Published online Aug 27, 2020. doi: 10.4254/wjh.v12.i8.519
Peer-review started: March 31, 2020
First decision: July 5, 2020
Revised: July 8, 2020
Accepted: July 26, 2020
Article in press: July 26, 2020
Published online: August 27, 2020
Processing time: 146 Days and 17.4 Hours
Non-islet cell tumor hypoglycemia (NICTH) is a rare cause of persistent hypoglycemia seen in patients with hepatocellular carcinoma (HCC). It is likely to be underdiagnosed especially in the patients with poor hepatic function and malnutrition. Herein, we report a rare case of NICTH as the initial presentation of HCC in a patient with chronic hypoglycemia due to end-stage liver cirrhosis.
A 62-year-old male with chronic fasting hypoglycemia secondary to end-stage hepatitis C-related cirrhosis, presented with altered mental status and dizziness. He was found to have severe hypoglycemia refractory to glucose supplements. Imaging studies and biopsy discovered well differentiated HCC without metastasis. Further evaluation showed low insulin, C-peptide and beta-hydroxybutyrate along with a high insulin-like growth factor-2/insulin-like growth factor ratio, consistent with the diagnosis of NICTH. As patient was not a candidate for surgical resection or chemotherapy, he was started on prednisolone with some improvements in the glucose homeostasis, but soon decompensated after a superimposed hospital acquired pneumonia.
NICTH can occur as the sole initial presentation of HCC and is often difficult to correct without tumor removal. Clinicians should maintain high clinical suspicion for early recognition of paraneoplastic NICTH in patients at risk for HCC, even those with chronic fasting hypoglycemia in the setting of severe hepatic failure and malnutrition.
Core tip: Paraneoplastic Non-islet cell tumor hypoglycemia can occur as an initial presentation in patients with hepatocellular carcinoma and is often difficult to correct. It is tend to be underdiagnosed because patients often developed tolerance to chronic fasting hypoglycemia secondary to advanced liver cirrhosis. A ratio of insulin-like growth factor-2/insulin-like growth factor-1 above 10 is often found if non-islet cell tumor hypoglycemia is induced by overproduction of incompletely processed insulin-like growth factor-2. Oral corticosteroids and frequent high carbohydrate meals are often recommended but the outcome is unfavorable in general if tumor removal is not possible.
- Citation: Yu B, Douli R, Suarez JA, Gutierrez VP, Aldiabat M, Khan M. Non-islet cell tumor hypoglycemia as an initial presentation of hepatocellular carcinoma coupled with end-stage liver cirrhosis: A case report and review of literature. World J Hepatol 2020; 12(8): 519-524
- URL: https://www.wjgnet.com/1948-5182/full/v12/i8/519.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i8.519
Non-islet cell tumor hypoglycemia (NICTH) is a rare paraneoplastic complication associated with malignancies of both epithelial and mesenchymal origin. One of the most common epithelial tumors is hepatocellular carcinoma (HCC)[1,2]. Hypoglycemia is induced either by tumor consumption of glucose (type A) or overproduction of incompletely processed insulin-like growth factor-2 (IGF-2) (type B), while levels of insulin, C-peptide, pro-insulin, and beta-hydroxybutyrate are suppressed[3]. NICTH occurs in 4% to 27% of patients with HCC[4]. However the actual prevalence might be underestimated due to limited availability of testing for IGF-2. In addition, the etiologies of hypoglycemia in HCC patients are often multifactorial. Many patients might have developed tolerance to chronic fasting hypoglycemia due to long term poor hepatic function and nutritional status at the time of discovery of HCC. Here we report a case of persistent NICTH as the initial presentation in a patient with newly diagnosed HCC overlapped with end-stage liver cirrhosis.
A 62-year-old Hispanic male with long-standing hepatitis C-related cirrhosis was brought to the emergency room on December 7, 2019 due to 2 episodes of altered mental status and non-vertiginous dizziness witnessed by his family. He also reported an unintentional 1-kg weight loss over the past 1 mo.
There was no history of loss of consciousness, falls, or head trauma. He was first found to have hepatitis C infection with concurrent liver cirrhosis and portal hypertension in 2015. Viral load became undetectable after the completion of antiviral therapy but the patient lost follow-up ever since July 2018. Child-Pugh score during the last outpatient visit was 8 (class B). AFP was within the normal limit. No signs of malignancy were found on liver ultrasound.
On physical exam, he was all the time conscious and had full ability to communicate. Vital signs were within normal limits. Rest of the physical exam was significant for cachectic appearance, jaundice, and bilateral lower extremity edema up to the knee.
In the emergency room, his blood glucose was detected to be 26 mg/dL. He denied poor oral intake or history of diabetes, alcohol abuse or illicit drug use. Of note, his blood glucose level tended to be on the lower side (75-85 mg/dL) seen in the records of several outpatient visits before he lost follow-up. The blood glucose level was corrected by two immediate intravenous 50% dextrose pushes, but dropped again down to 10 mg/dL in 2 h for which continuous 10% dextrose infusion was started and the patient was instructed to consume frequent carbohydrate-rich snacks. However, recurrent hypoglycemic attacks still occurred since admission that required multiple IV 50% dextrose and glucagon pushes.
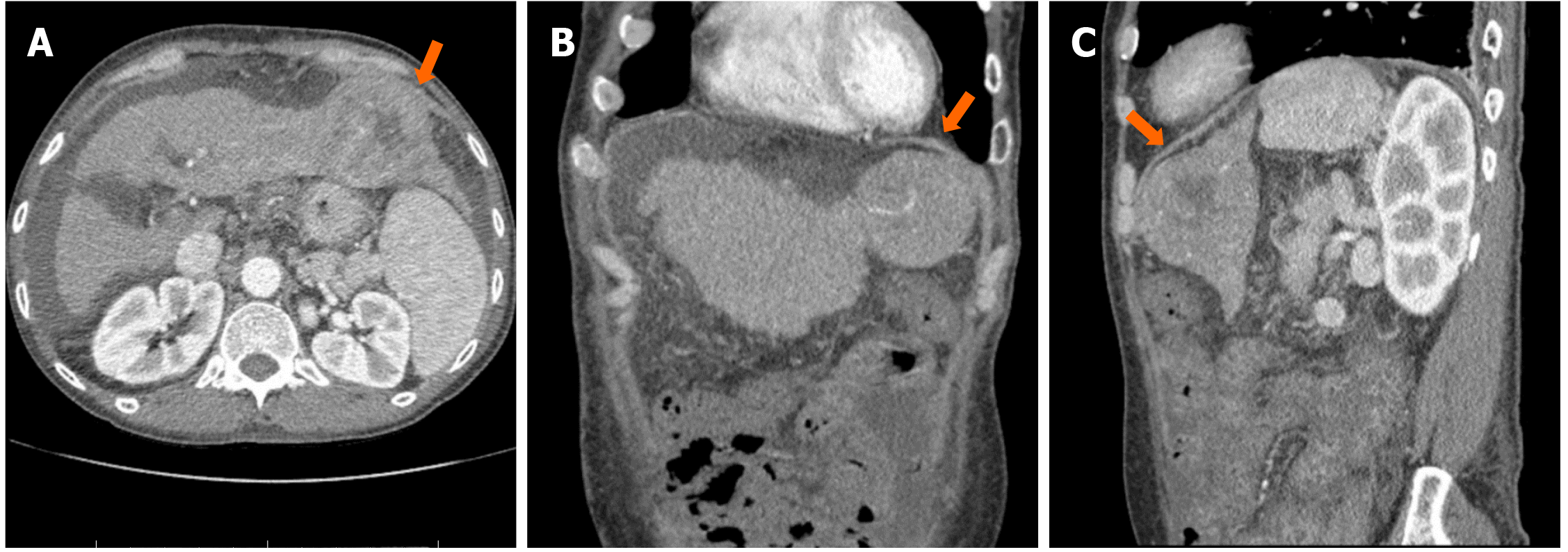
Laboratory evaluation of hypoglycemia showed undetectable insulin [< 0.4 µU/mL (2.6-24.9 µU/mL)], low C-peptide [0.2 ng/mL (1.1-4.4 ng/mL)], lower normal pro-insulin [1.3 pmol/L (0-10.0 pmol/L)], and undetectable beta-hydroxybutyrate [< 0.1 mg/dL (0.2-2.8 mg/dL)], excluding the possibility of insulinoma. Sulfonylurea screen test was negative. Adrenal insufficiency was also unlikely due to a high serum cortisol concentration. His hepatic function deteriorated [INR 2.8; albumin 2.9 g/dL (3.5-5.2 g/dL); total bilirubin 3.76 mg/dL (0.2-1.2 mg/dL); aspartate transaminase 145 U/L (< 40 U/L); alanine transaminase 93 U/L (< 41 U/L); alkaline phosphatase 263 U/L (40-130 U/L)]. Hepatic encephalopathy was also suspected due to high ammonia level [101 µmol/L (16-60 µmol/L)]. Child-Pugh score was calculated to be 11 (class C). AFP level was found to be elevated [108 ng/mL (< 8.3 ng/mL)]. Computed tomography of the abdomen with contrast showed cirrhosis and there was a centrally necrotic mass in the left hepatic lobe, measuring 6.7 cm × 6.5 cm (Figure 1). Three-phase liver computed tomography scan demonstrated suboptimal arterial phase enhancement due to the timing of the contrast with washout on delayed phase of the study. A subsequent biopsy confirmed the diagnosis of well differentiated HCC. No metastasis was found on bone scan. Therefore, NICTH was suspected. To establish the diagnosis, serum insulin-like growth factor-1 (IGF-1), IGF-2, and insulin-like growth factor-binding protein 3, the major binding protein for IGF-2 were measured. IGF-1 was suppressed [14 ng/mL (49-214 ng/mL)], IGF-2 was lower normal [303 ng/mL (300-960 ng/mL)], insulin-like growth factor-binding protein 3 was slightly decreased [2.2 µg/mL (2.6-4.8 µg/mL)], and the IGF-2/IGF-1 ratio was 21.6 (> 10), consistent with the diagnosis of NICTH.
NICTH (type B); well differentiated HCC, Barcelona Clinic Liver Cancer Stage D; decompensated liver cirrhosis, Child-Pugh Class C.
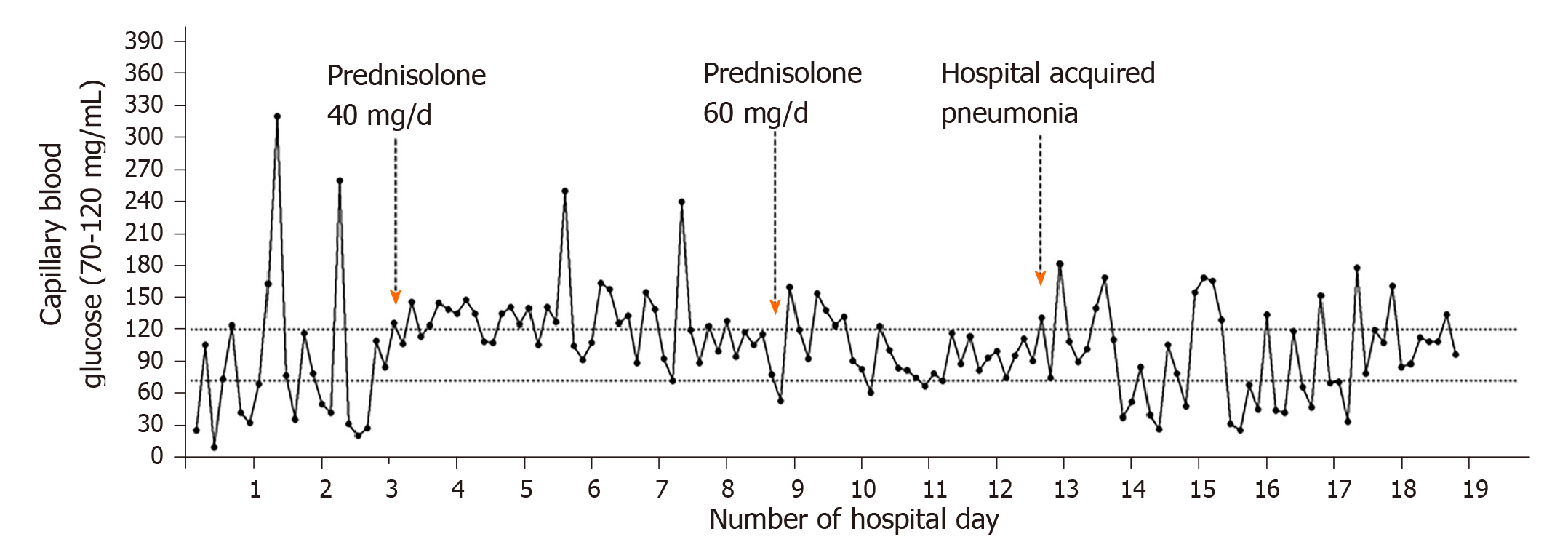
The patient was not a candidate of transplant, surgical resection, or palliative chemotherapy due to the baseline poor hepatic function. He was started on oral prednisolone with a dose titrated up to 60 mg daily. There were less hypoglycemic episodes and the patient showed improvements in the severity of hypoglycemia. However, he still required on and off glucose supplements to maintain glucose homeostasis. While waiting for the trial of trans-arterial chemoembolization and radiotherapy, he developed hospital-acquired pneumonia. And concurrently his plasma glucose dropped and became difficult to correct again (Figure 2).
The patient opted for inpatient hospice care and died of septic shock on day 19 of hospitalization.
NICTH is a rare complication seen in patients with HCC. In the present case, the patient had advanced cirrhosis without regular follow up for a year, so it’s unclear when the HCC first developed. Interestingly, acute hypoglycemic encephalopathy occurred as the sole initial clinical symptom prior to the diagnosis of HCC. Different from a few previously reported cases[5-9], our patient had a very poor hepatic function and nutritional status which could both contribute to his hypoglycemia to some extent. He might have developed chronic hypoglycemia with diminished awareness during the past year since there were no signs of sympathetic activation. This might obscure other underlying causes of hypoglycemia if patient was not assessed thoroughly. However, his glucose level fluctuated drastically and was very difficult to correct. Further investigation for NICTH is merited given high risk of malignancy.
Two types of NICTH (type A and B) are seen in HCC patients[10]. Type A often occurs at the terminal stage of disease when there is an increased glucose consumption by the tumor on top of a progressive reduction in glucose supply due to hepatic failure on the residual liver tissue and in part due to malnutrition. The tumor mass is usually rapid growing and poorly differentiated, associated with severe anorexia, muscle wasting and weight loss. But hypoglycemia is often mild and relatively easier to correct[11,12]. Type B, less common than type A, is related to an overproduction of IGF-2 and its precursors by the tumor. It often occurs at the earlier course of the disease and is thought to be a paraneoplastic syndrome. The severity of hypoglycemia is predominant and is often difficult to control. Glucose utilization by the tumor might also contribute to the hypoglycemia but is not a significant pathway[9]. The excess of IGF-2 Messenger RNA overwhelms the enzyme transforming pro-IGF-2 to mature IGF-2, thus producing various sizes of incompletely processed and unprocessed pro-IGF-2, the so called “big IGF-2”[3,13]. Normally most of serum IGF-2 is transported in the form of a 150 kDa ternary complex together with insulin-like growth factor-binding protein 3 and acid-labile sub-unit. But the “big IGF-2” mainly forms a 50 kDa binary complex with only insulin-like growth factor-binding protein 3. These binary complexes have a higher biological activity and can readily cross the capillary membrane to interact with insulin receptors in the liver, adipose tissue, and skeletal muscle due to their smaller size, leading to more glucose uptake and inhibition of gluconeogenesis[14,15]. By interacting with the IGF-1 receptors in the hypothalamus, the excess of pro-IGF-2 and IGF-2 inhibits the secretion of growth hormone, which in turn suppresses the production of IGF-1, insulin-like growth factor-binding protein 3, and acid-labile sub-unit. Therefore, more amount of free IGF-2 might gain access to the target tissue[16-19].
As to our patient, IGF-2 was inappropriately normal for the extremely low IGF-1 level. An IGF-2/IGF-1 ratio greater than 10 has been proposed to be enough to confirm the diagnosis of NICTH[16,20,21]. IGF-2 might be falsely normal in our patient because the sample was collected after the first dose of prednisolone was administered, which was able to inhibit the production of IGF-2[22]. In addition, serum IGF-2 levels in NICTH are often not elevated partially because most “big IGF-2” are not measured by common commercially available assay[16,23,24]. It has also been found by a few case reports that the levels of serum IGF-2 were decreased or normal in contrast to an increased pro-IGF-2 in NICTH[25,26]. Pro-IGF-2 was not measured in this patient because the test was not available in our setting. Although we are not able to entirely exclude the possibility of excessive glucose consumption by the tumor, the tumor mass was not extensive, only occupying part of the left lobe, and the level of AFP was not significantly elevated, indicating mild biological activities. The hepatic failure was more likely due to his advanced cirrhosis rather than the tumor. Therefore, we believe that our case fits more into type B rather than type A NICTH.
Priority of management of NICTH is still tumor resection. In inoperable patients, several treatment options of local tumor cytoreduction are recommended, including percutaneous ethanol injection and trans-arterial chemoembolization[27,28]. Systemic chemotherapy, such as Sorafenib or FOLFOX (oxaliplatin and 5-fluorouracil/leucovorin), has also been showed to be effective[29]. In addition, emerging drugs that directly inhibit the IGF signals (PI3K-AKT-TOR or RAF-MEK-ERK) are under investigation[30]. In case that the primary malignancy cannot be treated, palliative medical management can be chosen. Glucocorticoid together with frequent high carbohydrate meals and IV glucose infusions is an ideal option to achieve long-term prevention of hypoglycemia. Glucocorticoid, on one hand, stimulates hepatic gluconeogenesis and inhibits peripheral glucose uptake; on the other hand, can reduce the level of “big IGF-2” either by decreasing tumor production or by promoting the maturation of pro-IGF-2 and the formation of normal ternary complexes[22,31-33]. Other than glucocorticoid, glucagon, growth hormone, and octreotide infusion are also recommended, but their effects are transient and limited[2,8,34,35]. Our patient initially showed responses to high-dose prednisolone, but it failed to last for a long time mainly because of a poor hepatic reserve from cirrhosis. And the concurrent sepsis and pneumonia further destroyed patient’s ability to maintain the euglycemic status.
In conclusion, paraneoplastic NICTH should be considered in the evaluation of refractory hypoinsulinemic hypoglycemia in patients with risk factors of HCC, even in the setting of chronic fasting hypoglycemia induced by severe hepatic failure and malnutrition. NICTH can occur as the only initial presentation of HCC. Oral corticosteroids and frequent high carbohydrate meals are often recommended but the outcome is unfavorable in general if tumor removal is not possible.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tanaka Y S-Editor: Zhang L L-Editor: A P-Editor: Li JH
| 1. | Marks V, Teale JD. Tumours producing hypoglycaemia. Diabetes Metab Rev. 1991;7:79-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Bodnar TW, Acevedo MJ, Pietropaolo M. Management of non-islet-cell tumor hypoglycemia: a clinical review. J Clin Endocrinol Metab. 2014;99:713-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 143] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 3. | Ishida S, Noda M, Kuzuya N, Kubo F, Yamada S, Yamanaka T, Isozaki O, Hizuka N, Kanazawa Y. Big insulin-like growth factor II-producing hepatocellular carcinoma associated with hypoglycemia. Intern Med. 1995;34:1201-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13557] [Article Influence: 677.9] [Reference Citation Analysis (1)] |
| 5. | Lau CI, Wang HC, Hsu WC. Hypoglycemic encephalopathy as the initial presentation of hepatic tumor: a case report. Neurologist. 2010;16:206-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Jha V, Borpujari P. Hypoglycaemia presenting as sole manifestation of hepatocellular carcinoma. Med J Armed Forces India. 2012;68:75-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Tsai CY, Chou SC, Liu HT, Lin JD, Lin YC. Persistent hypoglycemia as an early, atypical presentation of hepatocellular carcinoma: A case report and systematic review of the literature. Oncol Lett. 2014;8:1810-1814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Sharma M, Reddy DN, Kiat TC. Refractory Hypoglycemia Presenting as First Manifestation of Advanced Hepatocellular Carcinoma. ACG Case Rep J. 2014;2:50-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Zhou S, Jiang L, Sun M. Recurrent hypoglycemic coma as the initial and single clinical manifestation of advanced hepatocellular carcinoma. J Gastrointest Cancer. 2015;46:64-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | McFadzean AJ, Yeung RT. Further observations on hypoglycaemia in hepatocellular carcinoma. Am J Med. 1969;47:220-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 66] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Yeung RT. Hypoglycaemia in hepatocellular carcinoma: a review. Hong Kong Med J. 1997;3:297-301. [PubMed] |
| 12. | Sorlini M, Benini F, Cravarezza P, Romanelli G. Hypoglycemia, an atypical early sign of hepatocellular carcinoma. J Gastrointest Cancer. 2010;41:209-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Daughaday WH, Emanuele MA, Brooks MH, Barbato AL, Kapadia M, Rotwein P. Synthesis and secretion of insulin-like growth factor II by a leiomyosarcoma with associated hypoglycemia. N Engl J Med. 1988;319:1434-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 219] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Eastman RC, Carson RE, Orloff DG, Cochran CS, Perdue JF, Rechler MM, Lanau F, Roberts CT, Shapiro J, Roth J. Glucose utilization in a patient with hepatoma and hypoglycemia. Assessment by a positron emission tomography. J Clin Invest. 1992;89:1958-1963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Daughaday WH, Kapadia M. Significance of abnormal serum binding of insulin-like growth factor II in the development of hypoglycemia in patients with non-islet-cell tumors. Proc Natl Acad Sci USA. 1989;86:6778-6782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Teale JD, Marks V. Inappropriately elevated plasma insulin-like growth factor II in relation to suppressed insulin-like growth factor I in the diagnosis of non-islet cell tumour hypoglycaemia. Clin Endocrinol (Oxf). 1990;33:87-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Frohman LA, Downs TR, Chomczynski P. Regulation of growth hormone secretion. Front Neuroendocrinol. 1992;13:344-405. [PubMed] |
| 18. | Dynkevich Y, Rother KI, Whitford I, Qureshi S, Galiveeti S, Szulc AL, Danoff A, Breen TL, Kaviani N, Shanik MH, Leroith D, Vigneri R, Koch CA, Roth J. Tumors, IGF-2, and hypoglycemia: insights from the clinic, the laboratory, and the historical archive. Endocr Rev. 2013;34:798-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 19. | Rana P, Kim B. A Unique Case of IGF-2 Induced Hypoglycemia Associated with Hepatocellular Carcinoma. Case Rep Endocrinol. 2019;2019:4601484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER, Service FJ; Endocrine Society. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009;94:709-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 746] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 21. | Garla V, Sonani H, Palabindala V, Gomez-Sanchez C, Subauste J, Lien LF. Non-islet Cell Hypoglycemia: Case Series and Review of the Literature. Front Endocrinol (Lausanne). 2019;10:316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Teale JD, Marks V. Glucocorticoid therapy suppresses abnormal secretion of big IGF-II by non-islet cell tumours inducing hypoglycaemia (NICTH). Clin Endocrinol (Oxf). 1998;49:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Yonei Y, Tanaka M, Ozawa Y, Miyazaki K, Tsukada N, Inada S, Inagaki Y, Miyamoto K, Suzuki O, Okawa H. Primary hepatocellular carcinoma with severe hypoglycemia: involvement of insulin-like growth factors. Liver. 1992;12:90-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Forde JJ, Ewelukwa O, Brar T, Cabrera R. Intractable Fasting Hypoglycemia as a Manifestation of Hepatocellular Carcinoma. Case Reports Hepatol. 2017;2017:7465025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Zapf J, Futo E, Peter M, Froesch ER. Can "big" insulin-like growth factor II in serum of tumor patients account for the development of extrapancreatic tumor hypoglycemia? J Clin Invest. 1992;90:2574-2584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 116] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | van den Berg SAA, Krol CG. Pro-IGF2-induced hypoglycaemia associated with hepatocellular carcinoma. Endocrinol Diabetes Metab Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Saigal S, Nandeesh HP, Malhotra V, Sarin SK. A case of hepatocellular carcinoma associated with troublesome hypoglycemia: management by cytoreduction using percutaneous ethanol injection. Am J Gastroenterol. 1998;93:1380-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Whitsett M, Lindenmeyer CC, Shaw CM, Civan JM, Fenkel JM. Transarterial chemoembolization for palliation of paraneoplastic hypoglycemia in a patient with advanced hepatocellular carcinoma. J Vasc Interv Radiol. 2013;24:1918-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Huang JS, Chang PH. Refractory hypoglycemia controlled by systemic chemotherapy with advanced hepatocellular carcinoma: A case report. Oncol Lett. 2016;11:898-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Gualberto A, Pollak M. Emerging role of insulin-like growth factor receptor inhibitors in oncology: early clinical trial results and future directions. Oncogene. 2009;28:3009-3021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 223] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 31. | Baxter RC, Holman SR, Corbould A, Stranks S, Ho PJ, Braund W. Regulation of the insulin-like growth factors and their binding proteins by glucocorticoid and growth hormone in nonislet cell tumor hypoglycemia. J Clin Endocrinol Metab. 1995;80:2700-2708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Thipaporn T, Bubpha P, Varaphon V. Hepatocellular carcinoma with persistent hypoglycemia: successful treatment with corticosteroid and frequent high carbohydrate intake. J Med Assoc Thai. 2005;88:1941-1946. [PubMed] |
| 33. | de Groot JW, Rikhof B, van Doorn J, Bilo HJ, Alleman MA, Honkoop AH, van der Graaf WT. Non-islet cell tumour-induced hypoglycaemia: a review of the literature including two new cases. Endocr Relat Cancer. 2007;14:979-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 34. | Wing JR, Panz VR, Joffe BI, Kalk WJ, Seftel HC, Zapf J, Kew MC. Hypoglycemia in hepatocellular carcinoma: failure of short-term growth hormone administration to reduce enhanced glucose requirements. Metabolism. 1991;40:508-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |