Published online Aug 27, 2020. doi: 10.4254/wjh.v12.i8.525
Peer-review started: April 13, 2020
First decision: April 22, 2020
Revised: July 5, 2020
Accepted: July 19, 2020
Article in press: July 19, 2020
Published online: August 27, 2020
Processing time: 133 Days and 15.4 Hours
The “six-and-twelve” (6&12) score is a new hepatocellular carcinoma (HCC) prognostic index designed for recommended transarterial chemoembolization (TACE) candidates. Quick and easy to use by the sum of tumor size (cm) and number, this model identifies three groups with different survival time (the sum is ≤ 6; or > 6 but ≤ 12; or > 12); a survival benefit with TACE can be expected for HCC patients with a score not exceeding twelve. Recently, Wang ZW et al showed that the “6&12” model was the best system correlated with radiological response after the first TACE. Thus, we wanted to assess its survival prediction ability as well as its prognostic value and compared it to other systems (Barcelona Clinic Liver Cancer, Hong Kong Liver Cancer (HKLC) staging, Albumin-Bilirubin grade, tumor nodularity, infiltrative nature of the tumor, alpha-fetoprotein, Child-Pugh class, and Performance Status score, Cancer of the Liver Italian Program, Model to Estimate Survival for HCC scores, up-to-seven criteria) different from Wang ZW et al study in a multicenter French cohort of HCC including only recommended TACE candidates retrospectively enrolled. As previously demonstrated, we show that the "6&12” score can classify survival within this French cohort, with a prognostic value comparable to that of other systems, except HKLC staging. More importantly, the “6&12” score simplicity and ability in patients’ stratification outperform other systems for a routine clinical practice.
Core tip: Not all-intermediate stage hepatocellular carcinoma (HCC) benefit from transarterial chemoembolization (TACE). The recent “six-and-twelve” (6&12) score is an easy to use prognostic model that ensure a quick and appropriate patient’ selection before the first TACE in Chinese cohorts. In this multicenter French cohort of HCC, the “6&12” score can also classify survival among recommended TACE candidates with a good prognostic performance. It may help clinicians in routine clinical practice.
- Citation: Adhoute X, Pénaranda G, Raoul JL, Bronowicki JP, Anty R, Bourlière M. “Six-and-twelve” score for outcome prediction of hepatocellular carcinoma following transarterial chemoembolization. In-depth analysis from a multicenter French cohort. World J Hepatol 2020; 12(8): 525-532
- URL: https://www.wjgnet.com/1948-5182/full/v12/i8/525.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i8.525
We have read with great interest the study by Wang et al[1] who assessed and compared different prognostic models for hepatocellular carcinoma (HCC) patients undergoing transarterial chemoembolization (TACE) treatment, especially the latest “six-and-twelve” (6&12) score[2] within a nationwide Chinese HCC cohort (n = 1107). Increased survival after TACE is correlated with radiological response[3,4] and this study shows that the “6&12” index is the best system correlated with radiological response after the first TACE. The study population was more heterogeneous than the population used to develop the score, including patients with slightly altered performance status (PS) and logically a model like the 3rd version of the hepatoma arterial-embolization prognostic score[5] (which include liver function parameters) had a higher predictive value for survival. However, simplicity (using two cut-off values for risk stratification) and presumed reliability of the “6&12” score have convinced us to assess once again[6] the reproducibility and the predictive value of this new model in a multicenter French cohort of HCC patients including only recommended TACE candidates (n = 324) ie intermediate and early unresectable stages according to the treatment stage migration concept. We compared it to other systems different from Wang et al[1]’s study (Barcelona Clinic Liver cancer[7] (BCLC) staging, Child-Pugh (CP) class, Albumin-Bilirubin[8] (ALBI) grade, NIACE[9] [tumor nodularity, infiltrative nature of the tumor, alpha-fetoprotein (AFP), CP class, and PS] score (Table 1)) using time-dependent area under receiver operating characteristic curve (AUROC) values and C-indices.
| CLIP (0 to 7 points) | MESH (0 to 6 points) | NIACE (0 to 7 points) | ||||
| Portal vein thrombosis | 1 point | Tumor extent: Beyond Milan criteria | 1 point | Tumor nodules ≥ 3 | 1 point | |
| AFP ≥ 400 ng/mL | 1 point | Vascular invasion and/or Extrahepatic spread | 1 point | Infiltrative HCC | 1.5 points | |
| Nodular HCC | 0 point | |||||
| Child-Pugh grade | A | 0 point | PS ≥ 2 | 1 point | AFP ≥ 200 ng/mL | 1.5 points |
| B | 1 point | Child-Pugh grade ≥ A6 | 1 point | |||
| C | 2 points | |||||
| Tumor extent | Unidolar and extension ≤ 50% | 0 point | AFP ≥ 20 ng/mL | 1 point | Child-Pugh grade A | 0 point |
| Multinodular and extension ≤ 50% | 1 point | Alkaline phosphatase ≥ 200 IU/l | 1 point | Child-Pugh grade B | 1.5 points | |
| Massive or extension > 50% | 2 points | PS ≥ 1 | 1.5 points | |||
Patients were retrospectively enrolled over a six years period in two centers (Marseille, Nancy). Demographic and clinical characteristics of HCC patients are shown in Table 2. HCC patients were mostly male (85%), with a median of age of 68 years. Cirrhosis was present in 96% of cases, CP class A (77%), CP class B7 (23%). Underlying liver disease was mostly related to alcohol abuse (38%) or viral C hepatitis (40%). Patients were BCLC stage B (n = 179), BCLC stage A (n = 145). HCC were multinodular in 71% of cases and the median tumor diameter was 35 mm (25-50). The mean session number of conventional TACE was 2.7 ± 1.8.
| Demographic variables | Marseille/Nancy cohort, n = 324 | Marseille cohort1, n = 252 |
| Age - Median [Q1-Q3], year | 68 [62-74] | 68 [60-73] |
| Gender Male/female | 276 (85)/48 (15) | 214 (85)/38 (15) |
| Liver disease HCV/HBV/Alcoholism/MS/other | 129 (40)/14 (4)/122 (38)/42 (13)/17 (5) | 109 (43)/12 (5)/84 (33)/37 (15)/10 (4) |
| ECOG (PS-0) | 324 (100) | 252 (100) |
| Cirrhosis | 311 (96) | 243 (96) |
| Tumor variables: | ||
| Tumor Size – mm - median [q1-q3] | 35 [25-50] | 32 [25-44] |
| Nodule (s): 1/2/3/4/≥ 5 | 95 (29)/72 (22)/80 (25)/38 (12)/39 (12) | 83 (33)/67 (27)/34 (13)/31 (12)/37 (15) |
| Laboratory variables | ||
| AFP – ng/mL, median [q1-q3] | 16.3 [6.0-120.3] | 11.2 [5.0-77.7] |
| PT (%), median [q1-q3] | 76 [64-88] | 78 [68-88] |
| Albumin (g/L), median [q1-q3] | 35 [28-38] | 36.6 [32.7-41.0] |
| Total bilirubin (mcmol/L), median [q1-q3] | 19.0 [13.7-28.7] | 17 [11-27] |
| Child - Pugh grade A/B7 | 249 (77)/75 (23) | 180 (71)/72 (29) |
| ALBI1 class | 64 (20)/230 (71)/30 (9) | 37 (15)/175 (73)/29 (12) |
| BCLC1 stage A/B | 145 (45%)/179 (55%) | 134 (56)/107 (44) |
| “6&12”1 score allocation n ≤ 6/> 6 - ≤ 12/> 12 | 154 (48)/163 (50)/7 (2) | 130 (54)/106 (44)/5 (2) |
| NIACE score allocation ≤ 1/1.5 - 3/> 3 | 168 (52)/134 (41)/22 (7) | |
| CLIP1 score allocation 0/1/2/≥ 3 | - | 55 (23)/135 (56)/45 (19)/ 6 (2) |
| MESH1 score allocation 0/1/2/3/4 | - | 41 (17)/77 (32)/78 (32)/37 (15)/8 (4) |
| Up-to-Seven model1 (In/Out) | - | 176 (73)/65 (27) |
| HKLC1 stage 1/2a/2b/3a/3b | - | 89 (37)/43 (17)/65 (27)/24 (10)/21 (9) |
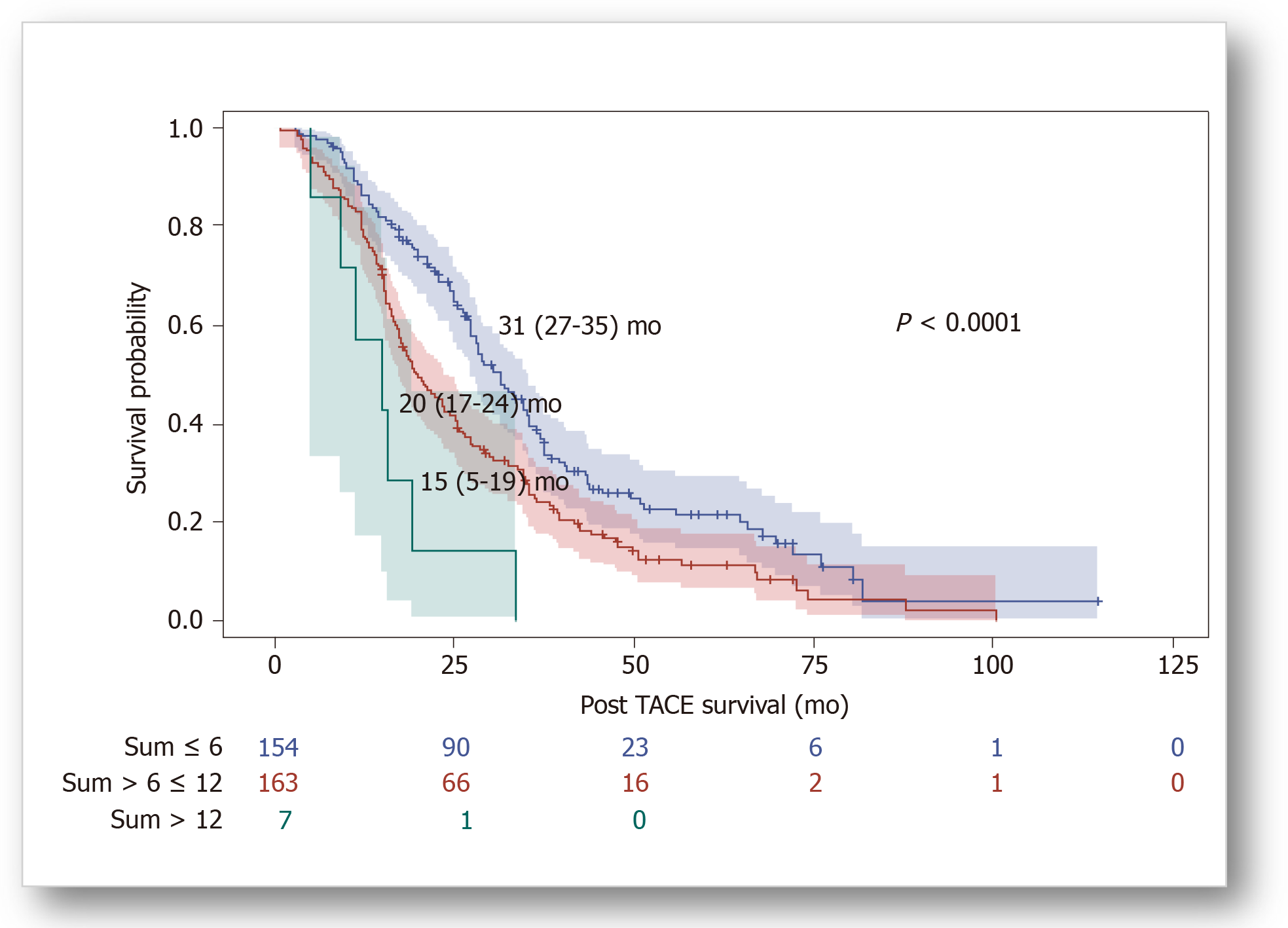
After a median follow-up duration of 24.4 (15.0-36.8) mo, eighty one percent of patients died. Kaplan-Meier analyses showed significant differences in overall survival (OS) distributions across subgroups of BCLC staging, “6&12” (Figure 1) and NIACE scores within this cohort (P < 0.05) (Table 3). Liver function at baseline also had an impact on survival; median OS was significantly different according to the CP class [CP-A, 27 (25-31) mo; CP-B7, 21 (15-24) mo (P = 0.0003)], or ALBI grade [grade 1, 35 (25-43) mo; grade 2, 26 (22-28) mo; grade 3, 16 (12-24) mo (P = 0.0029)].
| Scoring/stage systems | OS [95%CI], mo | P value (log-rank) | Sidak1 | Hazard ratio [95%CI] | P value |
| “6&12” score | < 0.0001 | ||||
| sum ≤ 6 (n = 154) | 31 [27-35] | Ref | Ref | ||
| sum > 6 ≤ 12 (n = 163) | 20 [17-24] | 0.0009 | 1.55 [1.21-1.99] | 0.0005 | |
| sum > 12 (n = 7) | 15 [5-19] | < 0.0001 | 3.80 [1.76-8.21] | 0.0007 | |
| BCLC staging | < 0.0001 | ||||
| A (n = 145) | 35 [29-38] | NR | Ref | ||
| B (n = 179) | 19 [17-23] | NR | 1.88 [1.47-2.41] | < 0.0001 | |
| NIACE score | < 0.0001 | ||||
| ≤ 1 (n = 168) | 35 [28-36] | Ref | Ref | ||
| 1.5 - 3 (n= 134) | 20 [16-23] | < 0.0001 | 1.92 [1.49-2.48] | < 0.0001 | |
| > 3 (n = 22) | 11 [5-16] | < 0.0001 | 6.23 [3.87-10.02] | < 0.0001 | |
| Child-Pugh class | 0.0003 | ||||
| A (n = 249) | 27 [25-31] | NR | Ref | ||
| B (n = 75) | 21 [15-24] | NR | 1.66 [1.26-2.19] | 0.0003 | |
| ALBI grade | 0.0029 | ||||
| Grade 1 (n = 64) | 35 [25-43] | Ref | Ref | ||
| Grade 2 (n = 230) | 26 [22-28] | 0.1228 | 1.50 [1.06-2.11] | 0.0216 | |
| Grade 3 (n = 30) | 16 [12-24] | 0.0016 | 2.30 [1.41-3.75] | 0.0009 |
Performances of the “6&12” score and other systems for survival prediction are indicated in Table 4. Time-dependent AUROC values and C-indices of the “6&12” score was not significantly different from those of other systems. We checked our results within the main cohort from Marseille (n = 252) (Table 2) by comparing the “6&12” score to other staging scoring systems (Hong Kong Liver Cancer[10] (HKLC), Cancer of the Liver Italian Program[11] (CLIP), Model to Estimate Survival for HCC[12] (MESH), up-to-seven criteria[13]). Significant differences in survival distributions were also found across subgroups of the “6&12” score and other systems within this single center cohort (P < 0.05) (Table 5). Its predictive value remained comparable to that of other systems [C-index “6&12” 0.63 (0.56-0.70) vs CLIP 0.70 (0.62-0.78) vs “up-to-seven” 0.61 (0.56-0.66) vs MESH 0.71 (0.63-0.78), not significant] except for HKLC staging, which provides a better prognostication ability [3-year AUROC (“6&12”) 0.56 (0.44-0.68) vs (HKLC) 0.69 (0.65-0.74), P = 0.0325] using a more complex stratification into five subgroups.
| Scoring/stage systems | 1-yr AUROC | P (vs ref) | 2-yr AUROC | P (vs ref) | 3-yr AUROC | P (vs ref) | C-index | P (vs ref) |
| “6&12” score | 0.65 [0.57-0.74] | Ref | 0.65 [0.59-0.71] | Ref | 0.64 [0.58-0.71] | Ref | 0.66 [0.58-0.74] | |
| BCLC staging | 0.61 [0.54-0.67] | 0.1827 | 0.64 [0.59-0.70] | 0.7079 | 0.61 [0.55-0.68] | 0.2317 | 0.61 [0.54-0.68] | NS |
| NIACE score | 0.75 [0.68-0.83] | 0.0134 | 0.69 [0.64-0.75] | 0.2368 | 0.69 [0.63-0.74] | 0.2827 | 0.70 [0.64-0.77] | NS |
| Child-Pugh class | 0.56 [0.49-0.63] | 0.1057 | 0.56 [0.51-0.60] | 0.0217 | 0.55 [0.50-0.59] | 0.0304 | 0.59 [0.55-0.64] | NS |
| ALBI grade | 0.63 [0.57-0.69] | 0.6835 | 0.56 [0.51-0.61] | 0.0479 | 0.55 [0.49-0.61] | 0.1033 | 0.62 [0.55-0.68] | NS |
| Scoring/stage systems | OS [95%CI], mo | P value (log-rank) | Sidak1 | Hazard ratio [95%CI] | P value |
| “6&12” score | 0.0004 | ||||
| sum ≤ 6 (n = 130) | 32 [28-36] | Ref | Ref | ||
| sum > 6 ≤ 12 (n = 106) | 20 [17-25] | 0.0017 | 1.61 [1.21-2.14] | 0.0010 | |
| sum > 12 (n = 5) | 16 [5-34] | 0.0003 | 3.34 [1.35-8.25] | 0.0092 | |
| CLIP | < 0.0001 | ||||
| 0 (n = 55) | 35 [30-68] | Ref | Ref | ||
| 1 (n = 135) | 28 [25-32] | 0.0724 | 1.81 [1.23-2.67] | 0.0028 | |
| 2 (n = 45) | 18 [15-23] | < 0.0001 | 2.86 [1.81-4.54] | < 0.0001 | |
| 3 (n = 6) | 10 [1-27] | < 0.0001 | 8.12 [3.35-19.67] | < 0.0001 | |
| HKLC | < 0.0001 | ||||
| 1 (n = 89) | 36 [30-40] | Ref | Ref | ||
| 2a (n = 42) | 25 [19-35] | 0.0024 | 1.79 [1.18-2.72] | 0.0060 | |
| 2b (n = 65) | 26 [19-34] | 0.0749 | 1.45 [1.01-2.10] | 0.0450 | |
| 3a (n = 24) | 17 [11-23] | < 0.0001 | 3.30 [2.03-5.36] | < 0.0001 | |
| 3b (n = 21) | 14 [11-16] | < 0.0001 | 4.55 [2.73-7.58] | < 0.0001 | |
| Up-to-Seven | 0.0001 | ||||
| In (n = 176) | 30 [27-35] | NA | Ref | ||
| Out (n = 65) | 18 [15-24] | NA | 1.81 [1.34-2.46] | 0.0001 | |
| MESH | < 0.0001 | ||||
| 0 (n = 41) | 43 [35-70] | Ref | Ref | ||
| 1 (n = 77) | 30 [25-35] | 0.1291 | 2.16 [1.33-3.48] | 0.0017 | |
| 2 (n = 78) | 26 [19-34] | 0.0490 | 2.30 [1.41-3.74] | 0.0008 | |
| 3 (n = 37) | 15 [10-21] | < 0.0001 | 6.02 [3.51-10.33] | < 0.0001 | |
| 4 (n = 8) | 13 [4-24] | < 0.0001 | 9.69 [3.86-24.36] | < 0.0001 |
Firstly, our findings confirm previously published results[1,2], the “6&12” score can classify survival among recommended TACE candidates. Its prognostic performance was similar within our cohort compared to Wang et al[2] original study [3-year AUROC values: 0.64 (0.58-0.71) vs 0.65 (0.61, 0.70); C-indices: 0.66 (0.58-0.74) vs 0.66 (0.63, 0.69) (Table 4)], and higher than that observed in this nationwide Chinese cohort[1] [c-index: 0.58 (0.56, 0.60)]. Moreover, HCC patients with the highest tumor burden [sum of largest tumor size (cm) and number exceeding 12] have a median survival of 15 mo similar to Wang et al[1]’s manuscript. Thus, this model can also identify within our population a subgroup of patients with poor prognosis who may not achieve benefit from TACE. The “6&12” risk stratification into three subgroups is relevant. Indeed, the first one (sum of tumor size and number not exceeding six) identifies TACE candidates with long-term survival especially those who may achieve a complete necrosis after this treatment[14,15]. Moreover, TACE is also an effective therapy for the second subgroup (sum of tumor size and number above six and not exceeding twelve), which has clear boundaries unlike intermediate stage subclassifications[16,17] that divide tumor burden according to the up-to-seven criteria (within/out).
Secondly, in our study the “6&12” score prognostic value is comparable to that of other systems, but most of these models cannot be used to guide treatment decision directly. “6&12” simplicity outweighs other systems for a current clinical practice including models with online calculator[5]. Indeed, therapeutic management is determined using a multidisciplinary approach and control of different published prognostic scores for TACE by clinicians (surgeons, oncologists, hepatologists and radiologists) is very unusual. By adding “the sum of largest tumor size and number”, it is true that consensus is easy to achieve among all clinicians. Moreover, other scores[9] encompass other baseline features that are likely to impact OS such as morphology of the tumor[18], but those parameters are not routinely recorded, which limits their use.
Thirdly, TACE should be limited to HCC patients with preserved liver function, and our results also highlight the importance of liver function in our population that included only recommended TACE candidates. Our patients are older, with more cirrhotic patients, and more alcohol-related diseases. This probably explains the differences in survival observed between this multicenter French cohort and Wang et al[2] original study, with OS ranging from 31.0 to 15.0 mo compared to 43.3 to 16.8 mo (according to “6&12” score), respectively. However, OS observed in our cohort was comparable to that of this nationwide Chinese cohort[1] including a more heterogeneous population with OS ranging from 31.3 to 18.5 mo.
Fourthly, Wang et al[19] findings on ABCR score are not surprising. This model designed for further TACE combines four parameters (AFP serum level, BCLC stage, change in Child-Pugh grade, and radiological tumor Response), but unlike ART[20,21] (assessment for re-treatment with TACE) model the highest coefficient is assigned to radiological tumor response.
In summary, in this multicenter French HCC cohort different staging/scoring systems classify survival among recommended TACE candidates with a similar predictive power. However, “6&12” score simplicity and ability in patients’ stratification outperform other systems for a routine clinical practice.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Montasser IF S-Editor: Liu M L-Editor: A P-Editor: Li JH
| 1. | Wang ZX, Wang EX, Bai W, Xia DD, Mu W, Li J, Yang QY, Huang M, Xu GH, Sun JH, Li HL, Zhao H, Wu JB, Yang SF, Li JP, Li ZX, Zhang CQ, Zhu XL, Zheng YB, Wang QH, Li J, Yuan J, Li XM, Niu J, Yin ZX, Xia JL, Fan DM, Han GH, On Behalf Of China Hcc-Tace Study Group. Validation and evaluation of clinical prediction systems for first and repeated transarterial chemoembolization in unresectable hepatocellular carcinoma: A Chinese multicenter retrospective study. World J Gastroenterol. 2020;26:657-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Wang Q, Xia D, Bai W, Wang E, Sun J, Huang M, Mu W, Yin G, Li H, Zhao H, Li J, Zhang C, Zhu X, Wu J, Li J, Gong W, Li Z, Lin Z, Pan X, Shi H, Shao G, Liu J, Yang S, Zheng Y, Xu J, Song J, Wang W, Wang Z, Zhang Y, Ding R, Zhang H, Yu H, Zheng L, Gu W, You N, Wang G, Zhang S, Feng L, Liu L, Zhang P, Li X, Chen J, Xu T, Zhou W, Zeng H, Zhang Y, Huang W, Jiang W, Zhang W, Shao W, Li L, Niu J, Yuan J, Li X, Lv Y, Li K, Yin Z, Xia J, Fan D, Han G; China HCC-TACE Study Group. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: A multicentre observational study. J Hepatol. 2019;70:893-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 3. | Memon K, Kulik L, Lewandowski RJ, Wang E, Riaz A, Ryu RK, Sato KT, Marshall K, Gupta R, Nikolaidis P, Miller FH, Yaghmai V, Senthilnathan S, Baker T, Gates VL, Abecassis M, Benson AB, Mulcahy MF, Omary RA, Salem R. Radiographic response to locoregional therapy in hepatocellular carcinoma predicts patient survival times. Gastroenterology. 2011;141:526-535, 535.e1-535.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Kim BK, Kim KA, Park JY, Ahn SH, Chon CY, Han KH, Kim SU, Kim MJ. Prospective comparison of prognostic values of modified Response Evaluation Criteria in Solid Tumours with European Association for the Study of the Liver criteria in hepatocellular carcinoma following chemoembolisation. Eur J Cancer. 2013;49:826-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Cappelli A, Cucchetti A, Cabibbo G, Mosconi C, Maida M, Attardo S, Pettinari I, Pinna AD, Golfieri R. Refining prognosis after trans-arterial chemo-embolization for hepatocellular carcinoma. Liver Int. 2016;36:729-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Bourlière M, Pénaranda G, Adhoute X, Bronowicki JP. The "six-and-twelve score" for TACE treatment: Does it really help us? J Hepatol. 2019;71:1051-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4111] [Article Influence: 587.3] [Reference Citation Analysis (6)] |
| 8. | Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1962] [Cited by in RCA: 2017] [Article Influence: 201.7] [Reference Citation Analysis (0)] |
| 9. | Adhoute X, Pénaranda G, Raoul JL, Bollon E, Pol B, Letreut YP, Perrier H, Bayle O, Monnet O, Beaurain P, Muller C, Hardwigsen J, Lefolgoc G, Castellani P, Bronowicki JP, Bourlière M. NIACE score for hepatocellular carcinoma patients treated by surgery or transarterial chemoembolization. Eur J Gastroenterol Hepatol. 2017;29:706-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146:1691-700.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 543] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 11. | A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 963] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 12. | Liu PH, Hsu CY, Hsia CY, Lee YH, Huang YH, Su CW, Lee FY, Lin HC, Huo TI. Proposal and validation of a new model to estimate survival for hepatocellular carcinoma patients. Eur J Cancer. 2016;63:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P; Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1574] [Article Influence: 92.6] [Reference Citation Analysis (1)] |
| 14. | Golfieri R, Cappelli A, Cucchetti A, Piscaglia F, Carpenzano M, Peri E, Ravaioli M, D'Errico-Grigioni A, Pinna AD, Bolondi L. Efficacy of selective transarterial chemoembolization in inducing tumor necrosis in small (<5 cm) hepatocellular carcinomas. Hepatology. 2011;53:1580-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 15. | Allard MA, Sebagh M, Ruiz A, Guettier C, Paule B, Vibert E, Cunha AS, Cherqui D, Samuel D, Bismuth H, Castaing D, Adam R. Does pathological response after transarterial chemoembolization for hepatocellular carcinoma in cirrhotic patients with cirrhosis predict outcome after liver resection or transplantation? J Hepatol. 2015;63:83-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 16. | Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, Raoul JL, Sangro B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 303] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 17. | Kudo M, Arizumi T, Ueshima K, Sakurai T, Kitano M, Nishida N. Subclassification of BCLC B Stage Hepatocellular Carcinoma and Treatment Strategies: Proposal of Modified Bolondi's Subclassification (Kinki Criteria). Dig Dis. 2015;33:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 18. | Kim HY, Park JW, Joo J, Jung SJ, An S, Woo SM, Kim HB, Koh YH, Lee WJ, Kim CM. Severity and timing of progression predict refractoriness to transarterial chemoembolization in hepatocellular carcinoma. J Gastroenterol Hepatol. 2012;27:1051-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Adhoute X, Penaranda G, Naude S, Raoul JL, Perrier H, Bayle O, Monnet O, Beaurain P, Bazin C, Pol B, Folgoc GL, Castellani P, Bronowicki JP, Bourlière M. Retreatment with TACE: the ABCR SCORE, an aid to the decision-making process. J Hepatol. 2015;62:855-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 20. | Sieghart W, Hucke F, Pinter M, Graziadei I, Vogel W, Müller C, Heinzl H, Trauner M, Peck-Radosavljevic M. The ART of decision making: retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology. 2013;57:2261-2273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 270] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 21. | Adhoute X, Penaranda G, Castellani P, Perrier H, Bourliere M. Recommendations for the use of chemoembolization in patients with hepatocellular carcinoma: Usefulness of scoring system? World J Hepatol. 2015;7:521-531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |