Published online Sep 27, 2020. doi: 10.4254/wjh.v12.i9.641
Peer-review started: March 9, 2020
First decision: May 5, 2020
Revised: July 10, 2020
Accepted: July 18, 2020
Article in press: July 18, 2020
Published online: September 27, 2020
Processing time: 196 Days and 13.1 Hours
Gallbladder cancer (GBC) is the most common biliary malignancy and has the worst prognosis, but aggressive surgeries [e.g., resection of the extrahepatic bile duct (EHBD), major hepatectomy and lymph node (LN) dissection] may improve long-term survival. GBC may be suspected preoperatively, identified intraoperatively, or discovered incidentally on histopathology.
To present our data together with a discussion of the therapeutic strategies for GBC.
We retrospectively investigated nineteen GBC patients who underwent surgical treatment.
Nearly all symptomatic patients had poor outcomes, while suspicious or incidental GBCs at early stages showed excellent outcomes without the need for two-stage surgery. Lymph nodes around the cystic duct were reliable sentinel nodes in suspicious/incidental GBCs. Intentional LN dissection and EHBD resection prevented metastases or recurrence in early-stage GBCs but not in advanced GBCs with metastatic LNs or invasion of the nerve plexus. All patients with positive surgical margins (e.g., the biliary cut surface) showed poor outcomes. Hepatectomies were performed in sixteen patients, nearly all of which were minor hepatectomies. Metastases were observed in the left-sided liver but not in the caudate lobe. We may need to reconsider the indications for major hepatectomy, minimizing its use except when it is required to accomplish negative bile duct margins. Only a few patients received neoadjuvant or adjuvant chemoradiation. There were significant differences in overall and disease-free survival between patients with stages ≤ IIB and ≥ IIIA disease. The median overall survival and disease-free survival were 1.66 and 0.79 years, respectively.
Outcomes for GBC patients remain unacceptable, and improved therapeutic strategies, including neoadjuvant chemotherapy, optimal surgery and adjuvant chemotherapy, should be considered for patients with advanced GBCs.
Core Tip: Gallbladder cancer (GBC) has a poor prognosis. Our GBC patients who underwent surgeries were retrospectively evaluated. Lymphadenectomy and resection of the extrahepatic bile duct prevented metastases or recurrence in early-stage GBCs but not in advanced GBCs with metastatic lymph nodes or invasion of the nerve plexus. We should reconsider the indications for major hepatectomy, when it is required to achieve negative bile duct margins. There were significant differences in overall and disease-free survival between patients with stages ≤ IIB and ≥ IIIA disease. The median overall survival and disease-free survival were 1.66 and 0.79 years, respectively. Postoperative outcome remains unacceptable.
- Citation: Kamada Y, Hori T, Yamamoto H, Harada H, Yamamoto M, Yamada M, Yazawa T, Tani M, Sato A, Tani R, Aoyama R, Sasaki Y, Zaima M. Surgical treatment of gallbladder cancer: An eight-year experience in a single center. World J Hepatol 2020; 12(9): 641-660
- URL: https://www.wjgnet.com/1948-5182/full/v12/i9/641.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i9.641
Gallbladder cancer (GBC) remains a relatively rare malignancy with a variable presentation[1-7], but it is the most common biliary malignancy and has the worst prognosis[3,4,7]. Although GBC generally carries a poor prognosis[1-4,7-15], complete surgical resection is associated with improved outcomes in GBC patients[1,2,11,14,16-23]. Some surgeons have suggested that aggressive surgeries [e.g., resection of the extrahepatic bile duct (EHBD), extended hepatectomy and intentional lymph node dissection (lymphadenectomy) of the para-aortic lymph nodes (LNs)] may improve long-term survival in patients with advanced GBC[1,2,9,11,13,14,16,18-25]. Metastatic LNs, invasion into the peribiliary nerve plexuses and positive surgical margins of the biliary tract are important prognostic factors[1,7,11,15,18,21].
Although radical resection is considered by many to be the ideal management strategy for GBC[1,2,11,14,16-23], selecting the appropriate surgical procedure based on the depth of the primary tumor and the clinical stage of GBC is still controversial[1-4,10,11,14,16,17,19,20,23,24,26]. Optimal treatment for advanced GBC is unclear[1,2,24], especially in patients with T2 disease according to the tumor-node-metastasis (TNM) classification[27]. The use of radical cholecystectomy and extended procedures with EHBD resection are a matter of debate[1,2,10,14,16,20,24,25]. Radical cholecystectomy involves an extended cholecystectomy with a full-thickness resection and a wedge resection with partial hepatectomy of the gallbladder bed[28-31]. The impact of routine resection of the EHBD, extended LN dissection, and major hepatectomy on outcomes still lacks consensus[1,11,16,20,24].
We retrospectively investigated our patients who underwent surgical treatment for incidentally or non-incidentally diagnosed GBC; their data are presented together with a discussion of the therapeutic strategies for GBC and a literature review.
A total of nineteen GBC patients who underwent surgical treatment at our institution from January 2011 to March 2019 were enrolled in this study. The patients comprised five men and fourteen women, with a mean age of 72.5 ± 11.5 years. Two patients had a past history of other cancers, and one patient had cholelithiasis as a comorbidity. None had viral hepatitis, alcoholic hepatitis or non-alcoholic steatohepatitis. The GBCs were staged according to the TNM classification[27].
This retrospective study was approved by the ethics review committee for clinical studies of our institution. This study was performed in accordance with the ethical guidelines of the Declaration of Helsinki. Informed consent was obtained from all patients before enrollment.
All results are shown as mean ± SD or median (range). Survival rates were calculated using the Kaplan–Meier method, and the log-rank test was used for between-group comparisons. All calculations were performed using SPSS Software (SPSS Inc., Chicago, IL, United States). Values of P < 0.05 were considered statistically significant.
Preoperative profiles are summarized in Table 1. Six patients presented with fever and abdominal pain, and two patients had obstructive jaundice; the remaining eleven patients (57.9%) were asymptomatic (Table 1). The eight symptomatic patients were categorized as stage ≥ IIB (Table 1). Biliary drainage was required in two symptomatic patients with stage IVB disease (Cases 14 and 17) due to obstructive jaundice and acute cholangitis (Table 1).
| Case | Symptomatic (Yes or No) | Preoperative biliary drainage (Yes or No) | PBM (Yes or No) | Preoperative diagnosis (GBC or benign) | Preoperative stage1 | Neoadjuvant chemotherapy (Yes or No) | Preoperative radiation (Yes or No) | Conversion to surgery (Yes or No) | The final stage1 | Adjuvant chemotherapy (Yes or No) | Chemotherapy for metastases/recurrences (Yes or No) |
| 1 | No | No | No | Benign | - | No | No | No | IA | No | No |
| 2 | No | No | Yes | Benign | - | No | No | No | IB | No | No |
| 3 | No | No | No | GBC | IIIB | No | No | No | IIA | No | No |
| 4 | No | No | No | Benign | - | No | No | No | IIA | No | No |
| 5 | No | No | No | GBC | IIA | No | No | No | IIA | No | No |
| 6 | Yes | No | No | Benign | IIB | No | No | No | IIB | No | No |
| 7 | Yes | No | No | Benign | IIB | No | No | No | IIB | No | Yes |
| 8 | No | No | No | Benign | IIIA | No | No | No | IIIA | No | No |
| 9 | Yes | No | No | GBC | IVB | No | No | No | IIIA | No | No |
| 10 | Yes | No | No | GBC | IVA | No | No | Yes | IIIA | No | Yes |
| 11 | No | No | No | GBC | IIA | No | No | No | IIIA | Yes | No |
| 12 | Yes | No | No | GBC | IIIA | No | No | No | IIIA | No | No |
| 13 | No | No | No | GBC | IVA | No | No | No | IIIB | No | No |
| 14 | Yes | Yes | No | GBC | IVB | No | No | No | IIIB | No | No |
| 15 | No | No | No | GBC | IIA | No | No | No | IIIB | No | No |
| 16 | No | No | Yes | GBC | IVB | No | No | No | IIIB | No | No |
| 17 | Yes | Yes | No | GBC | IVB | No | No | No | IVA | No | No |
| 18 | Yes | No | No | GBC | IIIB | No | No | No | IVB | No | No |
| 19 | No | No | No | GBC | IVB | No | No | No | IVB | No | Yes |
Pancreaticobiliary maljunction was observed in two patients (Cases 2 and 16; Table 1). No patients had occupational risk factors for GBC. Six patients were initially diagnosed with benign diseases (two stage IIB patients and one each with stage IA, IB, IIA and IIIA disease) (Table 1), and these patients were classified as having suspicious or incidental GBCs. The preoperative stages of patients who received radical surgery were stage IVB (five patients); stage IIA (three patients); and stages IIB, IIIA, IIIB and IVA (two of each stage; Table 1).
None of the patients received neoadjuvant chemotherapy or radiation. One patient received chemotherapy [four courses with gemcitabine (GEM) + cisplatin (CDDP)] and radiation (60 Gy) for unresectable GBC; this patient underwent conversion surgery (Case 10).
Operative factors are summarized in Table 2. Eleven patients underwent primary extended cholecystectomy with full-thickness resection and partial hepatectomy of the gallbladder bed, and three patients underwent wedge resection of the gallbladder bed as a two-stage surgery (Table 2). Although partial hepatectomy and/or wedge resection of the gallbladder bed were performed in fourteen patients, only one patient received a systemic hepatectomy (right-lobe hepatectomy accompanied by partial caudate lobectomy; Case 17).
| Case | Surgical approach (Primary or two-stage) | Surgical procedures | Caudate | Graphic/Surgical R03 | Postoperative complications | |||
| Resected area1 | Intentional LN dissection2 (Yes or No) | EHBD resection (Yes or No) | ||||||
| POD | Grade4 | |||||||
| 1 | Primary | LC | No | No | No | Yes | - | - |
| 2 | Primary | LC | No | No | No | Yes | - | - |
| 3 | Primary | Extended cholecystectomy | Yes | Yes | No | Yes | Intraperitoneal | 3a |
| 4 | Primary | LC | No | No | No | Yes | - | - |
| 5 | Primary | Extended cholecystectomy | No | No | No | Yes | - | - |
| 6 | Two-stage | Wedge resection | Yes | No | No | Yes | - | - |
| 7 | Two-stage | Wedge resection | Yes | Yes | No | Yes | - | - |
| 8 | Two-stage | Wedge resection | Yes | No | No | Yes | Intraperitoneal | 2 |
| 9 | Primary | Extended cholecystectomy | No | No | No | No | Intraperitoneal | 3a |
| 10 | Primary | Extended cholecystectomy | No | No | No | No | - | - |
| 11 | Primary | Extended cholecystectomy | Yes | Yes | No | No | - | - |
| 12 | Primary | Extended cholecystectomy | No | No | No | Yes | - | - |
| 13 | Primary | Extended cholecystectomy + SSpPD | Yes | Yes | No | Yes | Intraperitoneal | 2 |
| 14 | Primary | Extended cholecystectomy + SSpPD + partial resection of the portal vein | Yes | Yes | No | Yes | - | - |
| 15 | Primary | LC | No | No | No | Yes | - | - |
| 16 | Primary | Extended cholecystectomy + SSpPD | Yes | Yes | No | Yes | Pancreatitis (21) | 2 |
| 17 | Primary | Right hepatectomy + SSpPD | Yes | Yes | Yes | No | Pancreatic | 3b |
| 18 | Primary | Extended cholecystectomy+partial hepatectomy | Yes | No | No | Yes | - | - |
| 19 | Primary | Extended cholecystectomy + SSpPD | Yes | Yes | No | Yes | - | - |
A total of eight patients (three with stage IIB disease and one each with stage IIA, IIB, IIIA, IVA and IVB disease) underwent intentional resections of the EHBD, including five who underwent pancreaticoduodenectomy (Table 2). Lymphadenectomy of the para-biliary, para-arterial and peri-portal venous LNs was performed in eleven patients (57.9%; three with stage IIIB disease; two each with stage IIB, IIIA, IIIB and IVB disease; and one each with stage IIA and IVA disease). The para-aortic LNs were dissected in one stage IVB patient (Case 19).
Laparoscopic cholecystectomy was initially performed in seven patients with a preoperative diagnosis of benign disease, and three of these patients (two with stage IIB disease and one with stage IIIA disease) received two-stage surgery based on pathological findings. All three underwent wedge resection, and one patient received resection of the EHBD (Cases 6, 7 and 8; Table 2). Three patients (stages IA, IB and IIA) did not receive two-stage surgery (Cases 1, 2 and 4).
Curative resections, evaluated as graphic and surgical R0 according to the Japanese guidelines (General rules for clinical and pathological studies on cancer of the biliary tract[32]), were accomplished in fifteen patients. A total of four patients (three with stage IIIA and one with stage IVA disease; Cases 9, 10, 11 and 17) received non-curative resections; the biliary cut surface or surgical surface margins were each positive in two patients (Table 3).
| Case | Tumor occupation1 | Depth2 (invaded organ) | Invasions into the neck and/or cystic duct (Yes or No) | Invasions | Positive margin at the cut end (Yes or No) | Metastatic LNs (Positive LNs/total LNs) | Metastatic LN #12c3 (The LN around the cystic duct) (Positive LNs/sampling LNs) | |||
| Lymphoid duct (Yes or No) | Vessels (Yes or No) | Nerve plexus (Yes or No) | ||||||||
| Ventral or liver side | ||||||||||
| 1 | Gb | Ventral side | m | No | No | No | No | No | - | - |
| 2 | Gf | Ventral side | mp | No | No | No | No | No | 0 / 1 | 0 / 1 |
| 3 | Gf | Ventral side | ss | No | No | No | No | No | 0 / 13 | - |
| 4 | Gf | Ventral side | ss | No | No | No | No | No | - | - |
| 5 | Gf | Ventral side | ss | No | No | Yes | No | No | - | - |
| 6 | Gfbn | Liver side | ss | Yes | No | No | No | No | 0 / 20 | - |
| 7 | Gfbn | Liver side | ss | Yes | No | No | No | No | 0 / 19 | 0 / 1 |
| 8 | Gfb | Liver side | ss | No | No | Yes | No | No | 0 / 16 | - |
| 9 | Gfbn | Liver side | si (liver) | Yes | No | Yes | No | Yes | - | - |
| 10 | Gfb | Liver side | ss | No | No | No | No | No | - | - |
| 11 | Gfbn | Ventral side | si (liver) | Yes | Yes | Yes | Yes | Yes | 0 / 6 | - |
| 12 | Gfbn | Liver side | si (liver, EHBD) | Yes | Yes | No | No | Yes | - | - |
| 13 | Gfb | Liver side | si (liver) | No | Yes | Yes | Yes | No | 2 / 35 | - |
| 14 | Gnb | Liver side | si (liver, EHBD) | Yes | No | Yes | Yes | No | 3 / 13 | - |
| 15 | Gf | Ventral side | ss | No | No | No | No | No | 1 / 1 | 1 / 1 |
| 16 | Gfbn | Ventral side | ss | Yes | Yes | Yes | No | No | 2 / 6 | 1 / 1 |
| 17 | Gfbn | Ventral side | si (EHBD) | Yes | Yes | Yes | Yes | Yes | 0 / 4 | - |
| 18 | Gfbn | Liver side | si (liver) | Yes | No | Yes | No | No | 0 / 4 | - |
| 19 | Gfbn (b) | Liver side | si (liver) | No | Yes | Yes | Yes | No | 18 / 29 | - |
The median operative time was 269 min (range, 32-775 min). Median blood loss was 430 mL (range, 0-3700 mL), and blood transfusions were needed in four patients. Intraoperative histopathological examination was performed in eight patients to assess the primary tumors, cut surface of the biliary tract, nerve plexus and LNs. Although carcinomas were correctly identified in all cases, the extension (i.e., oncological depth) of the primary tumor was misdiagnosed by intra-operative examination in one patient (Case 6).
Postoperative complications were observed in six patients (four cases of intraperitoneal abscess and one each of pancreatitis and pancreatic fistula), and these complications were categorized as grade 2 (n = 3), grade 3a (n = 2) and grade 3b (n = 1) according to the Clavien–Dindo classification[33] (Table 2).
The pathological findings are summarized in Table 3. Diagnoses of tubular, papillary, and poorly-differentiated adenocarcinomas were made in 12, 3 and 2 cases, respectively. Additionally, one adenosquamous cell carcinoma and one neuroendocrine carcinoma were diagnosed. No satellite lesions of dysplasia and/or neoplasia were observed. Only one patient (Case 1) was diagnosed with mucosal cancer (a so-called “m cancer”), and neither a second-stage surgery after laparoscopic cholecystectomy nor an extended cholecystectomy was chosen for this patient (Table 2).
The primary tumor was located in the liver in 10 patients and the ventral side in nine (Table 3). Invasions into the lymphoid duct, vessels and peribiliary nerve plexus were pathologically assessed, respectively. These invasions occurred in six, ten and five cases, respectively (Table 3). Notably, nine GBCs invaded into the GB neck or cystic duct (Table 3).
Positive margins at the biliary cut surface were observed in two patients (Cases 11 and 17), and positive margins at the ventral, dorsal and/or hepatic surfaces were identified in two patients (Cases 10 and 12; Table 3).
The final TNM stage based on pathology[27] was stage IA, IB or IVA in one patient each; stage IIB or IVB in two patients each; stage IIA in three patients; stage IIIB in four patients and stage IIIA in five patients (Table 1).
The median number of harvested LNs was 13 (range, 1–35). Actual LN metastases were observed on pathology in five patients, with a median of two metastatic LNs per patient (range, 1–18) (Table 3). In one patient, six of the seven para-aortic LNs were metastatic (Case 19).
Histopathological assessment of the LNs around the cystic duct (i.e., the 12c LNs according to the Japanese guideline[32]) was performed in four patients with suspicious or incidental GBC, and metastasis was detected in two patients. These two cases had other LN metastases (Cases 15 and 16; Table 3).
Invasion of the nerve plexus was pathologically observed in five patients, and three of these cases (60.0%) were GBCs with invasion into the GB neck and/or cystic duct (Table 3). Although these five patients also underwent resection of the EHBD (Tables 2 and 3), all five patients died from metastases and/or recurrences (Table 4). In two patients with advanced GBC, positive margins were observed at the cut surface of the biliary tract in spite of EHBD resection (Cases 11 and 17; Table 3). Only one of eight patients who received EHBD resection (12.5%) survived without any metastases and/or recurrences (Case 3; Tables 2 and 4), and this patient was categorized as stage IIA (Table 1).
| Case | Metastasis and/or recurrence (Yes or No) | The target sites of metastases/recurrences1 | Postoperative liver metastases (Right and/or left side) | Disease-free survival (yr) | Overallsurvival(yr) | Follow-up term (yr) | Prognosis (Dead or alive) |
| 1 | No | - | - | 2.4 | 2.4 | 2.4 | Alive |
| 2 | No | - | - | 2.3 | 5.0 | 5.0 | Alive |
| 3 | No | - | - | 3.6 | 3.6 | 3.6 | Alive |
| 4 | No | - | - | 3.6 | 3.6 | 3.6 | Alive |
| 5 | Yes | Liver | Right side | 0.4 | 0.8 | 0.8 | Alive |
| 6 | No | - | - | 8.0 | 8.0 | 8.0 | Alive |
| 7 | Yes | Peritoneal cavity | - | 2.0 | 2.8 | 2.8 | Dead |
| 8 | Yes | Liver | Right side | 1.4 | 2.0 | 2.0 | Dead |
| 9 | Yes | Peritoneal cavity | - | 0.1 | 0.2 | 0.2 | Dead |
| 10 | Yes | Local site, hepatic hilum | - | 1.2 | 2.6 | 2.6 | Dead |
| 11 | Yes | Perineal cavity | - | 0.8 | 1.0 | 1.0 | Dead |
| 12 | Yes | Local site, liver | Left side | 0.1 | 0.2 | 0.2 | Dead |
| 13 | Yes | Peritoneal cavity, paraaortic LN | - | 0.4 | 1.7 | 1.7 | Dead |
| 14 | Yes | Local site | - | 0.9 | 3.4 | 3.4 | Dead |
| 15 | Yes | Paraaortic LN | - | 4.0 | 5.4 | 5.4 | Alive |
| 16 | Yes | Bone, paraaortic LN | - | 2.0 | 7.5 | 7.5 | Alive |
| 17 | Yes | Liver remnant | Left side | 0.6 | 0.7 | 0.7 | Dead |
| 18 | Yes | Liver | Right and left sides | 0.2 | 0.3 | 0.3 | Dead |
| 19 | Yes | Liver, local site, paraaortic LN | Left side | 0.6 | 1.0 | 1.0 | Dead |
A total of fifteen patients received hepatectomies (Table 2). Pathological examination of the resected liver specimens revealed direct invasion into the liver in seven patients (Table 3). Liver metastases occurred postoperatively in six patients (three in segment 4, one each in segments 5 and 7, and one in the majority of the liver); four of the targeted segments were located in the left side of the liver (66.7%) (Table 4). Four of these six patients’ primary tumors showed vessel invasion (Table 3). Four metastatic tumors occurred in the left lobe (Table 4), and no metastases were observed in the caudate lobe.
Only one patient (stage IIIA) received adjuvant chemotherapy: Six courses of S-1 (Case 11). Three patients received chemotherapy after detection of metastases and/or recurrences. The regimens employed were GEM + CDDP, GEM + CDDP followed by GEM + S1, and CDDP + vinblastine, respectively (Cases 7, 10 and 19).
The clinical courses of all patients were followed for a median of 2.03 years (0.15–5.05 years). Metastasis and/or recurrence was observed in fourteen patients (74.7%); the five patients without metastasis/recurrence had stage IIA disease (two patients) and stage IA, IB or IIB disease (one of each) (Tables 1 and 4). Metastasis occurred in the liver (six patients), para-aortic LNs (four patients) intraperitoneal dissemination (four patients), and local recurrence (four patients) (Table 4).
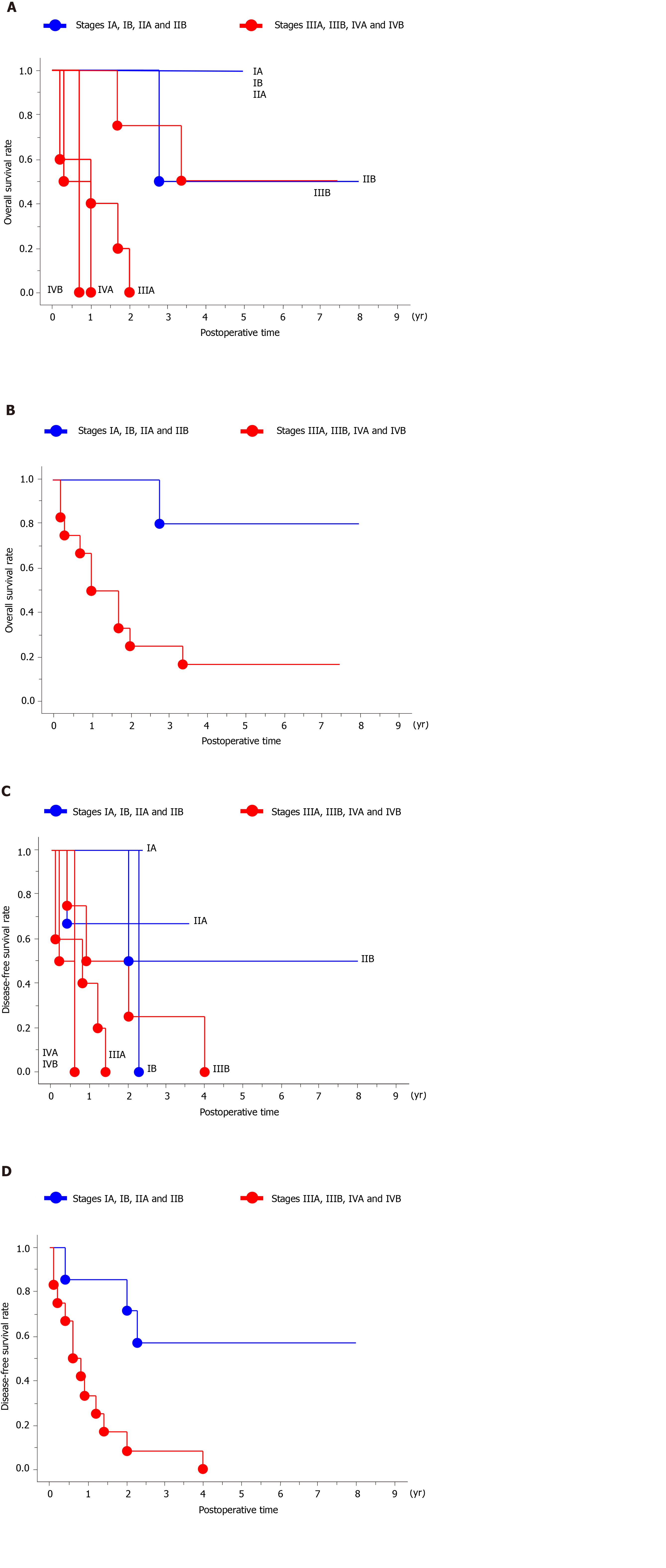
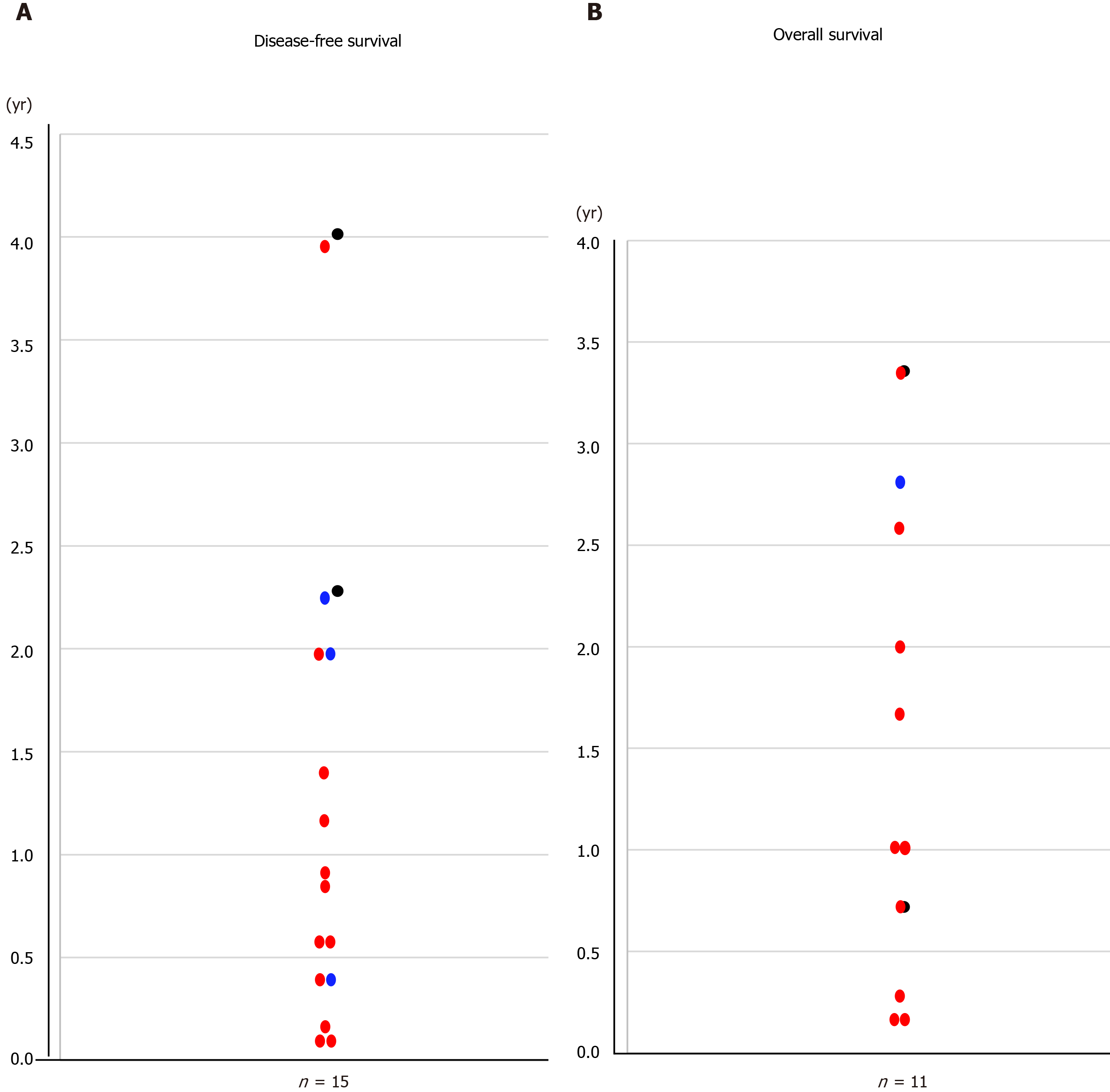
The overall survival curves by stage are shown in Figure 1A; there was a significant difference in overall survival between patients with stage ≤ IIB and stage ≥ IIIA disease (P = 0.0080; Figure 1B). The overall median survival in the eleven patients with oncological death was 1.66 years (range, 0.16-3.36 years; Figure 2A).
The disease-free survival curves by stage are shown in Figure 1C; there was a significant difference in disease-free survival between patients with stage ≤ IIB and ≥ IIIA disease (P = 0.0054; Figure 1D). The disease-free interval of the fourteen patients with recurrences was 0.79 years (0.12–4.01 years; Figure 2B).
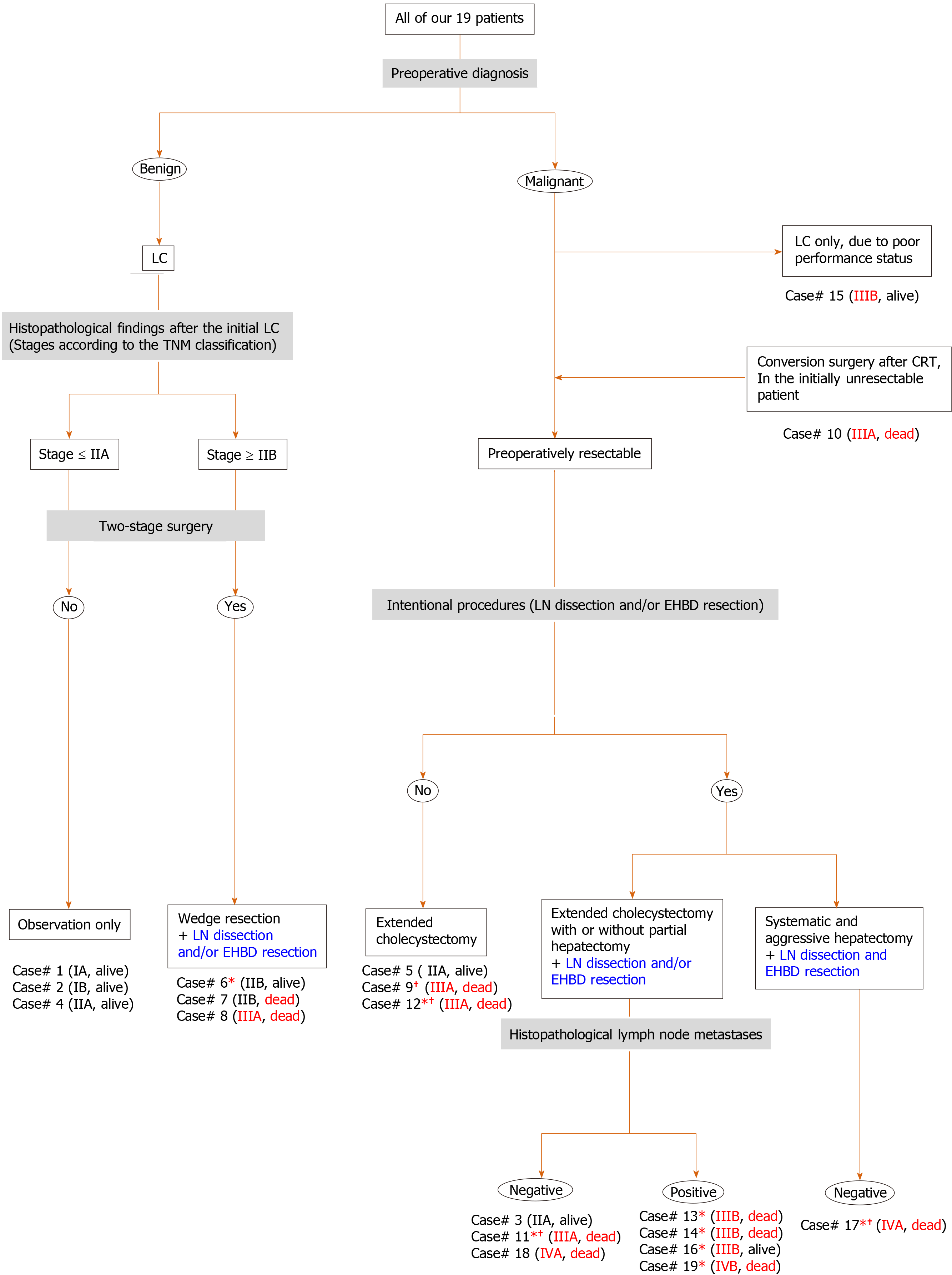
The specific characteristics of each patient may be difficult to understand. Characteristic findings and important points (e.g., preoperative diagnosis, surgical treatment, histopathological assessments, stage according to the TNM classification and prognosis) are summarized in Figure 3.
In GBC patients, the presence of associated symptoms is considered a relative contraindication to radical resection as these patients have a poor prognosis and high postoperative morbidity[1-3,9]. Of our eight symptomatic patients (Table 1), all were categorized as stage ≥ IIB, and seven (87.5%) showed poor outcomes (Table 4). Although jaundiced patients with advanced GBC should be considered as candidates for surgical resection, careful evaluation is important before undertaking aggressive surgery in this population[2,9].
Certain factors (e.g., liver injury and occupational history) are associated with an increased risk of developing GBC[12]. In particular, data support a relationship between pancreaticobiliary maljunction and GBC[34]. Among our patient population, only two cases (10.5%) had pancreaticobiliary maljunction.
Gallbladder cancer can be suspected preoperatively, identified intraoperatively, or discovered incidentally on final pathology[2-4,26,35]. In cases with suspicious or incidental GBCs, lesions tend to be stage T2 or T3 by the TNM classification[26,27]. Once GBC is diagnosed, a two-stage surgery should be considered[26], although simple or extended cholecystectomy produces comparable survival outcomes in GBC patients with T1 lesions[17]. Among our patients, the three with suspicious/incidental GBC of lower stages showed excellent outcomes without two-stage surgery (Cases 1, 2 and 4). Perforation during the initial surgery carries a higher risk of dissemination[35], although extended resection of adjacent organs may not be necessary in order to achieve radicality even in this instance[25]. Therefore, radical cholecystectomy (e.g., full-thickness resection and extended cholecystectomy) should be considered in the absence of unexpected rupture among patients with suspicious and incidental GBCs. In this study, we assessed the importance of metastasis to the 12c LNs[32] in suspicious and incidental GBCs. Of the four patients with suspicious/incidental GBCs in which the 12c LNs were histologically assessed, the two patients with 12c LN metastasis (Cases 15 and 16) had other metastatic LNs, while the two patients without 12c LN metastasis did not have other LN metastases (Cases 2 and 7). Even though the number of sampled LNs was small (1 or 2), the 12c LNs seem to be useful sentinel nodes in patients with suspicious and incidental GBCs. For patients with suspicious/incidental GBCs, full-thickness cholecystectomy and sampling of the 12c LNs may be suitable for the initial surgery, although two-stage surgery may be required based on histological findings.
GBCs tend to invade adjacent structures, including the liver parenchyma, bile duct, major vessels, nerve plexuses and regional LNs[1,11,14,20]. Metastatic LNs and bile duct margins are important prognostic factors[1,7,11,15,18-21], and invasion into the nerve plexuses is also a significant independent prognostic factor[11]. Intentional dissection of the LNs and nerve plexuses is still controversial[1,2,11,15,16,19,20,22,24,25]. Extended dissection of LNs and/or nerve plexuses should involve en bloc resection of the EHBD[1,11,16,20,24,36]. From the viewpoint of achieving curative resection, EHBD resection may have some advantages[1,11,16,20]. However, although routine EHBD resection in GBCs without bile duct invasion is associated with improvements in harvested LNs and local recurrence rate, this procedure does not improve the survival rate and is associated with a higher morbidity rate[14,16,24]. Among our patients, four of the five patients with metastatic LNs received intentional extended LN dissection, but all these patients developed metastases and/or recurrences (Cases 13, 14, 16 and 19). Extended LN dissection worked well in only one patient with early stage disease (Case 3). Moreover, nerve plexus invasion was observed in five patients, and all five of these patients succumbed to oncological death despite EHBD resection (Cases 11, 13, 14, 17 and 19). Of patients undergoing EHBD resection, only one patient with early stage disease survived without any metastases and/or recurrences (Case 3). Unfortunately, extended dissection of the nerve plexuses was not beneficial for advanced GBC patients with invasion into the GB neck and/or cystic duct in our study. Similar to previous reports[1,9,11,15,19,20,24], our advanced GBC patients with metastatic LNs and invasion into the nerve plexus experienced poor outcomes even after aggressive surgery including removal of the EHBD.
Major hepatectomy for biliary hilar malignancy may involve intraoperative risks and/or postoperative mortality[14,37]. Extended resection with partial hepatectomy and dissection of the regional LNs may be an option for GBC patients with T2 lesions[1,2,21], but major hepatectomy should only be performed in select cases[1,10]. Minimal hepatectomy should be performed to achieve curative resection whenever possible[1]. Drainage from the gallbladder is an important metastatic pathway[6,36], especially for liver metastases[6,36]. Previous publications have suggested that biliary drainage into the left-sided liver, including the caudate lobe, has an impact on metastasis[6,21,23,36,38,39]. Some physicians have documented that the caudate lobe is an important target site for metastases[21,23,26,38,39] and recommend complete resection of the caudate lobe, including Spiegel’s lobe[21,36,38,39]. Among our patient population, liver resections were performed in fifteen patients, but a systematic hepatectomy was only performed in one patient (Case 17). Despite hepatic resection, the liver was preserved as a target site of metastases in our patients. We observed metastases in the left-sided liver (Cases 12, 17, 18 and 19) but did not detect metastases in the caudate lobe in any of the eighteen patients with caudate lobe remnants. Accordingly, we suggest that complete resection of the caudate lobe may not be necessary in all cases. Positive surgical bile duct margins should be considered a strong negative prognostic factor[1], and our two patients with positive biliary tract margins showed very poor outcomes (Cases 11 and 17). Therefore, although minimal hepatectomy should be considered for some GBC patients if complete resection can be accomplished by this method[1,10], aggressive hepatectomy is mandatory if needed to accomplish negative biliary margins[1,20].
Overall survival and disease-free survival are shown in Figure 1, both of which clearly differ for patients with stage ≤ IIB vs stage ≥ IIIA disease. The median overall and disease-free survival times for patients were 1.66 and 0.79 years, respectively. Aggressive treatment, including extended surgeries, may be beneficial for early-stage GBC[1,25], especially in populations with disease stages ≤ IIB. However, the poor prognosis of advanced GBC has been documented even after extended and/or aggressive procedures[1,9,15,20,24]. Despite this poor prognosis, the role of chemotherapy and radiotherapy for GBCs remains controversial[4]. Among our patients, only one patient (who became resectable) received chemotherapy and radiation before conversion surgery (Case 10), and adjuvant chemotherapy was only performed in one patient with a non-curative resection (Case 11). Some chemotherapy regimens have been developed for GBC[1,10], and certain regimens (e.g., S-1 + cisplatin + GM, S-1 + GM, GM + cisplatin, nanoparticle albumin-bound-paclitaxel + cisplatin + GM and capecitabine) are considered to be useful[1,10,40-42]. Considering the frequency of early recurrence and/or metastases, the timing of surgery and use of neoadjuvant chemotherapy may need to be re-evaluated. Aggressive adjuvant chemotherapy should be considered[10], and surgical resection of metastases and/or recurrences may offer a better chance of long-term survival in select patients[18]. As other physicians have previously documented[1,2,4,7,8,10,21], standard therapeutic strategies need to be established for advanced GBC.
Here, we reviewed the main literature in this field[20,43-71], and crucial remarks in each are summarized in Table 5. The highest volume center in Japan is Nagoya University (Nagoya, Japan), and they continuously documented their decennial results[14,18,20,22,51,72-75]. Also, we analyzed the existing guidelines in Europe (in 2017)[76,77], United States (in 2019)[78] and Japan (in 2019)[79] in detail. Especially for a comparison purpose, important comments on therapeutic strategies for GBCs in each guideline are summarized in Table 5. Central inferior bisegmentectomy (i.e., hepatectomy of segments 4b and 5) is suitable for GBC[80-82], although we employed radical or extended cholecystectomy. Skillful surgeons conversed on the topic of minimally invasive approaches to GBC[83-87], and we all may have to focus on these advanced manipulations in the near future.
| Ref. | Country | Year | Remarks | Comments in each guideline |
| [43] | Germany | 2013 | Prognosis of incidental GBC was not influenced by the primary access technique. | Conventional open surgery is recommended for suspicious GBCs. |
| [44] | Japan | 2003 | Preoperative information indicated strategies for surgical treatment of GBCs. | Conventional open surgery is recommended for suspicious GBCs. |
| [45] | Japan | 1996 | Outcome of radical surgery for GBCs was evaluated according to the TNM classification. | Intentional LN dissection and prophylactic EHBD resection are considered for potential pathological invasions. |
| [46] | Japan | 2004 | Strong consideration should be given to intentional LN dissection and EHBD resection. | Intentional LN dissection and prophylactic EHBD resection are considered for potential pathological invasions. |
| [47] | Japan | 2013 | Hepatectomy of segments 4a and 5 was not superior to extended cholecystectomy in patients with pathological T2. | The EHBD resection does not improve the prognosis in patients with T2N0. |
| [48] | Korea | 2013 | Radical resection (R0 surgery) including EHBD resection should be considered in patients with T2 and T3 (A single-centre retrospective study). | The EHBD resection does not improve the prognosis in patients with T2N0. |
| [49] | Japan | 2014 | Surgery might not be indicated for patients with advanced invasion to the EHBD and visible paraaortic LN metastasis (A single-centre retrospective study). | The EHBD resection does not improve the prognosis in patients with T2N0. |
| [50] | Korea | 2015 | Two-stage surgery was highly recommended for patients with pathological T2 (A single-centre retrospective study). | The EHBD resection does not improve the prognosis in patients with T2N0. |
| [51] | Japan | 2015 | Combined treatment of intentional LN dissection and prophylactic EHBD resection had no survival impact for patients without the EHBD invasion (A single-centre retrospective study). | The EHBD resection does not improve the prognosis in patients with T2N0. |
| [52] | Japan | 2012 | Hepatectomy procedures (e.g., systematic, segmental and partial resections) did not significantly affect surgical outcomes | Radical resection (R0 surgery) is the most important prognostic factor |
| [53] | United States | 2008 | GBC was commonly diagnosed incidentally, and two-stage surgery revealed a high incidence of residual disease. | Overall prognosis is poor. |
| [54] | United States | 2004 | Surgeries were not routinely indicated for advanced GBCs with jaundice. | Jaundice is common in patients with advanced GBC. |
| [55] | France | 2011 | EHBD resection increased postoperative morbidity but did not improve survival. | Partial hepatectomy without EHBD resection indicates incidental GBC. |
| [56] | Korea | 2011 | Extended cholecystectomy was not advantageous over simple cholecystectomy for patients with T1b. | Simple cholecystectomy is adequate therapy for patients with T1a. |
| [57] | United States | 2007 | Radical resection for patients with T2 and T3 resulted in a significant survival advantage compared with simple cholecystectomy. | Advantages of radical resection including extended hepatectomy for incidental GBC and patients with T1b are controversial. |
| [58] | Canada | 2008 | Intentional LN dissection and EHBD resection may have stage-specific effects on survival. | Radical resection improves survivals in patients with T1b and T2 (not in patients with T3). |
| [59] | Korea | 2008 | Cholecystectomy with intentional LN dissection without EHBD resection was recommended for patients with T1b. | Advantages of radical resection including extended hepatectomy for incidental GBC and patients with T3b are controversial. |
| [60] | United States | 2009 | Radical resection had survival advantage for patients with T1b and T2. | Radical resection improves survival in patients with T1b and T2 (not in patients with T3). |
| [61] | United States | 2011 | Extended surgery including intentional LN dissection improved survival for incidental GBC | Aggressive surgeries including hepatectomy, LN dissection and EHBD resection are indicated for patients with T3, localized hepatic invasion and regional LN metastases. |
| [62] | Japan | 2012 | Extended cholecystectomy was adequate for patients with T2, and more aggressive surgeries were indicated for patients with T3, localized hepatic invasion and regional LN metastases. | Aggressive surgeries including hepatectomy, LN dissection and EHBD resection are indicated for patients with T3, localized hepatic invasion and regional LN metastases. |
| [63] | United States | 2009 | Major hepatectomy and EHBD resection were significantly associated with perioperative morbidity, and were not mandatory in all cases. | Independent prognostic factors associated with survival are T factor, N factor, pathological poor differentiation and EHBD involvement. |
| [64] | United States | 2007 | EHBD resection did not yield a greater count of LNs. Over one-third had residual disease in the EHBD at two-stage surgery. | During two-stage surgery, EHBD resection is indicated for negative cystic duct margins. |
| [65] | United States | 2000 | Radical resection can provide long-term survival, even for large tumors with extensive liver invasion. | Aggressive surgeries including hepatectomy, LN dissection and EHBD resection are indicated for patients with T3, localized hepatic invasion and regional LN metastases. |
| [66] | United States | 2007 | Incidental GBCs during laparoscopic cholecystectomy did not indicate immediate conversion to open surgery, and these patients should be referred to a tertiary care center for further surgery. | There was no difference in surgical deficit between immediate resection at the initial hospital and delayed resection at tertiary care center. |
| [67] | France | 2011 | Jaundice was a poor prognostic factor, but radical resection had survival benefit especially in highly selected patients with N0. | Radical resection improves survival in patients with N0. |
| [20] | Japan | 2011 | Patients with advanced GBCs were candidates for EHBD resection, if radical resection (R0) was achievable. | Radical resection improves survival in patients with EHBD invasion. |
| [68] | India | 2016 | Chemoradiotherapy in unresectable GBCs resulted in the resectability, and subsequent radical surgery (R0) had survival benefit. LN regression could serve as a predictor of response to radiochemotherapy. | Chemoradiotherapy in unresectable GBCs may result in the resectability, and conversion surgery (R0) has survival benefit. |
| [69] | United States | 2017 | Radical surgeries after favorable responses to neoadjuvant chemotherapies were associated with long-term survival in selected patients. | Chemoradiotherapy in unresectable GBCs may result in the resectability, and conversion surgery (R0) has survival benefit. |
| [70] | United States | 2011 | Pathological assessment of at least 6 LNs was important. | Patients with incidental GBC and T2 associated with residual tumor, and should undergo surgery to reflect the adverse outcome. |
| [71] | Canada | 2012 | Adjuvant radiochemotherapy had the greatest benefit in patients with positive LNs and R1 disease. | Adjuvant radiochemotherapy is beneficial. |
This study was as a comparative, observational and retrospective study performed at a single institution, and our sample size was small. Also, this study was not a randomized controlled trial. Accordingly, we cannot rule out bias and other potential limitations. Of course, we understood that our study’s conclusions must be interpreted with extreme caution. However, we hope this report will inform the management of GBCs in future patients.
In conclusion, even in the current era, the survival of GBC patients remains unacceptable. Improved therapeutic strategies, including neoadjuvant chemotherapy, optimal surgery, and adjuvant chemotherapy, should be developed to better treat patients with advanced GBC.
Gallbladder cancer (GBC) is the most common biliary malignancy with the worst prognosis, but aggressive surgeries may improve long-term survival.
We evaluated our own data along with a discussion of therapeutic strategies for GBC.
Nineteen GBC patients who underwent surgical treatment were enrolled in this study.
We retrospectively investigated our patients with incidentally or non-incidentally diagnosed GBC.
Suspicious or incidental GBCs at earlier stages showed excellent outcomes without the need for two-stage surgery. Lymph nodes (LNs) around the cystic duct were reliable sentinel nodes in suspicious/incidental GBCs. Extended lymphadenectomy and resection of the extrahepatic bile duct (EHBD) prevented metastases or recurrence in early-stage GBCs but not in advanced GBCs with metastatic LNs or invasion of the nerve plexus. All patients with positive surgical margins showed poor outcomes, and we may need to reconsider the indications for major hepatectomy, minimizing its use except when it is required to accomplish negative bile duct margins. There were significant differences in overall and disease-free survival between patients with stages ≤ IIB and ≥ IIIA disease. The median overall survival and disease-free survival were 1.66 and 0.79 years, respectively.
Outcomes for GBC patients remain unacceptable.
Improved therapeutic strategies should be considered for patients with advanced GBCs.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: American Association for the Study of Liver Diseases (Member), American College of Surgeons (Fellow), Japanese Board of Cancer Therapy (Certified Physician), The Japanese Society for Transplantation (Certified Physician), The Japan Society of Hepatology (Certified Surgeon), The Japanese Society of Gastroenterological Surgery (Certified Surgeon), The Japanese Society of Gastroenterology (Certified Surgeon), Japan Surgical Society (Certified Surgeon).
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gumbs A, Qin R, Ramia JM S-Editor: Wang JL L-Editor: Webster JR P-Editor: Wang LL
| 1. | Ebata T, Ercolani G, Alvaro D, Ribero D, Di Tommaso L, Valle JW. Current Status on Cholangiocarcinoma and Gallbladder Cancer. Liver Cancer. 2016;6:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | PDQ Adult Treatment Editorial Board. Gallbladder cancer treatment (PDQ): Health Professional Version. PDQ Cancer Information Summaries. Rockville: Bethesda, 2002. |
| 3. | Wernberg JA, Lucarelli DD. Gallbladder cancer. Surg Clin North Am. 2014;94:343-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 4. | Shukla SK, Singh G, Shahi KS, Bhuvan, Pant P. Staging, Treatment, and Future Approaches of Gallbladder Carcinoma. J Gastrointest Cancer. 2018;49:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Yokoyama Y, Nishio H, Ebata T, Abe T, Igami T, Oda K, Nimura Y, Nagino M. New classification of cystic duct carcinoma. World J Surg. 2008;32:621-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Ko K, Kamiya J, Nagino M, Oda K, Yuasa N, Arai T, Nishio H, Nimura Y. A study of the subvesical bile duct (duct of Luschka) in resected liver specimens. World J Surg. 2006;30:1316-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Ertel AE, Bentrem D, Abbott DE. Gall Bladder Cancer. Cancer Treat Res. 2016;168:101-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Mehrotra R, Tulsyan S, Hussain S, Mittal B, Singh Saluja S, Singh S, Tanwar P, Khan A, Javle M, Hassan MM, Pant S, De Aretxabala X, Sirohi B, Rajaraman P, Kaur T, Rath GK. Genetic landscape of gallbladder cancer: Global overview. Mutat Res. 2018;778:61-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Dasari BVM, Ionescu MI, Pawlik TM, Hodson J, Sutcliffe RP, Roberts KJ, Muiesan P, Isaac J, Marudanayagam R, Mirza DF. Outcomes of surgical resection of gallbladder cancer in patients presenting with jaundice: A systematic review and meta-analysis. J Surg Oncol. 2018;118:477-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Zaidi MY, Maithel SK. Updates on Gallbladder Cancer Management. Curr Oncol Rep. 2018;20:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Yamaguchi R, Nagino M, Oda K, Kamiya J, Uesaka K, Nimura Y. Perineural invasion has a negative impact on survival of patients with gallbladder carcinoma. Br J Surg. 2002;89:1130-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Sharma A, Sharma KL, Gupta A, Yadav A, Kumar A. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: Recent update. World J Gastroenterol. 2017;23:3978-3998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 212] [Cited by in RCA: 266] [Article Influence: 33.3] [Reference Citation Analysis (4)] |
| 13. | Fairweather M, Balachandran VP, D'Angelica MI. Surgical management of biliary tract cancers. Chin Clin Oncol. 2016;5:63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Kondo S, Nimura Y, Hayakawa N, Kamiya J, Nagino M, Uesaka K. Extensive surgery for carcinoma of the gallbladder. Br J Surg. 2002;89:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Kondo S, Nimura Y, Hayakawa N, Kamiya J, Nagino M, Uesaka K. Regional and para-aortic lymphadenectomy in radical surgery for advanced gallbladder carcinoma. Br J Surg. 2000;87:418-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 151] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Shukla PJ, Barreto SG. Systematic review: should routine resection of the extra-hepatic bile duct be performed in gallbladder cancer? Saudi J Gastroenterol. 2010;16:161-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Lee H, Kwon W, Han Y, Kim JR, Kim SW, Jang JY. Optimal extent of surgery for early gallbladder cancer with regard to long-term survival: a meta-analysis. J Hepatobiliary Pancreat Sci. 2018;25:131-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 18. | Takahashi Y, Ebata T, Yokoyama Y, Igami T, Sugawara G, Mizuno T, Nimura Y, Nagino M. Surgery for Recurrent Biliary Tract Cancer: A Single-center Experience With 74 Consecutive Resections. Ann Surg. 2015;262:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Sasaki E, Nagino M, Ebata T, Oda K, Arai T, Nishio H, Nimura Y. Immunohistochemically demonstrated lymph node micrometastasis and prognosis in patients with gallbladder carcinoma. Ann Surg. 2006;244:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Nishio H, Ebata T, Yokoyama Y, Igami T, Sugawara G, Nagino M. Gallbladder cancer involving the extrahepatic bile duct is worthy of resection. Ann Surg. 2011;253:953-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Nagino M, Kamiya J, Nishio H, Ebata T, Arai T, Nimura Y. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg. 2006;243:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 404] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 22. | Nishio H, Nagino M, Ebata T, Yokoyama Y, Igami T, Nimura Y. Aggressive surgery for stage IV gallbladder carcinoma; what are the contraindications? J Hepatobiliary Pancreat Surg. 2007;14:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Nagino M, Kamiya J, Kanai M, Uesaka K, Sano T, Yamamoto H, Hayakawa N, Nimura Y. Right trisegment portal vein embolization for biliary tract carcinoma: technique and clinical utility. Surgery. 2000;127:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 115] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Nigri G, Berardi G, Mattana C, Mangogna L, Petrucciani N, Sagnotta A, Aurello P, D'Angelo F, Ramacciato G. Routine extra-hepatic bile duct resection in gallbladder cancer patients without bile duct infiltration: A systematic review. Surgeon. 2016;14:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Sternby Eilard M, Lundgren L, Cahlin C, Strandell A, Svanberg T, Sandström P. Surgical treatment for gallbladder cancer - a systematic literature review. Scand J Gastroenterol. 2017;52:505-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Choi KS, Choi SB, Park P, Kim WB, Choi SY. Clinical characteristics of incidental or unsuspected gallbladder cancers diagnosed during or after cholecystectomy: a systematic review and meta-analysis. World J Gastroenterol. 2015;21:1315-1323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (2)] |
| 27. | Union for International Cancer Control (UICC). TNM classification of malignant tumors. 8th edition. New York: Wiley Blackwell, 2017. |
| 28. | Kasumova GG, Tabatabaie O, Najarian RM, Callery MP, Ng SC, Bullock AJ, Fisher RA, Tseng JF. Surgical Management of Gallbladder Cancer: Simple Versus Extended Cholecystectomy and the Role of Adjuvant Therapy. Ann Surg. 2017;266:625-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Tewari M, Kumar S, Shukla S, Shukla HS. Analysis of wedge resection of gallbladder bed and lymphadenectomy on adequate oncologic clearance for gallbladder cancer. Indian J Cancer. 2016;53:552-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Goetze TO. Gallbladder carcinoma: Prognostic factors and therapeutic options. World J Gastroenterol. 2015;21:12211-12217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 173] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (1)] |
| 31. | Goetze TO, Paolucci V. [Immediate radical re-resection of incidental T1b gallbladder cancer and the problem of an adequate extent of resection (results of the German Registry "Incidental Gallbladder Cancer")]. Zentralbl Chir. 2014;139 Suppl 2:e43-e48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Japanese Society of Hepato-Biliary-Pancreatic Surgery. General rules for clinical and pathological studies on cancer of the biliary tract. 6th edition. Tokyo: Kanehara, 2013. |
| 33. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24805] [Article Influence: 1181.2] [Reference Citation Analysis (0)] |
| 34. | Hori T, Aisu Y, Yamamoto M, Yasukawa D, Iida T, Yagi S, Taniguchi K, Uemoto S. Laparoscopic approach for choledochojejunostomy. Hepatobiliary Pancreat Dis Int. 2019;18:285-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Søreide K, Guest RV, Harrison EM, Kendall TJ, Garden OJ, Wigmore SJ. Systematic review of management of incidental gallbladder cancer after cholecystectomy. Br J Surg. 2019;106:32-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 36. | Uesaka K, Yasui K, Morimoto T, Torii A, Yamamura Y, Kodera Y, Hirai T, Kato T, Kito T. Visualization of routes of lymphatic drainage of the gallbladder with a carbon particle suspension. J Am Coll Surg. 1996;183:345-350. [PubMed] |
| 37. | Nagino M, Kamiya J, Arai T, Nishio H, Ebata T, Nimura Y. One hundred consecutive hepatobiliary resections for biliary hilar malignancy: preoperative blood donation, blood loss, transfusion, and outcome. Surgery. 2005;137:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 38. | Nimura Y, Hayakawa N, Kamiya J, Kondo S, Shionoya S. Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg. 1990;14:535-43; discussion 544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 320] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 39. | Kosuge T, Yamamoto J, Shimada K, Yamasaki S, Makuuchi M. Improved surgical results for hilar cholangiocarcinoma with procedures including major hepatic resection. Ann Surg. 1999;230:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 242] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 40. | Moriwaki T, Ishida H, Araki M, Endo S, Yoshida S, Kobayashi M, Hamano Y, Sugaya A, Shimoyamada M, Hasegawa N, Imanishi M, Ito Y, Sato D, Hyodo I. Phase I study of gemcitabine, cisplatin, and S-1 combination therapy for patients with untreated advanced biliary tract cancer. J Hepatobiliary Pancreat Sci. 2015;22:669-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Shroff RT, Javle MM, Xiao L, Kaseb AO, Varadhachary GR, Wolff RA, Raghav KPS, Iwasaki M, Masci P, Ramanathan RK, Ahn DH, Bekaii-Saab TS, Borad MJ. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol. 2019;5:824-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 344] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 42. | Abdel-Rahman O, Elsayed Z, Elhalawani H. Gemcitabine-based chemotherapy for advanced biliary tract carcinomas. Cochrane Database Syst Rev. 2018;4:CD011746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 43. | Goetze TO, Paolucci V. Prognosis of incidental gallbladder carcinoma is not influenced by the primary access technique: analysis of 837 incidental gallbladder carcinomas in the German Registry. Surg Endosc. 2013;27:2821-2828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Kokudo N, Makuuchi M, Natori T, Sakamoto Y, Yamamoto J, Seki M, Noie T, Sugawara Y, Imamura H, Asahara S, Ikari T. Strategies for surgical treatment of gallbladder carcinoma based on information available before resection. Arch Surg. 2003;138:741-50; discussion 750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 45. | Tsukada K, Hatakeyama K, Kurosaki I, Uchida K, Shirai Y, Muto T, Yoshida K. Outcome of radical surgery for carcinoma of the gallbladder according to the TNM stage. Surgery. 1996;120:816-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 132] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 46. | Shimizu Y, Ohtsuka M, Ito H, Kimura F, Shimizu H, Togawa A, Yoshidome H, Kato A, Miyazaki M. Should the extrahepatic bile duct be resected for locally advanced gallbladder cancer? Surgery. 2004;136:1012-7; discussion 1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 47. | Horiguchi A, Miyakawa S, Ishihara S, Miyazaki M, Ohtsuka M, Shimizu H, Sano K, Miura F, Ohta T, Kayahara M, Nagino M, Igami T, Hirano S, Yamaue H, Tani M, Yamamoto M, Ota T, Shimada M, Morine Y, Kinoshita H, Yasunaga M, Takada T. Gallbladder bed resection or hepatectomy of segments 4a and 5 for pT2 gallbladder carcinoma: analysis of Japanese registration cases by the study group for biliary surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2013;20:518-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 48. | Choi SB, Han HJ, Kim WB, Song TJ, Suh SO, Choi SY. Surgical strategy for T2 and T3 gallbladder cancer: is extrahepatic bile duct resection always necessary? Langenbecks Arch Surg. 2013;398:1137-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Higuchi R, Ota T, Araida T, Kajiyama H, Yazawa T, Furukawa T, Yoshikawa T, Takasaki K, Yamamoto M. Surgical approaches to advanced gallbladder cancer : a 40-year single-institution study of prognostic factors and resectability. Ann Surg Oncol. 2014;21:4308-4316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 50. | Ha TY, Yoon YI, Hwang S, Park YJ, Kang SH, Jung BH, Kim WJ, Sin MH, Ahn CS, Moon DB, Song GW, Jung DH, Lee YJ, Park KM, Kim KH, Lee SG. Effect of reoperation on long-term outcome of pT1b/T2 gallbladder carcinoma after initial laparoscopic cholecystectomy. J Gastrointest Surg. 2015;19:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Igami T, Ebata T, Yokoyama Y, Sugawara G, Mizuno T, Yamaguchi J, Shimoyama Y, Nagino M. Combined extrahepatic bile duct resection for locally advanced gallbladder carcinoma: does it work? World J Surg. 2015;39:1810-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Wakai T, Shirai Y, Sakata J, Tsuchiya Y, Nomura T, Hatakeyama K. Surgical outcomes of minor hepatectomy for locally advanced gallbladder carcinoma. Hepatogastroenterology. 2012;59:2083-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 53. | Duffy A, Capanu M, Abou-Alfa GK, Huitzil D, Jarnagin W, Fong Y, D'Angelica M, Dematteo RP, Blumgart LH, O'Reilly EM. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J Surg Oncol. 2008;98:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 314] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 54. | Hawkins WG, DeMatteo RP, Jarnagin WR, Ben-Porat L, Blumgart LH, Fong Y. Jaundice predicts advanced disease and early mortality in patients with gallbladder cancer. Ann Surg Oncol. 2004;11:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 55. | Fuks D, Regimbeau JM, Le Treut YP, Bachellier P, Raventos A, Pruvot FR, Chiche L, Farges O. Incidental gallbladder cancer by the AFC-GBC-2009 Study Group. World J Surg. 2011;35:1887-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 56. | Lee SE, Jang JY, Lim CS, Kang MJ, Kim SW. Systematic review on the surgical treatment for T1 gallbladder cancer. World J Gastroenterol. 2011;17:174-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 130] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 57. | Foster JM, Hoshi H, Gibbs JF, Iyer R, Javle M, Chu Q, Kuvshinoff B. Gallbladder cancer: Defining the indications for primary radical resection and radical re-resection. Ann Surg Oncol. 2007;14:833-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 58. | Coburn NG, Cleary SP, Tan JC, Law CH. Surgery for gallbladder cancer: a population-based analysis. J Am Coll Surg. 2008;207:371-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 59. | You DD, Lee HG, Paik KY, Heo JS, Choi SH, Choi DW. What is an adequate extent of resection for T1 gallbladder cancers? Ann Surg. 2008;247:835-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 60. | Jensen EH, Abraham A, Habermann EB, Al-Refaie WB, Vickers SM, Virnig BA, Tuttle TM. A critical analysis of the surgical management of early-stage gallbladder cancer in the United States. J Gastrointest Surg. 2009;13:722-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 61. | Downing SR, Cadogan KA, Ortega G, Oyetunji TA, Siram SM, Chang DC, Ahuja N, Leffall LD, Frederick WA. Early-stage gallbladder cancer in the Surveillance, Epidemiology, and End Results database: effect of extended surgical resection. Arch Surg. 2011;146:734-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 62. | Shirai Y, Sakata J, Wakai T, Ohashi T, Hatakeyama K. "Extended" radical cholecystectomy for gallbladder cancer: long-term outcomes, indications and limitations. World J Gastroenterol. 2012;18:4736-4743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 63. | D'Angelica M, Dalal KM, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol. 2009;16:806-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 164] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 64. | Pawlik TM, Gleisner AL, Vigano L, Kooby DA, Bauer TW, Frilling A, Adams RB, Staley CA, Trindade EN, Schulick RD, Choti MA, Capussotti L. Incidence of finding residual disease for incidental gallbladder carcinoma: implications for re-resection. J Gastrointest Surg. 2007;11:1478-86; discussion 1486-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 65. | Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg. 2000;232:557-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 311] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 66. | Shih SP, Schulick RD, Cameron JL, Lillemoe KD, Pitt HA, Choti MA, Campbell KA, Yeo CJ, Talamini MA. Gallbladder cancer: the role of laparoscopy and radical resection. Ann Surg. 2007;245:893-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 67. | Regimbeau JM, Fuks D, Bachellier P, Le Treut YP, Pruvot FR, Navarro F, Chiche L, Farges O. Prognostic value of jaundice in patients with gallbladder cancer by the AFC-GBC-2009 study group. Eur J Surg Oncol. 2011;37:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 68. | Agrawal S, Mohan L, Mourya C, Neyaz Z, Saxena R. Radiological Downstaging with Neoadjuvant Therapy in Unresectable Gall Bladder Cancer Cases. Asian Pac J Cancer Prev. 2016;17:2137-2140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 69. | Creasy JM, Goldman DA, Dudeja V, Lowery MA, Cercek A, Balachandran VP, Allen PJ, DeMatteo RP, Kingham TP, D'Angelica MI, Jarnagin WR. Systemic Chemotherapy Combined with Resection for Locally Advanced Gallbladder Carcinoma: Surgical and Survival Outcomes. J Am Coll Surg. 2017;224:906-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 70. | Ito H, Ito K, D'Angelica M, Gonen M, Klimstra D, Allen P, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Accurate staging for gallbladder cancer: implications for surgical therapy and pathological assessment. Ann Surg. 2011;254:320-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 71. | Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol. 2012;30:1934-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 513] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 72. | Nagino M. Fifty-year history of biliary surgery. Ann Gastroenterol Surg. 2019;3:598-605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 73. | Mizuno T, Ebata T, Yokoyama Y, Igami T, Yamaguchi J, Onoe S, Watanabe N, Ando M, Nagino M. Major hepatectomy with or without pancreatoduodenectomy for advanced gallbladder cancer. Br J Surg. 2019;106:626-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 74. | Yokoyama Y, Ebata T, Igami T, Sugawara G, Ando M, Nagino M. Predictive power of prothrombin time and serum total bilirubin for postoperative mortality after major hepatectomy with extrahepatic bile duct resection. Surgery. 2014;155:504-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 75. | Ebata T, Nagino M, Nishio H, Arai T, Nimura Y. Right hepatopancreatoduodenectomy: improvements over 23 years to attain acceptability. J Hepatobiliary Pancreat Surg. 2007;14:131-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 76. | European Society for Medical Oncology. Biliary cancer: ESMO Clinical practice guidelines. Lugano: European Society for Medical Oncology, 2017. |
| 77. | Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D; ESMO Guidelines Committee. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v28-v37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 483] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 78. | The National Comprehensive Cancer Network. Clinical practice guidelines in oncology. Plymouth Meeting: NCCN Health Information Technology, 2019. |
| 79. | Japanese Society of Hepato-Biliary-Pancreatic Surgery. Clinical practice guidelines for management of biliary tract cancers. Tokyo: Igakutosho Shuppan, 2019. |
| 80. | Machado MA, Makdissi FF, Surjan RC. Totally Laparoscopic Hepatic Bisegmentectomy (s4b+s5) and Hilar Lymphadenectomy for Incidental Gallbladder Cancer. Ann Surg Oncol. 2015;22 Suppl 3:S336-S339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 81. | Nag HH, Raj P, Sisodia K. The technique of laparoscopic hepatic bisegmentectomy with regional lymphadenectomy for gallbladder cancer. J Minim Access Surg. 2018;14:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 82. | Costa SR, Horta SH, Miotto MJ, Costas MC, Godinho CA, Henriques AC. [Central inferior bisegmentectomy (S4B+S5) for gallbladder carcinoma treatment: a series of seven resectable cases]. Arq Gastroenterol. 2008;45:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 83. | Han HS, Yoon YS, Agarwal AK, Belli G, Itano O, Gumbs AA, Yoon DS, Kang CM, Lee SE, Wakai T, Troisi RI. Laparoscopic Surgery for Gallbladder Cancer: An Expert Consensus Statement. Dig Surg. 2019;36:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 84. | Yoon YS, Han HS, Agarwal A, Belli G, Itano O, Gumbs AA, Yoon DS, Kang CM, Lee SE, Wakai T, Troisi RI. Survey Results of the Expert Meeting on Laparoscopic Surgery for Gallbladder Cancer and a Review of Relevant Literature. Dig Surg. 2019;36:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 85. | Gumbs AA, Jarufe N, Gayet B. Minimally invasive approaches to extrapancreatic cholangiocarcinoma. Surg Endosc. 2013;27:406-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 86. | de Aretxabala X, Oppliger F, Solano N, Rencoret G, Vivanco M, Carvajal D, Hepp J, Roa I. Laparoscopic management of incidental gallbladder cancer. Surg Endosc. 2018;32:4251-4255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 87. | Piccolo G, Ratti F, Cipriani F, Catena M, Paganelli M, Aldrighetti L. Totally Laparoscopic Radical Cholecystectomy for Gallbladder Cancer: A Single Center Experience. J Laparoendosc Adv Surg Tech A. 2019;29:741-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |