Published online Nov 27, 2021. doi: 10.4254/wjh.v13.i11.1484
Peer-review started: February 25, 2021
First decision: May 3, 2021
Revised: May 17, 2021
Accepted: August 30, 2021
Article in press: August 30, 2021
Published online: November 27, 2021
Processing time: 271 Days and 22.4 Hours
Knowledge about the connective-tissue framework of the liver is not systematized, the terminology is inconsistent and some perspectives on the construction of the hepatic matrix components are contradictory. In addition, until the last two decades of the 20th century, the connective-tissue sheaths of the portal tracts and the hepatic veins were considered to be independent from each other in the liver and that they do not make contact with each other. The results of the research carried out by Professor Shalva Toidze and his colleagues started in the 1970s in the Department of Operative Surgery and Topographic Anatomy at the Tbilisi State Medical Institute have changed this perception. In particular, Chanukvadze I showed that in some regions where they intersect with each other, the connective tissue sheaths of the large portal complexes and hepatic veins fuse. The areas of such fusion are called porta-caval fibrous connections (PCFCs). This opinion review aims to promote a systematic understanding of the hepatic connective-tissue skeleton and to demonstrate the hitherto underappreciated PCFC as a genuine structure with high biological and clinical significance. The components of the liver connective-tissue framework — the capsules, plates, sheaths, covers — are described, and their intercommunication is discussed. The analysis of the essence of the PCFC and a description of its various forms are provided. It is also mentioned that analogs of different forms of PCFC are found in different mammals.
Core Tip: In the places of spatial intersection of the Glissonean pedicles with the main hepatic veins, the fusion of their connective tissue sheaths is described. The sites of the above-mentioned fusion are called porta-caval fibrous connections. Various forms of porta-caval fibrous connections are discussed as well as their clinical and scientific implications.
- Citation: Patarashvili L, Gvidiani S, Azmaipharashvili E, Tsomaia K, Sareli M, Kordzaia D, Chanukvadze I. Porta-caval fibrous connections — the lesser-known structure of intrahepatic connective-tissue framework: A unified view of liver extracellular matrix. World J Hepatol 2021; 13(11): 1484-1493
- URL: https://www.wjgnet.com/1948-5182/full/v13/i11/1484.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i11.1484
The extracellular matrix — the connective-tissue framework of the liver — determines the shape of the organ and creates specialized compartments for the liver cell populations and blood and lymph circulations, the synergy of which determines the diverse functioning of the organ. The structure and components of the human liver extracellular matrix were comprehensively analyzed in a series of studies performed in the 1980s and the 90s[1,2].
The last five years saw a new wave of studies on hepatic connective-tissue stru
The emergence of endoscopic anatomic liver resections strengthened the need to specify the anatomy and interrelationship of the connective-tissue structures within the liver[8-11]. Additionally, the prospects for the use of human and animal liver matrices as scaffolds for the creation of bioartificial livers (thanks to the development of stem cells and bioengineering technologies)[11-14] also contribute to the resurgence of interests in the hepatic connective-tissue structures.
However, upon reviewing these studies, we noticed that knowledge on the connective-tissue skeleton of the liver were not systematized, the terminology was inconsistent, and the literature concerning the construction of one or another com
Until the last two decades of the 20th century, the branches of the portal vein and the hepatic veins were considered to be independent from each other in the liver and that their connective-tissue sheaths did not make contact with each other[16-18]. Modern hepatology textbooks usually perpetuate this notion that the Glissonean portal pedicles and the main hepatic veins intersect spatially, but some liver parenchyma always remains between them. Thus, it was believed that they are anatomically independent of each other[19].
The results of the research carried out by Professor Shalva Toidze and his colleagues started in the 1970s in the Department of Operative Surgery, and the Topographic Anatomy of Tbilisi State Medical Institute changed this perception. In particular, Chanukvadze[20] showed that in some regions where they intersect with each other, the connective-tissue sheaths of the main portal complex and a hepatic vein fuse. The regions of such fusion he called porta-caval fibrous connections (PCFCs). Several forms of PCFC have been described. It has also been revealed that PCFC, as an anatomical formation, develops in the 11th-12th weeks of gestation. Despite numerous publications, these data have not yet received proper acknowledgement in scientific discourse and, as a result, in clinical hepatology. This opinion review aims to promote a systematic understanding of the connective-tissue skeleton of the liver, standardize the definition and the nomenclature of its structural components, and highlight the importance of the hitherto underappreciated PCFC as a genuine structure.
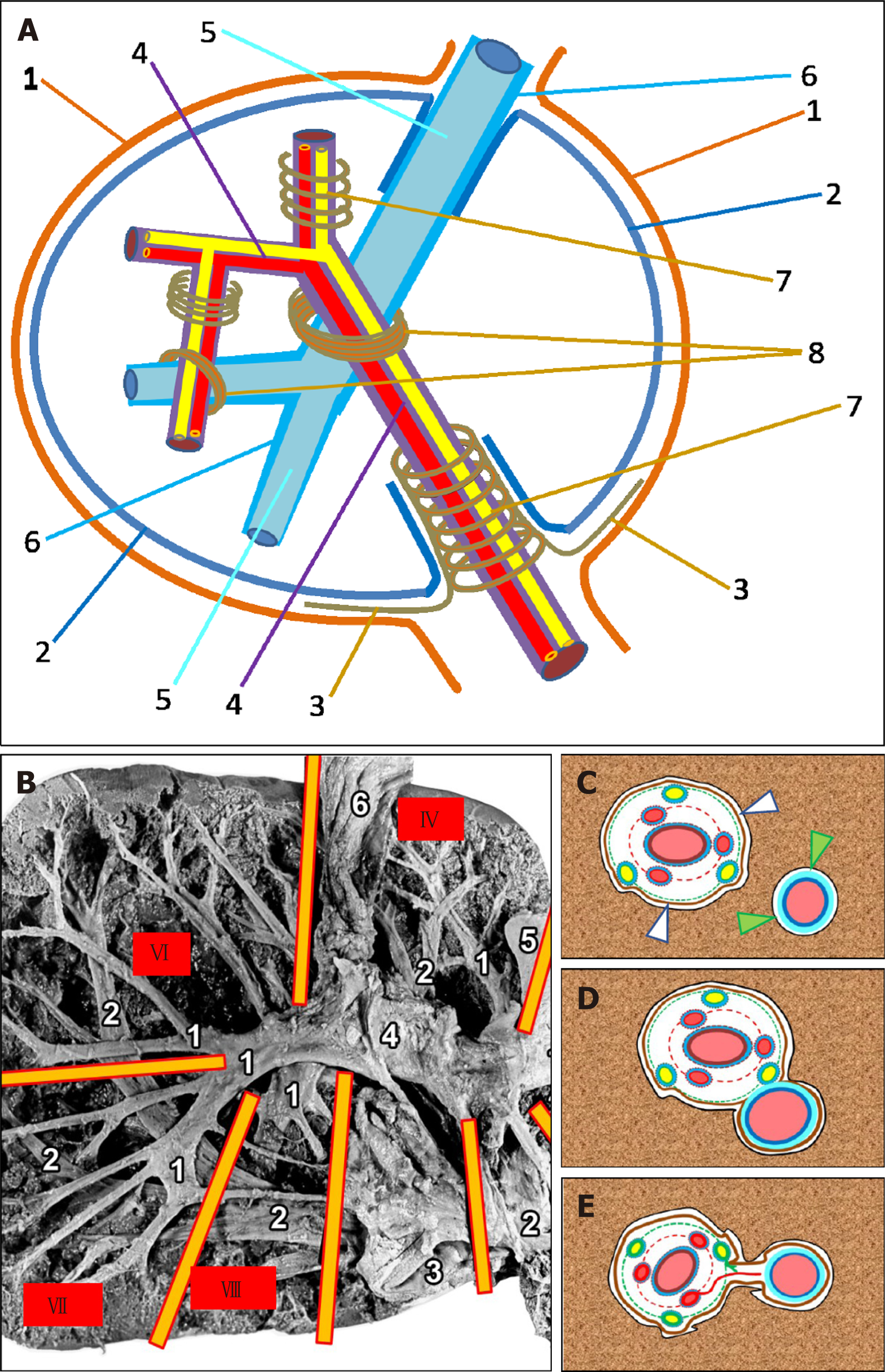
Since the same connective-tissue structure of the liver is often referred to by different names, we have tried to standardize the terms used throughout this article. The following terms will be used in the ensuing discussion: (1) Liver capsule is the same as Laennec's capsule (but not Glisson’s capsule); (2) Hilar plate is the same as Walaeus vasculo-biliary sheath (but not Glisson’s plate); (3) Perivascular fibrous capsule is the same as Glisson’s capsule; (4) Proper hepatic capsule (PHC) is the same as the intrahepatic part of Laennec’s capsule covering the liver parenchyma; (5) Portal hilus is the same as portal port; (6) Caval port is the same as hepatic venous port (where the inferior vena cava adjoins to the liver and incorporates the hepatic veins); and (7) Glissonean pedicle is the same as the portal tract surrounded by Glisson's capsule.
Laennec's capsule (liver capsule) covers the entire liver surface, including its bare area (aperitoneal area). In the portal hilus and venous port of the liver, Laennec's capsule around the Glissonean pedicles and the hepatic veins enters the hepatic parenchyma, covers it, and separates it from the portal tracts and hepatic vein tributaries[21].
In the hepatic hilus, the liver capsule directly touches the hilar plate (also known as Walaeus vascular-biliary sheath) covering the portal vein, the hepatic artery, and the bile ducts, while within the liver, the intrahepatic part of the liver capsule — PHC — covering the parenchyma, sets against the perivascular fibrous capsule (Glisson’s capsule), which is a direct extension of the Walaeus sheath and envelops the lobar, sectoral, and segmental portal tracts[15,22]. These two fibrous fascial structures — PHC and Glisson’s capsule — are separated by a narrow fissure[10] (Figure 1A and C). The individual fibers of the connective tissue (or their bundles) are located in this fissure and connect the outer side of Gleason’s capsule with the PHC. On the other hand, soft collagen fibers (type I and III collagen) separate from the internal side of PHC and extend within the liver lobule (Disse's spaces), fusing to the intralobular matrix[3].
In the region of the thinner portal tracts (subsegmental, zonal), Glisson's capsule tapers off, and cross-banded collagen fibers from portal spaces are in continuity with similar fibers in the immediately adjacent lobular interstitium, which in turn are in continuity with those in central spaces; in this manner, collagen type I fibers and bundles form the structural scaffold of the liver lobule[2]. Meanwhile, the portal, extralobular and intralobular matrices of the liver are united by creating a complex labyrinth that represents the circulation area for tissue fluid and prelymph[23,24].
Laenneс's capsule covering the liver parenchyma is related to the adventitia of the hepatic veins and their tributaries, represented by type I and type III collagen fibers and single muscle fibers, mainly running along the veins. Thick collagen fibers were found external to thin elastic fibers, which were intimately related to smooth muscle. The above-mentioned features are consistent with the observation that all veins of the infracardiac region in humans are mainly propulsive veins[25]. The increase in collagen content on the adventitial side of the interface may strengthen it and prevent rupture of the vein during extreme liver movements[26].
The PHC is often separated from the adventitia of the hepatic veins and their large tributaries by a narrow slit (similar to that described in relation to Glissonean pedicles), in which the tissue fluid and prelymph circulate[23,24] (Figure 1C). The average distance between the PHC and the Glissonean pedicle is 32 ± 8.7 μm, while that between the PHC and the hepatic veins is 26 ± 6.3 μm[8]. Some authors suggest that Laennec’s capsule, Glisson’s capsule and the sheath for the hepatic vein tributaries can be characterized by a high content of thin, wavy elastic fibers. The Waleaus vasculo-biliary sheath of the thick vessels and ducts does not contain elastic fibers[15]. However, some researchers believe that there is no fibrous sheath around the hepatic veins and that the adventitia of the hepatic veins is in direct contact with the PHC covering the liver parenchyma[27]. With the reduction of the diameter (caliber) of the tributaries of the hepatic veins, the adventitia of these veins thins out, PHC tapers off, and intralobular connective-tissue fibers connect directly to the connective-tissue fibers of adventitia of the small tributaries of the hepatic veins[2,28]. Such a relationship further reinforces the notion that the merger of the intralobular and extralobular connective-tissue fibers and that of the capsule covering the organ create a complex, yet well-regulated, structure of the extracellular matrix, which is the connective-tissue skeleton of the liver, coordinating the synergy between the cell populations and the neural and circulatory tubular structures. The PHC is mainly composed of reticular fibers (RFs) that cover the hepatic lobules. The ring of hepatocytes abutting the connective tissue of the portal region is called the periportal limiting plate. The RF bordering the hepatocytes constituting the limiting plate forms a capsule. This capsule covers the hepatic lobule from one side and abuts the perivascular fibrous capsule (Glisson’s capsule) enveloping the portal tract, from another side[3].
Based on computer software analysis of liver specimens (histotopograms), the same authors distinguish loose fiber construction (and not the fissure described above) between Glisson's capsule and the PHC and called it the private hepatic ligament (PHL). The PHL is a structure in which collagen fibers have invaded from the portal region into the lattice-like or mesh-like RF that originally surrounded the lost hepatocytes[3]. However, it should be noted that the existence of such a formation has to be confirmed by additional studies.
There is a system of connective-tissue plates in the area of the hepatic port, whose origin and structure continue to be the subject of debate. This system includes a cystic plate, a round ligament plate, an Arantial plate, and a hilar plate (Walaeus vasculo-biliary sheath)[27,29].
The names of the plates are determined by their location: the gallbladder bed, round ligament gutter, Arantial ligament (obliterated venous duct) gutter, and hilus of the liver[30,31]. Several researchers have further described the caval plate, the connective-tissue sheath situated between the hepatic parenchyma and the adventitia of the hepatic part of the inferior vena cava[26,32]. Some researchers believe that these plates are derivatives of Laennec's capsule, which is attached to the liver capsule as an additional outer layer in the above-mentioned areas[30]. Other researchers indicate that the plate complexes, especially the hilar plate (which has special functional and clinical significance), is not an embryological derivative of Laennec's capsule and is connected with the fibrous part of the hepatoduodenal ligament and the connective tissues surrounding the blood vessels and bile ducts located in the portal area[27]. However, another group of researchers believes that the hilar plate does not exist at all as an independent entity; it is part of the liver capsule, which thickens in the area of the hepatic port due to a large number of thin-walled bile ducts (so-called "vaginal ductuli"). During surgery and dissection, it should be kept in mind that the hilar plate is likely to be artificially generated when, the surgeon unintentionally bundles collagenous fibers around the vaginal ductuli[15,29,33]. Taken together, the origin of the plates located on the visceral surface of the liver requires additional studies. Furthermore, we can state with confidence that the hilar plate (Walaeus vasculo-biliary sheath) covers the structures entering or exiting the liver at the hepatic port — the branches of the portal vein, hepatic artery, and bile ducts and accompanying lymphatic vessels and nerve cords. In combination with the accompanying connective-tissue fibers, afore-mentioned structures form the portal tracts that branch inside the liver. Large portal tracts, such as lobar, sectoral, segmental, and sometimes subsegmental tracts, are enveloped by a perivascular fibrous capsule (Glisson's capsule), which forms the so-called Glissonean pedicle[30]. Glisson's capsule is an intrahepatic extension of the hilar plate (Walaeus sheath). Thus, the portal tracts at the hepatic port are surrounded by the Walaeus sheath and inside the liver with Glisson's capsule. As mentioned above, Glisson's capsule is prominent around the large-caliber portal tracts but tapers off or completely disappears in thinner tracts[7].
Taking all of the above into consideration, Hu et al[8] concluded that the plate system represented a fibrous, thickened part of the Walaeus vasculo-biliary sheath and that Laennec’s capsule had no continuity with the Glissonean pedicle. However, Laennec’s capsule, which is dissociated from the main Glissonean capsule, extends to the peripheral portal tracts, where the structural integrity loosens and directly continues into the intralobular connective tissue fibers.
Laennec's capsule is the critical structure for understanding the comprehensive surgical anatomy of the liver and standardizing extrahepatic Glissonean pedicle isolation in anatomical liver resection[21]. Its precise understanding may rewrite the descriptions in the hepatology textbooks on the relationship between the hepatic capsule and intrahepatic and extrahepatic portal pedicle sheaths as follows: the connective tissue that constitutes the hepatic capsule wraps around the portal vein, hepatic artery, bile duct, lymphatics, and nerves that enter and exit the liver from the hilar part and then enters the liver where it is distributed as a skeleton in the parenchyma[34].
The blood vessels, bile ducts and nerves located in the portal tracts are covered by their own fascial connective tissue. These structures are individually encased by a typical membrane containing laminin, collagen type IV, entactin, and heparan sulfate proteoglycan. The surrounding portal interstitium contains collagen types I, III, V, and VI, fibronectin and tenascin[2]. The fibrous covers are separated from the blood vessel walls by a space called the conceptual paravasal body[35].
In the liver hilus and adjacent proximal part of the hepatoduodenal ligament, the connective tissue cover of the portal vein is well distinguished. It surrounds the blood vessel in the form of a sheath, inside of which there is the aforementioned paravasal fissure, which contains connective tissue fibers running in different directions, connecting the portal vein adventitia with the inner surface of its fibrous cover. Likewise, in the same regions, the hepatic artery is also surrounded by a layer of fibrous connective tissue called the fibrous cover. It is separated from the blood vessel wall by a well-defined fissure containing the bundles of connective-tissue fibers connecting the inner wall of the fibrous cover with the adventitia of the artery[20,32].
The Brisbane Meeting of the International Society of Hepatobiliary-Pancreatic Surgery in 2000 formed a consensus on the uniform anatomical term/terminology classification to remedy the confusion that was present at that time. Their consensus was that first-order divisions of the elements of the portal triad were those that supplied the right and left halves of the liver, second-order divisions were those that supplied the liver sectors, and third-order divisions were those that supplied the segments[36].
The perivascular fibrous capsule abruptly appears in the area of the sectoral portal tract. It is dense and easily separates from the liver tissue, which in turn is covered by the PHC (the intrahepatic part of Laennec’s capsule)[3].
The perivascular fibrous capsule is formed by collagen fibers running in various directions (elastic fibers are relatively rare). In addition, the outer layer of the capsule is denser. The relatively loose inner layer is contiguous to the connective tissue that surrounds the covers of individual elements of the portal triad. The thickness of the sectoral perivascular fibrous capsule is 45-110 μm (average 70-75 μm). Gradually, with the decrease in the caliber of the portal tract, the perivascular fibrous capsule also becomes thinner. The perivascular fibrous capsule of the 2-3 mm caliber subsegmental portal tract loses its sheath-like structure and transforms into loose connective tissue located between the individual elements of the portal triad.
The thickness of the proper cover of sectoral and segmental branches of the portal vein ranges from 50 μm to 150 μm (on average 90-100 μm) and it is directly proportional to the caliber of the blood vessel. The portal vein cover, within the subsegmental tract, gradually becomes thinner and looser. In addition, studies have shown that in 15% of cases, the identification of the connective tissue cover of the portal vein is hampered, even around the sectoral and segmental branches[32,37].
The number of bile ducts in sectoral and segmental portal tracts always exceeds three. Bile ducts are enveloped by the fibrous parabiliary sheath. The sheath has circularly oriented internal bundles, while the external bundles form septa oriented in various directions and connect closely to both the adjacent bile duct wall and the perivascular fibrous capsule. Bile ducts are accompanied by the peribiliary glands, which are connected to the ducts mainly along their opposite edges. The glands can be distinguished between intramural and extramural parts. The extramural part of the glands is several times larger in size than the intramural part. It is covered by the fibers of the fibrous parabiliary sheath, extends a considerable distance from the duct wall, is closely related to the connective tissue sheaths of other elements of the portal complex, and sometimes directly attaches to the perivascular fibrous capsule. Occasionally, the fibers covering the peribiliary glands and that of the internal surface of the perivascular capsule are so intertwined that no border can be identified between them[32,38-40].
The number of branches of the hepatic artery with a caliber larger than 1 mm varies from 2 to 5 in each sectoral and/or segmental portal tract. They are located more centrally (closer to the portal vein branch) than the bile ducts. The covers of the hepatic artery are not as distinct in sectoral and segmental tracts as in the hepatic hilus or hepatoduodenal ligament. The paravasal fissure is invisible as the adventitia is virtually contiguous with its own cover. The covers of the arteries at the peripheral edges of the blood vessels extend into the septa, which often interconnect and create the circular layer of para-arterial connective tissue located between the portal vein and the parabiliary fibrous sheath (Figure 1C). The degree of differentiation of the connective tissue covers of the arteries strongly depends on the caliber of the portal tract. In the small (subsegmental and thinner) portal tracts, the arteries have no connective tissue covers at all, and they are surrounded only by loose connective tissue that forms a bed for all elements of the portal triad[32,37]. Therefore, a combination of paravasal and parabiliary connective-tissue formations concentrated around the portal vein makes the skeletons of the hepatic portal tracts. The perivascular fibrous capsule, with adjacent parabiliary tissue with bile ducts and peribiliary glands, is located on the periphery of Glissonean pedicles[32,37].
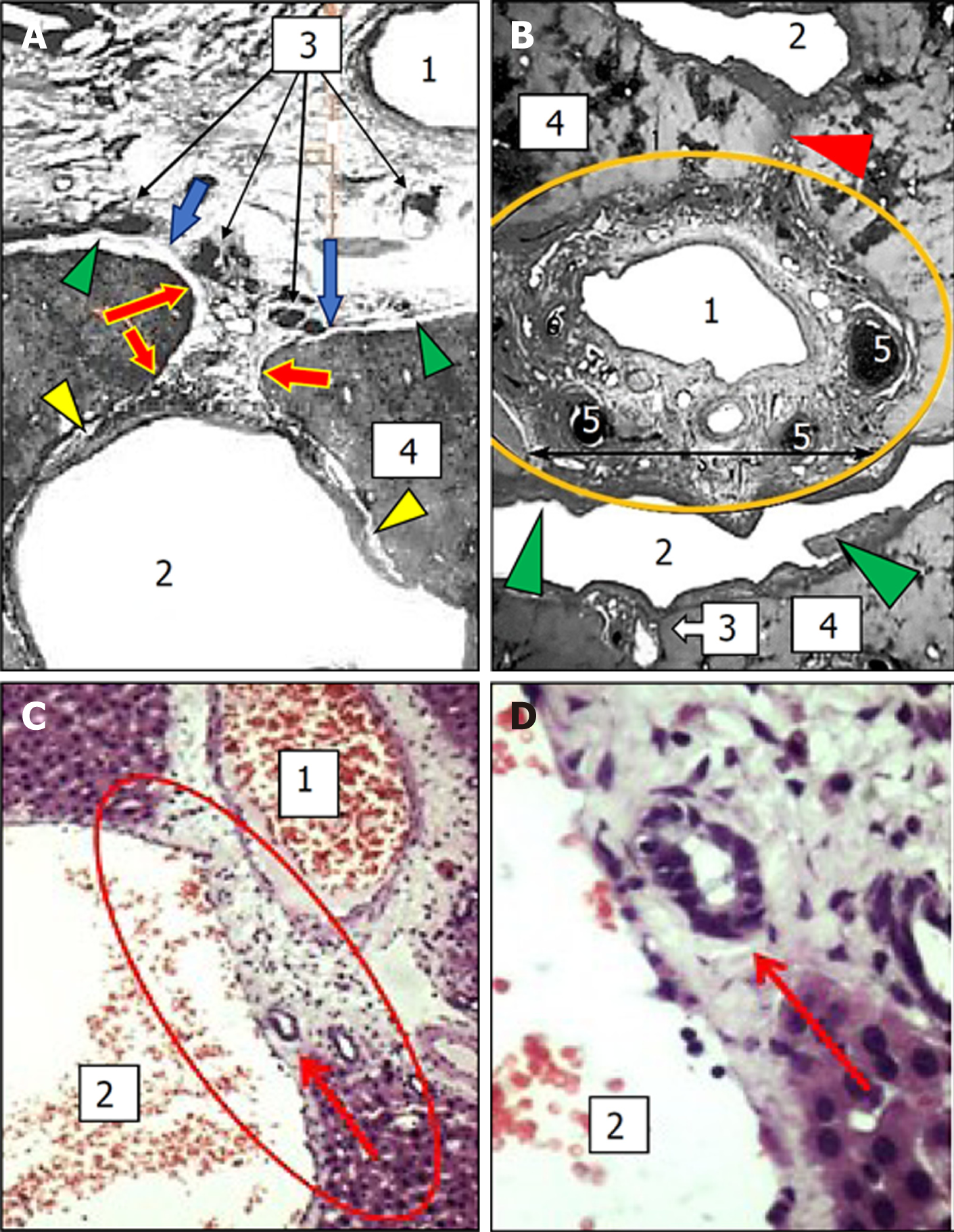
In the liver, at the site of the spatial intersection of the main portal tracts and the hepatic veins, there is a little-known anatomical formation generated by the fusion of the connective-tissue fibrous sheaths of the portal tracts and the hepatic veins where these two structures come into contact with each other. The perivascular fibrous capsule extends from the portal complex to the wall of the hepatic vein and it becomes an additional element (Figure 1A, B, D and E). An anatomical formation created by the fusion of the sheaths of portal tracts and hepatic veins is called the intrahepatic PCFC[20,32].
Various forms of PCFC are distinguished.
Complete fusion: This type of porta-caval connection is characterized by the complete fusion of the surfaces of connective tissue sheaths of the portal tract and hepatic vein directed towards each other (Figure 1D and 2B). This type of connection is mainly found in segments II and III of the liver. The connective tissue sheaths of the hepatic veins are highly developed in the PCFC area, and its thickness reaches 90 μm. It represents a thick network of the collagen fibers running in various directions and the spiral bundles of elastic fibers and separate cellular elements are located between them. At the same time, irrespective of the density of the elements of the portal triad that merge with the hepatic veins in the area of the PCFC, there is always a narrow gap between them, filled with loose connective tissue. Small blood vessels (up to 1.5 mm in diameter), which are separated from the branches of the hepatic artery located in the portal tract, might pass through this place. They extend to the wall of the hepatic vein and supply it with blood.
Touching connection: This type of PCFC occurs when the perivascular fibrous capsule and the sheath of the hepatic vein merge only with the parts of the surface facing each other, while the rest of the space between them is filled with liver tissue. Similar to complete fusion, this form of PCFC also contains small blood vessels, but rarely the nerves or lymphatics. Touching PCFCs are often found within segments II, III, VI, and VII of the liver.
Fan-shaped connection: The fan-shaped connection, a special form of connection, is formed when the 2-5 mm caliber portal tract touches the wall of the inferior vena cava or large hepatic vein and immediately splits into thinner branches. The fan-shaped PCFC is constantly found within segment I (caudal lobe), including the inferior vena cava wall. The branches feeding the wall of the inferior vena cava or large caliber hepatic veins are separated from the arteries of the portal tract within this connection[20,32]. Within the complete fusion, touching and fan-shaped PCFCs, the hepatic vein is most often bordered by the bile ducts and their peribiliary glands. Such direct contacts may facilitate the spread of the inflammatory process from the bile ducts to the liver[32].
Plate and thread-shaped connections: The plate or thread-shaped PCFCs are represented by a fibrous plate or a cone that stretches between the perivascular fibrous capsule and the hepatic venous sheath. The plate may contain small blood and lymphatic vessels[20,32] (Figure 1E and 2A).
It should also be noted that the presence of various forms of PCFC has been confirmed in other mammals (pigs, sheep, dogs, rats). In the histological liver specimens of these animals, the sites of the crossing of different size portal tracts and hepatic vein tributaries with integration (fusion) of their connective-tissue sheaths were described. At the same time, in rat livers, the translocation of biliary structures from the portal tract toward hepatic veins was shown. This translocation causes the appearance of ductular profiles accompanying hepatic veins and their tributaries on histological specimens[32] (Figure 2C and D).
Today, among the modern methods of surgical treatment of portal hypertension complicated by bleeding from varicose veins, the transjugular method of intrahepatic porta-caval anastomosis, which has a palliative effect, is widely used[41]. However, this method is often accompanied by complications; the most common ones are thrombotic or proliferative occlusion of the endoprosthetic shunt implanted between the branches of portal and hepatic veins, as well as stent migration-transposition[20]. This is exacerbated by the fact that the tubular shunt-prosthesis is often placed between the right branch of the portal vein and the right hepatic vein, which are significantly separated from each other (from 2 cm to 9 cm). The longer the shunting prosthesis is, the higher the likelihood of thrombosis, suppression and/or tran
It is quite probable that the endovascular method may be more successful in developing portocaval anastomoses in the area of PCFCs, where parenchyma-free areas of direct contact between the walls of large branches (5 mm to 20 mm) of the hepatic and portal veins already exist. It is preferable to perform endovascular intervention on liver segments II and III, where the left hepatic vein passes below the main portal complex and is in direct contact with the portal vein branch, as well as between the right hepatic vein and the portal vein branch of segment VII. The various types of branching of the portal and caval veins determine a large variation in the number of PCFCs — from 4 to 20; however, despite this, the above-mentioned PCFCs in segments III and VII are characterized by high stability. In addition, the sites of integration within the connective-tissue sheaths of the large portal tracts and hepatic veins with the standard topography can be visualized by magnetic resonance imaging[20].
In the human liver where the portal tracts and hepatic veins spatially intersect (spatial crossing), the fusion of their connective-tissue sheaths develops an anatomical structural element in the form of a nodal fibrous connection — “porta-caval fibrous connection” — allowing the hepatic vein to interact closely with the elements of the portal complex. The PCFC is a stable structure, whose formation begins at the 11th-12th week of embryogenic development. Based on the above discussion, intrahepatic PCFC can be considered an independent anatomical element of the liver, which deserves to be reflected in international anatomical nomenclature. Knowledge of the existence and features of PCFC enhance our understanding of the liver connective tissue framework and support the development of new surgical approaches for the treatment of various liver pathologies.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Georgia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Karamarkovic AR, Kim JM S-Editor: Gao CC L-Editor: Webster JR P-Editor: Zhang YL
| 1. | Rojkind M, Ponce-Noyola P. The extracellular matrix of the liver. Coll Relat Res. 1982;2:151-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 105] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Martinez-Hernandez A, Amenta PS. The hepatic extracellular matrix. I. Components and distribution in normal liver. Virchows Arch A Pathol Anat Histopathol. 1993;423:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 140] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Ikeda T, Okano S, Hashimoto N, Kimura K, Kudo K, Tsutsumi R, Sasaki S, Kawasaki J, Miyashita Y, Wada H. Histomorphological investigation of intrahepatic connective tissue for surgical anatomy based on modern computer imaging analysis. J Hepatobiliary Pancreat Sci. 2021;28:76-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Lada E, Anna M, Patrik M, Zbynek T, Miroslav J, Hynek M, Richard P, Sarah L, Vaclav L. Porcine Liver Anatomy Applied to Biomedicine. J Surg Res. 2020;250:70-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Al-Samawy ER, Waad SK, Hashim WS, Alabbas G. Comparative Histology of Human, Rats and Rabbits Liver. Indian J Public Heal Res Dev. 2019;10:1441. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Ota N, Hirose H, Kato H, Maeda H, Shiojiri N. Immunohistological analysis on distribution of smooth muscle tissues in livers of various vertebrates with attention to different liver architectures. Ann Anat. 2021;233:151594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Patarashvili L, Azmaipharashvili E, Jandieri K, Gvidiani S, Tsomaia K, Kikalishvili L, Sareli M, Chanukvadze I, Kordzaia D. Liver extracellular matrix peculiarities in mammals and avians. Georgian Med News. 2021;124-133. [PubMed] |
| 8. | Hu Y, Shi J, Wang S, Zhang W, Sun X, Sun B, Yu D. Laennec's approach for laparoscopic anatomic hepatectomy based on Laennec's capsule. BMC Gastroenterol. 2019;19:194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Morimoto M, Tomassini F, Berardi G, Mori Y, Shirata C, Abu Hilal M, Asbun HJ, Cherqui D, Gotohda N, Han HS, Kato Y, Rotellar F, Sugioka A, Yamamoto M, Wakabayashi G; Study group of Precision Anatomy for Minimally Invasive Hepato-Biliary-Pancreatic surgery (PAM-HBP surgery). Glissonean approach for hepatic inflow control in minimally invasive anatomic liver resection: A systematic review. J Hepatobiliary Pancreat Sci. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | Morimoto M, Matsuo Y, Ueda G, Kato T, Aoyama Y, Hayashi Y, Omi K, Imafuji H, Saito K, Tsuboi K, Ogawa R. Exploring the Fine-Layer Structure Around a Glissonean Pedicle in Cadaveric Models. Surg Gastroenterol Oncol. 2020;25:67-72. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Navarro-Tableros V, Herrera Sanchez MB, Figliolini F, Romagnoli R, Tetta C, Camussi G. Recellularization of rat liver scaffolds by human liver stem cells. Tissue Eng Part A. 2015;21:1929-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Liu Y, Yang R, He Z, Gao WQ. Generation of functional organs from stem cells. Cell Regen. 2013;2:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Lee JS, Shin J, Park HM, Kim YG, Kim BG, Oh JW, Cho SW. Liver extracellular matrix providing dual functions of two-dimensional substrate coating and three-dimensional injectable hydrogel platform for liver tissue engineering. Biomacromolecules. 2014;15:206-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 14. | Felgendreff P, Schindler C, Mussbach F, Xie C, Gremse F, Settmacher U, Dahmen U. Identification of tissue sections from decellularized liver scaffolds for repopulation experiments. Heliyon. 2021;7:e06129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Yamamoto M, Katagiri S, Ariizumi S, Kotera Y, Takahashi Y. Glissonean pedicle transection method for liver surgery (with video). J Hepatobiliary Pancreat Sci. 2012;19:3-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | ELIAS H, PETTY D. Gross anatomy of the blood vessels and ducts within the human liver. Am J Anat. 1952;90:59-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Ostroverkhov, G Zabrodskaya V. Surgical anatomy of the liver and biliary tract. In: Maksimenkov A. Surgical anatomy of the abdomen. Leningrad: 1972: 297-380. |
| 18. | Fegershanu N, Ionescu-Bujar K, Aloman D, Albu A. Surgery of the liver and intrahepatic biliary tract. Bucharest: 1976. |
| 19. | Dancygier H. Clinical Hepatology. Berlin, Heidelberg: Springer Berlin Heidelberg, 2010. |
| 20. | Chanukvadze I. Portacaval Fibrous Connections: Little Known Anatomical Structures of Liver. Rom Med J. 2017;64:43-48. |
| 21. | Sugioka A, Kato Y, Tanahashi Y. Systematic extrahepatic Glissonean pedicle isolation for anatomical liver resection based on Laennec's capsule: proposal of a novel comprehensive surgical anatomy of the liver. J Hepatobiliary Pancreat Sci. 2017;24:17-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 22. | Kawarada Y, Das BC, Taoka H. Anatomy of the hepatic hilar area: the plate system. J Hepatobiliary Pancreat Surg. 2000;7:580-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Poonkhum R, Pisetpaisan K, Wang BJ, Anupunpisit V, Ohtani Y, Ohtani O. Origins and pathways of fluid entering sublobular lymphatic vessels in cat livers. Arch Histol Cytol. 2003;66:317-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Ohtani O, Ohtani Y. Lymph circulation in the liver. Anat Rec (Hoboken). 2008;291:643-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 25. | Medeiros de Mello J, Orsi AM, Piffer CR, Torrejais MM. Architecture of the caudal vena cava wall of the dog at the level of the liver caudate lobe. Ann Anat. 2000;182:525-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 26. | Karau PB, Ogeng’o JA, Hassanali J, Odula PO. The histormorphological organization of the hepato-caval interface in the human. J Morphol Sci. 2010;27:148-151. |
| 27. | Takasaki K, Yamamoto M. Surgical Anatomy of the Liver in the Glissonean Pedicle Approach: What We Need to Know. In: Madoff D, Makuuchi M, Nagino M, Vauthey JN. Venous Embolization of the Liver. London: Springer London, 2011: 23-27. |
| 28. | Crawford AR, Lin XZ, Crawford JM. The normal adult human liver biopsy: a quantitative reference standard. Hepatology. 1998;28:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 168] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 29. | Hayashi S, Murakami G, Ohtsuka A, Itoh M, Nakano T, Fukuzawa Y. Connective tissue configuration in the human liver hilar region with special reference to the liver capsule and vascular sheath. J Hepatobiliary Pancreat Surg. 2008;15:640-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Couinaud C. Surgical anatomy of the liver revisited. Paris: Semantic Scholar, 1989: 29-39. |
| 31. | Couinaud C. Le foie; études anatomiques et chirurgicales. Masson, 1957. |
| 32. | Kordzaia D, Chanukvadze I, Jangavadze M. Functional Anatomy of Intrahepatic Biliary System (Clinical and Experimental Data). In: Miguel Ángel Mercado. Bile Duct: Functional Anatomy, Disease and Injury Classification and Surgical Management. Nova Science Publishers Inc, 2014: 1-87. |
| 33. | Guglielmi A, Ruzzenente A, Iacono C. Surgical Anatomy of the Hepatic Hilus. In: Guglielmi A, Ruzzenente A, Iacono C. Surgical Treatment of Hilar and Intrahepatic Cholangiocarcinoma. Milano: Springer, 2008: 101-111. |
| 34. | Kuntz E, Kuntz H-D. Hepatology Textbook and Atlas. Hepatol Textb Atlas. 2008;. [DOI] [Full Text] |
| 35. | Kovanov V, Anikina M. Surgical anatomy of human paravasal connective tissue structures [in Russian]. Moscow Med. 1985;. |
| 36. | Strasberg SM, Belghiti J, Clavien P-A, Gadzijev E, Garden JO, Lau W-Y, Makuuchi M, Strong RW. The Brisbane 2000 Terminology of Liver Anatomy and Resections. HPB. 2000;2:333-339. [DOI] [Full Text] |
| 37. | Chanukvadze I. Vasculo-fibrous architecture of the magistral portal complexes. Rom Med J. 2016;11:214-218. |
| 38. | de Jong IE, van Leeuwen OB, Lisman T, Gouw AS, Porte RJ. Repopulating the biliary tree from the peribiliary glands. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1524-1531. |
| 39. | Nakanuma Y, Katayanagi K, Terada T, Saito K. Intrahepatic peribiliary glands of humans. I. Anatomy, development and presumed functions. J Gastroenterol Hepatol. 1994;9:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Nakanuma Y, Sasaki M, Terada T, Harada K. Intrahepatic peribiliary glands of humans. II. Pathological spectrum. J Gastroenterol Hepatol. 1994;9:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Nolte W, Manke H, Schndler C. Dopplerronographsche kurz und landzeitunterrsuchunger der patalen Hamodynamik nach transjugular intrahepatischem portosistemischem Stent-Shunt. Ztsch fur Gastroenterol. 1998;491-499. |
| 42. | Richter GM, Roeren T, Brado M, Theilmann L, Sauer P, Kauffmann GW. [Portal hypertension and percutaneous transjugular portasystemic stent shunt]. Chirurg. 1995;66:555-565. [PubMed] |
| 43. | Kordzaia D, Jangavadze M. Unknown bile ductuli accompanying hepatic vein tributaries (experimental study). Georgian Med News. 2014;121-129. [PubMed] |