Published online Sep 27, 2020. doi: 10.4254/wjh.v12.i9.628
Peer-review started: March 9, 2020
First decision: April 3, 2020
Revised: June 3, 2020
Accepted: August 15, 2020
Article in press: August 15, 2020
Published online: September 27, 2020
Processing time: 195 Days and 23.4 Hours
Recurrent hepatitis C virus (HCV) infection of transplanted liver allografts is universal in patients with detectable HCV viremia at the time of transplantation. Direct-acting antiviral (DAA) therapy has been adopted as the standard of care for recurrent HCV infection in the post-transplant setting. However, there are insufficient data regarding its efficacy in liver transplant (LT) recipients with a history of hepatocellular carcinoma (HCC), and the risk of HCC recurrence after DAA therapy is unknown.
To demonstrate predictors of DAA treatment failure and HCC recurrence in LT recipients.
A total of 106 LT recipients given DAAs for recurrent HCV infection from 2015 to 2019 were identified (68 with and 38 without HCC). Descriptive statistics and logistic regression models were used to estimate the multivariate odds ratios and respective 95% confidence intervals for predictors of treatment failure and HCC recurrence.
Six patients (6%) experienced DAA therapy failure post-LT and 100 (94%) had a sustained virologic response at follow-up week 12. A high alanine transaminase level > 35 U/L at treatment week 4 was a significant predictor of treatment failure. Relapse to pre-LT DAA therapy is a predictor of post-LT HCC recurrence, P = 0.04. DAA relapse post-LT was also associated with post-transplantation HCC recurrence, P = 0.05.
DAAs are effective and safe in the treatment of recurrent HCV infection in LT recipients with history of HCC. Relapse to pre- and post-LT DAA therapy is associated with post-transplantation HCC recurrence.
Core Tip: Our study is the first to find an association between direct-acting antiviral relapse and hepatocellular carcinoma (HCC) recurrence in patients with past history of HCC pre-transplant. Also, our study is the first to highlight high sustained virologic response in patients with past history of HCC after liver transplantation which is similar to patients without past history of HCC as we removed the tumor-harboring liver.
- Citation: Ismail MS, Hassan M, Khaderi SA, Yousry WA, Kamal El-Din MM, Bahaa El-Din MM, El Sayed OA, Kaseb AO, Goss JA, Kanwal F, Jalal PK. Clinical efficacy of direct-acting antiviral therapy for recurrent hepatitis C virus infection after liver transplantation in patients with hepatocellular carcinoma. World J Hepatol 2020; 12(9): 628-640
- URL: https://www.wjgnet.com/1948-5182/full/v12/i9/628.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i9.628
Hepatitis C virus (HCV) infection is the leading indication for liver transplantation[1]. Recurrent HCV infection of transplanted liver allografts is universal in patients with detectable HCV viremia at the time of transplantation[2]. Evidence of recurrent HCV infection of an allograft occurs as early as 4 wk after transplantation, with development of hepatitis within 6-12 mo in 70%-90% of HCV-infected liver transplant (LT) recipients[3,4]. Furthermore, allograft cirrhosis with a poor outcome occurs within 5 years after transplantation in 20%-30% of these patients[5].
Direct-acting antiviral (DAA) therapy has been adopted as the standard of care for recurrent HCV infection after transplantation[6]. Patients with sustained virologic responses to antiviral therapy may experience fibrosis regression and reduced mortality rates[7,8]. In pre-transplant studies, there are conflicting data regarding the impact of DAA treatment on de-novo or recurrent hepatocellular carcinoma (HCC) in HCV-infected patients[9-12].
Researchers have investigated the safety and efficacy of DAA treatment in LT recipients[13-15]. However, the efficacy in LT recipients with a history of HCC has not been investigated. Moreover, there are no data regarding HCC recurrence in LT recipients following DAA therapy, especially patients who experienced treatment failure. Little is known about predictors of DAA treatment response after LT for HCC. Therefore, we performed this study to (1) Highlight the efficacy and safety of DAA therapy in LT recipients with HCV infections and history of HCC; and (2) Investigate the impact of DAA use on post-transplantation HCC recurrence.
A total of 106 LT recipients given DAAs for recurrent HCV infection from January 1, 2015, to March 1, 2019, at Baylor College of Medicine were retrospectively identified.
Criteria for study inclusion were (1) Age of 18 years or older; (2) History of liver transplantation; (3) Positive anti-HCV and HCV ribonucleic acid prior to and after transplantation; and (4) Treatment with an oral DAA regimen with or without ribavirin. We excluded (1) Patients with active hepatitis B virus (HBV) infection evident by positive hepatitis B surface antigens or HBV deoxyribonucleic acid; (2) Patients with human immunodeficiency virus infection; and (3) Any other metabolic, viral or genetic causes of chronic liver disease. Patients were classified into two groups: Those with pre-transplantation HCC (PHCC; n = 68) and those without pre-transplantation HCC (PnHCC; n = 38). The initiation of DAA treatment and the regimen type and duration were determined by the treating transplant hepatologists. Demographic and clinical information for each participant were documented and stored in a secure database under a Baylor College of Medicine Institutional Review Board-approved protocol. All patients gave written informed consent for data collection prior to their LTs.
Clinical data: The collected information included the following baseline characteristics: Age, sex, ethnicity, body mass index (BMI), medical history (comorbid conditions, stage of liver disease), adverse events, baseline (at time of DAA initiation) and on-treatment laboratory values obtained every 4 wk, including HCV ribonucleic acid levels, until the end of treatment (EOT) and at follow-up visits. BMI was calculated at treatment initiation, the EOT, and the last follow-up visit (normal BMI, < 25 kg/m2; overweight, 25-30 kg/m2; obese, > 30 kg/m2).
Laboratory data: The following laboratory test results were analyzed: Anti-HCV and HCV ribonucleic acid levels; HCV genotypes; Hepatitis B surface antigens; Anti-hepatitis B surface antibodies; Hepatitis B core antibodies; Anti-human immunodeficiency virus antibodies; Epstein-Barr virus, cyotmegalovirus, iron studies, ceruloplasmin, auto-antibodies, serum albumin, alkaline phosphatase, creatinine, sodium, alanine aminotransferase, and total bilirubin levels; Prothrombin time and international normalized ratio; Hemoglobin levels; and Platelet counts. Test results were analyzed to exclude other viral, metabolic, autoimmune causes of liver disease. Estimated glomerular filtration rates were calculated using the Modification of Diet in Renal Disease equation. The model for end-stage liver disease-sodium score was calculated using serum bilirubin, creatinine, and sodium levels and the international normalized ratio.
Clinical efficacy: Treatment efficacy was demonstrated by sustained virologic response at follow-up week 12, defined as an undetectable HCV ribonucleic acid level in the blood at week 12 after the EOT. Accordingly, patients were classified as having DAA responses or failures.
Clinical safety: Adverse events were defined as any events that required an HCV medication dose reduction or treatment discontinuation or the addition of a concomitant medication for management. Events of special interest in this study were HCC recurrence, HBV reactivation and acute allograft rejection.
Stata software (Stata Corp, College Station, TX, United States) was used for statistical analysis. Descriptive statistics were employed, with Student t-test and Mann-Whitney-Wilcoxon test statistics used to assess the significance of mean and median differences in continuous variables between study groups. The Pearson χ2 or Fisher exact test was used to test for differences in the distribution of categorical data between groups. To adjust for the small sample size, multivariate exact unconditional logistic regression analyses were performed. For each risk factor, the adjusted odds ratios and respective 95% confidence intervals were calculated. All odds ratios were adjusted for age, sex, and significant factors from univariate analysis. P value less than 0.05 was defined as significant.
Most patients in the PHCC and PnHCC groups were male and white (Table 1). The patients with PHCC were significantly older (P = 0.01), with a significantly more extensive past history of smoking than the patients with PnHCC (P < 0.01). Baseline comorbidities and cirrhosis were similar in both groups. However, diabetes mellitus was significantly higher in the PHCC group (P < 0.01).
| Number (%) | |||
| Characteristic | Non-HCC (n = 38) | HCC (n = 68) | P value |
| Demographics | |||
| mean (± SD) age at treatment initiation, years | 58.2 ± 11.1 | 62.2 ± 5.9 | 0.01 |
| Median age at treatment initiation, yr (range) | 62 (28-74) | 62.5 (47.0-78.0) | 0.2 |
| Male | 25 (66) | 50 (74) | 0.5 |
| Female | 13 (34) | 18 (26) | |
| White | 19 (50) | 37 (54) | 0.4 |
| Black | 11 (29) | 11 (16) | |
| Hispanic | 6 (16) | 17 (25) | |
| Asian | 2 (5) | 3 (4) | |
| Epidemiologic characteristics | |||
| mean (± SD) baseline BMI | 27.9 ± 5.5 | 28.5 ± 4.5 | 0.6 |
| Median baseline BMI, (range) | 27.0 (21.0-48.5) | 28.5 (17.0-46.0) | 0.3 |
| Alcohol drinking | 11 (29) | 32 (47) | 0.08 |
| Cigarette smoking | 18 (47) | 51 (75) | 0.01 |
| Baseline co-morbidities | |||
| Diabetes Mellitus | 20 (53) | 53 (78) | 0.01 |
| CKD | 20 (53) | 23 (34) | 0.06 |
| eGFR < 30 mL/min/1.73 m2 | 7 (18) | 2 (3) | 0.01 |
| Cirrhosis | 5 (15) | 4 (8) | 0.5 |
| HCV-positive allograft | 0 | 4 (6) | 0.3 |
| Anti-HBc-positive allograft | 1 (3) | 10 (15) | 0.09 |
| Pre-transplant relapse to antiviral treatment | 6 (16) | 23 (34) | 0.06 |
| mean (± SD) time from LT to DAA initiation, mo | 99.3 ± 79.7 | 39.4 ± 40.5 | 0.0001 |
| Median time from LT to DAA initiation, months (range) | 91.5 (2.0-331.0) | 28 (1-171) | 0.0001 |
| Baseline laboratory values | |||
| mean (± SD) creatinine level, mg/dL | 1.9 ± 1.9 | 1.2 ± 0.8 | 0.08 |
| Median creatinine level, mg/dL (range) | 1.2 (0.7-8.7) | 1.0 (0.7-7.0) | 0.01 |
| mean (± SD) AFP level, ng/mL | 10.2 ± 30.5 | 6.8 ± 12.8 | 0.5 |
| Median AFP level, ng/mL (range) | 3.2 (2.0-149.0) | 3.1 (1.3-88.0) | 0.5 |
| mean (± SD) MELD-Na score | 13 ± 5 | 10 ± 4 | 0.01 |
| Median MELD-Na score (range) | 13 (6-24) | 9 (6-24) | 0.01 |
| Clinical outcomes | |||
| Relapse at follow-up week 12 | 1 (3) | 5 (7) | 0.4 |
| SVR12 | 37 (97) | 63 (93) | 0.4 |
| HCC recurrence after DAA treatment | 0 | 6 (9) | 0.08 |
HCV genotypes 1a and 1b were present in 50%, and 21% of the PHCC patients, respectively, and 66% and 21% of the PnHCC patients, respectively (P = 0.1). Although not statistically significant, more PHCC patients had HCV genotype 3a than PnHCC patients (22% vs 11%; P = 0.1). The mean ± SD BMIs at DAA treatment initiation were comparable in the PHCC and PnHCC patients (28.5 ± 4.5 kg/m2vs 27.9 ± 5.5 kg/m2; P = 0.6).
Pre-transplant relapse rate to antiviral treatment was higher in the PHCC patients than in the PnHCC patients (34% vs 16%; P = 0.06). Although there was no statistically significant difference, more PHCC patients received HCV- or anti-HBc-positive allografts than PnHCC patients [6% vs 0% (P = 0.3) and 15% vs 3% (P = 0.09), respectively].
Table 1 shows significant variations in the mean and median laboratory and clinical values between the two groups. Baseline (at time of DAA initiation) laboratory values were similar in the PHCC and PnHCC patients except for the serum creatinine level, estimated glomerular filtration rate, and the model for end-stage liver disease-sodium score, which were markedly lower in the PHCC patients (Table 1).
The median time from liver transplantation to DAA treatment initiation in the entire cohort was 34 mo (range, 1-331 mo), and the median follow-up duration after DAA treatment completion was 20 mo (range, 3-46 mo). The median time from liver transplantation to DAA treatment initiation in the PHCC patients was 28 mo (range, 1-171 mo) which is significantly shorter than in the PnHCC patients, 91.5 mo (range, 2.0-331.0 mo) (P < 0.0001). One hundred patients (94%) experienced sustained virologic response at follow-up week 12 (SVR12) (DAA response), whereas 6 patients (6%) did not (DAA failure). The median times from transplantation to DAA therapy initiation were 32.5 mo (range, 1.0-331.0 mo) in the DAA response group and 54.5 mo (range, 8.0-148.0 mo) in those who had DAA failure (P = 0.7).
All patients who did not experience SVR12 were non-cirrhotic. Two patients had relapses at follow-up week 12, three patients did so at follow-up week 4, and one patient suffered viral breakthrough at treatment week 12.
In the DAA response group, the rates of concordance between SVR12 and SVR at follow-up week 24, and between SVR12 and quantitative HCV polymerase chain reaction at the last follow-up visit were both 100%, as no patients had relapses after experiencing SVR12.
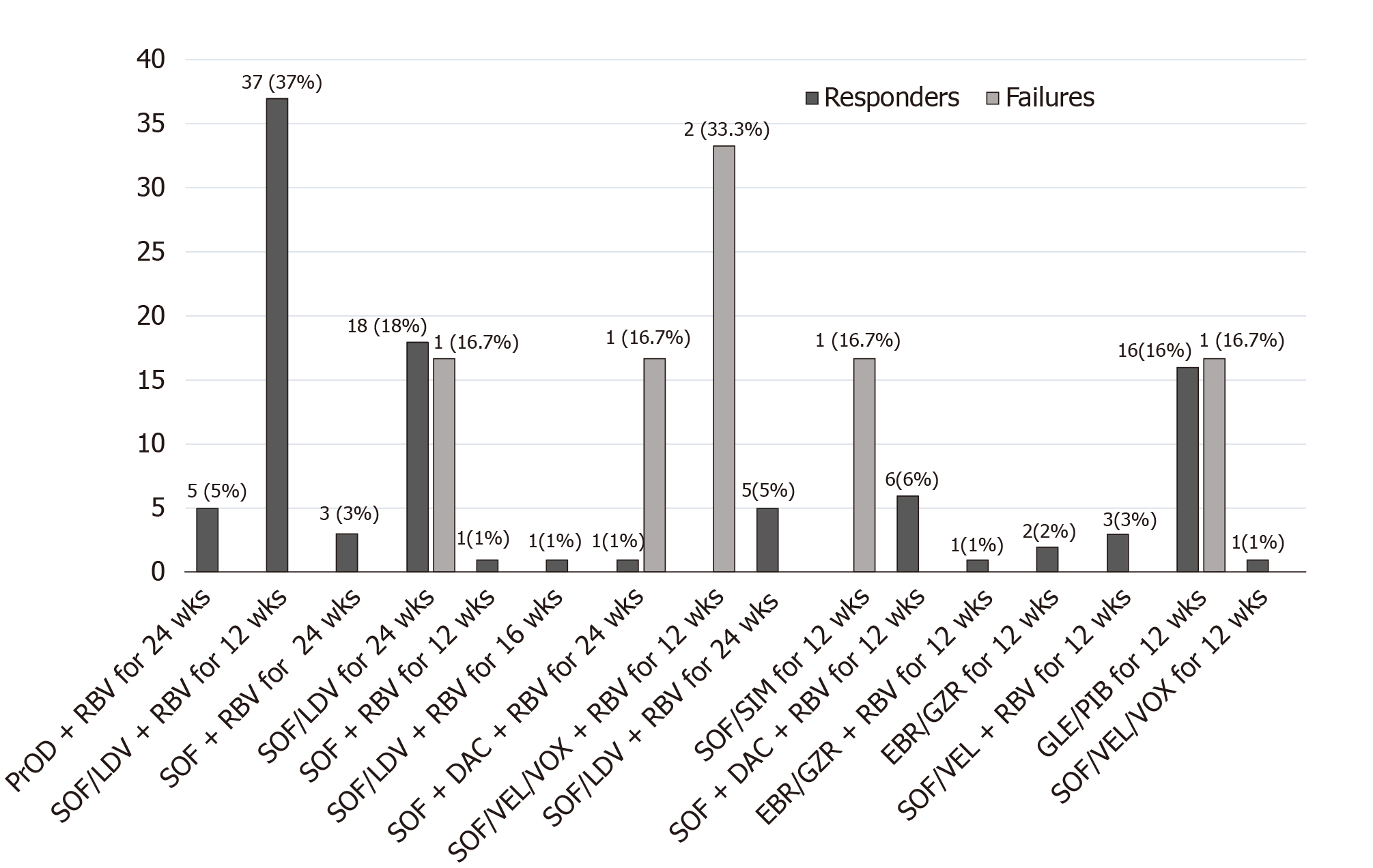
Figure 1 shows the numbers of patients who had responses to and failures of the 16 DAA regimens used in this study. We observed no significant variations in the treatment distribution between the PHCC and PnHCC groups (P = 0.3). The six patients who had DAA failures received five DAA regimens: (1) Sofosbuvir (SOF)/velpatasvir /voxilaprevir plus ribavirin (RBV) for 12 wk (n = 2); (2) SOF, daclatasvir, and RBV for 24 wk (n = 1); (3) SOF/ledipasvir for 24 wk (n = 1); (4) SOF/simeprevir for 12 wk (n = 1); and (5) Glecaprevir /pibrentasvir for 12 wk (n = 1).
Table 2 lists the univariate and multivariate odds ratios (ORs) and 95% confidence interval (CI) for potential predictors of post-LT DAA therapy failure. Univariate analysis showed a significant risk of failure (P < 0.05) in patients with HCV-positive allografts; HCV genotype 3a; high alanine aminotransferase (ALT) level > 35 U/L and or quantitative HCV polymerase chain reaction > 15 IU/mL at treatment week 4. However, multivariate analysis after adjustment for these predictors demonstrated a high ALT level > 35 U/L at treatment week 4 was significantly associated with DAA therapy failure (P = 0.04) after adjustment for the confounding effect of the significant factors described above. Pre-transplant relapse to DAA treatment and history of pre-transplant HCC did not impact SVR12 rates in patients treated with DAA post-LT.
| Variable | Number of DAA therapy responses/failures (n = 100/6) | Univariate OR (95%CI) | P value | Multivariate OR (95%CI) | P value |
| Age > 60 yr | 60/4 | 1.3 (0.2-15.4) | 0.8 | ||
| Male | 70/5 | 2.1 (0.2-104.7) | 0.9 | ||
| Baseline BMI (≥ 25 kg/m2) | 76/5 | 1.6 (0.2-77.7) | 0.9 | ||
| Alcohol drinking | 39/4 | 3.1 (0.4-35.7) | 0.4 | ||
| Cigarette smoking | 64/5 | 2.8 (0.3-136.6) | 0.6 | ||
| Diabetes Mellitus | 70/3 | 0.4 (0.1-3.4) | 0.5 | ||
| CKD | 42/1 | 0.30 (0.01-2.60) | 0.4 | ||
| Cirrhosis | 9/0 | - | |||
| Pre-LT relapse to DAA | 25/4 | 5.9 (0.8-68.7) | 0.09 | ||
| Anti-HBc-positive allograft | 9/2 | 4.9 (0.4-40.4) | 0.2 | ||
| HCV-positive allograft | 2/2 | 22.0 (1.3-381.3) | 0.02 | 3.2 (0.1-856.8) | 0.8 |
| HCV genotype 3a | 15/4 | 10.9 (1.4-130.9) | 0.03 | 1.40 (0.02-89.10) | 0.9 |
| Presence of HCC before LT | 63/5 | 2.9 (0.3-142.6) | 0.6 | ||
| Rejection during/after DAA therapy | 5/0 | - | - | ||
| ALT level at treatment week 4 (> 35 IU/L) | 16/4 | 1.02 (1.00-1.05) | 0.001 | 1.04 (1.00-1.10) | 0.04 |
| Quantitative HCV PCR at treatment week 4 (> 15 IU/mL) | 8/3 | 10.9 (1.3-96.4) | 0.01 | 3.6 (0.1-172.3) | 0.5 |
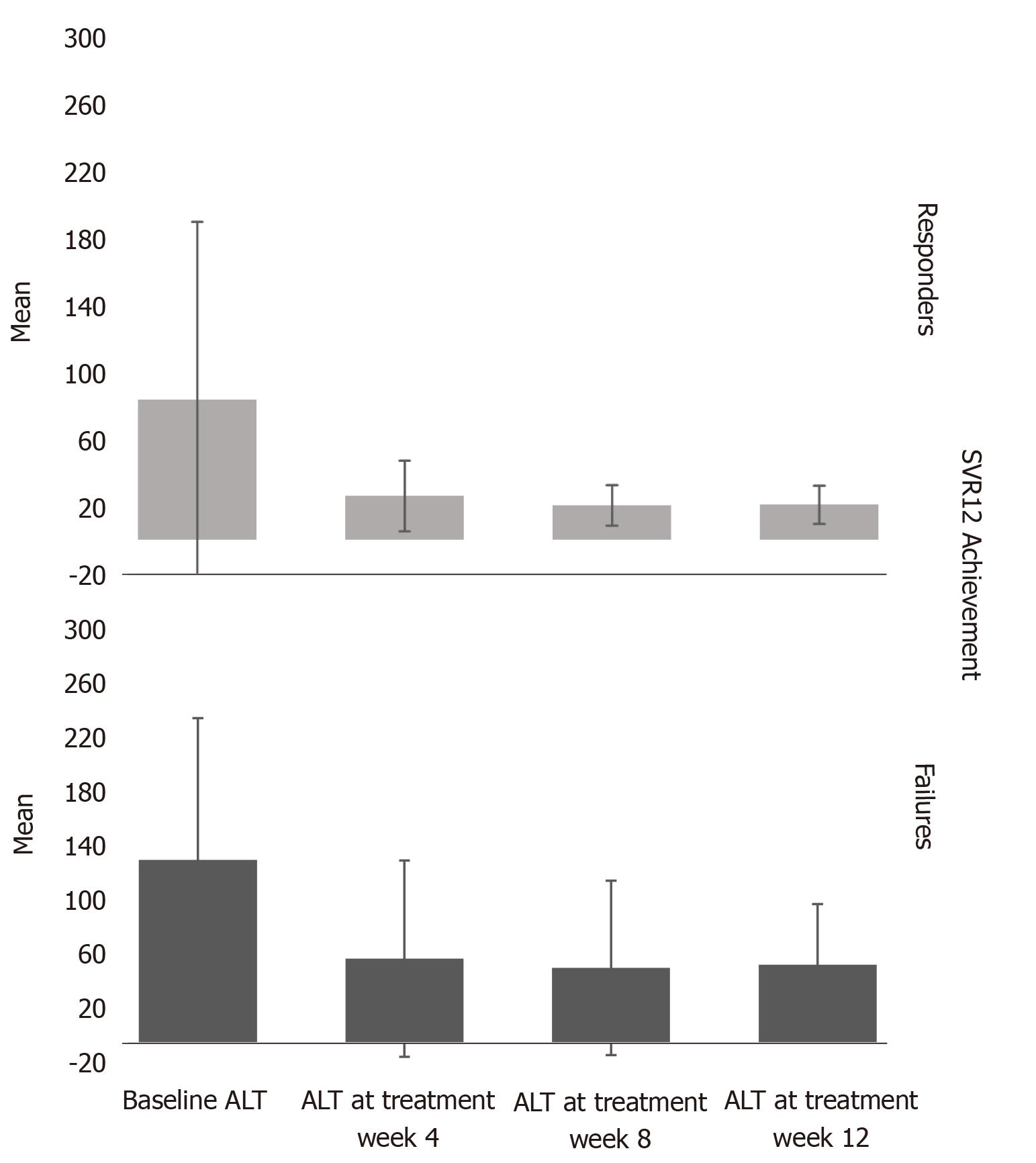
We observed normalization of ALT levels ≤ 35 U/L at treatment week 4 in 85% of the patients (Figure 2 and Table 3). Moreover, the mean platelet count in all patients improved significantly over the follow-up period with a mean difference of -36 (-47.9 to -6), P < 0.001 (Figure 3 and Table 4).
| SVR12 Achievement | Baseline ALT, | ALT at treatment week 4, mean (± SD) | ALT at treatment week 8, mean (± SD) | ALT at treatment week 12, mean (± SD) |
| Responders | 80.67 (± 102.447) | 25.21 (± 20.416) | 19.78 (± 11.651) | 20.21 (± 10.994) |
| Failures | 133.00 (±103.057) | 61.17 (± 71.337) | 54.50 (± 63.385) | 56.83 (± 43.938) |
| P values | 0.2 | 0.001 | 0.0001 | 0.0001 |
| SVR12 Achievement | Baseline PLT, | PLT at treatment week 4, mean (± SD) | PLT at treatment week 8, mean (± SD) | PLT at treatment week 12, mean (± SD) |
| Responders | 152.54 (± 55.880) | 172.33 (± 72.156) | 164.94 (± 53.580) | 169.42 (± 59.499) |
| Failures | 150.00 (± 35.157) | 153.00 (± 58.258) | 167.25 (± 57.058) | 160.67 (± 60.902) |
| P values | 0.9 | 0.5 | 0.9 | 0.7 |
In the PHCC group, six patients (9%) experienced HCC recurrence after DAA treatment initiation. The median time from DAA therapy initiation to HCC recurrence was 8.5 mo (range, 1.0-29.0 mo).
The demographic characteristics, HCC risk factors, and clinical features were similar in the patients who had HCC recurrences after DAA treatment (n = 6) and those who did not (n = 62), demonstrating a lack of a significant impact of these factors on prediction of recurrence after the treatment (Table 5). Of the patients who experienced HCC recurrence after DAA treatment, 5 patients (83%) had relapses to pre-transplant DAA treatment, suggesting pre-transplant relapse is a predictor of post-transplant HCC recurrence. The estimated adjusted OR was 14.9 (95%CI: 1.1-219.3).
| Variable | No HCC recurrence/HCC recurrence | Univariate OR(95%CI) | P value | Multivariate OR (95%CI) | P value |
| Age > 60 yr | 39/3 | 0.6 (0.1-4.8) | 0.8 | ||
| Male | 45/5 | 1.9 (0.2-94.5) | 0.9 | ||
| White | 34/3 | 0.8 (0.1-6.7) | 0.9 | ||
| Baseline BMI | 50/4 | 0.5 (0.1-5.9) | 0.7 | ||
| EOT BMI (< 25 kg/m2) | 11/4 | 8.8 (1.1-109.3) | 0.03 | 12.3 (1.2-131.0) | 0.03 |
| Alcohol drinking | 28/4 | 2.4 (0.3-28.3) | 0.6 | ||
| Cigarette smoking | 48/3 | 0.3 (0.1-2.5) | 0.3 | ||
| Cirrhosis | 4/0 | - | - | ||
| Pre-LT relapse to DAA | 18/5 | 11.7 (1.2-590.7) | 0.006 | 14.9 (1.1-219.3) | 0.04 |
| Anti-HBc-positive allograft | 8/2 | 3.3 (0.3-27.8) | 0.4 | ||
| HCV-positive allograft | 4/0 | - | - | ||
| HCV genotype 3a | 12/3 | 4.1 (0.5-34.2) | 0.2 | ||
| Post-LT relapse to DAA | 2/3 | 26.2 (2.2-432.9) | 0.002 | 10.6 (1.0-121.6) | 0.05 |
In addition, we identified relapse to the post-transplantation DAA therapy as a potential predictor of HCC recurrence (P = 0.05), with a 10-fold greater risk of recurrence in patients who had treatment failures than in responders. The estimated adjusted OR was 10.6 (95%CI: 1.0-121.6) (Table 5).
We also categorized patients into two groups according to use of RBV (Table 6). Demographic characteristics and baseline laboratory values were similar for patients given DAA therapy with and without RBV except for the serum creatinine and hemoglobin levels, the model for end-stage liver disease-sodium score, estimated glomerular filtration rate, and presence of chronic kidney disease.
| Number (%) | |||
| Characteristic | RBV-containing DAA regimens (n = 68) | Non-RBV containing DAA regimens (n = 38) | P value |
| Age ≥ 60 yr | 38 (58) | 26 (70) | 0.2 |
| Male | 51 (75) | 24 (63) | 0.3 |
| Female | 17 (25) | 14 (37) | |
| White | 37 (54) | 19 (50) | 0.8 |
| Black | 15 (22) | 7 (18) | |
| Hispanic | 13 (19) | 10 (26) | |
| Asian | 3 (4) | 2 (5) | |
| Diabetes Mellitus | 45 (66) | 28 (74) | 0.5 |
| CKD | 22 (32) | 21 (55) | 0.02 |
| eGFR < 30 mL/min/1.73 m2 | 1 (2) | 8 (21) | 0.001 |
| Cirrhosis | 4 (8) | 5 (16) | 0.3 |
| mean (± SD) creatinine level, mg/dL | 1.2 ± 1.0 | 1.9 ± 1.8 | 0.02 |
| mean (± SD) hemoglobin level, g/dL | 14.0 ± 1.7 | 12.0 ± 2.0 | 0.0001 |
| mean (± SD) MELD-Na score | 11 ± 4 | 13 ± 6 | 0.01 |
| SVR12 | 65 (95.5) | 35 (92.1) | 0.7 |
Patients who received DAA regimens with and without RBV had similar SVR12 rates (95.5% vs 92.1%; P = 0.7).
A high baseline BMI (≥ 25 kg/m2) did not have a more significant impact on SVR12 or HCC recurrence after DAA treatment than a low BMI (< 25 kg/m2) (Tables 2 and 5). However, among those with PHCC, weight loss by the end of DAA treatment was a significant predictor of HCC recurrence (P = 0.03).
Only one patient in the PnHCC group had to discontinue DAA treatment SOF/ ledipasvir at 16 weeks due to adverse events, which consisted of significant fatigue and headache, but achieved SVR12. However, of the patients receiving RBV-containing DAA regimens, 26 (38%) needed RBV discontinuation or dose modification due to adverse events, including anemia, fatigue, nausea, and elevated serum creatinine level.
No patients experienced decompensation during or after DAA treatment until the last follow-up visit. Also, none of anti-HBc-positive patients (30%) experienced HBV reactivation during or after DAA therapy until the last follow-up visit.
Physicians administered tacrolimus-containing regimens to 83% of the patients during DAA therapy. We found no significant changes in immunosuppressive drug dosages or levels during DAA treatment. Five patients (5%) experienced mild acute rejection episodes within a median time of 11 mo (range, 5-28 mo) after DAA treatment completion, all of whom experienced SVR12 and rejection episodes were controlled.
The present study is the first to demonstrate that pre-transplant relapse to DAA therapy is a significant predictor of post-transplant HCC recurrence. Post-LT DAA therapy relapse is also associated with HCC recurrence in LT recipients. High ALT level > 35 U/L at treatment week 4 is a predictor of DAA treatment failure.
All oral DAA treatments were recently adopted as the standard of care for treatment of recurrent HCV infection in LT recipients. However, contradictory data regarding the increased risk of HCC recurrence in pre-transplant patients after DAA therapy, and HCC influence on DAA SVR rates may affect the decision to treat unique populations of patients as LT recipients with past histories of HCC.
There is increasing evidence of interaction between DAA therapy and HCC in the recent literature. Patients with HCC tend to have low rates of SVR12, whereas those who do not experience SVR12 have increased risk of HCC[16-18]. In an editorial, Hollande and Pol[19] recommended intensive surveillance for HCC in patients who had DAA therapy failure. Yet researchers did not investigate these findings in LT recipients with PHCC[17]. Consistently, we observed high risk of HCC recurrence in LT recipients who had DAA treatment failure. However, history of pre-transplant HCC did not impact SVR12 rates in patients treated with DAA post-LT as we removed tumor-affected livers.
LT recipients may be a reliable population to study the interaction between DAAs and HCC, especially in those with a failed DAA therapy after transplantation. This is driven by two factors. First, LT recipients have the benefit of receiving non-cirrhotic liver allografts with low prevalence of non-characterized nodules, which investigators have pointed to as the cause of de-novo and recurrent HCC in patients undergoing DAA treatment[20]. Moreover, LT recipients are not decompensated, as some authors attributed increased HCC risk in the DAA era to less selection bias when compared with that in the interferon era, as they excluded interferon-based treatment from decompensated patients with Child-Pugh class B or C[21]. Second, the use of explant pathology to confirm the diagnosis of HCC rather than relying on imaging modalities that can misdiagnose HCC supports the use of LT recipients in investigating the interaction between DAA therapy and HCC.
None of the patients in the present study who had DAA therapy failure or recurrence of HCC were cirrhotic. This contradicts the suggestion that HCC recurrence or DAA failure risk is greater in cirrhotic than non-cirrhotic populations.
Whereas a specific explanation for the interaction between HCC and DAA therapy has yet to be established, some studies suggested that cross-talk among inflammatory, immune response, and angiogenesis pathways modulates the impact of DAAs on progression of HCC and the risk of its recurrence[22,23]. Additionally, HCC may interact biologically with DAA therapy, which may influence pre-transplant SVR12 achievement and increases the risk of HCC recurrence in patients who failed DAA therapy with a prior history of HCC irrespective of HCC treatment response or liver transplantation[17,19]. Genetic susceptibility and variability in the genes involved in HCC risk or DAA metabolism may also play a role in the interaction between DAAs and HCC. Integration of the use of molecular markers with DAA therapy should be explored in future studies.
There is no consensus regarding the optimum time to start DAA treatment after an LT. We found no difference in the SVR12 rate between patients who received early (< 1 year) and late (≥ 1 year) DAA therapy after LTs. Nevertheless, five of six patients who did not experience SVR12 received DAA therapy more than 1 year after their LTs.
We have no explanation why patients with PHCC received DAA treatment earlier than patients with PnHCC in our study. This may be attributed to more rigorous follow-up, better compliance, and fear of decompensation or HCC recurrence after liver transplantation in patients with PHCC.
During post-treatment follow-up, no patients had relapses after SVR12, with 100% concordance between SVR12 rates and SVR rates at follow-up week 24 and between SVR12 rates and quantitative HCV polymerase chain reaction at the last follow-up visit. This establishes SVR12 as an appropriate endpoint for demonstrating the clinical efficacy of all-oral DAA treatment similar to that in previous trials of sofosbuvir/RBV with and without interferon[24].
Consistent with a previous study[25], we showed that failure of the ALT level to normalize at treatment week 4 was a strong predictor of DAA treatment failure. This may result in enhancement of patient management via (1) Intensification of DAA treatment, either with RBV or extension of the treatment period, and (2) Thorough evaluation for other causes of a persistently elevated ALT level at treatment week 4, such as alcoholism, high body mass index, and type 2 diabetes mellitus.
Multivariate analysis showed no impact of HCV genotype 3 or an HCV-positive allograft on the risk of failure or HCC recurrence after DAA treatment.
Similar to other studies[13,14], we did not observe a significant difference in SVR12 rates between LT recipients who received DAA regimens with and without RBV. However, such an observation should be interpreted conservatively, as these patients were not randomized to receive RBV. Moreover, patients who received RBV were either cirrhotic or had a history of failure to antiviral therapy or quantitative HCV polymerase chain reaction > 15 IU/mL at treatment week 4, with RBV added to their regimens to intensify them and achieve SVR12. Use of RBV therefore may have helped this population to have an SVR12 rate similar to those in other favorable populations.
Overweight or obesity at baseline did not influence SVR12 achievement or HCC recurrence after DAA treatment. We observed no significant changes in body mass index from baseline to the end of DAA treatment. However, among those with PHCC, weight loss by the end of DAA treatment was a significant predictor of HCC recurrence, which may be attributed to release of inflammatory cytokines[26].
Tacrolimus-containing regimens were the most commonly used immunosuppressive medications in DAA therapy. Acute cellular rejection occurred in 5% of the patients after DAA treatment (median time of 11 mo after therapy), which is comparable with rejection rates in the literature[13].
HBV reactivation with DAA treatment is rare, with an estimated risk of 1.4% in patients with resolved HBV infections[27]. In our study, none of anti-HBc-positive patients experienced HBV reactivation during or after DAA therapy until the last follow-up visit.
This study has several strengths. It is the first to highlight predictors of HCC recurrence in patients in whom DAA treatment failed after an LT while adjusting for potential confounding factors. Furthermore, pre-transplant relapse to DAA therapy may predict post-transplant HCC recurrence. Given the uniqueness of LT recipients with recurrent HCV infection and HCC, we acknowledge the limitation of a single-center, retrospective study with a small sample size. Accordingly, we applied a rigid exact method of logistic regression analysis to overcome this limitation.
In conclusion, our results support the safety and efficacy of DAA treatment in LT recipients with history of HCC. Relapse to pre-transplant DAA therapy is a significant predictor of HCC recurrence following transplantation. Furthermore, relapse to post-transplant DAA therapy is associated with HCC recurrence. ALT level > 35 U/L at treatment week 4 is a significant predictor of treatment failure. Future validation of our findings in a multicenter study of a larger sample is warranted.
Recent studies have shown lower sustained virologic response in patients with hepatocellular carcinoma (HCC) pre-transplant. Moreover, there are conflicting data regarding HCC recurrence in patients treated with direct-acting antivirals (DAAs). However, there are insufficient data regarding the efficacy of DAA in liver transplant (LT) recipients with past history of HCC.
To identify risk factors for DAA relapse and HCC recurrence after LT.
To highlight the efficacy and safety of DAA therapy in LT recipients with hepatitis C virus infections and history of HCC and to investigate the impact of DAA use on post-transplantation HCC recurrence.
We retrospectively analyzed the data of our center of LT recipients who received DAA therapy for hepatitis C virus recurrence and their relapse and HCC recurrence outcomes.
Six patients (6%) experienced DAA therapy failure post-LT and 100 (94%) had a sustained virologic response at follow-up week 12. DAA relapse post-LT was associated with post-transplantation HCC recurrence, P = 0.05. Pre-LT DAA relapse is a predictor of post-LT HCC recurrence, P = 0.04.
DAA is safe and effective in LT recipients with history of HCC. High alanine aminotransferase > 35 U/L at treatment week 4 and pre-LT DAA relapse are predictors of HCC recurrence after transplant. Relapse to DAA therapy post-LT is a potential risk factor for HCC recurrence.
Performing a multicenter study of a larger sample to validate our findings.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: American Gastroenterological Association.
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fiore M, Ho CM S-Editor: Zhang L L-Editor: A P-Editor: Li JH
| 1. | Kim WR, Terrault NA, Pedersen RA, Therneau TM, Edwards E, Hindman AA, Brosgart CL. Trends in waiting list registration for liver transplantation for viral hepatitis in the United States. Gastroenterology. 2009;137:1680-1686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 2. | Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002;122:889-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 829] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 3. | König V, Bauditz J, Lobeck H, Lüsebrink R, Neuhaus P, Blumhardt G, Bechstein WO, Neuhaus R, Steffen R, Hopf U. Hepatitis C virus reinfection in allografts after orthotopic liver transplantation. Hepatology. 1992;16:1137-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Gane E. The natural history and outcome of liver transplantation in hepatitis C virus-infected recipients. Liver Transpl. 2003;9:S28-S34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 164] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 5. | Gane EJ, Portmann BC, Naoumov NV, Smith HM, Underhill JA, Donaldson PT, Maertens G, Williams R. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med. 1996;334:815-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 736] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 6. | Crespo G, Trota N, Londoño MC, Mauro E, Baliellas C, Castells L, Castellote J, Tort J, Forns X, Navasa M. The efficacy of direct anti-HCV drugs improves early post-liver transplant survival and induces significant changes in waiting list composition. J Hepatol. 2018;69:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 7. | Berenguer M, Schuppan D. Progression of liver fibrosis in post-transplant hepatitis C: mechanisms, assessment and treatment. J Hepatol. 2013;58:1028-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Picciotto FP, Tritto G, Lanza AG, Addario L, De Luca M, Di Costanzo GG, Lampasi F, Tartaglione MT, Marsilia GM, Calise F, Cuomo O, Ascione A. Sustained virological response to antiviral therapy reduces mortality in HCV reinfection after liver transplantation. J Hepatol. 2007;46:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S, Díaz A, Vilana R, Darnell A, Varela M, Sangro B, Calleja JL, Forns X, Bruix J. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 803] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 10. | Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, Foschi FG, Lenzi M, Mazzella G, Verucchi G, Andreone P, Brillanti S. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 698] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 11. | Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology. 2017;153:996-1005.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 684] [Article Influence: 85.5] [Reference Citation Analysis (0)] |
| 12. | ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER, CO12 CirVir and CO23 CUPILT cohorts). Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts. J Hepatol. 2016;65:734-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 330] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 13. | Saxena V, Khungar V, Verna EC, Levitsky J, Brown RS, Hassan MA, Sulkowski MS, O'Leary JG, Koraishy F, Galati JS, Kuo AA, Vainorius M, Akushevich L, Nelson DR, Fried MW, Terrault N, Reddy KR. Safety and efficacy of current direct-acting antiviral regimens in kidney and liver transplant recipients with hepatitis C: Results from the HCV-TARGET study. Hepatology. 2017;66:1090-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 14. | Kwok RM, Ahn J, Schiano TD, Te HS, Potosky DR, Tierney A, Satoskar R, Robertazzi S, Rodigas C, Lee Sang M, Wiegel J, Patel N, Gripshover J, Hassan MA, Branch A, Smith CI. Sofosbuvir plus ledispasvir for recurrent hepatitis C in liver transplant recipients. Liver Transpl. 2016;22:1536-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Brown RS, O'Leary JG, Reddy KR, Kuo A, Morelli GJ, Burton JR, Stravitz RT, Durand C, Di Bisceglie AM, Kwo P, Frenette CT, Stewart TG, Nelson DR, Fried MW, Terrault NA; Hepatitis C Therapeutic Registry Research Network Study Group. Interferon-free therapy for genotype 1 hepatitis C in liver transplant recipients: Real-world experience from the hepatitis C therapeutic registry and research network. Liver Transpl. 2016;22:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 16. | Prenner SB, VanWagner LB, Flamm SL, Salem R, Lewandowski RJ, Kulik L. Hepatocellular carcinoma decreases the chance of successful hepatitis C virus therapy with direct-acting antivirals. J Hepatol. 2017;66:1173-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 17. | Radhakrishnan K, Di Bisceglie AM, Reddy KR, Lim JK, Levitsky J, Hassan MA, Darling JM, Feld JJ, Akushevich L, Vainorius M, Nelson DR, Fried MW, Brown RS, Terrault NA. Treatment Status of Hepatocellular Carcinoma Does Not Influence Rates of Sustained Virologic Response: An HCV-TARGET Analysis. Hepatol Commun. 2019;3:1388-1399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, Duarte-Rojo A, Heathcote EJ, Manns MP, Kuske L, Zeuzem S, Hofmann WP, de Knegt RJ, Hansen BE, Janssen HL. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584-2593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1165] [Cited by in RCA: 1167] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 19. | Hollande C, Pol S. Editorial: reciprocal interaction between HCV direct-acting anti-virals (DAA) and hepatocellular carcinoma (HCC)-a negative impact of HCC on sustained virologic response not of DAA on HCC. Aliment Pharmacol Ther. 2019;50:227-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Mariño Z, Darnell A, Lens S, Sapena V, Díaz A, Belmonte E, Perelló C, Calleja JL, Varela M, Rodriguez M, Rodriguez de Lope C, Llerena S, Torras X, Gallego A, Sala M, Morillas RM, Minguez B, Llaneras J, Coll S, Carrion JA, Iñarrairaegui M, Sangro B, Vilana R, Sole M, Ayuso C, Ríos J, Forns X, Bruix J, Reig M. Time association between hepatitis C therapy and hepatocellular carcinoma emergence in cirrhosis: Relevance of non-characterized nodules. J Hepatol. 2019;70:874-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 21. | Pol S, Fontaine H, Carrat F; on the behalf of the ANRS/AFEF Hepather Study group. Time association between hepatitis C therapy and hepatocellular carcinoma emergence in cirrhosis: Relevance of non-characterized nodules - A response. J Hepatol. 2019;71:446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Debes JD, van Tilborg M, Groothuismink ZMA, Hansen BE, Schulze Zur Wiesch J, von Felden J, de Knegt RJ, Boonstra A. Levels of Cytokines in Serum Associate With Development of Hepatocellular Carcinoma in Patients With HCV Infection Treated With Direct-Acting Antivirals. Gastroenterology. 2018;154:515-517.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 23. | Faillaci F, Marzi L, Critelli R, Milosa F, Schepis F, Turola E, Andreani S, Vandelli G, Bernabucci V, Lei B, D'Ambrosio F, Bristot L, Cavalletto L, Chemello L, Sighinolfi P, Manni P, Maiorana A, Caporali C, Bianchini M, Marsico M, Turco L, de Maria N, Del Buono M, Todesca P, di Lena L, Romagnoli D, Magistri P, di Benedetto F, Bruno S, Taliani G, Giannelli G, Martinez-Chantar ML, Villa E. Liver Angiopoietin-2 Is a Key Predictor of De Novo or Recurrent Hepatocellular Cancer After Hepatitis C Virus Direct-Acting Antivirals. Hepatology. 2018;68:1010-1024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 24. | Yoshida EM, Sulkowski MS, Gane EJ, Herring RW, Ratziu V, Ding X, Wang J, Chuang SM, Ma J, McNally J, Stamm LM, Brainard DM, Symonds WT, McHutchison JG, Beavers KL, Jacobson IM, Reddy KR, Lawitz E. Concordance of sustained virological response 4, 12, and 24 weeks post-treatment with sofosbuvir-containing regimens for hepatitis C virus. Hepatology. 2015;61:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 25. | Raschzok N, Schott E, Reutzel-Selke A, Damrah I, Gül-Klein S, Strücker B, Sauer IM, Pratschke J, Eurich D, Stockmann M. The impact of directly acting antivirals on the enzymatic liver function of liver transplant recipients with recurrent hepatitis C. Transpl Infect Dis. 2016;18:896-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Aoyagi T, Terracina KP, Raza A, Matsubara H, Takabe K. Cancer cachexia, mechanism and treatment. World J Gastrointest Oncol. 2015;7:17-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 298] [Cited by in RCA: 302] [Article Influence: 30.2] [Reference Citation Analysis (2)] |
| 27. | Mücke MM, Backus LI, Mücke VT, Coppola N, Preda CM, Yeh ML, Tang LSY, Belperio PS, Wilson EM, Yu ML, Zeuzem S, Herrmann E, Vermehren J. Hepatitis B virus reactivation during direct-acting antiviral therapy for hepatitis C: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2018;3:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |