Published online Nov 27, 2021. doi: 10.4254/wjh.v13.i11.1791
Peer-review started: February 9, 2021
First decision: May 13, 2021
Revised: May 18, 2021
Accepted: October 12, 2021
Article in press: October 12, 2021
Published online: November 27, 2021
Processing time: 287 Days and 20.7 Hours
Patients with cirrhosis are at risk of cirrhotic cardiomyopathy, with resulting cardiac dysfunction and exercise limitations. Six minute walking test (6MWT) assesses functional status and predicts morbidity and mortality in cardiopulmonary diseases.
To determine if it associates with mortality by analyzing 6MWT performance in patients with liver cirrhosis.
A cohort of 106 cirrhotic patients was evaluated in the outpatient setting with echocardiogram and 6MWT and follow up for one year to document hepatic decompensation and mortality. The distance in meters was recorded at the end of 6 min (6MWD).
This cohort had a mean age of 51 years and 56% male; patients were staged as Child A in 21.7%, B 66% and C 12.3%. Walk distance inversely correlated with Child scores, and was significantly reduced as Child stages progresses. Patients who died (10.4%) showed shorter mean 6MWD (P = 0.006). Low 6MWD was an independent predictor of mortality (P = 0.01).
6MWT is a noninvasive inexpensive test whose result is related to Child scores and mortality. It is useful to identify patients with liver cirrhosis at high risk of mortality for closer monitoring and potential early intervention.
Core Tip: Our study proposes that six-minute walking test, a simple exercise test, can be applicable in the evaluation of cirrhotic patients. This is a well-none routine assessment in patients with cardiopulmonary diseases, where it is used to predict mortality in this population. Its use in liver cirrhosis is limited. Patients with chronic hepatic insufficient are at risk of progressively muscle loss, frailty, and exercise limitation, all factors directly associated with poor survival. We propose by using six-minute walk test a practical and simple manner of assess this risks and provide a better understanding of how exercise limitation can directly affect survival.
- Citation: Pimentel CFMG, Amaral ACC, Gonzalez AM, Lai M, Mota DO, Ferraz MLG, Junior WM, Kondo M. Six-minute walking test performance is associated with survival in cirrhotic patients. World J Hepatol 2021; 13(11): 1791-1801
- URL: https://www.wjgnet.com/1948-5182/full/v13/i11/1791.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i11.1791
Liver cirrhosis is related to functional impairment leading to reduction in physical fitness[1,2]. Some possible factors implicated in this process are profound muscle wasting (or cirrhotic myopathy)[3], cardiac dysfunction (cirrhotic cardiomyopathy)[4], autonomic dysfunction (chronotropic incompetence) and concurrent pulmonary disease (portopulmonary hypertension and hepatopulmonary syndrome). Recently studies reinforce the importance of frailty scores as a prediction of mortality in liver transplantation list[5,6], giving emphasis in sarcopenia and physical fitness as important factors associated with mortality[7].
The six-minute walk test (6MWT) is a practical simple inexpensive test that provides a global assessment of all systems involved during exercise[8]. Although it does not give information about specific organ impairment, it evaluates overall exercise capacity and has been shown, in patients with cardiac disease, to correlate with the maximal oxygen consumption (VO2) and survival[9].
Some studies demonstrated that short distance during 6MWT (6MWD) predicted poorer prognosis and disease outcome in patients with heart failure[10] and chronic obstructive pulmonary disease[11]. In addition, this test can be used to assess the overall functional status and quantify response to a certain intervention[8] in a variety of other chronic diseases and in the elderly population[9-12].
Previous studies highlight the importance of 6MWD in predicting survival in cirrhotic and non-cirrhotic patients[13-16]. There are also evidences suggesting an association between exercise performance and increase risk of death on the waiting liver transplantation list[15-18]. Despite its role in long term survival in different chronic diseases, the impact in mortality prediction in cirrhotic patients is underestimated over years.
The aim of this study was to analyze the association between 6MWT and long-term mortality in a cohort of cirrhotic patients.
A total of 106 outpatients with liver cirrhosis (57 male, mean age 51.2 ± 12.9 years) was included in the present study. Cirrhosis was defined by clinical history, physical examination, laboratory analysis and at least one imaging data. Disease prognosis and severity were established based on Child and MELD scores, according to original scores definitions[19,20]. Exclusion criteria were any previous or current cardiovascular or pulmonary disease, heart failure or diagnosis of hemochromatosis (when cardiac involvement was documented). Patients who had a history of alcohol abuse (more than 20 g and 60 g of ethanol per day for women and men, respectively)[21] were included if they had abstained from alcohol use for at least 6 mo prior to enrollment. Patients with non-sinus rhythm, decompensated arterial hypertension, low peripheral oxygen saturation (SpO2 < 90%), recent history (less than 3 mo) of new liver related decompensation or hospitalizations were also excluded (patients with previous ascites or encephalopathy were included, those characterized with chronic decompensated patients). Patients with neuromuscular diseases, myopathy, balance deficits or orthopedic disorders were also excluded. Patients who have previously received a liver transplant were not included. No paracentesis was performed within at least one week prior to exercise, avoiding volume depletion or electrolyte imbalances.
One hundred and sixty-four patients were consecutively screened from two liver transplantation centers between October 2014 and December 2014, 58 out of 164 were excluded according to previous criteria, most of the due to cardiovascular disorders (26%) or active alcohol consumption (19%). On the day of enrollment, patients provided written informed consent and had blood samples collected and 6MWT done. Electrocardiogram and transthoracic bubble echocardiogram were performed within 1 mo of enrollment.
Patients were followed-up by clinical visits, hospital records or telephone calls to patients to capture deaths and their causes. Patients were stratified according to their ability to complete 6MWT, whether they achieved or not predicted distance according to gender and age, and pattern of symptom secondary to physical effort due to the test. Patients included were follow-up to one year, main outcomes were defined as death or liver transplantation.
The study has been performed in accordance with the Declaration of Helsinki (2000) and approved by the Ethics Committee of our institution.
The 6MWT was conducted according to American Thoracic Society guidelines[8] and supervised by a qualified physician. The test was performed indoors, along a 30 m flat, straight corridor with a hard surface and free of any type of obstacles. Before starting the test, all patients were provided instructions by the evaluator, encouraged to walk as far as possible within 6 min, and instructed to stop if pain, dyspnea, or other symptoms. The distance in meters was recorded at the end of the six minutes (6MWD). Predicted distances were computed according to specific equations for gender, weight, height and age[22]. Predicted distance achieved percentage (%6MWD) is then derived by dividing the actual 6MWD divided by the predicted distance.
Data were analyzed using a statistical software program (IBM® SPSS® Statistics, version 22.0). Logistic regressions were performed to evaluate the independent association between 6MWD and death. Receiver operating curves (ROC) and the area under ROC (AUROC) were computed to estimate sensitivity, specificity and cut-off points for 6MWD used in regression models, selected by Youden’s index. COX regression analysis and Kaplan-Meier curves were performed and significant differences between the later were assessed by means of the log-rank test. We performed subgroup analysis according achievement of liver transplantation in order to evaluated 6MWT distance as a predictor of death.
The main demographic, clinical, and laboratory characteristics of the patients are presented in Table 1. One hundred and six patients were selected from two liver transplantation centers in Sao Paulo, Brazil. The majority was male (56%), and non-alcoholic etiology of the liver disease was the most common (69.8%). The mean MELD was 11.1, Child B more common (66%), and 74% of patients presented a history of at least one liver related decompensation. Ascites was identified in 32.1% and hepatic encephalopathy in 10.4% of patients on the day of the test.
| Characteristic | n (%) or means ± SD |
| Gender M/F | 59/47 (56/44) |
| Age (yr) | 51 ± 13 |
| BMI (kg/m2) | 25.7 ± 4.7 |
| PASP (mmHg) | 25.4 ± 8.0 |
| Cirrhosis etiology | |
| Virus | 36 (33.9) |
| Alcohol | 32 (30.2) |
| NASH | 8 (7.5) |
| Others | 30 (28.4) |
| Child-Pugh class n | 7.1 ± 1.8 |
| A | 23 (21.7) |
| B | 70 (66) |
| C | 13 (12.3) |
| MELD | 11.1 ± 3.1 |
| Previous history of liver related decompensation | 76 (73.8) |
| Hypertension | 19 (17.9) |
| Diabetes | 26 (24.5) |
| Tobacco smoking | 12 (11.4) |
| Beta-blocker use | 32 (30.2) |
| Hepatic decompensation on the day of the test | |
| Ascites | 34 (32.1) |
| (Grade 1, 2, and 3) | (11.3, 17, 5) |
| Peripheral edema | 13 (12.3) |
| Hepatic encephalopathy | 13 (12.3) |
| (Grade 1, 2, 3, and 4) | (10.4, 1.9, 0, 0) |
| Hepatocellular carcinoma | 5 (4.7) |
| Patient on the liver transplantation waiting list | 35 (33) |
| Baseline laboratory1 | |
| Hemoglobin (mg/dL) | 13.1 ± 1.9 |
| Hematocrit (%) | 39.3 ± 5.4 |
| Albumin (g/dL)Bilirubin (mg/dL)INR | 3.5 ± 0.62.0 ± 1.51.2 ± 0.2 |
| Creatinine (mg/dL) | 0.8 ± 0.3 |
| Na (mmol/L) | 137.8 ± 2.1 |
| K (mmol/L) | 4.1 ± 0.5 |
| Mg (mg/dL) | 1.8 ± 0.2 |
| Ca (mmol/L) | 1.2 ± 0.1 |
All patients were followed until death, time of transplantation or end of study follow-up (12 mo). During the study period, 11 patients died and 3 underwent liver transplantation. All deaths were related to hepatic decompensation.
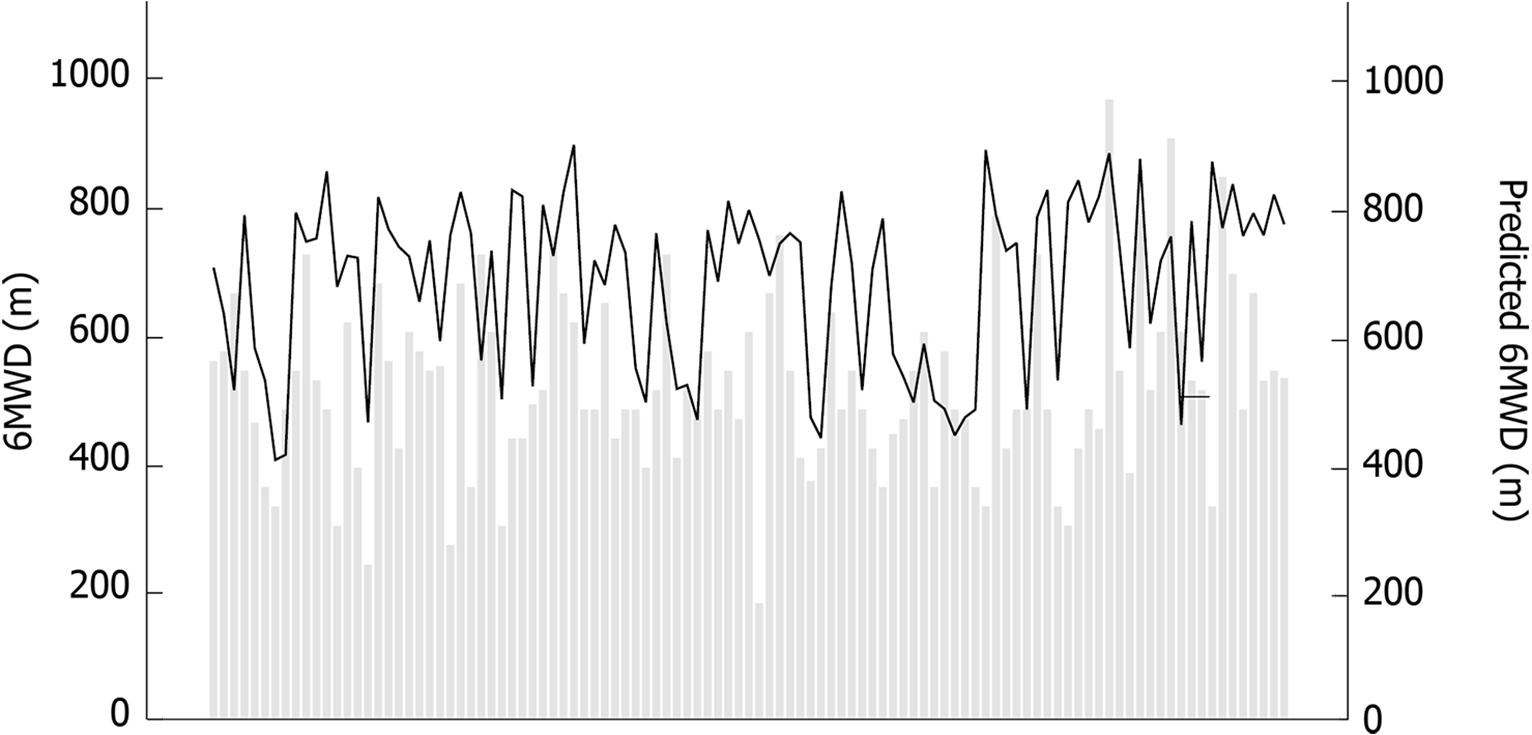
The majority of this cohort (71.7%) did not achieve the predicted distance adjusted for age and gender according to standardized equations[22] (678 ± 131m, 402-890 m) (see Figure 1). 6MWT performance is demonstrated in Table 2. The mean 6MWD of this cohort was 515 ± 138 m, 180-960 m. Not surprisingly, older patients with higher Child score, worse hepatic synthetic function (lower albumin) and anemia performed worse. It was found to be inversely correlated with age (r = -0.391, P < 0.001) and Child score (r = -0.228, P = 0.019), and positively correlated with albumin (r = 0.242, P = 0.012), creatinine (r = 0.242, P = 0.018) and hemoglobin (r = 0.192, P = 0.048). Patients with a history of at least one hepatic decompensation in the past (74.5%) presented with significant shorter 6MWD (496 ± 141 m vs 571 ± 115 m, P = 0.015).
| Variable | 6MWD (m) | P | 6MWD (%) | P |
| (t-test when applicable) | (t-test when applicable) | |||
| Mean 6MWD (m) | 515 ± 138 | |||
| Mean 6MWD (%) | 0.91 ± 2.3 | |||
| 6MWD according to Child classes | ||||
| A | 570 ± 144 | 0.97 ± 0.22 | ||
| B | 504 ± 137 | 0.88 ± 0.21 | ||
| C | 471 ± 115 | 0.82 ± 0.25 | ||
| 6MWD according to | ||||
| Liver decompensation | ||||
| Ascites (w vs wo) | 473 ± 20 vs 535 ± 17 | 0.03 | 0.86 ± 0.22 vs 0.95 ± 0.21 | 0.028 |
| Hepatic encephalopathy (w vs wo) | 435 ± 34 vs 525 ± 14 | 0.04 | 0.87 ± 0.25 vs 0.91 ± 0.21 | 0.87 |
| History of previous hepatic decompensation (w vs wo) | 496 ± 141 vs 571 ± 115 | 0.02 | 0.86 ± 0.22 vs 1.02 ± 0.17 | 0.004 |
| Hospital admission during follow-up (w vs wo) | 444 ± 172 vs 531 ± 125 | 0.01 | 0.77 ± 0.25 vs 0.92 ± 0.20 | 0.004 |
| Survival (died vs survived) | 423 ± 122 vs 526 ± 137 | 0.02 | 0.72 ± 0.21 vs 0.93 ± 0.21 |
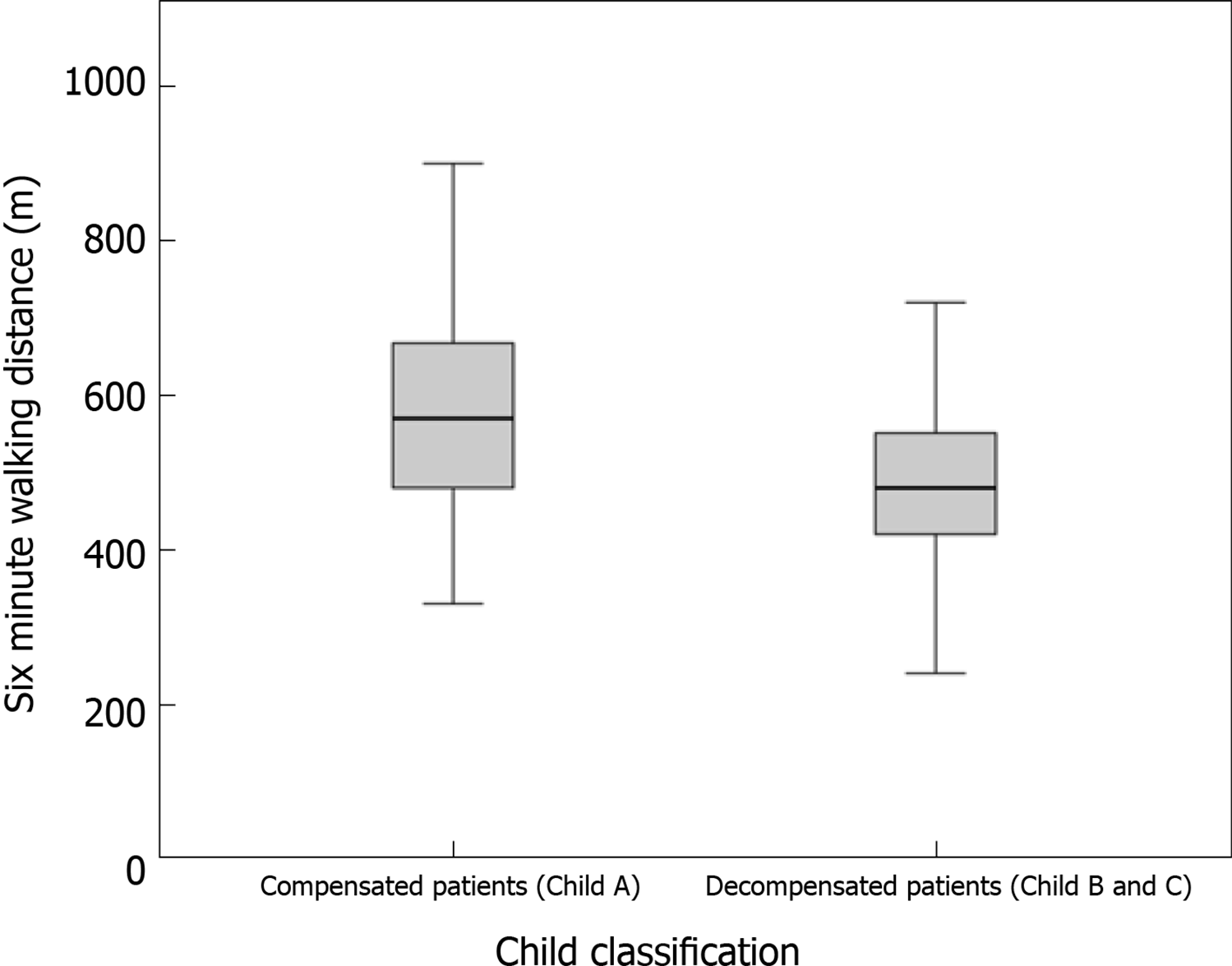
The mean 6MWD was progressively shorter among Child classes (A = 570 ± 144 m, B = 504 ± 137 m and C = 471 ± 115 m) and statistical significance was demonstrated between Child A and C (P = 0.04) and when Child A was compared with more advanced stages (B and C), P = 0.02. 6MWD was different among compensated (Child Pugh A) and decompensated (Child Pugh B and C) patients (P = 0.031) (see Figure 2). Patients decompensated with ascites or hepatic encephalopathy on the day of the test achieved shorter distances than those who did not have ascites or hepatic encephalopathy (472 vs 534m, P = 0.03; 440 vs 525m, P = 0.04, respectively). All patients previously included were submitted to 6MWT, even those with hepatic decompensation at the moment of evaluation, ascites or encephalopathy. 6MWD did not differ according to the etiologies of cirrhosis (P = 0.08), past history of alcohol abuse (P = 0.58), use of beta-blocker (P = 0.19), tobacco (P = 0.97) and presence of anemia (P = 0.84).
None of the patient presented with liver related decompensation within 2 wk following the exercise, meaning no detectable clinically significant portal hypertension increase induced by exercise. All patients were able to perform exercise adequately, without help, interruptions, or any significantly adverse effect.
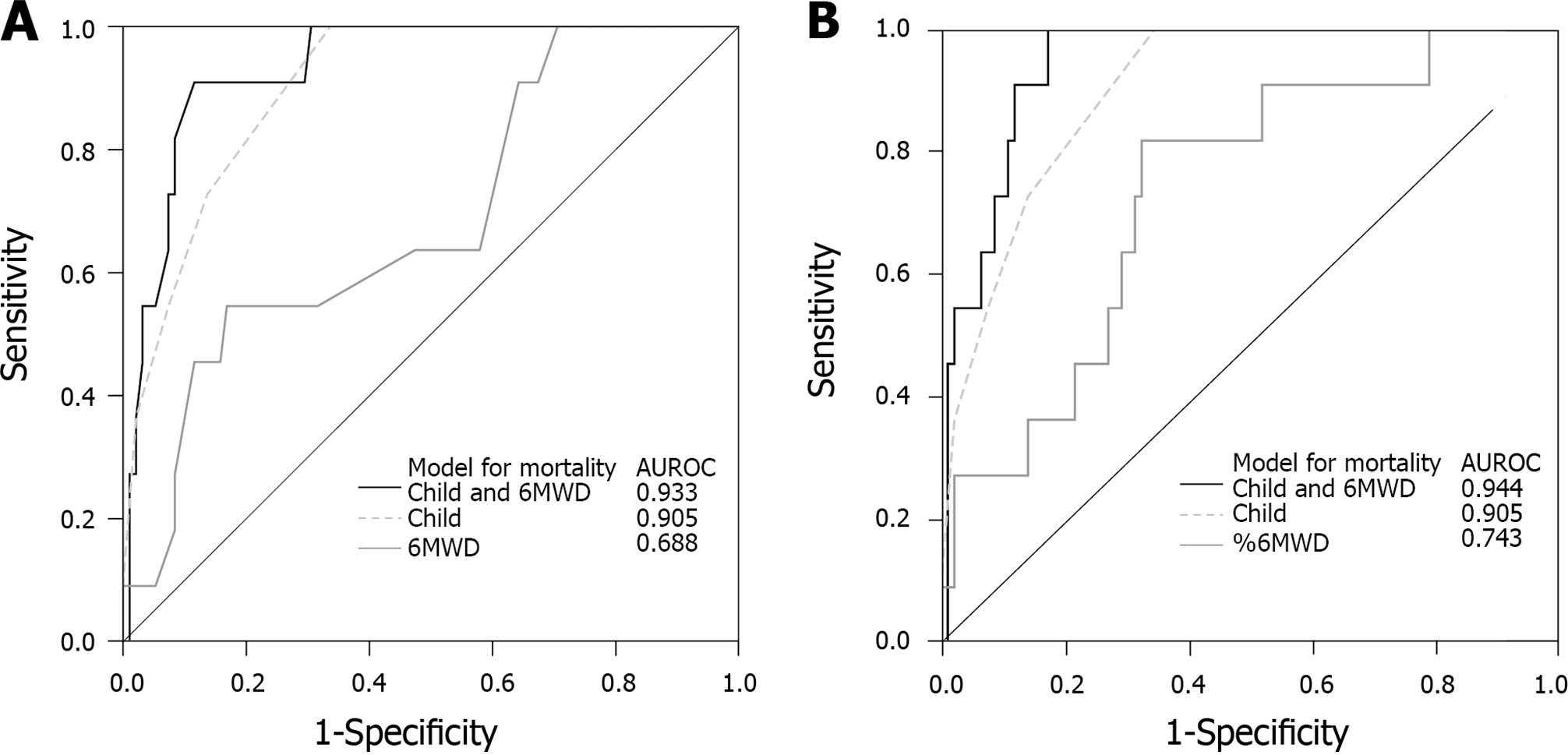
To emphasize the role of 6MWD and %6MWD in the prediction mortality, as an additional factor besides liver disease severity, logistic regression models were designed to evaluate if the inclusion of 6MWT parameters improves the model performance and increases the AUROC computed using regression models. MELD and Child score were used to quantify the severity of liver disease. When 6MWT parameters were added to the models designed to predicted mortality using MELD or Child score, we observed an improvement in model performance, defined as a significant difference according to Omnibus Chi-square test (P = 0.01) and higher AUROCs in combining models (see Figure 3).
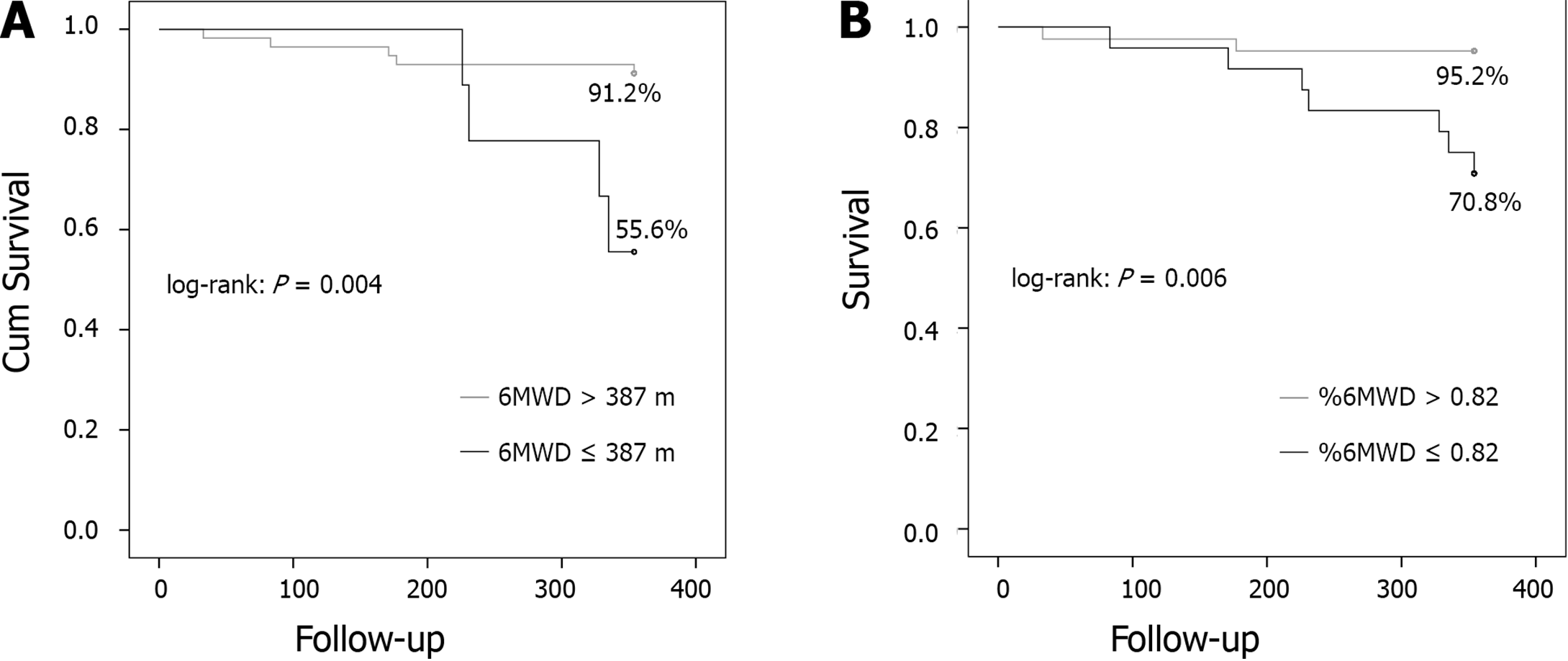
Cutoff points associated with mortality was 387 m for 6MWD (sensibility 90.9 and specificity 88.4) and 0.82 for %6MWD (sensibility 100 and specificity 83.2). After exclusion of patients who were submitted to liver transplantation, patients who died (11, 10.4%) had a shorter mean 6MWD (423 m vs 526 m, P = 0.006) and lower %6MWD (0.72 vs 0.92, P = 0.004). Just one of them achieved the predicted distance during 6MWT. 6MWD and %6MWD were independent predictors of mortality, after adjusted for Child scores, according to multivariate regression model analysis (Table 3). Patients who achieved distances shorter than 387 m or %6MWD < 0.82 presented higher mortality, and statistical difference according to Kaplan-Meier and log-rank analysis (P = 0.004 and P = 0.006, respectively) (Figure 4).
| Hospital Admission | Mortality | ||||||||||||
| Predictors | Univariate | Multivariate | Univariate | Multivariate | |||||||||
| b | p | OR | b | p | OR | b | p | OR | b | p | OR | ||
| Child score | 0.74 | < 0.01 | 2.1 | 0.72 | < 0.01 | 2.05 | 1.01 | < 0.01 | 2.75 | 1.03 | < 0.01 | 2.8 | |
| 6MWD | -0.005 | < 0.01 | 0.99 | -0.005 | 0.24 | 0.99 | -0.007 | 0.01 | 0.99 | -0.007 | 0.04 | 0.99 | |
| %6MWD | -0.04 | 0.01 | 0.96 | -0.03 | 0.03 | 0.96 | -0.05 | 0.02 | 0.95 | -0.05 | 0.03 | 0.95 | |
| 6MWD ≤ 444 m | -1.395 | 0.007 | 0.3 | -1.462 | 0.01 | 0.2 | - | - | - | - | - | - | |
| 6MWD ≤ 387 m | - | - | - | - | - | - | 1.659 | 0.004 | 5.25 | -1.17 | 0.2 | 0.31 | |
6MWT is a safe, easy-to-administer, and inexpensive test to determine the functional capacity of cirrhotic patients and also has prognostic value. We found that a decreased 6MWD, as a marker of impaired exercise capacity, is associated with hepatic dysfunction. In addition, 6MWD and %6MWD performed as independent predictors of mortality, becoming an important tool during risk evaluation of severe complications and death in liver cirrhosis. Also, this study reinforces the key importance of physical evaluation during cirrhotic patients, especially those referred to liver transplantation team.
Basal exercise capacity was significantly impaired in our patients, as only 28.3% achieved the pre-test predicted distance. The 6MWD results in our cohort of patients was similar to previous studies in patients with cirrhosis which found a significantly lower 6MWD values than expected for healthy population[22]. Our cohort had a mean 89.7% (34.8%-149%) of predicted 6MWD (vs 63% found by Román et al[18], and a mean 6MWD of 515 m (180-960 m), compared to 306 m in Alameri et al[14]‘s cohort of 98 patients with cirrhosis. The poor performance during 6MWT meets with the current knowledge about the abnormal exercise capacity in cirrhotic patients. Future studies should verify those findings and evaluate if 6MWD can be used as a more general tool able to evaluate outcomes and quality of life in this group[15].
We reported a weak inverse correlation between 6MWD and Child scores (r = -0.228, P = 0.019), although it was clear the tendency in walk distance reduction along Child classes. Carey et al[15], studying 121 cirrhotic patients, showed a strong correlation with MELD. In this particular study, all patients were listed for liver transplant, denoting a population with more advanced disease, making us understand that this stronger correlation reflects a major prevalence of their patient’s overall disability when comparing to our study group. In the same way, by comparing subjects with advanced disease (Child B and C) and those without it (Child A), we detected a significant difference between these groups (P = 0.02), supporting the previous interpretation. Furthermore, patients with a history of at least one hepatic decompensation in the past, presented shorter 6MWD (P = 0.015) and subjects presenting with ascites or encephalopathy at the moment of evaluation performed worse, these facts highlight the relationship between shorter distances and severity of liver disease in our study. Similarly, Wong et al[23] reported that patients with decompensated cirrhosis with ascites performed worse during cycle ergometer evaluation when compared to well compensated patients, however, no specific data is available regarding 6MWT.
Although the gold standard measurement of exercise capacity is maximal VO2[24] measurement during treadmill or cycle ergometer tests, 6MWT is a cheap and simple test found to correlate with oxygen consumption that can be administered without special equipment or skilled staff that you can perform in clinic to give an immediate result. Noticeable that all patients in our study completed the full test, independently of the presence of ascites or encephalopathy, demonstrating one great advantage above other exercise tests, that sometimes require a more complex adaptation and comprehension about the technique. Cahalin et al[9] performed 6MWT and symptom-limited cardiopulmonary exercise testing in patients with heart failure during cardiac transplant evaluation. The authors described a significant correlation between 6MWD and peak VO2 (r = 0.64, P < 0.001), concluding that 6MWT is a valuable tool to predict VO2 and short-term survival. These results should be validated in cirrhotic population, but represent a good evidence that 6MWT could be introduced in routine practice without loss of diagnostic accuracy in exercise capacity estimation. While our study did not evaluate the association between VO2 and 6MWD, it did show the safety and practicality of this procedure. García-Pagàn et al[25] reported that moderate exercise (30% of the maximum) significantly increases portal pressure in patients with portal hypertension, and, therefore, could increase the risk of variceal bleeding, ascites and encephalopathy. Although 6MWT is a submaximal exercise, we did not identify any clinical event directly associated with it during the period following the test. Recent studies do not mention the prevalence of adverse events induced by exercise, and more studies designed to respond this issue should be carried out.
Previously studies who reported the relationship between 6MWT and mortality were conducted with small populations and during a short period of followup[11,12]. Poor performance during 6MWT may warrant that the at-risk patients should be followed more closely due to the risk of adverse events. Notwithstanding, 6MWT has been proposed as a tool during frailty status evaluation, giving emphasis in this role as a practical and cheap method for this proposal. This study reinforces this importance, adding more powerful results due to our long period of follow-up, demonstrating how physical exercise evaluation may be an interesting long predictor of prognosis in cirrhotic patients.
In our study, 6MWD was an independent predictor of death, consistent with findings from previous studies by Alameri et al[14], and Carey et al[15]. In the first study, mortality was evaluated in the whole group, including patients with non-cirrhotic chronic hepatitis, which may bias the interpretation about causality between 6MWD and cirrhosis. Also, Carey et al[15] studied a population with more advanced disease, all of them on the liver transplant waiting list with a high frequency of liver transplantation (50.4%) performed in a short period of time (5-6 mo). The statistical power of 6MWT in predicting mortality could be affected by pulling out so many patients after transplant from this cohort.
The role of 6MWD and %6MWD in the prediction of mortality were independently of Child scores as demonstrated by multivariate logistic regression analysis. These facts highlight the association of 6MWT parameters with disease progression and adverse outcomes, despite the severity of liver disease.
There are several limitations to our study. First, we did not proceed an external validation of 6MWD cutoffs used in our study, although our main objectives were focused in the transversal and descriptive characterization of study population. Second, we did neither evaluated nutritional status nor calculate the Frailty score of our patients. When study was designed there were no clear parameters specific settle for this diagnosis and a retrospective evaluation was not possible due to lack of complete data. Although recent studies suggest a close relationship between malnou
In summary, 6MWT is a very simple, inexpensive, well tolerated, noninvasive test to assess exercise capacity and the result of which is related to MELD and Child scores. The present study showed that 6MWD is an independent predictor of mortality in this population. 6MWT is a promising prognostic marker in patients with liver cirrhosis and should be considered as part of liver transplantation evaluation especially in those referred for the liver transplantation team.
Patients with cirrhosis are at risk of exercise limitations due to progressive limitations related to liver dysfunction. Sarcopenia and cirrhotic cardiomyopathy may be possible related factors. The six-minute walking test (6MWT) is a known simple and practical tool used to evaluate patients with cardiopulmonary disease.
In face of limited diagnosis tools focused on exercise capacity, we purposed to evaluate the role of 6MWT in this population.
The aim of our study was to analyzed 6MWT performance in patients with liver cirrhosis to determine if it associates with mortality.
We analyzed 6MWT performance in 106 cirrhotic patients. They were evaluated in the outpatient setting with 6MWT and follow up for one year. Hepatic decompensation and mortality were documented.
This cohort had a mean age of 51 years and 56% male; patients were staged as Child A in 21.7%, B 66%, and C 12.3%. Walk distance inversely correlated with Child scores, and was significantly reduced as Child stages progress. Patients who died (10.4%) showed a shorter mean 6MWD (P = 0.006). Low 6MWD was an independent predictor of mortality (P = 0.01).
6MWT is a noninvasive inexpensive test whose result is related to Child scores and mortality.
It is a useful, simple, practical test that can be incorporated into cirrhotic evaluation due to its relation with mortality for closer monitoring and potential early intervention.
This study was supported by Sao Luis Hospital, D'Or Institute of Research and Education (IDOR), and Fleury Institution. The content is solely the responsibility of the authors and does not necessarily represent the official views of these institutions.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng L, Cojocariu C, Ielasi L, Moghadam BA, Payance A S-Editor: Liu M L-Editor: A P-Editor: Liu M
| 1. | Jones JC, Coombes JS, Macdonald GA. Exercise capacity and muscle strength in patients with cirrhosis. Liver Transpl. 2012;18:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 2. | Campillo B, Fouet P, Bonnet JC, Atlan G. Submaximal oxygen consumption in liver cirrhosis. Evidence of severe functional aerobic impairment. J Hepatol. 1990;10:163-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Bunchorntavakul C, Reddy KR. Review article: malnutrition/sarcopenia and frailty in patients with cirrhosis. Aliment Pharmacol Ther. 2020;51:64-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 4. | Izzy M, VanWagner LB, Lin G, Altieri M, Findlay JY, Oh JK, Watt KD, Lee SS; Cirrhotic Cardiomyopathy Consortium. Redefining Cirrhotic Cardiomyopathy for the Modern Era. Hepatology. 2020;71:334-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 233] [Article Influence: 46.6] [Reference Citation Analysis (2)] |
| 5. | Montano-Loza AJ. Muscle wasting: a nutritional criterion to prioritize patients for liver transplantation. Curr Opin Clin Nutr Metab Care. 2014;17:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Lai JC, Volk ML, Strasburg D, Alexander N. Performance-Based Measures Associate With Frailty in Patients With End-Stage Liver Disease. Transplantation. 2016;100:2656-2660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Bhanji RA, Montano-Loza AJ, Watt KD. Sarcopenia in Cirrhosis: Looking Beyond the Skeletal Muscle Loss to See the Systemic Disease. Hepatology. 2019;70:2193-2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6981] [Cited by in RCA: 8299] [Article Influence: 360.8] [Reference Citation Analysis (0)] |
| 9. | Cahalin LP, Mathier MA, Semigran MJ, Dec GW, DiSalvo TG. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest. 1996;110:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 494] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 10. | Roul G, Germain P, Bareiss P. Does the 6-minute walk test predict the prognosis in patients with NYHA class II or III chronic heart failure? Am Heart J. 1998;136:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 162] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, Pinto Plata V, Cabral HJ. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2409] [Cited by in RCA: 2549] [Article Influence: 121.4] [Reference Citation Analysis (0)] |
| 12. | Troosters T, Gosselink R, Decramer M. Six minute walking distance in healthy elderly subjects. Eur Respir J. 1999;14:270-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 627] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 13. | Enright PL, McBurnie MA, Bittner V, Tracy RP, McNamara R, Arnold A, Newman AB; Cardiovascular Health Study. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. 2003;123:387-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 484] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 14. | Alameri HF, Sanai FM, Al Dukhayil M, Azzam NA, Al-Swat KA, Hersi AS, Abdo AA. Six Minute Walk Test to assess functional capacity in chronic liver disease patients. World J Gastroenterol. 2007;13:3996-4001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Carey EJ, Steidley DE, Aqel BA, Byrne TJ, Mekeel KL, Rakela J, Vargas HE, Douglas DD. Six-minute walk distance predicts mortality in liver transplant candidates. Liver Transpl. 2010;16:1373-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 16. | Dharancy S, Lemyze M, Boleslawski E, Neviere R, Declerck N, Canva V, Wallaert B, Mathurin P, Pruvot FR. Impact of impaired aerobic capacity on liver transplant candidates. Transplantation. 2008;86:1077-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Veloso-Guedes CA, Rosalen ST, Thobias CM, Andreotti RM, Galhardo FD, Oliveira da Silva AM, Araujo O, Boin IF. Validation of 20-meter corridor for the 6-minute walk test in men on liver transplantation waiting list. Transplant Proc. 2011;43:1322-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Román E, Torrades MT, Nadal MJ, Cárdenas G, Nieto JC, Vidal S, Bascuñana H, Juárez C, Guarner C, Córdoba J, Soriano G. Randomized pilot study: effects of an exercise programme and leucine supplementation in patients with cirrhosis. Dig Dis Sci. 2014;59:1966-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 155] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 19. | Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1-85. [PubMed] |
| 20. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2069] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 21. | O'Shea RS, Dasarathy S, McCullough AJ; Practice Guideline Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology. 2010;51:307-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 853] [Article Influence: 56.9] [Reference Citation Analysis (2)] |
| 22. | Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1246] [Cited by in RCA: 1364] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 23. | Wong F, Girgrah N, Graba J, Allidina Y, Liu P, Blendis L. The cardiac response to exercise in cirrhosis. Gut. 2001;49:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 194] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 24. | McArdle W, Katch F, Katch V. Exercise Physiology. 7th ed. The Point, editor. Lippincott Williams & Wilkins; 2010: 192–247. |
| 25. | García-Pagàn JC, Santos C, Barberá JA, Luca A, Roca J, Rodriguez-Roisin R, Bosch J, Rodés J. Physical exercise increases portal pressure in patients with cirrhosis and portal hypertension. Gastroenterology. 1996;111:1300-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 3.9] [Reference Citation Analysis (0)] |