Published online Nov 27, 2021. doi: 10.4254/wjh.v13.i11.1766
Peer-review started: April 27, 2021
First decision: June 15, 2021
Revised: July 5, 2021
Accepted: September 19, 2021
Article in press: September 19, 2021
Published online: November 27, 2021
Processing time: 210 Days and 23.9 Hours
While primary liver cancer (PLC) is one of the most common cancers around the world, few large-scale population-based studies have been reported that evaluated the clinical survival outcomes among peripartum and postmenopausal women with PLC.
To investigate whether peripartum and postmenopausal women with PLC have lower overall survival rates compared with women who were not peripartum and postmenopausal.
The Taiwan National Health Insurance claims data from 2000 to 2012 was used for this propensity-score-matched study. A cohort of 40 peripartum women with PLC and a reference cohort of 160 women without peripartum were enrolled. In the women with PLC with/without menopause study, a study cohort of 10752 menopausal females with PLC and a comparison cohort of 2688 women without menopause were enrolled.
Patients with peripartum PLC had a non-significant risk of death compared with the non-peripartum cohort [adjusted hazard ratios (aHR) = 1.40, 95% confidence intervals (CI): 0.89-2.20, P = 0.149]. The survival rate at different follow-up durations between peripartum PLC patients and those in the non-peripartum cohort showed a non-significant difference. Patients who were diagnosed with PLC younger than 50 years old (without menopause) had a significant lower risk of death compared with patients diagnosed with PLC at or older than 50 years (postmenopausal) (aHR = 0.64, 95%CI: 0.61-0.68, P < 0.001). The survival rate of women < 50 years with PLC was significantly higher than older women with PLC when followed for 0.5 (72.44% vs 64.16%), 1 (60.57% vs 51.66%), 3 (42.92% vs 31.28%), and 5 year(s) (37.02% vs 21.83%), respectively (P < 0.001).
Peripartum females with PLC have no difference in survival rates compared with those patients without peripartum. Menopausal females with PLC have worse survival rates compared with those patients without menopause.
Core Tip: This is the first nationwide study to evaluate the survival rate of peripartum and postmenopausal women with primary liver cancer (PLC) using the National Health Insurance Research Database in Taiwan. The results showed that patients with peripartum PLC had a non-significant risk of death compared with those in the non-peripartum cohort. Patients who were diagnosed with PLC younger than 50 years (without menopause) had a significantly lower risk of death compared with patients diagnosed with PLC at 50 years or older (after menopause). We believe that the results presented in this study provide important information on clinical applications.
- Citation: Tseng GW, Lin MC, Lai SW, Peng CY, Chuang PH, Su WP, Kao JT, Lai HC. Do peripartum and postmenopausal women with primary liver cancer have a worse prognosis? A nationwide cohort in Taiwan. World J Hepatol 2021; 13(11): 1766-1776
- URL: https://www.wjgnet.com/1948-5182/full/v13/i11/1766.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i11.1766
Primary liver cancer (PLC), the sixth most common cancer, and the fourth leading cause of cancer-related death around the world in 2018, put a heavy burden on global health[1,2]. Hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma, account for 70%-75% and 15% of cases, respectively, and comprise most primary liver malignancies[3]. The common risk factors of PLC are male gender, excess body fat, type II diabetes mellitus, chronic infection with hepatitis B virus (HBV) and/or hepatitis C virus (HCV), cigarette smoking, aflatoxin, and heavy alcohol consumption[4,5]. Men appear to have a higher occurrence and worse outcomes, with two to three times higher incidence and mortality compared with women[1,6]. Thus, most studies have included too few women to draw accurate conclusions.
Animal studies indicated that the primary etiology behind the protective effect of the female sex hormone might involve the anti-inflammatory modulation of estrogen, as chronic inflammation was a major contributor to carcinogenic processes[7-9]. Nevertheless, controversial results were obtained in research targeting women of reproductive age. Despite the rarity, PLC diagnosed during pregnancy generally caused a shorter survival compared with non-pregnant patients with inoperable PLC[10-12]. Several early reports suggested that the adverse influence of pregnancy for the development of PLC was probably due to an alteration of the hormonal milieu[13,14]. In contrast, other recent papers attributed the consequence to delayed diagnosis[11,15]. However, the latest cohort analysis needs further interpretation, as most of the published articles were case reports, with the largest including 48 cases published in 2011[12,16]. In addition, evidence implied that the downturn in ovarian function in menopause is related to the spontaneous elevation in pro-inflammatory cytokines[17-19], which may have an undesirable effect on PLC development and progression. While there were limited epidemiologic statistics with the survival outcome among females, the research indicated that there was a reduced risk. It increased overall survival times of PLCs in postmenopausal patients receiving hormone replacement therapy (HRT)[20]. It is estimated that 1.2 billion women worldwide will be menopausal or postmenopausal by the year 2030[21]. Therefore, there is a growing necessity to make a thorough exploration of the morbidity and mortality of PLCs among this sector of the population.
To date, few large-scale population-based studies have been conducted to elucidate the relationship between pregnancy, menopause, and survival outcomes among women with PLCs. Our primary aim was to determine if pregnant and postmenopausal female patients with PLCs have a lower survival rate relative to population-based controls using a nationwide database in Taiwan.
Taiwan government built a nationwide health record-related database named the National Health Insurance Research Database (NHIRD) in 1995. The database contains comprehensive health information, representative study subjects, and long-term follow-up periods. This study was conducted using the population-based hospitalization file, including all hospitalization records of Taiwan citizens. The identification was encrypted before the database released the records for medical research to protect the privacy of each patient.
All previous diagnoses in the database were coded according to the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM). The Research Ethics Committee of China Medical University and Hospital in Taiwan approved the study (CMUH-104-REC2-115-R3).
According to the study objective, we would like to confirm the association between peripartum PLC and survival. We selected patients with peripartum PLC (ICD-9-CM: 155) who were diagnosed between 10 mo before and six months after delivery, during 2000-2012, as the exposed cohort. We defined the date of newly diagnosed PLC as the index date. The unexposed group was defined as patients with PLC who were diagnosed outside of the pregnancy period and selected by 4:1 propensity score matching with the exposed cohort. The matching variables included age, index year, and comorbidities, such as HBV, unspecified chronic hepatitis, alcoholic liver disease, cirrhosis, biliary stones, cholecystitis, and cholangitis. To further realize the correlation between menopause and PLC prognosis, we defined women aged 50 and beyond as postmenopausal period. While natural menopause may occur from 45 to 55 of age[22], a recent cohort analysis including 36931 postmenopausal women indicated that the mean age at menopause is 50.2 years in Taiwan[23]. Propensity score matching and matching variables mentioned above were applied. Patients with PLC before the index date were excluded from the study. The study population was followed up until death, withdrawn from NHIRD, or until December 31, 2013.
The comorbidities of concern in this study were HBV (ICD-9-CM: 070.2, 070.3, and V02.61), unspecified chronic hepatitis (ICD-9-CM: 070.9, 571.4, 571.8, 571.9), alcoholic liver disease (ICD-9-CM: 571.0, 571.1, 571.2, 571.3), cirrhosis (ICD-9-CM: 571.5, 571.6), biliary stones (ICD-9-CM: 574), cholecystitis (ICD-9-CM: 575), and cholangitis (ICD-9-CM: 576). The comorbidities above were defined as at least one hospitalization before the index date.
This study included demographic and comorbidities variables. The continuous and the categorical variables were shown by mean ± SD and number (%), and to compare the difference of each variable in two groups, a t-test and chi-square test were used, respectively. To calculate the risk of death in the exposed and the unexposed cohorts, Cox proportional hazard models were used and presented using hazard ratios, adjusted hazard ratios (aHR) and 95% confidence intervals (CIs). The survival rate of death in the two cohorts was presented by the Kaplan-Meier method. The log-rank test was used to compare the difference between two survival curves. All statistical analyses were performed with SAS statistical software version 9.4 (SAS Institute Inc., Cary, NC). The Figure of the cumulative incidence curve was plotted by R software. The significance criteria were set up as a two-sided test with a P value of less than 0.05.
Of 200 eligible subjects in this study (Table 1), 40 were diagnosed with peripartum PLC, and the other 160 were selected as the unexposed cohort. Among patients with peripartum PLC, the dominant age group was younger than 30 years old (47.5%), 11 (27.5%) with HBV, one (2.5%) with unspecified chronic hepatitis, one with alcoholic liver disease, three (7.5%) with cirrhosis, two (5%) with biliary stone, four (10%) with cholecystitis, and three (7.5%) with cholangitis. The mean age of the exposed and unexposed cohort was 30.9 and 31.3 years, respectively. The characteristics and comorbidities showed a non-significant difference between the two cohorts after propensity matching (P > 0.05).
| Characteristics | Total, N | Peripartum primary liver cancer | P value | |
| No, n = 160 | Yes, n = 40 | |||
| n (%) / mean ± SD | n (%) / mean ± SD | |||
| Age | 0.788 | |||
| < 30 | 88 | 69 (43.1) | 19 (47.5) | |
| 30-34 | 64 | 53 (33.1) | 11 (27.5) | |
| 35-49 | 48 | 38 (23.8) | 10 (25) | |
| mean ± SD1 | 31.3 ± 5.1 | 30.9 ± 4.8 | 0.673 | |
| Baseline comorbidity | ||||
| HBV | 58 | 47 (29.4) | 11 (27.5) | 0.815 |
| Unspecified chronic hepatitis | 2 | 1 (0.6) | 1 (2.5) | 0.286 |
| Alcoholic liver disease | 6 | 5 (3.1) | 1 (2.5) | 0.836 |
| Cirrhosis | 9 | 6 (3.8) | 3 (7.5) | 0.306 |
| Biliary stone | 6 | 4 (2.5) | 2 (5) | 0.407 |
| Cholecystitis | 12 | 8 (5) | 4 (10) | 0.234 |
| Cholangitis | 10 | 7 (4.4) | 3 (7.5) | 0.417 |
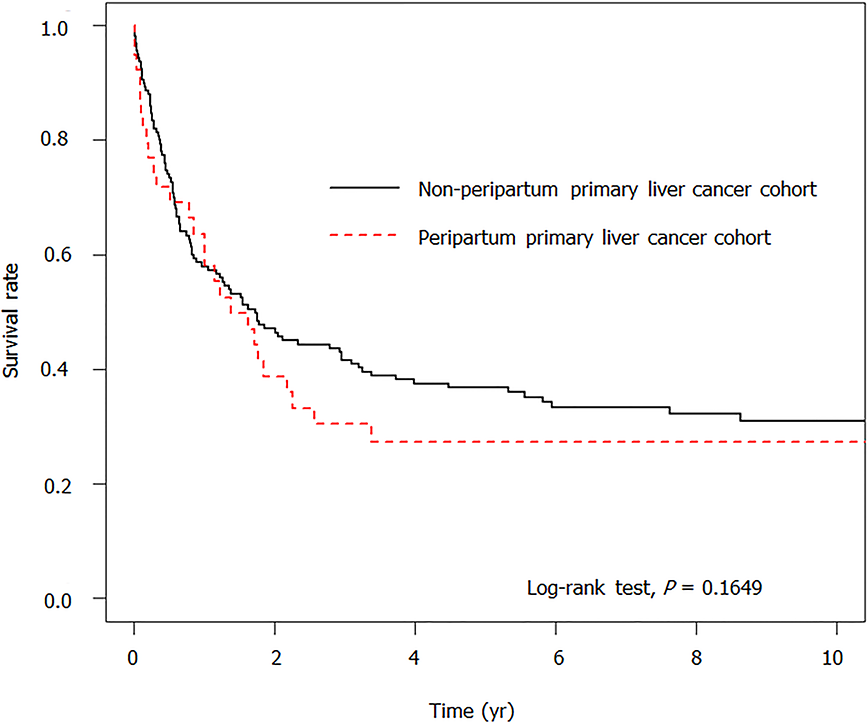
Table 2 presents the risk factors of death associated with and without peripartum PLC. Patients with peripartum PLC had a non- significant risk of death compared with the unexposed cohort (aHR = 1.40, 95%CI: 0.89-2.20, P = 0.149). Considering their older age and comorbidities, patients with HBV (aHR = 0.48, 95%CI: 0.30-0.77, P = 0.002) and cholecystitis (aHR = 0.30, 95%CI: 0.12-0.75) showed a decreased risk of death; patients with cholangitis showed a significantly higher risk of death (aHR = 3.34, 95%CI: 1.49-7.47, P = 0.003). Figure 1 illustrates the non-significant difference in the survival curves between the two cohorts (P = 0.1649).
| Characteristics | Event, n = 124 | Person, yr | IR | Crude | Adjusted | ||
| HR (95%CI) | P value | HR (95%CI) | P value | ||||
| Peripartum primary liver cancer | |||||||
| No | 97 | 587 | 16.53 | Ref. | Ref. | ||
| Yes | 27 | 99 | 27.17 | 1.35 (0.88-2.08) | 0.166 | 1.40 (0.89-2.20) | 0.149 |
| Age at baseline | |||||||
| < 30 | 54 | 350 | 15.44 | Ref. | Ref. | ||
| 30-34 | 39 | 169 | 23.08 | 1.21 (0.80-1.84) | 0.359 | 1.47 (0.95-2.28) | 0.083 |
| 35-49 | 31 | 167 | 18.52 | 1.29 (0.83-2.00) | 0.266 | 1.13 (0.69-1.85) | 0.617 |
| Baseline comorbidity | |||||||
| HBV | 27 | 230 | 11.72 | 0.52 (0.34-0.80) | 0.003 | 0.48 (0.30-0.77) | 0.002 |
| Unspecified chronic hepatitis | 1 | 11 | 9.04 | 0.86 (0.12-6.17) | 0.882 | 0.56 (0.08-4.10) | 0.565 |
| Alcoholic liver disease | 6 | 8 | 73.37 | 2.85 (1.25-6.49) | 0.013 | 2.15 (0.73-6.36) | 0.165 |
| Cirrhosis | 7 | 40 | 17.55 | 1.12 (0.52-2.40) | 0.773 | 1.49 (0.57-3.90) | 0.411 |
| Biliary stone | 4 | 11 | 35.47 | 1.23 (0.45-3.33) | 0.686 | 0.64 (0.17-2.35) | 0.499 |
| Cholecystitis | 5 | 80 | 6.26 | 0.43 (0.18-1.06) | 0.066 | 0.30 (0.12-0.75) | 0.010 |
| Cholangitis | 7 | 4 | 179.30 | 3.76 (1.71-8.26) | < 0.001 | 3.34 (1.49-7.47) | 0.003 |
The survival rate at different follow-up durations between patients with peripartum PLC and the unexposed cohort (Table 3) revealed a non-significant difference. When followed for less than 0.5 years, 1 year, 3 years, or 5 years, the survival rate in patients with peripartum PLC was lower than that in the unexposed cohort (71.79% vs 78.94%; 60.84 vs 63.61%; 30.42 vs 44.85%; 27.38 vs 39.59%), but without a significant difference between the two cohorts (P > 0.05).
| Follow-up duration | Survival rate (%) | P value | |
| Non-peripartum primary liver cancer | Peripartum primary liver cancer | ||
| ≤ 0.5 | 78.94 | 71.79 | 0.254 |
| ≤ 1 | 63.61 | 60.84 | 0.611 |
| ≤ 3 | 44.85 | 30.42 | 0.111 |
| ≤ 5 | 39.59 | 27.38 | 0.117 |
We enrolled 13440 study subjects to learn more about the influence of age and menopause on survival outcomes. Of these women, 2688 were diagnosed with PLC, younger than 50 years, and without menopause (Table 4). The other group comprised 10752 women who were PLC patients, aged 50 years and older, and with menopause (postmenopausal). The mean ages were 39.7 and 69.1 years, respectively. The percentage of comorbidities had no significant difference between the two cohorts after propensity score matching by age and comorbidities (P > 0.05), except alcoholic liver disease (P = 0.041).
| Characteristics | Total, N = 13440 | Liver cancer | P value | |
| ≥ 50 yr, n = 10752 | < 50 yr, n = 2688 | |||
| n (%)/mean ± SD | n (%)/mean ± SD | |||
| Age | ||||
| mean ± SD1 | 69.1 ± 9.6 | 39.7 ± 10.5 | < 0.001 | |
| Baseline comorbidity | ||||
| HBV | 2971 | 2358 (21.9) | 613 (22.8) | 0.329 |
| HCV | 1168 | 931 (8.7) | 237 (8.8) | 0.795 |
| Unspecified chronic hepatitis | 780 | 619 (5.8) | 161 (6) | 0.645 |
| Alcoholic liver disease | 211 | 157 (1.5) | 54 (2) | 0.041 |
| Cirrhosis | 4142 | 3321 (30.9) | 821 (30.5) | 0.730 |
| Biliary stone | 1269 | 1012 (9.4) | 257 (9.6) | 0.813 |
| Cholecystitis | 626 | 489 (4.5) | 137 (5.1) | 0.227 |
| Cholangitis | 818 | 649 (6) | 169 (6.3) | 0.626 |
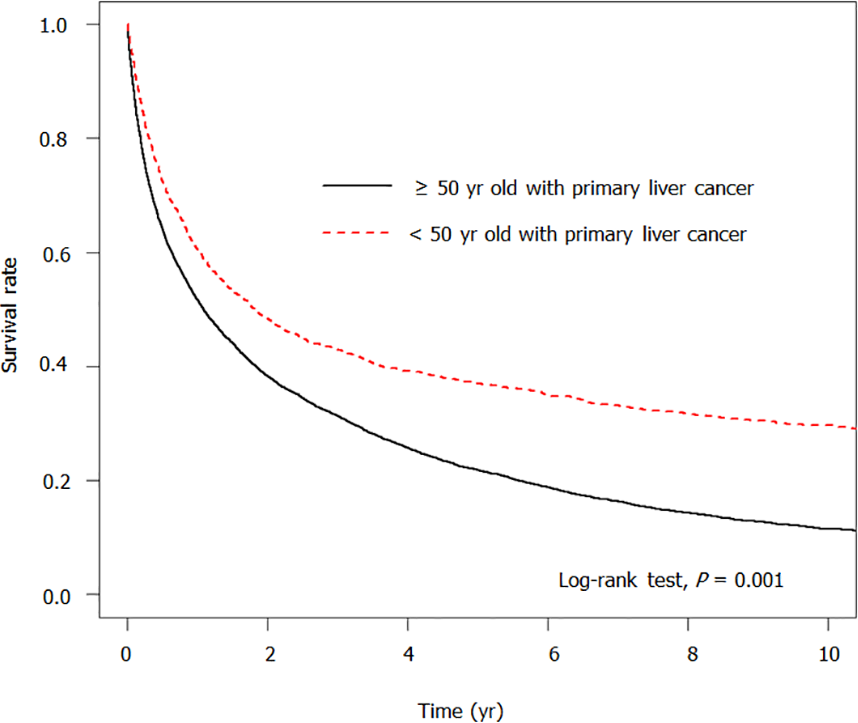
Table 5 shows the risk factors for developing death. Patients who were diagnosed with PLC at less than 50 years old had a substantially lower risk of death compared with patients diagnosed with PLC at 50 years or older (aHR = 0.64, 95%CI: 0.61-0.68, P < 0.001). Patients with HBV (aHR = 0.76, 95%CI: 0.72–0.80, P < 0.001), HCV (aHR = 0.72, 95%CI: 0.67-0.78, P < 0.001) and cholecystitis (aHR = 0.71, 95%CI: 0.64-0.78, P < 0.001) showed a significantly lower risk of developing death. patients with comorbidities such as cirrhosis (aHR = 1.18, 95%CI: 1.13-1.24, P < 0.001), and cholangitis (aHR = 1.77, 95%CI: 1.63-1.92, P < 0.001) had a notably higher risk of death. Figure 2 shows that the survival rate was significantly higher in women younger than 50 years old with PLC than in the older cohort (P < 0.001).
| Characteristics | Event, N = 9982 | Person, yr | IR | Crude | Adjusted | ||
| HR (95%CI) | P value | HR (95%CI) | P value | ||||
| Liver cancer | |||||||
| ≥ 50 yr | 8279 | 23410 | 35.37 | Ref. | Ref. | ||
| < 50 yr | 1703 | 9149 | 18.61 | 0.65 (0.61-0.68) | < 0.001 | 0.64 (0.61-0.68) | < 0.001 |
| Baseline comorbidity | |||||||
| HBV | 2049 | 7552 | 27.13 | 0.81 (0.77-0.85) | < 0.001 | 0.76 (0.72-0.80) | < 0.001 |
| HCV | 831 | 3513 | 23.65 | 0.75 (0.70-0.81) | < 0.001 | 0.72 (0.67-0.78) | < 0.001 |
| Unspecified chronic hepatitis | 584 | 2224 | 26.25 | 0.90 (0.83-0.98) | 0.015 | 0.96 (0.88-1.05) | 0.349 |
| Alcoholic liver disease | 165 | 449 | 36.72 | 1.04 (0.89-1.21) | 0.640 | 1.07 (0.91-1.25) | 0.408 |
| Cirrhosis | 3186 | 9924 | 32.10 | 1.01 (0.97-1.05) | 0.739 | 1.18 (1.13-1.24) | < 0.001 |
| Biliary stone | 955 | 2730 | 34.98 | 1.08 (1.01-1.15) | 0.024 | 0.98 (0.91-1.05) | 0.562 |
| Cholecystitis | 416 | 2162 | 19.25 | 0.70 (0.63-0.77) | < 0.001 | 0.71 (0.64-0.78) | < 0.001 |
| Cholangitis | 687 | 989 | 69.45 | 1.76 (1.63-1.91) | < 0.001 | 1.77 (1.63-1.92) | < 0.001 |
Table 6 presents the survival rates at different follow-up durations. The survival rate in women < 50 years with PLC was significantly higher than in older women with PLC when followed for 0.5 year (72.44% vs 64.16%), 1 year (60.57% vs 51.66%), 3 years (42.92% vs 31.28%), and 5 years (37.02% vs 21.83%), respectively (P < 0.001).
| Follow-up duration | Survival rate (%) | P value | |
| ≥ 50 yr | < 50 yr | ||
| ≤ 0.5 | 64.16 | 72.44 | < 0.001 |
| ≤ 1 | 51.66 | 60.57 | < 0.001 |
| ≤ 3 | 31.28 | 42.92 | < 0.001 |
| ≤ 5 | 21.83 | 37.02 | < 0.001 |
To our knowledge, this large-scale, population-based, cohort study is one of the pioneering research investigations that focused on women under different conditions to determine the relationship between peripartum and postmenopause and the risk of death from liver cancer. Based on our results, despite no significant difference, overall low survival was found in PLCs diagnosed either within or outside of the peripartum period among women of reproductive age (15-49 years old). Our data revealed that five-year survival rates in non-peripartum and peripartum PLCs were 39.59% and 27.38% (aHR = 1.40, 95%CI: 0.89-2.20, P = 0.149), respectively. However, postmenopausal women (> 50 years old) with PLCs have a considerable decrease in survival rates (five-year survival rates in fertile and postmenopausal women were 37.02% and 21.83%, respectively), compared with a significantly higher risk of death in premenopausal female patients (aHR = 0.64; 95%CI: 0.61-0.68). Although the molecular mechanisms underlying this protective effect are complicated, previous research suggested that the inhibitory role of estrogen was responsible for the gender disparity of PLCs partly via micro RNA, DNA repair, and obesity-associated pathways[7]. Moreover, the number of estrogen receptors (ERs) correlated with the risk of tumor occurrence and invasion. Some research proposed that ERs suppressed the proliferation and progression of liver cancer by decreasing the peroxisome proliferator-activated receptor γ and transcription of metastatic tumor antigen 1[24,25]. In the time of limited estrogen supply (e.g., Postmenopause), sex hormone binding globulin (SHBG), a plasma protein that involved in the maintenance of a reservoir of sex steroid hormones, played a crucial role in potentiating estrogenic action[26].
We focused on women of childbearing age to gain a deeper understanding of the influence of reproductive hormones. Because of the elevation of estrogen and progesterone during pregnancy, the diagnosis of PLCs within this period is rare. Nevertheless, among the 62 cases reported to date worldwide, all ended with poor outcomes when compared with non-pregnant women with PLCs[10]. As early as in 1995, Lau and his colleague[27] concluded that pregnancy has an adverse effect on the prognosis of patients with HCC, and therefore measurement of AFP level is recommended for screening HCC in pregnant women at high risk. The largest retrospective review published by Choi et al[12] demonstrated poor yet improving survival rates over time (median survivals of the groups before and during/after 1995 were 18 and 25.5 mo, respectively) among all 48 HCC cases in pregnancy. Contrary to prior research, our analysis of the nationwide database revealed an overall unpleasant prognosis among women of childbearing age. There was no significant difference in survival rates between parous and non-parous women with PLCs. This could probably be explained by the limited number of cases and the nationwide coverage of health insurance. Since almost all women received check-ups during the prenatal and postnatal period under the national health insurance program, proper management could be provided in time to improve outcomes.
Because menopause represents a state of gradual estrogen deficiency in the setting of physiologic aging, we also divided the study population into two groups by age, either younger or older than 50 years. According to Yang's research[28] investigating patients with HCC, women of 18 years old to 64 years old were noted as having longer survival than men of the same age, with the largest difference in survival among women aged 18 years to 44 years. Furthermore, Shimizu et al[29] reported that hepatic ER levels, which were inversely related to the progression of HCC, were significantly higher in premenopausal women compared with postmenopausal women. While El Mahdy Korah et al[30] stated that there was no clear relationship between sex hormone and HCC development or progression by analyzing total testosterone, estrogen, progesterone and prolactin levels among 40 selected HCC patients, Petrick’s cohort study in 2019[31] indicated that higher levels of SHBG and circulating estradiol were associated with an increased risk of HCC and ICC, respectively, among women after menopause. These data suggest that climacteric status may adversely mediate the outcomes of PLCs. Our results are consistent with those of previous studies, that implied a negative interplay between age and hormonal factors in the disease course since women beyond reproductive age (> 50 years old) with PLCs were found to have lower half-year, one-year, three-year, and five-year survival rates. Although it is difficult to distinguish how the two factors account for the consequence individually, it is certain that they interact with each other. This interaction results in diminishing immunologic responses to injury, and the imbalance between antioxidant formation and oxidative stress.
The use of a broad, representative, nationwide, population-based sample to observe the survival outcome of PLC in reproductive and postmenopausal female patients increased the validity of the results. Nevertheless, these results should be interpreted with caution because of several limitations in this study. First, detailed information related to the risk of PLC is not available. This information includes data on body mass index, smoking and alcohol use, high-fat diet, lower physical activity lifestyle, history of receiving HRT, and family history of PLC. Second, tumor burden, staging, and management strategies of PLC are not accessible from the NHIRD and therefore cannot be analyzed. Third, defining menopause by age alone may not be comprehensive enough since it is hard to make an optimal covariate adjustment. Fourth, the generalization of the findings to Western or non-Taiwanese populations is a concern. For instance, the high incidence of PLC warrants further follow-up in other populations. Fifth, the small number of cases during the peripartum period may lead to biased findings. Hence, future studies with an improved design, larger sample sizes, and better control of confounding factors are required to enable a more thorough understanding.
In summary, among female patients with PLC, we found a trend for older age to be associated with increased risk for both incidence and mortality of PLC. In contrast, no apparent relationship was noted between pregnancy and prognosis. Even though subsequent clinical studies are necessary for further validation, the present research demonstrates that age and hormonal factors have a protective influence on the occurrence and deterioration of PLCs. Moreover, patients with more risk factors are recommended to follow up regularly to achieve a better prognosis.
Primary liver cancer (PLC), the sixth most common cancer, accounts for the fourth leading cause of cancer-related death worldwide. Given the continuous rise of the global burden, there are increasing concerns about PLC outcomes in different populations.
For a long time, most studies about PLC put their focus on men due to higher incidence and riskier morbidities compared to women. Even with growing evidence on the protective effects of female sex hormones in animal research, few clinical cohorts pay attention to women with PLCs. Therefore, we are interested in the issue of how female reproductive status is related to the prognosis of PLCs.
This study aimed to assess whether peripartum and postmenopausal women with PLC have lower overall survival rates in a large cohort of subjects in Taiwan.
This is a retrospective cohort of the PLC prognosis among peripartum, non-peripartum, premenopausal, and postmenopausal women using the Taiwan National Health Insurance Research Database from 2000-2012. There were 200 eligible subjects enrolled in the study of peripartum PLC, whereas 13440 subjects enrolled in the research of menopausal PLC. 4:1 Propensity score matching was applied to adjust the covariates.
While the survival rate was overall lower in patients with peripartum PLC, there was no significant difference in the risk of death and the survival rate at different follow-up durations among patients with/without peripartum PLC. In the menopausal PLC cohort, significantly lower risk of death (aHR = 0.64, 95%CI: 0.61-0.68, P < 0.001) and higher survival rate when followed for 0.5 year (72.44% vs 64.16%), 1 year (60.57% vs 51.66%), 3 years (42.92% vs 31.28%), and 5 years were seen in patients diagnosed with PLC younger than 50 years old (without menopause) compared with patients diagnosed with PLC at or older than 50 years (with menopause).
According to our dataset, it is concluded that younger age and female hormonal factors may reduce the occurrence and deterioration of PLCs. Females with paripartum PLC have no difference in survival rates compared with those patients without peripartum. Menopausal females with PLC have worse survival rates compared with those patients without menopause.
To further clarify the association between sexual hormone and PLC outcome, future studies with more detailed information and better-controlled confounders are required.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koller T S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55765] [Article Influence: 7966.4] [Reference Citation Analysis (132)] |
| 2. | Lin L, Yan L, Liu Y, Qu C, Ni J, Li H. The Burden and Trends of Primary Liver Cancer Caused by Specific Etiologies from 1990 to 2017 at the Global, Regional, National, Age, and Sex Level Results from the Global Burden of Disease Study 2017. Liver Cancer. 2020;9:563-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 3. | Massarweh NN, El-Serag HB. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control. 2017;24:1073274817729245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 432] [Article Influence: 54.0] [Reference Citation Analysis (1)] |
| 4. | Rawla P, Sunkara T, Muralidharan P, Raj JP. Update in global trends and aetiology of hepatocellular carcinoma. Contemp Oncol (Pozn). 2018;22:141-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 5. | Petrick JL, Freedman ND, Demuth J, Yang B, Van Den Eeden SK, Engel LS, McGlynn KA. Obesity, diabetes, serum glucose, and risk of primary liver cancer by birth cohort, race/ethnicity, and sex: Multiphasic health checkup study. Cancer Epidemiol. 2016;42:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Global Burden of Disease Liver Cancer Collaboration, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, Artaman A, Ayele TA, Barac A, Bensenor I, Berhane A, Bhutta Z, Castillo-Rivas J, Chitheer A, Choi JY, Cowie B, Dandona L, Dandona R, Dey S, Dicker D, Phuc H, Ekwueme DU, Zaki MS, Fischer F, Fürst T, Hancock J, Hay SI, Hotez P, Jee SH, Kasaeian A, Khader Y, Khang YH, Kumar A, Kutz M, Larson H, Lopez A, Lunevicius R, Malekzadeh R, McAlinden C, Meier T, Mendoza W, Mokdad A, Moradi-Lakeh M, Nagel G, Nguyen Q, Nguyen G, Ogbo F, Patton G, Pereira DM, Pourmalek F, Qorbani M, Radfar A, Roshandel G, Salomon JA, Sanabria J, Sartorius B, Satpathy M, Sawhney M, Sepanlou S, Shackelford K, Shore H, Sun J, Mengistu DT, Topór-Mądry R, Tran B, Ukwaja KN, Vlassov V, Vollset SE, Vos T, Wakayo T, Weiderpass E, Werdecker A, Yonemoto N, Younis M, Yu C, Zaidi Z, Zhu L, Murray CJL, Naghavi M, Fitzmaurice C. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1459] [Cited by in RCA: 1491] [Article Influence: 186.4] [Reference Citation Analysis (0)] |
| 7. | Li Y, Xu A, Jia S, Huang J. Recent advances in the molecular mechanism of sex disparity in hepatocellular carcinoma. Oncol Lett. 2019;17:4222-4228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Sukocheva OA. Estrogen, estrogen receptors, and hepatocellular carcinoma: Are we there yet? World J Gastroenterol. 2018;24:1-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Ma WL, Lai HC, Yeh S, Cai X, Chang C. Androgen receptor roles in hepatocellular carcinoma, fatty liver, cirrhosis and hepatitis. Endocr Relat Cancer. 2014;21:R165-R182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 10. | Matsuo M, Furukawa K, Shimizu H, Yoshitomi H, Takayashiki T, Kuboki S, Takano S, Suzuki D, Sakai N, Kagawa S, Nojima H, Ohsuka M. Novel treatment strategy with radiofrequency ablation and surgery for pregnant patients with hepatocellular carcinoma: a case report. Surg Case Rep. 2018;4:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Li AJ, Zhou WP, Lu JH, Cui LJ, Yang XY, Yin L, Wu MC. Surgery for pregnancy-associated primary hepatocellular carcinoma: Report of four cases. Int J Surg Case Rep. 2014;5:882-885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Choi KK, Hong YJ, Choi SB, Park YN, Choi JS, Lee WJ, Kim KS. Hepatocellular carcinoma during pregnancy: is hepatocellular carcinoma more aggressive in pregnant patients? J Hepatobiliary Pancreat Sci. 2011;18:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Giannitrapani L, Soresi M, La Spada E, Cervello M, D'Alessandro N, Montalto G. Sex hormones and risk of liver tumor. Ann N Y Acad Sci. 2006;1089:228-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 14. | Hsing AW, McLaughlin JK, Hoover RN, Co Chien HT, Blot WJ, Fraumeni JF Jr. Parity and primary liver cancer among young women. J Natl Cancer Inst. 1992;84:1118-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Garko SB, David OS, Mohammed T, Isah MS, Bakari AG, Oguntayo AO, Shehu MS, Aminu SM. Hepatocellular carcinoma in pregnancy. Ann Afr Med. 2009;8:284-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Russell P, Sanjay P, Dirkzwager I, Chau K, Johnston P. Hepatocellular carcinoma during pregnancy: case report and review of the literature. N Z Med J. 2012;125:141-145. [PubMed] |
| 17. | Shimizu I. Impact of oestrogens on the progression of liver disease. Liver Int. 2003;23:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 143] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Brady CW. Liver disease in menopause. World J Gastroenterol. 2015;21:7613-7620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 87] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Zhong GC, Liu Y, Chen N, Hao FB, Wang K, Cheng JH, Gong JP, Ding X. Reproductive factors, menopausal hormone therapies and primary liver cancer risk: a systematic review and dose-response meta-analysis of observational studies. Hum Reprod Update. 2016;23:126-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Hassan MM, Botrus G, Abdel-Wahab R, Wolff RA, Li D, Tweardy D, Phan AT, Hawk E, Javle M, Lee JS, Torres HA, Rashid A, Lenzi R, Hassabo HM, Abaza Y, Shalaby AS, Lacin S, Morris J, Patt YZ, Amos CI, Khaderi SA, Goss JA, Jalal PK, Kaseb AO. Estrogen Replacement Reduces Risk and Increases Survival Times of Women With Hepatocellular Carcinoma. Clin Gastroenterol Hepatol. 2017;15:1791-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 21. | Research on the menopause in the 1990s: report of a WHO scientific group. [cited 27 December 2020]. Available from: https://apps.who.int/iris/handle/10665/41841. |
| 22. | Feasey R. Infertility and Non-Traditional Family Building: From Assisted Reproduction to Adoption in the Media, 1st ed.; Palgrave Macmillan: London, U.K., 2019. [DOI] [Full Text] |
| 23. | Shen TY, Strong C, Yu T. Age at menopause and mortality in Taiwan: A cohort analysis. Maturitas. 2020;136:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 24. | Lin YM, Velmurugan BK, Yeh YL, Tu CC, Ho TJ, Lai TY, Tsai CH, Tsai FJ, Huang CY. Activation of estrogen receptors with E2 downregulates peroxisome proliferator-activated receptor γ in hepatocellular carcinoma. Oncol Rep. 2013;30:3027-3031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Deng L, Yang H, Tang J, Lin Z, Yin A, Gao Y, Wang X, Jiang R, Sun B. Inhibition of MTA1 by ERα contributes to protection hepatocellular carcinoma from tumor proliferation and metastasis. J Exp Clin Cancer Res. 2015;34:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Lee SR, Lee YH, Yang H, Lee HW, Lee GS, An BS, Jeung EB, Park BK, Hong EJ. Sex hormone-binding globulin suppresses NAFLD-triggered hepatocarcinogenesis after menopause. Carcinogenesis. 2019;40:1031-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Lau WY, Leung WT, Ho S, Lam SK, Li CY, Johnson PJ, Williams R, Li AK. Hepatocellular carcinoma during pregnancy and its comparison with other pregnancy-associated malignancies. Cancer. 1995;75:2669-2676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Yang D, Hanna DL, Usher J, LoCoco J, Chaudhari P, Lenz HJ, Setiawan VW, El-Khoueiry A. Impact of sex on the survival of patients with hepatocellular carcinoma: a Surveillance, Epidemiology, and End Results analysis. Cancer. 2014;120:3707-3716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 29. | Shimizu I, Inoue H, Yano M, Shinomiya H, Wada S, Tsuji Y, Tsutsui A, Okamura S, Shibata H, Ito S. Estrogen receptor levels and lipid peroxidation in hepatocellular carcinoma with hepatitis C virus infection. Liver. 2001;21:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | El Mahdy Korah T, Abd Elfatah Badr E, Mohamed Emara M, Ahmed Samy Kohla M, Gamal Saad Michael G. Relation between sex hormones and hepatocellular carcinoma. Andrologia. 2016;48:948-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Petrick JL, Florio AA, Zhang X, Zeleniuch-Jacquotte A, Wactawski-Wende J, Van Den Eeden SK, Stanczyk FZ, Simon TG, Sinha R, Sesso HD, Schairer C, Rosenberg L, Rohan TE, Purdue MP, Palmer JR, Linet MS, Liao LM, Lee IM, Koshiol J, Kitahara CM, Kirsh VA, Hofmann JN, Guillemette C, Graubard BI, Giovannucci E, Gaziano JM, Gapster SM, Freedman ND, Engel LS, Chong DQ, Chen Y, Chan AT, Caron P, Buring JE, Bradwin G, Beane Freeman LE, Campbell PT, McGlynn KA. Associations Between Prediagnostic Concentrations of Circulating Sex Steroid Hormones and Liver Cancer Among Postmenopausal Women. Hepatology. 2020;72:535-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |