Published online Nov 27, 2021. doi: 10.4254/wjh.v13.i11.1653
Peer-review started: March 25, 2021
First decision: June 15, 2021
Revised: June 24, 2021
Accepted: August 30, 2021
Article in press: August 30, 2021
Published online: November 27, 2021
Processing time: 243 Days and 14.8 Hours
With increasing morbidity and mortality from chronic liver disease and acute liver failure, the need for liver transplantation is on the rise. Most of these patients are extremely vulnerable to infections as they are immune-compromised and have other chronic co-morbid conditions. Despite the recent advances in practice and improvement in diagnostic surveillance and treatment modalities, a major portion of these patients continue to be affected by post-transplant infections. Of these, fungal infections are particularly notorious given their vague and insidious onset and are very challenging to diagnose. This mini-review aims to discuss the incidence of fungal infections following liver transplantation, the different fungi involved, the risk factors, which predispose these patients to such infections, associated diagnostic challenges, and the role of prophylaxis. The population at risk is increasingly old and frail, suffering from various other co-morbid conditions, and needs special attention. To improve care and to decrease the burden of such infections, we need to identify the at-risk population with more robust clinical and diagnostic parameters. A more robust global consensus and stringent guidelines are needed to fight against resistant microbes and maintain the longevity of current antimicrobial therapies.
Core Tip: Fungal infections post liver transplant remains the predominant source of morbidity and mortality despite the incidence being low. This is because of evasive clinical features coupled with difficulty to isolate and culture these pathogens. Therefore, appropriate patients are selected for prophylactic regimen based on specific risk factors to curb the rise of drug-resistant species. Traditional regimens include fluconazole or liposomal amphotericin with a shift towards echinocandins based on recently published and promising data.
- Citation: Khalid M, Neupane R, Anjum H, Surani S. Fungal infections following liver transplantation. World J Hepatol 2021; 13(11): 1653-1662
- URL: https://www.wjgnet.com/1948-5182/full/v13/i11/1653.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i11.1653
Liver transplantation is one of the principal treatment modalities for the treatment of many hepatic diseases, mainly but not limited to chronic and end-stage liver disease. Despite advances in the field of transplantation, invasive fungal infections remain a major source of morbidity and mortality. This is attributed to delay in diagnosis, nonspecific clinical features[1], fastidious nature of these organisms, lack of consensus on prophylactic regimens, and rise of antifungal resistant species.
Moreover, with an increase in the number of grafts being offered, there is a trend towards recipients being older, debilitated, and having more non hepatic comor
In this article, we aim to discuss the incidence and trend of invasive fungal infections (IFI) in liver transplant (LT) patients, associated risk factors, diagnostic challenges, and data on prophylaxis.
IFIs, according to the Invasive Fungal Infections Cooperative Group in Europe and the Mycoses Study Group in the United States, are divided into 3 categories: proven, probable and possible.
Proven IFI is defined as a positive fungal culture or histological proof of fungal or hyphal elements in a sterile site biopsy. This also includes positive cryptococcal antigen in cerebrospinal fluid.
Probable and possible IFIs have a wider definition and inclusion criteria. This is based on several host factors along with various clinical and mycological criteria[3].
Some studies evaluating prophylactic regimens, in this regard have been a focus of criticism as their IFI’s were considered colonization rather than infection[4].
The incidence of IFI after LT has decreased in recent years and this is attributable to advancement and improvement in surgical techniques along with more aggressive post-operative care. Previously, in one study by Fung et al[5], the incidence of IFI after LT was reported to be 6.6% with a mortality of 54.5%. The ninety-day cumulative mortality after invasive candidiasis has been reported to be 26% and 1-year survival after invasive aspergillosis is about 59% according to TRANSNET in 2010[6].
More recently, according to some cohort studies, the overall incidence of IFI after solid organ transplant is about 1%-4%[7-9]. 1-year cumulative probability of IFI in LT was 1.8%[7]. This shows a promising trend and is related to improvise surgical techniques and timely recognition of risk factors that make certain patients more susceptible to IFIs.
However, in underdeveloped nations, it remains higher at 14.7% with an in-hospital mortality rate of 77%[10]. A future streamlined approach to the problem with specific guidelines might be one of the ways to improve these numbers.
The three major fungi involved are Candida spp., Cryptococcus, and Aspergillus spp. Candida predominates with 81% followed by Aspergillus (16%) and Cryptococcus (3%). Non-Albicans Candida accounted for 68% of all Candida infections[11]. The rise of resistant non-Albicans Candida especially C. parapsilosis was felt to coincide with the increased use of fluconazole[11]. C. parapsilosis is associated with increased mortality in these patients. This increase in resistant fungal species indicates a dire need for a patient-specific prophylactic regimen based on risk factors vs a universal approach.
The distribution of the fungal species remains similar in the East with Candida representing 64.1% and Aspergillus 35.8% of the IFIs in LT patients.
Despite the highly variable clinical presentation, these pathogens most commonly affect the respiratory system followed by renal and gastrointestinal tract[10]. According to a retrospective study in 2015 by Eschenauer and colleagues, intra-abdominal candidiasis (73%) was the most common IFI[12]. The common clinical manifestations of various fungal organisms are shown in Table 1.
| Clinical manifestations | |
| Candida | Intra-abdominal abscesses |
| Recurrent cholangitis | |
| Peritonitis | |
| Fungemia | |
| Aspergillus | Invasive pulmonary Aspergillosis |
| Brain abscess | |
| Endophthalmitis | |
| Osteomyelitis | |
| Endocarditis | |
| Cryptococcus | CNS infection |
| Focal lesions on imaging | |
| Meningeal enhancement |
There has been a shift in the time duration between the developments of IFIs after LT. It was initially thought to occur in the early post-operative phase most commonly within the first couple of months.
Grauhan et al[13] in 1994 reported a median time from LT to IFI of 2 mo.
According to Husain et al[14] in 2003, the median time to infection for invasive candidiasis was 13.5 d with 72% of the IFIs happening within the first month after LT.
Aspergillus tends to present later as compared to Candida. Results from one study by Singh and colleagues in 2003 reported 55% of their Aspergillus IFI occurring after 90 d[15] and Gravalda et al[16] also described 43% of their IFIs as late onset Aspergillus.
In transplant centers with a higher risk of Aspergillus based on epidemiology, this delayed time to presentation is important to consider while deciding on the length of prophylactic regimen in high-risk patients. Moreover, clinicians need to be mindful of this time frame while diagnosing an already difficult-to-diagnose disease.
Multiple factors have been observed over time to be associated with the development of fungal infections in LTs. Identifying patients that are at high risk for developing IFI can be of immense help as that can aide in decreasing the diagnostic delay and assure appropriate prophylaxis. By adopting this targeted method of prophylaxis vs universal approach, we can also potentially reduce the incidence of drug-resistant fungi, lower the morbidity due to side effects and interactions of these medications particularly with immunosuppressants, and mitigate the overall cost.
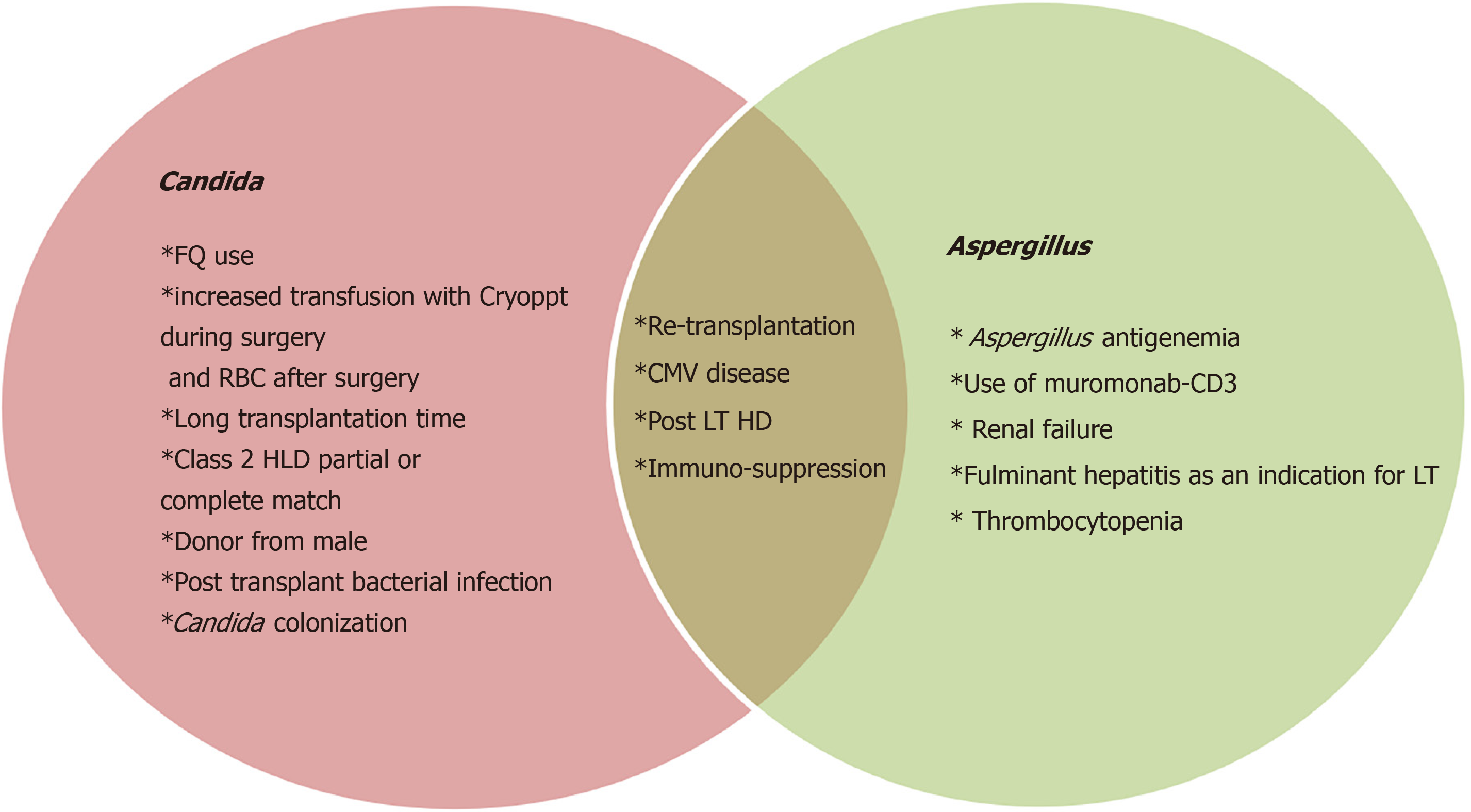
Many scientists over the past few decades have worked on identifying these attributes. These can be categorized into pre-operative, operative, and post-operative factors as shown in Table 2. Risk factors for Aspergillus specifically seem to depend more on post-operative factors as highlighted in Figure 1.
| Risk factors | |
| Pre-operative | SBP prophylaxis with fluoroquinolone |
| Operative | Retransplantation |
| Long transplantation time | |
| Long transplantation time | |
| Class 2 partial or complete match | |
| Donor from male | |
| Post-operative | Post-transplant HD |
| High number of RBC units transfused | |
| Post-transplant bacterial infection | |
| Cytomegalovirus infection | |
| Use of muromonab-CD3 | |
| Aspergillus antigenemia |
Collins et al[17] in 1994 identified the following as potential risk factors: renal insufficiency, length of transplant operation, rate of re-transplantation, abdominal or intra-thoracic reoperation, and cytomegalovirus infection.
Other studies showed that model for end-stage liver disease (MELD) scores > 25, post-transplant acute kidney injury (Cr > 2 or risk, injury, failure, loss of kidney function, and end-stage n criteria I- or F-) and pre-transplant fungal colonization seem to be the culprits identified with IFIs[11,18].
One of these was an important and common risk factor of daily prophylactic fluconazole dose of < 200 mg, which was thought to cause a rise in drug-resistant non-Albicans Candida spp[11].
Although very rare, a French study also identified contamination during organ procurement as a risk factor with a 1.33% prevalence of Candida spp. in preservation fluid. This was associated with a higher rate of IFI and impaired survival[19].
Alongside predictable risk factors like diabetes and hemodialysis dependence, Verma et al[10] pointed out prior antibiotic use, cerebral and respiratory organ failures, chronic liver failure (CLIF) organ failure/CLIF-consortium acute-on-chronic liver failure as predictors of IFIs. Non-survivors in their study also had higher levels of 1.3-beta D glucan (BDG) levels. BDG levels have been studied as a diagnostic marker and look promising.
There has been a general shift in the trend of risk factors over the last 2 decades, which is attributable to better surgical techniques. Singh et al[20] studied 190 liver transplants during 1990 and 2000 and demonstrated improvement in length of operation, intraoperative transfusion requirements, use of roux-en-Y biliary anastomosis, re-transplantation, rate of rejection over time, and cold ischemic time. This led to a decrease in the incidence of invasive candidiasis in this study population from 9%-1.7% without any use of antifungal prophylaxis.
In 2015, Eschenauer and colleagues identified bile leaks within the first 30 d post-transplant and living donor liver transplants as new independent risk factors for IFIs. This is because Candida has an affinity for growth in the biliary tract. Moreover, living donor liver transplants are highly technical procedures that are not commonly performed in the United States. The increased length and complexity of these procedures along with higher disruption of the biliary tract is responsible for these findings. The authors recommended instituting antifungal prophylaxis in all living donor liver transplants[12].
A small study recently in 2020 by Jorgenson et al[21] studied the effects of pre-transplant roux-en Y gastric bypass on liver transplant outcomes. There were increased rates of fungal infection in patients with bariatric surgery before transplant and might be associated with loss of defense provided by gastric acid. This study is limited by its retrospective nature and its size.
In general, fungal infections do not present themselves vividly and are increasingly difficult to grow in culture media. It makes it even more challenging in patients who have chronic liver disease, are immunosuppressed, or have other underlying comorbidities. They are difficult to detect clinically and also objectively in laboratories. Hence, prevention becomes essential, and it has significantly improved in the last decade with the advancement in surgical techniques, intense pre-operative evaluation, and appropriate use of antifungal prophylactic agents in high-risk patients.
Distinguishing between colonization and true infection can be challenging for the clinician. Apart from 'proven IFI' as discussed above, the other two categories are vague and have plenty of variable factors. In these clinical scenarios, the use of newer diagnostic tools like BDG and galactomannan (GM) can be helpful. Polymerase chain reaction fungal assays are promising but not yet approved by the Food and Drug Administration.
BDG has been studied and looks promising as a diagnostic marker in serum. In a study from 2017, with 271 transplant patients, weekly BDG was tested and monitored for IFIs. 95% of the patients with IFI had positive BDG and a very promising negative predictive value of 96% was seen. The sensitivity of BDG was 75% and specificity was 65%, making it a very good tool to rule out IFIs[22].
The GM test is an enzyme-linked immunosorbent assay that detects the GM antigen released by Aspergillus hyphae when they invade host cells.
The patient’s epidemiological risk factors should be considered strongly which would help guide better towards increasing clinical suspicion and ordering appro
Fungal infections in liver transplant recipients are mostly attributed to Aspergillus and Candida. Three agents are mainly used in prophylaxis–fluconazole, liposomal amphotericin B, and itraconazole. The studies involving these agents have been confounded by the difficulty of differentiating colonization and a true infection, the variability between patient selection, therapeutic agent(s) used in comparison with placebo or each other, and variable duration of treatment.
Data on the effectiveness of antifungal prophylaxis in LT over the past 10 years have been summarized in the Table 3 below.
| Ref. | Trials | Patients | Regimens | Infection reduction | Comments |
| (95%CI) | |||||
| Cruciani et al[25], 2006 | 6 | 698 | AmB vs Pla (1) | Total proven fungal infections RR 0.31 (0.21-0.46), IFI RR 0.33 (0.18-0.59) | Patients receiving prophylaxis had higher number of non-Albicans proven fungal infections. Mostly C. glabrata. |
| Flu vs nonsystemic AF (1) | |||||
| Flu vs Pla (2) | |||||
| Itra vs Pla(1) | |||||
| Amb-Itra vs Flu-itra vs Pla (1) | |||||
| Playford et al[24], 2006 | 7 | 793 | Flu vs Pla (2) | Proven IFI RR 0.39 (0.18-0.85), fungal colonization RR 0.51 (0.41-0.62), fungal colonization with C. glabrata/C. krusei, RR 1.57 (0.76-3.24) | Formulated algorithm in which patients with < 2 RF deemed low risk (4%incidence) for IFI and those with ≥ 2 at high risk (25% incidence) for IFI. |
| Flu vs nonsystemic AF (2) | |||||
| Itra vs Pla (2) | |||||
| AmB vs Pla (1) | |||||
| Evans et al[26], 2014 | 14 | 1633 | Flu vs Pla/nonabs AF (4) | Proven IFI OR 0.37 (0.19-0.72), P = 0.003, Bayesian MTC, AmB vs Pla OR 0.21 (0.05-0.71), Flu vs Pla OR 0.21 (0.06-0.57) | Benefit of AmB is of similar magnitude to that previously described for fluconazole. |
| Itra vs Pla (1) | |||||
| AmB vs Pla (1) | |||||
| 3 arm study with Pla/AmB/Flu (1) | |||||
| Flu vs AmB (3) | |||||
| Liposomal + Flu vs standard AmB + Flu | |||||
| Itra vs Flu (2) | |||||
| Micafungin vs standard care (1) | |||||
| Clo vs Nys (1) | |||||
There have been three meta-analyses as summarized in Table 3. Playford et al[24] and Cruciani et al[25] published two in 2006, with 10 and 6 studies respectively. These summarized that universal fungal prophylaxis leads to a reduction in proven IFIs without any mortality benefit. This universal approach leads to a significantly higher proportion of episodes of non-Albicans Candida infection.
In 2014, Evans et al[26] published a meta-analysis of studies on prophylaxis to prevent IFIs after LT and concluded that the odds of proven IFI and IFI related mortality were lower in patients receiving antifungal prophylaxis, even if the overall mortality did not change. It was also demonstrated that the efficacy of fluconazole compared to liposomal amphotericin was similar with the latter having the benefit of not altering the cytochrome P450 system and therefore not affecting the calci-neurin inhibitor levels. However, fluconazole is favored because of its cost-effectiveness and safety profile. This meta-analysis did not reveal any information on echinocandins, however, it was different from their counterparts in that they did a mixed treatment comparison and was more recent of the few meta-analyses already on the subject matter.
Studies since 2014 (after the last meta-analysis) on prophylaxis are summarized in Table 4.
| Ref. | Design | Regimen | Outcomes |
| Antunes et al[29], 2014 | Single center. Retrospective (n = 461) | High risk group: AmB vs nystatin; Low risk group: nystatin | Higher IFI in high risk patients who did not receive AmB |
| Winston et al[30], 2014 | Randomized, double-blind. 2010-2011 (n = 200) | Group 1: Andulafugin; Group 2: Flu | 1:1 randomized. Similar cumulative IFI occurrence and equal 3 mo mortality |
| Saliba et al[27], 2015 | Randomized, open label. 2009-2012 (n = 347) | Micafungin vs center specific standard care (Flu/AmB/Caspo) | Micafungin was non-inferior to standard of care |
| Giannella et al[31], 2015 | Prospective, non-randomized. 2009-2013. Safety of high dose AmB (n = 76) | Amb 10 mg/kg Q weekly until hospital discharge for a minimum of 2 wk | 10 patients discontinued therapy. (6 for AmB related AEs and 4 for IFI) |
| Eschenauer et al[12], 2015 | Single center study. 2008-2012. Effectiveness of targeted prophylaxis (n = 381) | Universal ppx: Vori. Targeted: Group1: Vori, 30 d. Group 2: Flu during icu sta. Group3: No ppx | Cumulative IFI occurrence 5.2% (targeted vs universal group). Similar 100 day mortality between targeted and universal ppx gp. 40% breakthrough IFI |
| Balogh et al[32], 2016 | Single center study. 2008-2014 (n = 314) | Voriconazole vs oral nystatin or Flu | No episodes of IA occurred. No difference in graft and patient survival curves between the two groups |
| Perrella et al[33], 2016 | Single center study. 2006-2012. Comparative observational study for targeted prophylaxis (n = 54) | Group 1: AmB 3 mg/kg/day; Group2: Caspofungin 70 mg loading→50 mg/day | No episodes of IFI in both groups |
| Fortún et al[28], 2016 | Multicenter. 2005-2012. Comparative observational study for targeted prophylaxis (n = 195) | Group 1: Caspofungin 50 mg/d; Group 2: Flu median 200 mg/day | Similar 6 m IFI occurrence [5.2% b (G1) vs 12.2% (G2)]. Reduced risk of IA in LT receiving caspofungin. Similar overall mortality |
| Chen et al[34], 2016 | Single center study. 2005-2014. Effectiveness of targeted prophylaxis (n = 402) | Group 1: Anidulafungin 100 mg/day or micafungin 100 mg/day; Group 2: No prophylaxis | High risk patients MELD > 20; Similar IFI occurrence lower cumulative mortality in group 1 (P = 0.001) |
| Giannella et al[35], 2016 | Retrospective, single center. 2010-2014. Evaluation of RF for a targeted prophylaxis (n = 303) | Group 1: No RF. No prophylaxis; Group 2: 1RF IC, Flu; Group3: High risk, anti mould agent | Antifungal prophylaxis administered to 45.9% patients. Cumulative IFI prevalence 6.3%. Flu independently associated with IFI development |
| Lavezzo et al[36], 2018 | Single center study. 2011-2015. Effectiveness of targeted prophylaxis | Group 1 high risk: AmB; Group 2 low risk: No prophylaxis | Overall IFI prevalence 2.8%. 1 yr mortality higher in prophylaxis group (P = 0.001). 1 yr mortality higher in IFI patients (P < 0.001) |
| Jorgenson et al[37], 2019 | Single center study. 2009-2016. Effectiveness of fixed dose prophylaxis (n = 189) | Group 1: Flu 400 mg/day for 14 d for high risk patients; Group 2: unsupervised antifungal protocols | Reduction in 1 yr IFI among high risk group (12.5% vs 26.6%). Similar 1 yr patient and graft survival |
| Kang et al[38], 2020 | Multicenter, randomized, open label. Living donor LT. 2012-2015 (n = 144) | Group 1: Micafungin | Group 1 vs Group 2: 69 vs 75 pts. IFI occurrence in 3 wk: 1/69 vs 0/75. Micafungin was noninferior to Flu |
| 100 mg/d; Group 2: Flu 100-200 mg/day | |||
In 2015, Eschenauer and colleagues performed a retrospective study involving liver transplant patients that were divided into three main groups. Group 1 included 145 patients who received targeted prophylaxis with either voriconazole in 54%, fluconazole in 5% or no antifungal which was the case of 38% of these patients. This was compared to a group of 237 patients, who received universal prophylaxis with voriconazole. These regimens were continued for a median time of 11 d in the targeted group and for 6 d in the universal group, with a significant P value. There was no statistical difference between incidence of IFI between both groups (6.8% in targeted and 4.2% in universal). Similarly, the P value was not statistically significant for the mortality rates over 100 d from IFIs in both groups (10% for targeted and 7% for universal group). They, therefore concluded that targeted approach to antifungal use in liver transplant patients was a safe, cost effective strategy and prevented unnec
With regards to echinocandins, Saliba et al[27] in 2015 compared micafungin vs standard treatment and found them equally effective. Standard therapy was center-specific and included IV fluconazole, liposomal amphotericin, or IV caspofungin.
Similarly, in a study from Spain in 2016, caspofungin was compared to fluconazole in high-risk patients and similar efficacy was reported to prevent global IFIs. In this study caspofungin was related to decrease in breakthrough IFIs and also led to a lower rate of invasive aspergillosis[28].
Echinocandins should be considered as prophylactic agents, where appropriate, especially in areas of increased prevalence of drug-resistant non-Albicans Candida. Unfortunately, these too come with a higher price tag compared to fluconazole which can affect their use, especially in non-affluent countries.
According to the Infectious Disease Society of America guidelines, patients who meet 2 or more of the following risk factors to be considered for prophylaxis: creat
However, since the current data suggest that the incidence and risk of fungal infection overall in the general liver transplantation population is low, these agents should be utilized for higher-risk patients as unguided use is associated with drug-resistant non-Albicans Candida infection and higher mortality in these patients[23].
Fungal infections following liver transplantation remain an influential cause of morbidity and mortality in these patients, despite the low incidence. Identification of high-risk patients based on risk factors discussed above and starting an appropriate prophylactic antifungal regimen based on epidemiology, calcineurin inhibitor use, and renal function is the first step in avoiding dealing with this evasive disease.
Prophylactic antifungals are generally well tolerated but can lead to drug-resistant Candida spp., hence the importance of selecting the appropriate patient and agent. Using BDG as a negative predictive tool and having a high degree of suspicion, even if the time from transplant exceeds 2 mo, can prevent diagnostic delays.
Further randomized controlled trials comparing azoles, amphotericin, and echino
Provenance and peer review: Invited article; Externally peer reviewed.
Corresponding Author's Membership in Professional Societies: American College of Physician; American College of Chest Physician.
Specialty type: Medicine, general and internal
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ankrah AO S-Editor: Zhang H L-Editor: A P-Editor: Yu HG
| 1. | Liu X, Ling Z, Li L, Ruan B. Invasive fungal infections in liver transplantation. Int J Infect Dis. 2011;15:e298-e304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Toniutto P, Zanetto A, Ferrarese A, Burra P. Current challenges and future directions for liver transplantation. Liver Int. 2017;37:317-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE; European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813-1821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4096] [Cited by in RCA: 3957] [Article Influence: 232.8] [Reference Citation Analysis (0)] |
| 4. | Huprikar S. Reply: Effect of prophylaxis on fungal infection and costs for high-risk liver transplant recipients. Liver Transpl. 2008;14:709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Fung JJ. Fungal infection in liver transplantation. Transpl Infect Dis. 2002;4 Suppl 3:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, Anaissie EJ, Brumble LM, Herwaldt L, Ito J, Kontoyiannis DP, Lyon GM, Marr KA, Morrison VA, Park BJ, Patterson TF, Perl TM, Oster RA, Schuster MG, Walker R, Walsh TJ, Wannemuehler KA, Chiller TM. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis. 2010;50:1101-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1047] [Cited by in RCA: 1125] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 7. | Hosseini-Moghaddam SM, Ouédraogo A, Naylor KL, Bota SE, Husain S, Nash DM, Paterson JM. Incidence and outcomes of invasive fungal infection among solid organ transplant recipients: A population-based cohort study. Transpl Infect Dis. 2020;22:e13250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 8. | Andes DR, Safdar N, Baddley JW, Alexander B, Brumble L, Freifeld A, Hadley S, Herwaldt L, Kauffman C, Lyon GM, Morrison V, Patterson T, Perl T, Walker R, Hess T, Chiller T, Pappas PG; TRANSNET Investigators. The epidemiology and outcomes of invasive Candida infections among organ transplant recipients in the United States: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Transpl Infect Dis. 2016;18:921-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 9. | van Delden C, Stampf S, Hirsch HH, Manuel O, Meylan P, Cusini A, Hirzel C, Khanna N, Weisser M, Garzoni C, Boggian K, Berger C, Nadal D, Koller M, Saccilotto R, Mueller NJ; Swiss Transplant Cohort Study. Burden and Timeline of Infectious Diseases in the First Year After Solid Organ Transplantation in the Swiss Transplant Cohort Study. Clin Infect Dis. 2020;71:e159-e169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 10. | Verma N, Singh S, Taneja S, Duseja A, Singh V, Dhiman RK, Chakrabarti A, Chawla YK. Invasive fungal infections amongst patients with acute-on-chronic liver failure at high risk for fungal infections. Liver Int. 2019;39:503-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 11. | Raghuram A, Restrepo A, Safadjou S, Cooley J, Orloff M, Hardy D, Butler S, Koval CE. Invasive fungal infections following liver transplantation: incidence, risk factors, survival, and impact of fluconazole-resistant Candida parapsilosis (2003-2007). Liver Transpl. 2012;18:1100-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Eschenauer GA, Kwak EJ, Humar A, Potoski BA, Clarke LG, Shields RK, Abdel-Massih R, Silveira FP, Vergidis P, Clancy CJ, Nguyen MH. Targeted versus universal antifungal prophylaxis among liver transplant recipients. Am J Transplant. 2015;15:180-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Grauhan O, Lohmann R, Lemmens P, Schattenfroh N, Keck H, Klein E, Raakow R, Jonas S, Langrehr JM, Bechstein W. Fungal infections in liver transplant recipients. Langenbecks Arch Chir. 1994;379:372-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Husain S, Tollemar J, Dominguez EA, Baumgarten K, Humar A, Paterson DL, Wagener MM, Kusne S, Singh N. Changes in the spectrum and risk factors for invasive candidiasis in liver transplant recipients: prospective, multicenter, case-controlled study. Transplantation. 2003;75:2023-2029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Singh N, Avery RK, Munoz P, Pruett TL, Alexander B, Jacobs R, Tollemar JG, Dominguez EA, Yu CM, Paterson DL, Husain S, Kusne S, Linden P. Trends in risk profiles for and mortality associated with invasive aspergillosis among liver transplant recipients. Clin Infect Dis. 2003;36:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 175] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Gavalda J, Len O, San Juan R, Aguado JM, Fortun J, Lumbreras C, Moreno A, Munoz P, Blanes M, Ramos A, Rufi G, Gurgui M, Torre-Cisneros J, Montejo M, Cuenca-Estrella M, Rodriguez-Tudela JL, Pahissa A; RESITRA (Spanish Network for Research on Infection in Transplantation). Risk factors for invasive aspergillosis in solid-organ transplant recipients: a case-control study. Clin Infect Dis. 2005;41:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 223] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 17. | Collins LA, Samore MH, Roberts MS, Luzzati R, Jenkins RL, Lewis WD, Karchmer AW. Risk factors for invasive fungal infections complicating orthotopic liver transplantation. J Infect Dis. 1994;170:644-652. [PubMed] |
| 18. | Utsumi M, Umeda Y, Yagi T, Nagasaka T, Shinoura S, Yoshida R, Nobuoka D, Kuise T, Fuji T, Takagi K, Takaki A, Fujiwara T. Risk Analysis for Invasive Fungal Infection after Living Donor Liver Transplantation: Which Patient Needs Potent Prophylaxis? Dig Surg. 2019;36:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Levesque E, Paugam-Burtz C, Saliba F, Khoy-Ear L, Merle JC, Jung B, Stecken L, Ferrandiere M, Mihaila L, Botterel F. Fungal complications after Candida preservation fluid contamination in liver transplant recipients. Transpl Int. 2015;28:1308-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Singh N, Wagener MM, Marino IR, Gayowski T. Trends in invasive fungal infections in liver transplant recipients: correlation with evolution in transplantation practices. Transplantation. 2002;73:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Jorgenson MR, Gracon AS, Hanlon B, Leverson GE, Parajuli S, Smith JA, Al-Adra DP. Pre-transplant bariatric surgery is associated with increased fungal infection after liver transplant. Transpl Infect Dis. 2021;23:e13484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Levesque E, Rizk F, Noorah Z, Aït-Ammar N, Cordonnier-Jourdin C, El Anbassi S, Bonnal C, Azoulay D, Merle JC, Botterel F. Detection of (1,3)-β-d-Glucan for the Diagnosis of Invasive Fungal Infection in Liver Transplant Recipients. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Eschenauer GA, Lam SW, Carver PL. Antifungal prophylaxis in liver transplant recipients. Liver Transpl. 2009;15:842-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Playford EG, Webster AC, Sorrell TC, Craig JC. Systematic review and meta-analysis of antifungal agents for preventing fungal infections in liver transplant recipients. Eur J Clin Microbiol Infect Dis. 2006;25:549-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Cruciani M, Mengoli C, Malena M, Bosco O, Serpelloni G, Grossi P. Antifungal prophylaxis in liver transplant patients: a systematic review and meta-analysis. Liver Transpl. 2006;12:850-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | Evans JD, Morris PJ, Knight SR. Antifungal prophylaxis in liver transplantation: a systematic review and network meta-analysis. Am J Transplant. 2014;14:2765-2776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Saliba F, Pascher A, Cointault O, Laterre PF, Cervera C, De Waele JJ, Cillo U, Langer RM, Lugano M, Göran-Ericzon B, Phillips S, Tweddle L, Karas A, Brown M, Fischer L; TENPIN (Liver Transplant European Study Into the Prevention of Fungal Infection) Investigators; TENPIN Liver Transplant European Study Into the Prevention of Fungal Infection Investigators. Randomized trial of micafungin for the prevention of invasive fungal infection in high-risk liver transplant recipients. Clin Infect Dis. 2015;60:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Fortún J, Muriel A, Martín-Dávila P, Montejo M, Len O, Torre-Cisneros J, Carratalá J, Muñoz P, Fariñas C, Moreno A, Fresco G, Goikoetxea J, Gavaldá J, Pozo JC, Bodro M, Vena A, Casafont F, Cervera C, Silva JT, Aguado JM; Grupo de Estudio de Infección en Pacientes Trasplantados-Grupo de Estudio de Micología Médica (Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica), and Red Española de Investigación en Patología Infecciosa. Caspofungin versus fluconazole as prophylaxis of invasive fungal infection in high-risk liver transplantation recipients: A propensity score analysis. Liver Transpl. 2016;22:427-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Antunes AM, Teixeira C, Corvo ML, Perdigoto R, Barroso E, Marcelino P. Prophylactic use of liposomal amphotericin B in preventing fungal infections early after liver transplantation: a retrospective, single-center study. Transplant Proc. 2014;46:3554-3559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Winston DJ, Limaye AP, Pelletier S, Safdar N, Morris MI, Meneses K, Busuttil RW, Singh N. Randomized, double-blind trial of anidulafungin versus fluconazole for prophylaxis of invasive fungal infections in high-risk liver transplant recipients. Am J Transplant. 2014;14:2758-2764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 31. | Giannella M, Ercolani G, Cristini F, Morelli M, Bartoletti M, Bertuzzo V, Tedeschi S, Faenza S, Puggioli C, Lewis RE, Pinna AD, Viale P. High-dose weekly liposomal amphotericin b antifungal prophylaxis in patients undergoing liver transplantation: a prospective phase II trial. Transplantation. 2015;99:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Balogh J, Gordon Burroughs S, Boktour M, Patel S, Saharia A, Ochoa RA, McFadden R, Victor DW, Ankoma-Sey V, Galati J, Monsour HP Jr, Fainstein V, Li XC, Grimes KA, Gaber AO, Aloia T, Ghobrial RM. Efficacy and cost-effectiveness of voriconazole prophylaxis for prevention of invasive aspergillosis in high-risk liver transplant recipients. Liver Transpl. 2016;22:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Perrella A, Esposito C, Amato G, Perrella O, Migliaccio C, Pisaniello D, Calise F, Cuomo O, Santaniello W. Antifungal prophylaxis with liposomal amphotericin B and caspofungin in high-risk patients after liver transplantation: impact on fungal infections and immune system. Infect Dis (Lond). 2016;48:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Chen YC, Huang TS, Wang YC, Cheng CH, Lee CF, Wu TJ, Chou HS, Chan KM, Lee WC, Soong RS. Effect of Prophylactic Antifungal Protocols on the Prognosis of Liver Transplantation: A Propensity Score Matching and Multistate Model Approach. Biomed Res Int. 2016;2016:6212503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Giannella M, Bartoletti M, Morelli M, Cristini F, Tedeschi S, Campoli C, Tumietto F, Bertuzzo V, Ercolani G, Faenza S, Pinna AD, Lewis RE, Viale P. Antifungal prophylaxis in liver transplant recipients: one size does not fit all. Transpl Infect Dis. 2016;18:538-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Lavezzo B, Patrono D, Tandoi F, Martini S, Fop F, Ballerini V, Stratta C, Skurzak S, Lupo F, Strignano P, Donadio PP, Salizzoni M, Romagnoli R, De Rosa FG. A simplified regimen of targeted antifungal prophylaxis in liver transplant recipients: A single-center experience. Transpl Infect Dis. 2018;20:e12859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Jorgenson MR, Descourouez JL, Marka NA, Leverson GE, Smith JA, Andes DR, Fernandez LA, Foley DP. A targeted fungal prophylaxis protocol with static dosed fluconazole significantly reduces invasive fungal infection after liver transplantation. Transpl Infect Dis. 2019;21:e13156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Kang WH, Song GW, Lee SG, Suh KS, Lee KW, Yi NJ, Joh JW, Kwon CHD, Kim JM, Choi DL, Kim JD, Kim MS. A Multicenter, Randomized, Open-Label Study to Compare Micafungin with Fluconazole in the Prophylaxis of Invasive Fungal Infections in Living-Donor Liver Transplant Recipients. J Gastrointest Surg. 2020;24:832-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |