Published online Nov 27, 2021. doi: 10.4254/wjh.v13.i11.1802
Peer-review started: March 21, 2021
First decision: August 18, 2021
Revised: August 31, 2021
Accepted: October 14, 2021
Article in press: October 14, 2021
Published online: November 27, 2021
Processing time: 247 Days and 16.9 Hours
The use of umbilical venous catheters (UVCs) in the perinatal period may be associated with severe complications, including the occurrence of portal vein thrombosis (PVT).
To assess the incidence of UVC-related PVT in infants with postnatal age up to three months.
A systematic and comprehensive database searching (PubMed, Cochrane Library, Scopus, Web of Science) was performed for studies from 1980 to 2020 (the search was last updated on November 28, 2020). We included in the final analyses all peer-reviewed prospective cohort studies, retrospective cohort studies and case-control studies. The reference lists of included articles were hand-searched to identify additional studies of interest. Studies were considered eligible when they included infants with postnatal age up to three months with UVC-associated PVT. Incidence estimates were pooled by using random effects meta-analyses. The quality of included studies was assessed using the Newcastle-Ottawa scale. The systematic review was performed according to the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines.
Overall, 16 studies were considered eligible and included in the final analyses. The data confirmed the relevant risk of UVC-related thrombosis. The mean pooled incidence of such condition was 12%, although it varied across studies (0%-49%). In 15/16 studies (94%), diagnosis of thrombosis was made accidentally during routine screening controls, whilst in 1/16 study (6%) targeted imaging assessments were carried out in neonates with clinical concerns for a thrombus. Tip position was investigated by abdominal ultrasound (US) alone in 1/16 (6%) studies, by a combination of radiography and abdominal US in 14/16 (88%) studies and by a combination of radiography, abdominal US and echocardiography in 1/16 (6%) studies.
To the best of our knowledge, this is the first systematic review specifically investigating the incidence of UVC-related PVT. The use of UVCs requires a high index of suspicion, because its use is significantly associated with PVT. Well-designed prospective studies are required to assess the optimal approach to prevent UVC-related thrombosis of the portal system.
Core Tip: Portal vein thrombosis (PVT) is a dreadful complication that can occur after umbilical vein catheterization in neonates. Although previous observational studies have provided a general overview about the risk of this complication, the present systematic review specifically investigates the incidence catheter-related PVT and identifies relevant gaps in knowledge about the optimal diagnostic approach highlighting the need for prospective randomized studies and updated guidelines.
- Citation: Bersani I, Piersigilli F, Iacona G, Savarese I, Campi F, Dotta A, Auriti C, Di Stasio E, Garcovich M. Incidence of umbilical vein catheter-associated thrombosis of the portal system: A systematic review and meta-analysis. World J Hepatol 2021; 13(11): 1802-1815
- URL: https://www.wjgnet.com/1948-5182/full/v13/i11/1802.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i11.1802
The placement of an umbilical venous catheter (UVC) is a common procedure in neonatology and has multiple clinical indications driven by the need for quick and secure access for medication administration[1]. During placement, the UVC should run through the umbilical vein, pass the medial portion of the left portal vein at the umbilico-portal confluence, join the direct communication existing between the umbilical vein and the ductus venosus and, through it, bypass the liver and join the inferior vena cava[2,3]. The UVC has to be placed in a central position, ideally at the junction between the inferior vena cava and the right atrium. If a central position is not achieved, then the tip of the catheter can be left below the liver, i.e., below the level of umbilical-portal confluence (peripheral position). The UVC in peripheral position can be used as an emergency access, but it has to be replaced as soon as possible by a central venous catheter. To prevent UVC-related complications, a proper assessment of catheter tip position is mandatory before its use. In fact, if the tip of the catheter is too deep, it can cause complications such as thrombo-embolic disorders, arrhythmias, and pericardial effusion. On the other hand, if the tip of the UVC is too low, then it can be associated with necrotizing enterocolitis, colon perforation, hepatic abscess, and portal vein thrombosis (PVT)[1,4-9]. Furthermore, if the ductus venosus is not perfectly aligned to the umbilical vein, the UVC may unintentionally enter the portal system through the left portal vein during placement and possibly lead to severe complications involving both the hepatic vasculature and parenchyma[1,2,5-8,10-16]. Such liver complications may arise from multiple mechanisms including thrombosis of the portal system vasculature, infusion of irritating drugs and/or hypertonic solutions within the UVC leading to hepatic necrotizing direct mechanical injury[3,17-19]. Besides individual hereditary or acquired predisposing factors (such as prematurity, hereditary prothrombotic disorders, sepsis, the need of transfusions, hyper-viscosity syndrome, dehydration, asphyxia, congenital malformations etc.), whose actual role is still debated[3,10,19-26], umbilical venous catheterization itself represents a risk factor for the development of PVT[18]. In fact, multiple factors may explain the association between UVC and PVT: The introduction of a foreign surface with thrombogenic properties in a small diameter vessel, endothelial damage, and the well-known pro-thrombotic predisposition typical of the neonatal period[27-29]. Symptoms/signs suggestive of PVT may include unexplained thrombocytopenia, catheter-obstructed fluid delivery, increased UVC in-line pressure, impaired lower body/extremity perfusion, although PVT may remain completely asymptomatic[30,31]. When persisting, PVT may inflict substantial damage to the liver leading to portal hypertension, mainly related to the increased vascular resistance in the portal venous system, and to liver atrophy[11,19,32].
In the present systematic review, we specifically focused our search attention on the risk of UVC-related PVT. Although multiple observational studies have provided an overview about the risk of PVT after UVC positioning, to the best of our knowledge no reviews explored systematically this issue. Our aim was to investigate the most accurate information about the actual incidence of UVC-related PVT in the neonatal setting, and to assess if any particular risk factor was systematically associated with the development of such complication.
The review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines[33].
The PICOS strategy was used, which comprised the following (PRISMA): Population: Infants with less than three months of postnatal age; Intervention (or exposure): Umbilical venous catheter; Comparison: No catheter; Outcome (primary): Incidence of PVT; Outcome (secondary): Association with a specific risk factor; Study type: Peer-reviewed observational, cohort and case-control studies.
There was no funding agency for this study. The systematic review did not require ethical approval/informed consent since there was no direct contact with individual patients, and only previously published data were included in the analyses.
The primary outcome was the incidence of PVT related to the use of UVCs (UVC only/attempted UVC/UVC + umbilical artery catheters) in infants with postnatal age up to three months. The secondary outcome was the identification of any risk factor associated with the development of UVC-related PVT.
The following search strategy was used: (portal OR vein OR system OR hepatic) AND (thrombosis) AND (neonat* OR newborn OR pediatric*) AND (catheter* OR umbilical). For reliability, three review authors (Bersani I, Iacona G and Piersigilli F) independently analyzed the currently available literature through systematic and comprehensive database searching (PubMed, Cochrane Library, Scopus, Web of Science) from 1980 to 2020 (the search was last updated on November 28, 2020). Reviews, in vitro studies, animal studies, autopsy studies and conference abstracts were excluded. The reference lists of the included articles were hand-searched to identify additional studies of interest. We obtained the full texts of all the potentially eligible studies.
Three review authors independently undertook eligibility assessment (Bersani I, Iacona G and Piersigilli F). Any disagreement about study eligibility was resolved by discussion with a fourth review author (Garcovich M) until consensus. We considered the studies eligible if they investigated the incidence of UVC-related PVT in infants with postnatal age up to three months. For articles resulting eligible based on the title or abstract, the full paper was retrieved. Case reports were considered not eligible for the final analyses being the calculation of an incidence not possible for such study design. Non-English studies were considered not eligible for the final analyses. We finally included all peer-reviewed, English-language, prospective/retrospective cohort studies and case-control studies.
To assess the risk of bias, two authors (Bersani I and Garcovich M) independently used the Newcastle-Ottawa Scale for comparative nonrandomized studies corresponding to each study’s design (cohort/cross-sectional)[34]. Such scale is a validated quality assessment instrument for non-randomized trials which evaluates three parameters of study quality: selection, comparability and exposure assessment. The scale assigns a maximum score of 4 for selection, 2 for comparability, and 3 for exposure, for a maximum total score of 9. Studies with a total score of ≥ 5 or ≥ 7 were considered to be of moderate or high quality, whereas those with a score of less than 5 were considered low-quality studies with high risk of bias. The scale results were tabulated in Table 1.
| Ref. | Selection | Comparability | Outcome | Total score |
| Levit et al[42], 2020 | 4 | 2 | 3 | 9 |
| Dubbink-Verheij et al[31], 2020 | 4 | 2 | 3 | 9 |
| Chen et al[15], 2020 | 4 | 0 | 3 | 7 |
| Hwang et al[46], 2020 | 4 | 2 | 3 | 9 |
| Çakır et al[38], 2020 | 4 | 0 | 3 | 7 |
| Cabannes et al[32], 2018 | 4 | 2 | 3 | 9 |
| Derinkuyu et al[5], 2018 | 4 | 0 | 3 | 7 |
| Chandrashekhar et al[45], 2015 | 4 | 0 | 3 | 7 |
| Michel et al[37], 2012 | 4 | 2 | 3 | 9 |
| Gharehbaghi et al[39], 2011 | 4 | 2 | 3 | 9 |
| Sakha et al[41], 2007 | 4 | 2 | 3 | 9 |
| Turebylu et al[21], 2007 | 4 | 2 | 3 | 9 |
| Kim et al[30], 2001 | 4 | 2 | 3 | 9 |
| Boo et al[44], 1999 | 4 | 2 | 3 | 9 |
| Schwartz et al[40], 1997 | 4 | 0 | 3 | 7 |
| Yadav et al[43], 1993 | 4 | 0 | 2 | 6 |
Three review authors independently performed data extraction (Bersani I, Iacona G and Piersigilli F). Disagreements about data extraction were resolved by discussion with a fourth review author (Garcovich M) until consensus. Pertinent findings from the included studies were tabulated in Table 2 and assessed according to pre-specified subgroups analyses: (1) Year of publication: 1980-2000 or 2001-2020; (2) Indication for thrombosis assessment: Abdominal US as systematic screening or abdominal ultrasound (US) only in case of a clinical concern for thrombosis; (3) Type of diagnostic technique to detect tip position: Radiography or/and (US) evaluation; (4) UVC model: UVC material, size (French), single or double lumen; (5) Thrombosis localization and type: Exact localization within the portal system, complete or partial; (6) Dwell time: Mean UVC in situ persistence (in days); and (7) Prophylaxis: None or heparin infusion or other.
| Ref. | Study design | UVC with PVT | UVC without PVT | Dwel time UVC with PVT | Dwel time UVC without PVT | Indication to UVC control | Type of imaging | Country/territory |
| Levit et al[42], 2020 | Prospective | 1 | 2016 | N/A | N/A | Clinical Suspicion | X-ray + US | United States |
| Dubbink-Verheij et al[31], 2020 | Prospective | 13 | 27 | N/A | N/A | Screening | X-ray + US | The Netherlands |
| Chen et al[15], 2020 | Retrospective | 7 | 1320 | N/A | N/A | Screening | X-ray + US | Taiwan |
| Hwang et al[46], 2020 | Retrospective | 15 | 54 | N/A | N/A | Screening | X-ray + US | South Korea |
| Çakır et al[38], 2020 | Prospective | 13 | 83 | 10.5 ± 4.31 | 12.2 ± 4.11 | Screening | X-ray + US | Turkey |
| Cabannes et al[32], 2018 | Prospective | 51 | 53 | N/A | N/A | Screening | X-ray + US | France |
| Derinkuyu et al[5], 2018 | Prospective | 15 | 229 | N/A | N/A | Screening | X-ray + US | Turkey |
| Chandrashekhar et al[45], 2015 | Prospective | 3 | 27 | N/A | N/A | Screening | X-ray + US | India |
| Michel et al[37], 2012 | Prospective | 2 | 59 | N/A | N/A | Screening | X-ray + US + Echocardiography | France |
| Gharehbaghi et al[39], 2011 | Prospective | 5 | 159 | N/A | N/A | Screening | X-ray + US | Iran |
| Sakha et al[41], 2007 | Prospective | 17 | 33 | 2 ± 1.121 | N/A | Screening | US | Iran |
| Turebylu et al[21], 2007 | Prospective | 2 | 26 | N/A | 6 | Screening | X-ray + US | United States |
| Kim et al[30], 2001 | Prospective | 43 | 57 | > 6 d in 23/43 | > 6 d in 6/57 | Screening | X-ray + US | South Korea |
| Boo et al[44], 1999 | Prospective | 0 | 57 | N/A | N/A | Screening | X-ray + US | Malaysia |
| Schwartz et al[40], 1997 | Prospective | 1 | 99 | 3 | 4 (0-12)2 | Screening | X-ray + US | United States |
| Yadav et al[43], 1993 | Prospective | 7 | 15 | N/A | N/A | Screening | X-ray + US | India |
Because of high heterogeneity, pooled data on the incidence of UVC-related PVT were analyzed using a random effects (DerSimonian and Laird method) model approach. Statistical heterogeneity among studies was assessed with Cochran’s Q and quantified with Higgins I2 statistic[35,36]. We considered an I2 of < 25% as low heterogeneity, I2 of 25% to 75% as moderate heterogeneity and I2 > 75% as high heterogeneity. Publication bias was assessed graphically using funnel plots and qualitatively using Egger’s regression and Begg rank correlation method. Statistical analysis was performed by using the Statistical Package for Social Science (SPSS 22.0; SPSS Inc, Chicago, IL, United States) and Microsoft Excel (Version 16.45).
The searches identified 2460 potentially relevant papers, 1835 after duplicates were removed. After title and abstract screening, 53 full-text studies were considered potentially eligible for inclusion and 37 studies were then excluded for the following reasons: (1) Not relevant comparators (n = 23); (2) Non-English language (n = 3); and (3) Wrong study design (n = 11) (Figure 1). Since the design/methodologies varied among different studies, information was not uniformly available for all analyses. For example, some studies could not be considered eligible, although pertinent, since the exact incidence UVC-associated PVT and/or the exact site of a catheter-related thrombosis and/or the exact age of patients with PVT could not be clearly extrapolated from the results.
According to the Newcastle-Ottawa Scale assessing the risk of bias, all the included studies were of moderate-high quality (Table 1). The characteristics and most relevant findings of the included studies are summarized in Table 2[5,21,30-32,37-45]. Of the 16 included studies, 14 were prospective and 2 were retrospective[15,46]. In some cases, the information about the clinical features of the included population was generically related to the overall cohort rather than specifically to neonates with UVC-related PVT and could not be extrapolated.
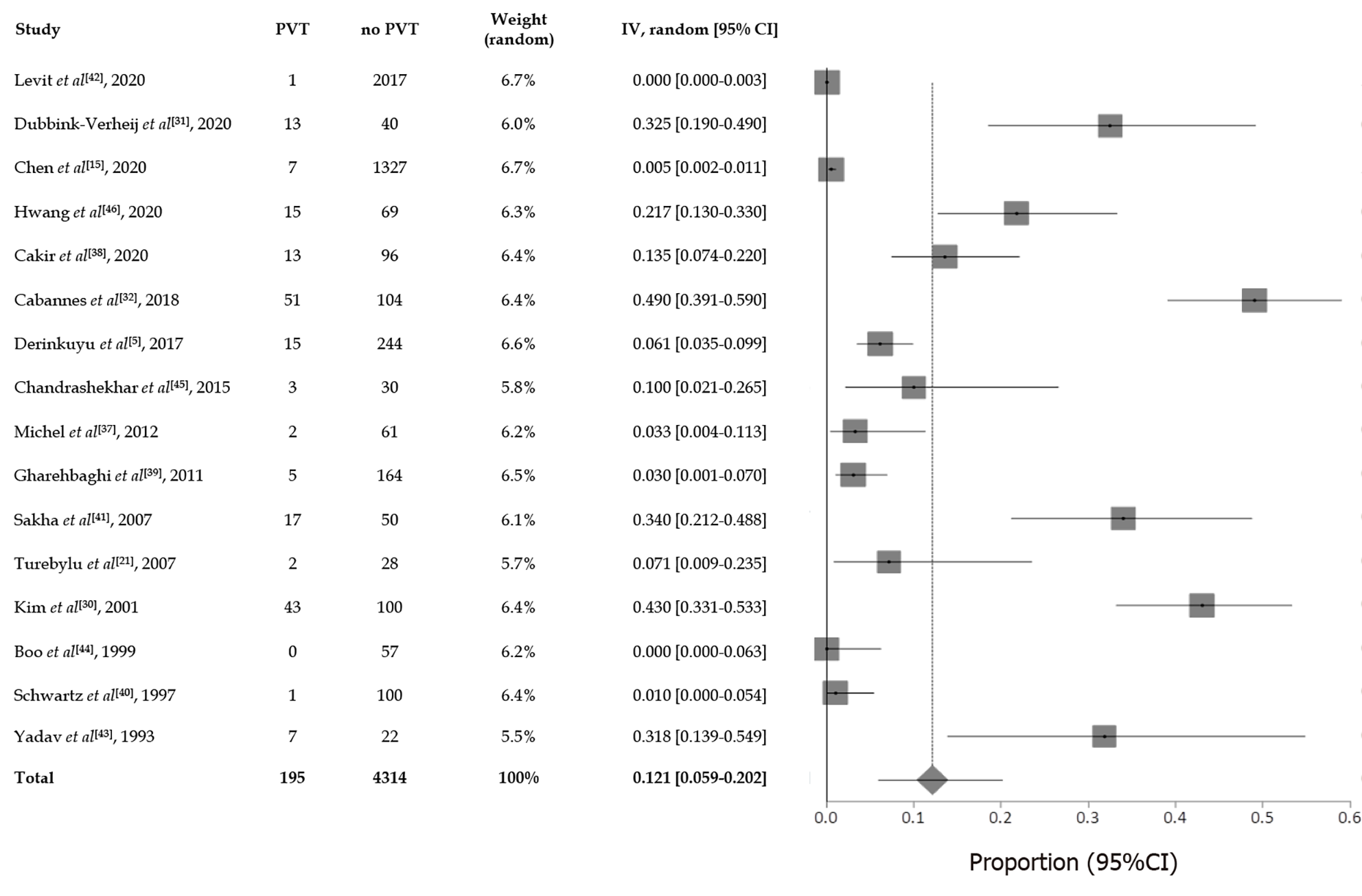
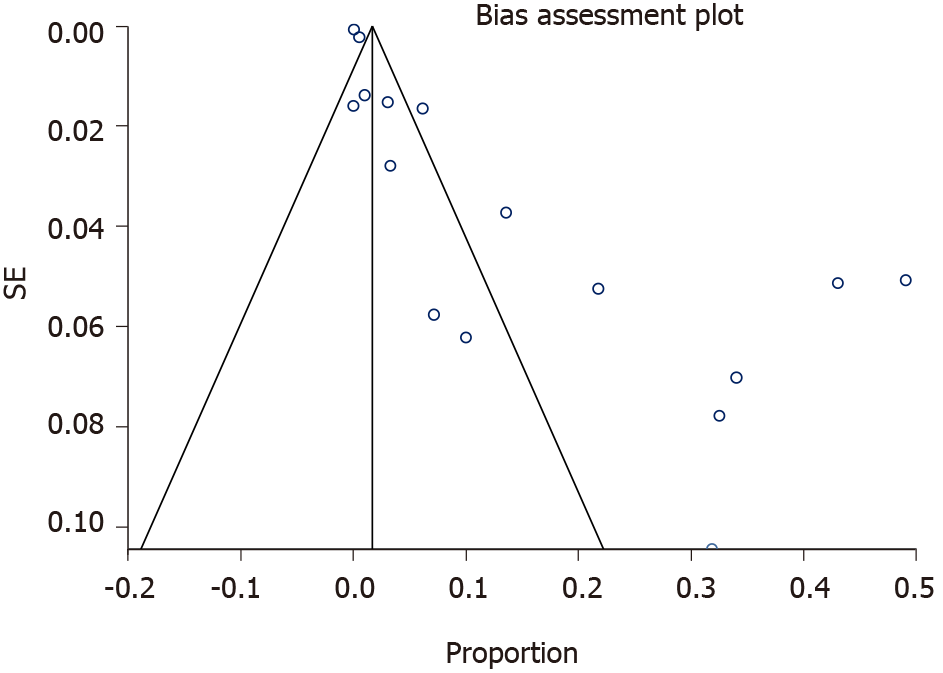
In the present review a total pooled sample of 4509 of neonates aged less than three months with UVC was included, 195 of whom experienced UVC-related PVT. The sample sizes ranged widely across studies (median, 83 patients; range, 22-2017). Mean gestational age and birth weight were 30.9 wk and 1738 g respectively, but it was not possible to extrapolate these data from each study, since neonates with PVT sometimes only represented a subgroup, whilst the available data mostly referred to the overall cohort. Figure 2 presents the results of overall meta-analysis with a random effects overall pooled-estimated incidence of UVC-related PVT of 12% [95% confidence interval (CI): 5.91-20.16], with high heterogeneity [I2 = 97.5% (95%CI: 97.1%-97.9%)]. Figure 3 shows evidence of publication bias, as indicated by visual inspection of the funnel plot and by the Egger test for small study effects for the primary outcome [bias coefficient for the main analysis, 3.5309 (95%CI: 1.983176-5.078624); P = 0.0002].
When investigating the pre-specified subgroups analyses, we found the following data (Table 2): (1) Year of publication: Overall, 3/16 (19%) studies were published between 1980 and 2000, whereas 13/16 (81%) between 2001 and 2020; (2) Indication for thrombosis assessment: In 15/16 studies (94%), the diagnosis of thrombosis was made accidentally during routine screening controls, whilst in 1/16 study (6%) targeted imaging assessments were carried out in neonates with clinical concerns for a thrombus. In most studies it was not possible to extrapolate mean age at the time of PVT diagnosis (Table 2); (3) Type of diagnostic technique used to assess tip position: Tip position was never assessed exclusively by radiography or echocardiography alone, while it was investigated by abdominal US alone in 1/16 (6%) studies, by a combination of radiography and abdominal US in 14/16 (88%) studies and by a combination of radiography, abdominal US and echocardiography in 1/16 (6%) studies. Only a minority of studies (3/16 studies, with a total number of 39/195 neonates) explicitly specified wrong tip position at the first imaging assessment, in UVC-related PVT cases[32,37,39]. However, most of the studies did not provide such information specifically for neonates who developed PVT, but rather for the overall population. Follow-up imaging controls were scheduled differently across studies; (4) UVC model: Information about UVC material, size and lumen number was only specified by a minority of studies. When the information was available, the studies reported the use of polyvinyl UVCs (n = 3/16) or polyurethane (n = 3/16) UVCs. When described, UVC size varied from 2.5 French to 5 French; (5) Thrombosis localization and type: Only a minority of studies specified PVT exact localization within the portal system. When reported, the left portal vein was the most frequently involved. Similarly, only a minority of studies (in a total number of 84/195 neonates) specified if PVT was complete or partial[5,30,38-41]. According to the available data, PVT was complete in 27/84 (32%) cases and partial in 57/84 (68%) cases; (6) Dwell time: Only a minority of studies reported explicitly the mean UVC dwelling time in neonates with PVT (since most of the studies provided mean dwelling time for the overall population); and (7) Prophylaxis: Only 6/16 (37%) studies reported a prophylactic administration of heparin[21,38,39,42,44,46].
To the best of our knowledge, this is the first systematic review specifically investigating the issue of UVC-related PVT. One of the most important limitations that emerged when reviewing the scientific literature was the extreme heterogeneity of study designs across the investigated studies (Table 2 and Figure 3).
As a whole, the data achieved by our systematic review confirmed the relevant risk of PVT associated with umbilical catheterization. The mean reported pooled incidence of neonatal UVC-related PVT among studies was 12%, with a range which varied from 0% to 49% from study to study (Figure 2). Such large difference might be attributed to multiple factors, including the different indication to imaging diagnostics, the different imaging time schedules, the heterogeneous UVC size/position/duration, and the proportion of preterm/term neonates[30,40,43]. Moreover, the time frame of research and publication may have influenced the incidence of UVC-related PVT as well. In fact, across literature, PVT was more frequently reported in the most recent studies. For example, a large multicenter registry assessing all thrombotic events occurring between 1989 and 1992 in 22 Canadian and 42 international centers from Europe, Australia and United States, recorded only 97 thrombotic events but did not explicitly report any case of PVT at all[47]. In contrast, a more recent large multicenter survey which included 187 children with a diagnosis of PVT (mean age at diagnosis: 4 years) reported a history of neonatal UVC placement in 65% of cases[19]. The higher incidence of PVT in recent years might be explained by the fact that clinicians are more aware of the thrombotic risk associated with the use of UVC and are more attentive to its detection. Furthermore, advances in US techniques make the detection of PVT easier.
The scientific literature emphasizes that UVC-related PVT is mostly related to improper tip position. Considering the small distance required for an UVC to become dislodged, UVC may migrate into the portal vein even following an initial proper positioning[2,15,16,42,48-52]. Therefore, tip location must be verified with accuracy not only soon after placement but also at regular intervals throughout time[30,31]. For this purpose, US is the ideal tool to check the position of the tip, since it is easy to perform for clinicians, it can be done at bedside and is not invasive for the patient.
When reviewing the literature, we found differences regarding the indication for US assessment, i.e., systematic surveillance in asymptomatic neonates with history of UVCs vs targeted diagnostic test in neonates with clinical concerns for a thrombus. However, in the studies which were finally included in the analyses, UVC-related PVT was mostly asymptomatic and only detected thanks to systematic imaging surveillance. Levit et al[42] found that in their neonatal unit, where routine US screening for PVT was not conducted, the rate of clinically identified thrombi was only 0.15% of all UVCs placed and 1.1% of all UVC-associated complications. On the other hand, Kim et al[30] found clinically silent PVT after UVC placement in 43% of critically ill neonates undergoing systematic US assessment. This indicates that UVC-related PVT might be largely underestimated if not properly investigated[42], once more confirming the need for routine imaging screenings in all neonates with UVC to exactly determine the incidence of UVC related PVT. Notably, PVT might also be associated with short- and long-term severe complications, deserving meticulous clinical evaluation[5,15].
According to the results of our systematic review, UVC-related PVT was reliably investigated by US assessment. Nevertheless, we found large discrepancies across studies concerning data presentation. As described above in the text, only a minority of studies reported the exact thrombus position/extension within the portal system and if the occlusion was partial/complete. After PVT detection, imaging follow-up controls were performed with heterogeneous time schedules across studies. As a whole, however, the data confirmed that US is a valid, non-invasive, bed-side diagnostic technique for PVT detection. But whereas assessment of tip position is easy, requires a minimal training, and can be performed by the neonatologist bedside, detection of PVT at an early stage usually warrants a higher degree of US expertise. Besides the skill level of the radiologist/neonatologist, correct US examination might also depend on further technical factors (neonatal cooperation, abdominal gas distension, clinical instability, small-sized anatomical structures etc.) which may influence the assessment.
A meticulous assessment of UVC tip position is needed to decrease catheter-related complications. Radiography is the most widely used technique to assess and follow-up UVC tip location[53,54]. However, most of the studies used only the anteroposterior view to assess tip location, although such view alone is not able to safely define the correct UVC tip position[54]. In case of wrong tip position within the portal system, radiography may show: (1) The tip below the diaphragm (below the vertebral body T10), overlying the liver; (2) Portal venous gas; and (3) Hypodiaphan lesions in the liver if fluid extravasation into liver parenchyma occurred[2,9,10,12,13]. However, radiographic assessments expose neonates to repeated ionizing radiations. US evaluation can be used in daily practice to check UVC tip position as well as the possible occurrence of UVC-associated hepatic complications. In fact, point-of-care US is able to assess in real-time UVC navigation and tip position during catheter placement[55]. Once UVC is correctly in place, US is the technique of choice to detect the development of UVC-related liver complications[5,30,31,53,56,57]. US and Doppler findings demonstrating hepatic complications include: (1) Detection of air in the portal venous system; (2) Portal venous thrombosis with impaired vascular patency; and (3) Liver parenchymal lesions presenting as nodular echogenic lesions/branched echogenic lesions/wide irregular heterogeneous lesions with laceration and the presence of peri-hepatic fluid[2,5,9,10,32]. Data exist comparing the ability of radiography and sonography to assess UVC positioning. A recent study found that US testing of UVC placement was able to identify catheter location in 100% of cases when compared to radiographic assessment[57]. Moreover, US is more accurate in the assessment of tip position compared to an estimation of catheter position achieved by its relationship to external structures on a radiograph[9,37,54,58]. Echocardiographic evaluation of UVC tip position was also assessed with success in recent years, although most studies focused on its ability to detect intra-cardiac abnormal tip position or atrial/inferior vena cava thrombosis, considering its limited ability to detect thrombi outside of the thoracic great vessels[24,59-62].
To date, the latest guidelines recommend the removal of UVCs after 7-10 d, although some authors reported an UVC in situ duration up to 28 d, once more proving how the management of UVCs is highly heterogeneous[4,22,24,38,42,61,63,64]. Unfortunately, the mean UVC dwell time in neonates with PVT was explicitly reported only by a minority of the included studies. Some authors found comparable UVC duration both in neonates with or without PVT[38-40], whilst in a large prospective study Kim et al[30] found an increased risk of PVT with a dwell time longer than 6 d. Noteworthy, PVT occurrence may develop soon after UVC position, as demonstrated by studies describing its detection already 12 h after placement[37]. It could be put forward that the presence of an UVC may itself represent a trigger for PVT development, presumably by raising vascular pressure in the ductus venosus and slowing down blood flow[18], and that such risk may eventually increase if catheterization persists. Such hypothesis deserves proper validation and large randomized controlled trials are warranted to achieve conclusive data about the benefits of early UVC removal.
Only a minority of studies described the occurrence of difficult or failed umbilical catheterization[30,65]. Considering that traumatic catheterization and/or failed insertion may induce vasculature injury and predispose to PVT by damaging the endothelial wall and decreasing portal flow[8], also the occurrence and number of failed attempts to UVC placement may play a role in PVT development and should be therefore considered either when programming diagnostic/follow-up controls for PVT or in the design of future studies.
The studies included in the final analyses reported the use of different models of UVCs, but unfortunately several studies did not specify the UVC model at all. Today, the most used UVC are dedicated catheters in polyurethane or in polyvinyl chloride but in the past several units used nasogastric tubes for venous umbilical catheterization. Furthermore, most of the studies did not specify the size and the number of lumens of the catheters that have been used. The use of different UVC models/materials may have influenced the incidence of UVC-related PVT in each study.
Concerning the presence of hereditary risk factors, the literature is, once more, quite vague and inconclusive. Turebylu et al[21] evaluated prospectively the prevalence of hereditary prothrombotic mutations in neonates with umbilical catheterization developing thrombotic lesions (including two cases of PVT). Interestingly, the authors found no increase in the risk of catheter-related thrombosis in patients carrying such prothrombotic mutations. In contrast, Heller et al[25] found that among 65 neonates, 24 of whom had PVT, the rate of genetic prothrombotic risk factors was higher than healthy, age-/sex-matched controls.
Sepsis was suggested as possible risk factor for pediatric PVT development[3,66,67]. However, only a minority of patients affected by PVT presented with infection[3]. Furthermore, as for the studies included in the present review, only a minority of authors explicitly reported the presence of sepsis in case of PVT.
Recently, Hwang et al[46] reported for the first time significantly higher serum calcium concentrations in infants with umbilical catheter-related thrombosis. The authors assessed that such finding may reflect a possible role of calcium as a clotting factor leading to a hypercoagulable state. Further evidence is however required to confirm these results.
Only a minority of the studies included in our review reported a prophylactic treatment with heparin which, moreover, varied in terms of dosage[21,38,39,42,46]. After UVC-related PVT development, spontaneous resolution may often occur in UVC-related PVT, but this warrants close monitoring to determine either progression or resolution of the thrombus[21,30,32,40,46,64,68-70]. However, in case of thrombus extension with occlusion of the portal venous tract or clinical deterioration, antithrombotic therapy with unfractionated or low molecular weight heparin can be considered[64,68,70,71]. Kim et al[30] investigated prospectively the occurrence of UVC-related PVT in 100 neonates by subsequent US assessment. The authors found that 43% of neonates had a clinically silent PVT and reported complete resolution in 56% of neonates at follow-up controls, with recanalization being more frequent in neonates with partial rather than occlusive thrombi. Cabannes et al[32] investigated prospectively the occurrence of PVT in a cohort of patients including preterm neonates. PVT occurred in 53/123 of which 51 had an UVC. In these cases, the authors reported a spontaneous favorable evolution of left PVT in 95% of cases. In a prospective observational study, Dubbink-Verheij et al[31] investigated by serial US evaluations the incidence of catheter-related thrombosis in neonates with UVCs compared to a control group of neonates without UVC. The authors found the presence of thrombotic lesions in the UVC route in 30/40 cases (75%), of which 13 in the portal vein system. Most of the thrombotic lesions were asymptomatic and regressed spontaneously, whilst a minority required treatment with heparin. In contrast, Derinkuyu et al[5] treated with low-molecular-weight heparin all neonates with a diagnosis of UVC-related PVT (all described as asymptomatic). This heterogeneous approach may reflect the absence of solid evidence about safety/efficacy of antithrombotic therapy specifically addressing the neonatal period.
Our systematic review has multiple limitations, mostly attributable to the heterogeneity across studies. First, the intrinsic limitation of having included either retrospective studies or “old” studies (from 1980 onwards), i.e., performed at time-points during which clinical approach to patients and awareness about PVT was presumably different compared to more recent studies. Second, the lack of correlation between PVT and UVC tip position in most studies. Third, the different study designs regarding the indication and time schedule for imaging assessment. Fourth, the different approach of clinicians about the use of prophylactic/therapeutic treatment in neonates with indwelling UVCs.
In conclusion, the use of umbilical lines requires a high index of suspicion for PVT development, especially if considering that the need for an UVC obviously preselects ill newborns in whom multiple risk factors for the development of thrombotic disorders may coexist. To avoid or minimize the risk of PVT, some crucial key-points have to be followed, as checking the correct position before infusing in the catheter, checking again the correct tip position every 48 h, and removing the UVC after a maximum of 7 d.
As a whole, this systematic review revealed relevant gaps also in knowledge about the optimal diagnostic approach and treatment for UVC-related PVT, maybe related to the lack of updated, evidence-based guidelines addressing step-by-step all the aspects of what the best approach to the management of this complication should be. According to our opinion, this represents a call to action addressed to researchers and clinicians to design large prospective randomized studies and to draft specific, concrete and updated guidelines.
The use of umbilical venous catheters (UVCs) in the perinatal period may be associated with severe complications, including the occurrence of portal vein thrombosis (PVT).
Although multiple observational studies have provided an overview about the risk of PVT after UVC positioning, no studies/reviews explored systematically this issue.
The main goal was to investigate the most accurate information about the actual incidence of UVC-related PVT in the neonatal setting, and to assess if any particular risk factor was systematically associated with the development of such complication.
A systematic and comprehensive database searching (PubMed, Cochrane Library, Scopus, Web of Science) was performed for prospective cohort studies, retrospective cohort studies and case-control studies from 1980 to 2020. Incidence estimates were pooled by using random effects meta-analyses. The quality of included studies was assessed using the Newcastle-Ottawa scale.
Sixteen studies were considered eligible and included in the final analyses. The data confirmed the relevant risk of UVC-related thrombosis with a mean pooled incidence of 12%, although it varied across studies (0%-49%).
This is the first systematic review specifically investigating the incidence of UVC-related PVT. The use of UVCs requires a high index of suspicion, because its use is significantly associated with PVT.
Large prospective randomized studies and updated guidelines are warranted in order to define the best management of this dreaded complication.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Karatza AA S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
| 1. | El Ters N, Claassen C, Lancaster T, Barnette A, Eldridge W, Yazigi F, Brar K, Herco M, Rogowski L, Strand M, Vachharajani A. Central vs Low-Lying Umbilical Venous Catheters: A Multicenter Study of Practices and Complications. Am J Perinatol. 2019;36:1198-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Hagerott HE, Kulkarni S, Restrepo R, Reeves-Garcia J. Clinical-radiologic features and treatment of hepatic lesions caused by inadvertent infusion of parenteral nutrition in liver parenchyma due to malposition of umbilical vein catheters. Pediatr Radiol. 2014;44:810-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Williams S, Chan AK. Neonatal portal vein thrombosis: diagnosis and management. Semin Fetal Neonatal Med. 2011;16:329-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Gordon A, Greenhalgh M, McGuire W. Early planned removal of umbilical venous catheters to prevent infection in newborn infants. Cochrane Database Syst Rev. 2017;10:CD012142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Derinkuyu BE, Boyunaga OL, Damar C, Unal S, Ergenekon E, Alimli AG, Oztunali C, Turkyilmaz C. Hepatic Complications of Umbilical Venous Catheters in the Neonatal Period: The Ultrasound Spectrum. J Ultrasound Med. 2018;37:1335-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Goh SSM, Kan SY, Bharadwaj S, Poon WB. A review of umbilical venous catheter-related complications at a tertiary neonatal unit in Singapore. Singapore Med J. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Mutlu M, Aslan Y, Kul S, Yılmaz G. Umbilical venous catheter complications in newborns: a 6-year single-center experience. J Matern Fetal Neonatal Med. 2016;29:2817-2822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Sulemanji M, Vakili K, Zurakowski D, Tworetzky W, Fishman SJ, Kim HB. Umbilical Venous Catheter Malposition Is Associated with Necrotizing Enterocolitis in Premature Infants. Neonatology. 2017;111:337-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Selvam S, Humphrey T, Woodley H, English S, Kraft JK. Sonographic features of umbilical catheter-related complications. Pediatr Radiol. 2018;48:1964-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Schlesinger AE, Braverman RM, DiPietro MA. Pictorial essay. Neonates and umbilical venous catheters: normal appearance, anomalous positions, complications, and potential aid to diagnosis. AJR Am J Roentgenol. 2003;180:1147-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Morag I, Epelman M, Daneman A, Moineddin R, Parvez B, Shechter T, Hellmann J. Portal vein thrombosis in the neonate: risk factors, course, and outcome. J Pediatr. 2006;148:735-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Grizelj R, Vukovic J, Bojanic K, Loncarevic D, Stern-Padovan R, Filipovic-Grcic B, Weingarten TN, Sprung J. Severe liver injury while using umbilical venous catheter: case series and literature review. Am J Perinatol. 2014;31:965-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Auriti C, Ronchetti MP, Bersani I, Gennari F, Piersigilli F. Intrahepatic Administration of Liposomal Amphotericin B (Ambisome) for the Management of a Liver Abscess from Candida albicans in a Preterm Infant. Antimicrob Agents Chemother. 2018;62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Türk E, Soylar R, Akca T, Serter S, Karaca İ. Venobiliary fistula related to umbilical venous catheter in a newborn. Pediatr Int. 2015;57:478-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Chen HJ, Chao HC, Chiang MC, Chu SM. Hepatic extravasation complicated by umbilical venous catheterization in neonates: A 5-year, single-center experience. Pediatr Neonatol. 2020;61:16-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Yiğiter M, Arda IS, Hiçsönmez A. Hepatic laceration because of malpositioning of the umbilical vein catheter: case report and literature review. J Pediatr Surg. 2008;43:E39-E41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Macartney CA, Chan AK. Thrombosis in children. Semin Thromb Hemost. 2011;37:763-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Sulemanji MN, Azpurua H, Suh M, Potanos K, Cauley R, Kunisaki SM, Modi B, Zurakowski D, Fishman SJ, Kim HB. Ductus venosus closure results in transient portal hypertension--is this the silent trigger for necrotizing enterocolitis? J Pediatr Surg. 2013;48:2067-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Di Giorgio A, De Angelis P, Cheli M, Vajro P, Iorio R, Cananzi M, Riva S, Maggiore G, Indolfi G, Calvo PL, Nicastro E, D'Antiga L. Etiology, presenting features and outcome of children with non-cirrhotic portal vein thrombosis: A multicentre national study. Dig Liver Dis. 2019;51:1179-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Ferri PM, Rodrigues Ferreira A, Fagundes ED, Xavier SG, Dias Ribeiro D, Fernandes AP, Borges KB, Liu SM, de Melo Mdo C, Roquete ML, Penna FJ. Evaluation of the presence of hereditary and acquired thrombophilias in Brazilian children and adolescents with diagnoses of portal vein thrombosis. J Pediatr Gastroenterol Nutr. 2012;55:599-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Turebylu R, Salis R, Erbe R, Martin D, Lakshminrusimha S, Ryan RM. Genetic prothrombotic mutations are common in neonates but are not associated with umbilical catheter-associated thrombosis. J Perinatol. 2007;27:490-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Revel-Vilk S, Ergaz Z. Diagnosis and management of central-line-associated thrombosis in newborns and infants. Semin Fetal Neonatal Med. 2011;16:340-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Albisetti M, Moeller A, Waldvogel K, Bernet-Buettiker V, Cannizzaro V, Anagnostopoulos A, Balmer C, Schmugge M. Congenital prothrombotic disorders in children with peripheral venous and arterial thromboses. Acta Haematol. 2007;117:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Narang S, Roy J, Stevens TP, Butler-O'Hara M, Mullen CA, D'Angio CT. Risk factors for umbilical venous catheter-associated thrombosis in very low birth weight infants. Pediatr Blood Cancer. 2009;52:75-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Heller C, Schobess R, Kurnik K, Junker R, Günther G, Kreuz W, Nowak-Göttl U. Abdominal venous thrombosis in neonates and infants: role of prothrombotic risk factors - a multicentre case-control study. For the Childhood Thrombophilia Study Group. Br J Haematol. 2000;111:534-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Avila ML, Amiri N, Stanojevic S, Vu TT, Barron K, Krol P, Yue N, Williams S, Brandão LR. Can thrombophilia predict recurrent catheter-related deep vein thrombosis in children? Blood. 2018;131:2712-2719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Sobczak A, Kruczek P, Homa M, Kwinta P. A new microscopic insight into the thrombogenicity of umbilical catheters. Thromb Res. 2018;168:80-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Pottecher T, Forrler M, Picardat P, Krause D, Bellocq JP, Otteni JC. Thrombogenicity of central venous catheters: prospective study of polyethylene, silicone and polyurethane catheters with phlebography or post-mortem examination. Eur J Anaesthesiol. 1984;1:361-365. [PubMed] |
| 29. | Thornburg C, Pipe S. Neonatal thromboembolic emergencies. Semin Fetal Neonatal Med. 2006;11:198-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Kim JH, Lee YS, Kim SH, Lee SK, Lim MK, Kim HS. Does umbilical vein catheterization lead to portal venous thrombosis? Radiology. 2001;219:645-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Dubbink-Verheij GH, Visser R, Roest AA, van Ommen CH, Te Pas AB, Lopriore E. Thrombosis after umbilical venous catheterisation: prospective study with serial ultrasound. Arch Dis Child Fetal Neonatal Ed. 2020;105:299-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Cabannes M, Bouissou A, Favrais G, Sembély-Taveau C, Morales L, Favreau A, Bertrand P, Saliba E, Sirinelli D, Morel B. Systematic ultrasound examinations in neonates admitted to NICU: evolution of portal vein thrombosis. J Perinatol. 2018;38:1359-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47107] [Article Influence: 2944.2] [Reference Citation Analysis (0)] |
| 34. | Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. [cited 28 November 2020]. In: The Ottawa Hospital Research Institute [Internet]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
| 35. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 25760] [Article Influence: 1120.0] [Reference Citation Analysis (0)] |
| 36. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46429] [Article Influence: 2110.4] [Reference Citation Analysis (3)] |
| 37. | Michel F, Brevaut-Malaty V, Pasquali R, Thomachot L, Vialet R, Hassid S, Nicaise C, Martin C, Panuel M. Comparison of ultrasound and X-ray in determining the position of umbilical venous catheters. Resuscitation. 2012;83:705-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 38. | Çakır SÇ, Özkan H, Dorum BA, Köksal N, Kudretoğlu P, Baytan B, Sezgin M, Güneş AM. The danger awaiting premature babies: Portal vein thrombosis. Turk Pediatri Ars. 2020;55:257-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 39. | Gharehbaghi MM, Nemati M, Hosseinpour SS, Taei R, Ghargharechi R. Umbilical vascular catheter associated portal vein thrombosis detected by ultrasound. Indian J Pediatr. 2011;78:161-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Schwartz DS, Gettner PA, Konstantino MM, Bartley CL, Keller MS, Ehrenkranz RA, Jacobs HC. Umbilical venous catheterization and the risk of portal vein thrombosis. J Pediatr. 1997;131:760-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Sakha SH, Rafeey M, Tarzamani MK. Portal venous thrombosis after umbilical vein catheterization. Indian J Gastroenterol. 2007;26:283-284. [PubMed] |
| 42. | Levit OL, Shabanova V, Bizzarro MJ. Umbilical catheter-associated complications in a level IV neonatal intensive care unit. J Perinatol. 2020;40:573-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 43. | Yadav S, Dutta AK, Sarin SK. Do umbilical vein catheterization and sepsis lead to portal vein thrombosis? J Pediatr Gastroenterol Nutr. 1993;17:392-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Boo NY, Wong NC, Zulkifli SS, Lye MS. Risk factors associated with umbilical vascular catheter-associated thrombosis in newborn infants. J Paediatr Child Health. 1999;35:460-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 85] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Chandrashekhar C, Krishnegowda S, Vikas VM, Bhaktavatsala HR. Portal vein thrombosis following umbilical vein catheterization in neonates. Pediatr Rev: Int J Pediatr Res. 2015;2:135-137. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 46. | Hwang JH, Chung ML, Lim YJ. Incidence and risk factors of subclinical umbilical catheter-related thrombosis in neonates. Thromb Res. 2020;194:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Schmidt B, Andrew M. Neonatal thrombosis: report of a prospective Canadian and international registry. Pediatrics. 1995;96:939-943. [PubMed] |
| 48. | Hoellering A, Tshamala D, Davies MW. Study of movement of umbilical venous catheters over time. J Paediatr Child Health. 2018;54:1329-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 49. | Abiramalatha T, Kumar M, Shabeer MP, Thomas N. Advantages of being diligent: lessons learnt from umbilical venous catheterisation in neonates. BMJ Case Rep. 2016;2016. [PubMed] |
| 50. | Plooij-Lusthusz AM, van Vreeswijk N, van Stuijvenberg M, Bos AF, Kooi EMW. Migration of Umbilical Venous Catheters. Am J Perinatol. 2019;36:1377-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Dubbink-Verheij GH, Visser R, Tan RNGB, Roest AAW, Lopriore E, Te Pas AB. Inadvertent Migration of Umbilical Venous Catheters Often Leads to Malposition. Neonatology. 2019;115:205-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Franta J, Harabor A, Soraisham AS. Ultrasound assessment of umbilical venous catheter migration in preterm infants: a prospective study. Arch Dis Child Fetal Neonatal Ed. 2017;102:F251-F255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 53. | Sharma D, Farahbakhsh N, Tabatabaii SA. Role of ultrasound for central catheter tip localization in neonates: a review of the current evidence. J Matern Fetal Neonatal Med. 2019;32:2429-2437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 54. | Guimarães AF, Souza AA, Bouzada MC, Meira ZM. Accuracy of chest radiography for positioning of the umbilical venous catheter. J Pediatr (Rio J). 2017;93:172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 55. | Kishigami M, Shimokaze T, Enomoto M, Shibasaki J, Toyoshima K. Ultrasound-Guided Umbilical Venous Catheter Insertion With Alignment of the Umbilical Vein and Ductus Venosus. J Ultrasound Med. 2020;39:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 56. | Simanovsky N, Ofek-Shlomai N, Rozovsky K, Ergaz-Shaltiel Z, Hiller N, Bar-Oz B. Umbilical venous catheter position: evaluation by ultrasound. Eur Radiol. 2011;21:1882-1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 57. | Saul D, Ajayi S, Schutzman DL, Horrow MM. Sonography for Complete Evaluation of Neonatal Intensive Care Unit Central Support Devices: A Pilot Study. J Ultrasound Med. 2016;35:1465-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 58. | Greenberg M, Movahed H, Peterson B, Bejar R. Placement of umbilical venous catheters with use of bedside real-time ultrasonography. J Pediatr. 1995;126:633-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 59. | Ades A, Sable C, Cummings S, Cross R, Markle B, Martin G. Echocardiographic evaluation of umbilical venous catheter placement. J Perinatol. 2003;23:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 60. | Unal S, Ekici F, Cetin İİ, Bilgin L. Heparin infusion to prevent umbilical venous catheter related thrombosis in neonates. Thromb Res. 2012;130:725-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 61. | Butler-O'Hara M, Buzzard CJ, Reubens L, McDermott MP, DiGrazio W, D'Angio CT. A randomized trial comparing long-term and short-term use of umbilical venous catheters in premature infants with birth weights of less than 1251 g. Pediatrics. 2006;118:e25-e35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 62. | Wever ML, Liem KD, Geven WB, Tanke RB. Urokinase therapy in neonates with catheter related central venous thrombosis. Thromb Haemost. 1995;73:180-185. [PubMed] |
| 63. | O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML, Raad II, Randolph AG, Rupp ME, Saint S; Healthcare Infection Control Practices Advisory Committee. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39:S1-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 731] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 64. | Park CK, Paes BA, Nagel K, Chan AK, Murthy P; Thrombosis and Hemostasis in Newborns (THiN) Group. Neonatal central venous catheter thrombosis: diagnosis, management and outcome. Blood Coagul Fibrinolysis. 2014;25:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 65. | Aiyagari R, Song JY, Donohue JE, Yu S, Gaies MG. Central venous catheter-associated complications in infants with single ventricle: comparison of umbilical and femoral venous access routes. Pediatr Crit Care Med. 2012;13:549-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 66. | Sethi SK, Dewan P, Faridi MM, Aggarwal A, Upreti L. Liver abscess, portal vein thrombosis and cavernoma formation following umbilical vein catherisation in two neonates. Trop Gastroenterol. 2007;28:79-80. [PubMed] |
| 67. | Shah I, Bhatnagar S. Liver abscess in a newborn leading to portal vein thrombosis. Indian J Pediatr. 2009;76:1268-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 68. | Monagle P, Chan AKC, Goldenberg NA, Ichord RN, Journeycake JM, Nowak-Göttl U, Vesely SK. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e737S-e801S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 1028] [Article Influence: 79.1] [Reference Citation Analysis (0)] |
| 69. | Bhatt MD, Patel V, Butt ML, Chan AKC, Paes B; Thrombosis and Hemostasis in Newborns (THiN) Group. Outcomes following neonatal portal vein thrombosis: A descriptive, single-center study and review of anticoagulant therapy. Pediatr Blood Cancer. 2019;66:e27572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 70. | Chalmers E, Ganesen V, Liesner R, Maroo S, Nokes T, Saunders D, Williams M; British Committee for Standards in Haematology. Guideline on the investigation, management and prevention of venous thrombosis in children. Br J Haematol. 2011;154:196-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 71. | Demirel N, Aydin M, Zenciroglu A, Bas AY, Yarali N, Okumus N, Cinar G, Ipek MS. Neonatal thrombo-embolism: risk factors, clinical features and outcome. Ann Trop Paediatr. 2009;29:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |