Published online Oct 27, 2021. doi: 10.4254/wjh.v13.i10.1439
Peer-review started: April 25, 2021
First decision: June 15, 2021
Revised: June 19, 2021
Accepted: September 2, 2021
Article in press: September 2, 2021
Published online: October 27, 2021
Processing time: 180 Days and 1.4 Hours
Management of single small hepatocellular carcinoma (HCC) is straightforward with curative outcomes achieved by locoregional therapy or resection. Liver transplantation is often considered for multiple small or single large HCC. Management of two small HCC whether presenting synchronously or sequentially is less clear.
To define the outcomes of patients presenting with two small HCC.
Retrospective review of HCC databases from multiple institutions of patients with either two synchronous or sequential HCC ≤ 3 cm between January 2000 and March 2018. Primary outcomes were overall survival (OS) and transplant-free survival (TFS).
104 patients were identified (male n = 89). Median age was 63 years (interquartile range 58-67.75) and the most common aetiology of liver disease was hepatitis C (40.4%). 59 (56.7%) had synchronous HCC and 45 (43.3%) had sequential. 36 patients died (34.6%) and 25 were transplanted (24.0%). 1, 3 and 5-year OS was 93.0%, 66.1% and 62.3% and 5-year post-transplant survival was 95.8%. 1, 3 and 5-year TFS was 82.1%, 45.85% and 37.8%. When synchronous and sequential groups were compared, OS (1,3 and 5 year synchronous 91.3%, 63.8%, 61.1%, sequential 95.3%, 69.5%, 64.6%, P = 0.41) was similar but TFS was higher in the sequential group (1,3 and 5 year synchronous 68.5%, 37.3% and 29.7%, sequential 93.2%, 56.6%, 48.5%, P = 0.02) though this difference did not remain during multivariate analysis.
TFS in patients presenting with two HCC ≤ 3 cm is poor regardless of the timing of the second tumor. All patients presenting with two small HCC should be considered for transplantation.
Core Tip: Transplant-free survival in patients with 2 small hepatocellular carcinomas is poor, whether presenting synchronously or sequentially, and so should be considered for transplantation.
- Citation: Pham AD, Vaz K, Ardalan ZS, Sinclair M, Apostolov R, Gardner S, Majeed A, Mishra G, Kam NM, Patwala K, Kutaiba N, Arachchi N, Bell S, Dev AT, Lubel JS, Nicoll AJ, Sood S, Kemp W, Roberts SK, Fink M, Testro AG, Angus PW, Gow PJ. Clinical outcomes of patients with two small hepatocellular carcinomas. World J Hepatol 2021; 13(10): 1439-1449
- URL: https://www.wjgnet.com/1948-5182/full/v13/i10/1439.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i10.1439
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide and is the fourth leading cause of cancer-related mortality globally[1]. With uptake of standardized HCC surveillance programs, a greater number of patients are being diagnosed at earlier stages of disease when curative treatment is still possible[2-5]. In patients presenting with small tumors the probability of survival has progressively improved over recent decades with 5-year survival rates greater than 50% now frequently reported[6,7].
Curative therapies for HCC include surgical resection, percutaneous thermal ablation and liver transplantation. Within widely adopted eligibility criteria, transplantation may be considered when up to three individual HCC are present[8,9]. For solitary HCC, selection of therapy is based upon tumor size and location, in addition to severity of underlying hepatic dysfunction and portal hypertension. Surgical resection and ablative therapies have comparable survival rates in patients with solitary HCC less than 3 cm in diameter[10-13].
Whilst the guidelines are relatively clear for management of patients presenting with a single HCC ≤ 3 cm or three small HCC, there is little data to guide decision-making in patients who present with two small HCC, particularly when a second lesion appears sequentially after the index lesion. In this present study we sought to define the outcome of patients presenting with two HCC each up to 3 cm, in addition to exploring whether outcomes vary depending on whether tumors present either synchronously or sequentially (metachronously).
Retrospective data of all HCC diagnosed between 1st of January 2000 to 31st of March 2018 from four tertiary referral centres in Melbourne, Victoria were reviewed. Data were retrieved from site-specific prospectively collected electronic health records. Institutional ethics committee approval was obtained from participating sites prior to commencement at each centre.
Patients ≥ 18 years old with either two synchronous or two sequential HCC each up to 3 cm in size were identified. Patients with and without cirrhosis were included. Cirrhosis was established on standardized clinical, biochemical and radiologic grounds with or without histologic confirmation. In non-cirrhotic patients, HCC diagnosis was established histologically in all cases. HCC diagnoses between 2001 and 2012 were made according to 2001 European Association for the Study of the Liver (EASL) guidelines; all other lesions outside of these criteria required biopsy for diagnosis[14]. Diagnoses made beyond 2012 were in accordance with revised EASL criteria[2].
Patients who only ever had a single HCC or more than two tumors at diagnosis were excluded. Patients were also excluded if either of their first two HCC exceeded 3 cm or if they had radiologic evidence of vascular invasion or distant metastasis. Patients managed at more than one centre were only included once. After inclusion and exclusion criteria were applied, 104 patients were included in the study for analysis.
Data was collated from patient records into a central database and included demographics (age, gender), aetiology of chronic liver disease, the presence of or absence of cirrhosis, Child-Turcotte-Pugh (CTP) and model for end-stage liver disease (MELD)[15] scores, α-feto protein (AFP) level and radiologic tumor characteristics (total diameter of both lesions and diameter of largest individual lesion). Date of disease progression, the nature of progression (local recurrence, new disease, portal vein invasion or metastases) and date of death were recorded.
Treatment modalities and number of treatments were recorded. Treatment was administered according to multidisciplinary consensus at each institution. Locoregional therapies included percutaneous ablation (inclusive of microwave and radiofrequency ablation), percutaneous ethanol injection (PEI), transarterial chemoembolization (TACE) and irreversible electroporation. All cases being considered for transplantation were referred to the Victorian Liver Transplantation Unit at Austin Health. Patients with HCC waitlisted for transplantation in Victoria are not granted MELD exception points, with decisions on timing of transplant made at twice-weekly multidisciplinary meetings and priority given to patients with active tumor rather than cumulative time on the waitlist.
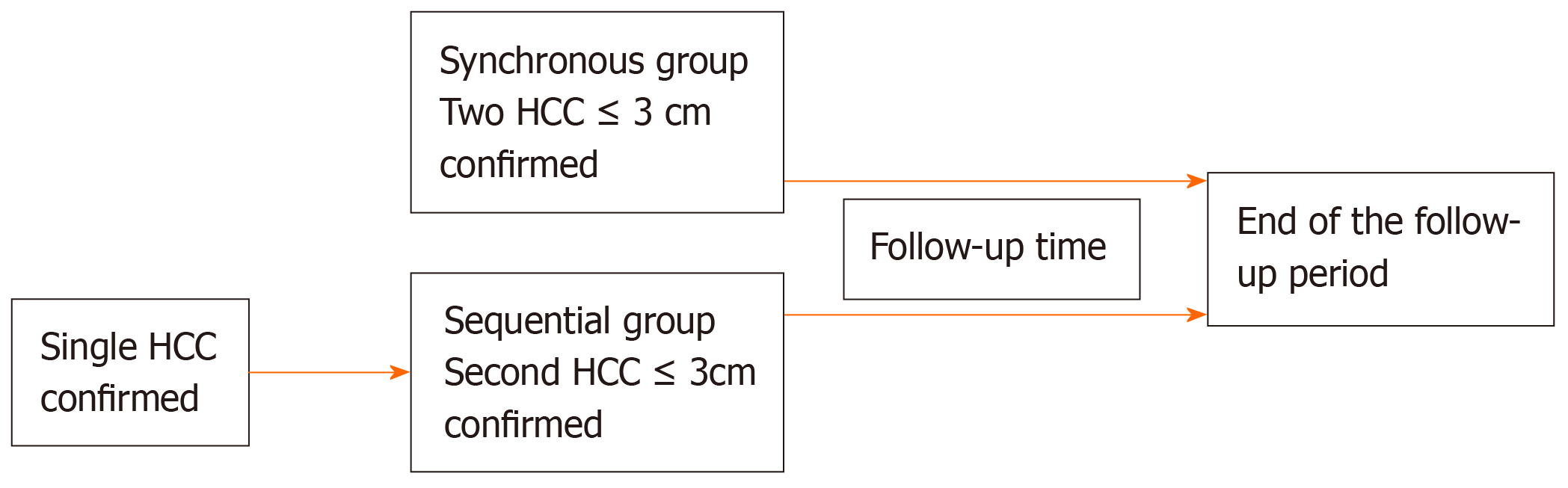
For the synchronous group, follow-up time began at the date two HCC were confirmed radiologically (Figure 1). For the sequential group, records of patients presenting with a single lesion were reviewed for occurrence of a second lesion. Follow-up time in the sequential group began at the time the second HCC was diagnosed (the first lesion may have received treatment; response to treatment whether it be partial or complete was not a requirement for inclusion). The primary outcome was overall survival (OS) which was calculated from the date of meeting inclusion criteria until death. Transplant-free survival (TFS) was calculated from the date of meeting inclusion criteria until liver transplantation or death without transplantation. Progression-free survival was from date of meeting inclusion criteria until either disease progression according to mRECIST[16] criteria or death without confirmed radiologic progression.
Demographic and continuous variables were assessed for normality and were accordingly presented as mean ± SD or median and interquartile range (IQR). Categorical variables were presented as frequencies with percentages. Baseline characteristics were compared between groups using one-way ANOVA and Mann-Whitney U test for normally-distributed and non-normally-distributed continuous variables, respectively. Pearson chi square test was used to compare categorical variables.
Survival was calculated by Kaplan-Meier analysis with all patients alive at the end of the follow-up period or transplanted before confirmed radiological progression being censored from survival analysis. Univariate analysis of prognostic factors was performed by log-rank testing; group comparisons included age ≤ 70 vs > 70 years, male vs female, aetiology of underlying liver disease, CTP class, MELD ≤ 14 vs > 14, AFP at diagnosis <10 or ≥ 10 μg/L, presentation with synchronous or sequential lesions both ≤ 3 cm and transplanted vs non-transplanted. Multivariate Cox proportional hazard analysis of univariate variables with a P value < 0.10 was performed and reported as hazard ratios (HR) with 95%CI. Significance tests were two-tailed with a P value < 0.05 considered statistically significant. All analyses were performed using SPSS version 22 (Armonk, NY: IBM Corp).
One hundred and four patients were identified as having two HCC and were followed up for a median of 2.54 years (IQR 2.73 years, range 0.08-13.67); only six patients (5.8%) had less than six months follow-up. Eighty-nine (85.6%) were male and the median age was 63 years (IQR 58-68). The most common cause of liver disease was chronic hepatitis C (n = 42, 40.4%) followed by chronic hepatitis B (n = 15, 14.4%). The majority were CTP score A (n = 66, 63.7%) and median MELD at diagnosis was 9.5 (IQR 7-13).
Baseline characteristics comparing synchronous vs sequential tumors are shown in Table 1. Fifty-nine patients (56.7%) had two synchronous HCC at inclusion, whilst forty-five (43.3%) had sequential lesions with the median time between index and sequential lesions 14 mo (IQR 7.5-29.5). There was no difference in follow-up time between the two groups (P = 0.54). Mean MELD score at diagnosis was the only statistically significant difference between the two groups, higher in the synchronous cohort (11 ± 7 vs 8 ± 5, P = 0.01). The median combined diameter of the two tumors in the synchronous group was not significantly different from the sequential group (3.8 cm vs 3.4 cm, P = 0.28).
| All (n = 104) | Synchronous group (n = 59) | Sequential group (n = 45) | P value | |
| Age, yr, median (IQR) | 63 (10) | 63 (10) | 63 (9) | 0.41 |
| Gender, n (%) | 0.08 | |||
| Male | 89 (85.6) | 51 (86.4) | 38 (84.4) | |
| Female | 15 (14.4) | 8 (13.6) | 7 (15.6) | |
| Aetiology, n (%) | 0.73 | |||
| Alcohol | 12 (11.5) | 8 (13.6) | 4 (8.9) | |
| HCV | 42 (40.4) | 22 (37.3) | 20 (44.4) | |
| HBV | 15 (14.4) | 8 (13.6) | 7 (15.6) | |
| NASH | 5 (4.8) | 4 (6.8) | 1 (2.2) | |
| Alcohol and HCV | 18 (17.3) | 9 (15.3) | 9 (20.0) | |
| Other1 | 12 (11.5) | 8 (13.6) | 4 (8.9) | |
| Cirrhosis status, n (%) | 0.07 | |||
| Non-cirrhotic | 10 (9.6) | 3 (5.1) | 7 (15.6) | |
| Cirrhotic | 94 (90.4) | 56 (94.9) | 38 (84.4) | |
| CTP class, n (%) | 0.1 | |||
| A | 66 (63.5) | 35 (59.3) | 31 (68.9) | |
| B | 25 (24.0) | 13 (22.0) | 12 (26.7) | |
| C | 13 (12.5) | 11 (18.6) | 2 (4.4) | |
| MELD, median (IQR) | 9.6 (6) | 11 (7) | 8 (5) | 0.01 |
| AFP (μg/L), median (IQR) | 9.6 (24.0) | 8.6 (26.0) | 10.4 (22.8) | 0.61 |
| Combined tumour diameter (cm), median (IQR) | 3.5 (1.7) | 3.8 (1.7) | 3.4 (1.2) | 0.28 |
| Transplanted, n (%) | 25 (24) | 18 (30.5) | 7 (15.6) | 0.08 |
| Death, n (%) | 36 (34.6) | 23 (39.0) | 13 (28.9) | 0.28 |
The most common single treatment for patients with synchronous HCC was TACE (32.2%) followed by percutaneous ablation (20.3%), whilst two patients (3.4%) had unsuccessful locoregional therapy due to technical limitations and received transplantation as their primary treatment modality (Supplementary Table 1). Percutaneous ablation was the commonest single treatment for index lesions in the sequential group (57.8%) followed by surgical resection (17.8%). As first line treatment, TACE was more commonly utilized in the synchronous group (32.2% vs 8.9%, P < 0.01), whilst percutaneous ablation was more common in the sequential group (57.8% vs 20.3%, P < 0.01). There was no significant difference in the rate of PEI or resection between the two groups (P = 0.25 and P = 0.16, respectively). Synchronous lesions were more frequently treated with two modalities upfront (30.5% vs 13.3%, P = 0.04). The second lesion in the sequential group was most frequently treated by percutaneous ablation (31.1%) followed by TACE (28.9%), with only three patients (6.67%) undergoing transplantation (Supplementary Table 2).
During the follow-up period, 25 patients (24%) were transplanted with median time to transplantation 12 mo (IQR 2.83). The only significant differences between transplanted and non-transplanted patients were CTP and MELD score (P < 0.01 for both) (Supplementary Table 3). Although a higher proportion of patients with synchronous HCC were transplanted compared to the sequential group (30.5% vs 15.6%), this did not reach statistical significance (P = 0.08).
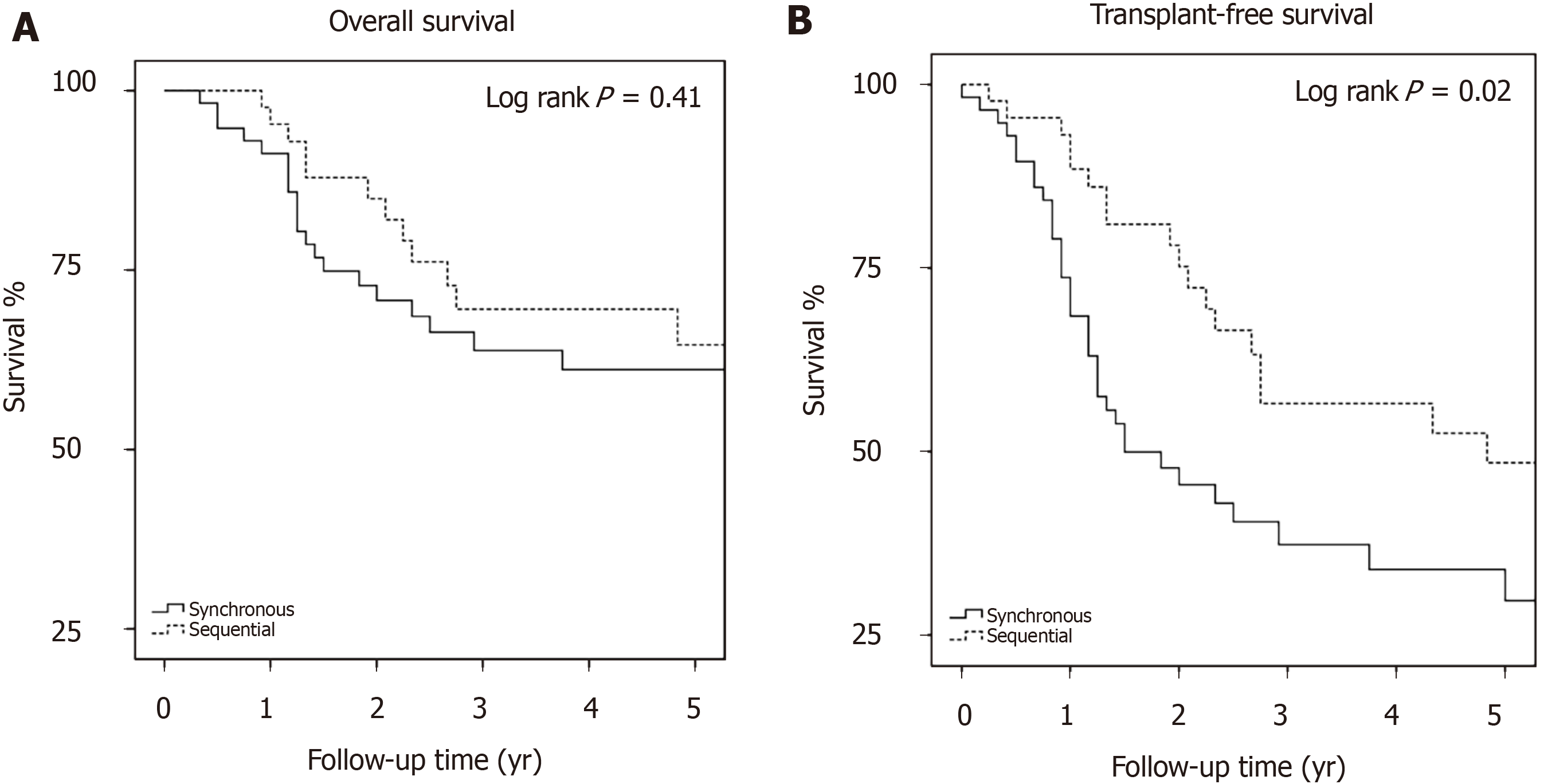
Overall survival: Thirty-six (34.6%) patients died during the study period with median time to death 1.45 years (IQR 1.17-2.63) (Supplementary Figure 1). OS at 1-, 3- and 5-years was 93%, 66.1% and 62.3%, respectively (Table 2). There was no difference in OS between the synchronous and sequential groups (P = 0.41, Figure 2A). On univariate analysis (Supplementary Table 4), only age ≥ 70 years was associated with increased risk of mortality (HR 2.19, 95%CI: 1.08-4.45, P = 0.03), whilst only transplantation was associated with reduced mortality (HR 0.19, 95%CI: 0.07-0.55, P < 0.01). On multivariate analysis, only transplantation remained significant with HR 0.20, 95%CI: 0.07-0.61, P < 0.01 (Supplementary Table 5).
| Overall survival (n = 104) | Overall survival | Overall survival | Transplanted survival (n = 25) | Transplant-free survival (n = 104) | Transplant-free survival | Transplant-free survival | |
| Synchronous group (n = 59) | Sequential group (n = 45) | Synchronous group (n = 59) | Sequential group (n = 45) | ||||
| 1-yr survival (%) | 93 | 91.3 | 95.3 | 100 | 77.1 | 68.5 | 93.2 |
| 3-yr survival (%) | 66.1 | 63.8 | 69.5 | 95.8 | 45.4 | 37.3 | 56.6 |
| 5-yr survival (%) | 62.3 | 61.1 | 64.6 | 95.8 | 37.8 | 29.7 | 48.5 |
TFS: TFS was 77.1%, 45.4% and 37.8% at 1-, 3 and 5-years, respectively (Table 2, Supplementary Figure 2). TFS was significantly different between the synchronous and sequential groups, with five-year transplant-free survival of 29.7% in the synchronous group and 48.5% in the sequential group (P = 0.02, Figure 2B). Univariate analysis identified CTP C status (HR 5.17, 95%CI: 2.59-10.29, P < 0.01) and MELD > 14 (HR 4.07 95%CI: 2.27-7.32, P < 0.01) as predictors of mortality (Supplementary Table 6), whilst the sequential tumor was associated with survival (HR 0.53, 95%CI: 0.31-0.92, P = 0.03). After multivariate analysis (Table 3), the difference between the sequential and synchronous groups did not remain significant (HR 0.70, 95%CI: 0.38-1.27, P = 0.24) and only MELD > 14 remained a significant predictor of death (HR 2.51, 95%CI: 1.15-5.46 P = 0.02).
| n (%) | HR | 95%CI | P value | |
| CTP class | ||||
| A | 66 (63.5) | - | - | - |
| B | 25 (24.0) | 1.51 | 0.81-2.82 | 0.19 |
| C | 13 (12.5) | 2.26 | 0.90-5.65 | 0.8 |
| MELD at diagnosis | ||||
| ≤ 14 | 85 (81.7) | - | - | - |
| > 14 | 19 (18.3) | 2.51 | 1.15-5.46 | 0.02 |
| Lesion group | ||||
| Synchronous | 59 (56.7) | - | - | - |
| Sequential | 45 (43.3) | 0.7 | 0.38-1.27 | 0.24 |
Transplanted patients: 1-, 3- and 5-year survival in transplanted patients was 100%, 95.8% and 95.8% (Table 2) with median time to death after transplant 6.42 years (IQR 1.33-6.67 years). Four transplanted patients (16%) died; three from recurrent HCC and the fourth from complications of motor neurone disease. All three transplanted patients with recurrent HCC had initially presented with synchronous lesions.
Progressive disease in the entire cohort was seen in 71 patients (68%) by five years. Median time to progression was 1.58 years (IQR 1-3). Amongst those with disease progression, recurrence with new lesions was the commonest form of progression, occurring in 30 patients (42.2%). Progression-free survival was not significantly different between the synchronous and sequential groups (P = 0.19). Subgroup analysis showed that the sequential group had longer progression-free survival without local recurrence (P < 0.01, Supplementary Figure 3) and without new lesions (P < 0.01, Supplementary Figure 4). No differences were seen in survival without progression, survival without failure of primary treatment or survival without metastatic spread (data not shown).
This study provides novel data on the clinical outcome of patients who develop two HCC up to 3 cm in diameter and explores the question of whether small HCC behave differently when presenting synchronously compared to sequentially. We found that regardless of whether HCC are diagnosed synchronously or sequentially, transplant-free survival is poor, with 5-year transplant free survival being only 37.8%. This suggests that liver transplantation should be considered earlier amongst the treatment options for patients with two HCC regardless of the timing of the second HCC. This is supported by the excellent five-year survival of transplanted patients in our cohort of 95.8%.
Our five-year OS of 62.3% was similar to that reported elsewhere. A retrospective survival analysis of an international, multi-institution HCC cohort of 814 patients that underwent hepatectomy with curative intent identified a five-year OS of 69% in patients with BCLC stage A disease[17]. Whilst this encompasses patients with two small HCC ≤ 3cm, the target group in our study, their cohort also included patients with single lesions ranging 2-5 cm in size or 3 lesions ≤ 3 cm each and therefore represented a broader range of patients. Additionally, we included patients that received a heterogeneous array of therapies in contrast to this study that looked only at surgical outcomes. The authors identified AFP > 400 ng/mL as being associated with poorer survival, in line with data elsewhere on surgical outcomes in low volume disease[18], yet our study did not find this association at AFP thresholds of 10 μg/L nor 400 μg/L (latter data not shown). Rather, we identified transplantation as the single independent variable that influenced survival.
We had excellent outcomes in patients who underwent transplantation for two small HCC, with 5-year survival 95.8%. The reported five year survival for transplantation with HCC is in the order of 70%[7]. For early HCC, a recent meta-analysis of low volume disease showed post-transplant survival to be 61.26% at 5 years[19]. Our higher post-transplant survival is likely due to the selection criteria for inclusion in this study, with patients only included if they had two small HCC. Despite excellent survival data, we note that in four deaths amongst transplanted patients, three were from recurrent HCC and all three of these patients had synchronous HCC.
The only independent factor impacting TFS in this study was MELD score. This suggests that in patients with two HCC, the severity of liver disease is an important factor in defining outcome, rather than lesion synchronous or sequential presentation, a similar finding to other series that examined the prognostic value of MELD scores in non-transplant HCC survival[20]. It is noteworthy that the non-transplant outcomes in patients with MELD ≤ 14 remained poor in our cohort, with five-year survival of only 45.9%. This indicates that many patients with two small HCC would benefit from consideration of transplantation.
Strengths of this study include robust and comprehensive follow-up data, with only 5.8% of patients having less than 6 mo follow-up, and real-world data from four large tertiary centres. The data in this series was prospectively collected onto HCC databases at treating institutions. Given that all transplants occur in a single centre, we are confident that all transplant records are complete with accurate data and outcome of transplantation. The primary methodological limitation of this study is that it was not randomized, which can lead to inherent biases in the groups transplanted and not transplanted that may have influenced outcomes. Some patients who were deemed not appropriate for transplantation may have had other co-factors that influenced survival, such as severe non-liver comorbidities or ongoing substance abuse. There are also differing treatment algorithms and techniques between institutions involved in our study. The index presentation of a single small HCC tends to be treated by thermal ablative techniques, rather than transarterial chemoembolization, which was the treatment of choice for unresectable synchronous tumors[21].
Our study was also limited by being focused on tumor number and size as surrogate markers for tumor biology. We were not able to evaluate the impact of histology on outcomes as the majority of diagnoses were made according to radiological criteria, in line with international guidelines[2,14]. As reported previously, transplantation according histological tumor grade leads to improved outcomes beyond selection by Milan criteria alone[22]. However, a single-centre series found that pre-transplant liver biopsy did not affect outcomes when selecting patients that are within Milan criteria, as our patients were[23]. Additionally, we recognize that amongst both groups it is not possible to determine which patients experienced intrahepatic metastasis compared to multi-centric hepatocarcinogenesis as both scenarios may lead to presentation with ‘two’ lesions. However, our study was focused purely on the number of lesions and whether this clinical determinant could guide our multidisciplinary meeting treatment decisions.
Choice of curative vs non-curative locoregional therapies may also have affected survival time between the two groups. The synchronous group had a higher rate of TACE as initial therapy compared to the sequential group, which more frequently received ablative therapies as first line treatment. This in part may explain the difference seen in TFS between the two groups.
Our data collection period spanned almost two decades and it is recognized that survival of patients diagnosed at the beginning of the observation period may not be directly comparable to patients diagnosed towards the latter portion. In an analysis of HCC cases from the Australian Cancer Registry, a national database that began in 1982, the median OS of patients doubled from 6.15 mo in those diagnosed between 2000-2004 to 12.07 mo for those diagnosed 2010-2014[6]. These data represent all patients and due to this heterogeneity, identification of the causes of improved survival are difficult but potentially attributable to better patient selection, earlier detection through HCC screening, widespread adoption of multidisciplinary decision-making, evolving locoregional treatments along with emergence of palliative therapies for advanced disease, such as oral multi tyrosine kinase inhibitors.
In conclusion we report for the first-time data specifically pertaining to patients presenting with two small HCC 3 cm in size or smaller. Our results demonstrate that the non-transplant survival of patients presenting with two small HCC is poor. Survival was similarly poor in patients presenting with two synchronous HCC as compared to sequential HCC. We therefore recommend that patients that develop a second small HCC after their first should be considered for early liver transplantation. Further larger-scale studies are required to validate these results in other populations and determine broader implications for liver transplantation waitlist management.
Hepatocellular carcinoma (HCC) is one of the most common malignancies world-wide, and is a growing cause for cancer-related mortality globally. Curative therapies include ablation for small tumors, surgical resection, and liver transplantation.
At present, there is clear evidence underpinning the guidelines for management of small tumors (≤ 3 cm in maximal diameter) and three small tumors (i.e., all ≤ 3 cm), however a scarcity of literature surrounding the optimal management of two small tumors. In addition, it is unclear if synchronous (i.e., occurring at the same time) and sequential (i.e., occurring at different points in time) tumors have differing prognoses.
This study aimed to assess the outcome of two small tumors (i.e., ≤ 3 cm in maximal diameter), and whether there was a difference in prognosis between those occurring synchronously and sequentially. This is to help guide future guidelines for manage
This was a retrospective multicenter study conducted in Victoria, Australia, including all patients diagnosed with two small HCCs between 1st January 2000 and 31st March 2018. Review of the medical record for patient demographics, liver disease, tumor-specific details, treatment and outcome was collected. Diagnosis of HCC was based on accepted radiographic and/or histologic criteria. Primary outcomes were overall survival (OS) and transplant-free survival (TFS).
One-hundred and four patients, majority male (n = 89, 86%), with a median age of 63 years-old (interquartile range 58-67.75), and predominantly suffering from viral chronic liver disease (n = 57, 55%) were included in the final analysis and followed up for a median of 2.54 years. There was a slight majority in those presenting synchronously (n = 59, 57%) compared with those diagnosed sequentially (n = 45, 43%), with the only difference between these two groups being more severe liver disease on the basis of model for end stage liver disease (MELD) (11 vs 8, P = 0.01). 1-, 3-, and 5-year OS was similar between the two groups (P = 0.41), however TFS was higher in the sequential group (1-, 3- and 5-year TFS 93.2%, 56.6% and 48.5%, compared with 68.5%, 37.3% and 29.7% in the synchronous group, P = 0.02). This difference did not persist in multivariate analysis (P = 0.24), with only MELD > 14 being predictive of mortality in the model (hazard ratio 2.51, 95%CI: 1.15-5.46, P = 0.02).
Transplant-free survival in patients with two HCCs ≤ 3 cm is poor irrespective if diagnosed synchronously or sequentially, and so all patients with two small tumors should be assessed and considered for liver transplantation.
Given limited availability of liver transplantation, future research should aim to define the molecular carcinogenetic signature in multifocal tumors, which can occur from multi-centric hepatocarcinogenesis or intrahepatic metastases, and whether this impacts recurrence, prognosis, and response to curative therapy.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shamaa MM S-Editor: Wu YXJ L-Editor: A P-Editor: Yu HG
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55843] [Article Influence: 7977.6] [Reference Citation Analysis (132)] |
| 2. |
|
| 3. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3031] [Article Influence: 433.0] [Reference Citation Analysis (3)] |
| 4. | Fitzmorris P, Singal AK. Surveillance and Diagnosis of Hepatocellular Carcinoma. Gastroenterol Hepatol (NY). 2015;11:38-46. |
| 5. | Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11:e1001624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 516] [Cited by in RCA: 604] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 6. | Wallace MC, Preen DB, Short MW, Adams LA, Jeffrey GP. Hepatocellular carcinoma in Australia 1982-2014: Increasing incidence and improving survival. Liver Int. 2019;39:522-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 665] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 8. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5313] [Article Influence: 183.2] [Reference Citation Analysis (0)] |
| 9. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [DOI] [Full Text] |
| 10. | Pompili M, Saviano A, de Matthaeis N, Cucchetti A, Ardito F, Federico B, Brunello F, Pinna AD, Giorgio A, Giulini SM, De Sio I, Torzilli G, Fornari F, Capussotti L, Guglielmi A, Piscaglia F, Aldrighetti L, Caturelli E, Calise F, Nuzzo G, Rapaccini GL, Giuliante F. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤ 3 cm. Results of a multicenter Italian survey. J Hepatol. 2013;59:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 235] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 11. | Cho YK, Rhim H, Noh S. Radiofrequency ablation vs surgical resection as primary treatment of hepatocellular carcinoma meeting the Milan criteria: a systematic review. J Gastroenterol Hepatol. 2011;26:1354-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Li L, Zhang J, Liu X, Li X, Jiao B, Kang T. Clinical outcomes of radiofrequency ablation and surgical resection for small hepatocellular carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2012;27:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | Xu XL, Liu XD, Liang M, Luo BM. Radiofrequency Ablation vs Hepatic Resection for Small Hepatocellular Carcinoma: Systematic Review of Randomized Controlled Trials with Meta-Analysis and Trial Sequential Analysis. Radiology. 2018;287:461-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 14. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J; EASL Panel of Experts on HCC. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3244] [Article Influence: 135.2] [Reference Citation Analysis (0)] |
| 15. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3678] [Article Influence: 153.3] [Reference Citation Analysis (0)] |
| 16. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3303] [Article Influence: 220.2] [Reference Citation Analysis (36)] |
| 17. | Tsilimigras DI, Bagante F, Sahara K, Moris D, Hyer JM, Wu L, Ratti F, Marques HP, Soubrane O, Paredes AZ, Lam V, Poultsides GA, Popescu I, Alexandrescu S, Martel G, Workneh A, Guglielmi A, Hugh T, Aldrighetti L, Endo I, Pawlik TM. Prognosis After Resection of Barcelona Clinic Liver Cancer (BCLC) Stage 0, A, and B Hepatocellular Carcinoma: A Comprehensive Assessment of the Current BCLC Classification. Ann Surg Oncol. 2019;26:3693-3700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 18. | Ho CM, Hu RH, Lee PH, Wu YM, Ho MC. Long-term survival in patients with T2 hepatocellular carcinoma after primary curative resection can be further stratified by tumor size. Medicine (Baltimore). 2014;93:e203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Schoenberg MB, Bucher JN, Vater A, Bazhin AV, Hao J, Guba MO, Angele MK, Werner J, Rentsch M. Resection or Transplant in Early Hepatocellular Carcinoma. Dtsch Arztebl Int. 2017;114:519-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Marrero JA, Fontana RJ, Barrat A, Askari F, Conjeevaram HS, Su GL, Lok AS. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41:707-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 474] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 21. | Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, Kudo M, Johnson P, Wagner S, Orsini LS, Sherman M. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155-2166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 569] [Cited by in RCA: 945] [Article Influence: 94.5] [Reference Citation Analysis (0)] |
| 22. | Cillo U, Vitale A, Bassanello M, Boccagni P, Brolese A, Zanus G, Burra P, Fagiuoli S, Farinati F, Rugge M, D'Amico DF. Liver transplantation for the treatment of moderately or well-differentiated hepatocellular carcinoma. Ann Surg. 2004;239:150-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 258] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 23. | DuBay D, Sandroussi C, Sandhu L, Cleary S, Guba M, Cattral MS, McGilvray I, Ghanekar A, Selzner M, Greig PD, Grant DR. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg. 2011;253:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 229] [Article Influence: 16.4] [Reference Citation Analysis (0)] |