Published online Sep 27, 2020. doi: 10.4254/wjh.v12.i9.685
Peer-review started: April 22, 2020
First decision: May 15, 2020
Revised: July 24, 2020
Accepted: August 1, 2020
Article in press: August 1, 2020
Published online: September 27, 2020
Processing time: 152 Days and 10.2 Hours
Myotonic dystrophy (MD) is sometimes accompanied by metabolic/endocrine disorders, including dyslipidemia, central obesity, and hypogonadism. Due to considerable individual differences in the severity and progression of myopathy, MD patients with minimal-to-mild muscle symptoms might be followed as having other diseases, such as non-alcoholic fatty liver disease (NAFLD).
A 40-year-old non-obese man without a history of regular ethanol consumption was referred to our hospital due to persistent liver dysfunction and hyperlipidemia. His body mass index was 23.4 kg/m2. Liver histology demonstrated macrovesicular steatosis, ballooned hepatocytes with eosinophilic inclusion bodies, and perisinusoidal fibrosis, leading to the diagnosis of non-alcoholic steatohepatitis (NASH). Although he had no discernable muscle pain or weakness, persistently high serum creatine kinase (CK) and myoglobin levels as well as the presence of frontal baldness, a hatched face, history of cataract surgery, and grip myotonia indicated the possibility of MD. Southern blotting of the patient’s DNA revealed the presence of CTG repeats, confirming the diagnosis.
When gastroenterologists encounter NAFLD/NASH patients, serum CK should be verified. If hyperCKemia, frontal baldness, a hatched face, history of cataract surgery, and grip myotonia are noted, the possibility of MD may be considered.
Core Tip: We describe a patient with non-alcoholic steatohepatitis (NASH) who was later diagnosed as having myotonic dystrophy (MD). Some MD patients with minimal-to-mild muscle symptoms may be misdiagnosed as having non-alcoholic fatty liver disease (NAFLD). Therefore, when gastroenterologists encounter patients with NAFLD/NASH, serum creatine kinase (CK) should be verified. If high serum CK levels persist in the presence of frontal baldness, a hatched face, history of cataract surgery, and grip myotonia, the possibility of MD may be considered.
- Citation: Tanaka N, Kimura T, Fujimori N, Ichise Y, Sano K, Horiuchi A. Non-alcoholic fatty liver disease later diagnosed as myotonic dystrophy. World J Hepatol 2020; 12(9): 685-692
- URL: https://www.wjgnet.com/1948-5182/full/v12/i9/685.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i9.685
Non-alcoholic fatty liver disease (NAFLD) is a common cause of persistent liver dysfunction and is defined as the presence of hepatic steatosis without regular consumption of ethanol or drugs[1,2]. For the diagnosis of NAFLD, other causes of chronic liver injury, such as hepatitis virus infection, bacterial and parasitic infection, autoimmunity, drugs and toxicants, hemochromatosis, Wilson disease, and citrin deficiency should be excluded. Additionally, the possibility of secondary NAFLD, including gastrointestinal/pancreatic surgery, hypothyroidism, hyperadrenalism, and pancreatic exocrine insufficiency, needs to be carefully surveyed[3,4].
Although it is accepted that endocrine/metabolic disorders are closely associated with the development of NAFLD, the relationship between myopathy and NAFLD has not been fully addressed. Among several types of myopathy, myotonic dystrophy (MD) is often accompanied by metabolic/endocrine disorders, such as dyslipidemia, central obesity, insulin resistance, and hypogonadism[5]. Due to considerable variation in the severity and progression of myopathy, MD patients with minimal-to-mild muscle symptoms may be followed as having other diseases. We herein describe a patient with non-alcoholic steatohepatitis (NASH), a severe phenotype of NAFLD, later diagnosed as having MD.
A 40-year-old non-obese man without a history of regular ethanol consumption was referred to our hospital due to persistent hypertransaminasemia and hyperlipidemia.
It was pointed out that he had liver dysfunction and hyperlipidemia at annual health checkups from 35 years of age. He had noticed easy fatigability, but presumed that it was caused by overwork and insufficient sleep. He had no history of regular ethanol, drugs, or supplements consumption, or smoking.
He had no history of past blood transfusion, surgical treatment, or acupuncture.
His body mass index was 23.4 kg/m2. He was asymptomatic with no signs of hepatomegaly, xanthoma, or Achilles tendon thickening.
Laboratory findings revealed significant increases in serum aspartate aminotransferase (65 U/L, normal 13-30), alanine aminotransferase (103 U/L, normal 7-23), total cholesterol (298 mg/dL, normal 142-220), and triglycerides (TG; 318 mg/dL, normal 30-150). Hyperinsulinemia and greater index of homeostasis model assessment for insulin resistance indicated the presence of insulin resistance. Hepatitis virus markers and autoantibodies were all negative and immunoglobulins, ferritin and ceruloplasmin were within normal ranges. Laboratory data at the time of liver biopsy are shown in Table 1.
| Item | Value | Item | Value | Item | Value |
| WBC | 4450 /μL | BUN | 10 mg/dL | IgG | 1512 mg/dL |
| RBC | 544 × 104 /μL | Cr | 0.8 mg/dL | IgA | 235 mg/dL |
| Hb | 17.0 g/dL | UA | 7.2 mg/dL | IgM | 131 mg/dL |
| Plt | 19.9 × 104/μL | Na | 146 mEq/L | ANA | (-) |
| K | 4.3 mEq/L | HBsAg | (-) | ||
| TP | 7.4 g/dL | Cl | 109 mEq/L | Anti-HCV | (-) |
| Alb | 4.2 g/dL | ||||
| T-Bil | 0.5 mg/dL | T-Chol | 257 mg/dL | TSH | 2.1 μIU/mL |
| AST | 74 U/L | TG | 274 mg/dL | FT3 | 3.2 pg/mL |
| ALT | 102 U/L | HDL-C | 76 mg/dL | FT4 | 1.0 ng/dL |
| LDH | 260 U/L | ||||
| ALP | 202 U/L | FBS | 87 mg/dL | HA | 27 ng/mL |
| GGT | 121 U/L | HbA1c | 5.0 % | 4C7S | 4.2 ng/mL |
| ChE | 390 IU/L | Insulin | 30 μU/mL | ||
| HOMA-IR | 6.4 | Fe | 91 μg/dL | ||
| Ferritin | 77 ng/mL | ||||
| Cu | 89 μg/dL | ||||
| Ceruloplasmin | 26 mg/dL |
Abdominal ultrasonography revealed hyperechogenic liver parenchyma with deep attenuation, indicating the presence of steatosis.
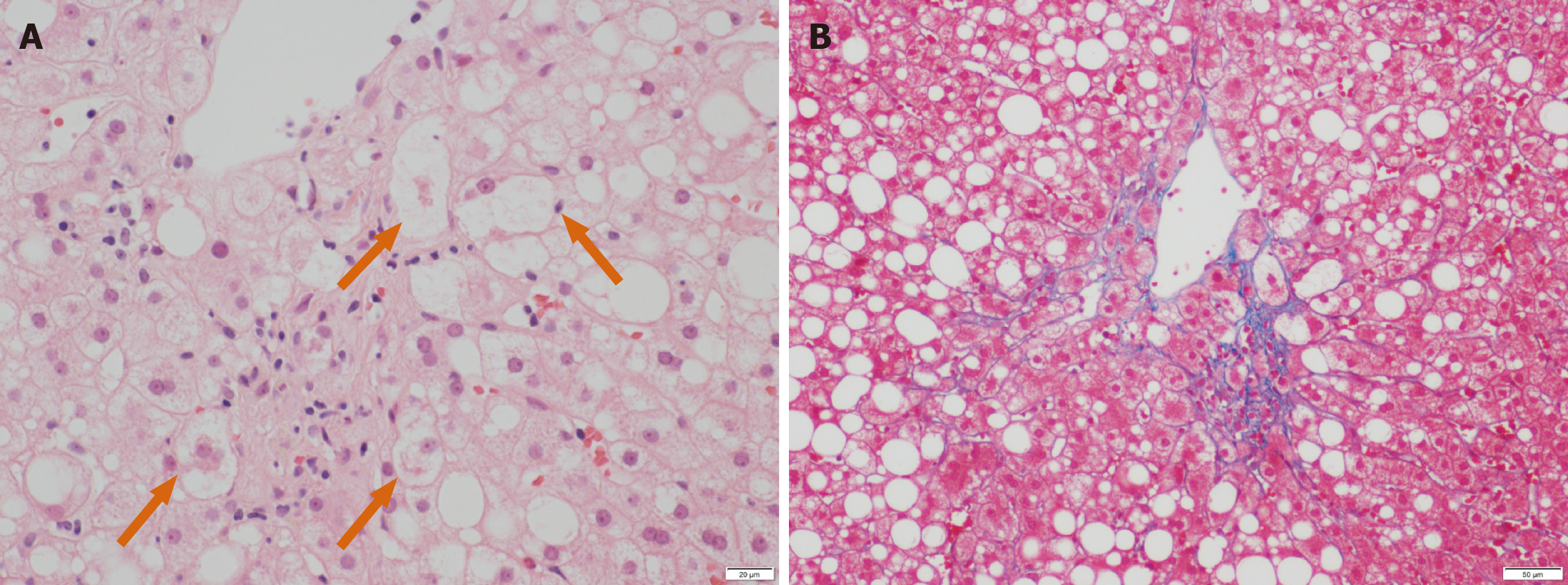
A percutaneous liver biopsy was conducted to evaluate his persistent liver dysfunction. Liver histology showed macrovesicular steatosis, ballooned hepatocytes with eosinophilic inclusion bodies (Figure 1A, arrows), and perisinusoidal fibrosis (Figure 1B), which led to the diagnosis of NASH. According to the criteria proposed by Kleiner et al[6], the score for steatosis, lobular inflammation, and hepatocyte ballooning were 2 (moderate), 1 (few), and 1 (few), respectively, for a total NAFLD activity score of 4. Bezafibrate (400 mg/d) was commenced for his hypertriglyceridemia.
A blood examination to assess the effects of fibrate treatment 1 mo later revealed elevated levels of creatine kinase (CK; 757 U/L, normal 62-287) and myoglobin. The patient denied any symptoms of muscle pain, weakness, or dark urine. The abnormalities in serum CK and myoglobin persisted despite immediate cessation of the fibrate. Thyroid function was normal.
The patient’s face was closely inspected again (Figure 2). He had frontal baldness at the age of 40 and a hatchet face. Manual muscle testing demonstrated that he was very slow opening his hands after grasping forcefully (i.e., grip myotonia). Careful history taking revealed a prior cataract operation. These findings indicated the possibility of MD.
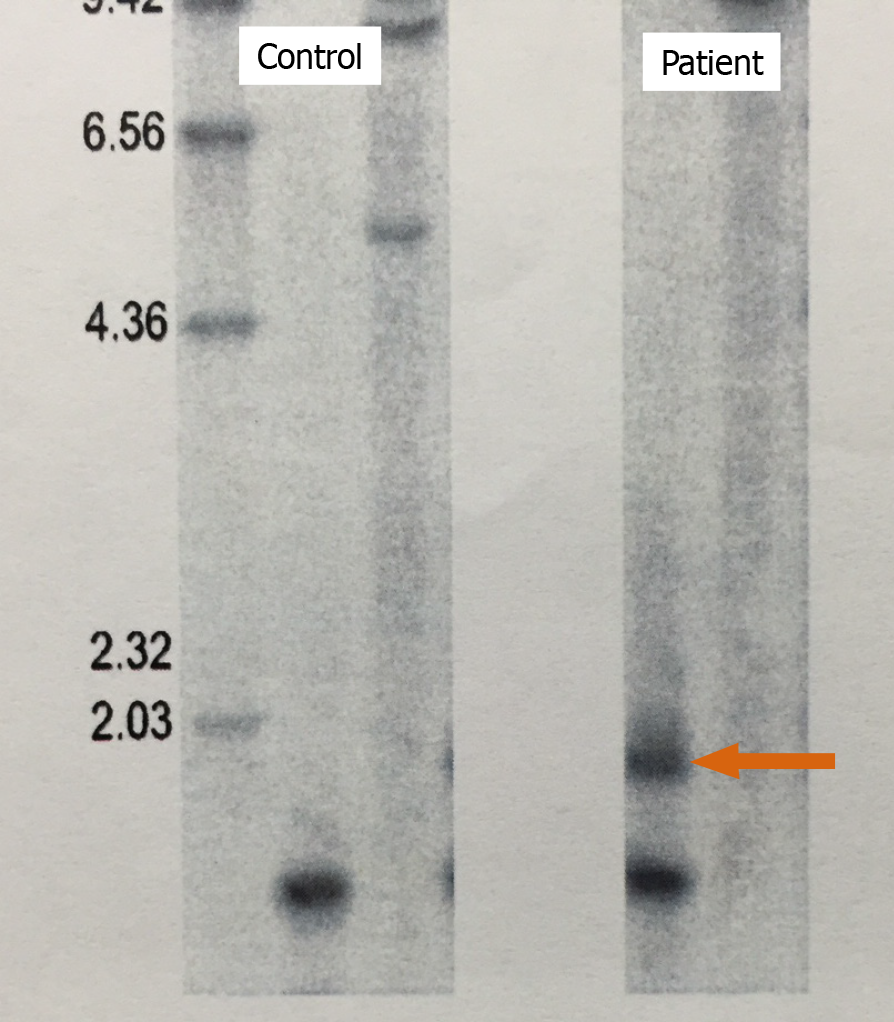
MD is an autosomal dominant disease that affects the dystrophia myotonica protein kinase (DMPK) gene on chromosome 19[5]. The expansion of CTG trinucleotide repeats in the 3’ untranslated region of this gene results in the retention of an expanded RNA repeat that is directly toxic to myocytes. Southern blotting of the patient’s DNA after BamHI digestion detected an additional band caused by CTG repeats (Figure 3), confirming the diagnosis of MD.
The final diagnosis in this patient case was NASH with underlying MD.
The patient is being treated with eicosapentaenoic acid (2700 mg/d) for dyslipidemia and is under calorie restriction by medical dieticians.
Since the diagnosis of MD, the patient has been followed for 14 years. His hepatosteatosis and mild elevation of serum aminotransferases have persisted, with no findings indicating liver cirrhosis or cancer. However, he has suffered from progressive muscle weakness and reduced physical activity.
MD is a relatively common myopathy with almost 100% penetrance that is estimated to afflict approximately 5-7 per 100000 persons worldwide[5]. The condition is characterized by two different mutations: An expansion of CTG repeats in the 3’ untranslated region of the DMPK gene on chromosome 19 and an expansion of CCTG repeats in intron 1 on zinc finger protein 9 on chromosome 3, which are classified as MD type 1 and type 2, respectively. Southern blotting of the present patient led to the diagnosis of MD type 1.
MD is a multisystemic disease exhibiting diverse clinical manifestations, including muscle weakness, myotonia, early cataracts/baldness, arrhythmia, neuropsychiatric symptoms, and gonadal atrophy. The common features of MD type 1 are myotonia and insulin resistance, which are caused by aberrant splicing of chloride channel 1 and insulin receptor RNA in skeletal muscle due to the toxic effect of retained CUG-expanded repeats. Whole-body glucose disposal is reduced by 15%-25% after insulin infusion in MD type 1 patients, and insulin sensitivity, as well as insulin receptor RNA and protein, in the skeletal muscle is significantly decreased. Since peripheral insulin resistance is commonly observed in MD patients, metabolic disturbances, such as dyslipidemia, diabetes, and NAFLD/NASH may co-exist.
Indeed, it was reported that liver test abnormalities were frequent in MD patients[7-10]. Shieh et al[11] prospectively evaluated the abnormalities in liver enzymes and metabolic parameters of 31 MD type 1 patients. The prevalence of diabetes, impaired fasting glucose, and metabolic syndrome (MetS) was approximately 12%, 21%, and 41%, respectively. Furthermore, 44% of MD patients had liver test abnormalities and 42% had NAFLD, as defined by abnormal liver chemistry tests and ultrasonography findings.
We reviewed the clinical data of 7 MD type 1 patients with NAFLD who underwent liver biopsy, including this case, and summarized the pathological features in Table
| Item | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 |
| Steatosis (0-3) | 3 | 1 | 1 | 2 | 1 | 3 | 2 |
| Lobular inflammation (0-3) | 0 | 0 | 0 | 1 | 1 | 3 | 1 |
| Ballooning (0-2) | 1 | 1 | 0 | ND | 1 | 2 | 1 |
| NAFLD activity score (0-8) | 4 | 2 | 1 | ND | 3 | 8 | 4 |
| Diagnosis | NASH | NASH | SS | ND | NASH | NASH | NASH |
| Fibrosis (0-4) | 2 | 1a | 1a | 1 | 1a | 4 | 1a |
| Ref. | Shieh et al[11] | Shieh et al[11] | Shieh et al[11] | Bhardwaj et al[12] | Yamada et al[13] | Ariake et al[14] | Our case |
Vujnic et al[16] examined the prevalence of MetS components in 66 MD type 1 patients and found dyslipidemia to be the most frequent [hypertriglyceridemia (67%) and low HDL cholesterol (35%)]. On the other hand, the prevalence of hypertension, central obesity, and hyperglycemia was relatively low (18%, 13%, and 9%, respectively). This observation indicated that hypertriglyceridemia often co-existed in MD type 1 patients and that some MD patients with minimal-to-mild muscle symptoms might be mistaken as having hypertriglyceridemia and/or NAFLD. Insulin resistance enhances hepatic TG synthesis and the secretion of very-low-density lipoprotein particles, but reduces the activity of lipoprotein lipase, consequently leading to raised circulating TG levels. Fibrates are commonly available for treating hypertriglyceridemia, but myotoxicity and rhabdomyolysis are possible adverse effects. Before commencing fibrate administration for NAFLD/NASH patients with hypertriglyceridemia, serum CK levels should be checked to rule out the possibility of latent MD.
Recent studies documented the contribution of small intestinal bacterial overgrowth and disrupted bile acid metabolism to the pathogenesis of NAFLD/NASH[1,2,17,18]. Tarnopolsky et al[19] documented that 65% of MD type 1 patients with gastrointestinal symptoms exhibited small intestinal bacterial overgrowth using glucose breath hydrogen testing. Additionally, it was reported that specific bile acids, such as dihydroxymono-oxocholanic acid and dihydroxycholanic acid, were detected in the serum of MD type 1 patients and biliary ursodeoxycholic acid was reduced[20], indicating bile acid abnormality accompanied by MD. Although the pathogenesis of NAFLD/NASH is multifactorial, these factors might be associated with NAFLD/NASH in MD.
MD is a relatively common congenital myopathy accompanied by peripheral insulin resistance. However, some MD patients with minimal-to-mild muscle symptoms might be followed as having other diseases associated with insulin resistance, such as hypertriglyceridemia, postprandial hyperglycemia, and NAFLD/NASH. In patients with abnormal liver function tests, muscular diseases might be hidden at all ages[21-24]. When gastroenterologists encounter NAFLD/NASH patients, serum CK should be verified. If hyperCKemia is detected, the possibility of MD may be considered and careful observation of the patient’s face, and history taking for cataracts may help uncover subclinical MD.
We thank Mr. Trevor Ralph for his editorial assistance.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vajro P S-Editor: Wang DM L-Editor: Webster JR P-Editor: Wang LL
| 1. | Tanaka N, Kimura T, Fujimori N, Nagaya T, Komatsu M, Tanaka E. Current status, problems, and perspectives of non-alcoholic fatty liver disease research. World J Gastroenterol. 2019;25:163-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 116] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (4)] |
| 2. | Tanaka N, Aoyama T, Kimura S, Gonzalez FJ. Targeting nuclear receptors for the treatment of fatty liver disease. Pharmacol Ther. 2017;179:142-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 169] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 3. | Komatsu M, Kimura T, Yazaki M, Tanaka N, Yang Y, Nakajima T, Horiuchi A, Fang ZZ, Joshita S, Matsumoto A, Umemura T, Tanaka E, Gonzalez FJ, Ikeda S, Aoyama T. Steatogenesis in adult-onset type II citrullinemia is associated with down-regulation of PPARα. Biochim Biophys Acta. 2015;1852:473-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | Tanaka N, Horiuchi A, Yokoyama T, Kaneko G, Horigome N, Yamaura T, Nagaya T, Komatsu M, Sano K, Miyagawa S, Aoyama T, Tanaka E. Clinical characteristics of de novo nonalcoholic fatty liver disease following pancreaticoduodenectomy. J Gastroenterol. 2011;46:758-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Brook JD, McCurrach ME, Harley HG, Buckler AJ, Church D, Aburatani H, Hunter K, Stanton VP, Thirion JP, Hudson T. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3' end of a transcript encoding a protein kinase family member. Cell. 1992;68:799-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1702] [Cited by in RCA: 1705] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 6. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8229] [Article Influence: 411.5] [Reference Citation Analysis (5)] |
| 7. | Achiron A, Barak Y, Magal N, Shohat M, Cohen M, Barar R, Gadoth N. Abnormal liver test results in myotonic dystrophy. J Clin Gastroenterol. 1998;26:292-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Heatwole CR, Miller J, Martens B, Moxley RT. Laboratory abnormalities in ambulatory patients with myotonic dystrophy type 1. Arch Neurol. 2006;63:1149-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Kalafateli M, Triantos C, Tsamandas A, Kounadis G, Labropoulou-Karatza C. Abnormal liver function tests in a patient with myotonic dystrophy type 1. Ann Hepatol. 2012;11:130-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Spaziani M, Semeraro A, Bucci E, Rossi F, Garibaldi M, Papassifachis MA, Pozza C, Anzuini A, Lenzi A, Antonini G, Radicioni AF. Hormonal and metabolic gender differences in a cohort of myotonic dystrophy type 1 subjects: a retrospective, case-control study. J Endocrinol Invest. 2020;43:663-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Shieh K, Gilchrist JM, Promrat K. Frequency and predictors of nonalcoholic fatty liver disease in myotonic dystrophy. Muscle Nerve. 2010;41:197-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Bhardwaj RR, Duchini A. Non-Alcoholic Steatohepatitis in Myotonic Dystrophy: DMPK Gene Mutation, Insulin Resistance and Development of Steatohepatitis. Case Rep Gastroenterol. 2010;4:100-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Yamada H, Kawanaka M, Okimoto N. A case of myotonic dystrophy during the clinical course of non-alcoholic steatohepatitis. Kawasaki Igaku Zasshi. 2013;93:95-99 (Japanese). |
| 14. | Ariake K, Miura M, Takahashi M, Ueno T, Sato S, Akada M, Maeda S, Fujisaka Y, Ohto T, Naito H. [A case of liver cirrhosis due to non-alcoholic steatohepatitis complicated by myotonic dystrophy]. Nihon Shokakibyo Gakkai Zasshi. 2013;110:1633-1639. [PubMed] |
| 15. | Finsterer J, Karpatova A, Rauschka H, Loewe-Grgurin M, Frank M, Gencik M. Myotonic dystrophy 2 manifesting with non-alcoholic and non-hepatitic liver cirrhosis. Acta Clin Belg. 2015;70:432-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Vujnic M, Peric S, Popovic S, Raseta N, Ralic V, Dobricic V, Novakovic I, Rakocevic-Stojanovic V. Metabolic syndrome in patients with myotonic dystrophy type 1. Muscle Nerve. 2015;52:273-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Bures J, Cyrany J, Kohoutova D, Förstl M, Rejchrt S, Kvetina J, Vorisek V, Kopacova M. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol. 2010;16:2978-2990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 383] [Cited by in RCA: 364] [Article Influence: 24.3] [Reference Citation Analysis (4)] |
| 18. | Tanaka N, Matsubara T, Krausz KW, Patterson AD, Gonzalez FJ. Disruption of phospholipid and bile acid homeostasis in mice with nonalcoholic steatohepatitis. Hepatology. 2012;56:118-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 212] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 19. | Tarnopolsky MA, Pearce E, Matteliano A, James C, Armstrong D. Bacterial overgrowth syndrome in myotonic muscular dystrophy is potentially treatable. Muscle Nerve. 2010;42:853-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Tanaka K, Takeshita K, Takita M. Abnormalities of bile acids in serum and bile from patients with myotonic muscular dystrophy. Clin Sci (Lond). 1982;62:627-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ. 2005;172:367-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1178] [Cited by in RCA: 1175] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 22. | Vajro P, Maddaluno S, Veropalumbo C. Persistent hypertransaminasemia in asymptomatic children: a stepwise approach. World J Gastroenterol. 2013;19:2740-2751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Paolella G, Pisano P, Albano R, Cannaviello L, Mauro C, Esposito G, Vajro P. Fatty liver disease and hypertransaminasemia hiding the association of clinically silent Duchenne muscular dystrophy and hereditary fructose intolerance. Ital J Pediatr. 2012;38:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Veropalumbo C, Del Giudice E, Esposito G, Maddaluno S, Ruggiero L, Vajro P. Aminotransferases and muscular diseases: a disregarded lesson. Case reports and review of the literature. J Paediatr Child Health. 2012;48:886-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |