Published online Jul 27, 2020. doi: 10.4254/wjh.v12.i7.399
Peer-review started: January 15, 2020
First decision: March 5, 2020
Revised: April 2, 2020
Accepted: May 19, 2020
Article in press: May 19, 2020
Published online: July 27, 2020
Processing time: 189 Days and 13.4 Hours
Percutaneous transluminal angioplasty and stenting represent an effective treatment for hepatic artery stenosis after liver transplantation. In the first year after stenting, approximately 22% of patients experience in-stent restenosis, increasing the risk of artery thrombosis and related complications, and 50% experience liver failure. Although angiography is an important tool for diagnosis and the planning of therapeutic interventions, it may raise doubts, especially in small-diameter arteries, and it provides low resolution rates compared with newer intravascular imaging methods, such as optical coherence tomography (OCT).
A 64-year-old male developed hepatic artery stenosis one year after orthotropic liver transplantation and was successfully treated with percutaneous transluminal angioplasty with stenting. Five months later, the Doppler ultrasound results indicated restenosis. Visceral arteriography confirmed hepatic artery tortuosity but was doubtful for significant in-stent restenosis (ISR) and intrahepatic flow reduction. To confirm ISR, identify the etiology and guide treatment, OCT was performed. OCT showed severe stenosis due to four mechanisms: Focal and partial stent fracture, late stent malapposition, in-stent neointimal hyperplasia, and neoatherosclerosis.
Intravascular diagnostic methods can be useful in evaluating cases in which initial angiography results are not sufficient to provide a proper diagnosis of significant stenosis, especially with regard to ISR. A wide range of diagnoses are provided by OCT, resulting in different treatment options. Interventional radiologists should consider intravascular diagnostic methods as additional tools for evaluating patients when visceral angiography results are unclear.
Core tip: This is the first case report of optical coherence tomography in the evaluation of in-stent restenosis in a transplant hepatic artery. In this case, optical coherence tomography proved to be valuable in grading the significance of stenosis, identifying its possible causes, and providing measures for choosing appropriate devices for re-treatment. In this case, this additional and modern tool helped in the diagnosis and the therapeutic planning after a doubtful angiography result.
- Citation: Galastri FL, Gilberto GM, Affonso BB, Valle LGM, Falsarella PM, Caixeta AM, Lima CA, Silva MJ, Pinheiro LL, Baptistella CDPA, Almeida MD, Garcia RG, Wolosker N, Nasser F. Diagnosis and management of hepatic artery in-stent restenosis after liver transplantation by optical coherence tomography: A case report. World J Hepatol 2020; 12(7): 399-405
- URL: https://www.wjgnet.com/1948-5182/full/v12/i7/399.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i7.399
Hepatic artery stenosis (HAS) is one of the most common vascular complications of liver transplantation and is a leading cause of hepatic artery thrombosis and allograft dysfunction[1]. HAS treatment consisting of percutaneous transluminal angioplasty with stenting (PTAS) has been largely adopted and proven to be effective, although it carries a risk of up to 22% for in-stent restenosis (ISR) in the first year and poses a therapeutic challenge related to the fact that up to 50% of cases result in liver failure[2].
ISR after liver transplantation is frequently attributed to neointimal proliferation. Doppler ultrasound (DUS) is the standard method for screening diagnosis combined with laboratory abnormalities[3]. Angiography is an important tool for confirming the diagnosis and planning optimum therapeutic intervention. However, because angiography only provides a two-dimensional view of a three-dimensional structure, it poorly estimates plaque volume, morphology and lesion severity. Moreover, angiography sometimes overestimates lumen dimensions and provides low resolution rates compared with newer intravascular imaging methods, such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT), especially in small-diameter arteries[4]. Additionally, considerable intra- and interobserver variability in the interpretation of stenosis severity has been observed with arteriography[5].
Several factors contribute to stent failures, and OCT can assess them with great imaging quality. One of these factors is neoatherosclerosis, which is observed by OCT as the presence of clusters of lipid-laden foamy macrophages with or without necrotic core formation and/or calcification within the neointimal tissue of stented segments[6]. Other possible findings are: Stent malapposition which is defined by the separation of at least one stent strut from the intimal surface of the arterial wall with evidence of blood behind the strut[7] and stent fracture which is complete separation of stent segments or separated stent struts without displacement[8].
OCT provides the highest resolution of all invasive imaging modalities, resulting in high-quality cross-sectional tomographic images of vessel architecture, and it demonstrates superiority compared with IVUS or angiography in terms of assessing lesions, particularly identifying thrombus, plaque erosion and rupture[9].
To our knowledge, this is the first case report of OCT used to evaluate ISR in a transplant hepatic artery. In this case, OCT proved to be valuable for grading the significance of stenosis, identifying its possible causes, and providing measures for choosing appropriate devices for retreatment.
A 64-year-old man developed, during clinical and ultrasound follow up, hepatic artery intra-stent restenosis.
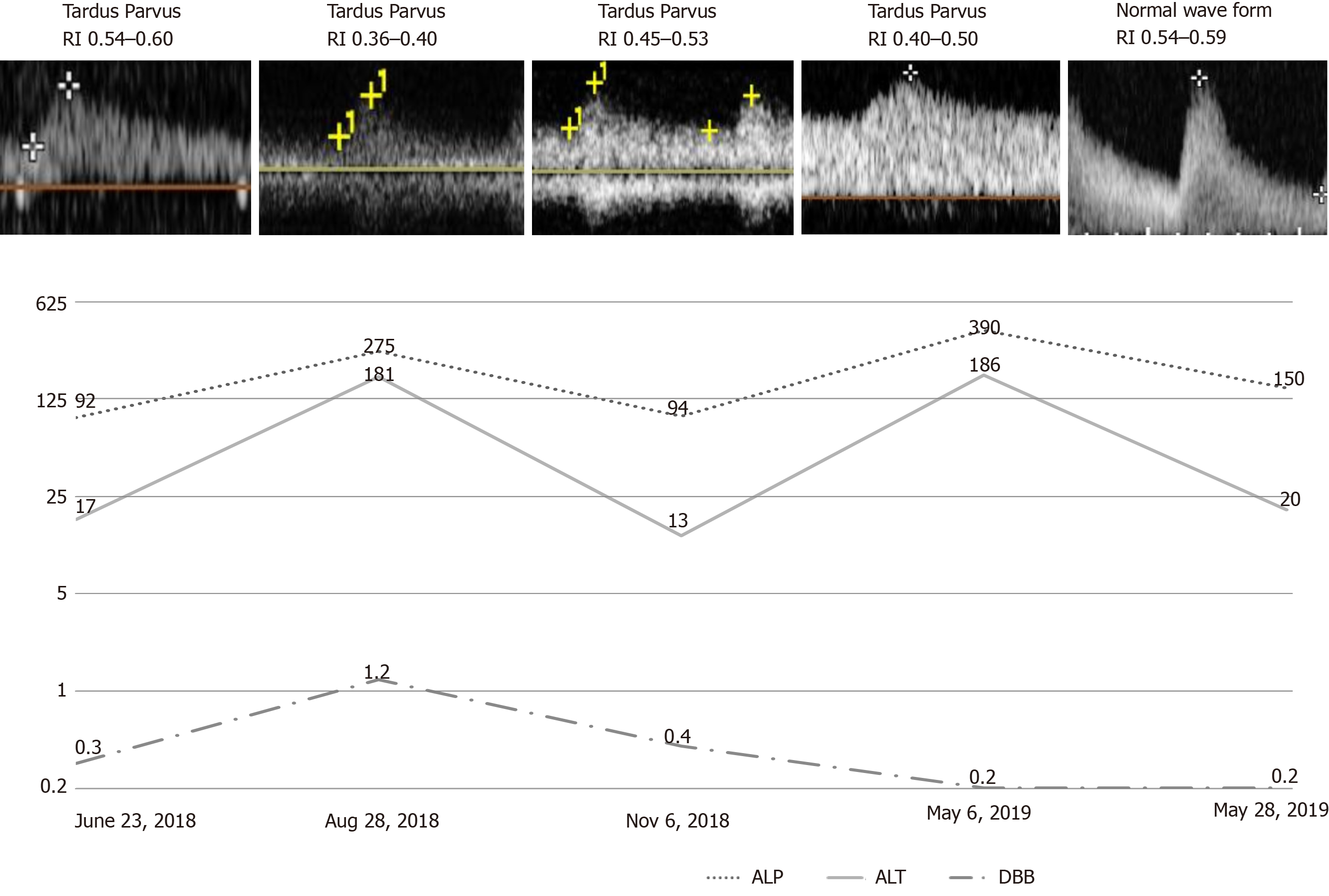
The patient had a history of orthotopic liver transplantation and was successfully treated with PTAS. Five months later, the patient presented with increased alkaline phosphatase and alanine aminotransferase levels and DUS indicated restenosis.
The patient had alcoholic liver cirrhosis previous to liver transplantation.
The patient was conscious, with good general health and normal vital signs, anicteric, eupneic and without fever. The abdomen was distended without signs of ascites.
Alkaline phosphatase level was 186 U/L and alanine aminotransferase level was 390 U/L.
DUS of the intrahepatic arteries showed a low resistance index, ranging from 0.4 to 0.5, indicating restenosis (Figure 1).
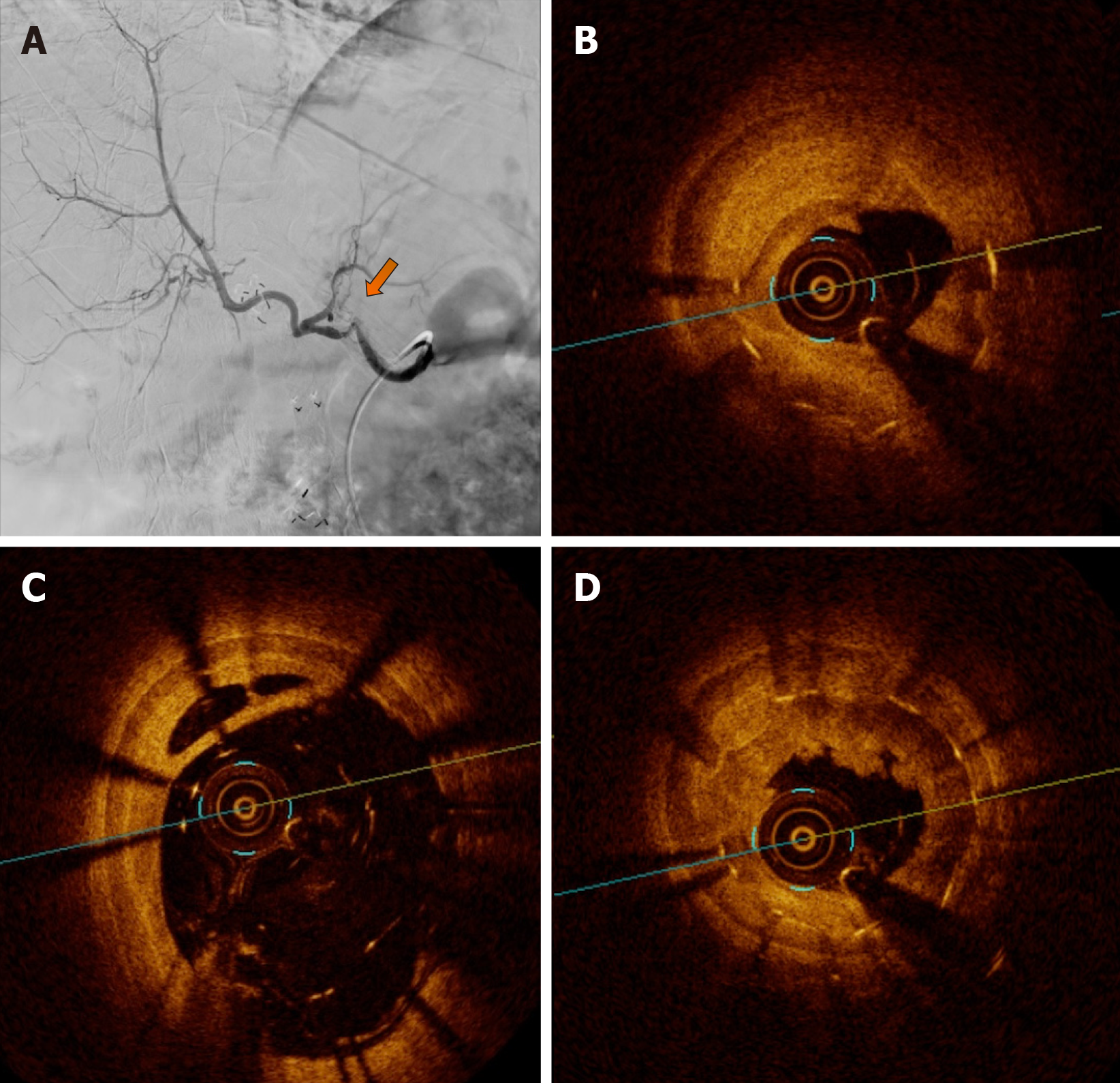
Visceral arteriography confirmed the tortuosity of the hepatic artery but showed doubtful significant intrastent diameter reduction and intrahepatic flow reduction (Figure 2). Two experienced interventional radiologists disagreed on the angiography results. To confirm ISR, identify the etiology and guide treatment, OCT was performed.
OCT showed severe stenosis (vessel luminal area: 9.8 mm2/minimal luminal area: 1.7 mm2/82% of area reduction) due to four mechanisms: Focal and partial stent fracture, late stent malapposition, in-stent neointimal hyperplasia, and neoatherosclerosis (Figure 2).
Hepatic artery intra-stent restenosis.
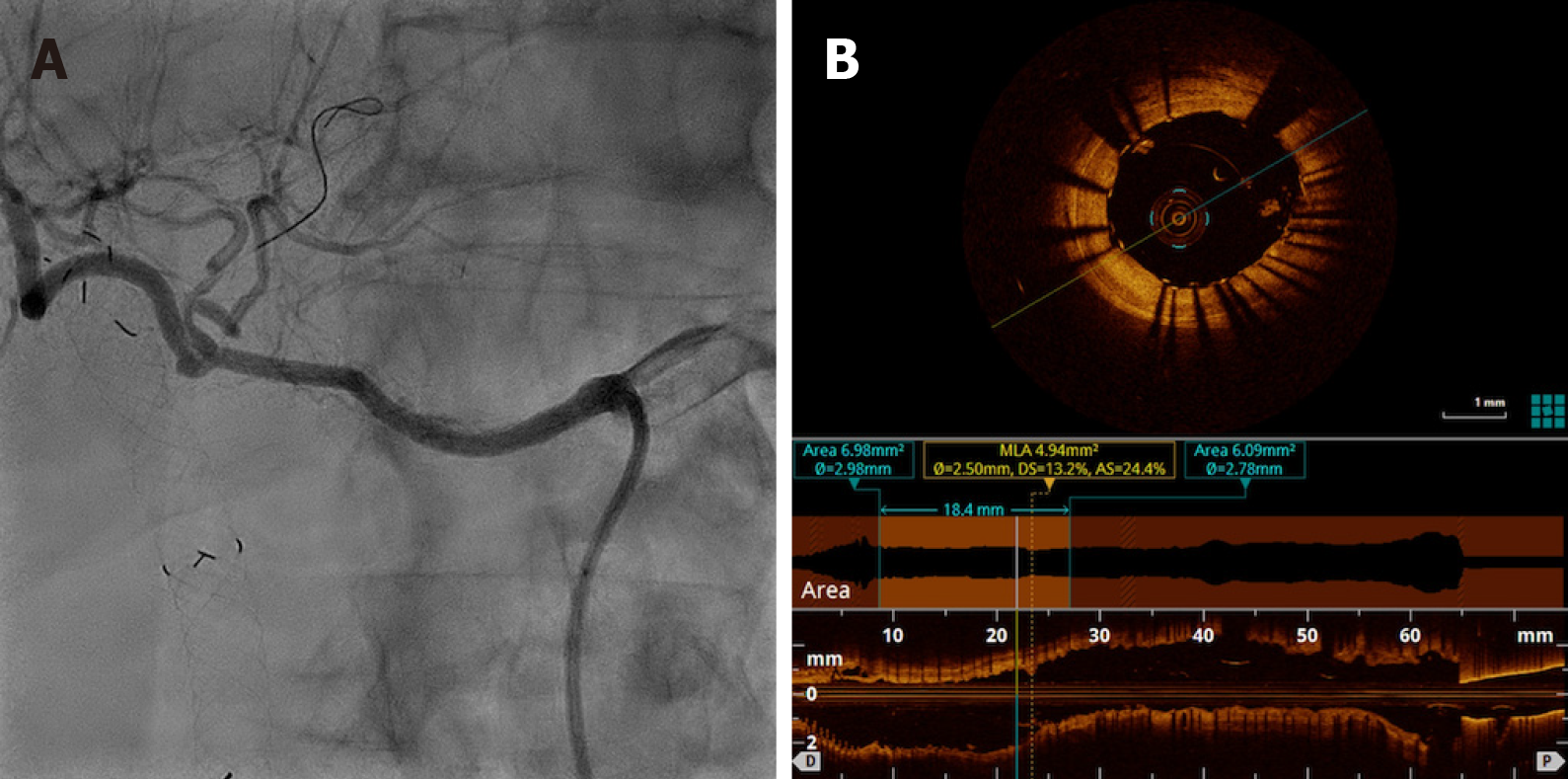
PTAS with a new drug-eluting stent (Sirolimus) Orsiro 3 mm × 30 mm (Biotronik) resulted in complete resolution of restenosis with improvements in intrahepatic perfusion (Figure 3).
Twenty days later, liver enzymes were normalized, and DUS waveforms were normal for the first time after liver transplantation. The resistance index ranged from 0.54 to 0.59 (Figure 1). In a 10-mo outpatient follow-up, the patient was free from symptoms, with normal liver enzymes.
The endovascular approach has become an alternative for the treatment of HAS due to its low morbidity, high patency rates similar to those of open surgery[10], low graft loss, and low mortality rates. Recent retrospective studies have shown that primary stenting may be the first option, with primary assisted patency rates up to 97% within 12 mo and 93% within 24 mo as well as a decrease in the need for reintervention[3,11].
ISR and occlusion are known complications of endovascular procedures that justify clinical follow-up and DUS in these patients. The development of intravascular diagnostic methods has shown that restenosis etiologies are not as simple as they were believed to be. IVUS and OCT enable better evaluation of ISR, and they should be considered for the detection of stent-related mechanical problems leading to restenosis in patients with coronary revascularization, therefore leading to class IIA and level C recommendations[12,13]. The wide range of differential diagnoses for ISR are due to endovascular diagnostic methods, including stent fracture, neoatherosclerosis, neointimal hyperplasia, late acquired stent malapposition, stent underexpansion, evagination, stent crush and edge dissection. Treatment options differ according to the diagnosis of ISR[13].
In our case, the patient presented with biochemical alterations and DUS parameters that suggested new arterial stenosis and probable ISR. Angiography showed tortuosity of the treated vessel, but this tool was limited in terms of measuring intrastent luminal reduction and intrahepatic contrast enhancement. Furthermore, angiography could not categorize the severity of the lesion, the presence of hemodynamic repercussion or the etiology of restenosis. OCT showed severe stenosis caused by stent fracture, late stent malapposition, neointimal hyperplasia and probable neoatherosclerosis tissue. These diagnoses and OCT measurements guided the treatment and stent choice.
Posttreatment angiography undoubtedly showed ISR resolution and improvements in hepatic perfusion with faster parenchymal enhancement and washout. Both interventional radiologists agreed with these findings. Similar to angiography, OCT confirmed an increased vessel area without residual stenosis and provided the finding of adequate stent apposition. DUS performed immediately postprocedure demonstrated normal intrahepatic wave forms and resistance index, and it was used as a basic follow-up test. Consistent with the results of the imaging exams, the patient progressed and demonstrated clinical and laboratory improvements.
The disagreement on the angiography evaluation by two experienced interventional radiologists and the urge to identify stent-related mechanical problems motivated the use of additional intravascular diagnostic methods. In our case, for the first time in the published medical literature, OCT has proven to be an effective additional method for elucidating doubtful lesions on visceral arteries after transplantation. Moreover, posttreatment OCT evaluation confirmed that the interventional radiologists were in agreement regarding the adequate results. Although OCT provides outstanding new information to the vascular territory, we understand that it has some limitations, such as the additional volume of contrast that is unavoidable, the imaging is not free from artifacts and it depends on the observer’s experience. In addition, it has a high cost and poor availability[14].
Angiography is the gold standard for evaluating patients with suspected restenosis; however, in some cases, this procedure can cast doubt. Intravascular diagnostic methods can be useful when evaluating such cases, especially with regard to in-stent restenosis. A wide range of diagnoses are provided by OCT, which results in different treatment options. Interventional radiologists should consider intravascular diagnostic methods as additional tools for evaluating patients when visceral angiography results are unclear.
OCT analysis assistance was by Eduardo Ufeni Sandes, BIOMED.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gerber DA, Govindarajan GK, Pan GD S-Editor: Dou Y L-Editor: Webster JR E-Editor: Wang LL
| 1. | Rostambeigi N, Hunter D, Duval S, Chinnakotla S, Golzarian J. Stent placement versus angioplasty for hepatic artery stenosis after liver transplant: a meta-analysis of case series. Eur Radiol. 2013;23:1323-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Sugi MD, Albadawi H, Knuttinen G, Naidu SG, Mathur AK, Moss AA, Oklu R. Transplant artery thrombosis and outcomes. Cardiovasc Diagn Ther. 2017;7:S219-S227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Pulitano C, Joseph D, Sandroussi C, Verran D, Strasser SI, Shackel NA, McCaughan GW, Crawford M. Hepatic artery stenosis after liver transplantation: is endovascular treatment always necessary? Liver Transpl. 2015;21:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Nakamura S, Mahon DJ, Maheswaran B, Gutfinger DE, Colombo A, Tobis JM. An explanation for discrepancy between angiographic and intravascular ultrasound measurements after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1995;25:633-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Thomas AC, Davies MJ, Dilly S, Dilly N, Franc F. Potential errors in the estimation of coronary arterial stenosis from clinical arteriography with reference to the shape of the coronary arterial lumen. Br Heart J. 1986;55:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 70] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Hong SJ, Lee SY, Hong MK. Clinical Implication of Optical Coherence Tomography-Based Neoatherosclerosis. J Korean Med Sci. 2017;32:1056-1061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Karalis I, Ahmed TA, Jukema JW. Late acquired stent malapposition: why, when and how to handle? Heart. 2012;98:1529-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Mohsen MK, Alqahtani A, Al Suwaidi J. Stent fracture: how frequently is it recognized? Heart Views. 2013;14:72-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Hamby BA, Ramirez DE, Loss GE, Bazan HA, Smith TA, Bluth E, Sternbergh WC. Endovascular treatment of hepatic artery stenosis after liver transplantation. J Vasc Surg. 2013;57:1067-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Le L, Terral W, Zea N, Bazan HA, Smith TA, Loss GE, Bluth E, Sternbergh WC. Primary stent placement for hepatic artery stenosis after liver transplantation. J Vasc Surg. 2015;62:704-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Vidjak V, Novačić K, Matijević F, Kavur L, Slavica M, Mrzljak A, Filipec-Kanižaj T, Leder NI, Škegro D. Percutaneous Endovascular Treatment for Hepatic Artery Stenosis after Liver Transplantation: The Role of Percutaneous Endovascular Treatment. Pol J Radiol. 2015;80:309-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Secco GG, Grattoni C, Parisi R, Oshoala K, Cremonesi A, Fattori R, Castriota F. Optical Coherence Tomography Guidance during Peripheral Vascular Intervention. Cardiovasc Intervent Radiol. 2015;38:768-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Juni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferović PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO. [2018 ESC/EACTS Guidelines on myocardial revascularization]. Kardiol Pol. 2018;76:1585-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 14. | Roleder T, Jąkała J, Kałuża GL, Partyka Ł, Proniewska K, Pociask E, Zasada W, Wojakowski W, Gąsior Z, Dudek D. The basics of intravascular optical coherence tomography. Postepy Kardiol Interwencyjnej. 2015;11:74-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |