS, Grossi PA, Shouval D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. 2021;74:944-951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 174] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 139. | Fix OK, Blumberg EA, Chang KM, Chu J, Chung RT, Goacher EK, Hameed B, Kaul DR, Kulik LM, Kwok RM, McGuire BM, Mulligan DC, Price JC, Reau NS, Reddy KR, Reynolds A, Rosen HR, Russo MW, Schilsky ML, Verna EC, Ward JW, Fontana RJ; AASLD COVID-19 Vaccine Working Group. American Association for the Study of Liver Diseases Expert Panel Consensus Statement: Vaccines to Prevent Coronavirus Disease 2019 Infection in Patients With Liver Disease. Hepatology. 2021;74:1049-1064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 140. | Than NN, Hodson J, Schmidt-Martin D, Taubert R, Wawman RE, Botter M, Gautam N, Bock K, Jones R, Appanna GD, Godkin A, Montano-Loza AJ, Lammert F, Schramm C, Manns MP, Swain M, Burak KW, Adams DH, Hirschfield GM, Oo YH. Efficacy of rituximab in difficult-to-manage autoimmune hepatitis: Results from the International Autoimmune Hepatitis Group. JHEP Rep. 2019;1:437-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 141. | Yri OE, Torfoss D, Hungnes O, Tierens A, Waalen K, Nordøy T, Dudman S, Kilander A, Wader KF, Ostenstad B, Ekanger R, Meyer P, Kolstad A. Rituximab blocks protective serologic response to influenza A (H1N1) 2009 vaccination in lymphoma patients during or within 6 mo after treatment. Blood. 2011;118:6769-6771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 142. | Bedognetti D, Zoppoli G, Massucco C, Zanardi E, Zupo S, Bruzzone A, Sertoli MR, Balleari E, Racchi O, Messina M, Caltabiano G, Icardi G, Durando P, Marincola FM, Boccardo F, Ferrarini M, Ansaldi F, De Maria A. Impaired response to influenza vaccine associated with persistent memory B cell depletion in non-Hodgkin's lymphoma patients treated with rituximab-containing regimens. J Immunol. 2011;186:6044-6055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 143. | Takata T, Suzumiya J, Ishikawa T, Takamatsu Y, Ikematsu H, Tamura K. Attenuated antibody reaction for the primary antigen but not for the recall antigen of influenza vaccination in patients with non-Hodgkin B-cell lymphoma after the administration of rituximab-CHOP. J Clin Exp Hematop. 2009;49:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 144. | van der Kolk LE, Baars JW, Prins MH, van Oers MH. Rituximab treatment results in impaired secondary humoral immune responsiveness. Blood. 2002;100:2257-2259. [PubMed] |
| 145. | Bingham CO 3rd, Looney RJ, Deodhar A, Halsey N, Greenwald M, Codding C, Trzaskoma B, Martin F, Agarwal S, Kelman A. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum. 2010;62:64-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 335] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 146. | Kim W, Kim SH, Huh SY, Kong SY, Choi YJ, Cheong HJ, Kim HJ. Reduced antibody formation after influenza vaccination in patients with neuromyelitis optica spectrum disorder treated with rituximab. Eur J Neurol. 2013;20:975-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 147. | Eisenberg RA, Jawad AF, Boyer J, Maurer K, McDonald K, Prak ET, Sullivan KE. Rituximab-treated patients have a poor response to influenza vaccination. J Clin Immunol. 2013;33:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 148. | Nazi I, Kelton JG, Larché M, Snider DP, Heddle NM, Crowther MA, Cook RJ, Tinmouth AT, Mangel J, Arnold DM. The effect of rituximab on vaccine responses in patients with immune thrombocytopenia. Blood. 2013;122:1946-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 149. | Marjot T, Webb GJ, Barritt AS, Ginès P, Lohse AW, Moon AM, Pose E, Trivedi P, Barnes E. SARS-CoV-2 vaccination in patients with liver disease: responding to the next big question. Lancet Gastroenterol Hepatol. 2021;6:156-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 150. | Alexander JL, Moran GW, Gaya DR, Raine T, Hart A, Kennedy NA, Lindsay JO, MacDonald J, Segal JP, Sebastian S, Selinger CP, Parkes M, Smith PJ, Dhar A, Subramanian S, Arasaradnam R, Lamb CA, Ahmad T, Lees CW, Dobson L, Wakeman R, Iqbal TH, Arnott I, Powell N; Inflammatory Bowel Disease section of the British Society of Gastroenterology and the the Inflammatory Bowel Disease Clinical Research Group. SARS-CoV-2 vaccination for patients with inflammatory bowel disease: a British Society of Gastroenterology Inflammatory Bowel Disease section and IBD Clinical Research Group position statement. Lancet Gastroenterol Hepatol. 2021;6:218-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 105] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 151. | Siegel CA, Melmed GY, McGovern DP, Rai V, Krammer F, Rubin DT, Abreu MT, Dubinsky MC; International Organization for the Study of Inflammatory Bowel Disease (IOIBD); International Organization for the Study of Inflammatory Bowel Diseases (IOIBD). SARS-CoV-2 vaccination for patients with inflammatory bowel diseases: recommendations from an international consensus meeting. Gut. 2021;70:635-640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 159] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 152. | Bailey LC, Razzaghi H, Burrows EK, Bunnell HT, Camacho PEF, Christakis DA, Eckrich D, Kitzmiller M, Lin SM, Magnusen BC, Newland J, Pajor NM, Ranade D, Rao S, Sofela O, Zahner J, Bruno C, Forrest CB. Assessment of 135 794 Pediatric Patients Tested for Severe Acute Respiratory Syndrome Coronavirus 2 Across the United States. JAMA Pediatr. 2021;175:176-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 168] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 153. | Swann OV, Holden KA, Turtle L, Pollock L, Fairfield CJ, Drake TM, Seth S, Egan C, Hardwick HE, Halpin S, Girvan M, Donohue C, Pritchard M, Patel LB, Ladhani S, Sigfrid L, Sinha IP, Olliaro PL, Nguyen-Van-Tam JS, Horby PW, Merson L, Carson G, Dunning J, Openshaw PJM, Baillie JK, Harrison EM, Docherty AB, Semple MG; ISARIC4C Investigators. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370:m3249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 405] [Cited by in RCA: 406] [Article Influence: 81.2] [Reference Citation Analysis (0)] |
| 154. | Su L, Ma X, Yu H, Zhang Z, Bian P, Han Y, Sun J, Liu Y, Yang C, Geng J, Gai Z. The different clinical characteristics of corona virus disease cases between children and their families in China - the character of children with COVID-19. Emerg Microbes Infect. 2020;9:707-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 224] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 155. | U S. Food and Drug Administration. Pfizer-BioNTech COVID-19 Vaccine. [cited December 22, 2020]. In: U.S. Food and Drug Administration [Internet]. Available from: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine. |
| 156. | Frenck RW Jr, Klein NP, Kitchin N, Gurtman A, Absalon J, Lockhart S, Perez JL, Walter EB, Senders S, Bailey R, Swanson KA, Ma H, Xu X, Koury K, Kalina WV, Cooper D, Jennings T, Brandon DM, Thomas SJ, Türeci Ö, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group. Safety, Immunogenicity, and Efficacy of the BNT162b2 Covid-19 Vaccine in Adolescents. N Engl J Med. 2021;385:239-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 551] [Cited by in RCA: 664] [Article Influence: 166.0] [Reference Citation Analysis (0)] |
| 157. | Rentsch CT, Beckman JA, Tomlinson L, Gellad WF, Alcorn C, Kidwai-Khan F, Skanderson M, Brittain E, King JT Jr, Ho YL, Eden S, Kundu S, Lann MF, Greevy RA Jr, Ho PM, Heidenreich PA, Jacobson DA, Douglas IJ, Tate JP, Evans SJW, Atkins D, Justice AC, Freiberg MS. Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: cohort study. BMJ. 2021;372:n311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 150] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 158. | Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2516] [Cited by in RCA: 2842] [Article Influence: 568.4] [Reference Citation Analysis (0)] |
| 159. | Wong MCS, Wong ELY, Huang J, Cheung AWL, Law K, Chong MKC, Ng RWY, Lai CKC, Boon SS, Lau JTF, Chen Z, Chan PKS. Acceptance of the COVID-19 vaccine based on the health belief model: A population-based survey in Hong Kong. Vaccine. 2021;39:1148-1156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 309] [Article Influence: 77.3] [Reference Citation Analysis (0)] |