Published online Nov 27, 2021. doi: 10.4254/wjh.v13.i11.1459
Peer-review started: February 26, 2021
First decision: July 18, 2021
Revised: July 19, 2021
Accepted: September 3, 2021
Article in press: September 3, 2021
Published online: November 27, 2021
Processing time: 271 Days and 8.4 Hours
The role of endoscopic ultrasound (EUS) as a diagnostic and therapeutic modality for the management of various gastrointestinal diseases has been expanding. The imaging or intervention for various liver diseases has primarily been the domain of radiologists. With the advances in EUS, the domain of endosonologists is rapidly expanding in the field of hepatology. The ability to combine endoscopy and sonography in one hybrid device is a unique property of EUS, together with the ability to bring its probe/transducer near the liver, the area of interest. Its excellent spatial resolution and ability to provide real-time images coupled with several enhancement techniques, such as contrast-enhanced (CE) EUS, have facilitated the growth of EUS. The concept of “Endo-hepatology” encompasses the wide range of diagnostic and therapeutic procedures that are now gradually becoming feasible for managing various liver diseases. Diagnostic advancements can enable a wide array of techniques from elastography and liver biopsy for liver parenchymal diseases, to CE-EUS for focal liver lesions to portal pressure measurements for managing various liver conditions. Similarly, therapeutic advancements range from EUS-guided eradication of varices, drainage of bilomas and abscesses to various EUS-guided modalities of liver tumor management. We provide a comprehensive review of all the different diagnostic and therapeutic EUS modalities available for the management of various liver diseases. A synopsis of all the technical details involving each procedure and the available data has been tabulated, and the future trends in this area have been highlighted.
Core Tip: The advancements in the field of endoscopic ultrasound (EUS) have enabled endosonologists to rapidly expand their wings in the field of hepatology. “Endo-hepatology” encompasses the wide range of diagnostic and therapeutic endoscopic procedures that can be used for the management of various liver diseases. Diagnostic advancements range from elastography for liver parenchymal diseases, contrast-enhanced EUS for a focal liver lesion to portal pressure measurements. Therapeutic advancements range from EUS-guided eradication of varices to drainage of abscesses to liver tumor ablation. In this comprehensive review, all the various diagnostic and therapeutic EUS modalities available for the management of liver diseases have been detailed.
- Citation: Dhar J, Samanta J. Role of endoscopic ultrasound in the field of hepatology: Recent advances and future trends. World J Hepatol 2021; 13(11): 1459-1483
- URL: https://www.wjgnet.com/1948-5182/full/v13/i11/1459.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i11.1459
The armamentarium of endoscopic ultrasound (EUS) has grown considerably in recent years, both as an investigative and a therapeutic modality. The established diagnostic tools for the study of liver diseases include trans-abdominal ultrasound (USG), computed tomography (CT) scan and magnetic resonance imaging (MRI). While in the past, interventions in liver disease have predominantly been performed by the percutaneous or vascular route, EUS is now more and more being used for both diagnostic and therapeutic purposes. The ability to combine endoscopy and sonography in one hybrid device is a unique property of EUS, together with the ability to bring its probe/transducer in close proximity to the liver, the area of interest. In addition, its excellent spatial resolution and ability to provide real-time images, along with additional techniques, such as contrast-enhanced (CE) EUS, have facilitated the growth of EUS.
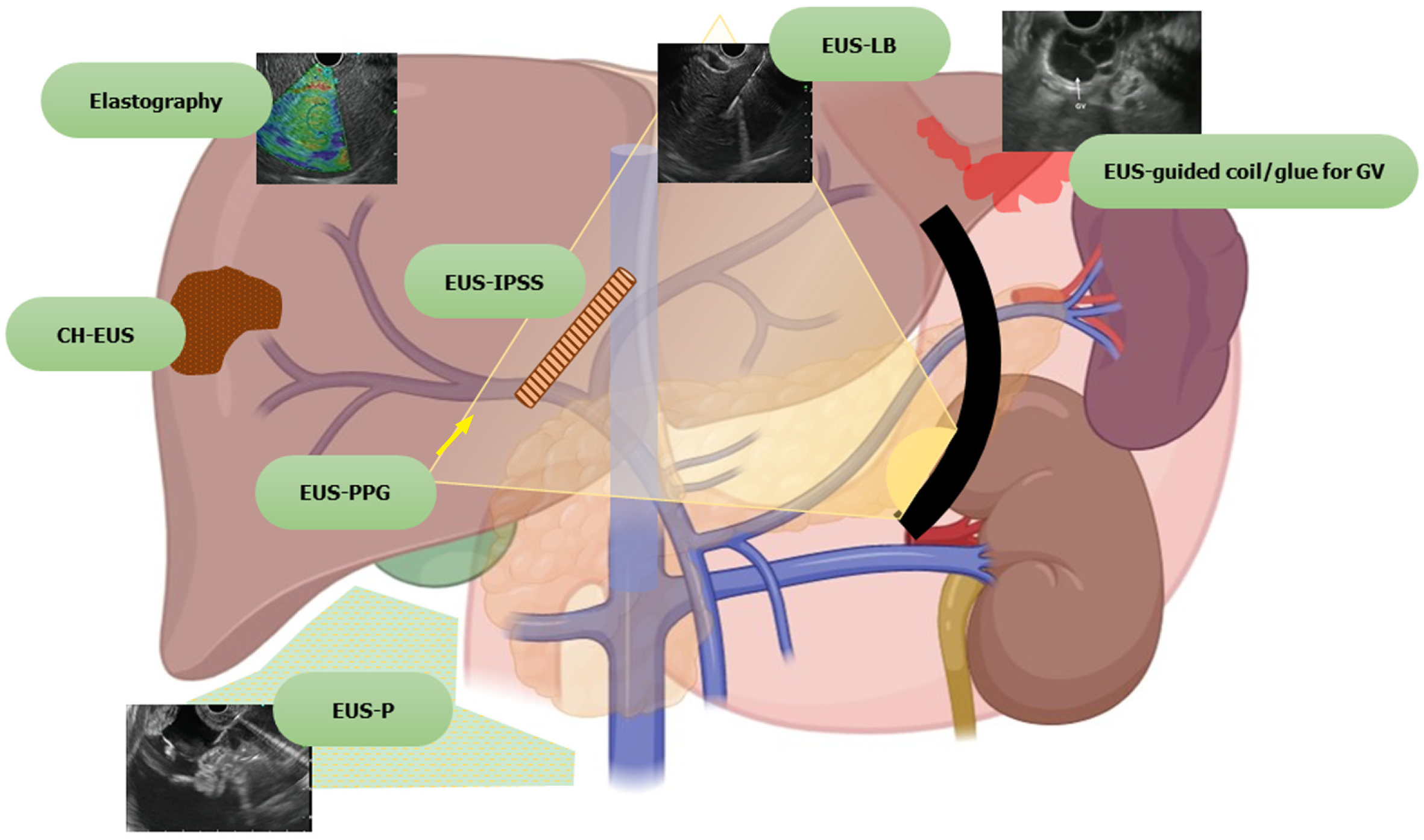
Furthermore, EUS guided intervention is also used as a rescue modality when the percutaneous approach is not favorable. EUS has opened doors to a variety of other procedures which are being explored, such as portal vein (PV) sampling for cancer cells, delivery of chemotherapy in the PV, measurement of portosystemic pressure gradient, and EUS guided transjugular intrahepatic portosystemic shunt (TIPS) creation. Harnessing its use in various liver-related interventions paves the way for a new zone of specialty, “Endo-hepatology.” Herein we provide a comprehensive review on the use of EUS in the field of hepatology, both diagnostic and therapeutic, discussing the various recent advances and future trends (Figure 1).
A search was performed in PubMed and Embase and the search strategy is outlined in Supplementary Doc 1. All studies such as case reports, series, clinical studies, animal models and reviews regarding EUS applications in liver disorders, including portal hypertension (PHTN), were reviewed. Non-English language literature was not included in the review. EUS applications for extrahepatic bile duct obstruction, gallbladder, etc., including their interventions, are beyond the scope of this review and have been excluded.
EUS can be used for the diagnosis, assessment and therapeutic management of ascites, liver parenchymal pathologies, space-occupying lesions (SOLs), liver biopsy, drainage of liver abscesses, bilomas and the management of hepatic tumors.
Ascites can be due to benign or malignant diseases. Although the differential diagnosis is broad, around 80%-90% of cases are attributed to underlying cirrhosis and PHTN[1]. Traditionally, routine paracentesis is performed bedside and sometimes with abdominal ultrasound guidance. However, abdominal paracentesis may become difficult in the presence of multiple abdominal scars, previous puncture marks, obesity, dilated bowel loops, dilated/tortuous veins, or the presence of omental or peritoneal nodules[1-3]. EUS guided paracentesis (EUS-P) is more sensitive than CT in detecting ascites[2,4]. The presence of ascites not visualized on imaging (CT/USG) as well as compartmentalization of fluid (such as benign etiologies like tuberculosis or tumor implants in peritoneal carcinomatosis) makes EUS-P a very promising tool in these areas[4,5]. With EUS-P, even small amounts of fluid (as little as 2.7 mL) can be aspirated and provide valuable diagnostic information[6]. In addition, EUS-P can be used as a rescue procedure in the case of previously failed percutaneous paracentesis or part of diagnostic workup during diagnostic EUS (Figure 2).
Additionally, EUS guided fine needle aspiration (EUS-FNA) of suspicious nodules in the omentum/peritoneum can be performed simultaneously while performing paracentesis for targeted cytological diagnosis[7]. Contrast-enhanced EUS (CE-EUS) has also been evaluated to identify enhancement patterns of peritoneal nodules or omental caking and differentiate benign or malignant causes of undiagnosed ascites[8].
The technique of EUS-P: The technique of EUS-P is detailed in Table 1.
| Pre-procedure requirements |
| (1) No recommendations exist for EUS-P, although most studies have been performed under the cover of pre/peri-procedural antibiotics; and (2) Patient is usually fasted for 4-6 h before the procedure |
| Technical aspects |
| (1) EUS-P is usually performed using a 22 G/25 G FNA needle. A specialized spring-loaded 22 G FNA needle can also be used for the same; (2) The approach can be transgastric or transduodenal. The tip of the needle is visualized under EUS guidance in the ascites; (3) At the time of puncture, care is taken to avoid a trajectory involving any tumor/vessels to avoid peritoneal seeding or bleeding; (4) For therapeutic paracentesis, a suction tube attached to a vacuum canister can be used; (5) Repositioning of the needle is carried out in case it gets blocked by the tumor or omentum; (6) Two and fro motion is usually not needed; (7) CE-EUS followed by FNA of the peritoneal/omental nodules can also be done for added diagnostic value; and (8) The sample aspirated is sent for routine cytological assessment and for any additional tests that might be needed |
| Post procedure |
| The administration of albumin post 5 L of paracentesis and post procedure observation are carried out as per standard recommendations (EASL, AASLD) |
Future trends: Since the first report of EUS-FNA of ascites and pleural fluid performed in 1995, various reports of EUS-P with/out FNA of peritoneal deposits have been published subsequently with excellent diagnostic capability and correlation with intraoperative findings[12]. Some cases of development of infectious complications (attributed to traversing the contaminated gastrointestinal wall) such as self-limited fever (3.3%) and bacterial peritonitis (4%) have been reported[5,10]. Recent develop
| Ref. | Study design | Patient population | Imaging | Age (yr) | Gender (M/F) | Needle | Route (TG/TD) | Amount of fluid aspirated | Diagnosis on EUS | Actual diagnosis | Complications |
| Chang et al[12], 1995 | Case report | 2 cases | CT (pleural effusion and ascites) | - | - | - | - | - | - | Malignant effusion and ascites | - |
| Romero-Castro et al[14], 2017 | Case series | 3 cases | DLBCL (1 case), HCC (2 cases) | 60/74/55 | 3/- | 19 G FNA (all cases) | TG (3 cases) | Double Pigtail placement (3 cases) | - | Malignant ascites (3 cases) | None |
| Wardeh et al[16], 2011 | Retrospective study | 101 | Ascites not detected in 6/9 cases on CT | 68.3 | 54/47 | 19 G FNA | NA | 10 mL (max) in 90 cases, 2 smears in 11 cases | 74 negative | 84 malignant | None |
| Suzuki et al[11], 2014 | Retrospective study | 11 cases | CT (no ascites in 4) | 66.4 | 7/4 | 22 G (automatedspring-loaded) | NA | 14.1 mL (range 0.5-38 mL) | Benign 5; malignant 6 | NA | None |
| Kaushik et al[10], 2006 | Retrospective study | 25 | NA | 66-70 | 16/9 | 22/25 G FNA | Both | 6.8 mL (range, 1-20 mL) | 64% malignant (benign 9; malignant 16) | Benign 8; malignant 17 | 1 cases (4%) (bacterial peritonitis) |
| Lee et al[4], 2005 | Retrospective study | 250 cases | CT in all | 60.3 | 160/90 | NA | NA | NA | 37% ascites, 28% peritoneal metastasis | All malignant | None |
| Dewitt et al[5], 2007 | Retrospective study | 60 | CT/MRI/USG in all (ascites 31 cases (51%) | 67 | 33/27 | 22 G | 55 (TG), 5 (TD) | 8.9 (1-40) mL | Benign 42; malignant/atypical 18 | Benign 15; malignant 45 | 2 cases fever |
| Köck et al[13], 2018 | Case report | 2 cases | Rectal cancer, ovarian cancer | 36, 56 | -/2 | 19 G | Both TG | Pigtail (plastic) placed | - | - | None |
| Nguyen and Chang[2], 2001 | Retrospective study | 31 cases (of 85) | CT had ascites in 14/79 (18%) | NA | NA | NA | NA | 7.9 (1-40 mL) | Malignant 5; benign 26 | NA | None |
| Varadarajulu and Drelichman[3], 2008 | Case report | 1 | SCC anus | 31 | -/1 | 19 G | TG (1) | 10 mL (diagnostic); 5 L (therapeutic) | Malignant ascites | NA | None |
Thus, EUS-P is an excellent tool (sensitivity 94%, specificity 100%) to detect a small quantity of ascites[10] and therapeutic drainage where the percutaneous approach is not amenable. Furthermore, FNA of peritoneal/omental nodules is an added advantage that can increase the diagnostic yield.
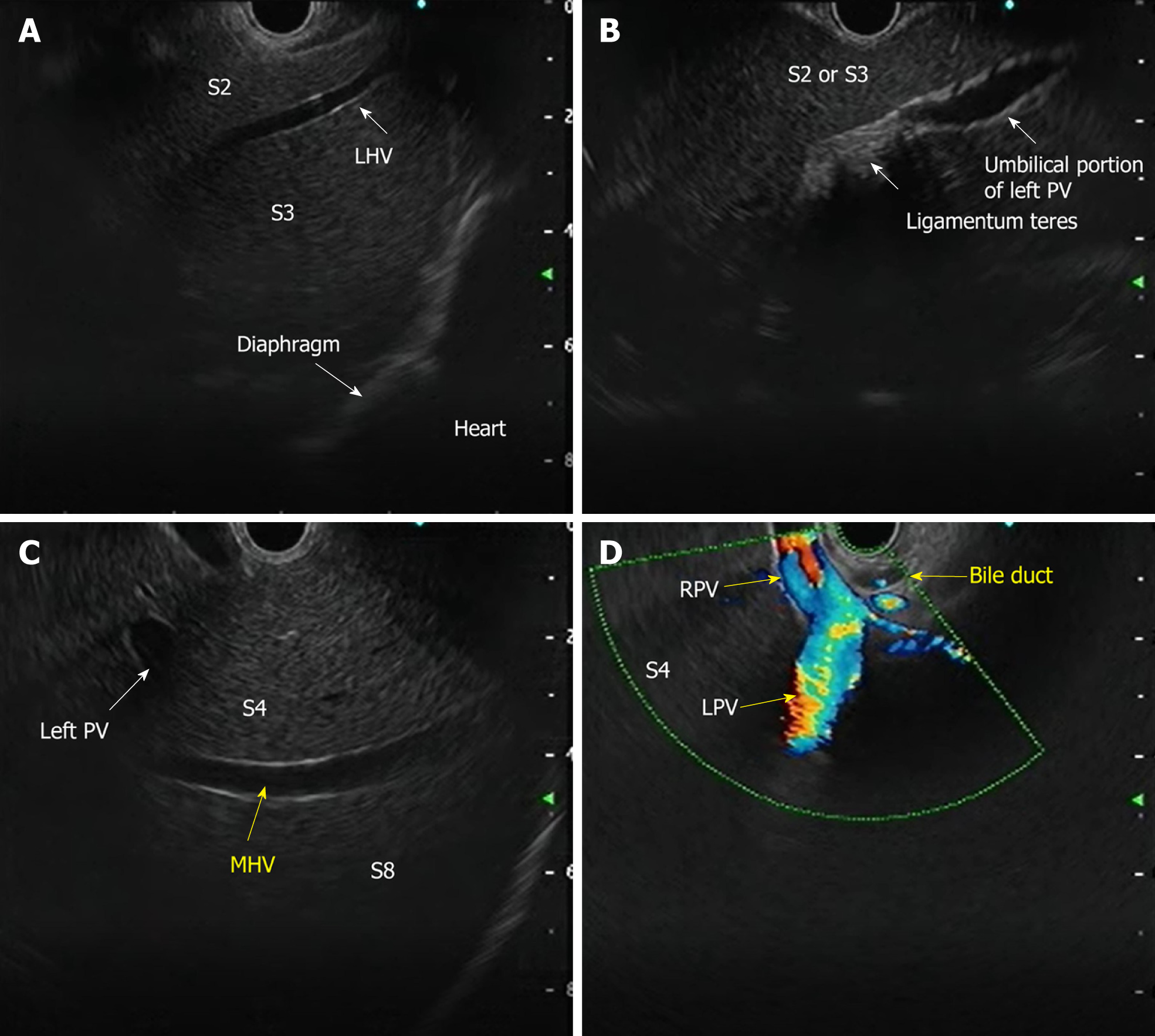
The requirement for three-dimensional conceptualization of the liver parenchyma makes EUS assessment of the liver and surrounding structures different from the conventional methods of USG/CT/MRI. Depending on the position of the EUS scope, either in the stomach or duodenum, various structures can be identified (Table 3 and Figure 4) such as[17]: (1) From the gastric end: Segments I (caudate lobe), left lobe segments (II, III, IV), right lobe (V, VIII), umbilical part of the left PV and ligamentum teres, ligamentum venosum, inferior vena cava, and hilum; and (2) From the duodenal bulb: Segments VI, VII; the hepatoduodenal ligament structures and PV and hepatic artery branches, the liver hilum and the segmental divisions of the right PV and hepatic artery.
| Structure | Features | Doppler |
| Portal vein branches | Thick and hyperechoic walls | Positive signal |
| Hepatic vein branches | Thin, non-reflective walls, straight course | Positive signal |
| Biliary radical | Hyperechoic walls, irregular course | Negative signal |
| Ligaments (teres and venosum) | Thick, hyperechoic (no lumen) (between vessels and Glisson’s capsule) | Negative signal |
| Gallbladder | Cystic structure, hyperechoic walls, anechoic content | Negative signal |
| Falciform ligament | Thick, hyperechoic (no lumen); on the left anterior to segment III, on the right anterior to segment IVa and IVb | Negative signal |
| Hepatic artery | Thick with reflective walls | Positive signal |
Although transabdominal USG or CT scan is the first-line approach for evaluation of liver parenchyma or focal lesions, EUS has additional features which can add to its diagnostic/therapeutic potential[18,19]: (1) Transducer proximity enables better identification of the structures; (2) Combination of real-time images with elastography enables semi-quantitative measurements of liver parenchymal stiffness; (3) Newer generation EUS machines with color, power and pulsed Doppler systems helps easy assessment of the vasculature; (4) CE-EUS or harmonic EUS increases the diagnostic performance of focal liver lesions; and (5) Simultaneous assessment and interventions such as management of varices and liver biopsy can be performed in a single setting.
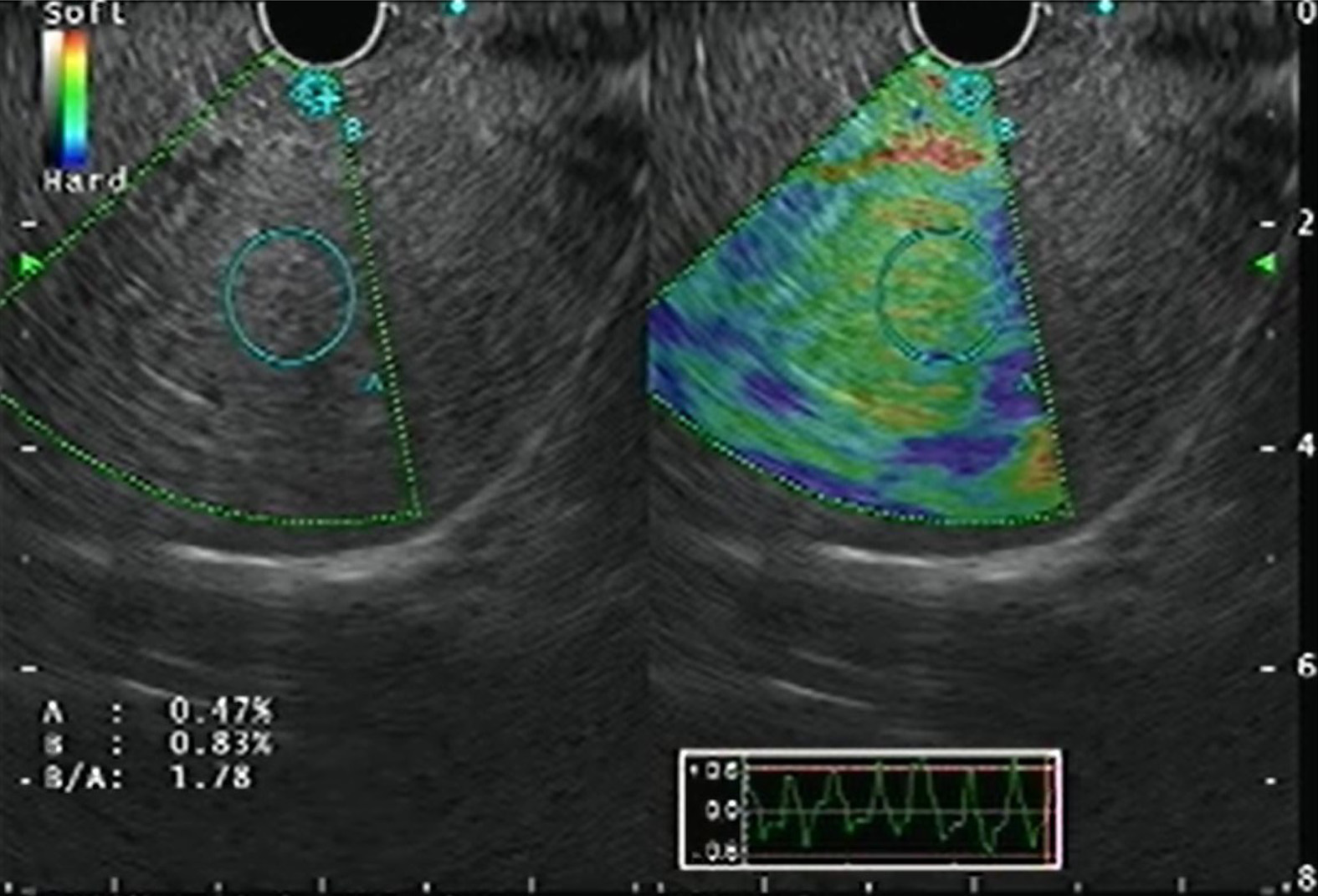
Real-time elastography (RTE) has been developed for the assessment and quantification of liver tissue stiffness. Qualitative RTE uses the degree of deformation by the compression of structures as an indicator of tissue stiffness and is depicted using a color map wherein hard tissue is blue, intermediate stiffness is green and soft tissue is red. Quantitative RTE, on the other hand, uses hue histogram and strain ratio. While the former is a graphical representation of the color distribution in a selected image field, the strain ratio is calculated as the ratio of the target area (A) by reference area (B) (Figure 5)[20].
CE-EUS is a more valuable technique to improve the diagnostic performance of focal liver lesions. It is of 2 types: CE-EUS with the Doppler method (CE-EUS-D) and CE-EUS with harmonic imaging (CE-EUS-H). The former helps distinguish vascular-rich and hypovascular areas of a liver SOL, whereas the latter helps provide a detailed roadmap of the vasculature of the same. Of the contrast agents available, Sonovue and Sonazoid are more commonly used[21].
The concept of CE-EUS depends on the dual blood supply of the liver and has 3 phases: arterial phase (20-45 s), portal venous phase (lasting up to 120 s), and the late phase (contrast agent clearance, around 6 min)[21].
The advantages of CE-EUS over CT and MRI are that: (1) It provides real-time imaging; (2) Contrast is not excreted by the kidneys, and thus can be used in cases with renal insufficiency; (3) Contrast is confined to the vascular space only and so has prolonged enhancement of vascular system; (4) Higher resolution helps in targeted biopsies; and (5) Can characterize lesions less than 1 cm.
Certain tests such as transient elastography (TE), Fibroscan, and RTE can aid in the diagnosis of the degree of liver fibrosis. However, these tests are fraught with limitations in people with obesity and ascites. EUS can be used similarly with probably better diagnostic sensitivity for the same. Schulman et al[22] reported that liver fibrosis index (LFI) correlated with abdominal imaging (LFI in normal, fatty liver and cirrhosis patients were 0.8, 1.4 and 3.2, respectively). Similar findings were replicated in liver fibrosis assessment for chronic hepatitis C cases (LFI of 2.38 had an area under the receiver operating characteristic curve of 0.73) compared with the gold standard of liver biopsy. Histogram acquisition was successful in 82% of patients[23]. A recent study by Tu et al[24] in early-stage cirrhosis showed that the accuracy of a combination of EUS, EUS-RTE, acoustic radiation force impulse (AFRI) and aspartate aminotransferase-to-platelet ratio (APRI) had the highest diagnostic rate (sensitivity 87%). Thus, EUS can provide a one-stop diagnostic modality to screen and rule out a host of conditions in patients with liver disease, from the screening of varices, pancreaticobiliary pathology to hepatic parenchymal/SOL assessment.
The diagnostic accuracy of EUS in detecting focal liver lesions, mostly less than 1 cm, exceeds that of USG, CT, and MRI[25,26]. Singh et al[27] addressed the diagnostic yield of EUS vs CT for hepatic metastasis (98% vs 92%), wherein EUS identified a significantly greater number of metastatic lesions (40 vs 19). Diagnostic criteria proposed by Fujii-Lau et al[28] can be used to differentiate between benign and malignant metastatic hepatic lesions based on EUS findings with a positive predictive value of 82%. Lesion shape, borders, echogenicity, homogeneity, and size are used to delineate malignant lesions. It is said to be neoplastic if it meets at least three criteria: (1) Lack of isoechoic/slightly hyperechoic center; (2) Post-acoustic enhancement; (3) Adjacent structures distortion; (4) Hypoechogenicity (slightly or distinctly); and (5) At least 10 mm in size.
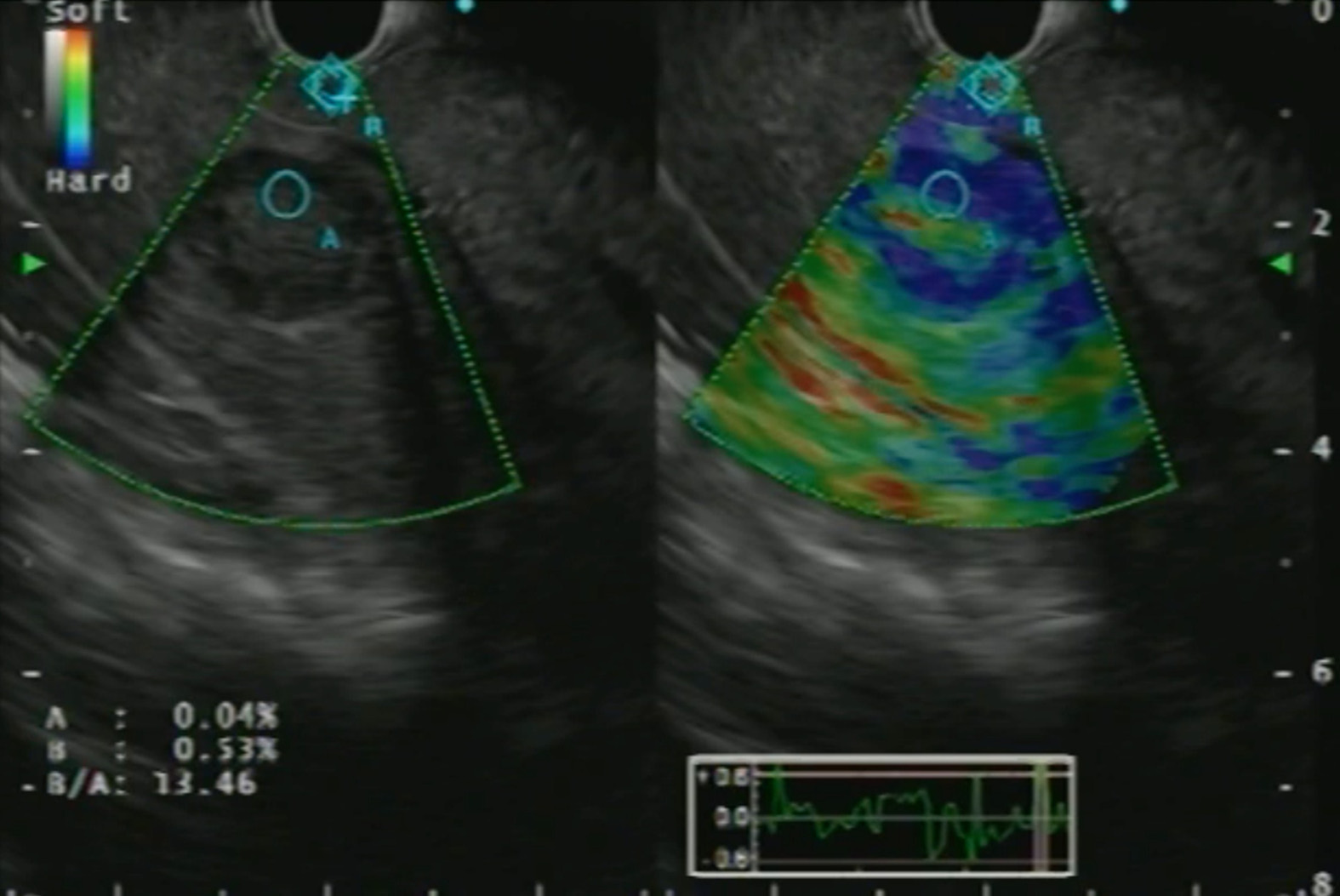
With the advent of EUS-RTE, the characterization of liver SOLs and their biopsies have become better (Figure 6). A study reported a hue histogram cutoff of 170 to discriminate between benign and malignant tumors (sensitivity 92.5%, accuracy 88.6%)[29]. In addition, the use of contrast agents in CE-EUS helps in differentiating primary tumors and metastasis[30]. CE-EUS has also been utilized for the assessment of treatment response in hepatocellular carcinoma (HCC) post-trans-arterial catheter embolization[31]. Hence, EUS with RTE, CE-EUS and CE-EUS-H might be a promising tool for diagnosing focal liver lesions and targeted intervention.
Several studies exist on the use of EUS-FNA/FNB (fine needle biopsy) for solid liver lesions with a complication rate of 0%-6% (Table 4). A recent systematic review by Ichim et al[42] showed the diagnostic yield of EUS-FNA to be 80%-100%.
| Ref. | Design | Patients | Diagnostic yield (%) | Needle passes (median) | Complications |
| EUS-FNA | |||||
| Nguyen et al[32] | Prospective | 14 | 100 | 2 | 0 |
| TenBerge et al[33] | Retrospective | 26 | 88.6 | - | 3.8% (fever) |
| DeWitt et al[34] | Retrospective | 77 | 91 | 3.4 (mean) | 0 |
| Hollerbach et al[35] | Prospective | 33 | 94 | 1.4 ± 0.6 | 6.1% (self-limited bleeding) |
| McGrath et al[36] | Prospective | 7 | 100 | 2 | 0 |
| Singh et al[26] | Prospective | 9 | 88.9 | 2 | 0 |
| Singh et al[27] | Prospective | 26 | 96 | 2.1 | 0 |
| Crowe et al[37] | Retrospective | 16 | 75 | 3 (minimum) | 0 |
| Prachayakul et al[38] | Retrospective | 14 | 100 | 0 | |
| Oh et al[39] | Prospective | 47 | 90.5 | 3 | 0 |
| Ichim et al[25] | Prospective | 48 | 98 | 2 | 0 |
| EUS-FNB | |||||
| Lee et al[40] | Prospective | 21 | 90.5 | 2 | 0 |
| Chon et al[41] | Retrospective | 58 | 89.7 | 2 | 1.7% (bleed) |
Studies have reported additional assessment of KRAS mutation in inconclusive cytological samples, which has resulted in an improved diagnostic yield from 89.3% to 96.4%[43]. Similarly, an animal study has evaluated the art of in vivo cytological observation using a high-resolution micro-endoscopy (HRME) system under EUS guidance[44] to decrease the number of needle-passes and subsequent adverse events. Recently, Minaga et al[45] have reported the additive role of CE-EUS-H in the detection of left lobe liver metastasis from pancreatic ductal adenocarcinoma. The diagnostic accuracy of CH-EUS was 98.4% compared to 90.6% with CECT.
Despite the advances in various non-invasive testing available to determine the degree of fibrosis, liver biopsy remains the gold standard method for accurate assessment in diagnosis and staging. As first described in 1883 by Dr. Paul Ehlrich, percutaneous liver biopsy (PC-LB) has evolved from a mere percussion method to an “image-guided” technique in the last ten years using ultrasound/CT imaging to accomplish it. However, despite image guidance, the risk of bleeding persists, occurring in up to 0.6% of cases, including other adverse events like pneumothorax and gallbladder puncture and even death in a few cases[46]. The transjugular technique of liver biopsy, introduced in 1973, can help reduce this risk, especially in patients with underlying coagulopathy. However, this method also carried added risks of local site hematoma, intraperitoneal bleeding, arrhythmia and carotid puncture[47].
EUS guided liver biopsy (EUS-LB) initiated as early as 2007 is currently emerging as a cost-effective, safe and well-tolerated procedure and helps in more representative sampling. The American Association for the Study of Liver Diseases recommends a tissue length of at least 2-3 cm with ≥ 11 or more complete portal tracts (CPTs) for determining the adequacy of liver biopsy samples[48]. The mean tissue length and CPTs for EUS-LB, PC-LB and TJLB, as shown in various studies is 36.9, 9 and 17.7 mm, and 7.7, 13.5 and 6.8 mm, respectively[49,50]. This can be achieved with a regular 19 G EUS-FNA needle (71). Similarly, a meta-analysis on EUS-LB revealed that pooled successful histological diagnosis was achieved in 93.9% of cases. Adverse event rates with EUS-LB, PC-LC and TJLB were 2.3%, 0.09%-3.1% and 0.56%-6.5%, respectively[48,51,52]. A recent meta-analysis between the three techniques revealed that EUS-LB was comparable to PC-LB in terms of CPT, but tissue length was better with the former with no complication rates[53].
EUS-LB has been used in the setting where patients undergo other endoscopic procedures such as screening of the biliary tree, assessment of surrounding structures and lymph nodes and variceal screening in those not affected with ascites and obesity[50], thereby saving time and resources. Furthermore, EUS-LB is theoretically less painful as it does not require skin puncture, eliminates the need for breath-hold and allows visualization and avoidance of blood vessels even 1 mm in size and is suitable for anxious patients by using adequate sedation (Figure 7). Moreover, bilobar biopsy can be achieved, reducing sampling error and helping in better assessment of disease activity and fibrosis[54].
Technique: The technique of EUS-LB is described in Table 5.
| Pre-biopsy: The following workup is needed in all cases of liver biopsy |
| (1) Coagulation work up including platelet count, PT/INR and BT/CT; (2) Prior to the biopsy, the medications should be stopped as follows: anti-platelet medications 7 d, warfarin 5 d, heparin and related products discontinued 12-24 h prior to biopsy; and (3) Use of conscious sedation such as midazolam and nalbuphine or propofol as per operator’s preference or patient comfort |
| Procedural details of EUS-LB |
| (1) A linear array echoendoscope (Olympus GF-UCT180, Center Valley, United States) is generally used for the procedure; (2) Prior to the procedure, Doppler imaging is done to ensure that no vascular structures are present along the expected trajectory of the needle; (3) The EUS-LB can be performed using a 19 G EUS-FNA/FNB needle; (4) The left lobe is identified first, as that liver parenchyma which is a few centimeters below the gastro-esophageal junction with the scope torqued clockwise. The right lobe if needed to be biopsied, is accessed from the duodenal bulb. Two site biopsy can be undertaken at the discretion of the endosonographer; (5) A preferably long vessel free trajectory allowing free passage of the needle to a depth of at least 3 cm or more is usually selected; (6) For wet heparin suction, the stylet is removed and the needle is primed with a heparin flush and the suction syringe is reattached to the needle hub; (7) The needle is then introduced into the echoendoscope channel; (8) Once liver parenchymal penetration is achieved with the needle (around 1-2 cm), full suction is applied with the 20 mL vacuum syringe with fluid column; (9) One pass consists of a total of 4-5 to-and-fro needle motions using the fanning technique under direct EUS guided visualization of the tip of the needle. Two such passes are usually taken (maximum 10 actuations); and (10) The specimen is pushed from the needle directly into the formalin solution using the stylet or saline flush |
| Post-liver biopsy: The following instructions are to be followed in all cases post liver biopsy |
| (1) The patient post biopsy, irrespective of the type of procedure, is transferred to the post procedure recovery room and monitored as per the AASLD protocol[69]; (2) The minimum observation period is 2-4 h; (3) Post-procedure pain and need for analgesics to be noted and provided; and (4) Patient is asked to report adverse events at specific time intervals (as per institute policy) |
Future trends: In attempts to acquire better quality and quantity of specimens, various studies have been published on different needles and methods of executing a EUS-LB procedure. A recent RCT comparing a 19 G FNB needle (fork-tip) vs 19 G standard FNA needle yielded better results with the former (pre-processing length 2.09 cm vs 1.47 cm and more CPTs)[55]. In contrast, a recent meta-analysis showed the superiority of FNA needles over core biopsy needles in terms of better tissue acquisition[51]. Thus, 19 G FNA needle may be used for EUS-LB procedures except for the cases where immunohistochemistry and architecture characterization are warranted, in whom core biopsy needle may be used.
Mok et al[56] showed that the “wet heparin” suction technique had greater tissue yield compared to “dry suction” (aggregate specimen length 49.2 mm vs 23.9 mm; mean CPT count 7 vs 4). Thus, the combination of wet-heparinized suction and a 19-G second-generation (FNA/FNB) needle might help achieve better specimens with minimal fragmentation.
The various studies using EUS-LB (FNA/FNB) in patients with chronic liver disease are highlighted in Table 6. The average technical success and diagnostic yield for EUS-FNA and EUS-FNB-guided liver biopsy are 100% and 89.8%, respectively, with a complication rate of 3.3%, consisting entirely of minor events[70]. In addition, studies reporting the use of EUS-LB in patients with NAFLD (overall technical success rate 100%, yield 96.8% with 7.7% complication rate) are reported in Supplementary Table 1.
| Ref. | Design of the study | Patients | Technical success (%) | Diagnostic yield (%) | Specimen length (median, range) (mm) | CPT (median, range) | Needle used for EUS-LB | Needle passes (median) | Complications, n (%) |
| EUS-FNA guided liver biopsy | |||||||||
| Pineda et al[57] | Retrospective | 110 | 100 | 98 | 38 (24-81) | 14 (9-27) | 19 G | - | 0 |
| Shuja et al[58] | Retrospective | 69 | 100 | 100 | 45.8 (mean) | 10.84 (mean) | 19 G | 3 | 0 |
| Stavropoulos et al[50] | Prospective case series | 22 | 100 | 91 | 36.9 (2-184.6) | 9 (1-73) | 19 G | 2 (1-3) | 0 |
| Diehl et al[59] | Prospective non randomized | 110 | 100 | 98 | 38 (0-203) | 14 (0-68) | 19 G | 1.5 (1-2) | 1 (0.9) (mild bleeding) |
| Gor et al[60] | Retrospective case series | 10 | 100 | 100 | 13 (6-23) | 8 (6-15) | 19 G | - | 0 |
| EUS-FNB guided liver biopsy | |||||||||
| Shah et al[61] | Retrospective | 24 | 100 | 96 | 65.6 (17-167.4) | 32.5 (5-85) | 19 G (SharkCore) | 2 (1-3) | 2 (8.3) |
| Nieto et al[62] | Retrospective | 165 | 100 | 100 | 60 (43-80) | 18 (13-24) | 19 G (SharkCore) | 1 | 3 (1.8) |
| Mathew[63] | Case report | 2 | 100 | 100 | - | - | 19 G (QuickCore) | - | 0 |
| Ching et al[55] | Prospective (RCT) | 20; 20 | 100; 100 | 100; 100 | 114 (mean); 153.2 (mean) | 16.5 (6-38); 38 (0-81) | 19 G (FNA); 19 G (Acquire) | -- | 8 (40); 7 (35) |
| Mok et al[56] | Prospective (RCT) | 40; 40 | 100; 100 | 88; 68 | -; - | -; - | 19 G (FNA); 22 G (SharkCore) | -; - | 0; 1 (2.5) |
| Patel et al[64] | Retrospective | 30; 50; 28; 27 | 100; 100; 100; 100 | 66.7; 46; 82.1; 81.5 | 1.8 (mean); 4.7 (mean); 1.9 (mean); 8.4 (mean) | 6.9 (mean); 3 (mean); 7.3 (mean); 16.9 (mean) | Acquire 22 G; QuickCore 19 G; ProCore 19 G; Expect 19 G | -; -; -; - | -; -; -; - |
| Gleeson et al[65] | Retrospective | 9 | 100 | 100 | 13 (8-28) | 7 (5-8) | 19 G (QuickCore) | 2 (1-3) | 0 |
| DeWitt et al[66] | Prospective case series | 21 | 100 | 90.5 | 9 (1-23) | 2 (0-10) | 19 G (QuickCore) | 3 (1-4) | 0 |
| Nakai et al[67] | Case report | 1 | 100 | 100 | 15 | 8 | ProCore 19 G | 0 | |
| Sey et al[68] | Prospective cross sectional study | 45; 30 | 100; 100 | 73.3; 96.7 | 9 (0-25); 20 (5-60) | 2 (0-15); 5 (0-24) | QuickCore 19 G; ProCore 19 G | 3; 2 | 2 (4.4); 0 |
| Hasan et al[69] | Prospective (RCT) | 40 | 100 | 100 | 55 (44.5-68) | 42 (28.5-53) | Acquire 22 G | - | 6 (15) |
Symptomatic liver cysts, abscesses and bilomas may require drainage. Traditionally, these were approached through surgical or interventional radiology using percutaneous catheter drainage (PCD). Recently, EUS guidance has been used to drain simple intrahepatic cysts of varied etiologies, liver abscesses and bilomas. EUS guided drainage may be superior to PCD as it enables a one-step approach, leading to internal drainage and thus avoiding the complications of catheter dislodgement, pericatheter leak, multiple interventions and movement restrictions.
EUS guided treatment of hepatic cysts: The most frequent liver cysts encountered for drainage via EUS include simple hepatic cysts and intrahepatic pancreatic pseudocysts. Those located in the left lobe of the liver or the caudate lobe can be drained via EUS guidance. PCD would be preferred for right lobe cysts as it is difficult to access the right lobe in the duodenal bulb with an unstable scope position. Therapies offered by EUS include fine-needle aspiration, ethanol lavage and transmural stent placement.
In a retrospective study by Lee et al[71], 19 cases of hepatic cysts were treated by PCD and EUS guided ethanol lavage and reported a 97.5% reduction in cyst volume at 11.5 mo of follow-up in the PCD group and a 100% reduction at 15 mo in the EUS arm. The studies on EUS guided treatment of hepatic cysts are outlined in Supplemen
EUS guided drainage of liver abscess: Traditionally, pyogenic and amoebic liver abscesses have been drained by PCD with a high technical success rate. However, EUS guided drainage of liver abscesses is a promising new approach, especially for difficult-to-reach locations. Additionally, the advantage of internal drainage with a single-step procedure and easy access from the stomach makes transmural drainage of left and caudate lobe abscess convenient.
The technique was first described by Seewald et al[72], who reported complete resolution 4 weeks post-procedure. Literature on EUS guided drainage is limited to retrospective case series only in which the majority have been drained with double pigtail plastic stents[73-75]. Recently, data are emerging on the use of fully covered self-expandable metal stents (SEMS)[76] for the same. Ogura et al[77] reported retrospective comparative data on EUS vs PCD guided abscess drainage wherein EUS guided abscess drainage (EUS-AD) cases showed greater clinical success (100% vs 89%) with shorter hospital stay (21 d vs 41 d). Studies on EUS-AD are listed in Sup
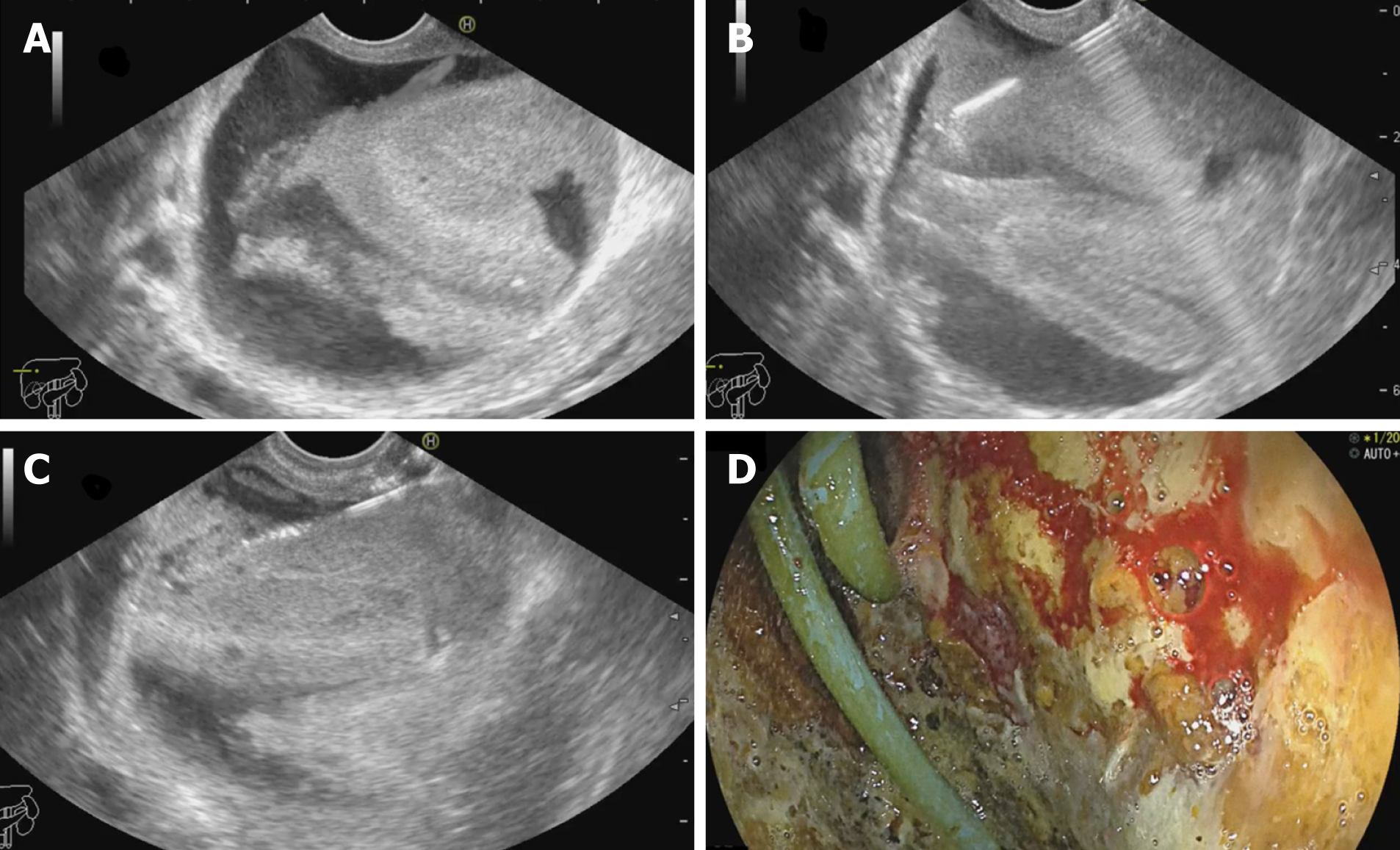
EUS guided drainage of biloma: Biloma is defined as a well-demarcated collection of bile outside the biliary tree, which can be extrahepatic or intrahepatic, encapsulated or without a capsule[78]. It is most frequently caused by iatrogenic biliary tree injury during cholecystectomy. It has been traditionally managed with PCD or surgery. However, large bilomas in opposition to the gastric wall can be taken up for transmural drainage (Figure 8). Similar to EUS-AD, earlier plastic stents were utilized for the same, but now SEMS has been in vogue for biloma drainage with excellent results. Post drainage, such patients should be evaluated to determine the need for endoscopic retrograde cholangiopancreatography, or sphincterotomy with/out biliary stenting or surgery[79]. Studies on EUS guided drainage of bilomas are described in Supplementary Table 4.
Despite it being a point of contention, EUS guided drainage of intrahepatic lesions (cysts, abscesses and bilomas) is an upcoming promising technique and may be considered in conditions where PCD is not amenable or has failed.
A thrilling offshoot of EUS guided therapeutic interventions has been EUS guided local treatment of tumor lesions (both pancreatic and hepatic tumors)[80]. EUS-guided tumor management is a new experimental application that has shown promise in reaching difficult lesions (left lobe, caudate lobe), provided a rescue option in refractory cases, and has potential to improve quality of life by minimizing systemic side effects[81,82]. This procedure has been extensively studied in cases of pancreatic neoplasm, but its role in hepatic tumors (primary or metastatic) is still in its infancy.
Various techniques of EUS guided liver tumor management have been described.
Percutaneous injection of ablative injections is most commonly used worldwide to manage HCC, although EUS guided fine needle injection can be performed using acetic acid or ethanol (pure alcohol 95%-99%)[83]. Its advantage is that it enables real-time imaging during delivery of ethanol to the tumorous lesion and thus can help avoid collateral damage.
Initial case reports using 22 G and 25 G FNA needles have been reported with excellent technical success and complete resolution of HCC[84-87]. For example, Nakaji et al[87] reported a high-resolution rate at 31 mo in 12 cases of caudate lobe HCC, whereas Jiang et al[88] only showed 30% complete resolution at 12 mo. This technique has also been evaluated for the treatment of hepatic metastasis from pancreatic adenocarcinoma[89].
Radiofrequency ablation: Radiofrequency ablation (RFA) uses a high-frequency alternating current (375 kHz to 500 kHz) and is minimally invasive with good tolerability[90]. It can be delivered percutaneously, intraoperatively, via an endoluminal approach or endosonographic (transmural) route. Emerging data on the latter have resulted in its application in cases wherein the percutaneous approach fails. Obesity, tumor nodules in the left lobe or caudate lobe, deep-seated and sub
Laser ablation by neodymium:yttrium-aluminum-garnet: Neodymium:yttrium-aluminum-garnet (Nd-YAG) is a type of LITT (laser interstitial thermotherapy) wherein laser waves are introduced through the EUS needle directly into the tumor tissue leading to cell apoptosis and eventual necrosis. Only two human studies have been published so far for the treatment of HCC. Di Matteo et al[95] reported complete HCC resolution in 2 mo in a case of previously failed caudate lobe HCC. Similarly, Jiang et al[96] reported resolution at 3 mo with an encouraging safety profile.
Cryotherapy ablation: Cryotherapy ablation (CYA) destroys tissue through multiple freezing-thawing cycles leading to osmotic dehydration and injury to the intracellular structures and cell death[90]. No human study exists for its use in liver lesions. However, a single animal study showed the efficacy of a hybrid EUS-RFA and cryoderm device in a porcine model[97].
High-intensity focused ultrasound: This is a non-invasive technique that causes tissue necrosis via heat generation and acoustic cavitation by the formation and collapse of bubbles produced by intense USG waves[90]. Its use in EUS has only been tested in animal models[98,99], showing complete necrosis of the lesions with no immediate side effects.
This treatment modality has been used for various cancers with the advantage of less toxicity to surrounding tissues over external beam radiotherapy[81,90]. For example, EUS guided brachytherapy with permanent seed placement of Iodine (I125) or palladium (Pd103) has been performed for head-neck, esophageal, and pancreatic cancer[100-102]. In addition, Jiang et al[88] have used EUS guided I125 seed im
Studies on EUS guided liver tumor treatments are outlined in Table 7.
| EUS guided treatment | Study design | Patients | Location of the lesion | Technical success (%) | Response to therapy | Complications |
| Ethanol ablation in HCC | ||||||
| Nakaji et al[84] | Case report | 1 | Segment 8 | 100 | Complete | 0 |
| Lisotti et al[85] | Case report | 1 | Segment 2 | 100 | Complete | 0 |
| Nakaji et al[86] | Case report | 1 | Segment 3 | 100 | Complete | 0 |
| Nakaji et al[87] | Retrospective | 12 | Caudate lobe | 100 | Complete | 2 (16.7%) |
| Jiang et al[88] | RCT | 10 | Left lobe | 92 | Partial (30%) | 0 |
| Alcohol ablation in liver metastasis | ||||||
| Barclay et al[89] | Case report | 1 | Left lobe | 100 | Complete | Self-limited sub-capsular hematoma |
| Hu et al[103] | Case report | 1 | Left lobe | 100 | Complete | Low grade fever |
| RFA (radiofrequency ablation) in HCC | ||||||
| Armellini et al[91] | Case report | 1 | Left lobe | 100 | Complete | None |
| Attili et al[92] | Case report | 1 | Segment 3 | 100 | Complete | None |
| de Nucci et al[93] | Case report | 1 | Segment 2-3-4b | 100 | 70% reduction | None |
| Ablation by Nd-YAG | ||||||
| Di Matteo et al[95] | Case report | 1 | Caudate lobe | 100 | Complete | 0 |
| Jiang et al[96] | Prospective | 10 | Left lobe | 100 | Complete | 0 |
| Brachytherapy (Iodine-125) | ||||||
| Jiang et al[88] | RCT | 13 | Left lobe | 92 | Near complete | 0 |
The presence of real-time, high-resolution sonographic imaging with Doppler, along with the relative proximity of the gastrointestinal tract to the major blood vessels in the abdomen and the mediastinum, has led to a growing interest to explore the role of EUS in the field of vascular interventions. EUS may be preferred over the percuta
EUS guided vascular intervention in patients with PHTN has been well established in managing varices (esophageal, gastric, duodenal, and ectopic).
Management of esophageal varices: Endoscopic variceal band ligation (EVL) has been the standard treatment of esophageal varices (EV) (both primary and secondary prophylaxis). However, re-bleeding rates of 15%-65% have been reported due to the failure to obliterate perforating veins and collaterals feeding the varices[105]. Lahoti et al[106] described the first report of EUS guided sclerotherapy in 5 cases, wherein sclerosant (sodium morrhuate) was injected under EUS guidance (2-4 mL per injection site) directed at the perforating vessels as determined by color Doppler with complete eradication of the varices. An RCT comparing EUS vs direct sclerotherapy revealed no difference in both arms[107]. Thus, although EUS carries a theoretical advantage for identifying the feeders, more studies are needed to assess its practical clinical benefit.
Management of gastric varices: In patients with PHTN, gastric varices (GV) are present in up to 20% with a 50%-65% re-bleeding rate[108]. Endoscopic injection of CYA glue for GVs has been the treatment of choice since its first description in 1986 but is still prone to a re-bleeding rate of 40%[109]. In the current era of EUS guided vascular interventions, management of GVs by EUS has many conceptual advantages, both diagnostic and therapeutic such as[110,111]: (1) A higher detection rate (6 times) over conventional endoscopy; (2) Greater success in differentiating varices from thick gastric folds; (3) Confirmation of the cessation of blood flow post-treatment; (4) Real-time varix visualization and hence accurate delivery of hemostatic agent to the varix; and (5) Targeted treatment for feeder vessels.
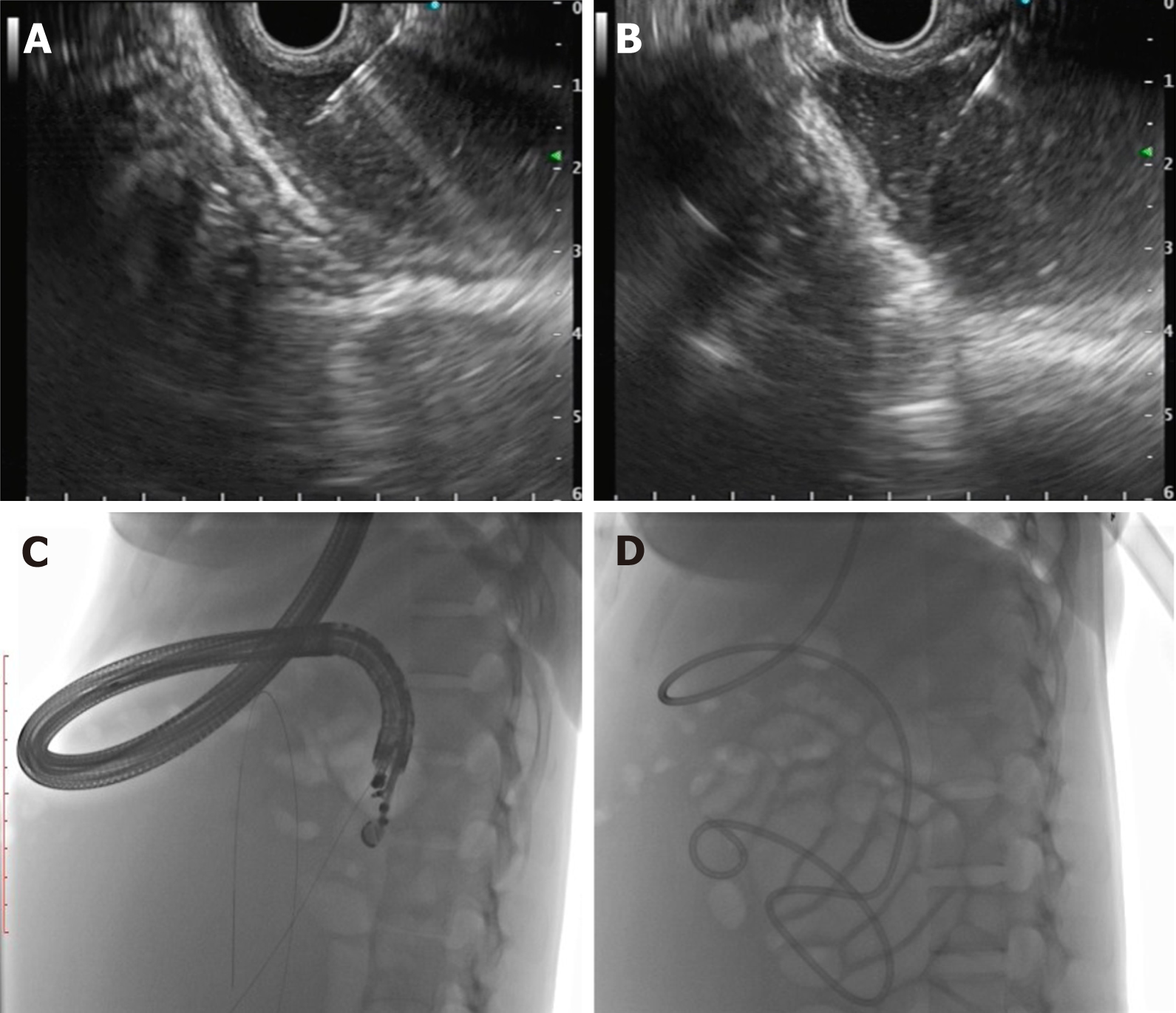
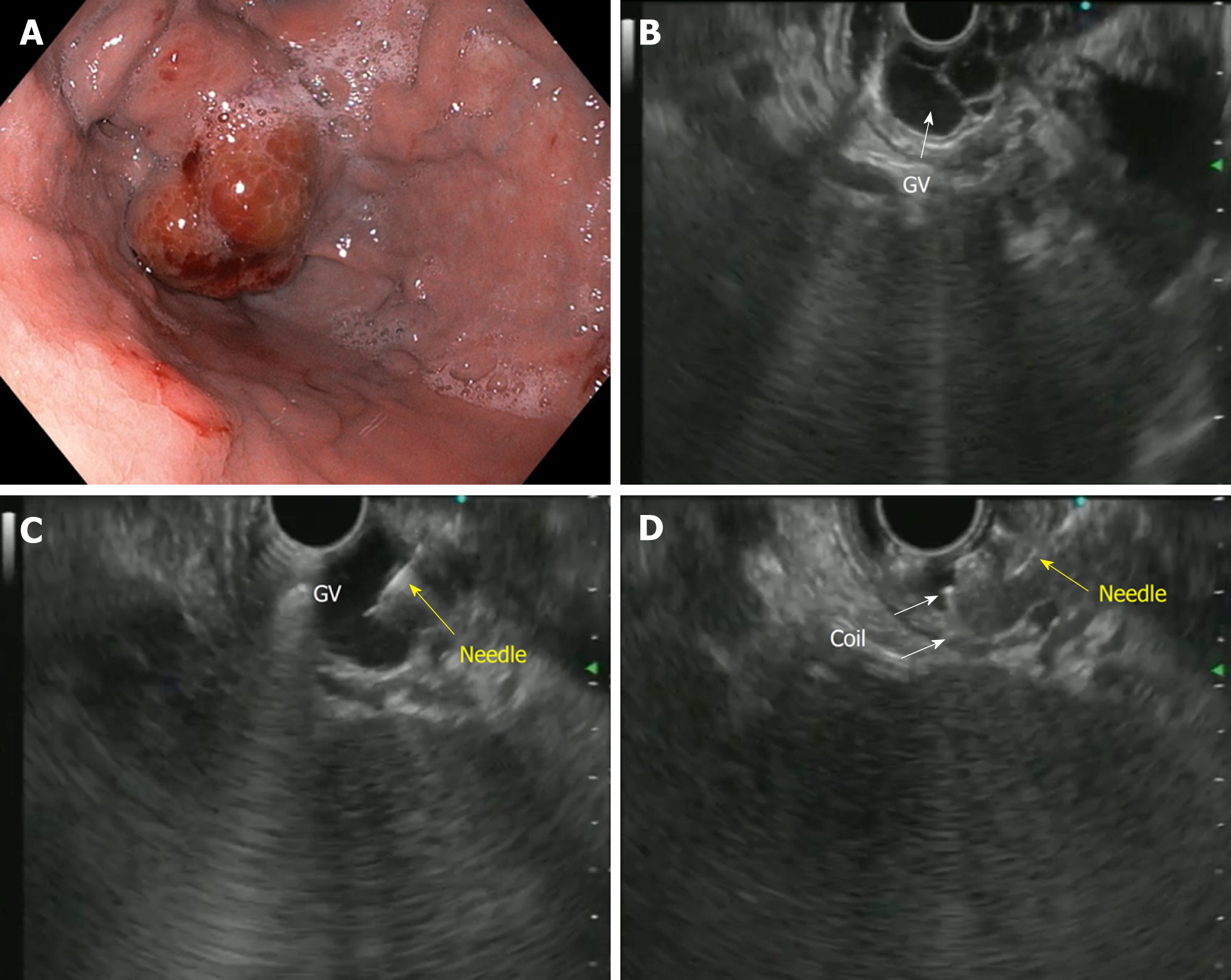
The first description of EUS guided CYA injection in GVs was given by Romero-Castro et al[111] and Lee et al[112]. To reduce the chances of embolization with CYA, stainless steel coils alone or in combination with CYA glue have been introduced. The advantage is three-pronged: additive hemostasis and varix obliteration, reducing the volume of glue needed and acting as a scaffold to retain the glue within the varix, thereby decreasing embolization. Various studies, including RCTs, have favored coil over glue. Bhat et al[113] reported a complete obliteration in 93% with only 3% re-bleeding rates using coils and glue combination. Similarly, two RCTs and a meta-analysis have reported the combination therapy of coil with glue to be superior to either agent alone[114-116]. Newer treatments of utilizing coils with gelatin sponge and sclerotherapy or isolated thrombin injection have been reported in various case series and have shown good results[117-119].
The technical steps of the EUS guided coil and glue placement for the obliteration of GV are outlined in Table 8 and Figure 9.
| Pre-procedure requirements |
| (1) All procedures are done under the cover of pre/peri-procedural antibiotics; (2) Patient is usually fasted for 4-6 h before the procedure; and (3) Adequate resuscitation of the patient, in case of active bleeding is ensured, prior to the procedure |
| Technical aspects |
| (1) The echoendoscope is usually positioned either in the distal esophagus or the gastric fundus; (2) Water is filled intra-luminally in the fundus. This enables a good acoustic coupling for better visualization of the gastric varices. Adequate examination of the fundus, the intramural varices and the feeder vessels is carried out; (3) The approach can be trans-esophageal or transgastric, wherein the trans-esophageal route is given preference; (4) EUS-guided coil and glue embolization is usually performed using a 22 G/19 G (gauge) FNA needle. The size of the coil is determined by the short axis of the diameter of the varix; (5) After puncture of the varix, blood is aspirated to confirm the location. This is followed by flushing of the needle with saline; (6) The coils are then deployed into the varix using the stylet as a pusher. Once the coils are deployed, flushing of the needle is done with normal saline; (7) After coil deployment, 1-2 mL of cyanoacrylate glue is injected over 30-45 s followed by rapid flushing with saline; and (8) Once, the varix is obliterated, visualized by absence of flow on color Doppler, the sheath of the needle is advanced beyond the endoscope tip for 2-3 cm before withdrawing the scope. This avoids contact of glue with the endoscope tip. The sample aspirated is sent for routine cytological assessment as well as for any additional tests that might be needed |
| Post procedure |
| (1) The patients are kept under observation for 12 h; (2) Repeat EUS can be done after 2 d to look for residual varices; and (3) Follow-up EUS can be performed at 1- and 3-mo intervals |
Use of EUS in the prediction of re-bleeding from EV/GV: EUS with Doppler has a higher sensitivity for detecting esophageal and GV than upper GI endoscopy and can also be used to predict re-bleeding. Certain parameters can help guide us in this direction[120,121]: (1) EUS can help in demonstrating collaterals or feeders, a strong indicator to a future occurrence of a re-bleed; (2) Hematocystic spots on EVs identified as saccular aneurysms on EUS is associated with a high risk of variceal rupture; (3) Digital image analysis on EUS can help to determine the cross-sectional area of EVs in the distal esophagus and a cutoff of 0.45 cm2 has a sensitivity of 83% for future re-bleeding; and (4) Para-esophageal diameter after EVL is a better recurrence predictor (cutoff 4 mm has a 70.6% sensitivity).
Thus, there is a huge prospect for using EUS in PHTN, namely in the evaluation of vascular changes of the digestive wall, hemodynamic assessment by measurement of PV pressure gradient, management of variceal bleeding and re-bleeding prediction and currently liquid biopsy via PV sampling. Nonetheless, despite the diversity of possible uses, more data on efficacy and safety are warranted.
The PV can be accessed from both the stomach and duodenum and is in very close juxtaposition with the tip of the echoendoscope. The most frequent location to target is the intrahepatic PV through the hepatic parenchyma. The other less commonly used technique is the extrahepatic PV via the duodenum[122,123].
Technique of the procedure: After confirming the vascular structure with color Doppler and pulse-wave verification, PV puncture is done via the EUS-FNA needle. Studies have shown that 25 G needle causes the least trauma. The trans-gastric, trans-hepatic approach is safer than the trans-duodenal approach. CO2 is better than using iodine as a contrast (allows better PV visualization and easier intravascular administration through the small-caliber FNA needle). After PV puncture, on withdrawal of the needle, the track is monitored with color Doppler to check for bleeding. In cases of blood flow being identified, the needle is kept in place until the flow has stopped[122,123].
Animal studies: The first case of PV access was reported in 2004 by Lai et al[124], wherein a EUS guided trans-duodenal access to extrahepatic PV was adopted with a 22 G FNA needle in 21 swine models, proving the technical feasibility of the procedure. Thereafter, PV angiography was reported for the first time in 2007 by Magno et al[125], wherein autopsies revealed no injuries with a 25 G needle and a hematoma with 19 G needle. Subsequently, Giday et al[123,126] reported trans-hepatic access to the PV with a 25 G needle.
Measurement of PHTN is useful in determining the stage, progression, prognosis and complications of cirrhosis. Currently, the standard practice of measuring the portal pressure gradient (PPG) is the percutaneous route. However, both direct PV access and hepatic venous pressure gradient (HVPG) measurement are invasive procedures and have high complication rates. Moreover, HVPG correlated poorly in presinusoidal PHTN cases. Therefore, EUS guided PPG can be performed to overcome these difficulties. Moreover, additional analyses such as assessment of varices and liver biopsy can be carried out in the same sitting. The technique of PPG measurement and the studies (human and animal models) on the same are shown in Supplementary Tables 5 and 6.
TIPS has an established role in managing PHTN-related complications like variceal bleeding (pre-emptive or rescue) and refractory ascites. EUS-guided TIPS creation in a live porcine model (8 cases) was first described by Buscaglia et al[127], wherein the hepatic vein (HV) and PV were sequentially punctured, and a metal stent was inserted with the distal end in the PV and proximal end in the HV. In addition, Binmoeller and Shah[128], and Schulman et al[129] have both reported using a lumen apposing metal stent (LAMS) in porcine models for the same purpose.
“Liquid biopsy” for hepatobiliary malignancies is gaining momentum in view of the PV harboring circulating tumor cells (CTCs) from the primary tumor. These CTCs are the forerunners of future metastasis of solid organ cancers and help predict the development of liver metastasis[130]. They have been inconsistently found in the peripheral blood due to hepatic sequestration. They reflect tumor signature, help in prognostic stratification, and potentially form organoids for future tumor study.
Catenacci et al[131] reported the first human study of PV sampling wherein a 19 G FNA needle was used to sample the PV as four 7.5 mL aliquots of blood. CTCs were detected in 100% cases from the PV vs 4 (22.2%) cases from peripheral blood. Liu et al[132] reported similar findings in cases of advanced pancreatic cancer (100% detection of CTCs in PV vs 54% in peripheral blood). Besides these, further studies are needed to establish the clinical utility of EUS guided liquid biopsies.
The presence of malignant PV thrombosis (PVT) usually portends a poor prognosis. Therefore, differentiating bland and malignant thrombus needs FNA confirmation. Various case reports have suggested the use of EUS guided FNA of the PVT by overcoming the complications encountered via the percutaneous route[133-135] with excellent results.
Both systemic palliative chemotherapy and transarterial microbead injection into the hepatic artery for diffuse liver metastasis are fraught with complications. However, Faigel et al[136] reported the feasibility of EUS guided PV injection chemotherapy in 24 porcine models using drug-eluting microbeads and nanoparticles. In comparison with systemic injection, systemic levels were halved, but the hepatic concentration of drugs was doubled. Human studies are warranted for the same.
Preoperative PV embolization before liver resection in hepatobiliary malignancies induces affected lobe atrophy and ultimately hypertrophy in the functional liver[137]. However, preliminary studies in the animal model by Matthes et al[138] and Park et al[139] using EUS guided PV embolization using ethylene-vinyl alcohol copolymer and coil with CYA glue embolization, respectively, reported high success rates.
EUS directed PV access has opened up avenues for stent placement via this route in PV occlusion or thrombosis. Park et al[140] reported 100% technical success (all uncovered stents) in 6 swine models.
Photodynamic therapy (PDT) is a commonly used modality for treating malignant biliary obstruction, requiring pretreatment with a photosensitizer followed by exposure to selective tissue wavelength of light-generating singlet oxygen species (tissue necrosis from 6-40 mm depth)[141]. Preliminary animal studies exist on the use of EUS guided PDT on the porcine pancreas[141,142] and pancreaticobiliary malignancies (with lesions in the caudate lobe)[143].
Stereotactic body radiation therapy demands high targeting accuracy to minimize toxicity to surrounding organs. Placement of fiducial markers can help localize and track the target and can be placed via a percutaneous or surgical approach. EUS guided fiducial marker placement has come into the forefront for targeting even deeper abdominal lesions not amenable via standard means[144,145]. However, no studies exist on its use in liver malignancies.
Artificial intelligence (AI) is a prediction technique using mathematical algorithms to create automated learning and recognize patterns in the fed data. Artificial neural network (ANN) and deep learning (DL) are powerful machine-learning-based tools used to provide high yield predictions and are being used more and more in the medical field to aid in diagnosis. Just like its widespread use in the field of endoscopic diagnosis of polyps and other lesions, AI has also found its place in the arena of diagnostic EUS. Studies have used ANN for the interpretation of EUS-elastography and CE-EUS[146]. However, to date, only two studies have used DL for EUS image analysis. With the availability of additional studies, AI can add to the diagnostic armamentarium of EUS and lead to much better accuracy.
Hepatologists have always turned to radiologists for imaging and intervention of various liver-related conditions. However, with the expansion of this intersection of endoscopy in EUS and hepatology, the field of “Endo-hepatology” may soon evolve into a sub-specialty with hepatologists trained in interventional EUS. Starting from EUS-guided liver biopsy to PV interventions, the merger of EUS and hepatology seems to show invigorating scope in the future. However, more studies are needed to establish the safety and efficacy of these newer modalities in regular mainstream practice.
Provenance and peer review: Invited article; Externally peer reviewed.
Corresponding Author's Membership in Professional Societies: American Society for Gastrointestinal Endoscopy, No. 159772; American College of Gastroenterology, No. 55899.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Matsubara S S-Editor: Gao CC L-Editor: Webster JR P-Editor: Zhang YL
| 1. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1812] [Article Influence: 258.9] [Reference Citation Analysis (2)] |
| 2. | Nguyen PT, Chang KJ. EUS in the detection of ascites and EUS-guided paracentesis. Gastrointest Endosc. 2001;54:336-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Varadarajulu S, Drelichman ER. EUS-guided therapeutic paracentesis. Gastrointest Endosc. 2008;67:758-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Lee YT, Ng EK, Hung LC, Chung SC, Ching JY, Chan WY, Chu WC, Sung JJ. Accuracy of endoscopic ultrasonography in diagnosing ascites and predicting peritoneal metastases in gastric cancer patients. Gut. 2005;54:1541-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | DeWitt J, LeBlanc J, McHenry L, McGreevy K, Sherman S. Endoscopic ultrasound-guided fine-needle aspiration of ascites. Clin Gastroenterol Hepatol. 2007;5:609-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Chin MA. EUS-guided paracentesis and ascitic fluid analysis. Endosc Ultrasound. 2018;7:223-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Levy MJ, Abu Dayyeh BK, Fujii LL, Clayton AC, Reynolds JP, Lopes TL, Rao AS, Clain JE, Gleeson FC, Iyer PG, Kendrick ML, Rajan E, Topazian MD, Wang KK, Wiersema MJ, Chari ST. Detection of peritoneal carcinomatosis by EUS fine-needle aspiration: impact on staging and resectability (with videos). Gastrointest Endosc. 2015;81:1215-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Que Y, Wang X, Tao C, Zhang Y, Wan W, Chen B. Peritoneal metastases: evaluation with contrast-enhanced ultrasound. Abdom Imaging. 2011;36:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | ASGE Standards of Practice Committee; Khashab MA, Chithadi KV, Acosta RD, Bruining DH, Chandrasekhara V, Eloubeidi MA, Fanelli RD, Faulx AL, Fonkalsrud L, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Shaukat A, Wang A, Cash BD. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2015;81:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 241] [Article Influence: 24.1] [Reference Citation Analysis (2)] |
| 10. | Kaushik N, Khalid A, Brody D, McGrath K. EUS-guided paracentesis for the diagnosis of malignant ascites. Gastrointest Endosc. 2006;64:908-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Suzuki R, Irisawa A, Bhutani MS, Hikichi T, Takagi T, Shibukawa G, Sato A, Sato M, Ikeda T, Watanabe K, Nakamura J, Annangi S, Tasaki K, Obara K, Ohira H. An automated spring-loaded needle for endoscopic ultrasound-guided abdominal paracentesis in cancer patients. World J Gastrointest Endosc. 2014;6:55-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Chang KJ, Albers CG, Nguyen P. Endoscopic ultrasound-guided fine needle aspiration of pleural and ascitic fluid. Am J Gastroenterol. 1995;90:148-150. [PubMed] |
| 13. | Köck R, Jürgensen C, Fischer-Lampsatis R. Endosonography-guided drainage of loculated malignant ascites using double-pigtail plastic stents. Endosc Int Open. 2018;6:E902-E906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Romero-Castro R, Jimenez-Garcia VA, Boceta-Osuna J, Castilla-Guerra L, Pellicer-Bautista F, Caunedo-Alvarez A, Herrerias-Gutierrez JM, Romero-Gómez M, Giovannini M. Endoscopic ultrasound-guided placement of plastic pigtail stents for the drainage of refractory malignant ascites. Endosc Int Open. 2017;5:E1096-E1099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Romero-Castro R. EUS-Guided Drainage of Refractory Malignant Ascites. [accessed 2021 Feb 15]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03550560 ClinicalTrials.gov Identifier: NCT03550560. |
| 16. | Wardeh R, Lee JG, Gu M. Endoscopic ultrasound-guided paracentesis of ascitic fluid: a morphologic study with ultrasonographic correlation. Cancer Cytopathol. 2011;119:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Bhatia V, Hijioka S, Hara K, Mizuno N, Imaoka H, Yamao K. Endoscopic ultrasound description of liver segmentation and anatomy. Dig Endosc. 2014;26:482-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Friedberg SR, Lachter J. Endoscopic ultrasound: Current roles and future directions. World J Gastrointest Endosc. 2017;9:499-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Campos S, Poley JW, van Driel L, Bruno MJ. The role of EUS in diagnosis and treatment of liver disorders. Endosc Int Open. 2019;7:E1262-E1275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Iglesias-García J, Lariño-Noia J, Domínguez-Muñoz JE. New Imaging Techniques: Endoscopic Ultrasound-Guided Elastography. Gastrointest Endosc Clin N Am. 2017;27:551-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Alvarez-Sánchez MV, Napoléon B. Contrast-enhanced harmonic endoscopic ultrasound imaging: basic principles, present situation and future perspectives. World J Gastroenterol. 2014;20:15549-15563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Schulman AR, Lin MV, Rutherford A, Chan WW, Ryou M. A Prospective Blinded Study of Endoscopic Ultrasound Elastography in Liver Disease: Towards a Virtual Biopsy. Clin Endosc. 2018;51:181-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Marques S, Carmo J, Túlio MA, Bispo M, Matos L, Chagas C. Diagnostic Performance of Real-Time Elastography in the Assessment of Advanced Fibrosis in Chronic Hepatitis C. GE Port J Gastroenterol. 2016;23:13-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Tu CH, Li J, Wang CY, Zhou L, Ma Y, Gao M, Wang J, Zeng QM, Lu W. [Diagnostic value of endoscopic ultrasonography, fibroscan, acoustic radiation pulse imaging, serological index, and their combination for early stage liver cirrhosis]. Zhonghua Gan Zang Bing Zazhi. 2019;27:615-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 25. | Ichim VA, Chira RI, Mircea PA, Nagy GA, Crisan D, Socaciu MA. Accuracy of endoscopic ultrasound-guided biopsy of focal liver lesions. Med Ultrason. 2020;22:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Singh P, Erickson RA, Mukhopadhyay P, Gopal S, Kiss A, Khan A, Ulf Westblom T. EUS for detection of the hepatocellular carcinoma: results of a prospective study. Gastrointest Endosc. 2007;66:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Singh P, Mukhopadhyay P, Bhatt B, Patel T, Kiss A, Gupta R, Bhat S, Erickson RA. Endoscopic ultrasound vs CT scan for detection of the metastases to the liver: results of a prospective comparative study. J Clin Gastroenterol. 2009;43:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Fujii-Lau LL, Abu Dayyeh BK, Bruno MJ, Chang KJ, DeWitt JM, Fockens P, Forcione D, Napoleon B, Palazzo L, Topazian MD, Wiersema MJ, Chak A, Clain JE, Faigel DO, Gleeson FC, Hawes R, Iyer PG, Rajan E, Stevens T, Wallace MB, Wang KK, Levy MJ. EUS-derived criteria for distinguishing benign from malignant metastatic solid hepatic masses. Gastrointest Endosc. 2015;81:1188-96.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Sandulescu L, Padureanu V, Dumitrescu C, Braia N, Streba CT, Gheonea DI, Cazacu S, Ciurea T, Rogoveanu I, Saftoiu A. A pilot study of real time elastography in the differentiation of focal liver lesions. Curr Health Sci J. 2012;38:32-35. [PubMed] |
| 30. | Kitano M, Kamata K. Contrast-enhanced harmonic endoscopic ultrasound: Future perspectives. Endosc Ultrasound. 2016;5:351-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Liu M, Lin MX, Lu MD, Xu ZF, Zheng KG, Wang W, Kuang M, Zhuang WQ, Xie XY. Comparison of contrast-enhanced ultrasound and contrast-enhanced computed tomography in evaluating the treatment response to transcatheter arterial chemoembolization of hepatocellular carcinoma using modified RECIST. Eur Radiol. 2015;25:2502-2511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Nguyen P, Feng JC, Chang KJ. Endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration (FNA) of liver lesions. Gastrointest Endosc. 1999;50:357-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 123] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | TenBerge J, Hoffman BJ, Hawes RH, Van Enckevort C, Giovannini M, Erickson RA, Catalano MF, Fogel R, Mallery S, Faigel DO, Ferrari AP, Waxman I, Palazzo L, Ben-Menachem T, Jowell PS, McGrath KM, Kowalski TE, Nguyen CC, Wassef WY, Yamao K, Chak A, Greenwald BD, Woodward TA, Vilmann P, Sabbagh L, Wallace MB. EUS-guided fine needle aspiration of the liver: indications, yield, and safety based on an international survey of 167 cases. Gastrointest Endosc. 2002;55:859-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 34. | DeWitt J, LeBlanc J, McHenry L, Ciaccia D, Imperiale T, Chappo J, Cramer H, McGreevy K, Chriswell M, Sherman S. Endoscopic ultrasound-guided fine needle aspiration cytology of solid liver lesions: a large single-center experience. Am J Gastroenterol. 2003;98:1976-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Hollerbach S, Willert J, Topalidis T, Reiser M, Schmiegel W. Endoscopic ultrasound-guided fine-needle aspiration biopsy of liver lesions: histological and cytological assessment. Endoscopy. 2003;35:743-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | McGrath K, Brody D, Luketich J, Khalid A. Detection of unsuspected left hepatic lobe metastases during EUS staging of cancer of the esophagus and cardia. Am J Gastroenterol. 2006;101:1742-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Crowe DR, Eloubeidi MA, Chhieng DC, Jhala NC, Jhala D, Eltoum IA. Fine-needle aspiration biopsy of hepatic lesions: computerized tomographic-guided vs endoscopic ultrasound-guided FNA. Cancer. 2006;108:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 38. | Prachayakul V, Aswakul P, Kachintorn U. EUS guided fine needle aspiration cytology of liver nodules suspicious for malignancy: yields, complications and impact on management. J Med Assoc Thai. 2012;95 Suppl 2:S56-S60. [PubMed] |
| 39. | Oh D, Seo DW, Hong SM, Jun JH, Song TJ, Park DH, Son BK, Lee SS, Lee SK, Kim MH. The usefulness of contrast-enhanced harmonic EUS-guided fine-needle aspiration for evaluation of hepatic lesions (with video). Gastrointest Endosc. 2018;88:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Lee YN, Moon JH, Kim HK, Choi HJ, Choi MH, Kim DC, Lee TH, Cha SW, Kim SG, Kim YS. Usefulness of endoscopic ultrasound-guided sampling using core biopsy needle as a percutaneous biopsy rescue for diagnosis of solid liver mass: Combined histological-cytological analysis. J Gastroenterol Hepatol. 2015;30:1161-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Chon HK, Yang HC, Choi KH, Kim TH. Endoscopic Ultrasound-Guided Liver Biopsy Using a Core Needle for Hepatic Solid Mass. Clin Endosc. 2019;52:340-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Ichim VA, Chira RI, Mircea PA. Diagnostic yield of endoscopic ultrasound-guided biopsy of focal liver lesions. Med Pharm Rep. 2019;92:15-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Choi HJ, Moon JH, Kim HK, Lee YN, Lee TH, Cha SW, Cho YD, Park SH. KRAS mutation analysis by next-generation sequencing in endoscopic ultrasound-guided sampling for solid liver masses. J Gastroenterol Hepatol. 2017;32:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Suzuki R, Shin D, Richards-Kortum R, Coghlan L, Bhutani MS. In vivo cytological observation of liver and spleen by using high-resolution microendoscopy system under endoscopic ultrasound guidance: A preliminary study using a swine model. Endosc Ultrasound. 2016;5:239-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Minaga K, Kitano M, Nakai A, Omoto S, Kamata K, Yamao K, Takenaka M, Tsurusaki M, Chikugo T, Matsumoto I, Chiba Y, Watanabe T, Kudo M. Improved detection of liver metastasis using Kupffer-phase imaging in contrast-enhanced harmonic EUS in patients with pancreatic cancer (with video). Gastrointest Endosc. 2021;93:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 46. | Tapper EB, Lok AS. Use of Liver Imaging and Biopsy in Clinical Practice. N Engl J Med. 2017;377:756-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 281] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 47. | Rösch J, Hanafee WN, Snow H. Transjugular portal venography and radiologic portacaval shunt: an experimental study. Radiology. 1969;92:1112-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 217] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 48. | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology. 2009;49:1017-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1449] [Cited by in RCA: 1580] [Article Influence: 98.8] [Reference Citation Analysis (1)] |
| 49. | Cholongitas E, Senzolo M, Standish R, Marelli L, Quaglia A, Patch D, Dhillon AP, Burroughs AK. A systematic review of the quality of liver biopsy specimens. Am J Clin Pathol. 2006;125:710-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 50. | Stavropoulos SN, Im GY, Jlayer Z, Harris MD, Pitea TC, Turi GK, Malet PF, Friedel DM, Grendell JH. High yield of same-session EUS-guided liver biopsy by 19-gauge FNA needle in patients undergoing EUS to exclude biliary obstruction. Gastrointest Endosc. 2012;75:310-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 51. | Mohan BP, Shakhatreh M, Garg R, Ponnada S, Adler DG. Efficacy and safety of EUS-guided liver biopsy: a systematic review and meta-analysis. Gastrointest Endosc. 2019;89:238-246.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 52. | Kalambokis G, Manousou P, Vibhakorn S, Marelli L, Cholongitas E, Senzolo M, Patch D, Burroughs AK. Transjugular liver biopsy--indications, adequacy, quality of specimens, and complications--a systematic review. J Hepatol. 2007;47:284-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 263] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 53. | Mony S, Shah I, Vyas N, Sofi A, Das A. Su1375 Meta-analysis of EUS guided liver biopsy in comparison with percutaneous and transjugular liver biopsy for the diagnosis of parenchymal liver disease. Gastrointest Endosc. 2018;87:AB328-AB329. |
| 54. | Khurana S, Butt W, Khara HS, Johal AS, West SF, Chen ZE, Berger AL, Diehl DL. Bi-lobar liver biopsy via EUS enhances the assessment of disease severity in patients with non-alcoholic steatohepatitis. Hepatol Int. 2019;13:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 55. | Ching-Companioni RA, Diehl DL, Johal AS, Confer BD, Khara HS. 19 G aspiration needle vs 19 G core biopsy needle for endoscopic ultrasound-guided liver biopsy: a prospective randomized trial. Endoscopy. 2019;51:1059-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 56. | Mok SRS, Diehl DL, Johal AS, Khara HS, Confer BD, Mudireddy PR, Kirchner HL, Chen ZE. A prospective pilot comparison of wet and dry heparinized suction for EUS-guided liver biopsy (with videos). Gastrointest Endosc. 2018;88:919-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 57. | Pineda JJ, Diehl DL, Miao CL, Johal AS, Khara HS, Bhanushali A, Chen EZ. EUS-guided liver biopsy provides diagnostic samples comparable with those via the percutaneous or transjugular route. Gastrointest Endosc. 2016;83:360-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 58. | Shuja A, Alkhasawneh A, Fialho A, Shukri A, Harris C, Smotherman C, Malespin M, de Melo SW Jr. Comparison of EUS-guided vs percutaneous and transjugular approaches for the performance of liver biopsies. Dig Liver Dis. 2019;51:826-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 59. | Diehl DL, Johal AS, Khara HS, Stavropoulos SN, Al-Haddad M, Ramesh J, Varadarajulu S, Aslanian H, Gordon SR, Shieh FK, Pineda-Bonilla JJ, Dunkelberger T, Gondim DD, Chen EZ. Endoscopic ultrasound-guided liver biopsy: a multicenter experience. Endosc Int Open. 2015;3:E210-E215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 60. | Gor N, Salem SB, Jakate S, Patel R, Shah N, Patil A. Histological adequacy of EUS-guided liver biopsy when using a 19-gauge non-Tru-Cut FNA needle. Gastrointest Endosc. 2014;79:170-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 61. | Shah ND, Sasatomi E, Baron TH. Endoscopic Ultrasound-guided Parenchymal Liver Biopsy: Single Center Experience of a New Dedicated Core Needle. Clin Gastroenterol Hepatol. 2017;15:784-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 62. | Nieto J, Khaleel H, Challita Y, Jimenez M, Baron TH, Walters L, Hathaway K, Patel K, Lankarani A, Herman M, Holloman D, Saab S. EUS-guided fine-needle core liver biopsy sampling using a novel 19-gauge needle with modified 1-pass, 1 actuation wet suction technique. Gastrointest Endosc. 2018;87:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 63. | Mathew A. EUS-guided routine liver biopsy in selected patients. Am J Gastroenterol. 2007;102:2354-2355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 64. | Patel HK, Saxena R, Rush N, Patel SK, Dasari CS, Mneimneh W, Quickery A, Rahal MA, Temnykh L, DeWitt J, Al-Haddad M. A Comparative Study of 22G vs 19G Needles for EUS-Guided Biopsies for Parenchymal Liver Disease: Are Thinner Needles Better? Dig Dis Sci. 2021;66:238-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 65. | Gleeson FC, Clayton AC, Zhang L, Clain JE, Gores GJ, Rajan E, Smyrk TC, Topazian MD, Wang KK, Wiersema MJ, Levy MJ. Adequacy of endoscopic ultrasound core needle biopsy specimen of nonmalignant hepatic parenchymal disease. Clin Gastroenterol Hepatol. 2008;6:1437-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 66. | Dewitt J, McGreevy K, Cummings O, Sherman S, Leblanc JK, McHenry L, Al-Haddad M, Chalasani N. Initial experience with EUS-guided Tru-cut biopsy of benign liver disease. Gastrointest Endosc. 2009;69:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 67. | Nakai Y, Samarasena JB, Iwashita T, Park DH, Lee JG, Hu KQ, Chang KJ. Autoimmune hepatitis diagnosed by endoscopic ultrasound-guided liver biopsy using a new 19-gauge histology needle. Endoscopy. 2012;44 Suppl 2 UCTN:E67-E68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 68. | Sey MS, Al-Haddad M, Imperiale TF, McGreevy K, Lin J, DeWitt JM. EUS-guided liver biopsy for parenchymal disease: a comparison of diagnostic yield between two core biopsy needles. Gastrointest Endosc. 2016;83:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 69. | Hasan MK, Kadkhodayan K, Idrisov E, Ali S, Rafiq E, Ben-Ami Shor D, Abdel-Jalil A, Navaneethan U, Bang J, Varadarajulu S, Hawes R, Pernicone P. Endoscopic ultrasound-guided liver biopsy using a 22-G fine needle biopsy needle: a prospective study. Endoscopy. 2019;51:818-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 70. | Sbeit W, Kadah A, Mahamid M, Pellicano R, Mari A, Khoury T. A State-of-the-Art Review on the Evolving Utility of Endoscopic Ultrasound in Liver Diseases Diagnosis. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 71. | Lee S, Seo DW, Paik WH, Park DH, Lee SS, Lee SK, Kim MH. Ethanol lavage of huge hepatic cysts by using EUS guidance and a percutaneous approach. Gastrointest Endosc. 2014;80:1014-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 72. | Seewald S, Imazu H, Omar S, Groth S, Seitz U, Brand B, Zhong Y, Sikka S, Thonke F, Soehendra N. EUS-guided drainage of hepatic abscess. Gastrointest Endosc. 2005;61:495-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 73. | Noh SH, Park DH, Kim YR, Chun Y, Lee HC, Lee SO, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided drainage of hepatic abscesses not accessible to percutaneous drainage (with videos). Gastrointest Endosc. 2010;71:1314-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 74. | Itoi T, Ang TL, Seewald S, Tsuji S, Kurihara T, Tanaka R, Itokawa F. Endoscopic ultrasonography-guided drainage for tuberculous liver abscess drainage. Dig Endosc. 2011;23 Suppl 1:158-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 75. | Medrado BF, Carneiro FO, Vilaça TG, Gouveia TS, Frazão MS, de Moura EG, Sakai P, Otoch JP, Artifon EL. Endoscopic ultrasound-guided drainage of giant liver abscess associated with transgastric migration of a self-expandable metallic stent. Endoscopy. 2013;45 Suppl 2:E331-E332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 76. | Alcaide N, Vargas-Garcia AL, de la Serna-Higuera C, Sancho Del Val L, Ruiz-Zorrilla R, Perez-Miranda M. EUS-guided drainage of liver abscess by using a lumen-apposing metal stent (with video). Gastrointest Endosc. 2013;78:941-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 77. | Ogura T, Masuda D, Saori O, Wataru T, Sano T, Okuda A, Miyano A, Kitano M, Abdel-Aal UM, Takeuchi T, Fukunishi S, Higuchi K. Clinical Outcome of Endoscopic Ultrasound-Guided Liver Abscess Drainage Using Self-Expandable Covered Metallic Stent (with Video). Dig Dis Sci. 2016;61:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 78. | Della Valle V, Eshja E, Bassi EM. Spontaneous biloma: a case report. J Ultrasound. 2015;18:293-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 79. | Sandha GS, Bourke MJ, Haber GB, Kortan PP. Endoscopic therapy for bile leak based on a new classification: results in 207 patients. Gastrointest Endosc. 2004;60:567-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 139] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 80. | Sbeit W, Kadah A, Mari A, Mahamid M, Khoury T. A Comprehensive Narrative Review on the Evolving Role of Endoscopic Ultrasound in Focal Solid Liver Lesions Diagnosis and Management. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 81. | Lakhtakia S, Seo DW. Endoscopic ultrasonography-guided tumor ablation. Dig Endosc. 2017;29:486-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 82. | Chua T, Faigel DO. Endoscopic Ultrasound-Guided Ablation of Liver Tumors. Gastrointest Endosc Clin N Am. 2019;29:369-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 83. | Schoppmeyer K, Weis S, Mössner J, Fleig WE. Percutaneous ethanol injection or percutaneous acetic acid injection for early hepatocellular carcinoma. Cochrane Database Syst Rev. 2009;CD006745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 84. | Nakaji S, Hirata N, Iwaki K, Shiratori T, Kobayashi M, Inase M. Endoscopic ultrasound (EUS)-guided ethanol injection for hepatocellular carcinoma difficult to treat with percutaneous local treatment. Endoscopy. 2012;44 Suppl 2 UCTN:E380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 85. | Lisotti A, Piscaglia F, Fusaroli P. Contrast-enhanced harmonic endoscopic ultrasound-guided ethanol injection for a small hepatocellular carcinoma. Endoscopy. 2019;51:E317-E318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 86. | Nakaji S, Hirata N, Kobayashi M, Shiratori T, Sanagawa M. Endoscopic ultrasonography-guided ethanol injection as a treatment for ruptured hepatocellular carcinoma in the left hepatic lobe. Endoscopy. 2015;47 Suppl 1:E558-E560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 87. | Nakaji S, Hirata N, Mikata R, Kobayashi M, Shiratori T, Ogasawara S, Ooka Y, Tsuyuguchi T, Yamaguchi T, Yokosuka O. Clinical outcomes of endoscopic ultrasound-guided ethanol injection for hepatocellular carcinoma in the caudate lobe. Endosc Int Open. 2016;4:E1111-E1115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 88. | Jiang TA, Deng Z, Tian G, Zhao QY, Wang WL. Efficacy and safety of endoscopic ultrasonography-guided interventional treatment for refractory malignant left-sided liver tumors: a case series of 26 patients. Sci Rep. 2016;6:36098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 89. | Barclay RL, Perez-Miranda M, Giovannini M. EUS-guided treatment of a solid hepatic metastasis. Gastrointest Endosc. 2002;55:266-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 90. | Nault JC, Sutter O, Nahon P, Ganne-Carrié N, Séror O. Percutaneous treatment of hepatocellular carcinoma: State of the art and innovations. J Hepatol. 2018;68:783-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 283] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 91. | Armellini E, Leutner M, Stradella D, Ballarè M, Occhipinti P. EUS-guided radiofrequency ablation: an option for the extrapancreatic region. Endosc Ultrasound. 2018;7:282-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 92. | Attili F, Boškoski I, Bove V, Familiari P, Costamagna G. EUS-guided radiofrequency ablation of a hepatocellular carcinoma of the liver. VideoGIE. 2018;3:149-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 93. | de Nucci G, Della Corte C, Reati R, Imperatore N, Arena I, Larghi A, Manes G. Endoscopic ultrasound-guided radiofrequency ablation for hepatocellular carcinoma in cirrhosis: a case report test for efficacy and future perspectives. Endosc Int Open. 2020;8:E1713-E1716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 94. | Hines-Peralta A, Hollander CY, Solazzo S, Horkan C, Liu ZJ, Goldberg SN. Hybrid radiofrequency and cryoablation device: preliminary results in an animal model. J Vasc Interv Radiol. 2004;15:1111-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 95. | Di Matteo F, Grasso R, Pacella CM, Martino M, Pandolfi M, Rea R, Luppi G, Silvestri S, Zardi E, Costamagna G. EUS-guided Nd:YAG laser ablation of a hepatocellular carcinoma in the caudate lobe. Gastrointest Endosc. 2011;73:632-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 96. | Jiang T, Tian G, Bao H, Chen F, Deng Z, Li J, Chai W. EUS dating with laser ablation against the caudate lobe or left liver tumors: a win-win proposition? Cancer Biol Ther. 2018;19:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 97. | Carrara S, Arcidiacono PG, Albarello L, Addis A, Enderle MD, Boemo C, Neugebauer A, Campagnol M, Doglioni C, Testoni PA. Endoscopic ultrasound-guided application of a new internally gas-cooled radiofrequency ablation probe in the liver and spleen of an animal model: a preliminary study. Endoscopy. 2008;40:759-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 98. | Pioche M, Lafon C, Constanciel E, Vignot A, Birer A, Gincul R, Lépilliez V, Prat F, Roman S, Chapelon JY, Saurin JC, Ponchon T. High-intensity focused ultrasound liver destruction through the gastric wall under endoscopic ultrasound control: first experience in living pigs. Endoscopy. 2012;44 Suppl 2 UCTN:E376-E377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 99. | Li T, Khokhlova T, Maloney E, Wang YN, D'Andrea S, Starr F, Farr N, Morrison K, Keilman G, Hwang JH. Endoscopic high-intensity focused US: technical aspects and studies in an in vivo porcine model (with video). Gastrointest Endosc. 2015;81:1243-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 100. | Lah JJ, Kuo JV, Chang KJ, Nguyen PT. EUS-guided brachytherapy. Gastrointest Endosc. 2005;62:805-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 101. | Martínez-Monge R, Subtil JC, López-Picazo JM. Transoesophageal endoscopic-ultrasonography-guided 125I permanent brachytherapy for unresectable mediastinal lymphadenopathy. Lancet Oncol. 2006;7:781-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 102. | Sun S, Xu H, Xin J, Liu J, Guo Q, Li S. Endoscopic ultrasound-guided interstitial brachytherapy of unresectable pancreatic cancer: results of a pilot trial. Endoscopy. 2006;38:399-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 103. | Hu YH, Tuo XP, Jin ZD, Liu Y, Guo Y, Luo L. Endoscopic ultrasound (EUS)-guided ethanol injection in hepatic metastatic carcinoma: a case report. Endoscopy. 2010;42 Suppl 2:E256-E257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 104. | Samarasena JB, Chang KJ. Endoscopic Ultrasound-Guided Interventions for the Measurement and Treatment of Portal Hypertension. Gastrointest Endosc Clin N Am. 2019;29:311-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 105. | Helmy A, Hayes PC. Review article: current endoscopic therapeutic options in the management of variceal bleeding. Aliment Pharmacol Ther. 2001;15:575-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 106. | Lahoti S, Catalano MF, Alcocer E, Hogan WJ, Geenen JE. Obliteration of esophageal varices using EUS-guided sclerotherapy with color Doppler. Gastrointest Endosc. 2000;51:331-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 107. | de Paulo GA, Ardengh JC, Nakao FS, Ferrari AP. Treatment of esophageal varices: a randomized controlled trial comparing endoscopic sclerotherapy and EUS-guided sclerotherapy of esophageal collateral veins. Gastrointest Endosc. 2006;63:396-402; quiz 463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 108. | Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16:1343-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 846] [Article Influence: 25.6] [Reference Citation Analysis (42)] |
| 109. | Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1229] [Cited by in RCA: 1209] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 110. | Iwase H, Suga S, Morise K, Kuroiwa A, Yamaguchi T, Horiuchi Y. Color Doppler endoscopic ultrasonography for the evaluation of gastric varices and endoscopic obliteration with cyanoacrylate glue. Gastrointest Endosc. 1995;41:150-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 111. | Romero-Castro R, Pellicer-Bautista FJ, Jimenez-Saenz M, Marcos-Sanchez F, Caunedo-Alvarez A, Ortiz-Moyano C, Gomez-Parra M, Herrerias-Gutierrez JM. EUS-guided injection of cyanoacrylate in perforating feeding veins in gastric varices: results in 5 cases. Gastrointest Endosc. 2007;66:402-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 112. | Lee YT, Chan FK, Ng EK, Leung VK, Law KB, Yung MY, Chung SC, Sung JJ. EUS-guided injection of cyanoacrylate for bleeding gastric varices. Gastrointest Endosc. 2000;52:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 128] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 113. | Bhat YM, Weilert F, Fredrick RT, Kane SD, Shah JN, Hamerski CM, Binmoeller KF. EUS-guided treatment of gastric fundal varices with combined injection of coils and cyanoacrylate glue: a large U.S. experience over 6 years (with video). Gastrointest Endosc. 2016;83:1164-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 114. | Robles-Medranda C, Oleas R, Valero M, Puga-Tejada M, Baquerizo-Burgos J, Ospina J, Pitanga-Lukashok H. Endoscopic ultrasonography-guided deployment of embolization coils and cyanoacrylate injection in gastric varices vs coiling alone: a randomized trial. Endoscopy. 2020;52:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 115. | Lôbo MRA, Chaves DM, DE Moura DTH, Ribeiro IB, Ikari E, DE Moura EGH. Safety and efficacy of EUS-guided coil plus cyanoacrylate versus conventional cyanoacrylate technique in the treatment of gastric varices: a randomized controlled trial. Arq Gastroenterol. 2019;56:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 116. | McCarty TR, Bazarbashi AN, Hathorn KE, Thompson CC, Ryou M. Combination therapy versus monotherapy for EUS-guided management of gastric varices: A systematic review and meta-analysis. Endosc Ultrasound. 2020;9:6-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (1)] |
| 117. | Irisawa A, Shibukawa G, Hoshi K, Yamabe A, Sato A, Maki T, Yoshida Y, Yamamoto S, Obara K. Endoscopic ultrasound-guided coil deployment with sclerotherapy for isolated gastric varices: Case series of feasibility, safety, and long-term follow-up. Dig Endosc. 2020;32:1100-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 118. | Bazarbashi AN, Wang TJ, Thompson CC, Ryou M. Endoscopic ultrasound-guided treatment of gastric varices with coil embolization and absorbable hemostatic gelatin sponge: a novel alternative to cyanoacrylate. Endosc Int Open. 2020;8:E221-E227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 119. | Frost JW, Hebbar S. EUS-guided thrombin injection for management of gastric fundal varices. Endosc Int Open. 2018;6:E664-E668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 120. | Carneiro FO, Retes FA, Matuguma SE, Albers DV, Chaves DM, Dos Santos ME, Herman P, Chaib E, Sakai P, Carneiro D'Albuquerque LA, Maluf Filho F. Role of EUS evaluation after endoscopic eradication of esophageal varices with band ligation. Gastrointest Endosc. 2016;84:400-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 121. | Kuramochi A, Imazu H, Kakutani H, Uchiyama Y, Hino S, Urashima M. Color Doppler endoscopic ultrasonography in identifying groups at a high-risk of recurrence of esophageal varices after endoscopic treatment. J Gastroenterol. 2007;42:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 122. | Hashimoto R, Chang KJ. Endoscopic ultrasound guided hepatic interventions. Dig Endosc. 2021;33:54-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 123. | Giday SA, Clarke JO, Buscaglia JM, Shin EJ, Ko CW, Magno P, Kantsevoy SV. EUS-guided portal vein catheterization: a promising novel approach for portal angiography and portal vein pressure measurements. Gastrointest Endosc. 2008;67:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 124. | Lai L, Poneros J, Santilli J, Brugge W. EUS-guided portal vein catheterization and pressure measurement in an animal model: a pilot study of feasibility. Gastrointest Endosc. 2004;59:280-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 125. | Magno P, Ko CW, Buscaglia JM, Giday SA, Jagannath SB, Clarke JO, Shin EJ, Kantsevoy SV. EUS-guided angiography: a novel approach to diagnostic and therapeutic interventions in the vascular system. Gastrointest Endosc. 2007;66:587-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 126. | Giday SA, Ko CW, Clarke JO, Shin EJ, Magno P, Jagannath SB, Buscaglia JM, Kantsevoy SV. EUS-guided portal vein carbon dioxide angiography: a pilot study in a porcine model. Gastrointest Endosc. 2007;66:814-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 127. | Buscaglia JM, Dray X, Shin EJ, Magno P, Chmura KM, Surti VC, Dillon TE, Ducharme RW, Donatelli G, Thuluvath PJ, Giday SA, Kantsevoy SV. A new alternative for a transjugular intrahepatic portosystemic shunt: EUS-guided creation of an intrahepatic portosystemic shunt (with video). Gastrointest Endosc. 2009;69:941-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 128. | Binmoeller KF, Shah JN. Sa1428 EUS-guided transgastric intrahepatic portosystemic shunt using the axios stent. Gastrointest Endosc. 2011;73:AB167. |
| 129. | Schulman AR, Ryou M, Aihara H, Abidi W, Chiang A, Jirapinyo P, Sakr A, Ajeje E, Ryan MB, Thompson CC. EUS-guided intrahepatic portosystemic shunt with direct portal pressure measurements: a novel alternative to transjugular intrahepatic portosystemic shunting. Gastrointest Endosc. 2017;85:243-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 130. | Chapman CG, Waxman I. Portal-vein blood samples as a new diagnostic entity for pancreatic cancer. Expert Rev Gastroenterol Hepatol. 2016;10:665-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 131. | Catenacci DV, Chapman CG, Xu P, Koons A, Konda VJ, Siddiqui UD, Waxman I. Acquisition of Portal Venous Circulating Tumor Cells From Patients With Pancreaticobiliary Cancers by Endoscopic Ultrasound. Gastroenterology. 2015;149:1794-1803.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 132. | Liu X, Li C, Li J, Yu T, Zhou G, Cheng J, Li G, Zhou Y, Lou W, Wang X, Gong G, Liu L, Chen Y. Detection of CTCs in portal vein was associated with intrahepatic metastases and prognosis in patients with advanced pancreatic cancer. J Cancer. 2018;9:2038-2045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 133. | Lai R, Stephens V, Bardales R. Diagnosis and staging of hepatocellular carcinoma by EUS-FNA of a portal vein thrombus. Gastrointest Endosc. 2004;59:574-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 134. | Storch I, Gomez C, Contreras F, Schiff E, Ribeiro A. Hepatocellular carcinoma (HCC) with portal vein invasion, masquerading as pancreatic mass, diagnosed by endoscopic ultrasound-guided fine needle aspiration (EUS-FNA). Dig Dis Sci. 2007;52:789-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 135. | Moreno M, Gimeno-García AZ, Corriente MM, Nicolás-Pérez D, Brito-García A, García-Castro C, Quintero E. EUS-FNA of a portal vein thrombosis in a patient with a hidden hepatocellular carcinoma: confirmation technique after contrast-enhanced ultrasound. Endoscopy. 2014;46 Suppl 1 UCTN:E590-E591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 136. | Faigel DO, Lake DF, Landreth TL, Kelman CC, Marler RJ. EUS-guided portal injection chemotherapy for treatment of hepatic metastases: feasibility in the acute porcine model. Gastrointest Endosc. 2016;83:444-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 137. | Loffroy R, Favelier S, Chevallier O, Estivalet L, Genson PY, Pottecher P, Gehin S, Krausé D, Cercueil JP. Preoperative portal vein embolization in liver cancer: indications, techniques and outcomes. Quant Imaging Med Surg. 2015;5:730-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 138. | Matthes K, Sahani D, Holalkere NS, Mino-Kenudson M, Brugge WR. Feasibility of endoscopic ultrasound-guided portal vein embolization with Enteryx. Acta Gastroenterol Belg. 2005;68:412-415. [PubMed] |
| 139. | Park TY, Seo DW, Kang HJ, Song TJ, Park DH, Lee SS, Lee SK, Kim MH. Feasibility and safety of EUS-guided selective portal vein embolization with a coil and cyanoacrylate in a live porcine model. Endosc Ultrasound. 2018;7:389-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 140. | Park TY, Seo DW, Kang HJ, Cho MK, Song TJ, Park DH, Lee SS, Lee SK, Kim MH. Endoscopic ultrasonography-guided placement of a transhepatic portal vein stent in a live porcine model. Endosc Ultrasound. 2016;5:315-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 141. | Chan HH, Nishioka NS, Mino M, Lauwers GY, Puricelli WP, Collier KN, Brugge WR. EUS-guided photodynamic therapy of the pancreas: a pilot study. Gastrointest Endosc. 2004;59:95-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 142. | Yusuf TE, Matthes K, Brugge WR. EUS-guided photodynamic therapy with verteporfin for ablation of normal pancreatic tissue: a pilot study in a porcine model (with video). Gastrointest Endosc. 2008;67:957-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 143. | Choi JH, Oh D, Lee JH, Park JH, Kim KP, Lee SS, Lee YJ, Lim YS, Song TJ, Seo DW, Lee SK, Kim MH, Park DH. Initial human experience of endoscopic ultrasound-guided photodynamic therapy with a novel photosensitizer and a flexible laser-light catheter. Endoscopy. 2015;47:1035-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 144. | DiMaio CJ, Nagula S, Goodman KA, Ho AY, Markowitz AJ, Schattner MA, Gerdes H. EUS-guided fiducial placement for image-guided radiation therapy in GI malignancies by using a 22-gauge needle (with videos). Gastrointest Endosc. 2010;71:1204-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 145. | Varadarajulu S, Trevino JM, Shen S, Jacob R. The use of endoscopic ultrasound-guided gold markers in image-guided radiation therapy of pancreatic cancers: a case series. Endoscopy. 2010;42:423-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 146. | Kuwahara T, Hara K, Mizuno N, Haba S, Okuno N, Koda H, Miyano A, Fumihara D. Current status of artificial intelligence analysis for endoscopic ultrasonography. Dig Endosc. 2021;33:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |