Published online Oct 27, 2021. doi: 10.4254/wjh.v13.i10.1394
Peer-review started: February 28, 2021
First decision: May 2, 2021
Revised: May 12, 2021
Accepted: September 19, 2021
Article in press: September 19, 2021
Published online: October 27, 2021
Processing time: 235 Days and 17 Hours
Increased gut permeability and bacterial translocation play an important role in liver cirrhosis. Zonulin is a recently recognized protein involved in the disintegration of the intestinal barrier.
To investigate possible differences in serum zonulin levels among patients with different cirrhosis stages and their potential prognostic implications.
Consecutive cirrhotic patients who attended our liver clinic were included in the study. Serum zonulin levels, clinical, radiological and biochemical data were collected at baseline. Patients who accepted participation in a regular surveillance program were followed-up for at least 12 mo.
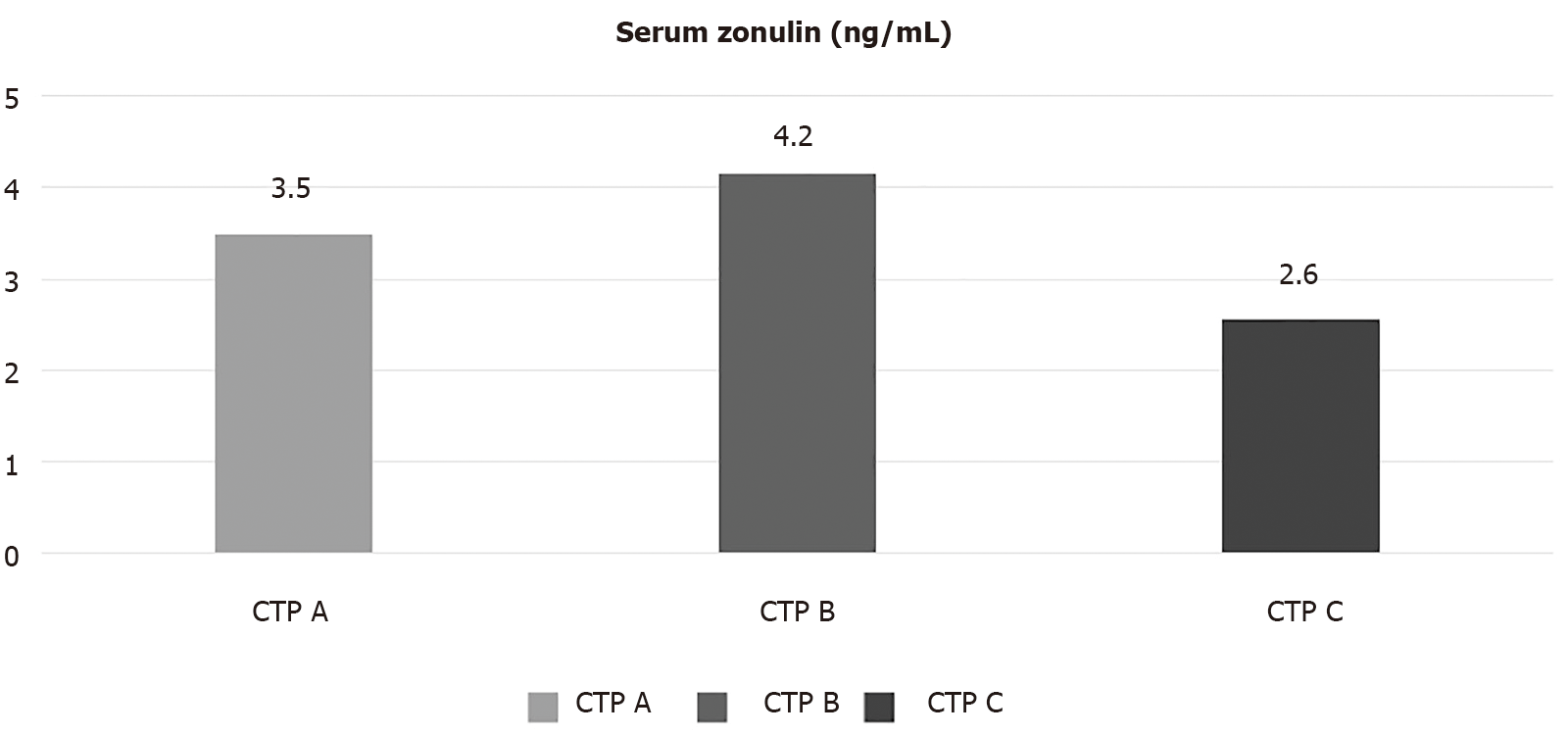
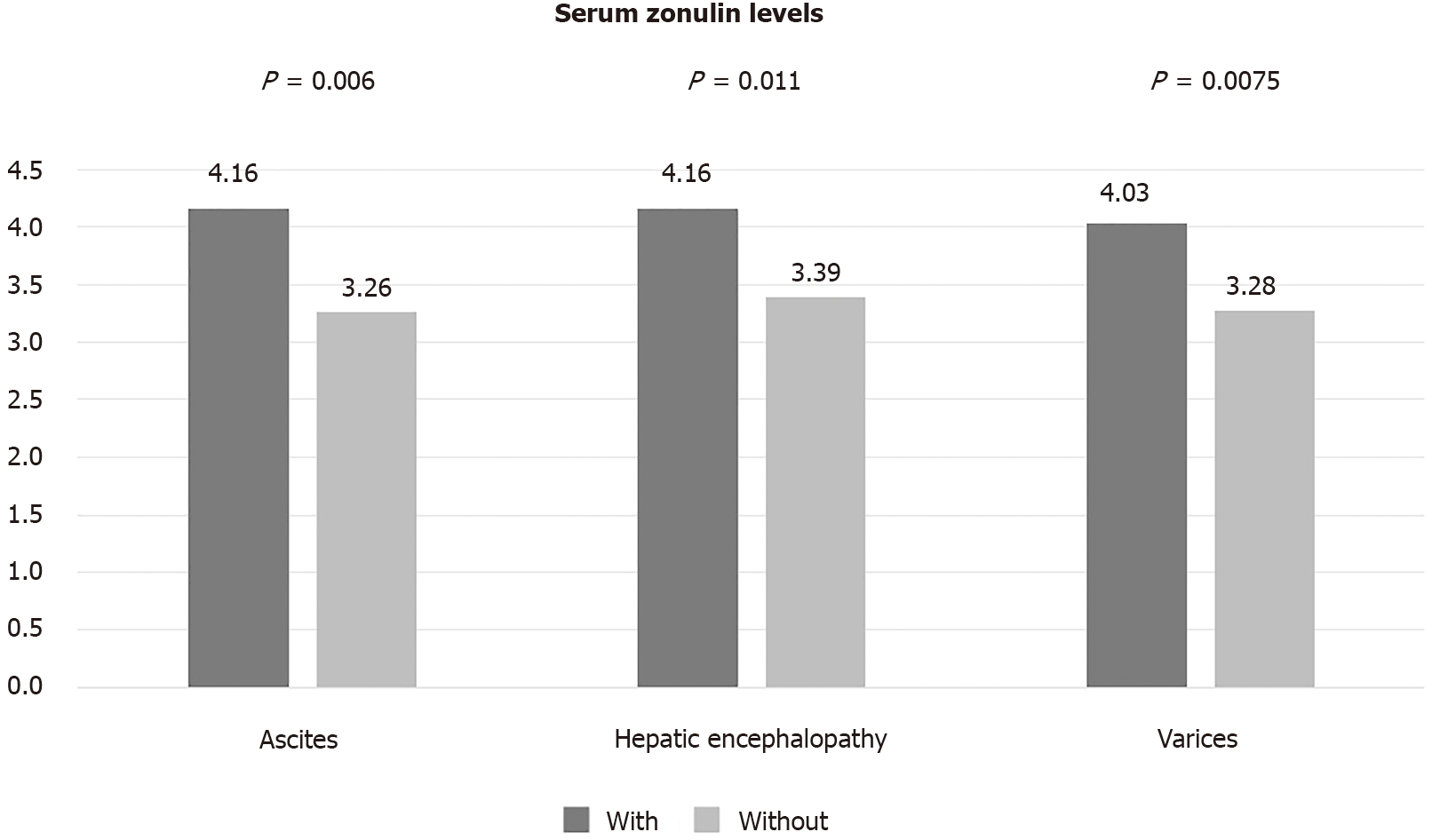
We enrolled 116 cirrhotics [mean Child-Turcotte-Pugh (CTP) score: 6.2 ± 1.6; model for end-stage liver disease score: 11 ± 3.9]. The causes of cirrhosis were viral hepatitis (39%), alcohol (30%), non-alcoholic fatty liver disease (17%), and other (14%). At baseline, 53% had decompensated cirrhosis, 48% had ascites, and 32% had history of hepatic encephalopathy. Mean zonulin levels were significantly higher in patients with CTP-B class than CTP-A class (4.2 ± 2.4 ng/dL vs 3.5 ± 0.9 ng/dL, P = 0.038), with than without ascites (P = 0.006), and with than without history of encephalopathy (P = 0.011). Baseline serum zonulin levels were independently associated with the probability of decompensation at 1 year (P = 0.039), with an area under the receiving operating characteristic of 0.723 for predicting hepatic decompensation. Higher CTP score (P = 0.021) and portal vein diameter (P = 0.022) were independent predictors of mortality.
Serum zonulin levels are higher in patients with more advanced chronic liver disease and have significant prognostic value in identifying patients who will develop decompensation.
Core Tip: Zonulin is a protein that appears to play a significant role in gut barrier integrity. Increased zonulin levels and deregulation of intestinal permeability have been demonstrated in patients suffering from celiac disease or type 2 diabetes. However, the role of zonulin as a promoting factor of intestinal barrier disruption in patients with liver cirrhosis has not been studiedadequately. We evaluated serum zonulin levels in patients with different stages of advanced liver disease. According to our findings, serum zonulin levels are increased in patients with more advanced liver disease and are independently associated with progression to decompensation.
- Citation: Voulgaris TA, Karagiannakis D, Hadziyannis E, Manolakopoulos S, Karamanolis GP, Papatheodoridis G, Vlachogiannakos J. Serum zonulin levels in patients with liver cirrhosis: Prognostic implications. World J Hepatol 2021; 13(10): 1394-1404
- URL: https://www.wjgnet.com/1948-5182/full/v13/i10/1394.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i10.1394
Bacterial translocation (BT) is defined as the passage of viable endogenous bacteria and endotoxins from the intestinal lumen through the mucosa into the mesenteric lymph nodes and other organs[1]. In patients with liver disease, ΒΤ has been demonstrated to play a pivotal role on the occurrence or aggravation of serious complications[2]. Bacterial overgrowth, decreased intestinal peristalsis with concomitant increased permeability, as well as immunological alterations that have been found in patients with chronic liver diseases appear to be the main causative factors of ΒΤ[3-6]. Among them, the exact pathophysiological mechanism leading to increased intestinal permeability is the most difficult to investigate and remains to be thoroughly explained.
Recently, Fasano[7] identified zonulin, a novel 47-kDa protein precursor of haptoglobin-2 (pre-HP2), which is synthesized by the intestinal and liver cells and mayplay a significant role in disruption of the gut barrier. Evidence exist to support that small intestine epithelial cells exposed to enteric bacteria, secret zonulin, which in turn attaches to special receptors located on the membrane of intestinal epithelial cells, leading to a disconnection of occludin from ZO-1. This disrupts the tight junctions and consequently increases the gut permeability[8].
Currently, the connection between increased zonulin levels and the deregulation of intestinal permeability has been observed in patients suffering from celiac disease, type 2 diabetes, obesity and inflammatory bowel disease (IBD)[9-13]. However, the role of zonulin and its possible involvement in the dysfunction of intestinal barrier function in patients with cirrhosis has not been studiedthoroughly.
The aim of our study was to assess the serum zonulin levels in patients with cirrhosis and investigate their possible impact on patients’ prognosis.
Over a period of 12 mo (February 2017-January 2018), all cirrhotic patients, aged from 18 years to 80 years, who attended our outpatient liver clinics were considered eligible for inclusion in the study, regardless of the etiology and severity of their liver disease. We excluded patients with alcoholic hepatitis, porto-splenic vein thrombosis, non-cirrhotic portal hypertension, hepatocellular carcinoma (HCC), transjugular intrahepatic portosystemic shunt (TIPS), chronic kidney disease, celiac disease, acute infection, IBD, or any other chronic intestinal disease.
The diagnosis of cirrhosis was based on clinical and laboratory findings, imaging studies or liver histology, when available. All patients had liver stiffness measurement (LSM) of ≥ 14 kPa (by elastography). At baseline, all patients underwent abdominal ultrasound with spleen and portal diameter measurements and baseline LSM and spleen stiffness measurement (SSM) by shear wave elastography (SWE). In addition, all patients underwent clinical examination and laboratory testing every 3 mo, and abdominal ultrasound every 6 mo.
The study protocol was approved by the Ethics Committee of “Laiko” General Hospital of Athens, Greece. A written consent was obtained from each patient with respect to all ethical guidelines issued by the 2000 revision (Edinburgh) of the 1975 Declaration of Helsinki.
Clinical and laboratory data, routine blood parameters, including platelet count, prothrombin time, serum albumin, serum creatinine, international normalized ratio (INR), serum aspartate aminotransferase, alanine aminotransferase, and bilirubin, were measured at the time of patient enrollment. Likewise, the existence of ascites or hepatic encephalopathy (HE) was noted. The severity of liver disease was determined by Child-Turcotte-Pugh (CTP) scoring, and the model for end-stage liver disease (MELD) score calculated according to the UNOS formula. Study end-points included death, liver transplantation and liver decompensation in patients with compensated cirrhosis at baseline.
All patients underwent LSM and SSM by two-dimensional (2D)-SWE performed by a single experienced operator (> 500-exam experience) in fasting patients. The Aixplorer® ultrasound system (Supersonic Imagine S.A., Aix-en-Provence, France) with an abdominal 3.5 MHz curved array probe was used, as recommended. 2D-SWE measurements were performed at each patient’s initial assessment. Ten reliable LSM and ten reliable SSM values were obtained from each patient and the mean values were then calculatedrespectively. The SD was < 20% of the mean values of LSM and SSM, respectively.
A venous blood sample was collected from each patient, with or without precooled anticoagulant (heparinized/EDTA)-coated tube. The serum or plasma was then separated from the blood by centrifugation at 3000 rpm for 10 min at room temperature. The samples were stored at -80 °C.
Serum levels of zonulin were measured using an enzyme-linked immune-sorbent assay (Immundiagnostik AG, Bensheim, Germany); the sensitivity of the assay was 0.01 ng/mL.
Statistical analysis was performed by SPSS V23 (IBM Corp., Armonk, NY, United States). Data were expressed as frequencies, mean with SD, or median with interquartile range, as appropriate. Quantitative variables were compared with Student’s t-test or Mann-Whitney test for normally distributed and non-normally distributed variables, respectively. Qualitative variables were compared with chi-squared test or Fisher’s exact test, as appropriate. The relationship between parameters was assessed by using Spearman’s correlation coefficient. Multivariate logistic regression analysis models were used to identify independent, significant, predictive factors of a poor outcome. Only parameters with a significant or a trend for significant associations (P < 0.10) with the dependent variable in the univariate analysis being included in the multivariate analysis models. The area under the receiving operating characteristic (AUROC) curves for zonulin predictability, as well as sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), were calculated. The c-statistics of AUROC curves were provided with their 95% confidence intervals (CIs). Diagnostic accuracy was considered to be poor when a c-statistic was 0.65-0.75, good when a c-statistic was 0.76-0.85, and excellent when a c-statistic was > 0.85. The optimal cut-off was selected from the AUROC curves as the point which provided the maximum sum of sensitivity and specificity. All tests were two-sided and P values < 0.05 were considered to be significant.
In total, 127 consecutive cirrhotic patients were initially assessed. Eleven patients were excluded, due to HCC (n = 5), acute infection (n = 4) or portal vein thrombosis (n = 2). Therefore, 116 patients were finally included in the study. Mean age was 59 ± 13 years, and 71 (61.2%) were male. Viral hepatitis was the main cause of liver disease (38.8%). Compensated and decompensated liver disease were marginally equally distributed in our cohort, while a significant proportion of patients had ascites at the time of enrollment. Esophageal or gastric varices were documented in 65 (55.2%) of the patients and 60 (51.7%) were under treatment with b-blockers. Patient characteristics are presented in Table 1.
| Examined parameter | Baseline value | |
| Sex as M/F, n (%) | 71/45 (61.2) | |
| Age in yr1 | 59 ± 13 | |
| BMI, kg/m2 | 27.5 ± 5.0 | |
| Liver disease etiology, n (%) | ||
| Chronic hepatitis B | 25 (21.6) | |
| Chronic hepatitis C | 20 (17.2) | |
| Alcohol abuse | 35 (30.2) | |
| NAFLD | 20 (17.2) | |
| Autoimmune hepatitis | 5 (4.3) | |
| Other | 11 (9.5) | |
| CTP class, n (%) | ||
| A | 78 (67.2) | |
| B | 33 (28.4) | |
| C | 5 (4.4) | |
| CTP score | 6.2 ± 1.6 | |
| MELD score | 11.0 ± 3.9 | |
| Decompensated cirrhosis, n (%) | 61 (52.6) | |
| History of HE, n (%) | 37 (31.9) | |
| Ascites, n (%) | 56 (48.3) | |
| Bilirubin in mg/dL | 1.3 ± 0.9 | |
| Creatinine in mg/dL | 1.0 ± 1.2 | |
| Albumin in g/L | 41.0 ± 4.0 | |
| Platelet count as × 109/L | 121 ± 49 | |
| INR | 1.3 ± 0.3 | |
Compared to patients with compensated liver disease, those with decompensated liver disease had significantly lower platelet counts (106 ± 37 × 109/L vs 137 ± 55 × 109/L, P = 0.006), higher INR values (1.3 ± 0.28 vs 1.2 ± 0.2, P = 0.003) and lower albumin levels (3.5 ± 0.5 g/Dl vs 4.8 ± 0.6 g/dL, P < 0.001) as well as higher MELD (12.6 ± 4.1 vs 9.2 ± 1.6, P < 0.001) and CTP scores (7 ± 1.7 vs 5.3 ± 0.5, P < 0.001).
Mean serum zonulin levels were 3.6 ± 1.5 ng/dL. Patients with CTP-B had significantly higher serum zonulin levels compared to those with CTP-A cirrhosis (4.2 ± 2.4 ng/dL vs 3.5 ± 0.9 ng/dL, P = 0.038). On the other hand, patients with CTP-C cirrhosis had lower levels of serum zonulin compared to the two other groups. Specifically, CTP-C patients had lower levels of zonulin than CTP-A (2.6 ± 0.7 ng/dL vs 3.5 ± 0.9 ng/dL, P = 0.035) or CTP-B patients, although the latter difference did not reach statistical significance (2.6 ± 0.7 ng/dL vs 4.2 ± 2.4 ng/dL, P = 0.157) (Figure 1).
Serum zonulin levels were higher in patients with than without ascites (4.16 ng/dL vs 3.26 ng/dL, P = 0.006). Similarly, patients with a history of HE had higher zonulin levels compared to those without history of HE (4.17 ng/dL vs 3.39 ng/dL, P = 0.011). The presence of varices was also associated with numerically higher levels of zonulin but this difference did not reach statistical significance (Figure 2).
No significant correlation was observed between serum zonulin levels and platelets, serum albumin, bilirubin, INR, MELD score, age or body mass index. Moreover, treatment with b-blockers was not found to affect the levels of zonulin (patients on treatment: 3.6 ± 1.5 ng/dL vs no treatment with b-blockers: 3.4 ± 0.9 ng/dL, P = 0.513).
Sixty-three out of the one-hundred and sixteen patients were followed for at least 12 mo or until death/Liver transplantation, whichever occurred first. Their mean age was 60 ± 15 years and 30 (48%) were male. The majority of patients (n = 36, 57%) had compensated cirrhosis at baseline. Forty-four (69.8%) patients had CTP-A and nineteen had CTP-B (30.2%) cirrhosis. Mean MELD score was 11.3 ± 3.2. Thirty-nine (61.9%) patients had no varices or small varices without red spots and twenty-four (38.1%) patients had high-risk varices (large varices or small varices with red spots). Twenty of the twenty-seven (74%) patients with decompensated cirrhosis had ascites at baseline. No patient with compensated liver disease who was in the follow-up group was under rifaximin treatment, while 8/27 patients with decompensated disease were receiving rifaximin. Patients with decompensated cirrhosis receiving rifaximin on baseline and followed up for at least 12 mo showed numerically higher serum zonulin levels at baseline, though not statistically significant (patients onrifaximin treatment: 4.49 ± 2.37 ng/dL vs no rifaximintreatment: 3.41 ± 1.08 ng/dL, P = 0.144)
Specific treatment was received by 32 (50.8%) of the 63 patients. Among them, mean baseline LSM was 22.9 ± 9.3 kPa and mean baseline SSM was 35.3 ± 8.6 kPa.
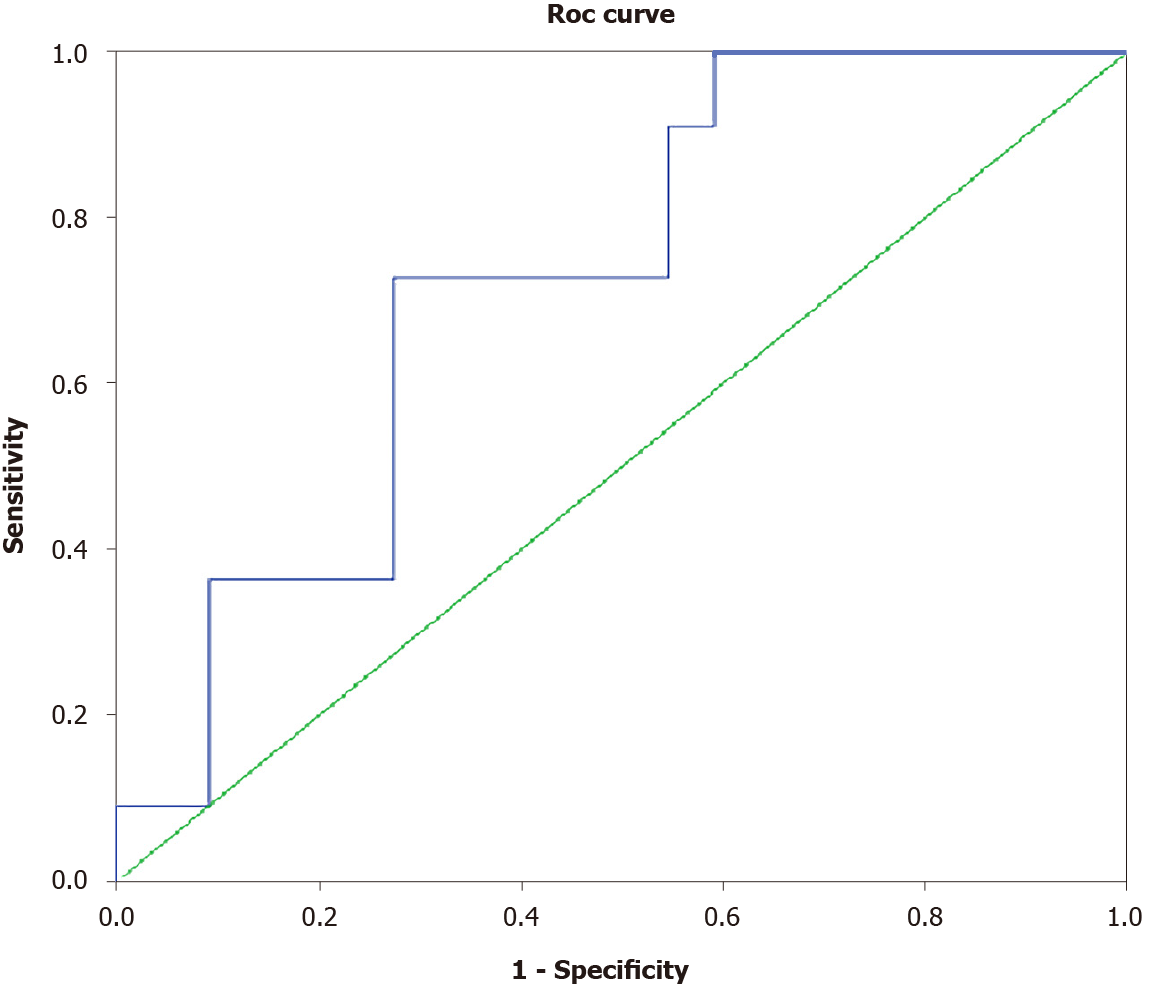
Twelve (33.3%) of the thirty-six patients with compensated cirrhosis at baseline progressed to decompensated disease [11/36 (30.5%) developed ascites and 1/36 (2.8%) developed variceal bleeding]. Patients who progressed to liver decompensation (n = 12) had higher baseline serum zonulin levels at (3.98 ± 0.79 ng/dL vs 3.18 ± 1.02 ng/dL, P = 0.011) and lower albumin levels (3.64 ± 0.53 g/dL vs 4.10 ± 0.51 g/dL, P = 0.013) as well as a trend for lower platelet counts (104 × 109/L vs 138 × 109/L, P = 0.094) and higher SSM (36.1 ± 9.3 kPa vs 31.1 ± 7.4 kPa, P = 0.087) compared to patients who remained compensated during follow-up (Table 2). In multivariate logistic regression analysis, progression to liver decompensation within 12 mo was independently associated with higher serum zonulin [odds ratio (OR): 6.53, 95%CI: 1.08-39.57, P = 0.041] and lower albumin at baseline (OR: 0.03, 95%CI: 0.002-0.92, P = 0.044). Baseline serum zonulin levels offered an AUROC of 0.723 (P = 0.039) for predicting development of decompensation within 1 year (Figure 3). The cut-off point that could better predict progression to decompensation was 3.65 ng/dL, with specificity 73%, sensitivity 73%, NPV 84% and PPV 57%.
| Examined parameter, baseline values | Patients who remained compensated during follow-up, n = 24 | Patients who proceed to decompensated disease during follow-up, n = 12 | P value |
| Sex as M/F | 13/11 | 3/9 | 0.157 |
| Age in yr1 | 59 ± 12 | 61 ± 14 | 0.710 |
| Liver-specific treatment, Y/N | 13/11 | 9/3 | 0.292 |
| High-grade varices, Y/N | 6/24 | 6/12 | 0.157 |
| Platelet count as × 109/L | 138 ± 54 | 105 ± 46 | 0.094 |
| Albumin in g/dL | 3.64 ± 0.53 | 4.13 ± 0.51 | 0.013 |
| Spleen diameter in cm | 13.1 ± 2.5 | 14.0 ± 2.3 | 0.325 |
| Portal diameter in cm | 1.29 ± 0.22 | 1.27 ± 0.21 | 0.768 |
| Liver stiffness in kPa | 19.7 ± 6.9 | 23.9 ± 9.2 | 0.139 |
| Spleen stiffness in kPa | 31.1 ± 7.4 | 36.1 ± 9.3 | 0.087 |
| Serum zonulin levels in ng/mL | 3.19 ± 1.02 | 4.15 ± 0.95 | 0.011 |
In total, 7 (11.3%) patients died (6 due to liver related causes and 1 due to non-liver related malignancy), while 2 patients (2.9%) underwent liver transplantation. Patients who died or underwent liver transplantation (n = 9) had lower baseline albumin levels compared to patients (n = 54) who survived (3.20 ± 0.62 g/dL vs 3.87 ± 0.62 g/dL, P = 0.010), higher CTP score (7.4 vs 5.9, P < 0.001) and greater portal vein diameter (1.55 cm vs 1.27 cm, P = 0.002) (Table 3). In multivariate logistic regression analysis, higher CTP score (OR: 2.06, 95%CI: 1.02-4.16, P = 0.021) and portal vein diameter (OR: 71.54, 95%CI: 1.56-329.52, P = 0.022) were independently associated with mortality.
| Examined parameter, baseline values | Patients alive/not transplanted at the end of the follow-up, n = 54 | Patients transplanted or dead, n = 9 | P value |
| Age in yr1 | 59 ± 15 | 66 ± 14 | 0.238 |
| Liver-specific treatment, Y/N | 28/26 | 4/5 | 0.474 |
| CTP score | 5.9 ± 1.0 | 7.4 ± 1.4 | < 0.001 |
| MELD score | 11.3 ± 3.0 | 11.7 ± 3.6 | 0.772 |
| High-risk varices, Y/N | 19/54 | 5/9 | 0.241 |
| Platelet count as × 109/L | 120 ± 52 | 109 ± 40 | 0.602 |
| Albumin in g/dL | 3.87 ± 0.62 | 3.20 ± 0.62 | 0.010 |
| Spleen diameter in cm | 13.7 ± 2.8 | 15.2 ± 2.5 | 0.154 |
| Portal diameter in cm | 1.27 ± 0.21 | 1.55 ± 0.25 | 0.002 |
| Liver stiffness in kPa | 22.5 ± 9.2 | 27.9 ± 10.3 | 0.127 |
| Spleen stiffness in kPa | 35.0 ± 8.8 | 37.9 ± 6.9 | 0.383 |
| Serum zonulin levels in ng/mL | 3.70 ± 1.36 | 3.17 ± 1.21 | 0.300 |
BT is increased in cirrhosis and seems to play a pivotal pathophysiological role in the development of complications related to end-stage liver disease, such as hepatorenal syndrome, HE, spontaneous bacterial peritonitis and acute-on-chronic liver failure[2,14]. Although many factors have been implicated in the pathophysiology of BT, the exact pathogenic mechanisms leading to gut epithelial disfunction in liver cirrhosis remain unclear[1,15,16]. To date, the role of zonulin as a promoting factor of the intestinal barrier’s disruption has been thoroughly investigated in several diseases, but in patients with cirrhosis there is only limited information.
In our cohort, we investigated whether serum zonulin levels have any impact on the prognosis of patients with cirrhosis. Initially, we found that mean serum zonulin levels were higher in patients with CTP-B than CTP-A class cirrhosis, supporting its possible contribution in the development of decompensated liver disease (CTP-B stage). Interestingly, serum zonulin levels were lowest in our few cases with CTP-C cirrhosis; although, the small number of CTP-C patients in our study weakens the validity of such a finding, as any type of statistical errors cannot be excluded. The latter finding is in contrast to the results of a recently published study, which reported increasing serum zonulin levels from CTP-A to -B and to -C class. However, only chronic HBV patients were included in the abovementioned study and, more importantly, the study also included patients with HCC, a fact that could have affected the result[17]. The role of zonulin has also been previously investigated by others in small cohorts of patients with chronic liver disease. Serum zonulin levels were reported to decrease progressively, as liver function deteriorated in 9 patients with chronic viral hepatitis[18]. Obviously, such an under-powered study cannot lead to any valuable conclusion. In another study, serum zonulin levels were found to be lower in 40 patients with chronic HBV infection compared to 17 controls, but besides the small sample size of the study, no data for stage of liver disease were provided[19]. A pivotal study in children and adolescents reported increased serum zonulin levels in cases with rather than without NASH[20]. In the latter study, zonulin levels were found to correlate with the severity of liver steatosis and not of liver fibrosis, but cases with cirrhosis were not included. Contrary to the previous studies, we recruited a larger number of cirrhotic patients, irrespective of liver disease etiology, while at the same time we excluded older patients or patients with HCC which could jeopardize our results.
Additionally, in our study, we found that patients with more advanced cirrhosis, as documented by the presence of ascites or history of HE, had higher serum zonulin levels compared to those without these complications. Unexpectedly, in our cohort, we found a numerical but not statistically significant difference in zonulin levels between patients with or without varices. Moreover, there was no correlation between serum zonulin and SSM, which by recent data is suggested to correlate wellwith hepatic venous pressure gradient levels and the presence of high-risk varices[21,22]. It could be argued that the secretion of zonulin is regulated by mechanisms acting locally in the gut and is not directly affected by changes in portal pressure. However, such a speculation, taking under consideration the complexity of mechanisms implicated in the regulation of gut permeability in liver cirrhosis carries a great level of uncertainty.
Finally, the potential association between serum zonulin levels and the development of liver decompensation is further supported by the predictive role of zonulin for such an outcome within 1year of follow-up. In particular, baseline serum zonulin levels in our patients with compensated cirrhosis were found to be independently associated with progression to decompensated liver disease within the next year. The predictability of serum zonulin levels to predict progression to decompensated liver disease was significant but suboptimal (AUROC: 0.723). In addition, serum zonulin levels < 3.65 ng/mL at baseline offered a NPV of 84% for progression to liver decompensation within the next year.
Our study has some limitations. A substantial proportion of patients did not participate in the follow-up study and we included a small number of patients with CTP-C stage disease. Furthermore, serum zonulin levels were measured in a single time frame. According to guidelines, in our department, no patient with compensated disease was under rifaximin treatment. Therefore, the effect of rifaximin or other antibiotic treatment (patients with acute infection were excluded from our study) in the transition from compensated towards decompensated disease and their correlation to zonulin levels were not assessed. Undoubtedly, serial measurements of zonulin levels and their fluctuations during the course of the liver disease would enforce its prognostic value.
In conclusion, we have clearly shown that serum zonulin levels are increased in patients with more advanced liver disease and are independently associated with the progression to decompensation. The results of our study may be of particular value as they reveal, for the first time, the adverse effect of a new agent, zonulin, on the deterioration of chronic liver disease. More studies are needed to confirm our findings and to further investigate the pathophysiological mechanisms by which zonulin is involved in alteration of intestinal barrier and gut permeability.Taking into consideration that zonulin antagonists are already being tested in phase IIb studies in diseases characterized by disrupted intestinal permeability, such as celiac disease, confirmation of our results may have significant clinical implications[23].
Gut permeability is distorted in patients with liver cirrhosis and the observed deregulation of the intestinal integrity plays a crucial role in the development of bacterial translocation. Bacterial translocation contributes to the occurrence or aggravation of serious complications in patients with liver cirrhosis. Zonulin is a recently recognized protein, synthesized by the intestinal and liver cells, and thought to play an important role in the regulation of tight junctions between intestinal cells.
Increased zonulin levels have been observed in such diseases as celiac disease and inflammatory bowel disease and have shown correlation to the impairment of intestinal permeability. The exact mechanism that leads to the deregulation of the intestinal integrity in liver cirrhosis is not thoroughly investigated. Zonulin may have a role in the observed alterations of the gut barrier in advanced chronic liver disease.
We aimed to investigate if serum zonulin levels are altered in patients with different stages of liver cirrhosis and investigate their possible impact on patients’ prognosis.
We included 116 cirrhotic patients who attended our outpatient clinic during a 12-mo period. Serum zonulin levels were measured, as were epidemiological, laboratory and clinical data, and data from elastography and ultrasonography at baseline. Sixty-three patients were followed up for at least 1year and data from clinical events (death, liver transplantation and liver disease decompensation) were collected.
Our study included mainly Child-Turcotte-Pugh (CTP)-A (67%) and CTP-B patients (28%). We observed that serum zonulin levels are increased in patients with more advanced liver disease, such as patients with CTP–B stage, patients with ascites, or those with history of hepatic encephalopathy. What is more, serum zonulin levels were independently associated with the probability of decompensation within the next year.
According to our study results, serum zonulin levels are increased in patients with advanced chronic liver disease. What is more, a new agent, zonulin, is found to be implicated in the progress towards advanced liver disease.
Our findings highlight once more the significance of gut barrier deregulation in the setting of liver cirrhosis and emphasize the need of further studies in the field, aiming to reveal the complex pathophysiological interplay which leads to bacterial translocation. Especially, the role of zonulin should be further investigated, due to its possible therapeutic implications, as a zonulin antagonist alreadyexists and is being tested in studies of celiac disease.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Forlano R S-Editor: Gao CC L-Editor: A P-Editor: Guo X
| 1. | Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 743] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 2. | Bosch J, Groszmann RJ, Shah VH. Evolution in the understanding of the pathophysiological basis of portal hypertension: How changes in paradigm are leading to successful new treatments. J Hepatol. 2015;62:S121-S130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (1)] |
| 3. | Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 562] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 4. | Du Plessis J, Vanheel H, Janssen CE, Roos L, Slavik T, Stivaktas PI, Nieuwoudt M, van Wyk SG, Vieira W, Pretorius E, Beukes M, Farré R, Tack J, Laleman W, Fevery J, Nevens F, Roskams T, Van der Merwe SW. Activated intestinal macrophages in patients with cirrhosis release NO and IL-6 that may disrupt intestinal barrier function. J Hepatol. 2013;58:1125-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 5. | Genescà J, Martí R, Rojo F, Campos F, Peribáñez V, Gónzalez A, Castells L, Ruiz-Marcellán C, Margarit C, Esteban R, Guardia J, Segura R. Increased tumour necrosis factor alpha production in mesenteric lymph nodes of cirrhotic patients with ascites. Gut. 2003;52:1054-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Gunnarsdottir SA, Sadik R, Shev S, Simrén M, Sjövall H, Stotzer PO, Abrahamsson H, Olsson R, Björnsson ES. Small intestinal motility disturbances and bacterial overgrowth in patients with liver cirrhosis and portal hypertension. Am J Gastroenterol. 2003;98:1362-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 144] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Fasano A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann N Y Acad Sci. 2012;1258:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 8. | El Asmar R, Panigrahi P, Bamford P, Berti I, Not T, Coppa GV, Catassi C, Fasano A. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology. 2002;123:1607-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 256] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 9. | Lammers KM, Lu R, Brownley J, Lu B, Gerard C, Thomas K, Rallabhandi P, Shea-Donohue T, Tamiz A, Alkan S, Netzel-Arnett S, Antalis T, Vogel SN, Fasano A. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology. 2008;135:194-204.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 384] [Cited by in RCA: 370] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 10. | Sapone A, de Magistris L, Pietzak M, Clemente MG, Tripathi A, Cucca F, Lampis R, Kryszak D, Cartenì M, Generoso M, Iafusco D, Prisco F, Laghi F, Riegler G, Carratu R, Counts D, Fasano A. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55:1443-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 381] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 11. | Caviglia GP, Dughera F, Ribaldone DG, Rosso C, Abate ML, Pellicano R, Bresso F, Smedile A, Saracco GM, Astegiano M. Serum zonulin in patients with inflammatory bowel disease: a pilot study. Minerva Med. 2019;110:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 12. | Moreno-Navarrete JM, Sabater M, Ortega F, Ricart W, Fernández-Real JM. Circulating zonulin, a marker of intestinal permeability, is increased in association with obesity-associated insulin resistance. PLoS One. 2012;7:e37160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 212] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 13. | Tripathi A, Lammers KM, Goldblum S, Shea-Donohue T, Netzel-Arnett S, Buzza MS, Antalis TM, Vogel SN, Zhao A, Yang S, Arrietta MC, Meddings JB, Fasano A. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci U S A. 2009;106:16799-16804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 319] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 14. | Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 505] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 15. | Ghosh G, Jesudian AB. Small Intestinal Bacterial Overgrowth in Patients With Cirrhosis. J Clin Exp Hepatol. 2019;9:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Tsiaoussis GI, Assimakopoulos SF, Tsamandas AC, Triantos CK, Thomopoulos KC. Intestinal barrier dysfunction in cirrhosis: Current concepts in pathophysiology and clinical implications. World J Hepatol. 2015;7:2058-2068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Wang X, Li MM, Niu Y, Zhang X, Yin JB, Zhao CJ, Wang RT. Serum Zonulin in HBV-Associated Chronic Hepatitis, Liver Cirrhosis, and Hepatocellular Carcinoma. Dis Markers. 2019;2019:5945721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Akao T, Morita A, Onji M, Miyake T, Watanabe R, Uehara T, Kawasaki K, Miyaike J, Oomoto M. Low Serum Levels of Zonulin in Patients with HCV-Infected Chronic Liver Diseases. Euroasian J Hepatogastroenterol. 2018;8:112-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Calgin MK, Cetinkol Y. Decreased levels of serum zonulin and copeptin in chronic Hepatitis-B patients. Pak J Med Sci. 2019;35:847-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Pacifico L, Bonci E, Marandola L, Romaggioli S, Bascetta S, Chiesa C. Increased circulating zonulin in children with biopsy-proven nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:17107-17114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Buechter M, Manka P, Theysohn JM, Reinboldt M, Canbay A, Kahraman A. Spleen stiffness is positively correlated with HVPG and decreases significantly after TIPS implantation. Dig Liver Dis. 2018;50:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Karagiannakis DS, Voulgaris T, Koureta E, Chloupi E, Papatheodoridis GV, Vlachogiannakos J. Role of Spleen Stiffness Measurement by 2D-Shear Wave Elastography in Ruling Out the Presence of High-Risk Varices in Cirrhotic Patients. Dig Dis Sci. 2019;64:2653-2660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Leffler DA, Kelly CP, Green PH, Fedorak RN, DiMarino A, Perrow W, Rasmussen H, Wang C, Bercik P, Bachir NM, Murray JA. Larazotide acetate for persistent symptoms of celiac disease despite a gluten-free diet: a randomized controlled trial. Gastroenterology. 2015;148:1311-9.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 180] [Article Influence: 18.0] [Reference Citation Analysis (0)] |